Abstract
Environmental enrichment (EE) for rodents is generally defined as providing subjects with an environment enhanced with access to conspecifics, novel and tactile stimuli, and in many preparations, more space. EE exposure, in particular as an “intervention” in adult rodents, decreases food and drug seeking and taking. This review focuses on the reduction of sucrose seeking and taking in rats assessed in operant-based procedures. The operant-based model provides a means to evaluate addiction-related behaviors. Findings using the model might translate to clinically-relevant addiction behaviors directed towards both drugs and food. Both overnight (acute) and one month (chronic) EE effects on behavior are described, including a recent evaluation of the persistence of EE effects following its removal. EE effects on neurobiology related to sucrose seeking using the model are outlined, with a special emphasis on meso-cortico-limbic terminals. Overall, our working hypothesis for how EE reduces sucrose seeking and taking is that EE alters processing of incentive valence. This may also be accompanied by changes in learning and affect. Anti-seeking and anti-taking effects of EE have translational implications for the prevention and treatment of both drug addiction and food-focused behaviors (“food addiction”).
Keywords: addiction, craving, environmental enrichment, food, relapse, sucrose
1. Introduction
Environmental enrichment (EE) has been evaluated in a variety of animal models, proving to be effective at enhancing learning (Hebb, 1947), facilitating recovery following brain lesions (Johansson, 2003), and postponing disease progression in models of age-related cognitive decline and Huntington’s disease (Skillings et al., 2014; Birch and Kelly, 2019). In addition, there is a growing literature demonstrating EE effects on incentive motivation. An early example was a report with rats where subjects housed in a large enclosure with conspecifics (“Rat Park”) were less likely to consume a morphine solution than subjects housed in isolation (Alexander et al., 1978). Subsequent studies identified anti-drug taking effects of EE in an operant model where rats housed in EE self-administered less amphetamine than Controls (Bardo et al., 2001). Translational implications of these findings for drug addiction are far-reaching and have been considered in-depth (Alexander, 2008). In the past decade studies have revealed that EE has effects on incentive motivation beyond the reduction of primary reinforcement effects. Our laboratory discovered in 2008 that EE reduced operant responding maintained by a cue previously associated with sucrose self-administration (Grimm et al., 2008). This effect generalized to rats with a history of cocaine self-administration (Thiel et al., 2009) and to mice in a cocaine conditioned place preference procedure (Solinas et al., 2008). These results were found in rodent models of craving, a subjective and largely unconsciously-driven behavior (Grimm, 2011). Drug or food cravings often precede relapse to drug or food taking (Robbins and Ehrman, 1998; Boswell and Kober, 2016). Therefore, a translational implication is that EE could suppress drug- or food-directed behavior before the individual even has access to drug or food.
This review is primarily of operant-based findings of EE reducing responding for a sucrose-paired cue (seeking), but also findings of decreased operant-based sucrose taking following EE. In addition, reference is made to a small collection of studies examining EE effects on sucrose intake assessed using bottle choice/intake, and on food-reinforced responding in operant models not specifically addressing craving. We consider the behavioral and neurobiological effects of EE, what remains to be examined, and the translational implications of the reviewed findings.
