Abstract
Platinum-based anticancer drugs, including cisplatin, carboplatin, oxaliplatin, nedaplatin, and lobaplatin, are heavily applied in chemotherapy regimens. However, the intrinsic or acquired resistance severely limit the clinical application of platinum-based treatment. The underlying mechanisms are incredibly complicated. Multiple transporters participate in the active transport of platinum-based antitumor agents, and the altered expression level, localization, or activity may severely decrease the cellular platinum accumulation. Detoxification components, which are commonly increasing in resistant tumor cells, can efficiently bind to platinum agents and prevent the formation of platinum–DNA adducts, but the adducts production is the determinant step for the cytotoxicity of platinum-based antitumor agents. Even if adequate adducts have formed, tumor cells still manage to survive through increased DNA repair processes or elevated apoptosis threshold. In addition, autophagy has a profound influence on platinum resistance. This review summarizes the critical participators of platinum resistance mechanisms mentioned above and highlights the most potential therapeutic targets or predicted markers. With a deeper understanding of the underlying resistance mechanisms, new solutions would be produced to extend the clinical application of platinum-based antitumor agents largely.
Keywords: platinum-based anticancer drugs, transporter, apoptosis, autophagy, DNA repair, resistance
Introduction
Ever since cisplatin, the first generation of platinum antitumor agents, was approved by the U.S. Food and Drug Administration for the treatment of testicular cancer, the development of platinum antitumor agents has explosively grown during the last forty years. The second-generation product carboplatin and the third-generation product oxaliplatin were approved worldwide in succession with enlarged spectrum or decreased toxicity (Wheate et al., 2010). Till today, cisplatin, carboplatin, and oxaliplatin are still extensively applied in the treatment of cancer.
Cisplatin (Figure 1) is a neutral, square planar coordination complex of platinum(II) coordinated to two chloride and two ammonia groups, where the chloride ligands are in the cis-geometry (Kartalou and Essigmann, 2001). Once inside the cell, cisplatin undergoes aquation to form [Pt(NH3)2Cl(OH2)]+ and [Pt(NH3)2(OH2)2]2+, therefore, it becomes more reactive to DNA (Jamieson and Lippard, 1999). It is approved for the treatment of advanced pancreatic cancer, breast cancer, non-small cell lung cancer (NSCLC), advanced bladder cancer et al. In the second-generation products, two chloride atoms are replaced by an oxygenated bidendate cyclobutane-dicarboxylate group to form carboplatin (cis-diammine-1,1′-cyclobutane dicarboxylate platinum II, Figure 1), which is easier to administrate and less toxic compared with cisplatin (McKeage, 1995). However, carboplatin has a similar anticancer spectrum with cisplatin, and the cross-resistance with cisplatin is observed in many cancer types. Oxaliplatin ({[oxalate(2-)-O, O′][1R,2R-cyclohexanediamine-N, N’] platinum-(II)}, Figure 1) belongs to the third generation of platinum-based antitumor agents, in which 1, 2-diaminocyclohexane (DACH) ligand substitutes for the amine groups of cisplatin. It was reported that oxaliplatin could produce fewer DNA adducts but caused higher cytotoxicity than cisplatin (Woynarowski et al., 2000), and it also shows stronger activity in colorectal and other gastrointestinal cancers, while cisplatin and carboplatin show no efficacy. Nedaplatin (cis-diammine (glycolato-O¹, O²)platinum, Figure 1), the second-generation platinum analogue, was first approved in Japan. It has approximately ten times as soluble in water as cisplatin and lower toxicity than cisplatin (Kuwahara et al., 2009; Zhong et al., 2018). Nedaplatin shows promising results in combination therapies (Li et al., 2017c; Peng et al., 2017; Takekuma et al., 2018). Lobaplatin (cis-[trans-1,2-cyclobutanebis(methylamine)][(S)-lactato-O1, O2]platinum(II), Figure 1), the third-generation platinum analogue, was approved in China for treatment of breast cancer, NSCLC, and chronic myelocytic leukemia (Wheate et al., 2010).
Figure 1.
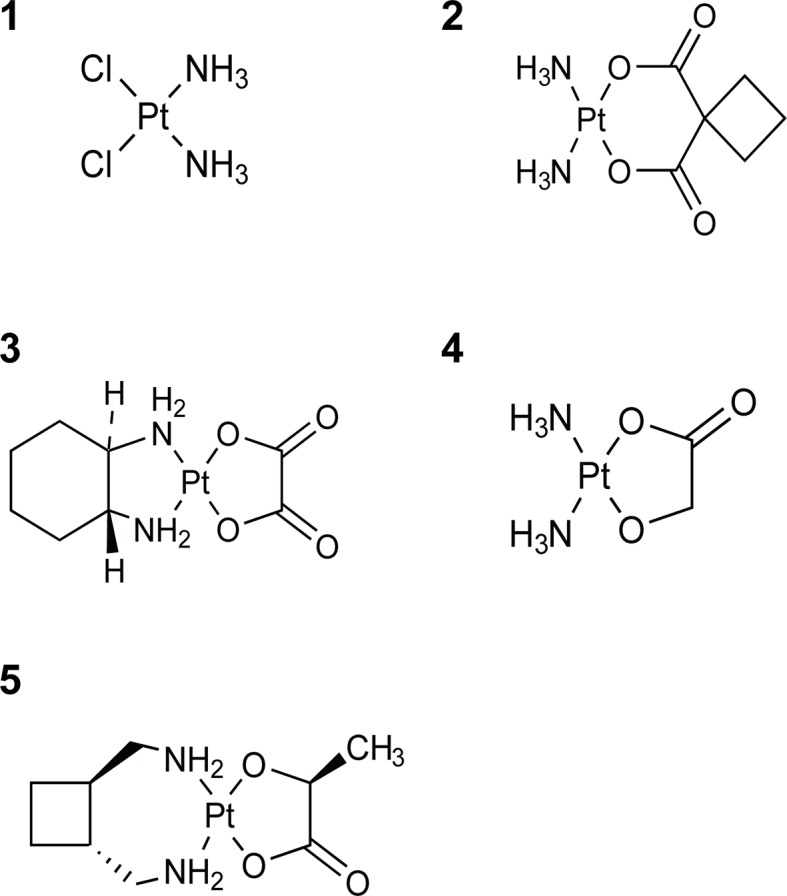
Chemical structures of platinum complexes. 1: Cisplatin; 2: Carboplatin; 3: Oxaliplatin; 4: Nedaplatin; 5: Lobaplatin.
It’s common to see patients who respond well to platinum treatment become resistant to platinum-based chemotherapy in a short time. The resistance result from (i) reduced cellular drug accumulation, (ii) increased detoxification system, (iii) increased DNA repair process, (iv) decreased apoptosis, and (v) autophagy as summarized in Figure 2. This work reviews the updated platinum-resistance mechanisms and the key participators.
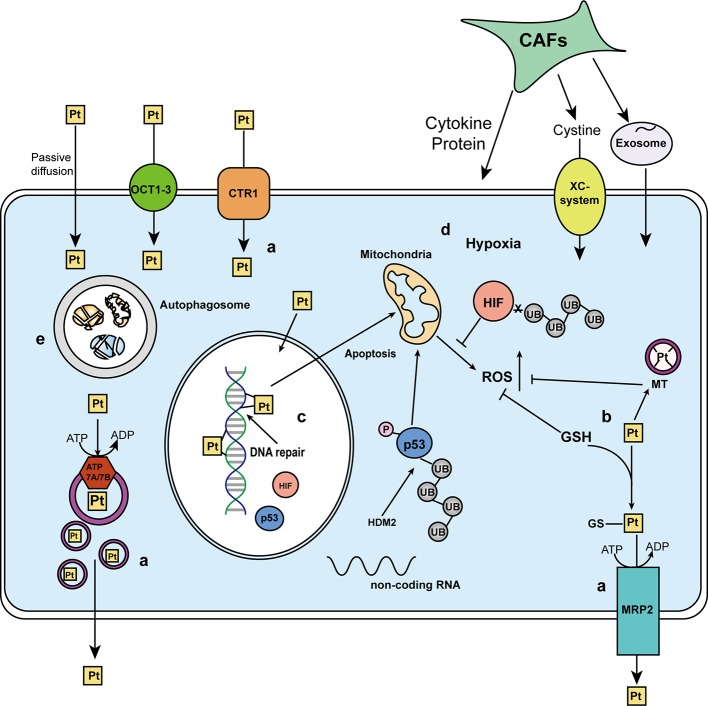
Figure 2.
A schematic of the mechanisms affecting platinum response. The response toward platinum-based antitumor agents can result from (a) cellular drug accumulation. Besides passive diffusion, the uptake of platinum agents is mediated by multiple transporters. Organic cation transporters (OCT1-3) and CTR1 mediated the influx, while ATP7A/7B and MRP2 participate in the isolation and efflux of platinum agents or GS-platinum complex. (b) Detoxification system. Platinum agents can be deactivated by binding to detoxification components, glutathione (GSH) and metallothionein (MT). (c) DNA repair process. The platinum atom can covalently bound to the N7 positions of purine bases to form the platinum-DNA adducts and induce cytotoxicity, but the DNA repair process could repair the damaged DNA lesion. (d) Apoptosis. Once the DNA repair fails or is overwhelmed by too many DNA lesions, apoptosis will be triggered. Mitochondria will generate excessed reactive oxygen species (ROS) to kill the cells, which might be neutralized by GSH and MT. p53 and tumor microenvironment [including hypoxia-induced hypoxia-inducible factor (HIF) and cancer-associated fibroblasts (CAFs)] play key regulatory roles in apoptosis. (e) Autophagy, a self-digestion process, has two sides in affecting platinum response.
Transporters Involved in Platinum Influx/Efflux
The accumulation of platinum antitumor agents inside the cells is the necessary assurance of cytotoxicity, so decreased influx or increased efflux is responsible for platinum resistance. For many years it has been assumed that platinum enters cells by passive diffusion and through gated channels (Gately and Howell, 1993). However, the role of active transport mediated by multiple transporters become prominent to platinum-uptake. Three urgent questions need explanations: (i) which transporters are responsible for platinum-uptake, (ii) how transporters change during drug-resistance development, and (iii) what can be done to reverse resistance by targeting transporters.
Solute Carrier Superfamily of Membrane Transporters
The solute carrier superfamily (SLCs) contains more than 300 members and 65 subfamilies (Perland and Fredriksson, 2017) such as the organic anion transporting polypeptides, organic anion transporters, and organic cation transporters (OCTs). Normally SLCs are expressed in the whole body to sustain the cellular homeostasis by mediating the transportation of endogenous substances and exogenous substances. However, the expression or the distribution of SLCs may change under the disease condition or due to the drug–drug interaction effect, and it will have a significant effect on the cellular uptake of therapeutic drugs then leads to the unsatisfied results (Zhou et al., 2017).
Cisplatin is a substrate of hOCT1 (SLC22A1), hOCT2 (SLC22A2), and hMATE1 (SLC47A1), and oxaliplatin is a substrate of hOCT2, hOCT3 (SLC22A3), hMATE1 (SLC47A1), and hMATE2-K (SLC47A2). Carboplatin and Nedaplatin are not transported by the transporters mentioned above (Yonezawa et al., 2006). However, some contradictory results were reported probably because of the different selection of verification models, as summarized in Table 1. The down-regulation, mislocation, or inhibited transport activity of OCTs can all reduce the intracellular platinum concentration. Gao et al. reported that omeprazole could decrease the protein level of OCT2, thus lead to reduced cellular accumulation of cisplatin (Gao et al., 2019). Buss et al. (2018) employed a novel fluorescent oxaliplatin derivative to discovered that OCT1 involves in oxaliplatin uptake in the sensitive but not in the resistant cell line, which may be a consequence of the altered localization of the transporter in resistant cells rather than the lower expression. Recent studies reported two crucial regulatory mechanisms of OCTs: phosphotyrosine-mediated activity regulation and epigenetic regulation. Sprowl and colleagues revealed that the phosphorylation status of Y362, mediated by the Src family kinase Yes1, might be essential for OCT2 function; hence tyrosine kinase inhibitor like dasatinib can inhibit oxaliplatin uptake and mitigate oxaliplatin-induced acute sensory neuropathy (Sprowl et al., 2016). Our previous work revealed the link between the epigenetic changes of OCT2 and oxaliplatin resistance in renal cell carcinoma (RCC) (Liu et al., 2016; Zhu et al., 2019). We found that hypermethylated CpG islands of OCT2 disrupted the interaction between MYC and the E-Box motif, further it would prevent MYC recruiting MLL1 to catalyze H3K4me3 at the OCT2 promoter. As a consequence, the transcriptional repression of OCT2 diminished oxaliplatin accumulation and failed the oxaliplatin treatment in RCC. But decitabine, a demethylating reagent, can efficiently reverse the hypermethylation of OCT2. Therefore, the combination therapy of decitabine and oxaliplatin is a promising treatment option to reactivate RCC to oxaliplatin. The results indicated that OCT2 demethylation cracks open oxaliplatin resistance in RCC (Aguilar, 2016) and it highlights the huge potentiality of targeting transporters in clinical application.
Table 1.
The influx/efflux transporters of cisplatin, carboplatin, oxaliplatin, nedaplatin, and lobaplatin.
| Solute carrier superfamily of membrane transporters | ATPases | ATP-binding cassette transporters |
References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLC22 families | SLC47 families | SCL31A families | |||||||||||
| OCT1 | OCT2 | OCT3 | MATE1 | MATE2K | CTR1 | CTR2 | ATP 7A | ATP 7B | MRP1 | MRP2 | MRP4 | (Holzer et al., 2006; Yonezawa et al., 2006); (Taniguchi et al., 1996; Borst et al., 2000; Samimi et al., 2004; Yokoo et al., 2007; Safaei et al., 2008; Blair et al., 2009; Beretta et al., 2010; Burger et al., 2010; Myint et al., 2015) | |
| Cisplatin | ± | ₊ | − | ₊ | − | ₊ | * | + | + | − | + | + | |
| Oxaliplatin | ± | + | + | + | + | + | * | + | + | + | + | + | |
| Carboplatin | − | − | − | − | − | + | * | + | + | / | / | / | |
| Nedaplatin | − | − | − | − | − | / | / | / | / | / | / | / | |
| Lobaplatin | / | / | / | / | / | / | / | / | / | / | / | / | |
+, substrate; −, not the substrate; ±, contradictory results; *, influenced accumulation but not proven transported substrates; /, not documented.
