Abstract
Despite an elaborate armamentarium to tackle microbes, emerging infectious diseases remain a crucial global challenge. Emerging infections can be defined as “infections that have newly appeared in a population or have existed previously but are rapidly increasing in incidence or geographic range.” Several factors like increase in international travel and trade, human encroachment on wild-life habitats, changes in agricultural practices and wild-life trade have contributed to the emergence of pathogens. Emergence/re-emergence of several viral infections has been reported from India in the past few decades; some of the important emerging viral infections are discussed in this review. They include infection due to Nipah, Hantaviruses, Chikungunya, Human Enterovirus-71, Influenza, Chandipura, Crimean Congo, SARS Coronavirus, Buffalopox, Dengue and Japanese Encephalitis viruses. Creating increased awareness and training of clinical microbiologists/virologists for identification of new/emerging pathogens, and prompt reporting and management of outbreaks is essential to tackle the threat posed by emerging/re-emerging infections.
Keywords: Emerging viral infections, Emerging infections in India
Introduction
It is time to close the book on infectious diseases and pay more attention to chronic ailments such as cancer and heart disease…. the war against infectious disease has been won.” This was the remark, made in 1967, by the then US Surgeon General William H. Stewart, buoyed up by the accelerating pace of scientific and technological progress, and the conquest of infectious diseases accomplished by use of “miracle drugs”-antibiotics, and vaccines. To write about infectious diseases, in 1962, said Macfarlane Burnet, Australian virologist and Nobel laureate “is almost to write of something that has passed into history.”
This over-optimism was soon shattered by the recognition of a new disease of infectious origin in 1981-AIDS, which remains the global killer today, threatening to outdo the Black Death of the fourteenth century and the Spanish Flu pandemic of 1918 which killed at least 50 million people, each. Indeed, despite an elaborate armamentarium to tackle infectious bugs, the continual evolution of emerging and re-emerging diseases, due to the astonishingly diverse microbial world and its adaptive capacity will remain a crucial global challenge in the twenty-first century.
Emerging infections can be defined as “infections that have newly appeared in a population or have existed previously but are rapidly increasing in incidence or geographic range.”[1] Emerging infections can be further classified as ‘newly emerging’, ‘re-emerging/resurging’ or ‘deliberately emerging’, since the underlying causes of emergence and preventive strategies differ between these groups. Newly emerging infections are those that have not previously been recognized in man. Re-emerging and resurging infections are those that existed in the past but are now rapidly increasing in incidence or in geographical or human host range. Deliberately emerging infections are infections due to microbes (naturally occurring or bio-engineered), deliberately introduced by man, usually for nefarious use [2].
Factors Contributing to Emergence of Viral Diseases
Several factors contribute to the emergence of viral infections and they are discussed below.
International Travel and Trade
Microbes know no geographical boundaries and their dissemination is accelerated by human mobility. Circumnavigation of the globe took more than a year a century ago; today it can be completed in a record 67 h! [3] At any given time, over a million people, each one a potential carrier of pathogens, are airborne around the planet. Transit times of people and other commercial goods which can be a potential source of pathogens and vectors are very short now, compared to the incubation times of diseases. Carriers of disease can reach their destination before the threat they harbour is detectable, reducing health quarantine to a near absurdity [4]. Rapid global spread of the recent 2009 H1N1 pandemic influenza, introduction of Aedes albopictus (Asian tiger mosquito) into the United States, Brazil and parts of Africa in shipments of used tyres from Asia [5] and the world-wide spread of hantaviruses by rats [6] can be attributed to international travel and commerce.
Human Behaviour and Demographics, and Advanced Medical Care
Mass migration of people due to war, natural calamities or economic hardships play a key role in disease emergence. Other aspects of modern human behaviour contribute to the emergence of novel infectious diseases, e.g., water shortage, leading to increased usage of open containers for water storage, creates a breeding ground for mosquito vectors (Dengue), social practices like tattooing in tribal areas in India (HBV, HCV and other blood borne viruses), rural urbanization, sexual behaviour (HIV), needle sharing among IV drug abusers (HIV, HCV), use of contaminated blood/tissue products (HIV, HBV, HCV, vCJD), contaminated vaccines (polio vaccines contaminated with SV40-linked to increased risk of non-Hodgkin’s Lymphoma). Immune deficiency associated with AIDS, and advanced medical care including cancer chemotherapy and organ transplantation, has contributed to an enormous increase in an immunocompromised population world-wide. ‘Opportunistic re-emerging Infections’ are frequently seen in such individuals due to reactivation of latent viruses or unrestrained replication of endogenous viruses, leading to enhanced virulence or changes in host or tissue tropism (CMV, EBV, HHV-6, Parvovirus B-19, JC, BK etc.) [2, 7].
Bio-Terrorism
Subsequent to the anthrax attacks through the US mail system in 2001, the possible use of viruses as weapons of bioterrorism has been considered. Potential viruses that can be deliberately introduced into susceptible human population as weapons of bio-terrorism include smallpox, Ebola, Marburg, Venezuelan equine encephalitis virus, Eastern equine encephalitis virus, Western equine encephalitis virus, Lassa fever virus etc. The potential use of genetically engineered viruses with enhanced virulence is also a possibility.
Microbial Adaptation
Microbes are constantly evolving to adapt to their environment. Many viruses like Influenza show a high mutation rate and rapidly evolve into new variants against which the immune system fails to mount a response, and vaccines against existing strains are ineffective. In certain viruses evolution of a new variant may result in new manifestation of disease, change in host or tissue tropism or altered virulence. Emergence of drug-resistant microbes by natural selection confers an added advantage to them. The molecular evolution of Chikungunya virus (CHIKV, A226V mutation in glycoprotein envelope gene) leading to a higher efficiency of replication and dissemination in the vector species Aedes albopictus, is reported to have contributed to resurgence of Chikungunya fever (CHIK) in Kerala state in 2007 [8].
Human Encroachment on Wild-Life Habitats
“When humans get infected, it’s not because the virus is ‘wily’ or ‘devious,’ adjectives applied to this complicated pathogen. Humans get infected, quite often, simply because they have put themselves in some virus’s way.” [9]
Deforestation, expansion of human habitat and mining activities have been suggested as risk factors associated with re-emergence of vampire bat rabies in the Amazon basin [10]. The dramatic increase of human cases of Kyasanur Forest Disease (KFD), a tick-borne flavivirus in south west India in 1983 can be attributed to deforestation and agricultural development, which increased exposure of humans to the virus and the vector (ticks). Usage of cleared areas in the forest for cattle grazing favoured the proliferation of the ticks (Haemaphysalis spinigera) which prefer to feed on bigger animals, including humans [11].
Changes in Agricultural Practices
The emergence of Argentine haemorrhagic fever and its expansion to more areas in Argentina, has been linked to agricultural activities like cultivation of corn, that sustain the corn mouse, the main reservoir of the Junin virus, causing the disease [12]. In India, Japanese encephalitis was introduced where dams and canals were built to bring in water for rice cultivation. A large pig population is conducive for outbreaks of JE, while a large cattle population may support mosquito feeding, but act as dampeners for spread of JE, since viraemia is not present [7]. The worst affected districts in Kerala during the 2007 Chikungunya outbreak, Kottayam and Pathanamthitta, are hilly and heavily forested, with vast rubber plantations. The rainwater that collects in the hemispherical containers fitted to the trunks of rubber trees for latex collection in the region supported prolific breeding of Aedes albopictus mosquitoes contributing to resurgence of the disease [8].
Wild-Life Trade/Ecotourism
Wild-life constitutes a large and often unknown reservoir of diseases; an estimated 75% of emerging infectious diseases are zoonotic, mainly of viral origin, and likely to be vector-borne [13]. Several factors related to wild-life, like wild-life trade and translocation, live animal and bush-meat markets, consumption of exotic foods, ecotourism, access to petting zoos and ownership of exotic pets contribute to emergence of infectious diseases [11]. Outbreaks of Marburg and Ebola have been associated with transport of animals. The rapid economic growth in southern China leading to an increasing demand for animal proteins including those from exotic game food animals such as civets, and many varieties of these wild game mammals in overcrowded cages in wet markets allowed the jumping of the novel SARS coronavirus from animals to human [14]. Currently very few countries have active wild-life disease surveillance and it is the need of the hour to monitor the emergence of novel microbes.
The Emergence of Viral Diseases: Indian scenario
Emerging infections can have a significant effect on human health and can cause enormous economic losses. Increased monitoring and surveillance by organizations such as the U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), and several other published reports of cases, outbreaks and epidemics have identified many unrecognized viral pathogens emerging globally with increasing frequency. The development of highly sensitive PCR based assays has also contributed to a dramatic increase in direct detection of viruses from clinical and environmental samples. Historically, Asia is believed to be the epicentre of several emerging viral diseases, (2 influenza pandemics in the previous millennium, SARS in China, avian influenza in Hong Kong etc.) that are of significant global public health importance. Emergence/re-emergence of several viral infections has been reported from India in the past few decades; some of the important emerging viral infections in India which may have serious health and economic implications in the future are discussed below (Fig. 1; Table 1).
Fig. 1.