2. General behavioral methods
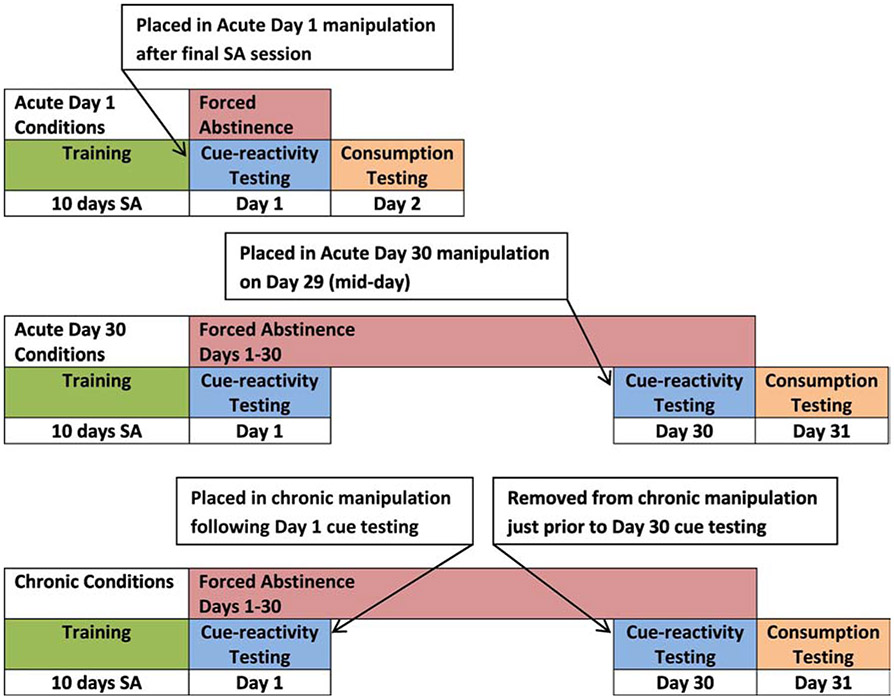
The operant conditioning approach allows assessment of several features of incentive-guided behavior, including rate of responding, with (taking) or without (seeking) primary reinforcement. Our studies have examined the effects of EE on both taking and seeking of sucrose including assessment of taking using the progressive ratio (PR) schedule of reinforcement. The PR schedule assesses motivational aspects of reinforcement including reinforcing efficacy (Roberts et al. 1989). Our general approach follows procedures developed for drug self-administration and drug cue-reactivity assessment (Meil and See, 1996). All research from our laboratory described in this review was conducted following NIH guidelines (PHS, 2015). In addition, unless otherwise stated, testing was conducted using food-sated, adult, male Long-Evans rats. In the vivarium, rats live single-housed from late adolescence (postnatal (PN) day 50) and are placed into EE conditions according to the particular study procedure, typically after PN 90. The single-housed condition is housing in standard, plastic ventilated cages with standard bedding materials. These are technically “isolated” subjects, but they are handled for weight measurement 3 times a week. Rats are not isolated in the wire-bottom cages typical of some housing manipulation studies (e.g. “Rat Park”; Alexander et al. 1978). The EE condition is a very large, multi-level cage designed for ferrets (Quality Cage Company, Portland, OR, USA) housing several subjects (typically 3 rats). A PVC pipe and a plastic shelter are provided for refuge. Toys are provided, and are replaced, every M, W, F for EE that lasts more than one day. For training, rats are placed in operant conditioning chambers for daily sucrose self-administration sessions. Rats respond on an “active” lever on a fixed ratio (FR) schedule in 10 daily sessions (sucrose taking) and are subsequently tested with either sucrose unavailable (seeking) or available (taking). Sucrose seeking, as with drug seeking (Shalev et al., 2002), has been argued to model subjective craving experienced by addicts confronting reward-paired cues (Grimm, 2011). Both seeking and taking assess the incentive value of sucrose, with seeking a measure of conditioned incentive value and taking a measure of primary incentive value. Basic methodology for examining effects of EE on sucrose seeking and taking is outlined in Figure 1. For the Figures in this review, the seeking or taking behaviors are presented as active lever responses. Also measured, but not presented in this review, are number of deliveries of sucrose (or of the sucrose-paired cue in seeking studies), inactive lever responses, and photobeam breaks.
Fig. 1.
General method for EE as an acute (overnight) or chronic (29 days) intervention following sucrose self-administration training. Cue-reactivity testing is responding on a lever that produces a tone+light cue previously associated with sucrose delivery (seeking) while consumption testing is responding that produces the cue along with sucrose (taking). Figure reproduced from “Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration,” by J.W. Grimm, R. Weber, J. Barnes, J. Koerber, K. Dorsey, and E. Glueck, 2013, PLoS One, 8:e54164. Copyright 2013 by the authors.