Copper Transporter 1/2
Copper transporter 1 (CTR1) ubiquitously expresses in tissues and is required for high-affinity copper uptake. It is widely acknowledging that CTR1 transports cisplatin, oxaliplatin, and carboplatin (Holzer et al., 2006). Deletion of the yeast CTR1 gene reduces the intracellular accumulation of cisplatin and leads to cisplatin-resistance (Ishida et al., 2002), while forced overexpression of hCTR1 sensitizes small cell lung cancer cells to cisplatin, carboplatin, and oxaliplatin (Song et al., 2004). Therefore, hCTR1 has the potentiality to become a hopeful biomarker for platinum-based chemotherapy. It can be down-regulated by intracellular copper (Howell et al., 2010) or cisplatin/oxaliplatin treatment which triggers a rapid loss of hCTR1 via macropinocytosis and proteasomal degradation in ovarian carcinoma cells (Holzer and Howell, 2006) and hepatocellular carcinoma cells (Li et al., 2016b). Ishida et al. shown that copper chelator tetrathiomolybdate could increase the uptake of cisplatin into tumor cells specifically (Ishida et al., 2010), and so did Fu and coworkers. They reported a combined therapy of carboplatin and trientine, a copper-lowering agent, partly reversed resistance to platinum therapy on five patients with platinum-resistant high-grade epithelial ovarian cancer (Fu et al., 2012). The proteasome inhibitors bortezomib (Al-Eisawi et al., 2013) and natural compound β-elemene were also discovered that could block CTR1 from degradation (Li et al., 2016b). However, a lot of conflicting conclusions of whether regulating CTR1 levels affects sensitivity to platinum-based drugs have emerged. Kristin et al. (Bompiani et al., 2016) knocked out the CTR1, CTR2, ATOX1, and CCS using CRISPR-Cas9 genome editing; and the results indicated that the loss of CTR1, CTR2, ATOX1, or CCS had little impact on cisplatin sensitivity in both human HEK-293T and ovarian carcinoma OVCAR8 cells. Another research found that overexpression of CTR1 (Akerfeldt et al., 2017) failed to increase platinum accumulation and had no effect on the sensitivity of cisplatin in DLD-1 cells. The clinical relevance of hCTR1 and platinum-based chemotherapy has been questioned as well (Kim et al., 2014).
CTR2 is a low-affinity transporter of copper that shares 41% amino acid homology and the similar essential domains for copper transport with CTR1 except for the extended N-terminal domain (Gupta and Lutsenko, 2009). It locates at late endosomes and lysosomes, although it had also been found on the plasma membrane (van den Berghe et al., 2007). The mRNA and protein levels of hCTR2 have significant correlations with the sensitivity of cisplatin (Blair et al., 2009). Knocking down CTR2 in some cells increases the cellular accumulation of cisplatin, yet overexpressing the CTR2 reduces the sensitivity to cisplatin (Huang et al., 2014). Blair et al. indicated that CTR2 regulated the accumulation of cisplatin through an effect on macropinocytosis, not by changing drug efflux or microsomal storage (Blair et al., 2011). Moreover, CTR2 can interact with CTR1 via stimulating CTR1 ectodomain cleavage resulting in less accumulation of cisplatin in cells (Ohrvik et al., 2016).
ATP7A and ATP7B
ATP7A/7B, which belongs to P-type ATPases, is responsible for copper homeostasis (Gupta and Lutsenko, 2009). After getting into the cells, platinum may bind to the CXXC motifs of ATP7A/B (Safaei et al., 2012), then the complex translocates into a vesicle in an ATP-dependent manner with the association of copper chaperone Atox1 (Boal and Rosenzweig, 2009). ATP7A/B resides in the trans-Golgi network under normal conditions (Hall et al., 2008; Kalayda et al., 2008), but in platinum-resistance cells, it distributes in more peripherally located vesicles in the cytosol. The altered localization may be caused by reduced lysosomal compartment, and it contributes to platinum-sequestration (Kalayda et al., 2008). ATP7A acts as an insulator, keeping cisplatin away from nuclear in resistance cells. Chisholm and coworkers observed that the cellular platinum intensity is low and is excluded from the nucleus (Chisholm et al., 2016) when it shows high expression. ATP7B is also regarded as a contributor to platinum resistance and may serve as a prognostic factor. Patients with the lowest mRNA expression levels of ATP7B presented a significantly longer time to progression and had the optimal curative effects from oxaliplatin/5FU treatment in colorectal cancer (Martinez-Balibrea et al., 2009). Given the above, it seems that downregulating ATP7A/B could be an effective way to overcome the resistance in tumor cells. By direct binding to 3′ untranslated region (3′ UTR) of the ATP7A/B mRNA, some microRNAs (miRNA, miR) regulates the cell response to cisplatin, carboplatin, and oxaliplatin such as miR-495, miR-139, and miR-133a (Song et al., 2014; Wang et al., 2016c; Xiao et al., 2018). The copper chelator ammonium tetrathiomolybdate ([(NH4)2MoS4], TM) can also restore the sensitivity to cisplatin by inducing dimerization of the metal-binding domain of ATP7B (Fang et al., 2019).
Multidrug Resistance Protein Subfamily
Multidrug resistance protein (MRP, ABCC) subfamily, which has seven subfamilies named A to G, belongs to the ATP-binding cassette transporter superfamily and functions as an ATP-dependent unidirectional efflux pump for anionic amphiphilic compounds (Jedlitschky et al., 1994; Yaneff et al., 2019). MRPs mediated the efflux of endogenous molecules, physiological substrate, and drugs (reviewed in Keppler, 2011). MRP2, not MRP1 or MDR1, has long been recognized as the efflux transporter of the platinum-GSH conjugate (Taniguchi et al., 1996; Borst et al., 2000). High-level expression of MRP2 is associated with intrinsic cisplatin resistance and clinical outcome in small cell lung carcinoma (Ushijima et al., 2007), ovarian cancer (Surowiak et al., 2006), and esophageal squamous cell carcinoma (Yamasaki et al., 2011). MRP4 has also been identified as a platinum resistance-associated candidate. The overexpression of MRP4 is related to cisplatin resistance (Zhang et al., 2010), and knocking down MRP4 instead of MRP1 increases the accumulation of oxaliplatin and cisplatin (Beretta et al., 2010; Zhang et al., 2010).
Detoxification System
Glutathione
Glutathione (L-γ-glutamyl-l-cysteinyl-glycine, GSH) plays a vital role in the cellular redox state via scavenging free radicals, defending cells against xenobiotics and maintaining the sulfhydryl groups of many proteins (Brozovic et al., 2010). The active SH-group of GSH has a high affinity to platinum, thus makes GSH become an easy, non-DNA-related target (Zimmermann and Burda, 2010). The GS-platinum complex catalyzed by glutathione-S-transferase can reach to about 60% of the intracellular platinum content after 12-h incubation in leukemia cells (Ishikawa and Ali-Osman, 1993), and the elevated expression of GSH and glutathione-S-transferase are often seen in the resistant-cells (Masters et al., 1996; Byun et al., 2005). Preventing the formation of the GS-platinum complex may reverse the platinum resistance efficiently. One choice is to use competitive inhibitors of GSH such as [Cu(phen)(H2O)2(ClO4)2] (C10) (Cadoni et al., 2017), another is to interfere with the synthesis of GSH. Two enzymes mediate the production of GSH, γ-glutamylcysteine ligase and GSH synthetase. In the first step, γ-glutamylcysteine ligase catalyze glutamate and cysteine to form γ-glutamylcysteine; then, in the second step, GSH synthetase catalyzes γ-glutamylcysteine and glycine to form GSH (Aoyama and Nakaki, 2013). GSH-depleting agents buthionine sulfoximine is a useful agent for inhibition of γ-glutamylcysteine ligase, and it reduced the resistance to cisplatin in malignant glioma (Rocha et al., 2015). However, non-specific GSH depletion can cause irreversible damage in most normal tissues. Hence selective tumor GSH depletion appears to be a better choice but remains as a superb challenge (reviewed in Estrela et al., 2006). Cystine is normally used as the precursor for GSH synthesis due to the chemical instability of cysteine, and the intracellular cysteine/cystine level is maintained by the xc− system which mediates the uptake of cystine; so finding ways to inhibit xc− system can block the synthesis of GSH. Sulfasalazine (Ma et al., 2015b) and salubrinal (Wang et al., 2018) are both reported as powerful blockers. Epigenetic interference also involves in the regulation of the GSH synthesis: miRNA-27a negatively regulates the xc- (Drayton et al., 2014); G9a, a transcriptional corepressor that catalyzes histone 3 lysine 9 dimethylation, transcriptional activates the glutamate-cysteine ligase catalytic subunit, and lead to the elevation of GSH level in head and neck squamous cell carcinoma (Liu et al., 2017a); Linc RNA (Lnc RNA, Lnc) H19 participates in the production and regeneration of GSH in high-grade serous ovarian cancer (Zheng et al., 2016).
GSH redox cycle also regulates platinum resistance. GSH can be oxidized into GSSG by GSH peroxidase using H2O2 as a substrate, whereas GSSG can be reduced back to GSH by GSSG reductase using NADPH as a cofactor (Chen and Kuo, 2010). Higher expression of glutathione reductase accompanied by lower levels of endogenous reactive oxygen species (ROS) contributes to the cisplatin-resistance (Zhu et al., 2018).
Metallothionein
Platinum antitumor drugs are also inactivated by chelating with metallothionein (MT) proteins. MTs are low-molecular-weight metal-binding proteins containing one-third cysteine residues, which make MTs become easy targets for platinum to chelate (Kimura and Kambe, 2016). Platinum agents may also bind to metal transcription inhibitor and release the metal transcription factor-1 to trigger the biosynthesis of MTs (Krizkova et al., 2010). It has been found that MTs level is increased not only in the tumor tissue but also in the serum of cancer patients (Tariba et al., 2015); RNA interference can inhibit the overexpression of MTs efficiently and reverse platinum resistance (Lee et al., 2015). Expressing level of the MTs has been considered as an important biological factor of platinum-based chemotherapy (Yamamoto et al., 1999), but rather than global MTs classes, Habel and colleagues suggested that MT isoforms are more valuable prognosis predictors markers (Habel et al., 2013). Pekarik et al. noted that MT-1 and MT-2 have redundant binding sites for many miRNAs, including miR23 and miR224 (Pekarik et al., 2013). It indicated that miRNAs may involve in the regulation of MTs, and could be a notable pointcut.
DNA Repair
The platinum atom can covalently bound to the N7 positions of purine bases to form the platinum–DNA adducts. Cisplatin can form mono adducts, intrastrand, or interstrand cross-links, but the 1,2-intrastrand crosslinks account for over 90% (reviewed in Jamieson and Lippard, 1999; Chaney et al., 2005). It is well acknowledged that forming DNA adducts is the determinant step for cytotoxicity of platinum-based antitumor agents because the platinum–DNA complexes influence the structure of DNA double helix (Kartalou and Essigmann, 2001) and nucleosomes (Danford et al., 2005). As a result, it causes replication and transcription inhibition and DNA double-strand breaks (DSBs), followed by the initiation of DNA repair. Once the DNA repair fails or is overwhelmed by too many DNA lesions, cell death will be triggered (reviewed in Jung and Lippard, 2007). The increased DNA repair process is considered as the most significant characteristic in platinum-resistance cells (Dijt et al., 1988; Wynne et al., 2007), with the exception of DNA mismatch repair whose deficiency gives rise to cisplatin/carboplatin resistance (Sawant et al., 2015), but has less influence to oxaliplatin (Goodspeed et al., 2019). Oxaliplatin has more potent cytotoxicity than cisplatin for inducing early secondary DSBs and massive apoptosis (Faivre et al., 2003). However, Bruno et al. reported that oxaliplatin induces ribosome biogenesis stress rather than DNA damage to kill cells (Bruno et al., 2017). Most intrastrand crosslinks are removed by nucleotide excision repair (NER) system via excising damaged nucleotides and synthesizing DNA to reconstitute genetic integrity, while other lesions are repaired by complex-combined mechanisms (Roos and Kaina, 2013). Only two key components of the DNA repair system will be discussed in this review.
Excision Repair Cross-Complementing
All excision repair cross-complementing (ERCC) members have their unique roles in DNA repair progress, and their expression level or SNP has a significant impact on platinum resistance. In a prospective study, Sullivan et al. analyzed the SNPs in eight DNA-repair related genes and found that after the treatment of platinum-based chemotherapy, the response of patients with stages III significantly associated with SNPs in ERCC1 and ERCC3 genes, while the response of patients with stage IV associated with a genetic variant in the ERCC4 gene (Sullivan et al., 2014). Many researchers highlight the relevance between ERCC1 and platinum-treatment response (Li et al., 2000; Ozcan et al., 2013). Liu et al. found that under DNA damage condition, p53 recruited CITED2/P300 along with chromatin relaxation H3K9Ac or H3K14Ac to bind with the ERCC1 promotor, and activated the DNA repair process (Liu et al., 2015b). However, Spada et al. reported that ERCC1 had no significant correlation with the clinical outcome with oxaliplatin-based chemotherapy in advanced neuroendocrine tumors (Spada et al., 2016). ERCC4 (XPF), a necessary component in NER, interstrand cross-links repair, homologous recombination repair (HRR) but not in non-homologous end-joining (Lehmann et al., 2017), contributes to the intrinsic resistance of cisplatin, and its expression level is tissue-specific (Zhang et al., 2016a). ERCC1 and XPF can form the human ERCC1–XPF complex which works as a nuclease in NER and the late stage of HRR (Gillet and Scharer, 2006; Al-Minawi et al., 2009). Arora et al. reported that knocking down ERCC1–XPF resulted in reduced intrastrand repair and interstrand crosslinks repair, which makes about fourfold to sixfold changes in IC50 value of cisplatin in NSCLC cells (Arora et al., 2010). Based on the above results, it is reasonable to believe that inhibiting ERCC1/XPF is a potential way to sensitize tumor cells to platinum-based chemotherapy, and many inhibitors are discovered: green tea polyphenol epigallocatechin-3-gallate, catechols, 3-hydroxypyridones, N-hydroxyimides, and hydroxypyrimidones (Chapman et al., 2015; Wang et al., 2015), but their clinical application still needs further investigation. Another nuclease function as ERCC1–XPF in NER is ERCC5 (XPG), which also relates to platinum sensitivity. Graf et al. reported that XPF and XPG knockdown increased the platinum-induced cytotoxicity in osteosarcoma cells (Graf et al., 2011). Interestingly, platinum-based chemotherapy is influenced by the 5′ noncoding mRNA element of ERCC5. Somers’s group discovered a common polymorphic variant rs751402 in the ERCC5 5′ untranslated region, which generated an upstream ORF, and the carriers with early childhood ependymoma were markedly resistant to platinum-based agents (Somers et al., 2015).