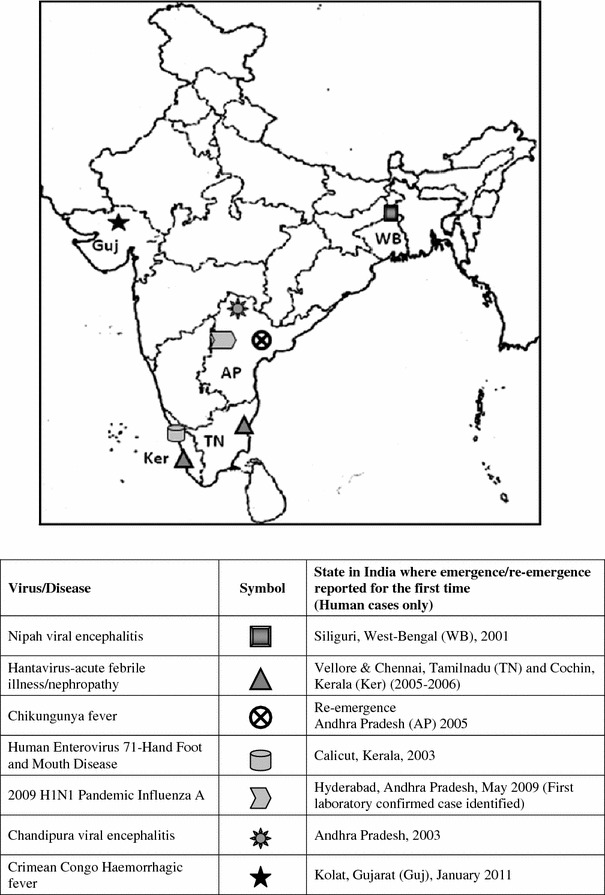
Emerging/re-emerging viral infections in India in the last decade
Table 1.
Emerging viruses in India
| Sl.No | Virus | Type of disease in humans | Host/Vector | Mode of infection | Clinical specimens/lab diagnosis | Outbreaks reported in India/Year (references in Indian context) |
|---|---|---|---|---|---|---|
| 1 |
Nipah Enveloped, ss RNA Family: Paramyxoviridae Genus: Henipavirus |
Encephalitis |
Affected Hosts-Pigs, humans Natural reservoir-Pteropid fruit bats |
a. Respiratory mode of transmission-by close contact with infected animal secretions and/or tissues, Human to human transmission b. Consumption of fruits/food contaminated with urine/saliva of infected fruit bats |
Detection of IgM/IgG antibodies in serum/CSF, viral RNA detection (RT-PCR) in serum/CSF/throat swabs, viral isolation-CSF/throat swabs, Histopathology/Immunostaining on brain tissue obtained at autopsy |
West Bengal 2001, 2007 [21, 25] |
| 2 |
Hantavirus Enveloped, ssRNA Family: Bunyaviridae Genus: Hantavirus |
Haemorrhagic fever with renal syndrome (HFRS), Hantavirus pulmonary syndrome (HPS) | Natural reservoir-Rodents |
a. Inhalation of aerosols of virus containing rodent faeces, urine or saliva b. infected rodent bites |
Serological diagnosis using IFA/ELISA/PRNT, detection of viral RNA in serum by PCR, immunohistochemistry for detection of viral antigen in tissues | Serological survey/Case reports from South India 1998–2000, 2005, 2006, Mumbai 2005, [35–39] |
| 3 |
Chikungunya Enveloped, ssRNA virus Family: Togaviridae Genus: Alphavirus |
Febrile illness with arthralgia, neurological complications (rare) | Vector-Mosquitoes of Aedes species |
Bite of infected Aedes mosquitoes, Mother-to-child transmission |
IgM antibodies in serum/CSF, virus isolation/detection of viral RNA by PCR in serum/CSF | Re-emergence in 2005 in several states of India after more than 30 years of quiescence. [43] |
| 4 |
Human enterovirus 71 (EV-71) Nonenveloped, ssRNA Family: Picornaviridae Genus: Enterovirus |
Hand-Foot and Mouth Disease (HFMD), Neurological complications | Humans- Only known host | Faeco-oral transmission | Isolation of the virus/Detection of viral RNA by RT-PCR from skin vesicle fluid, throat swabs and rectal swabs/faecal specimen | HFMD outbreaks Kerala 2003, West Bengal 2007, several reports of neurological complications due to EV-71 [53–57] |
| 5 |
Chandipura Enveloped, ssRNA Family: Rhabdoviridae Genus: Vesiculovirus |
Encephalitis | Vector-sandflies of Phlebotomus species | Bite of infected sand-fly | Detection of viral RNA by RT-PCR in serum/CSF, IgM antibodies in serum by ELISA | First identified in 1965 in 2 patients in Chandipura, Maharashtra. Emerged in 2003 at Andhra Pradesh, subsequently in Maharashtra and Gujarat. [64–71] |
| 6 |
Pandemic influenza 2009 H1N1 Enveloped ssRNA virus Family: Orthomyxoviridae Genus: Influenza A |
Influenza-Respiratory illness | Hosts- Humans, pigs, birds | Human to human transmission by infected droplets/aerosols released by coughing/sneezing by infected persons or through contaminated surfaces | Detection of viral RNA by RT-PCR in respiratory specimens | First case reported from Hyderabad, AP in May 2009. Pandemic spread to involve the whole country, cases continue to be reported. [59] |
| 7 |
Avian influenza H5N1 Enveloped ssRNA Family: Orthomyxoviridae Genus: Influenza A |
Influenza (Respiratory illness) | Hosts-Birds (wild and domestic) animals, humans |
Human infection acquired by close contact with infected birds or surfaces contaminated with secretion/excretions from infected birds Human-to-human transmission rare. |
Detection of viral RNA by RT-PCR in respiratory specimens | No human case reported from India so far |
| 8 |
Crimean Congo virus Enveloped, ssRNA Family: Bunyaviridae Genus: Nairovirus |
Febrile illness with haemorrhagic manifestations (Crimean Congo Haemorrhagic Fever-CCHF) | Reservoir and Vector-Ixodid ticks of genus Hyalomma, Hosts- several wild and domestic animals, Humans | Through bite of an infected tick, contact with blood or tissues of infected animals or humans, human-to-human transmission | Detection of viral antigen by ELISA/Immunohistochemical staining, detection of viral RNA by RT-PCR in the blood or in tissues collected from a fatal case | First report from Gujarat in January 2011, 3 deaths confirmed due to CCHF [78–80] |
| 9 |
SARS Coronavirus Enveloped ssRNA Family: Coronaviridae Genus: Coronavirus |
Severe Acute respiratory syndrome (SARS)-febrile illness with respiratory manifestations | Natural reservoir- horseshoe bats, amplification host-civets | Human-to-human transmission | Viral isolation from respiratory, fecal, and, occasionally, urine or tissue specimens, detection by nucleic acid amplification such as RT-PCR or antigen detection by EIA, a fourfold rise in the neutralizing antibody titer in paired serum samples | No confirmed SARS cases reported from India |
| 10 |
Buffalopox Enveloped, ds DNA virus Family: Poxviridae Genus: Orthopoxvirus |
Pox-like lesions on skin, fever, axillary lymphadenitis | Zoonotic infection from infected Buffaloes to humans | Close contact with infected buffaloes (e.g. Milkers, animal handlers etc.) | Isolation of virus/detection of viral DNA from skin scabs, skin scrapings, detection of antibodies in serum samples | Human cases reported from several states in India [89, 90] |
Nipah Virus
Nipah virus, a zoonotic paramyxovirus, is an emerging virus endemic in Southeast Asia. Beginning in September 1998 a major outbreak of disease in pigs and human beings appeared in the northern part of peninsular Malaysia, resulting in 265 infected individuals and 105 deaths, most of whom were farmers or abattoir workers who had come in close contact with infected pigs [15]. Surprisingly, while respiratory symptoms predominated in pigs, infected humans presented mostly with encephalitic symptoms. In March 1999, a novel paramyxovirus was isolated from the CSF of an encephalitic patient from Sungai Nipah village. This virus which was later named as the Nipah virus was found to be closely related to Hendra virus, another paramyxovirus, identified in 1994, which caused severe respiratory illness in horses and humans in Australia [15–17]. These two geographically remote, yet phylogenetically close viruses were classified into a new genus-Henipavirus [18, 19]. Fruit bats belonging to the genus Pteropus were identified as the natural hosts for Nipah virus, while pigs were considered the intermediary hosts. The most likely explanation for introduction of the virus into pigs appears to be related to the practice of growing fruit orchards near piggeries to allow efficient use of pig waste as fertilizer for the trees. With some trees overhanging the pig-pens, it is likely that pigs were exposed to virus laden excreta and masticated fruit pellets dropped from feeding fruit bats also called as flying foxes, who are symptomless carriers of the virus [15]. Pigs infected with the virus showed less mortality; however, due to their predominant respiratory involvement, they spread the infection to humans in close contact.
Mass culling of more than a million pigs contained the outbreak in Malaysia. However, subsequently several Nipah virus associated encephalitic outbreaks with a high mortality occurred in Bangladesh between 2001 and 2007 [20]. A similar outbreak which was retrospectively identified to be due to Nipah virus was reported in 2001 from Siliguri, West Bengal, not far from Bangladesh. All of the patients were residents of Siliguri and the outbreak occurred among hospitalized patients, family contacts of the patients, and medical staff of 4 hospitals. Sixty-six cases of encephalitis were identified, and the case-fatality ratio was 74%. Analysis of the limited sequence data suggested that the Nipah virus strains associated with the outbreak were more closely related to the virus isolated in Bangladesh than to the virus isolated in Malaysia, suggesting that viruses circulating in different areas have unique genetic signatures and may have co-evolved within local natural reservoirs [21].
Many of the epidemiologic features of the outbreak in Siliguri were comparable to those of the recent Nipah virus outbreaks in Bangladesh. In Bangladesh, no intermediate animal host was identified and many infected individuals were thought to have had direct contact with fruits eaten by bats or consumed contaminated date palm sap [22]. In the Siliguri outbreak, studies to detect an intermediate host were not conducted. However, serological evidence suggested Pteropus giganteus fruit bats as reservoirs of infections in Bangladesh [23] as well as in India [24]. In both outbreaks, human-to-human transmission occurred in healthcare settings through contact with infected persons, supporting nosocomial mode of transmission. Initiating adequate barrier nursing techniques helped to curtail further spread of infection [21].