3. EE reduces sucrose seeking and taking
3.1. Protective versus intervention effect of EE
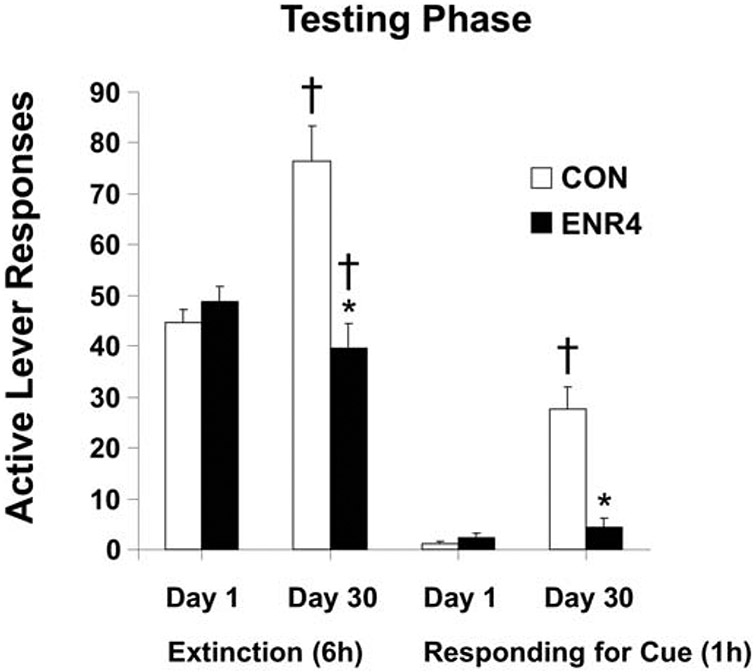
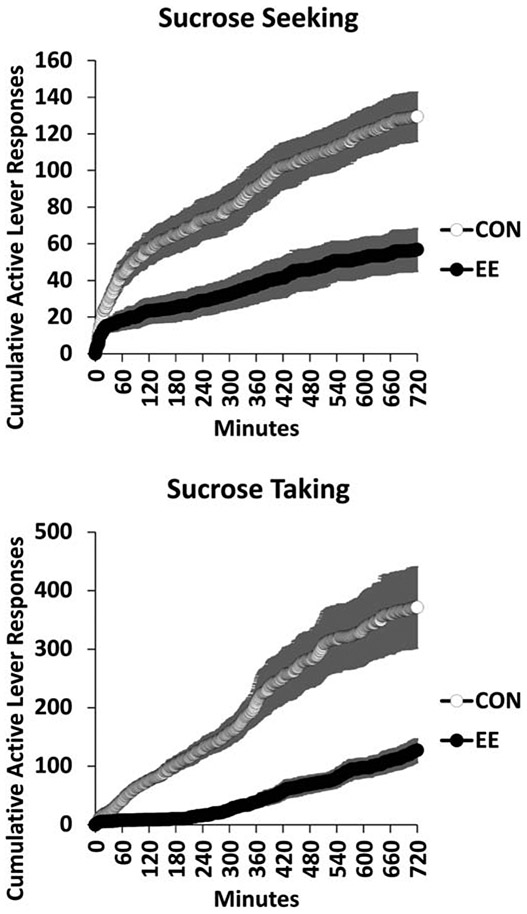
Our initial examination of the effects of EE on sucrose-seeking behavior followed the general design of EE studies where subjects live in either Control (isolated) or EE housing conditions for an extended period of early development (Grimm et al., 2008). We were curious as to whether EE would have a protective effect of reducing sucrose taking and subsequent seeking. In the experiment, rats were assigned to housing conditions two weeks after weaning (PN 38) and they remained in that condition for the remainder of the study. At PN 70, rats were trained to respond for sucrose in operant conditioning chambers on a FR schedule in daily 6-h sessions for 10 days. Rats housed in EE tended to respond at a higher rate for sucrose. After 30 days of abstinence, there were no significant differences in extinction behavior (without or with a sucrose-paired cue) between housing conditions. The taking results contrast with two studies subsequently reporting that rats assigned to EE housing for many weeks responded less than control-housed rats for sucrose (Green et al. 2010; Gill and Cain, 2011). Our EE condition (n=4) likely provided a different social environment from these other studies (n=8 or 12); perhaps this or other unidentified variables resulted in these different findings. In a second experiment, an “intervention” strategy was used where rats were only provided EE in adulthood following sucrose self-administration training (training began after PN 90). Just prior to EE, all rats were tested for baseline sucrose seeking; this was day 1 of abstinence. Seeking was assessed again on day 30 of abstinence as we were also interested in the effects of EE on the incubation of sucrose craving (Grimm et al. 2005). Incubation is the abstinence-dependent increase in sucrose seeking and taking (Harkness et al., 2010) and also in responding for drug-paired cues (reviewed by Venniro et al., 2016). Rats in EE for a month responded in extinction at half the rate of Controls, and then 80% less than Controls when responding for a tone+light cue previously paired with sucrose deliveries during training (Figure 2).
Fig. 2.