Breast Cancer Susceptibility Gene
Breast Cancer Susceptibility Genes (BRCAs) involve in the repair of DSB and DNA cross-linking damage induced by DNA-damaging agents through the HR pathway (reviewed in Foulkes and Shuen, 2013); BRCA1/2-deficient carcinomas have impaired HRR and become more sensitive to platinum agents (Dann et al., 2012; Muggia and Safra, 2014). The alterations or mutations of BRCA1 and BRCA2 lead to the impaired recognition of DNA damage, and therefore depending on the patients’ BRCA status to add platinum agents into neoadjuvant therapy before surgery could receive an excellent response (Golan et al., 2014; Soyano et al., 2018). However, in BRCA mutant cancers, platinum resistance still exists. To explain that phenomenon, Guillemette et al. conducted a genome-wide shRNA screen and found that loss of the nucleosome remodeling factor CHD4 might cause cisplatin resistance through a trans-lesion synthesis dependent manner rather than HRR in BRCA2 mutant cancer cells(Guillemette et al., 2015). In addition, a secondary mutation in BRCA1/2 may occur after patients receiving platinum-based chemotherapy treatment, and it restores the function of BRCA1/2, ending with platinum resistance (Norquist et al., 2011). Researchers recently found two regulators: Wwox and deubiquitination enzyme USP13. Wwox can interact with BRCA1 and tip the DSBs repair pathway choice, and its deficiency permits significantly increased resistance to cisplatin, so it could be an efficient predictor of response to cisplatin (Schrock et al., 2017). The knockdown of USP13 dramatically diminishes cisplatin-induced RAP80-BRCA1 foci formation, for its deficiency decreases the bind between RAP80 and K63-linked ubiquitin chain and fail the BRCA1 recruitment (Li et al., 2017b).
Other than ERCC and BRCA, many key components like FANCD2 (Martens-de Kemp et al., 2017), PCNA (Somasagara et al., 2017), XRCC1 (Xu et al., 2014), and RAD51 (Pan et al., 2013b; Xiao et al., 2017), also play different roles in multiple DNA repair pathway. A better understanding of the DNA repair regulation network will provide a more accurate guide to personalized platinum-based chemotherapy.
Apoptosis
After binding to the DNA, the core downstream events platinum agents triggered is apoptosis, which is also called programmed cell death. Two main apoptosis pathways have been well proposed: the extrinsic and the intrinsic pathways. The extrinsic pathway is activated after the tumor necrosis factor family binding to the cell surface receptors of the tumor necrosis factor receptor superfamily and leads to the self-activation of initiator caspase-8. The intrinsic pathway is switched by an imbalance between proapoptotic (i.e., BAX, BAK) and antiapoptotic proteins (i.e., BCL-2, BCL-XL, BCL-w). Upon activation, proapoptotic signaling leads to mitochondrial outer membrane permeabilization, and then cytochrome c will be released, initiating a series caspase cascade (Kim et al., 2002; Bai and Wang, 2014). Platinum-resistant tumor cells usually have a higher threshold for apoptosis induction, mostly due to the overexpression of anti-apoptotic proteins or the defect in mitochondrial signaling. Many factors contribute to these unwanted phenomenons, such as pro-survival signal pathways (Sun et al., 2016; Bao et al., 2017) [i.e., MAPK/ERK (Kong et al., 2015), PI3K/AKT pathway, NF-κB (Miow et al., 2015)], and tumor microenvironment (TME) and epigenetic regulation (Benard et al., 2014; Ramadoss et al., 2017). Researchers begin focusing on the critical role of non-coding RNA in diverse cellular processes; studies demonstrated that non-coding RNA broadly participated in proapoptotic/antiapoptotic proteins regulation and could work as therapeutic targets or predict markers (Chen et al., 2015; Zarogoulidis et al., 2015). We summarized recently found apoptosis-related non-coding RNA with details listed in Table 2. Among all the contributors, we specifically concentrate on the role of p53 and TME in platinum resistance.
Table 2.
The LncRNAs and miRNAs regulate platinum resistance through targeting apoptosis and autophagy in various cancer.
| Target | Platinum agents | Cancer type | References | |||
|---|---|---|---|---|---|---|
| Apoptosis | LncRNA | LncRNA UCA1 | miR-184/SF1 | Cisplatin | Oral squamous cell carcinoma | (Fang et al., 2017) |
| CREB-miR-196A-5P | Bladder cancer | (Pan et al., 2016) | ||||
| LncRNA AC023115.3 | miR-26a-GSK3β | Malignant glioma | (Ma et al., 2017) | |||
| LncRNA XIST | let-7i/BAG-1 | Lung adenocarcinoma | (Sun et al., 2017b) | |||
| LINC00473 | C/EBPβ IL24 |
Osteosarcoma | (Zhang et al., 2017) | |||
| HOMEOBOX A11 | miR‐454‐3p/Stat3 | Lung adenocarcinoma | (Zhao et al., 2018a) | |||
| HOTAIR | PI3K/Akt Wnt/β-catenin miR-34a |
Gastric cancer | (Cheng et al., 2018) | |||
| Lnc PVT1 | HIF1α | (Zhang et al., 2015) | ||||
| miRNA | miR−34a | MAGE-A/p53 | Carboplatin | Retinoblastoma | (Yang et al., 2019) | |
| miR-205 miR-218 |
Mcl-1 Survivin |
Lung cancer | (Zarogoulidis et al., 2015) | |||
| miR-634 | Ras-MAPK | Ovarian cancer | (van Jaarsveld et al., 2015) | |||
| miR-139-5p | MAPK | Cisplatin | Ovarian cancer | (Chen et al., 2018) | ||
| miR-5100 | Rab6 | Lung cancer | (Yang et al., 2018) | |||
| miR-873 | Bcl-2 | Gliomas | (Chen et al., 2015) | |||
| miR-199a-3p | ZEB1 | Melanoma | (Liang et al., 2018) | |||
| miR-195 | Prohibitin 1 | (Cirilo et al., 2017) | ||||
| miR-125a-5p | STAT3 | Esophageal carcinoma | (Zhao et al., 2018b) | |||
| miR-374a | PDCD4 | Nasopharyngeal carcinoma | (Zhen et al., 2017) | |||
| miR-146a | Cyclin J | Non-small cell lung cancer | (Shi et al., 2017) | |||
| miR-148b | DNMT1 | (Sui et al., 2015) | ||||
| miR-200c | AKT2 | Osteosarcoma | (Liu et al., 2017d) | |||
| miR-378 | Clusterin | Lung adenocarcinoma | (Chen et al., 2016) | |||
| miR-216b | PARP1 | Ovarian cancer | (Liu et al., 2017c) | |||
| miR-148a | Rab14 | Renal cell carcinoma | (Kim et al., 2017a) | |||
| miR-126 | SERPINE1 SLC7A5 mTOR/HIF |
(Liu et al., 2017b) | ||||
| miR-17-92 | AKT | Prostate cancer | (Zhou et al., 2016b) | |||
| miR-99a miR-491 |
CAPNS1 | Gastric cancer | (Zhang et al., 2016b) | |||
| Autophagy | LncRNA | BLACAT1 | miR-17/ATG7 | Cisplatin | Non−small cell lung cancer | (Huang et al., 2019) |
| MALAT1 | microRNA−30b/autophagy−related gene 5 | Gastric cancer | (Xi et al., 2019) | |||
| miRNA | miR-634 | APIP/XIAP/BIRC5/OPA1 NRF2 | Cisplatin | Esophageal squamo-us cell carcinoma | (Fujiwara et al., 2015) | |
| miR-148A-3p | RAB12 mTORC1 |
Gastric cancer | (Li et al., 2017a) | |||
| miR-874 | ATG16L1 | (Huang et al., 2018) | ||||
| miR-let-7f-1 | HMGB1 | Medulloblastoma | (Pannuru et al., 2014) | |||
| miR-181 | PTEN/PI3K/AKT/mTOR | Non-small cell lung cancer | (Liu et al., 2018) | |||
| miR-409-3p | Beclin-1 | Oxaliplatin | Colon cancer | (Tan et al., 2016) | ||
| miR-218 | YEATS4 | Colorectal cancer | (Fu et al., 2016) | |||
| miR-34a | TGF-β/Smad4 pathway | (Sun et al., 2017a) |
LncRNA, long non-coding RNA; miRNA, microRNA.
p53
The tumor suppressor p53 functions mainly as a transcriptional activator of many cellular programs, including checkpoint activation, DNA repair, and apoptosis, and therefore the status of p53 is crucial for the cytotoxicity of platinum agents (Gadhikar et al., 2013). Downstream genes of p53 related with apoptosis include FAS, BBC3, BAX, and BIRC5 (Riley et al., 2008). Besides the nuclear function, p53 located in cytoplasmic also promotes apoptosis through directly targeting mitochondria (reviewed in Marchenko and Moll, 2007). However, it is common to see the loss or mutation of p53 in the patients (Ahmed et al., 2010; Stransky et al., 2011), and the disfunction of p53 will cause the failure of checkpoint response, cell cycle arrest, programmed cell death (apoptosis), and permanent cell cycle arrest (senescence) (Martinez-Rivera and Siddik, 2012). Eventually, it leads to a poor outcome of platinum-based chemotherapy. A thought-provoking work reported a nonsynonymous single-nucleotide polymorphism Pro47Ser (rs1800371) in African descent; the carriers are more tumor-prone and show a significant defect in cisplatin-induced cell death due to the impaired trans-activation ability (Jennis et al., 2016). For this reason, more precision medicine approaches should be considered for different mutation carriers (Basu et al., 2016).
Antirepression, stabilization, DNA binding, and transcriptional activation are four key steps in p53’s activation along with an exquisite regulation network including phosphorylation, ubiquitination, methylation, sumoylation, neddylation, and acetylation (reviewed in Kruse and Gu, 2009). Ubiquitinate-dependent degradation of p53 is mediated by human double minute 2 (HDM2, MDM2); therefore, interference with the HDM2/p53 pathway is an optional strategy to rescue p53 from degradation and reactivate it. Two p53-MDM2 blockers, RITA (Issaeva et al., 2004) and nutlin (Vassilev et al., 2004), were discovered in 2004. Furthermore, recently Wanzel and coworkers used CRISPR-Cas9 based target validation to reveal the actual mechanisms of two p53-MDM2 blockers (Wanzel et al., 2016). Nutlin was designed to blocks the p53-binding pocket of Mdm2 and RITA was to bind the Mdm2-interacting N terminus of p53, but on the contrary, results showed that the activity of nutlin strictly depends on functional p53 while the sensitivity of RITA correlates with induction of DNA damage but not with p53 (Wanzel et al., 2016). The phosphorylation status is also vital to functional p53. DACH-diacetato-dichloro-Pt(IV) (DAP) and oxaliplatin circumvent cisplatin resistance for phosphorylating p53 at Ser20 in resistant cells while cisplatin cannot (Xie et al., 2017), and in cisplatin-resistant MCF-7 cells, resveratrol induces serine 20 phosphorylation to activate p53 target genes such as PUMA and BAX, restoring apoptosis (Hernandez-Valencia et al., 2018). All these results demonstrate that phosphorylation at Ser 20 site might be a critical event for p53 activation.
TME
The TME is a complex ecosystem and an active regulator in the progression of cancer development. The interaction between TME and cancer cells is mutual and dynamical. Tumor epithelial cells can gradually change the nature of the microenvironment, such as hypoxia, and conversely, the altered TME will regulate tumor development (Belli et al., 2018). TME consists of nonmalignant cells of the tumor such as cancer-associated fibroblasts (CAFs), endothelial cells and pericytes composing tumor vasculature, immune and inflammatory cells, bone marrow-derived cells, and the extracellular matrix (Belli et al., 2018). Some have been proven participating in the formation of drug resistance.
CAFs
The CAFs, which held the main proportion of tumor stroma, are perpetually activated, and neither revert to a normal phenotype nor undergoes apoptosis and elimination (Li et al., 2007). Emerging evidence demonstrates that non-malignant CAFs can contribute to tumor proliferation, metastasis, and chemoresistance. CAFs secrete specific cytokines, proteins, or exosomal miRNA to activate certain anti-apoptosis related signaling pathways like PI3K/Akt, ANXA3/JNK, and IL-11/IL-11R/STAT3, and consequently offer tumor cells the ability of resistance (Tao et al., 2016; Zhou et al., 2016a; Zheng et al., 2017b; Qin et al., 2019; Wang et al., 2019b). CAFs can also cause the aberrant remodeling of extracellular material and physical properties of the tumor altered, or release cysteine and GSH to limit the intracellular platinum concentration (Akkari and Joyce, 2016; Wang et al., 2016b). It is worth mention that most of these researches focus on the resistance change of tumor cells after platinum treatment. However, the innate chemoresistant of CAFs is poorly understood. Qin X et al. recently reported that HNC-derived CAFs were innately resistant to cisplatin, maybe due to the high expression of ERCC1 and ERCC4 (Qin et al., 2019).
Hypoxia
The imbalance between the rapid growth of tumor cells and inadequate supply from blood vessels alters the TME, and one of the significant characteristics is hypoxia (Bertout et al., 2008), which is strongly associated with a poor prognosis in different cancer types, such as hepatocellular carcinoma (Dai et al., 2009), ovarian cancer (Osada et al., 2007), NSCLC (Hung et al., 2009), etc. Hypoxia triggers the regulation of hypoxia-inducible factor (HIF) family at the transcription level and protein level (Pawlus and Hu, 2013). HIFs are heterodimeric transcription factors composed of two parts: an oxygen-labile alpha subunit (HIF1α, HIF2α, or HIF3α) and a stable beta subunit (HIF1β). HIF1α and HIF2α are widely expressed in various tissues and have been well understood, yet HIF3α is less known due to its high specificity of tissue distribution and the existence of multiple variants (Duan, 2016). So only HIF1α and HIF2α will be discussed in this review. Under normoxic conditions, two specific proline residues in HIF-1α are hydroxylated by HIF prolyl hydroxylase enzymes with the cofactor oxygen, iron, ascorbate, and 2-oxoglutarate. Then, the hydroxylated HIF1α protein is bound with the von Hippel–Lindau protein, and it will recruit E3 ubiquitin ligases and put HIF1α through polyubiquitination and degradation by the 26S proteasome. Whereas under hypoxic conditions, impaired hydroxylation process stabilizes the HIF1α protein, furthermore HIF1α will translocate into nuclear and dimerize with HIF1β to transactivate the target genes which relate with glucose metabolism, angiogenesis, cell proliferation, invasion and metastasis (reviewed in Pawlus and Hu, 2013; Soni and Padwad, 2017). HIF1α has a strong association with apoptosis; it regulates apoptosis-related genes (i.e., Bcl-2, Bax, caspase 3, caspase 8) (Hernandez-Luna et al., 2013; Zhao et al., 2016), and survival signaling pathways such as NF-κB (Rohwer et al., 2010). Zheng et al. recently found that HIF-1α could activate the PI3K/AKT by up-regulating Mxd1 expression to suppress the PTEN expression (Zheng et al., 2017a). Besides, HIF accumulation facilitates the secretion of several growth factors and further activates mTOR to form a positive feedback circuit. Deactivating these signaling pathways via reducing HIFs could be a promising approach.