In April 2007, 30 cases of fever with acute respiratory distress and/or neurological symptoms, with 5 deaths were reported from Nadia district of West Bengal. The cases presented mainly with fever, headache and body ache with a few cases having episodes of vomiting, disorientation and respiratory distress. Samples (including urine, CSF, brain and lung tissue) from the five fatal cases were found to be positive for Nipah virus by RT-PCR [25].
In northern India, as in Bangladesh, P. giganteus bats live in close association with the human population. The multiple outbreaks of Nipah encephalitis in Bangladesh, and the 2001/2007 outbreaks in West Bengal, show a perpetual risk for spillover infection between bats and humans in this region suggesting that more such outbreaks may continue to occur in future. Establishing appropriate surveillance systems in these areas will be necessary to initiate prompt infection control measures [21, 24].
Procedures for the laboratory diagnosis of Nipah virus include serology, histopathology, PCR and virus isolation. All work involving the propagation of Nipah virus must be done in containment level 4 facilities (BSL-4), considering the high virulence of virus, wide range of susceptible hosts and absence of therapeutic and prophylactic interventions. There is no effective treatment for Nipah virus disease, but ribavarin may alleviate the symptoms of nausea, vomiting, and convulsions [26]. Currently no vaccine is available for use; however, a few candidate vaccines include a recombinant sub-unit vaccine formulation which protects against lethal Nipah virus challenge in cats [27] and ALVAC Canarypox vectored Nipah F and G vaccine which appears to be a promising vaccine for swine and has potential as a vaccine for humans [28].
Hantaviruses
The occurrence of ‘hantavirus pulmonary syndrome’ in 1993 in the Southwestern United States due to the emergence of a disease caused by viruses known for decades, was attributed to the relatively abrupt and short-lived weather changes due to the El Nino effect. The El Nino effect provoked heavy rainfall across the southern states in US, leading to proliferation of vegetation and consequently the rodent population, the natural hantavirus hosts which are asymptomatic carriers of the virus. As the population of rodents, especially deer mice (Peromyscus maniculatus) exploded and they sought food and shelter in human dwellings, people were exposed to the aerosolized animal droppings, urine and saliva, which lead to the infection [29]. The outbreak has continued since and by December 1, 2009, a total of 534 cases of hantavirus pulmonary syndrome with 36% mortality have been reported in the United States [30].
Hantaviruses are the most widely distributed zoonotic rodent borne viruses known to cause two significant clinical syndromes-haemorrhagic fever with renal syndrome (HFRS) and Hantavirus pulmonary syndrome (HPS), also referred to as Hantavirus cardiopulmonary syndrome (HCPS). Hantaviruses belonging to family Bunyaviridae, genus Hantavirus are enveloped RNA viruses and currently up to 22 species and more than 30 genotypes have been described. Important species of hantaviruses include Hantaan virus, Seoul virus, Puumala virus, Sin Nombre virus and Dobrava-belgrade virus. HFRS and HPS are being increasingly reported from various geographical locations worldwide and are a cause of global concern.
Apart from inhalation of aerosols from virus containing rodent faeces, urine or saliva, transmission is reported to occur even through rodent bites [31]. Though considered unlikely, human-to-human transmission has been reported in an outbreak due to Andes virus in Argentina [32].
Thottapalayam virus, the first hantavirus to be isolated, is the only indigenous isolate from India, isolated from an insectivorous shrew, Suncus murinus captured in Vellore, South India during field studies of Japanese encephalitis in 1964 [33].. However, there is limited data to suggest that the Thottapalayam virus is pathogenic to humans [34]. Future studies on the epizootiology of the virus and exploration of new shrewborne hantaviruses will provide more insights into the evolutionary origin of hantaviruses in their rodent and insectivore reservoir hosts.
Clement et al. documented the first evidence of Seoul virus like and/or Puumala virus like antibodies in a serosurvey of leptospirosis-suspected cases from India in 1998-1999. Subsequently, they also reported nephropathy due to hantaviruses, mimicking leptospirosis in a prospective study in the Chennai and Cochin area in southern India, providing clinical evidence of disease due to hantaviruses in India. Seven (12%) and three (5%) of the 60 patients with suspected leptospirosis screened for IgG antibodies were positive for Seoul virus and Puumala virus, respectively. Nine of these cases were also positive for specific IgM antibodies confirming for the first time recent hantavirus infections in these Indian cases. Two deaths due to acute renal failure were reported. Wild rats which have a documented presence world-wide, including India are the reservoir of Seoul virus. They can cause thrombocytopenia with concomitant renal and hepatic involvement, thus mimicking leptospirosis. Interestingly, the bank vole, which spreads the Puumala virus, is not present in India. Therefore, the positive serological results for Puumala virus in Indian patients may represent cross-reactions with the Thottapalayam virus or yet another unknown hantavirus in India [35–37].
Chandy et al. found 14.7% of 152 serum samples from patients with febrile illness positive for anti-hantavirus IgM and in 5.7% of healthy blood donors in South India, indicating the presence of symptomatic and asymptomatic hantavirus infections in the Indian population [38].
Few cases of ocular involvement due to hantavirus were reported in patients admitted with pyrexia of unknown origin or hemorrhagic fever following exposure to flood waters in Mumbai in 2005 [39].
There are no specific anti-viral drugs for treatment of all hantavirus infections. Ribavirin used in clinical trials in China for HFRS has been reported to cause significant reduction in fatality [40]. Prevention of exposure to rodent excreta is the best preventive strategy. There is no WHO-approved hantavirus vaccine available. However, at least three different inactivated vaccines, developed in Korea and China, are being used only locally. Many other vaccine approaches, have been investigated using both inactivated virus and recombinant DNA technology [41].
Chikungunya Virus
Chikungunya fever (CHIK) caused by an arbovirus CHIKV a RNA virus belonging to family Togaviridae, genus Alphavirus, is a re-emerging viral disease. To date, a vertebrate reservoir or sylvan transmission cycle for CHIKV has only been identified in Africa; in Asia, the virus is presumed to exist in a human-mosquito-human cycle [42]. Re-emergence of CHIKV was recorded in India during 2005–2006 after a hiatus of 32 years, causing more than 1.38 million cases of CHIK in 14 states and Union Territories in India [43]. Several islands of the Indian Ocean reported similar outbreaks in the same period.
The illness is characterized by abrupt onset of fever with severe arthralgia followed by constitutional symptoms and rash lasting for 1–7 days. Most often the disease is a self-limiting febrile illness, however, atypical manifestations like cardiovascular, renal and neurological complications [44, 45], as well as mother to child transmission [46, 47] has been reported.
CHIKV is known to cause epidemics after a period of quiescence. The first recorded epidemic occurred in Tanzania in 1952–1953. In Asia, CHIKV activity was documented since its isolation in Bangkok, Thailand in 1958. In India, well-documented outbreaks occurred in 1963 and 1964 in Kolkata and southern India, respectively, and in Sholapur district, in Maharashtra in 1973. CHIKV emerged in the islands of South West Indian Ocean viz. French island of La Reunion, Mayotee, Mauritius and Seychelles in early 2005. After a hiatus of about three decades, CHIKV has re-emerged in India in the states of Andhra Pradesh, Karnataka, Maharashtra, Madhya Pradesh and Tamil Nadu since December, 2005. Cases have also been reported from various other states including Rajasthan, Orissa, Gujarat and Kerala. The outbreak is still continuing.
CHIKV is transmitted by Aedes species mosquitoes, primarily Aedes aegypti. However, unusually, the vector responsible for transmission between humans in the 2005–2006 CHIK epidemic on Reunion island was apparently the Asian tiger mosquito, Aedes albopictus. Interestingly, the same epidemic was associated with a strain of CHIKV with a mutation in the envelope protein gene (E1-A226V), which was reported to play a role in the adaptation of CHIKV to Aedes albopictus mosquitoes, providing an insight into how a genetic change in the pathogen can increase the host range and geographical distribution [48].
The precise reasons for the re-emergence of CHIKV in the Indian subcontinent remain an enigma. Lack of immunity may have possibly facilitated rapid spread of the infection [49]. Other factors include lack of efficient vector control activities, globalization, mutation of the virus and emergence of Aedes albopictus, in addition to Aedes aegypti as an efficient vector for CHIKV [8]. Molecular characterization has demonstrated two distinct lineages of strains which cause epidemics in Africa and Asia. The recent outbreaks were attributed to the African genotype of CHIKV.
Laboratory diagnosis can be achieved by detection of IgM antibodies in serum and CSF (in neurological involvement), virus isolation and direct detection of nucleic acids in the samples using PCR based techniques. To tackle this re-emerging virus, laboratory research aimed at the development of vaccine candidates, antiviral strategies and commercially available diagnostic kits is needed [42].
Human Enterovirus-71 (EV-71)
Even as we grapple with the challenge of eradicating poliomyelitis, another enterovirus EV-71, responsible for neurological disease has emerged [50].
Hand-Foot-and-Mouth Disease (HFMD) is a mild exanthematous illness seen worldwide, with characteristic lesions on the palms, soles and oral mucosa, affecting mainly children under 10 years of age. The causative agents were initially Coxsackie virus type A 16 (CA 16) and related serotypes. However, in recent years, a new aetiological agent of HFMD, EV-71, has emerged, causing large outbreaks with severe complications and many deaths, especially in the Asia pacific region [51]. This geographical predilection is unexplained but could be related to the frequency of intra- and inter-typic genetic recombinations of the virus, the genetic predisposition of host population, environmental hygiene, and standard of healthcare [52]. EV-71 is a member of the family Picornaviridae, and belongs to the genus Enterovirus (human enterovirus A). Faeco-oral route is the principal mode of transmission and humans are the only known natural host.