One month EE attenuates incubation of sucrose craving. After sucrose self-administration training, rats were tested for 6 h of sucrose seeking where lever presses had no consequence (extinction) followed by a 1-h session where a lever press delivered a tone+light cue previously paired with sucrose deliveries (responding for cue) after one and then 30 days of abstinence. During abstinence rats remained singly housed (CON) or were in EE with three conspecifics (ENR4). * indicates significant difference from CON on that day of testing; † indicates significant difference from Day one for that housing condition, P < .05. Means ± SEMs indicated on figure. Figure adapted with permission from, “Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats” by J.W. Grimm, D. Osincup, B. Wells, M. Manaois, A. Fyall, C. Buse, and J.H. Harkness, 2008, Behavioural Pharmacology, 19, p. 782. Copyright 2008 by Wolters Kluwer Health, Inc.
Experiment 1 indicated that a history of EE is not “protective” against developing a sucrose self-administration habit (but see Green et al. 2010; Gill and Cain, 2011), nor against developing sucrose cue-reactivity. Experiment 2, however, indicated that introduction of EE after the establishment of sucrose self-administration robustly decreases sucrose seeking. These results were subsequently identified in rats responding for a cocaine-paired cue (Thiel et al., 2009). As the EE manipulation in ours and Thiel et al.’s studies was in place for several weeks prior to seeking testing, it was not known if “chronic” EE was necessary for the “anti-craving” effect of EE. While not the main focus of a subsequent study with cocaine-experienced rats, Thiel et al. (2011) reported that responding in extinction was reduced after the first day of a two-week EE intervention.
3.2. EE parametrics
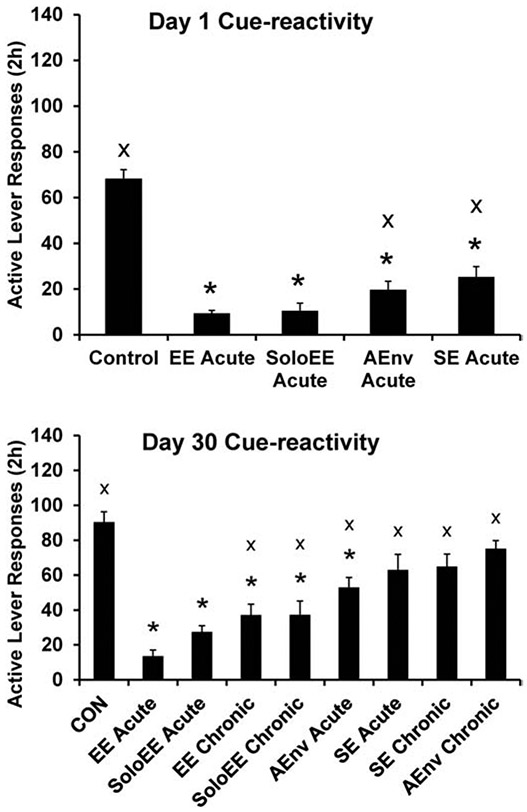
We investigated this “acute” EE effect on sucrose seeking in some detail in a parametric evaluation by including subjects that experienced EE for only a 22-h period immediately prior to seeking testing. To assess any potential interaction between incubation of craving and acute EE, we included conditions where rats were tested for seeking one or 30 days following the end of sucrose self-administration training (Grimm et al., 2013). Acute EE robustly decreased sucrose seeking both after one or 30 days of abstinence from sucrose self-administration (Figure 3).