Highly proliferative cancer cells switch glucose flux from oxidative phosphorylation to anaerobic glycolysis, which converts pyruvate into lactate even in the presence of oxygen (the Warburg effect). This shift will quench cytosolic ROS (Hervouet et al., 2007). However, recent evidence shows that in some cytotoxic antineoplastic drug-resistance tumor cells, aerobic glycolysis can shift back to oxidative phosphorylation (Xu et al., 2018). HIFs act as inducers to aerobic glycolysis and a suppressor to mitochondrial function; it can regulate many main enzymes in glycolysis and tricarboxylic acid cycle like GLUT1, LDH-A, PDH, and PDK1 (Nagao et al., 2019). Downregulating HIF-1α to redirect the aerobic glycolysis towards oxidative phosphorylation resulting in overexpressing ROS can resensitize the cisplatin-resistance ovarian cancer cells (Ai et al., 2016). ROS act as a double-bladed sword in the platinum treatment. On the one hand, platinum agents-induced cytotoxicity is strongly linked to excessed ROS generation (Brozovic et al., 2013; Choi et al., 2015; Jiang et al., 2015; Li et al., 2016c). Unlike cisplatin, oxaliplatin prefers to stimulate O2– production rather than H2O2 (Chen et al., 2017). Some essential components were newly discovered. Wang et al. reported that Scribble promotes cisplatin-induced apoptosis via protecting Nox2/ROS (Wang et al., 2019a). Inositol 1, 3, 4, 5-tetrakisphosphate, the product of inositol-trisphosphate 3-kinase B, inhibits NADPH oxidase 4, thereby inhibiting the generation of cisplatin-induced ROS (Pan et al., 2019). The imbalance between ROS production and elimination is often seen in resistance cells. Besides GSH and MTs, the elevated oxidative branch of the pentose phosphate pathway is another strong defensor against ROS (Xu et al., 2018). The key, it seems, is whether the ROS level can reach the threshold to trigger cell death. Stimulating the production or blocking the neutralization of ROS might be a promising way to reverse platinum resistance. On the other hand, the increased ROS level in cancer cells might accelerates the development of tumor aggressiveness and drug resistance (Kumar et al., 2008). Increased ROS can activate cellular survival pathways including c-Myc–miR-137–EZH2 pathway (Sun et al., 2019), IL-11–JAK2–STAT5 pathway (Zhou et al., 2018), ATR–Chk1 pathway (Meng et al., 2018) and AXL (Oien et al., 2017) et al. ROS may also mediate metabolism reprogramming (Cruz-Bermudez et al., 2019).
Classical strategies include direct/indirect HIF targeting and interference in upstream/downstream HIF regulators/signaling pathways. Many prodrugs, specific HIF inhibitors, and non-selective inhibitors are developed to inhibit HIFs with many different mechanisms including inhibition of HIF dimerization, mRNA or protein expression, transcriptional activity, and DNA binding capacity (reviewed in Wigerup et al., 2016). Recently found Trx-1 and KLF5 (Li et al., 2016a) are upstream regulators of HIF 1α. Trx-1 increase the expression and the binding capacity of HIF1α through enhanced interaction with Ref-1 and inhibition of Trx-1/Ref-1 axis can strengthen the oxidative phosphorylation, and reverse the resistance to cisplatin (Zhao et al., 2015). Gong et al. reported KLF5 knockdown suppressed hypoxia-induced cisplatin resistance by inhibiting HIF1α-dependent glycolysis through inactivation of the PI3K/Akt/mTOR pathway (Gong et al., 2018). Non-coding RNAs can also increase the expression of HIF1α, resulting in platinum resistance (Zhang et al., 2015; Liu et al., 2017b).
Besides HIF1α, HIF2α also associate with platinum response. GBM cells show an increase in cisplatin resistance after exposure to hypoxia, and downregulating HIF2α, not HIF1α, significantly sensitizes U251 and U87 cells (GBM cell lines) to cisplatin (Ahmed et al., 2018). Knocking down HIF2a significantly increases the sensitivity to cisplatin in A549 cells (Gao et al., 2018).
Autophagy
Autophagy, a “self-digestion” process, occurs in all eukaryotes. It is essential for nutrient regulation and intracellular quality control and homeostasis (Mizushima and Klionsky, 2007). Also, autophagy functions as a self-defense strategy by recycling macromolecules as an alternative energy source. However, if autophagy continuously or excessively proceeds, cell death will be triggered. There are generally three major types of autophagy, macroautophagy, microautophagy, and chaperone-mediated autophagy. In this review, only macroautophagy (referred to as autophagy) will be discussed. Macroautophagy seems to have a conflicting role in tumorigenesis and progression. It can act as a prodeath or prosurvival role at different cancer stages (reviewed in Dalby et al., 2010). After platinum treatment, the increased drug-induced autophagy is observed in platinum-resistant cells, along with the increased basal autophagy (Yu et al., 2014; Liu et al., 2015a; Wu et al., 2017; Gao and Wang, 2019; Wang et al., 2019c). Inhibition of autophagy via autophagy inhibitor or interference in regulatory elements or noncoding RNAs has been proven can reduce the platinum resistance. Using 3-methyladenine or chloroquine to inhibit early/later-stage autophagy can both enhance platinum-mediated cytotoxicity (Fukuda et al., 2015; Ma et al., 2015a). Autophagy is highly controlled by regulatory elements including PI3K–Akt–mTOR pathway, Beclin1, Bcl-2, Ras, p53, and noncoding RNAs [long non-coding RNAs (LncRNs) and miRNAs] (reviewed in Kumar et al., 2015). Recently, new regulators dual-specificity protein phosphatase 1 (DUSP1) (Wang et al., 2016a), heparinase (Shteingauz et al., 2015), and HMGB1 (Liu et al., 2015a), GFRA1 (Kim et al., 2017b) are reported. Targeting these regulatory elements can restore the efficiency of platinum agents. LncRNAs and miRNAs affect the platinum resistance by targeting key components like ATG7 or signaling pathways like PI3K/AKT/mTOR in autophagy. We summarized recently found autophagy-related non-coding RNA with details listed in Table 2.
Another big issue caused by autophagy is related to cancer stem cells (CSC). CSCs are a subpopulation of cancer cells within the tumor that have the ability to self-renew and to differentiate. CSCs have long been considered as the main culprit for drug resistance and relapse (MacDonagh et al., 2016; Steinbichler et al., 2018). Growing evidence indicated that autophagy protects CSCs for participating in the regulation of stem cell differentiation, somatic reprogramming and self-renewal capacity (Pan et al., 2013a). Yang et al. reported that oxaliplatin-induced autophagy enriches the population of colorectal CD44+ CSCs and maintain the stemness of colorectal CSCs, this protection eventually causes chemoresistance in colorectal CSCs (Yang et al., 2015), and so did Nail’s group. They found that autophagy regulates the expression of stemness surface marker CD44, drug resistance marker ABCB1 and invasion mediator ADAM17 to help maintain stemness and chemoresistance, but the mechanism remains unclear (Naik et al., 2018). Targeting autophagy to diminish the CSCs subpopulation might be a new direction to overcome platinum resistance.
In most aforementioned cases, increased autophagy has a negative relation to platinum response. However, some phytochemicals show anti-resistance effects via inducing autophagy. Zhu et al. reported that hyperoside sensitizes ovarian cancer cells to cisplatin relay on PGRMC1-dependent autophagy (Zhu et al., 2017); resveratrol can induce autophagy and apoptosis in cisplatin-resistant human oral cancer (Chang et al., 2017).
Further investigation about the changeful role of autophagy in tumor development and the complex regulatory mechanism involved in will provide more potential intervention targets and approaches for how and when to overcome platinum resistance.
Conclusions
The resistance of platinum agents is still an ordeal. Elucidating the molecular mechanisms underlying the resistance phenomena will largely extend the clinic application of platinum agents. The main mechanisms are summarized as changed cellular platinum accumulation, increased detoxification system, increased DNA repair, decreased apoptosis, and autophagy. As the relative technologies develop rapidly, new research means such as CRISPR-Cas9, RNAi-based functional genetic screening help researchers to reexamine some key regulators and illuminate their actual impact on platinum resistance. Another notable tendency is the deeper understanding of the epigenetic modification. Non-coding RNA shows extremely complicated regulation functions on all sides and the potential to be predictive markers. The intercrossed application among genome, epigenome, transcriptome, proteome, and metabolome systems will provide a new aspect to break through the bottlenecks. But the challenges still remain as to how to utilize the emerged candidate targets as predictive markers to assign patients to more promising therapy or as therapeutic targets to improve the curative effect of platinum-based antitumor agents.
Author Contributions
JZ, LC, and HW searched for articles, and JZ wrote the first draft of the review. JL made the figures. YK and LY revised the whole manuscript. SZ and LY supervised the process. All authors approved the submission of this manuscript.
Funding
This work was supported by the Leading Talent of "Ten Thousand Plan" - National High-Level Talents Special Support Plan and the grants from National Natural Science Foundation of China (81773805 and 81703616)
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aguilar A. (2016). Kidney cancer: OCT2 demethylation cracks open oxaliplatin resistance. Nat. Rev. Nephrol. 12 (10), 581. 10.1038/nrneph.2016.122 [DOI] [PubMed] [Google Scholar]
- Ahmed A. A., Etemadmoghadam D., Temple J., Lynch A. G., Riad M., Sharma R., et al. (2010). Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 221 (1), 49–56. 10.1002/path.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed E. M., Bandopadhyay G., Coyle B., Grabowska A. (2018). A HIF-independent, CD133-mediated mechanism of cisplatin resistance in glioblastoma cells. Cell Oncol. (Dordr.) 41 (3), 319–328. 10.1007/s13402-018-0374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Z., Lu Y., Qiu S., Fan Z. (2016). Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 373 (1), 36–44. 10.1016/j.canlet.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerfeldt M. C., Tran C. M., Shen C., Hambley T. W., New E. J. (2017). Interactions of cisplatin and the copper transporter CTR1 in human colon cancer cells. J. Biol. Inorg. Chem. 22 (5), 765–774. 10.1007/s00775-017-1467-y [DOI] [PubMed] [Google Scholar]
- Akkari L., Joyce J. A. (2016). Microenvironmental InterFereNce of metabolism regulates chemosensitivity. Cell Res. 26 (8), 867–868. 10.1038/cr.2016.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eisawi Z., Beale P., Chan C., Yu J. Q., Huq F. (2013). Carboplatin and oxaliplatin in sequenced combination with bortezomib in ovarian tumour models. J. Ovarian Res. 6 (1), 78. 10.1186/1757-2215-6-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Minawi A. Z., Lee Y. F., Hakansson D., Johansson F., Lundin C., Saleh-Gohari N., et al. (2009). The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 37 (19), 6400–6413. 10.1093/nar/gkp705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K., Nakaki T. (2013). Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 14 (10), 21021–21044. 10.3390/ijms141021021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Kothandapani A., Tillison K., Kalman-Maltese V., Patrick S. M. (2010). Downregulation of XPF–ERCC1 enhances cisplatin efficacy in cancer cells☆. DNA Repair 9 (7), 745–753. 10.1016/j.dnarep.2010.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Wang S. (2014). Targeting apoptosis pathways for new cancer therapeutics. Annu. Rev. Med. 65, 139–155. 10.1146/annurev-med-010713-141310 [DOI] [PubMed] [Google Scholar]
- Bao L., Wu J., Dodson M., Rojo de la Vega ,. E. M., Ning Y., Zhang Z., et al. (2017). ABCF2, an Nrf2 target gene, contributes to cisplatin resistance in ovarian cancer cells. Mol. Carcinog. 56 (6), 1543–1553. 10.1002/mc.22615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Barnoud T., Kung C. P., Reiss M., Murphy M. E. (2016). The African-specific S47 polymorphism of p53 alters chemosensitivity. Cell Cycle 15 (19), 2557–2560. 10.1080/15384101.2016.1215390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli C., Trapani D., Viale G., D’Amico P., Duso B. A., Della Vigna P., et al. (2018). Targeting the microenvironment in solid tumors. Cancer Treat Rev. 65, 22–32. 10.1016/j.ctrv.2018.02.004 [DOI] [PubMed] [Google Scholar]
- Benard A., Janssen C. M., van den Elsen P. J., van Eggermond M. C., Hoon D. S., van de Velde C. J., et al. (2014). Chromatin status of apoptosis genes correlates with sensitivity to chemo-, immune- and radiation therapy in colorectal cancer cell lines. Apoptosis 19 (12), 1769–1778. 10.1007/s10495-014-1042-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta G. L., Benedetti V., Cossa G., Assaraf Y. G., Bram E., Gatti L., et al. (2010). Increased levels and defective glycosylation of MRPs in ovarian carcinoma cells resistant to oxaliplatin. Biochem. Pharmacol. 79 (8), 1108–1117. 10.1016/j.bcp.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Bertout J. A., Patel S. A., Simon M. C. (2008). The impact of O2 availability on human cancer. Nat. Rev. Cancer 8 (12), 967–975. 10.1038/nrc2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair B. G., Larson C. A., Safaei R., Howell S. B. (2009). Copper transporter 2 regulates the cellular accumulation and cytotoxicity of Cisplatin and Carboplatin. Clin. Cancer Res. 15 (13), 4312–4321. 10.1158/1078-0432.ccr-09-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair B. G., Larson C. A., Adams P. L., Abada P. B., Pesce C. E., Safaei R., et al. (2011). Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol. Pharmacol. 79 (1), 157–166. 10.1124/mol.110.068411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boal A. K., Rosenzweig A. C. (2009). Crystal structures of cisplatin bound to a human copper chaperone. J. Am. Chem. Soc. 131 (40), 14196–14197. 10.1021/ja906363t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompiani K. M., Tsai C. Y., Achatz F. P., Liebig J. K., Howell S. B. (2016). Copper transporters and chaperones CTR1, CTR2, ATOX1, and CCS as determinants of cisplatin sensitivity. Metallomics 8 (9), 951–962. 10.1039/c6mt00076b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Evers R., Kool M., Wijnholds J. (2000). A family of drug transporters: the multidrug resistance-associated proteins. J. Natl. Cancer Inst. 92 (16), 1295–1302. 10.1093/jnci/92.16.1295 [DOI] [PubMed] [Google Scholar]