A major epidemic of HFMD induced by EV-71 was reported in Sarawak, Malaysia in 1997 followed by smaller outbreaks in Peninsular Malaysia. More than 2600 children, mostly under 6 years of age, were affected. Most of them presented with characteristic febrile illness and skin lesions, but severe neurological complications like aseptic meningitis, acute flaccid paralysis, fatal encephalomyelitis, and cardiopulmonary complications were also reported in a small proportion of patients, unlike the benign type of HFMD caused by CA 16 and other aetiologic agents. Subsequently, EV-71 associated HFMD has been reported from Taiwan in 1998, Perth in 1999, and Singapore, Korea, Malaysia, Japan and Taiwan in 2000, Vietnam in 2005 and China in 2008. Brainstem encephalitis, especially affecting the medulla, associated with cardiopulmonary dysfunction has become a notable feature in EV-71 epidemics in Asia, and is the primary cause of death.
In India, outbreaks of HFMD have been reported from Calicut, Kerala in 2003 and three districts of West Bengal in 2007, however, these outbreaks were not associated with neurological complications [53, 54]. Various case reports and hospital based or clinico-epidemiological studies from India have reported neurological complications caused by EV-71 [55–57]. Like all other enteroviruses, children are the most significant target as well as reservoir of EV-71. In a study of aetiology of viral encephalitis in children in Uttar Pradesh, the most common causative agent was EV-71, identified in 42% of the 87 patients enrolled in the study. Generalized convulsions with altered sensorium were a significant finding in these patients. The infection was associated with a significantly high morbidity and mortality; 50% of the children with EV-71 encephalitis died [57]. Nucleotide sequence analysis of an EV-71 isolate from a patient with acute flaccid paralysis (Guillain–Barré syndrome) from Haryana, revealed that the Indian isolate is genetically distinct from the EV-71 strains isolated from other outbreaks in the Asia pacific region [56].
Diagnosis of HFMD can be made clinically with certainty, if a strong index of clinical suspicion exists. Laboratory confirmation is dependent upon direct isolation of the virus from skin vesicle fluid, throat and rectal swabs/stool specimens in cell cultures, using neutralization with a type-specific antiserum, indirect fluorescent assay (IFA) and reverse transcriptase-polymerase chain reaction (RT-PCR) amplification of the viral RNA.
Prophylaxis and treatment with intravenous immunoglobulin (IVIG) has been used with some success in patients with complicated EV-71 infection and agammaglobulinaemic individuals prone to the development of chronic enteroviral meningoencephalitis. Vaccines being developed include inactivated whole-virus, live attenuated, subviral particle, and DNA vaccines.
Since the densely populated Asia Pacific region is the epicentre of emergence of this virus in the past decade, continuous monitoring of this disease and its epidemiology in India is warranted.
Influenza
Pandemic influenza, unarguably remains an emerging infection of global concern at all times. Influenza is an orthomyxovirus classified into three genera A, B and C. Influenza A is known to commonly cause epidemics and pandemics world-wide.
Influenza viruses are by nature unstable and unpredictable and have the unique capability of changing their antigenic characteristics by mutation. Occasionally, novel strains emerge, often through re-assortment or exchange of genetic material among influenza viruses from different animal, human or bird sources. Such major ‘antigenic shifts’ provide the virus with the capability of causing widespread disease across geographical borders in immunologically naïve populations to cause pandemics [58].
Pandemic 2009 H1N1 Influenza
Since the first outbreak of respiratory illness caused by a novel triple re-assortant, swine origin influenza A (2009 H1N1) was identified in Mexico in March 2009. Influenza A (2009 H1N1) cases have been documented throughout the world causing a tremendous impact on health and economy. The pandemic strain hit Indian shores on May 13, when a 23 year old passenger who traveled from USA to Hyderabad, Andhra Pradesh, tested positive for Influenza A (2009 H1N1). The first death due to 2009 H1N1 Influenza in India, of a 14 year old female student, was reported on August 3, 2009 from Pune, Maharashtra. As of November 30, 2009, samples from 87,417 persons were tested for Influenza A (2009 H1N1) in various laboratories designated by the government across the country and 18,198 (20.8%) of them were found positive and 575 (3.15%) deaths recorded [59]. In India, the state of Maharashtra was the worst affected, followed by Karnataka [58].
The mode of transmission is through droplets from coughing or sneezing, and through direct or indirect contact with the respiratory secretions of an infected person. The clinical spectrum of the illness varies from a mild respiratory tract illness to severe complications such as pneumonia, resulting in acute respiratory distress syndrome (ARDS), respiratory and multi-organ failure, and death. The risk of complications is higher among pregnant women and those with pre-existing diseases such as asthma, heart disease, obesity and kidney disease. Laboratory confirmation is achieved by detection of viral RNA by RT-PCR on respiratory samples. The drug of choice is Oseltamivir; Zanamivir can be used as an alternative. Indigenously developed vaccines have been approved for use in India.
On 10 Aug 2010, the WHO Director-General declared that the pandemic was over; by then 18,449 deaths had been reported worldwide. In the SEA Region 76,302 cases and 2,054 deaths had been reported. However, in the post-pandemic period it is likely that the pandemic H1N1 2009 virus can continue to circulate as a seasonal virus for some years. Several countries including Sri Lanka, India and Thailand continue to report cases each week although the trend is on a downswing [60].
With the continual circulation and interspecies transmission of human, swine, and avian influenza viruses in countries around the world, there is an increased need for influenza surveillance in pigs, birds, and other animals to aid in monitoring and assessing the risk of future pandemic virus emergence involving different species. Vaccination in humans and continued surveillance can curb the occurrence of recurrent waves of the pandemic.
Avian Influenza (H5N1)
Highly pathogenic H5N1 avian influenza emerged in Hong Kong in 1997. Infection was acquired by humans directly from chickens, without the involvement of an intermediate host. The outbreak was contained with effective culling of more than 1.5 million chickens [61]. Since December 2003, outbreaks have been reported in poultry from China, Vietnam and Thailand, and more than 70 countries across Asia, Europe, the Middle East, and Africa have reported the disease in poultry and wild birds. As of 11th April 2011, 549 confirmed human cases of Avian Influenza A/(H5N1) have been reported to WHO with 320 deaths [62].
Epidemics of the highly pathogenic Avian bird flu (H5N1) among Indian poultry birds were reported for the first time on a large scale during 2006 in the states of Maharashtra, Gujarat and Madhya Pradesh; subsequently several outbreaks in 2008–2009 were recorded in West Bengal, Sikkim, Assam and Tripura, in 2010. Recently, an outbreak of avian influenza has been reported from Tripura in February 2011 [63]. Mass culling of birds in affected farms led to the control of the disease spread. No suspected human cases of this deadly bird virus have appeared in India, where the mechanisms for spreading the disease and its potentially serious public health dangers in India are understood. However, as long as the avian influenza outbreaks due to H5N1 continue to occur from infected poultry in various parts of the world, the threat of an imminent pandemic looms large and hence a constant vigil is warranted.
Chandipura Virus
Chandipura virus is a member of the family Rhabdoviridae and genus Vesiculovirus that is associated with an encephalitic illness in humans. It was first identified in 1965 after isolation from the blood of two adult patients with febrile illness from Chandipura (Nagpur) in Maharashtra state, India [64] and subsequently from a child with acute encephalopathy syndrome in Raipur in central India [65].
However, the significance of Chandipura virus as a human pathogen was unresolved until 2003, when an outbreak of acute encephalitis in children in various districts of Andhra Pradesh with a case fatality of 55.6% was attributed to Chandipura virus [66]. Twenty-six suspected cases were investigated for the aetiological agent in another outbreak of encephalitis that affected children in tribal areas of Gujarat state in western India in 2004, with a case fatality rate of 78.3%. Demonstration of Chandipura viral RNA in 9 (45%) of 20 cases, IgM antibodies in 3 (15%), and isolation of the virus from one patient provided strong evidence for Chandipura virus as the aetiologic agent of the encephalitis outbreak. The virus sequences from the outbreak were closely related to prototype strain (1965) and isolates from the Andhra Pradesh, outbreak in 2003 [67]. Outbreaks of encephalitis due to Chandipura virus were reported in 2003 and 2005 from Maharashtra [68, 69] and in an hospital based surveillance of acute encephalitis among children from North Telangana in Andhra Pradesh [70]. Chandipura virus was confirmed as an aetiological agent of an acute encephalitis syndrome (AES) outbreak among children from Nagpur division in 2007, with a case fatality ratio of 43.6% [71].
Fever and neurological manifestations are the predominant features; bleeding tendencies have been reported in some cases of encephalitis due to Chandipura virus. The clinical course of Chandipura encephalitis is short with rapid progression to death, with 60% of the deaths occurring within 24 h [71].
Chandipura virus has been isolated from sandflies of Phlebotomus species in Andhra Pradesh and Maharashtra in India [71–73] and West Africa [74] and is probably spread through its bite. The presence of the virus in Africa indicates a wide distribution although no human cases have been observed outside of India. Laboratory studies indicated venereal transmission of this virus from infected male to female sand flies while mating [75]; the virus has also been found to be transmissible through Aedes aegypti mosquitoes in laboratory studies [76]. These findings may have important epidemiologic implications in spread of the infection.
Evaluation of a combination of recombinant glycoprotein (rGp) and a commercially available DPT vaccine (CHP-DPT) has shown promising results in a murine model [77].
Chandipura virus has emerged as an important encephalitis-causing pathogen with high mortality among paediatric population in India. The need for immediate comprehensive analysis of the transmission mechanisms of this virus, including the role of vectors and other ecologic factors, for the development of appropriate control strategies is warranted [67].
Crimean Congo Haemorrhagic Fever
In January 2011, the first ever report of an outbreak of Crimean Congo Haemorrhagic Fever (CCHF) has been confirmed from the village of Kolat, in Sanand Taluka, 30 kms south-west of Ahmedabad in Gujarat state in India. A 30 year old woman, and the doctor and nurse who treated her succumbed to the illness creating panic waves not only in the local population, but the country as well [78–80].