Fig. 3.
Parametric evaluation of components of EE that contribute to the anti-seeking effect of EE. Sucrose seeking (cue-reactivity) was assessed for 2 h either one or 30 days following sucrose self-administration training. Some subjects received EE or components of EE for the 22 h prior to seeking testing (Acute) or over 29 days of abstinence (Chronic). Components were experiencing the EE cage alone (SoloEE), being paired in a double-sized cage with a conspecific (SE), or being housed singly in a novel cage of similar size to Control housing (AEnv). * indicates significant difference from Control; x indicates significant difference from EE Acute, P < .05. Means ± SEMs indicated on figures. Figures reproduced from “Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration,” by J.W. Grimm, R. Weber, J. Barnes, J. Koerber, K. Dorsey, and E. Glueck, 2013, PLoS One, 8:e54164. Copyright 2013 by the authors.
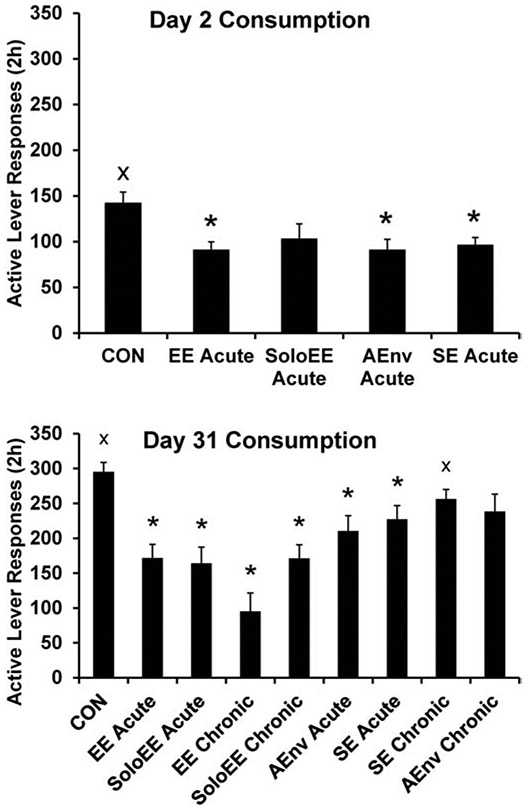
As noted, this was a parametric evaluation of EE including sucrose seeking and taking. The goal was to identify which aspect(s) of EE most contribute to its anti-sucrose seeking and taking effects. The “full” EE was three rats in the EE cage including sheltered spaces and toys. EE parametrics included using just the EE cage plus accessory items and one rat (SoloEE), housing in a novel environment (alternative environment AEnv), or social housing with a conspecific in a double-sized standard cage (SE). Controls remained single in standard housing. Sucrose self-administration training followed the procedures in Figure 1. All acute manipulations prior to day one of abstinence reduced sucrose seeking and most reduced sucrose taking (assessed the next day). Testing on day 30 included groups exposed to either acute or chronic housing conditions. Most, but not all, housing manipulations reduced sucrose seeking and then taking. Not surprisingly, the chronic AEnv condition in which novelty was habituated did not reduce sucrose seeking or taking. Unexpectedly, neither acute nor chronic social housing reduced sucrose seeking compared to Controls, although acute SE slightly reduced sucrose taking the next day compared to Controls. Examination of the results in full reveals that the most effective means to reduce sucrose cue-reactivity is to place individuals in the EE apparatus. The effect is larger if rats are housed in EE with conspecifics. For sucrose taking, the largest reduction was observed after 30 days of abstinence and this was by rats that had been in EE for one month with conspecifics (EE Chronic) (Figure 4). Further work is required to replicate this finding of acute EE being more effective at reducing seeking and chronic EE being more effect at reducing taking. An interesting recent development is that while these studies utilized a fixed-ratio schedule of reinforcement, we recently reported that acute EE is actually more effective than chronic EE at reducing responding for sucrose on a PR schedule of reinforcement (Grimm et al. 2019).