- Brozovic A., Ambriovic-Ristov A., Osmak M. (2010). The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit. Rev. Toxicol. 40 (4), 347–359. 10.3109/10408441003601836 [DOI] [PubMed] [Google Scholar]
- Brozovic A., Vukovic L., Polancac D. S., Arany I., Koberle B., Fritz G., et al. (2013). Endoplasmic reticulum stress is involved in the response of human laryngeal carcinoma cells to Carboplatin but is absent in Carboplatin-resistant cells. PloS One 8 (9), e76397. 10.1371/journal.pone.0076397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno P. M., Liu Y., Park G. Y., Murai J., Koch C. E., Eisen T. J., et al. (2017). A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat. Med. 23 (4), 461–471. 10.1038/nm.4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H., Zoumaro-Djayoon A., Boersma A. W., Helleman J., Berns E. M., Mathijssen R. H., et al. (2010). Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2). Br. J. Pharmacol. 159 (4), 898–908. 10.1111/j.1476-5381.2009.00569.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss I., Hamacher A., Sarin N., Kassack M. U., Kalayda G. V. (2018). Relevance of copper transporter 1 and organic cation transporters 1-3 for oxaliplatin uptake and drug resistance in colorectal cancer cells. Metallomics 10 (3), 414–425. 10.1039/c7mt00334j [DOI] [PubMed] [Google Scholar]
- Byun S. S., Kim S. W., Choi H., Lee C., Lee E. (2005). Augmentation of cisplatin sensitivity in cisplatin-resistant human bladder cancer cells by modulating glutathione concentrations and glutathione-related enzyme activities. BJU Int. 95 (7), 1086–1090. 10.1111/j.1464-410X.2005.05472.x [DOI] [PubMed] [Google Scholar]
- Cadoni E., Valletta E., Caddeo G., Isaia F., Cabiddu M. G., Vascellari S., et al. (2017). Competitive reactions among glutathione, cisplatin and copper-phenanthroline complexes. J. Inorg. Biochem. 173, 126–133. 10.1016/j.jinorgbio.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Chaney S. G., Campbell S. L., Bassett E., Wu Y. (2005). Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit. Rev. Oncol. Hematol. 53 (1), 3–11. 10.1016/j.critrevonc.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Chang C. H., Lee C. Y., Lu C. C., Tsai F. J., Hsu Y. M., Tsao J. W., et al. (2017). Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: A key role of AMPK and Akt/mTOR signaling. Int. J. Oncol. 50 (3), 873–882. 10.3892/ijo.2017.3866 [DOI] [PubMed] [Google Scholar]
- Chapman T. M., Gillen K. J., Wallace C., Lee M. T., Bakrania P., Khurana P., et al. (2015). Catechols and 3-hydroxypyridones as inhibitors of the DNA repair complex ERCC1-XPF. Bioorg. Med. Chem. Lett. 25 (19), 4097–4103. 10.1016/j.bmcl.2015.08.031 [DOI] [PubMed] [Google Scholar]
- Chen H. H., Kuo M. T. (2010). Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met. Based Drugs 2010, 430939. 10.1155/2010/430939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhang Y., Shi Y., Lian H., Tu H., Han S., et al. (2015). MiR-873 acts as a novel sensitizer of glioma cells to cisplatin by targeting Bcl-2. Int. J. Oncol. 47 (4), 1603–1611. 10.3892/ijo.2015.3143 [DOI] [PubMed] [Google Scholar]
- Chen X., Jiang Y., Huang Z., Li D., Chen X., Cao M., et al. (2016). miRNA-378 reverses chemoresistance to cisplatin in lung adenocarcinoma cells by targeting secreted clusterin. Sci. Rep. 6, 19455. 10.1038/srep19455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Solomides C., Simpkins F., Simpkins H. (2017). The role of Nrf2 and ATF2 in resistance to platinum-based chemotherapy. Cancer Chemother. Pharmacol. 79 (2), 369–380. 10.1007/s00280-016-3225-1 [DOI] [PubMed] [Google Scholar]
- Chen Y., Cao X. Y., Li Y. N., Qiu Y. Y., Li Y. N., Li W., et al. (2018). Reversal of cisplatin resistance by microRNA-139-5p-independent RNF2 downregulation and MAPK inhibition in ovarian cancer. Am. J. Physiol. Cell Physiol. 315 (2), C225–C235. 10.1152/ajpcell.00283.2017 [DOI] [PubMed] [Google Scholar]
- Cheng C., Qin Y., Zhi Q., Wang J., Qin C. (2018). Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/beta-catenin signaling pathways by up-regulating miR-34a. Int. J. Biol. Macromol. 107 (Pt B), 2620–2629. 10.1016/j.ijbiomac.2017.10.154 [DOI] [PubMed] [Google Scholar]
- Chisholm C. L., Wang H., Wong A. H., Vazquez-Ortiz G., Chen W., Xu X., et al. (2016). Ammonium tetrathiomolybdate treatment targets the copper transporter ATP7A and enhances sensitivity of breast cancer to cisplatin. Oncotarget 7 (51), 84439–84452. 10.18632/oncotarget.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. M., Kim H. K., Shim W., Anwar M. A., Kwon J. W., Kwon H. K., et al. (2015). Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PloS One 10 (8), e0135083. 10.1371/journal.pone.0135083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirilo P. D. R., de Sousa Andrade L. N., Correa B. R. S., Qiao M., Furuya T. K., Chammas R., et al. (2017). MicroRNA-195 acts as an anti-proliferative miRNA in human melanoma cells by targeting Prohibitin 1. BMC Cancer 17 (1), 750. 10.1186/s12885-017-3721-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Bermudez A., Laza-Briviesca R., Vicente-Blanco R. J., Garcia-Grande A., Coronado M. J., Laine-Menendez S., et al. (2019). Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1alpha in NSCLC which can be overcome by OXPHOS inhibition. Free Radic. Biol. Med. 135, 167–181. 10.1016/j.freeradbiomed.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Dai C. X., Gao Q., Qiu S. J., Ju M. J., Cai M. Y., Xu Y. F., et al. (2009). Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer 9, 418. 10.1186/1471-2407-9-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby K. N., Tekedereli I., Lopez-Berestein G., Ozpolat B. (2010). Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 6 (3), 322–329. 10.4161/auto.6.3.11625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danford A. J., Wang D., Wang Q., Tullius T. D., Lippard S. J. (2005). Platinum anticancer drug damage enforces a particular rotational setting of DNA in nucleosomes. Proc. Natl. Acad. Sci. U. S. A 102 (35), 12311–12316. 10.1073/pnas.0506025102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann R. B., DeLoia J. A., Timms K. M., Zorn K. K., Potter J., Flake D. D., 2nd, et al. (2012). BRCA1/2 mutations and expression: response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer. Gynecol. Oncol. 125 (3), 677–682. 10.1016/j.ygyno.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Dijt F. J., Fichtinger-Schepman A. M., Berends F., Reedijk J. (1988). Formation and repair of cisplatin-induced adducts to DNA in cultured normal and repair-deficient human fibroblasts. Cancer Res. 48 (21), 6058–6062. [PubMed] [Google Scholar]
- Drayton R. M., Dudziec E., Peter S., Bertz S., Hartmann A., Bryant H. E., et al. (2014). Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin. Cancer Res. 20 (7), 1990–2000. 10.1158/1078-0432.ccr-13-2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C. (2016). Hypoxia-inducible factor 3 biology: complexities and emerging themes. Am. J. Physiol. Cell Physiol. 310 (4), C260–C269. 10.1152/ajpcell.00315.2015 [DOI] [PubMed] [Google Scholar]
- Estrela J. M., Ortega A., Obrador E. (2006). Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 43 (2), 143–181. 10.1080/10408360500523878 [DOI] [PubMed] [Google Scholar]
- Faivre S., Chan D., Salinas R., Woynarowska B., Woynarowski J. M. (2003). DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem. Pharmacol. 66 (2), 225–237. 10.1016/s0006-2952(03)00260-0 [DOI] [PubMed] [Google Scholar]
- Fang Z., Zhao J., Xie W., Sun Q., Wang H., Qiao B. (2017). LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression. Cancer Med. 6 (12), 2897–2908. 10.1002/cam4.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T., Chen W., Sheng Y., Yuan S., Tang Q., Li G., et al. (2019). Tetrathiomolybdate induces dimerization of the metal-binding domain of ATPase and inhibits platination of the protein. Nat. Commun. 10 (1), 186. 10.1038/s41467-018-08102-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes W. D., Shuen A. Y. (2013). In brief: BRCA1 and BRCA2. J. Pathol. 230 (4), 347–349. 10.1002/path.4205 [DOI] [PubMed] [Google Scholar]
- Fu S., Naing A., Fu C., Kuo M. T., Kurzrock R. (2012). Overcoming platinum resistance through the use of a copper-lowering agent. Mol. Cancer Ther. 11 (6), 1221–1225. 10.1158/1535-7163.MCT-11-0864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Cheng J., Zhang J., Zhang Y., Chen X., Xie J., et al. (2016). Downregulation of YEATS4 by miR-218 sensitizes colorectal cancer cells to L-OHP-induced cell apoptosis by inhibiting cytoprotective autophagy. Oncol. Rep. 36 (6), 3682–3690. 10.3892/or.2016.5195 [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Inoue J., Kawano T., Tanimoto K., Kozaki K., Inazawa J. (2015). miR-634 Activates the Mitochondrial Apoptosis Pathway and Enhances Chemotherapy-Induced Cytotoxicity. Cancer Res. 75 (18), 3890–3901. 10.1158/0008-5472.CAN-15-0257 [DOI] [PubMed] [Google Scholar]
- Fukuda T., Oda K., Wada-Hiraike O., Sone K., Inaba K., Ikeda Y., et al. (2015). The anti-malarial chloroquine suppresses proliferation and overcomes cisplatin resistance of endometrial cancer cells via autophagy inhibition. Gynecol. Oncol. 137 (3), 538–545. 10.1016/j.ygyno.2015.03.053 [DOI] [PubMed] [Google Scholar]
- Gadhikar M. A., Sciuto M. R., Alves M. V., Pickering C. R., Osman A. A., Neskey D. M., et al. (2013). Chk1/2 inhibition overcomes the cisplatin resistance of head and neck cancer cells secondary to the loss of functional p53. Mol. Cancer Ther. 12 (9), 1860–1873. 10.1158/1535-7163.MCT-13-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Wang W. (2019). Knockdown of galectin-1 facilitated cisplatin sensitivity by inhibiting autophagy in neuroblastoma cells. Chem. Biol. Interact. 297, 50–56. 10.1016/j.cbi.2018.10.014 [DOI] [PubMed] [Google Scholar]
- Gao Z. J., Yuan W. D., Yuan J. Q., Yuan K., Wang Y. (2018). Downregulation of HIF-2alpha Reverse the Chemotherapy Resistance of Lung Adenocarcinoma A549 Cells to Cisplatin. Med. Sci. Monit. 24, 1104–1111. 10.12659/msm.906107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Zhang S., Hu T., Qu X., Zhai J., Zhang Y., et al. (2019). Omeprazole protects against cisplatin-induced nephrotoxicity by alleviating oxidative stress, inflammation, and transporter-mediated cisplatin accumulation in rats and HK-2cells. Chem. Biol. Interact. 297, 130–140. 10.1016/j.cbi.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Gately D. P., Howell S. B. (1993). Cellular accumulation of the anticancer agent cisplatin: a review. Br. J. Cancer 67 (6), 1171–1176. 10.1038/bjc.1993.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet L. C., Scharer O. D. (2006). Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem. Rev. 106 (2), 253–276. 10.1021/cr040483f [DOI] [PubMed] [Google Scholar]
- Golan T., Kanji Z. S., Epelbaum R., Devaud N., Dagan E., Holter S., et al. (2014). Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br. J. Cancer 111 (6), 1132–1138. 10.1038/bjc.2014.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T., Cui L., Wang H., Wang H., Han N. (2018). Knockdown of KLF5 suppresses hypoxia-induced resistance to cisplatin in NSCLC cells by regulating HIF-1alpha-dependent glycolysis through inactivation of the PI3K/Akt/mTOR pathway. J. Transl. Med. 16 (1), 164. 10.1186/s12967-018-1543-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodspeed A., Jean A., Costello J. C. (2019). A Whole-genome CRISPR Screen Identifies a Role of MSH2 in Cisplatin-mediated Cell Death in Muscle-invasive Bladder Cancer. Eur. Urol. 75 (2), 242–250. 10.1016/j.eururo.2018.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf N., Ang W. H., Zhu G., Myint M., Lippard S. J. (2011). Role of endonucleases XPF and XPG in nucleotide excision repair of platinated DNA and cisplatin/oxaliplatin cytotoxicity. Chembiochem 12 (7), 1115–1123. 10.1002/cbic.201000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette S., Serra R. W., Peng M., Hayes J. A., Konstantinopoulos P. A., Green M. R., et al. (2015). Resistance to therapy in BRCA2 mutant cells due to loss of the nucleosome remodeling factor CHD4. Genes Dev. 29 (5), 489–494. 10.1101/gad.256214.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Lutsenko S. (2009). Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med. Chem. 1 (6), 1125–1142. 10.4155/fmc.09.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel N., Hamidouche Z., Girault I., Patino-Garcia A., Lecanda F., Marie P. J., et al. (2013). Zinc chelation: a metallothionein 2A’s mechanism of action involved in osteosarcoma cell death and chemotherapy resistance. Cell Death Dis. 4, e874. 10.1038/cddis.2013.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. D., Okabe M., Shen D.-W., Liang X.-J., Gottesman M. M. (2008). The Role of Cellular Accumulation in Determining Sensitivity to Platinum-Based Chemotherapy. Annu. Rev. Pharmacol. Toxicol. 48 (1), 495–535. 10.1146/annurev.pharmtox.48.080907.180426 [DOI] [PubMed] [Google Scholar]
- Hernandez-Luna M. A., Rocha-Zavaleta L., Vega M. I., Huerta-Yepez S. (2013). Hypoxia inducible factor-1alpha induces chemoresistance phenotype in non-Hodgkin lymphoma cell line via up-regulation of Bcl-xL. Leuk. Lymphoma 54 (5), 1048–1055. 10.3109/10428194.2012.733874 [DOI] [PubMed] [Google Scholar]
- Hernandez-Valencia J., Garcia-Villa E., Arenas-Hernandez A., Garcia-Mena J., Diaz-Chavez J., Gariglio P. (2018). Induction of p53 phosphorylation at serine 20 by resveratrol is required to activate p53 target genes, restoring apoptosis in MCF-7 cells resistant to cisplatin. Nutrients 10 (9), 1148. 10.3390/nu10091148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervouet E., Simonnet H., Godinot C. (2007). Mitochondria and reactive oxygen species in renal cancer. Biochimie 89 (9), 1080–1088. 10.1016/j.biochi.2007.03.010 [DOI] [PubMed] [Google Scholar]
- Holzer A. K., Howell S. B. (2006). The Internalization and Degradation of Human Copper Transporter 1 following Cisplatin Exposure. Cancer Res. 66 (22), 10944–10952. 10.1158/0008-5472.can-06-1710 [DOI] [PubMed] [Google Scholar]