The RNA virus causing CCHF is a member of the genus Nairovirus of the family Bunyaviridae. Ixodid ticks of the genus Hyalomma specifically act as a vector and reservoir for the virus and numerous wild and domestic animals can serve as amplifying hosts. CCHF was first described as a clinical entity in 1944 when many soviet military personnel were infected in war affected Crimea. A similar illness was recognized in Congo and Uganda in 1967, resulting in the name Crimean Congo Haemorrhagic Fever. The virus is also distributed throughout the Mediterranean, northwestern China, central Asia, Africa, southern Europe, middle east and the Indian subcontinent [79, 80]. Outbreaks of CCHF have recently been reported from Iran in 2008 [81] Turkey in 2001–2003 [82] and Pakistan in 2010 [83].
Transmission of virus occurs through bite of an infected tick, contact with blood or tissues of infected animals or humans; nosocomial transmission also has been reported [79–81]. The virus causes predominantly haemorrhagic manifestations in humans, with high case fatality rates ranging from 15 to 70%. After an incubation period of 3–6 days, the onset is sudden, with high fever, chills, headache, joint pain, myalgia, vomiting and abdominal pain. The spectrum of haemorrhagic manifestations includes red eyes, flushing of face, throat congestion, petechiae over the palate, epistaxis, haematemesis, haematuria and intracerebral bleeds. The complications include jaundice, disseminated intravascular coagulation, hypovolumic shock and muti-organ failure.
Laboratory diagnosis of CCHF has to be performed in biological safety level-4 (BSL-4) facility. Immunohistochemical staining to demonstrate viral antigen in formalin fixed tissues, detection of viral antigen and antibodies by ELISA, virus isolation and detection of viral nucleic acids by PCR are used for laboratory diagnosis. Treatment of CCHF is mostly supportive, though Ribavirin has shown benefits in in vitro studies and is also recommended for post-exposure prophylaxis [79, 80].
In the recent outbreak in Gujarat, 20% of the samples collected from cattle around the affected area have tested positive for antibodies to CCHF. Earlier studies from India have also reported evidence of circulating antibodies both in humans and domestic animals, suggesting the presence of the virus in India since many years [84]. This necessitates constant surveillance for the virus and any new outbreaks to initiate immediate control measures. Animal handlers, abattoir workers and veterinarians are at high risk and preventive strategies include use of effective personal protective measures against tick bites.
Severe Acute Respiratory Syndrome (SARS) Coronavirus
Severe acute respiratory syndrome (SARS) is a form of atypical pneumonia that apparently originated in Guangdong Province of the People’s Republic of China in late 2002. A novel coronavirus, the SARS-associated coronavirus (SARS-CoV) was identified as the agent responsible for this first pandemic of the new millennium. After its recognition in early 2003, the infection was reported worldwide. As of July 31, 2003, 8,096 cases of ‘probable SARS’, have been reported in more than 30 countries world-wide with 774 (9.6%) deaths [85]. Human-to-human transmission of the virus, the lack of awareness in hospital infection control, and international air travel facilitated the rapid global dissemination of this agent. The findings that horseshoe bats are the natural reservoir for SARS-CoV-like virus and that civets are the amplification host emphasizes the importance of wildlife and biosecurity in farms and wet markets, which can serve as the source and amplification centers for emerging infections [86].
The typical clinical presentation of SARS is that of viral pneumonia with rapid respiratory deterioration. After 3–7 days of prodromal symptoms, the illness is characterized by an onset of dry, non-productive cough or dyspnoea, which may be accompanied by or progress to hypoxaemia. About 10–20% of the patients may develop acute respiratory distress requiring intubation and mechanical ventilation.
Laboratory testing requires a biosafety level 3 facility. A positive viral culture from respiratory, faecal, and, occasionally, urine or tissue specimens or a fourfold rise in the neutralizing antibody titer in paired serum samples is the most definitive evidence of infection. Rapid detection by nucleic acid amplification such as RT-PCR or antigen detection by EIA is the alternative [86].
Though a few suspected cases of SARS were reported from India, none of them were confirmed, and in the official declaration by the Government (as reported on May 2, 2003 in the media), India was declared by WHO as a “SARS free” country [87]. Absence of HLA B*46 in Indian population has been postulated as one of reasons for protection from SARS epidemic [88].
Coronaviruses are known to undergo genetic recombination which may lead to new genotypes and outbreaks. The possibility of the re-emergence of SARS and other novel viruses from animals or laboratories, and therefore the need for preparedness should not be ignored [86].
Buffalopox
Buffalopox is an important contagious viral infection of buffaloes of all ages, occurring in epidemic proportions in countries including India, where buffaloes are reared, causing considerable morbidity and reduction in milk yield. Buffalopox is an important emerging zoonosis affecting humans and in recent years several reports of infection in humans have been described from various states in India [89, 90]. Buffalopox virus is a member of the Orthopoxvirus, and is closely related to Vaccinia virus. Human beings, including smallpox-vaccines contract infection upon close contact with infected animals, but the extent of disease is greater in non-immunized individuals. Milking of affected animals is one of the major modes of spread; human to human transmission has not been reported. Milkers develop pox-like lesions on the shin of their fingers, arms, fore-head and face associated with fever and axillary lymphadenitis. Regular monitoring of buffalopox outbreaks in India in animals and humans is essential to curb economic losses as well as to reduce public health impact of the disease [90].
Flaviviruses: Re-Emergence and Changing Epidemiological Pattern of Disease
Dengue
Dengue is the most significant and widespread flavivirus disease to have emerged globally; the global epidemiology of dengue fever/dengue hemorrhagic fever (DF/DHF) is changing rapidly [91]. The Dengue virus belonging to family Flaviviridae, genus Flavivirus, consists of four serotypes (1–4). Despite extensive cross-reactivity among these viruses in serological tests, there is no cross protective immunity in humans; a person living in an endemic area can have as many as four infections, one with each serotype, during their life. The disease spectrum caused by Dengue virus varies from a subclinical infection to a mild self limiting disease-dengue fever (DF) and a severe disease that may be fatal, the dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). The Dengue viruses are the only known arboviruses that have fully adapted to humans, having lost the need for an enzootic cycle for maintenance. Usually transmitted by Aedes aegypti mosquitoes, the virus has been reported to be transmitted in recent years by Aedes albopictus, which may have significance with respect to dengue re-emergence [91].
Dengue infection has been known to be endemic in India for over two centuries as a benign and self limited disease. However, in recent years, dengue which has been an urban disease has spread to rural areas of India as well [92, 93]. Dengue and the vector have been reported in the arid zones of Rajasthan [94, 95]. The disease has changed its course manifesting in the severe form as DHF and with increasing frequency of outbreaks, with all four Dengue serotypes co-circulating and the predominant serotypes ever changing [96–98]. These severe complications are thought to occur as a result of evolution of the Dengue virus to escape high population immunity manifesting as increased viral virulence and human immunopathogenesis due to antibody-dependent enhancement of viral infection [99]. The resurgence of Dengue can be linked to urbanization and unprecedented population growth, increased density of the mosquito vector, reinfestation with Aedes aegypti of a new geographical area, warm and humid climate, water storage pattern in houses, storage of junk in open spaces, including tyres, coconut shells etc. that trap rain water, introduction of new serotype of the virus, lack of effective mosquito control and to the enormous increase in the international transport of people and commodities [100, 101]. Since vaccines or antiviral drugs are not available for Dengue viruses, the only effective way to prevent epidemic DF/DHF is to control the mosquito vector, Aedes aegypti and prevent its bite [101].
Japanese Encephalitis
Japanese encephalitis (JE) is one of the most important endemic encephalitis in the world especially in Eastern and Southeastern Asia. JE virus belonging to family Flaviviridae, is transmitted through a zoonotic cycle between Culex mosquitoes, pigs and water birds. Humans are accidentally infected and are a dead end host because of low level and transient viraemia. Japanese encephalitis kills more than 10,000 children in Asia and the Pacific every year, and it leaves one-third of its survivors with permanent neurological damage.
JE virus activity in India was first established by a serological survey in 1952 and clinical recognition of this disease was first made in 1955 at Vellore of North Arcot district in Tamilnadu state. Between 1955 and 2007, there had been an increase and spread of infection throughout the country, except in arid and high land regions. Outbreaks of JE occurred in West Bengal during 1973–1976 and in Andhra Pradesh, Bihar, Tamil Nadu and Uttar Pradesh during 1977–1979. Subsequently, outbreaks were reported in Assam, Karnataka and Pondicherry, and Uttar Pradesh in the 1980s [102]. In recent years, JEV has spread to new geographical locations within India-into Haryana and Kerala states in northwestern and southwestern India, respectively [103, 104]. A new focus of infection in Sangli district of Maharashtra was reported in 1999. Since 2000, JE occurrence has been reported from 16 States and Union Territories and among them Uttar Pradesh, Assam and Karnataka have accounted for more than 90% of the total incidence in India [102]. In 2005, Uttar Pradesh faced a devastating epidemic outbreak of JE, which surpassed in intensity and magnitude, all previous reported epidemic outbreaks in the country. The outbreak was mostly confined to Gorakhpur affecting 6,061 cases with 1,500 deaths followed by another outbreak in 2006 with 2,320 cases and 528 deaths [105].
Immunization in India was inspired by a pilot program that the government of Andhra Pradesh started with PATH (Program for Appropriate Technology in Health) with funding from the Bill and Melinda Gates Foundation. This partnership helped introduce the live, attenuated SA 14-14-2 JE vaccine that has been effectively and safely used in China for almost 20 years, to high-risk districts in the state at affordable costs, beginning in 2001. Subsequently, the government of India launched vaccination campaign in highly endemic states of Assam, Karnataka, West Bengal and Uttar Pradesh in 2006 and in Andhra Pradesh, Bihar, Haryana, Maharashtra, Tamil Nadu in 2007 and 2008, respectively, which has resulted in reduced incidence of JE in these states. By the end of 2009, more than 60 million Indian children have been immunized through these successful vaccination campaigns [106, 107].