- Holzer A. K., Manorek G. H., Howell S. B. (2006). Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharmacol. 70 (4), 1390–1394. 10.1124/mol.106.022624 [DOI] [PubMed] [Google Scholar]
- Howell S. B., Safaei R., Larson C. A., Sailor M. J. (2010). Copper Transporters and the Cellular Pharmacology of the Platinum-Containing Cancer Drugs. Mol. Pharmacol. 77 (6), 887–894. 10.1124/mol.109.063172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. P., Fofana M., Chan J., Chang C. J., Howell S. B. (2014). Copper transporter 2 regulates intracellular copper and sensitivity to cisplatin. Metallomics 6 (3), 654–661. 10.1039/c3mt00331k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Tang J., Zhang L., Bu Y., Zhang X. (2018). miR-874 regulates multiple-drug resistance in gastric cancer by targeting ATG16L1. Int. J. Oncol. 53 (6), 2769–2779. 10.3892/ijo.2018.4593 [DOI] [PubMed] [Google Scholar]
- Huang F. X., Chen H. J., Zheng F. X., Gao Z. Y., Sun P. F., Peng Q., et al. (2019). LncRNA BLACAT1 is involved in chemoresistance of nonsmall cell lung cancer cells by regulating autophagy. Int. J. Oncol. 54 (1), 339–347. 10.3892/ijo.2018.4614 [DOI] [PubMed] [Google Scholar]
- Hung J. J., Yang M. H., Hsu H. S., Hsu W. H., Liu J. S., Wu K. J. (2009). Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax 64 (12), 1082–1089. 10.1136/thx.2009.115691 [DOI] [PubMed] [Google Scholar]
- Ishida S., Lee J., Thiele D. J., Herskowitz I. (2002). Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. U. S. A 99 (22), 14298–14302. 10.1073/pnas.162491399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S., McCormick F., Smith-McCune K., Hanahan D. (2010). Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 17 (6), 574–583. 10.1016/j.ccr.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Ali-Osman F. (1993). Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J. Biol. Chem. 268 (27), 20116–20125. [PubMed] [Google Scholar]
- Issaeva N., Bozko P., Enge M., Protopopova M., Verhoef L. G., Masucci M., et al. (2004). Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat. Med. 10 (12), 1321–1328. 10.1038/nm1146 [DOI] [PubMed] [Google Scholar]
- Jamieson E. R., Lippard S. J. (1999). Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 99 (9), 2467–2498. 10.1021/cr980421n [DOI] [PubMed] [Google Scholar]
- Jedlitschky G., Leier I., Buchholz U., Center M., Keppler D. (1994). ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 54 (18), 4833–4836. [PubMed] [Google Scholar]
- Jennis M., Kung C. P., Basu S., Budina-Kolomets A., Leu J. I., Khaku S., et al. (2016). An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 30 (8), 918–930. 10.1101/gad.275891.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Zhang Q., Ren H., Ma S., Lu C., Liu B., et al. (2015). Dihydromyricetin Enhances the Chemo-Sensitivity of Nedaplatin via Regulation of the p53/Bcl-2 Pathway in Hepatocellular Carcinoma Cells. PloS One 10 (4), e0124994. 10.1371/journal.pone.0124994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Lippard S. J. (2007). Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 107 (5), 1387–1407. 10.1021/cr068207j [DOI] [PubMed] [Google Scholar]
- Kalayda G. V., Wagner C. H., Buß I., Reedijk J., Jaehde U. (2008). Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer 8 (1), 175. 10.1186/1471-2407-8-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartalou M., Essigmann J. M. (2001). Recognition of cisplatin adducts by cellular proteins. Mutat. Res. 478 (1-2), 1–21. 10.1016/s0027-5107(01)00142-7 [DOI] [PubMed] [Google Scholar]
- Keppler D. (2011). Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb. Exp. Pharmacol. 201, 299–323. 10.1007/978-3-642-14541-4_8 [DOI] [PubMed] [Google Scholar]
- Kim R., Tanabe K., Uchida Y., Emi M., Inoue H., Toge T. (2002). Current status of the molecular mechanisms of anticancer drug-induced apoptosis. The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother. Pharmacol. 50 (5), 343–352. 10.1007/s00280-002-0522-7 [DOI] [PubMed] [Google Scholar]
- Kim E. S., Tang X., Peterson D. R., Kilari D., Chow C. W., Fujimoto J., et al. (2014). Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer 85 (1), 88–93. 10.1016/j.lungcan.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. A., Kim T. G., Sung E. G., Song I. H., Kim J. Y., Doh K. O., et al. (2017. a). miR-148a increases the sensitivity to cisplatin by targeting Rab14 in renal cancer cells. Int. J. Oncol. 50 (3), 984–992. 10.3892/ijo.2017.3851 [DOI] [PubMed] [Google Scholar]
- Kim M., Jung J. Y., Choi S., Lee H., Morales L. D., Koh J. T., et al. (2017. b). GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy 13 (1), 149–168. 10.1080/15548627.2016.1239676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Kambe T. (2016). The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 17 (3), 336. 10.3390/ijms17030336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L. R., Chua K. N., Sim W. J., Ng H. C., Bi C., Ho J., et al. (2015). MEK Inhibition Overcomes Cisplatin Resistance Conferred by SOS/MAPK Pathway Activation in Squamous Cell Carcinoma. Mol. Cancer Ther. 14 (7), 1750–1760. 10.1158/1535-7163.MCT-15-0062 [DOI] [PubMed] [Google Scholar]
- Krizkova S., Fabrik I., Huska D., Adam V., Babula P., Hrabeta J., et al. (2010). An adsorptive transfer technique coupled with brdicka reaction to reveal the importance of metallothionein in chemotherapy with platinum based cytostatics. Int. J. Mol. Sci. 11 (12), 4826–4842. 10.3390/ijms11124826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J. P., Gu W. (2009). Modes of p53 regulation. Cell 137 (4), 609–622. 10.1016/j.cell.2009.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Koul S., Khandrika L., Meacham R. B., Koul H. K. (2008). Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 68 (6), 1777–1785. 10.1158/0008-5472.can-07-5259 [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh U. K., Chaudhary A. (2015). Targeting autophagy to overcome drug resistance in cancer therapy. Future Med. Chem. 7 (12), 1535–1542. 10.4155/fmc.15.88 [DOI] [PubMed] [Google Scholar]
- Kuwahara A., Yamamori M., Nishiguchi K., Okuno T., Chayahara N., Miki I., et al. (2009). Replacement of cisplatin with nedaplatin in a definitive 5-fluorouracil/cisplatin-based chemoradiotherapy in Japanese patients with esophageal squamous cell carcinoma. Int. J. Med. Sci. 6 (6), 305–311. 10.7150/ijms.6.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Chae J. W., Kim J. K., Kim H. J., Chung J. Y., Kim Y. H. (2015). Inhibition of cisplatin-resistance by RNA interference targeting metallothionein using reducible oligo-peptoplex. J. Control Release 215, 82–90. 10.1016/j.jconrel.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Lehmann J., Seebode C., Smolorz S., Schubert S., Emmert S. (2017). XPF knockout via CRISPR/Cas9 reveals that ERCC1 is retained in the cytoplasm without its heterodimer partner XPF. Cell Mol. Life Sci. 74 (11), 2081–2094. 10.1007/s00018-017-2455-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yu J. J., Mu C., Yunmbam M. K., Slavsky D., Cross C. L., et al. (2000). Association between the level of ERCC-1 expression and the repair of cisplatin-induced DNA damage in human ovarian cancer cells. Anticancer Res. 20 (2a), 645–652. [PubMed] [Google Scholar]
- Li H., Fan X., Houghton J. (2007). Tumor microenvironment: the role of the tumor stroma in cancer. J. Cell Biochem. 101 (4), 805–815. 10.1002/jcb.21159 [DOI] [PubMed] [Google Scholar]
- Li X., He Y., Xu Y., Huang X., Liu J., Xie M., et al. (2016. a). KLF5 mediates vascular remodeling via HIF-1alpha in hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 310 (4), L299–L310. 10.1152/ajplung.00189.2015 [DOI] [PubMed] [Google Scholar]
- Li X., Lin Z., Zhang B., Guo L., Liu S., Li H., et al. (2016. b). beta-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci. Rep. 6, 21010. 10.1038/srep21010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu B., Yang F., Yu Y., Zeng A., Ye T., et al. (2016. c). Lobaplatin induces BGC-823 human gastric carcinoma cell apoptosis via ROS- mitochondrial apoptotic pathway and impairs cell migration and invasion. BioMed. Pharmacother. 83, 1239–1246. 10.1016/j.biopha.2016.08.053 [DOI] [PubMed] [Google Scholar]
- Li B., Wang W., Li Z., Chen Z., Zhi X., Xu J., et al. (2017. a). MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 410, 212–227. 10.1016/j.canlet.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo K., Yin Y., Wu C., Deng M., Li L., et al. (2017. b). USP13 regulates the RAP80-BRCA1 complex dependent DNA damage response. Nat. Commun. 8, 15752. 10.1038/ncomms15752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zeng J., Huang M., An J., Bai P., Wu L., et al. (2017. c). A phase 2 study of nanoparticle albumin-bound paclitaxel plus nedaplatin for patients with advanced, recurrent, or metastatic cervical carcinoma. Cancer 123 (3), 420–425. 10.1002/cncr.30328 [DOI] [PubMed] [Google Scholar]
- Liang L., Zhang Z., Qin X., Gao Y., Zhao P., Liu J., et al. (2018). Gambogic Acid Inhibits Melanoma through Regulation of miR-199a-3p/ZEB1 Signalling. Basic Clin. Pharmacol. Toxicol. 123 (6), 692–703. 10.1111/bcpt.13090 [DOI] [PubMed] [Google Scholar]
- Liu W., Zhang Z., Zhang Y., Chen X., Guo S., Lei Y., et al. (2015. a). HMGB1-mediated autophagy modulates sensitivity of colorectal cancer cells to oxaliplatin via MEK/ERK signaling pathway. Cancer Biol. Ther. 16 (4), 511–517. 10.1080/15384047.2015.1017691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. C., Chang P. Y., Chao C. C. (2015. b). CITED2 silencing sensitizes cancer cells to cisplatin by inhibiting p53 trans-activation and chromatin relaxation on the ERCC1 DNA repair gene. Nucleic Acids Res. 43 (22), 10760–10781. 10.1093/nar/gkv934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zheng X., Yu Q., Wang H., Tan F., Zhu Q., et al. (2016). Epigenetic activation of the drug transporter OCT2 sensitizes renal cell carcinoma to oxaliplatin. Sci. Transl. Med. 8 (348), 348ra397. 10.1126/scitranslmed.aaf3124 [DOI] [PubMed] [Google Scholar]
- Liu C. W., Hua K. T., Li K. C., Kao H. F., Hong R. L., Ko J. Y., et al. (2017. a). Histone Methyltransferase G9a Drives Chemotherapy Resistance by Regulating the Glutamate-Cysteine Ligase Catalytic Subunit in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 16 (7), 1421–1434. 10.1158/1535-7163.MCT-16-0567-T [DOI] [PubMed] [Google Scholar]
- Liu W., Chen H., Wong N., Haynes W., Baker C. M., Wang X. (2017. b). Pseudohypoxia induced by miR-126 deactivation promotes migration and therapeutic resistance in renal cell carcinoma. Cancer Lett. 394, 65–75. 10.1016/j.canlet.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Niu Z., Lin X., Tian Y. (2017. c). MiR-216b increases cisplatin sensitivity in ovarian cancer cells by targeting PARP1. Cancer Gene Ther. 24 (5), 208–214. 10.1038/cgt.2017.6 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhu S. T., Wang X., Deng J., Li W. H., Zhang P., et al. (2017. d). MiR-200c regulates tumor growth and chemosensitivity to cisplatin in osteosarcoma by targeting AKT2. Sci. Rep. 7 (1), 13598. 10.1038/s41598-017-14088-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xing Y., Rong L. (2018). miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncol. Rep. 39 (4), 1631–1639. 10.3892/or.2018.6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Xu Y., Su J., Yu H., Kang J., Li H., et al. (2015. a). Autophagic flux promotes cisplatin resistance in human ovarian carcinoma cells through ATP-mediated lysosomal function. Int. J. Oncol. 47 (5), 1890–1900. 10.3892/ijo.2015.3176 [DOI] [PubMed] [Google Scholar]
- Ma M. Z., Chen G., Wang P., Lu W. H., Zhu C. F., Song M., et al. (2015. b). Xc- inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH-dependent mechanism. Cancer Lett. 368 (1), 88–96. 10.1016/j.canlet.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Ma B., Yuan Z., Zhang L., Lv P., Yang T., Gao J., et al. (2017). Long non-coding RNA AC023115.3 suppresses chemoresistance of glioblastoma by reducing autophagy. Biochim. Biophys. Acta Mol. Cell Res. 1864 (8), 1393–1404. 10.1016/j.bbamcr.2017.05.008 [DOI] [PubMed] [Google Scholar]
- MacDonagh L., Gray S. G., Breen E., Cuffe S., Finn S. P., O’Byrne K. J., et al. (2016). Lung cancer stem cells: The root of resistance. Cancer Lett. 372 (2), 147–156. 10.1016/j.canlet.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Marchenko N. D., Moll U. M. (2007). The role of ubiquitination in the direct mitochondrial death program of p53. Cell Cycle 6 (14), 1718–1723. 10.4161/cc.6.14.4503 [DOI] [PubMed] [Google Scholar]
- Martens-de Kemp S. R., Brink A., van der Meulen I. H., de Menezes R. X., Te Beest D. E., Leemans C. R., et al. (2017). The FA/BRCA Pathway Identified as the Major Predictor of Cisplatin Response in Head and Neck Cancer by Functional Genomics. Mol. Cancer Ther. 16 (3), 540–550. 10.1158/1535-7163.MCT-16-0457 [DOI] [PubMed] [Google Scholar]
- Martinez-Balibrea E., Martinez-Cardus A., Musulen E., Gines A., Manzano J. L., Aranda E., et al. (2009). Increased levels of copper efflux transporter ATP7B are associated with poor outcome in colorectal cancer patients receiving oxaliplatin-based chemotherapy. Int. J. Cancer 124 (12), 2905–2910. 10.1002/ijc.24273 [DOI] [PubMed] [Google Scholar]
- Martinez-Rivera M., Siddik Z. H. (2012). Resistance and gain-of-resistance phenotypes in cancers harboring wild-type p53. Biochem. Pharmacol. 83 (8), 1049–1062. 10.1016/j.bcp.2011.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters J. R., Thomas R., Hall A. G., Hogarth L., Matheson E. C., Cattan A. R., et al. (1996). Sensitivity of testis tumour cells to chemotherapeutic drugs: role of detoxifying pathways. Eur. J. Cancer 32a (7), 1248–1253. 10.1016/0959-8049(96)00033-0 [DOI] [PubMed] [Google Scholar]
- McKeage M. J. (1995). Comparative adverse effect profiles of platinum drugs. Drug Saf. 13 (4), 228–244. 10.2165/00002018-199513040-00003 [DOI] [PubMed] [Google Scholar]
- Meng Y., Chen C. W., Yung M. M. H., Sun W., Sun J., Li Z., et al. (2018). DUOXA1-mediated ROS production promotes cisplatin resistance by activating ATR-Chk1 pathway in ovarian cancer. Cancer Lett. 428, 104–116. 10.1016/j.canlet.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miow Q. H., Tan T. Z., Ye J., Lau J. A., Yokomizo T., Thiery J. P., et al. (2015). Epithelial-mesenchymal status renders differential responses to cisplatin in ovarian cancer. Oncogene 34 (15), 1899–1907. 10.1038/onc.2014.136 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Klionsky D. J. (2007). Protein turnover via autophagy: implications for metabolism. Annu. Rev. Nutr. 27, 19–40. 10.1146/annurev.nutr.27.061406.093749 [DOI] [PubMed] [Google Scholar]
- Muggia F., Safra T. (2014). ‘BRCAness’ and its implications for platinum action in gynecologic cancer. Anticancer Res. 34 (2), 551–556. [PMC free article] [PubMed] [Google Scholar]
- Myint K., Li Y., Paxton J., McKeage M. (2015). Multidrug Resistance-Associated Protein 2 (MRP2) Mediated Transport of Oxaliplatin-Derived Platinum in Membrane Vesicles. PloS One 10 (7), e0130727. 10.1371/journal.pone.0130727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao A., Kobayashi M., Koyasu S., Chow C. C. T., Harada H. (2019). HIF-1-Dependent Reprogramming of Glucose Metabolic Pathway of Cancer Cells and Its Therapeutic Significance. Int. J. Mol. Sci. 20 (2), 238. 10.3390/ijms20020238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P. P., Mukhopadhyay S., Panda P. K., Sinha N., Das C. K., Mishra R., et al. (2018). Autophagy regulates cisplatin-induced stemness and chemoresistance via the upregulation of CD44, ABCB1 and ADAM17 in oral squamous cell carcinoma. Cell Prolif 51 (1), e12411. 10.1111/cpr.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norquist B., Wurz K. A., Pennil C. C., Garcia R., Gross J., Sakai W., et al. (2011). Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 29 (22), 3008–3015. 10.1200/JCO.2010.34.2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrvik H., Logeman B., Turk B., Reinheckel T., Thiele D. J. (2016). Cathepsin Protease Controls Copper and Cisplatin Accumulation via Cleavage of the Ctr1 Metal-binding Ectodomain. J. Biol. Chem. 291 (27), 13905–13916. 10.1074/jbc.M116.731281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oien D. B., Garay T., Eckstein S., Chien J. (2017). Cisplatin and Pemetrexed Activate AXL and AXL Inhibitor BGB324 Enhances Mesothelioma Cell Death from Chemotherapy. Front. Pharmacol. 8, 970. 10.3389/fphar.2017.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada R., Horiuchi A., Kikuchi N., Yoshida J., Hayashi A., Ota M., et al. (2007). Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: nuclear expression of hypoxia-inducible factor 1alpha is an independent prognostic factor in ovarian carcinoma. Hum. Pathol. 38 (9), 1310–1320. 10.1016/j.humpath.2007.02.010 [DOI] [PubMed] [Google Scholar]
- Ozcan M. F., Dizdar O., Dincer N., Balci S., Guler G., Gok B., et al. (2013). Low ERCC1 expression is associated with prolonged survival in patients with bladder cancer receiving platinum-based neoadjuvant chemotherapy. Urol. Oncol. 31 (8), 1709–1715. 10.1016/j.urolonc.2012.06.014 [DOI] [PubMed] [Google Scholar]
- Pan H., Cai N., Li M., Liu G. H., Izpisua Belmonte J. C. (2013. a). Autophagic control of cell ‘stemness’. EMBO Mol. Med. 5 (3), 327–331. 10.1002/emmm.201201999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang Q., Atsaves V., Yang H., Claret F. X. (2013. b). Suppression of Jab1/CSN5 induces radio- and chemo-sensitivity in nasopharyngeal carcinoma through changes to the DNA damage and repair pathways. Oncogene 32 (22), 2756–2766. 10.1038/onc.2012.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Li X., Wu W., Xue M., Hou H., Zhai W., et al. (2016). Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 382 (1), 64–76. 10.1016/j.canlet.2016.08.015 [DOI] [PubMed] [Google Scholar]
- Pan C., Jin L., Wang X., Li Y., Chun J., Boese A. C., et al. (2019). Inositol-triphosphate 3-kinase B confers cisplatin resistance by regulating NOX4-dependent redox balance. J. Clin. Invest. 129 (6), 2431–2445. 10.1172/jci124550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannuru P., Dontula R., Khan A. A., Herbert E., Ozer H., Chetty C., et al. (2014). miR-let-7f-1 regulates SPARC mediated cisplatin resistance in medulloblastoma cells. Cell Signal. 26 (10), 2193–2201. 10.1016/j.cellsig.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Pawlus M. R., Hu C. J. (2013). Enhanceosomes as integrators of hypoxia inducible factor (HIF) and other transcription factors in the hypoxic transcriptional response. Cell Signal 25 (9), 1895–1903. 10.1016/j.cellsig.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarik V., Gumulec J., Masarik M., Kizek R., Adam V. (2013). Prostate cancer, miRNAs, metallothioneins and resistance to cytostatic drugs. Curr. Med. Chem. 20 (4), 534–544. 10.2174/0929867311320040005 [DOI] [PubMed] [Google Scholar]
- Peng P. J., Lv B. J., Wang Z. H., Liao H., Liu Y. M., Lin Z., et al. (2017). Multi-institutional prospective study of nedaplatin plus S-1 chemotherapy in recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-containing regimens. Ther. Adv. Med. Oncol. 9 (2), 68–74. 10.1177/1758834016675099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perland E., Fredriksson R. (2017). Classification Systems of Secondary Active Transporters. Trends Pharmacol. Sci. 38 (3), 305–315. 10.1016/j.tips.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Qin X., Guo H., Wang X., Zhu X., Yan M., Wang X., et al. (2019). Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 20 (1), 12. 10.1186/s13059-018-1604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss S., Sen S., Ramachandran I., Roy S., Chaudhuri G., Farias-Eisner R. (2017). Lysine-specific demethylase KDM3A regulates ovarian cancer stemness and chemoresistance. Oncogene 36 (46), 6508. 10.1038/onc.2017.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T., Sontag E., Chen P., Levine A. (2008). Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 9 (5), 402–412. 10.1038/nrm2395 [DOI] [PubMed] [Google Scholar]
- Rocha C. R., Garcia C. C., Vieira D. B., Quinet A., de Andrade-Lima L. C., Munford V., et al. (2015). Glutathione depletion sensitizes cisplatin- and temozolomide-resistant glioma cells in vitro and in vivo. Cell Death Dis. 6, e1727. 10.1038/cddis.2015.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer N., Dame C., Haugstetter A., Wiedenmann B., Detjen K., Schmitt C. A., et al. (2010). Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PloS One 5 (8), e12038. 10.1371/journal.pone.0012038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos W. P., Kaina B. (2013). DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 332 (2), 237–248. 10.1016/j.canlet.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Safaei R., Otani S., Larson B. J., Rasmussen M. L., Howell S. B. (2008). Transport of cisplatin by the copper efflux transporter ATP7B. Mol. Pharmacol. 73 (2), 461–468. 10.1124/mol.107.040980 [DOI] [PubMed] [Google Scholar]
- Safaei R., Adams P. L., Maktabi M. H., Mathews R. A., Howell S. B. (2012). The CXXC motifs in the metal binding domains are required for ATP7B to mediate resistance to cisplatin. J. Inorg. Biochem. 110, 8–17. 10.1016/j.jinorgbio.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimi G., Katano K., Holzer A. K., Safaei R., Howell S. B. (2004). Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol. Pharmacol. 66 (1), 25–32. 10.1124/mol.66.1.25 [DOI] [PubMed] [Google Scholar]
- Sawant A., Kothandapani A., Zhitkovich A., Sobol R. W., Patrick S. M. (2015). Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair (Amst.) 35, 126–136. 10.1016/j.dnarep.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrock M. S., Batar B., Lee J., Druck T., Ferguson B., Cho J. H., et al. (2017). Wwox-Brca1 interaction: role in DNA repair pathway choice. Oncogene 36 (16), 2215–2227. 10.1038/onc.2016.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Xu Z., Wu G., Chen X., Huang Y., Wang Y., et al. (2017). Up-regulation of miR-146a increases the sensitivity of non-small cell lung cancer to DDP by downregulating cyclin J. BMC Cancer 17 (1), 138. 10.1186/s12885-017-3132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteingauz A., Boyango I., Naroditsky I., Hammond E., Gruber M., Doweck I., et al. (2015). Heparanase Enhances Tumor Growth and Chemoresistance by Promoting Autophagy. Cancer Res. 75 (18), 3946–3957. 10.1158/0008-5472.CAN-15-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasagara R. R., Spencer S. M., Tripathi K., Clark D. W., Mani C., Madeira da Silva L., et al. (2017). RAD6 promotes DNA repair and stem cell signaling in ovarian cancer and is a promising therapeutic target to prevent and treat acquired chemoresistance. Oncogene 36 (48), 6680–6690. 10.1038/onc.2017.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J., Wilson L. A., Kilday J. P., Horvilleur E., Cannell I. G., Poyry T. A., et al. (2015). A common polymorphism in the 5’ UTR of ERCC5 creates an upstream ORF that confers resistance to platinum-based chemotherapy. Genes Dev. 29 (18), 1891–1896. 10.1101/gad.261867.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I. S., Savaraj N., Siddik Z. H., Liu P., Wei Y., Wu C. J., et al. (2004). Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol. Cancer Ther. 3 (12), 1543–1549. [PubMed] [Google Scholar]
- Song L., Li Y., Li W., Wu S., Li Z. (2014). miR-495 enhances the sensitivity of non-small cell lung cancer cells to platinum by modulation of copper-transporting P-type adenosine triphosphatase A (ATP7A). J. Cell Biochem. 115 (7), 1234–1242. 10.1002/jcb.24665 [DOI] [PubMed] [Google Scholar]
- Soni S., Padwad Y. S. (2017). HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta Oncol. 56 (4), 503–515. 10.1080/0284186X.2017.1301680 [DOI] [PubMed] [Google Scholar]
- Soyano A. E., Baldeo C., Kasi P. M. (2018). BRCA Mutation and Its Association With Colorectal Cancer. Clin. Colorectal Cancer 17 (4), e647–e650. 10.1016/j.clcc.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Spada F., Antonuzzo L., Marconcini R., Radice D., Antonuzzo A., Ricci S., et al. (2016). Oxaliplatin-Based Chemotherapy in Advanced Neuroendocrine Tumors: Clinical Outcomes and Preliminary Correlation with Biological Factors. Neuroendocrinology 103 (6), 806–814. 10.1159/000444087 [DOI] [PubMed] [Google Scholar]
- Sprowl J. A., Ong S. S., Gibson A. A., Hu S., Du G., Lin W., et al. (2016). A phosphotyrosine switch regulates organic cation transporters. Nat. Commun. 7, 10880. 10.1038/ncomms10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbichler T. B., Dudas J., Skvortsov S., Ganswindt U., Riechelmann H., Skvortsova ,. I. I. (2018). Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol. 53, 156–167. 10.1016/j.semcancer.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Stransky N., Egloff A. M., Tward A. D., Kostic A. D., Cibulskis K., Sivachenko A., et al. (2011). The mutational landscape of head and neck squamous cell carcinoma. Science 333 (6046), 1157–1160. 10.1126/science.1208130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui C., Meng F., Li Y., Jiang Y. (2015). miR-148b reverses cisplatin-resistance in non-small cell cancer cells via negatively regulating DNA (cytosine-5)-methyltransferase 1(DNMT1) expression. J. Transl. Med. 13, 132. 10.1186/s12967-015-0488-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan I., Salazar J., Majem M., Pallares C., Del Rio E., Paez D., et al. (2014). Pharmacogenetics of the DNA repair pathways in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Lett. 353 (2), 160–166. 10.1016/j.canlet.2014.07.023 [DOI] [PubMed] [Google Scholar]
- Sun Y., Guan Z., Liang L., Cheng Y., Zhou J., Li J., et al. (2016). NF-kappaB signaling plays irreplaceable roles in cisplatin-induced bladder cancer chemoresistance and tumor progression. Int. J. Oncol. 48 (1), 225–234. 10.3892/ijo.2015.3256 [DOI] [PubMed] [Google Scholar]
- Sun C., Wang F. J., Zhang H. G., Xu X. Z., Jia R. C., Yao L., et al. (2017. a). miR-34a mediates oxaliplatin resistance of colorectal cancer cells by inhibiting macroautophagy via transforming growth factor-beta/Smad4 pathway. World J. Gastroenterol. 23 (10), 1816–1827. 10.3748/wjg.v23.i10.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Pan L. M., Chen L. B., Wang Y. (2017. b). LncRNA XIST promotes human lung adenocarcinoma cells to cisplatin resistance via let-7i/BAG-1 axis. Cell Cycle 16 (21), 2100–2107. 10.1080/15384101.2017.1361071 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun J., Cai X., Yung M. M., Zhou W., Li J., Zhang Y., et al. (2019). miR-137 mediates the functional link between c-Myc and EZH2 that regulates cisplatin resistance in ovarian cancer. Oncogene 38 (4), 564–580. 10.1038/s41388-018-0459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surowiak P., Materna V., Kaplenko I., Spaczynski M., Dolinska-Krajewska B., Gebarowska E., et al. (2006). ABCC2 (MRP2, cMOAT) can be localized in the nuclear membrane of ovarian carcinomas and correlates with resistance to cisplatin and clinical outcome. Clin. Cancer Res. 12 (23), 7149–7158. 10.1158/1078-0432.ccr-06-0564 [DOI] [PubMed] [Google Scholar]
- Takekuma M., Shimokawa M., Nishio S., Omi H., Tabata T., Takei Y., et al. (2018). Phase II study of adjuvant chemotherapy with paclitaxel and nedaplatin for uterine cervical cancer with lymph node metastasis. Cancer Sci. 109 (5), 1602–1608. 10.1111/cas.13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Shi H., Ba M., Lin S., Tang H., Zeng X., et al. (2016). miR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int. J. Mol. Med. 37 (4), 1030–1038. 10.3892/ijmm.2016.2492 [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Wada M., Kohno K., Nakamura T., Kawabe T., Kawakami M., et al. (1996). A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 56 (18), 4124–4129. [PubMed] [Google Scholar]
- Tao L., Huang G., Wang R., Pan Y., He Z., Chu X., et al. (2016). Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci. Rep. 6, 38408. 10.1038/srep38408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariba B., Zivkovic T., Krasnici N., Marijic V. F., Erk M., Gamulin M., et al. (2015). Serum metallothionein in patients with testicular cancer. Cancer Chemother. Pharmacol. 75 (4), 813–820. 10.1007/s00280-015-2702-2 [DOI] [PubMed] [Google Scholar]
- Ushijima R., Takayama K., Izumi M., Harada T., Horiuchi Y., Uchino J., et al. (2007). Immunohistochemical expression of MRP2 and clinical resistance to platinum-based chemotherapy in small cell lung cancer. Anticancer Res. 27 (6c), 4351–4358. [PubMed] [Google Scholar]