Effective prevention and control strategies need to be continually implemented, since the number of JE epidemics has increased in the recent past and the disease has spread to new geographical areas. The occurrence of epidemic JE is attributed mainly to vagaries of climatic conditions and thereby warrants preparedness to implement an effective preventive strategy like JE immunization of target population in risk area. Resurgence of JE in endemic areas is largely determined by agricultural activities, irrigation and animal husbandry practices; an integrated approach using vector control and selective immunization of children has been recommended to tackle this problem [102].
Rotaviruses, Noroviruses, Bocaviruses, Parvovirus B-19
Rotaviruses cause acute viral gastroenteritis worldwide. The diversity of rotavirus strains reported and the high prevalence of mixed infections are unique features of rotavirus epidemiology in India and emphasize the need for surveillance of cocirculating strains, to follow the rapid changes in circulation and to detect novel strains. Group B rotaviruses have been reported from Kolkata and Pune, and human infections with strains G6, G8, G10, and G9P [19], which may occur as a result of zoonotic transmission of bovine and porcine rotaviruses, have been reported from western, southern, and eastern India. The occurrence of these unusual rotavirus strains which are natural reassortants of human and bovine rotaviruses, suggests that reassortment may be an important mechanism for generation of rotavirus strains of newborns. This is catalyzed by the age old traditions of calves and humans living in the same household and socio-economic conditions in India [108, 109]. The emergence of new variants/strains of Noroviruses, causative agents of acute gastroenteritis has been described in various studies from India [110, 111]. Human bocavirus, a new human parvovirus identified in children with respiratory tract disease in 2005, has been reported from India as well [112]. Red Baby Syndrome, a new disease characterised by abrupt onset of high fever and generalized erythema involving the entire skin, seen in infants and young children associated with human parvovirus B-19 infection was recently reported from India [113].
Disease Surveillance in India: The Challenge of Emerging and Re-Emerging Infections
With the adoption of the International Health Regulations [IHR(2005)] by the WHO, India has made considerable progress and continues to demonstrate a strong commitment for establishing and operating a disease surveillance programme. The Union Ministry of Health and Family Welfare launched an Integrated Disease Surveillance Project (IDSP) in 2004 to connect all district hospitals and state-run medical colleges to facilitate tele-education, training of health professionals and monitoring of disease trends. The project is implemented through the Central Surveillance Unit within the National Centre for Disease Control (NCDC) at New Delhi and aims to detect early warning signals of impending disease outbreaks and initiate effective and rapid health actions with the help of information and communication technology at the district, state and national levels. In addition to monitoring diseases like vector borne infections, diarrhoeal diseases, respiratory diseases, and vaccine preventable diseases, the IDSP also has a category called ‘unusual clinical syndromes,’ which would alert public health professionals to an intentional release of any biological-agent [114].
Laboratories in India played a major role in the management of the outbreak investigations during the recent 2009 H1N1 Influenza pandemic. Some laboratories geared their capacity by training manpower to carry out confirmation of index cases and test large number of samples. This activity may have helped build capacity of several state level laboratories to tackle similar emergencies, though, lessons learnt from managing one outbreak may not always suffice for another, as the agent, mode of transmission and susceptible population may differ. Two main factors for containment of an outbreak are isolation and contact tracing [115]. There is a need to move from conventional time consuming laboratory techniques to molecular technologies with rapid and sensitive detection capabilities and increased throughput for responding to the threats posed by emerging and re-emerging viruses in the recent past [115, 116]. There is also a need to create awareness among clinical microbiologists/virologists about existing outbreak reporting systems, laboratories designated for handling and processing samples for outbreak management, and for training and development of skills for testing and identifying new and emerging pathogens [115].
References
- 1.Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.http://www.thenakedscientists.com/HTML/articles/article/pandemic-where-do-new-viral-infections-come-from/
- 4.Lederberg J. Infectious history. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control (CDC) (1991) Aedes albopictus introduction into continental Africa, 1991. MMWR Morb Mortal Wkly Rep 40:836–838 [PubMed]
- 6.LeDuc JW, Childs JE, Glass GE. The Hantaviruses, etiologic agents of hemorrhagic fever with renal syndrome: a possible cause of hypertension and chronic renal disease in the United States. Annu Rev Public Health. 1992;13:79–98. doi: 10.1146/annurev.pu.13.050192.000455. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee K. Emerging viral infections with special reference to India. Indian J Med Res. 1996;103:177–200. [PubMed] [Google Scholar]
- 8.Kumar NP, Joseph R, Kamaraj T, Jambulingam P. A226 V mutation in virus during the 2007 chikungunya outbreak in Kerala, India. J Gen Virol. 2008;89:1945–1948. doi: 10.1099/vir.0.83628-0. [DOI] [PubMed] [Google Scholar]
- 9.Hening RM (1993) In: Dancing matrix; Vintage Books, New York, pp xii–xiii
- 10.Schneider MC, Belotto A, Ade MP, Leanes LF, Correa E, Tamayo H, Medina G, Rodrigues MJ. Epidemiologic situation of human rabies in Latin America in 2004. Epidemiol Bull. 2005;26:2–4. [PubMed] [Google Scholar]
- 11.Chomel BB, Belotto A, Meslin FX. Wildlife, exotic pets, and emerging zoonoses. Emerg Infect Dis. 2007;13:6–11. doi: 10.3201/eid1301.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charrel RN, de Lamballerie X. Arenaviruses other than Lassa virus. Antiviral Res. 2003;57:89–100. doi: 10.1016/S0166-3542(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 13.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo PC, Lau SK, Yuen KY. Infectious diseases emerging from Chinese wet-markets: zoonotic origins of severe respiratory viral infections. Curr Opin Infect Dis. 2006;19:401–407. doi: 10.1097/01.qco.0000244043.08264.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ, Mahy BW. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 16.Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, Zaki SR, Paul G, Lam SK, Tan CT. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 17.Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Harcourt BH, Yu M, Tamin A, Rota PA, Bellini WJ, Eaton BT. Molecular biology of Hendra and Nipah viruses. Microbes Infect. 2001;3:279–287. doi: 10.1016/S1286-4579(01)01381-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang LF, Yu M, Hansson E, Pritchard LI, Shiell B, Michalski WP, Eaton BT. The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. J Virol. 2000;74:9972–9979. doi: 10.1128/JVI.74.21.9972-9979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luby SP, Hossain MJ, Gurley ES, Ahmed BN, Banu S, Khan SU, Homaira N, Rota PA, Rollin PE, Comer JA, Kenah E, Ksiazek TG, Rahman MR. ecurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis. 2009;15:1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ, Ksiazek TG, Mishra A. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12:235–240. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E, Khan R, Ahmed BN, Rahman S, Nahar N, Kenah E, Comer JA, Ksiazek TG. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu VP, Hossain MJ, Parashar UD, Ali MM, Ksiazek TG, Kuzmin I, Niezgoda M, Rupprecht C, Bresee J, Breiman RF. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis. 2004;10:2082–2087. doi: 10.3201/eid1012.040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein JH, Prakash V, Smith CS, Daszak P, McLaughlin AB, Meehan G, Field HE, Cunningham AA. Henipavirus infection in fruit bats (Pteropus giganteus), India. Emerg Infect Dis. 2008;14:1309–1311. doi: 10.3201/eid1408.071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.http://www.searo.who.int/en/Section10/Section372_13452.htm
- 26.Chong HT, Kamarulzaman A, Tan CT, Goh KJ, Thayaparan T, Kunjapan SR, Chew NK, Chua KB, Lam SK. Treatment of acute Nipah encephalitis with ribavirin. Ann Neurol. 2001;49:810–813. doi: 10.1002/ana.1062. [DOI] [PubMed] [Google Scholar]
- 27.McEachern JA, Bingham J, Crameri G, Green DJ, Hancock TJ, Middleton D, Feng YR, Broder CC, Wang LF, Bossart KN. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine. 2008;26:3842–3852. doi: 10.1016/j.vaccine.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weingartl HM, Berhane Y, Caswell JL, Loosmore S, Audonnet JC, Roth JA, Czub M. Recombinant nipah virus vaccines protect pigs against challenge. J Virol. 2006;80:7929–7938. doi: 10.1128/JVI.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong LR, Zaki SR, Goldoft MJ, Todd RL, Khan AS, Khabbaz RF, Ksiazek TG, Peters CJ. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used, rodent-infested structures. J Infect Dis. 1995;172:1166. doi: 10.1093/infdis/172.4.1166. [DOI] [PubMed] [Google Scholar]
- 30.http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/caseinfo.htm
- 31.Douron E, Moriniere B, Matheron S, Girard PM, Gonzalez JP, Hirsch F, McCormick JB. HFRS after a wild rodent bite in the Haute-Savoie and risk of exposure to Hantaan-like virus in a Paris laboratory. Lancet. 1984;1:676–677. doi: 10.1016/S0140-6736(84)92187-1. [DOI] [PubMed] [Google Scholar]
- 32.Wells RM, Sosa Estani S, Yadon ZE, Enria D, Padula P, Pini N, Mills JN, Peters CJ, Segura EL. An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia. Emerg Infect Dis. 1997;3:171–174. doi: 10.3201/eid0302.970210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carey DE, Reuben R, Panicker KN, Shope RE, Myers RM. Thottapalayam virus: a presumptive arbovirus isolated from a shrew in India. Indian J Med Res. 1971;59:1758–1760. [PubMed] [Google Scholar]
- 34.Okumura M, Yoshimatsu K, Kumperasart S, Nakamura I, Ogino M, Taruishi M, Sungdee A, Pattamadilok S, Ibrahim IN, Erlina S, Agui T, Yanagihara R, Arikawa J. Development of serological assays for Thottapalayam virus, an insectivore-borne Hantavirus. Clin Vaccine Immunol. 2007;14:173–181. doi: 10.1128/CVI.00347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clement J, Hinrichsen S, Crescente J, Bigaignon G, Yersin C, Muthusethupathi M, Nainan G, Terpstra W, Lundkvist A, Van Ranst M. Hantavirus-induced hemorrhagic fever with renal syndrome (HFRS) has to be considered in the differential diagnosis of leptospirosis-suspected cases in the new and the old world. Am J Trop Med Hyg. 1999;61:316–317. [Google Scholar]
- 36.Clement J, Muthusethupathi M, Nainan G, van Ranst M. First fatal cases of hantavirus nephropathy in India. Clin Inf Dis. 2000;31:315. doi: 10.1093/ndt/gfi334. [DOI] [PubMed] [Google Scholar]
- 37.Clement J, Maes P, Muthusethupathi M, Nainan G, van Ranst M. First evidence of fatal hantavirus nephropathy in India, mimicking leptospirosis. Nephrol Dial Transplant. 2006;21:826–827. doi: 10.1093/ndt/gfi334. [DOI] [PubMed] [Google Scholar]
- 38.Chandy S, Mitra S, Sathish N, Vijayakumar TS, Abraham OC, Jesudason MV, Abraham P, Yoshimatsu K, Arikawa J, Sridharan G. A pilot study for serological evidence of hantavirus infection in human population in south India. Indian J Med Res. 2005;122:211–215. [PubMed] [Google Scholar]
- 39.Mehta S, Jiandani P. Ocular features of hantavirus infection. Indian J Ophthalmol. 2007;55:378–380. doi: 10.4103/0301-4738.33827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huggins JW, Hsiang CM, Cosgriff TM, Guang MY, Smith JI, Wu ZO, LeDuc JW, Zheng ZM, Meegan JM, Wang QN, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164:1119–1127. doi: 10.1093/infdis/164.6.1119. [DOI] [PubMed] [Google Scholar]
- 41.Maes P, Clement J, Van Ranst M. Recent approaches in hantavirus vaccine development. Expert Rev Vaccines. 2009;8:67–76. doi: 10.1586/14760584.8.1.67. [DOI] [PubMed] [Google Scholar]
- 42.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 43.http://www.searo.who.int/en/Section10/Section2246_13975.htm
- 44.Rajapakse S, Rodrigo C, Rajapakse A. Atypical manifestations of chikungunya infection. Trans R Soc Trop Med Hyg. 2010;104:89–96. doi: 10.1016/j.trstmh.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS, Morey SH, Purohit HJ, Taori GM, Daginawala HF. Neurological complications of Chikungunya virus infection. Neurol India. 2009;57:177–180. doi: 10.4103/0028-3886.51289. [DOI] [PubMed] [Google Scholar]
- 46.Ramful D, Carbonnier M, Pasquet M, Bouhmani B, Ghazouani J, Noormahomed T, Beullier G, Attali T, Samperiz S, Fourmaintraux A, Alessandri JL. Mother-to-child transmission of chikungunya virus infection. Pediatr Infect Dis J. 2007;26:811–815. doi: 10.1097/INF.0b013e3180616d4f. [DOI] [PubMed] [Google Scholar]
- 47.Gerardin P, Barau G, Michault A, Bintner M, Randrianaivo H, Choker G, Lenglet Y, Touret Y, Bouveret A, Grivard P, Le Roux K, Blanc S, Schuffenecker I, Couderc T, Arenzana-Seisdedos F, Lecuit M, Robillard PY. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Reunion. PLoS Med. 2008;5:e60. doi: 10.1371/journal.pmed.0050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravi V. Re-emergence of chikungunya virus in India. Indian J Med Microbiol. 2006;24:83–84. doi: 10.4103/0255-0857.25175. [DOI] [PubMed] [Google Scholar]
- 50.Abzug MJ. Enterovirus 71: emergence of the new poliomyelitis. South Afr J Epidemiol Infect. 2009;24:5–8. [Google Scholar]
- 51.Mizuta K, Abiko C, Murata T, Matsuzaki Y, Itagaki T, Sanjoh K, Sakamoto M, Hongo S, Murayama S, Hayasaka K. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J Clin Microbiol. 2005;43:6171–6175. doi: 10.1128/JCM.43.12.6171-6175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong SS, Yip CC, Lau SK, Yuen KY. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol Infect. 2010;138:1071–1089. doi: 10.1017/S0950268809991555. [DOI] [PubMed] [Google Scholar]
- 53.Sasidharan CK, Sugathan P, Agarwal R, Khare S, Lal S, Jayaram Paniker CK. Hand-foot-and-mouth disease in Calicut. Indian J Pediatr. 2005;72:17–21. doi: 10.1007/BF02760573. [DOI] [PubMed] [Google Scholar]
- 54.Sarma N, Sarkar A, Mukherjee A, Ghosh A, Dhar S, Malakar R. Epidemic of hand, foot and mouth disease in West Bengal, India in August, 2007: a multicentric study. Indian J Dermatol. 2009;54:26–30. doi: 10.4103/0019-5154.48982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karmarkar SA, Aneja S, Khare S, Saini A, Seth A, Chauhan BK. A study of acute febrile encephalopathy with special reference to viral etiology. Indian J Pediatr. 2008;75:801–805. doi: 10.1007/s12098-008-0150-2. [DOI] [PubMed] [Google Scholar]
- 56.Deshpande JM, Nadkarni SS, Francis PP. Enterovirus 71 isolated from a case of acute flaccid paralysis in India represents a new genotype. Curr Sci. 2003;84:1350–1353. [Google Scholar]
- 57.Beig FK, Malik A, Rizvi M, Acharya D, Khare S. Etiology and clinico-epidemiological profile of acute viral encephalitis in children of western Uttar Pradesh, India. Int J Infect Dis. 2010;14:e141–e146. doi: 10.1016/j.ijid.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 58.Narain JP, Rajesh K, Bhatia R. Pandemic (H1N1) 2009: Epidemiological, clinical and prevention aspects. Natl Med J India. 2009;22:e1–e6. [PubMed] [Google Scholar]
- 59.http://mohfw-h1n1.nic.in/
- 60.http://www.searo.who.int/LinkFiles/CDS_News_letter_vol-8_issue-1.pdf
- 61.Chan PK. Outbreak of avian influenza A (H5N1) virus infection in Hong Kong in 1997. Clin Infect Dis. 2002;34(Suppl 2):S58–64. doi: 10.1086/338820. [DOI] [PubMed] [Google Scholar]
- 62.http://www.who.int/csr/disease/avian_influenza/country/cases_table_2011_04_11/en/index.html
- 63.http://www.dahd.nic.in/
- 64.Bhatt PN, Rodrigues FM. Chandipura: a new arbovirus isolated in India from patients with febrile illness. Indian J Med Res. 1967;55:1295–1305. [PubMed] [Google Scholar]
- 65.Rodrigues JJ, Singh PB, Dave DS, Prasan R, Ayachit V, Shaikh BH, Pavri KM. Isolation of chandipura virus from the blood in acute encephalopathy syndrome. Indian J Med Res. 1983;77:303–307. [PubMed] [Google Scholar]
- 66.Rao BL, Basu A, Wairagkar NS, Gore MM, Arankalle VA, Thakare JP, Jadi RS, Rao KA, Mishra AC. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with chandipura virus. Lancet. 2004;364:869–874. doi: 10.1016/S0140-6736(04)16982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chadha MS, Arankalle VA, Jadi RS, Joshi MV, Thakare JP, Mahadev PV, Mishra AC. An outbreak of chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566–570. [PubMed] [Google Scholar]
- 68.Investigation of outbreak of encephalitis in Nanded district and some districts of Vidarbha region of Maharashtra, Annual Report 2003–2004. National Institute of Virology (NIV). NIV, Pune 2004, pp 3–4
- 69.Encephalitis in Bhandara and Nagpur, Maharashtra, Annual Report 2005–2006. National Institute of Virology (NIV), Pune 2006 p 8
- 70.Tandale BV, Tikute SS, Arankalle VA, Sathe PS, Joshi MV, Ranadive SN, Kanojia PC, Eshwarachary D, Kumarswamy M, Mishra AC. Chandipura virus: a major cause of acute encephalitis in children in North Telangana, Andhra Pradesh, India. J Med Virol. 2008;80:118–124. doi: 10.1002/jmv.21041. [DOI] [PubMed] [Google Scholar]
- 71.Gurav YK, Tandale BV, Jadi RS, Gunjikar RS, Tikute SS, Jamgaonkar AV, Khadse RK, Jalgaonkar SV, Arankalle VA, Mishra AC. Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian J Med Res. 2010;132:395–399. [PubMed] [Google Scholar]