- van den Berghe P. V., Folmer D. E., Malingre H. E., van Beurden E., Klomp A. E., van de Sluis B., et al. (2007). Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem. J. 407 (1), 49–59. 10.1042/bj20070705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Jaarsveld M. T., van Kuijk P. F., Boersma A. W., Helleman J., van I. W. F., Mathijssen R. H., et al. (2015). miR-634 restores drug sensitivity in resistant ovarian cancer cells by targeting the Ras-MAPK pathway. Mol. Cancer 14, 196. 10.1186/s12943-015-0464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L. T., Vu B. T., Graves B., Carvajal D., Podlaski F., Filipovic Z., et al. (2004). In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303 (5659), 844–848. 10.1126/science.1092472 [DOI] [PubMed] [Google Scholar]
- Wang X., Jiang P., Wang P., Yang C. S., Wang X., Feng Q. (2015). EGCG Enhances Cisplatin Sensitivity by Regulating Expression of the Copper and Cisplatin Influx Transporter CTR1 in Ovary Cancer. PloS One 10 (4), e0125402. 10.1371/journal.pone.0125402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhou J. Y., Kho D., Reiners J. J., Jr., Wu G. S. (2016. a). Role for DUSP1 (dual-specificity protein phosphatase 1) in the regulation of autophagy. Autophagy 12 (10), 1791–1803. 10.1080/15548627.2016.1203483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Kryczek I., Dostal L., Lin H., Tan L., Zhao L., et al. (2016. b). Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 165 (5), 1092–1105. 10.1016/j.cell.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhu W., Zhao X., Wang P. (2016. c). miR-133a enhances the sensitivity of Hep-2 cells and vincristine-resistant Hep-2v cells to cisplatin by downregulating ATP7B expression. Int. J. Mol. Med. 37 (6), 1636–1642. 10.3892/ijmm.2016.2569 [DOI] [PubMed] [Google Scholar]
- Wang S. F., Wung C. H., Chen M. S., Chen C. F., Yin P. H., Yeh T. S., et al. (2018). Activated integrated stress response induced by salubrinal promotes cisplatin resistance in human gastric cancer cells via enhanced xCT expression and glutathione biosynthesis. Int. J. Mol. Sci. 19 (11), 3389. 10.3390/ijms19113389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Song L., Xu Y., Zhang L., Wu Y., Guo J., et al. (2019. a). Loss of Scribble confers cisplatin resistance during NSCLC chemotherapy via Nox2/ROS and Nrf2/PD-L1 signaling. EBioMedicine 47, 65–77. 10.1016/j.ebiom.2019.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Deng Z., Zhu G. (2019. b). Emerging platinum(iv) prodrugs to combat cisplatin resistance: from isolated cancer cells to tumor microenvironment. Dalton Trans. 48 (8), 2536–2544. 10.1039/c8dt03923b [DOI] [PubMed] [Google Scholar]
- Wang Z., Liu G., Jiang J. (2019. c). Profiling of apoptosis- and autophagy-associated molecules in human lung cancer A549 cells in response to cisplatin treatment using stable isotope labeling with amino acids in cell culture. Int. J. Oncol. 54 (3), 1071–1085. 10.3892/ijo.2019.4690 [DOI] [PubMed] [Google Scholar]
- Wanzel M., Vischedyk J. B., Gittler M. P., Gremke N., Seiz J. R., Hefter M., et al. (2016). CRISPR-Cas9-based target validation for p53-reactivating model compounds. Nat. Chem. Biol. 12 (1), 22–28. 10.1038/nchembio.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheate N. J., Walker S., Craig G. E., Oun R. (2010). The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 39 (35), 8113–8127. 10.1039/c0dt00292e [DOI] [PubMed] [Google Scholar]
- Wigerup C., Pahlman S., Bexell D. (2016). Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 164, 152–169. 10.1016/j.pharmthera.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Woynarowski J. M., Faivre S., Herzig M. C., Arnett B., Chapman W. G., Trevino A. V., et al. (2000). Oxaliplatin-induced damage of cellular DNA. Mol. Pharmacol. 58 (5), 920–927. 10.1124/mol.58.5.920 [DOI] [PubMed] [Google Scholar]
- Wu B., Cui J., Yang X. M., Liu Z. Y., Song F., Li L., et al. (2017). Cytoplasmic fragment of CD147 generated by regulated intramembrane proteolysis contributes to HCC by promoting autophagy. Cell Death Dis. 8 (7), e2925. 10.1038/cddis.2017.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne P., Newton C., Ledermann J. A., Olaitan A., Mould T. A., Hartley J. A. (2007). Enhanced repair of DNA interstrand crosslinking in ovarian cancer cells from patients following treatment with platinum-based chemotherapy. Br. J. Cancer 97 (7), 927–933. 10.1038/sj.bjc.6603973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z., Si J., Nan J. (2019). LncRNA MALAT1 potentiates autophagyassociated cisplatin resistance by regulating the microRNA30b/autophagyrelated gene 5 axis in gastric cancer. Int. J. Oncol. 54 (1), 239–248. 10.3892/ijo.2018.4609 [DOI] [PubMed] [Google Scholar]
- Xiao M., Cai J., Cai L., Jia J., Xie L., Zhu Y., et al. (2017). Let-7e sensitizes epithelial ovarian cancer to cisplatin through repressing DNA double strand break repair. J. Ovarian Res. 10 (1), 24. 10.1186/s13048-017-0321-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Li Y., Wan Y., Xue M. (2018). MircroRNA-139 sensitizes ovarian cancer cell to cisplatin-based chemotherapy through regulation of ATP7A/B. Cancer Chemother. Pharmacol. 81 (5), 935–947. 10.1007/s00280-018-3548-1 [DOI] [PubMed] [Google Scholar]
- Xie X., He G., Siddik Z. H. (2017). Functional Activation of Mutant p53 by Platinum Analogues in Cisplatin-Resistant Cells Is Dependent on Phosphorylation. Mol. Cancer Res. 15 (3), 328–339. 10.1158/1541-7786.MCR-16-0257-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Chen Q., Wang Q., Sun Y., Wang S., Li A., et al. (2014). JWA reverses cisplatin resistance via the CK2-XRCC1 pathway in human gastric cancer cells. Cell Death Dis. 5, e1551. 10.1038/cddis.2014.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Gao W., Zhang Y., Wu S., Liu Y., Deng X., et al. (2018). ABT737 reverses cisplatin resistance by targeting glucose metabolism of human ovarian cancer cells. Int. J. Oncol. 53 (3), 1055–1068. 10.3892/ijo.2018.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Tsujinaka T., Shiozaki H., Doki Y., Tamura S., Inoue M., et al. (1999). Metallothionein expression correlates with the pathological response of patients with esophageal cancer undergoing preoperative chemoradiation therapy. Oncology 56 (4), 332–337. 10.1159/000011988 [DOI] [PubMed] [Google Scholar]
- Yamasaki M., Makino T., Masuzawa T., Kurokawa Y., Miyata H., Takiguchi S., et al. (2011). Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br. J. Cancer 104 (4), 707–713. 10.1038/sj.bjc.6606071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneff A., Sahores A., Gomez N., Carozzo A., Shayo C., Davio C. (2019). MRP4/ABCC4 As a New Therapeutic Target: Meta-Analysis to Determine cAMP Binding Sites as a Tool for Drug Design. Curr. Med. Chem. 26 (7), 1270–1307. 10.2174/0929867325666171229133259 [DOI] [PubMed] [Google Scholar]
- Yang H. Z., Ma Y., Zhou Y., Xu L. M., Chen X. J., Ding W. B., et al. (2015). Autophagy contributes to the enrichment and survival of colorectal cancer stem cells under oxaliplatin treatment. Cancer Lett. 361 (1), 128–136. 10.1016/j.canlet.2015.02.045 [DOI] [PubMed] [Google Scholar]
- Yang L., Lin Z., Wang Y., Gao S., Li Q., Li C., et al. (2018). MiR-5100 increases the cisplatin resistance of the lung cancer stem cells by inhibiting the Rab6. Mol. Carcinog. 57 (3), 419–428. 10.1002/mc.22765 [DOI] [PubMed] [Google Scholar]
- Yang G., Fu Y., Lu X., Wang M., Dong H., Li Q. (2019). miR34a regulates the chemosensitivity of retinoblastoma cells via modulation of MAGEA/p53 signaling. Int. J. Oncol. 54 (1), 177–187. 10.3892/ijo.2018.4613 [DOI] [PubMed] [Google Scholar]
- Yokoo S., Yonezawa A., Masuda S., Fukatsu A., Katsura T., Inui K. (2007). Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem. Pharmacol. 74 (3), 477–487. 10.1016/j.bcp.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Yonezawa A., Masuda S., Yokoo S., Katsura T., Inui K. (2006). Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family). J. Pharmacol. Exp. Ther. 319 (2), 879–886. 10.1124/jpet.106.110346 [DOI] [PubMed] [Google Scholar]
- Yu L., Gu C., Zhong D., Shi L., Kong Y., Zhou Z., et al. (2014). Induction of autophagy counteracts the anticancer effect of cisplatin in human esophageal cancer cells with acquired drug resistance. Cancer Lett. 355 (1), 34–45. 10.1016/j.canlet.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Zarogoulidis P., Petanidis S., Kioseoglou E., Domvri K., Anestakis D., Zarogoulidis K. (2015). MiR-205 and miR-218 expression is associated with carboplatin chemoresistance and regulation of apoptosis via Mcl-1 and Survivin in lung cancer cells. Cell Signal 27 (8), 1576–1588. 10.1016/j.cellsig.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Zhang Y. H., Wu Q., Xiao X. Y., Li D. W., Wang X. P. (2010). Silencing MRP4 by small interfering RNA reverses acquired DDP resistance of gastric cancer cell. Cancer Lett. 291 (1), 76–82. 10.1016/j.canlet.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Zhang X. W., Bu P., Liu L., Zhang X. Z., Li J. (2015). Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem. Biophys. Res. Commun. 462 (3), 227–232. 10.1016/j.bbrc.2015.04.121 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Shi J., Yuan F., Wang H., Fu W., Pan J., et al. (2016. a). Higher expression of XPF is a critical factor in intrinsic chemotherapy resistance of human renal cell carcinoma. Int. J. Cancer 139 (12), 2827–2837. 10.1002/ijc.30396 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu W., Ni P., Li A., Zhou J., Xu S. (2016. b). MiR-99a and MiR-491 Regulate Cisplatin Resistance in Human Gastric Cancer Cells by Targeting CAPNS1. Int. J. Biol. Sci. 12 (12), 1437–1447. 10.7150/ijbs.16529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang Y., Li X., Xia X., Li N., He R., et al. (2017). ZBTB7A Enhances Osteosarcoma Chemoresistance by Transcriptionally Repressing lncRNALINC00473-IL24 Activity. Neoplasia 19 (11), 908–918. 10.1016/j.neo.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Li W., Zhou Y., Zhang Y., Huang S., Xu X., et al. (2015). The overexpression and nuclear translocation of Trx-1 during hypoxia confers on HepG2 cells resistance to DDP, and GL-V9 reverses the resistance by suppressing the Trx-1/Ref-1 axis. Free Radic. Biol. Med. 82, 29–41. 10.1016/j.freeradbiomed.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Tan B. B., Li Y., Fan L. Q., Yang P. G., Tian Y. (2016). Enhancement of Drug Sensitivity by Knockdown of HIF-1alpha in Gastric Carcinoma Cells. Oncol. Res. 23 (3), 129–136. 10.3727/096504015x14500513118029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Li X., Zhou L., Ni J., Yan W., Ma R., et al. (2018. a). LncRNA HOXA11-AS drives cisplatin resistance of human LUAD cells via modulating miR-454-3p/Stat3. Cancer Sci. 109 (10), 3068–3079. 10.1111/cas.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Ma K., Yang S., Zhang X., Wang F., Zhang X., et al. (2018. b). MicroRNA-125a-5p enhances the sensitivity of esophageal squamous cell carcinoma cells to cisplatin by suppressing the activation of the STAT3 signaling pathway. Int. J. Oncol. 53 (2), 644–658. 10.3892/ijo.2018.4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y., Fang W., Zhao M., Luo R., Liu Y., Fu Q., et al. (2017). miR-374a-CCND1-pPI3K/AKT-c-JUN feedback loop modulated by PDCD4 suppresses cell growth, metastasis, and sensitizes nasopharyngeal carcinoma to cisplatin. Oncogene 36 (2), 275–285. 10.1038/onc.2016.201 [DOI] [PubMed] [Google Scholar]
- Zheng Z. G., Xu H., Suo S. S., Xu X. L., Ni M. W., Gu L. H., et al. (2016). The Essential Role of H19 Contributing to Cisplatin Resistance by Regulating Glutathione Metabolism in High-Grade Serous Ovarian Cancer. Sci. Rep. 6, 26093. 10.1038/srep26093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., Wu W., Dong N., Jiang X., Xu J., Zhan X., et al. (2017. a). Mxd1 mediates hypoxia-induced cisplatin resistance in osteosarcoma cells by repression of the PTEN tumor suppressor gene. Mol. Carcinog. 56 (10), 2234–2244. 10.1002/mc.22676 [DOI] [PubMed] [Google Scholar]
- Zheng P., Chen L., Yuan X., Luo Q., Liu Y., Xie G., et al. (2017. b). Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 36 (1), 53. 10.1186/s13046-017-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Z., Xu H. Y., Zhao Z. M., Zhang G. M., Lin F. W. (2018). Comparison of efficacy and toxicity between nedaplatin and cisplatin in treating malignant pleural effusion. Onco. Targets Ther. 11, 5509–5512. 10.2147/ott.s168391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Sun C., Li N., Shan W., Lu H., Guo L., et al. (2016. a). Cisplatin-induced CCL5 secretion from CAFs promotes cisplatin-resistance in ovarian cancer via regulation of the STAT3 and PI3K/Akt signaling pathways. Int. J. Oncol. 48 (5), 2087–2097. 10.3892/ijo.2016.3442 [DOI] [PubMed] [Google Scholar]
- Zhou P., Ma L., Zhou J., Jiang M., Rao E., Zhao Y., et al. (2016. b). miR-17-92 plays an oncogenic role and conveys chemo-resistance to cisplatin in human prostate cancer cells. Int. J. Oncol. 48 (4), 1737–1748. 10.3892/ijo.2016.3392 [DOI] [PubMed] [Google Scholar]
- Zhou F., Zhu L., Wang K., Murray M. (2017). Recent advance in the pharmacogenomics of human Solute Carrier Transporters (SLCs) in drug disposition. Adv. Drug Deliv. Rev. 116, 21–36. 10.1016/j.addr.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Zhou W., Sun W., Yung M. M. H., Dai S., Cai Y., Chen C. W., et al. (2018). Autocrine activation of JAK2 by IL-11 promotes platinum drug resistance. Oncogene 37 (29), 3981–3997. 10.1038/s41388-018-0238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Ji M., Han Y., Guo Y., Zhu W., Gao F., et al. (2017). PGRMC1-dependent autophagy by hyperoside induces apoptosis and sensitizes ovarian cancer cells to cisplatin treatment. Int. J. Oncol. 50 (3), 835–846. 10.3892/ijo.2017.3873 [DOI] [PubMed] [Google Scholar]
- Zhu Z., Du S., Du Y., Ren J., Ying G., Yan Z. (2018). Glutathione reductase mediates drug resistance in glioblastoma cells by regulating redox homeostasis. J. Neurochem. 144 (1), 93–104. 10.1111/jnc.14250 [DOI] [PubMed] [Google Scholar]
- Zhu Q., Yu L., Qin Z., Chen L., Hu H., Zheng X., et al. (2019). Regulation of OCT2 transcriptional repression by histone acetylation in renal cell carcinoma. Epigenetics 14 (8), 791–803. 10.1080/15592294.2019.1615354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann T., Burda J. V. (2010). Cisplatin interaction with amino acids cysteine and methionine from gas phase to solutions with constant pH. Interdiscip. Sci. 2 (1), 98–114. 10.1007/s12539-010-0094-x [DOI] [PubMed] [Google Scholar]