- 72.Geevarghese G, Arankalle VA, Jadi R, Kanojia PC, Joshi MV, Mishra AC. Detection of chandipura virus from sand flies in the genus Sergentomyia (Diptera: Phlebotomidae) at Karimnagar District, Andhra Pradesh, India. J Med Entomol. 2005;42:495–496. doi: 10.1603/0022-2585(2005)042[0495:DOCVFS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 73.Dhanda V, Rodrigues FM, Ghosh SN. Isolation of Chandipura virus from sandflies in Aurangabad. Indian J Med Res. 1970;58:179–180. [PubMed] [Google Scholar]
- 74.Fontenille D, Traore-Lamizana M, Trouillet J, Leclerc A, Mondo M, Ba Y, Digoutte JP, Zeller HG. First isolations of arboviruses from phlebotomine sand flies in West Africa. Am J Trop Med Hyg. 1994;50:570–574. doi: 10.4269/ajtmh.1994.50.570. [DOI] [PubMed] [Google Scholar]
- 75.Mavale MS, Fulmali PV, Geevarghese G, Arankalle VA, Ghodke YS, Kanojia PC, Mishra AC. Venereal transmission of chandipura virus by Phlebotomus papatasi (Scopoli) Am J Trop Med Hyg. 2006;75:1151–1152. [PubMed] [Google Scholar]
- 76.Rao TR, Singh KR, Dhanda V, Bhatt PN. Experimental transmission of chandipura virus by mosquitoes. Indian J Med Res. 1967;55:1306–1310. [PubMed] [Google Scholar]
- 77.Venkateswarlu CH, Arankalle VA. Evaluation of the immunogenicity of a recombinant glycoprotein-based chandipura vaccine in combination with commercially available DPT vaccine. Vaccine. 2010;28:1463–1467. doi: 10.1016/j.vaccine.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 78.http://www.ndtv.com/article/india/rare-deadly-virus-surfaces-in-gujarat-kills-three-80202
- 79.Prajapati DS, Patel KM, Patel RK, Sen DJ, Patel JS, Garg CS. Crimean Congo Haemorrhagic fever from tick-borne viral disease. Int J Compr Pharm. 2011;2:6. [Google Scholar]
- 80.Bajpai S, Nadkar MY. Crimean Congo haemorrhagic fever: requires vigilance and not panic. JAPI. 2011;59:164–167. [PubMed] [Google Scholar]
- 81.Chinikar S, Mojtaba Ghiasi S, Moradi M, Goya MM, Reza Shirzadi M, Zeinali M, Mostafavi E, Pourahmad M, Haeri A. Phylogenetic analysis in a recent controlled outbreak of Crimean-Congo haemorrhagic fever in the south of Iran. Euro Surveill. 2008;2010:15. doi: 10.2807/ese.15.47.19720-en. [DOI] [PubMed] [Google Scholar]
- 82.Bakir M, Ugurlu M, Dokuzoguz B, Bodur H, Tasyaran MA, Vahaboglu H. Crimean-Congo haemorrhagic fever outbreak in Middle Anatolia: a multicentre study of clinical features and outcome measures. J Med Microbiol. 2005;54:385–389. doi: 10.1099/jmm.0.45865-0. [DOI] [PubMed] [Google Scholar]
- 83.Outbreak news Cholera, Haiti, cholera, Pakistan, Crimean-Congo haemorrhagic fever (CCHF) and dengue fever, Pakistan. Wkly Epidemiol Rec. 2010;85:437–439. [PubMed] [Google Scholar]
- 84.Shanmugam J, Smirnova SE, Chumakov MP. Presence of antibody to arboviruses of the Crimean haemorrhagic fever-Congo (CHF-Congo) group in human beings and domestic animals in India. Indian J Med Res. 1976;64:1403–1413. [PubMed] [Google Scholar]
- 85.http://www.who.int/csr/sars/country/table2004_04_21/en/index.html
- 86.Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shaila MS. Severe acute respiratory syndrome (SARS): an old virus jumping into a new host or a new creation? J Biosci. 2003;28:359–360. doi: 10.1007/BF02705108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Umapathy S. Absence of HLA B*46 in Indian population: could it be the cause for protection from SARS epidemic? J Assoc Physicians India. 2004;52:760–761. [PubMed] [Google Scholar]
- 89.Singh RK, Hosamani M, Balamurugan V, Bhanuprakash V, Rasool TJ, Yadav MP. Buffalopox: an emerging and re-emerging zoonosis. Anim Health Res Rev. 2007;8:105–114. doi: 10.1017/S1466252307001259. [DOI] [PubMed] [Google Scholar]
- 90.Venkatesan G, Balamurugan V, Prabhu M, Yogisharadhya R, Bora DP, Gandhale PN, Sankar MS, Kulkarni AM, Singh RK, Bhanuprakash V. Emerging and re-emerging zoonotic buffalopox infection: a severe outbreak in Kolhapur (Maharashtra), India. Vet Ital. 2010;46:439–448. [PubMed] [Google Scholar]
- 91.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehendale SM, Risbud AR, Rao JA, Banerjee K. Outbreak of dengue fever in rural areas of Parbhani district of Maharashtra (India) Indian J Med Res. 1991;93:6–11. [PubMed] [Google Scholar]
- 93.Arunachalam N, Murty US, Kabilan L, Balasubramanian A, Thenmozhi V, Narahari D, Ravi A, Satyanarayana K. Studies on dengue in rural areas of Kurnool District, Andhra Pradesh, India. J Am Mosq Control Assoc. 2004;20:87–90. [PubMed] [Google Scholar]
- 94.Chouhan GS, Rodrigues FM, Shaikh BH, Ilkal MA, Khangaro SS, Mathur KN, Joshi KR, Vaidhye NK. Clinical & virological study of dengue fever outbreak in Jalore city, Rajasthan 1985. Indian J Med Res. 1990;91:414–418. [PubMed] [Google Scholar]
- 95.Angel B, Joshi V. Distribution and seasonality of vertically transmitted dengue viruses in Aedes mosquitoes in arid and semi-arid areas of Rajasthan, India. J Vector Borne Dis. 2008;45:56–59. [PubMed] [Google Scholar]
- 96.Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J. 2006;3:92. doi: 10.1186/1743-422X-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chakravarti A, Kumar A, Matlani M. Displacement of dengue virus type 3 and type 2 by dengue virus type 1 in Delhi during 2008. Indian J Med Microbiol. 2010;28:412. doi: 10.4103/0255-0857.71806. [DOI] [PubMed] [Google Scholar]
- 98.Dash PK, Sharma S, Srivastava A, Santhosh SR, Parida MM, Neeraja M, Subbalaxmi MV, Lakshmi V, Rao PV. Emergence of dengue virus type 4 (genotype I) in India. Epidemiol Infect. 2011;139:857–861. doi: 10.1017/S0950268810001706. [DOI] [PubMed] [Google Scholar]
- 99.Morens DM. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin Infect Dis. 1994;19:500–512. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- 100.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 101.Chaturvedi UC, Nagar R. Dengue and dengue haemorrhagic fever: Indian perspective. J Biosci. 2008;33:429–441. doi: 10.1007/s12038-008-0062-3. [DOI] [PubMed] [Google Scholar]
- 102.Sabesan S, Konuganti HKR, Perumal V. Spatial delimitation, forecasting and control of Japanese encephalitis: India: a case study. The open parasitology journal. 2008;2:59–63. doi: 10.2174/1874421400802010059. [DOI] [Google Scholar]
- 103.Prasad SR, Kumar V, Marwaha RK, Batra KL, Rath RK, Pal SR. An epidemic of encephalitis in Haryana: serological evidence of Japanese encephalitis in a few patients. Indian Pediatr. 1993;30:905–910. [PubMed] [Google Scholar]
- 104.Dhanda V, Thenmozhi V, Kumar NP, Hiriyan J, Arunachalam N, Balasubramanian A, Ilango A, Gajanana A. Virus isolation from wild-caught mosquitoes during a Japanese encephalitis outbreak in Kerala in 1996. Indian J Med Res. 1997;106:4–6. [PubMed] [Google Scholar]
- 105.Samuel PP, Ayanar K, Kannan M, Thenmozhi V, Paramasivan R, Balasubramanian A, Tyagi BK. Sero-entomological investigations on Japanese encephalitis outbreak in Gorakhpur division, Uttar Pradesh, India. Indian J Med Res. 2009;129:329–332. [PubMed] [Google Scholar]
- 106.Dhillon GP, Raina VK. Epidemiology of Japanese encephalitis in context with Indian scenario. J Indian Med Assoc. 2008;106:660–663. [PubMed] [Google Scholar]
- 107.http://www.path.org/projects/japanese_encephalitis_project.php
- 108.Kang G, Kelkar SD, Chitambar SD, Ray P, Naik T. Epidemiological profile of rotaviral infection in India: challenges for the 21st century. J Infect Dis. 2005;192(Suppl 1):S120–S126. doi: 10.1086/431496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Broor S, Ghosh D, Mathur P. Molecular epidemiology of rotaviruses in India. Indian J Med Res. 2003;118:59–67. [PubMed] [Google Scholar]
- 110.Nayak MK, Chatterjee D, Nataraju SM, Pativada M, Mitra U, Chatterjee MK, Saha TK, Sarkar U, Krishnan T. A new variant of Norovirus GII.4/2007 and inter-genotype recombinant strains of NVGII causing acute watery diarrhoea among children in Kolkata, India. J Clin Virol. 2009;45:223–229. doi: 10.1016/j.jcv.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 111.Chhabra P, Chitambar SD. Norovirus genotype IIb associated acute gastroenteritis in India. J Clin Virol. 2008;42:429–432. doi: 10.1016/j.jcv.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Bharaj P, Sullender WM, Kabra SK, Broor S. Human bocavirus infection in children with acute respiratory tract infection in India. J Med Virol. 2010;82:812–816. doi: 10.1002/jmv.21637. [DOI] [PubMed] [Google Scholar]
- 113.Sasidharan CK, Sugathan P, Mampilly N, Agarwal R, Khare S, Lal S, Jayaram Paniker CK. Red baby syndrome a new disease due to parvovirus B-19 observed in Kerala. Indian J Pediatr. 2009;76:309–312. doi: 10.1007/s12098-009-0057-6. [DOI] [PubMed] [Google Scholar]
- 114.Kant L, Krishnan SK. Information and communication technology in disease surveillance, India: a case study. BMC Public Health. 2010;10(Suppl 1):S11. doi: 10.1186/1471-2458-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanungo R. Managment of infectious disease outbreak: lessons learnt from the H1N1 outbreak. Indian J Med Microbiol. 2010;28:1. doi: 10.4103/0255-0857.58718. [DOI] [PubMed] [Google Scholar]
- 116.Parida MM. Rapid and real-time detection technologies for emerging viruses of biomedical importance. J Biosci. 2008;33:617–628. doi: 10.1007/s12038-008-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