Fig. 4.
Parametric evaluation of components of EE that contribute to the anti-taking effect of EE. Sucrose taking (consumption) was assessed for 2 h either two or 31 days following sucrose self-administration training. Some subjects received EE or components of EE for the 22 h prior to seeking testing (Acute) on Day one or 30 of abstinence or over 29 days of abstinence (Chronic) prior to seeking testing on Day 30. Components were experiencing the EE cage alone (SoloEE), being paired in a double-sized cage with a conspecific (SE), or being housed singly in a novel cage of similar size to Control housing (AEnv). * indicates significant difference from Control; x indicates significant difference from EE Acute, P < .05. Means ± SEMs indicated on figures. Figures reproduced from “Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration,” by J.W. Grimm, R. Weber, J. Barnes, J. Koerber, K. Dorsey, and E. Glueck, 2013, PLoS One, 8:e54164. Copyright 2013 by the authors.
The results of these intervention-based experiments indicate both acute and chronic EE to be robust means to reduce sucrose seeking and taking. As noted in section 3.1., other laboratories have found anti sucrose seeking or taking effects in a protective-based procedure (i.e. rats live in EE for many weeks, often starting just after weaning). Specifically, Green et al. 2010 and Gill and Cain (2011) observed that EE-reared rats responded less for sucrose than isolated rats. In addition, Stairs et al. (2006) found that EE-housed rats extinguished previously sucrose-maintained responding (seeking) faster than isolated-housed Controls. One additional intriguing finding by Green et al. and then Gill and Cain was that when EE-reared rats were food restricted, they actually responded more for sucrose than isolated rats. Gill and Cain hypothesized that, as the hunger of the rats interacts with EE, EE affects incentive motivation. Finally, Yates and colleagues (2019) conducted a behavioral economic analysis of responding for sucrose in rats with a history of EE and found that, like with cocaine, elasticity of demand for sucrose was increased in EE rats (EE-housed rats find things other than drug or food to be reinforcing).
The studies from these other laboratories, in addition to our own, are the only we are aware of examining EE effects on operant measures of sucrose seeking and/or taking. There are, however, several studies of housing manipulation effects on sucrose consumption using sucrose made available in a 2-bottle choice/intake procedure. This approach lacks certain advantages of the operant approach. For example, operant methods allow assessment of motivation and reinforcing efficacy. However, choice/intake provides a means to determine whether housing environment increases/decreases the incentive value of sucrose. Of the studies that included an EE manipulation, EE reduced sucrose choice/intake in a binge model with mice (Rodriguez-Ortega et al., 2019a, 2019b), reduced sucrose choice/intake by rats (Brenes and Fornaguera, 2008), and decreased onset of binging on vegetable fat by rats (Preston et al., 2018). Loss of EE using the two-bottle choice procedure resulted in a “rebound” effect where sucrose preference increased following removal of EE from mice (Holgate et al., 2017) and sucrose preference increased for males, but decreased for female, rats (Smith et al., 2017 Morano et al., 2019) following removal of EE. Interestingly, loss of EE has been observed to produce helplessness behaviors (Smith et al.; Morano et al.) indicating that, at least in these studies, loss of EE induced a state of negative affect.
3.3. EE persistence
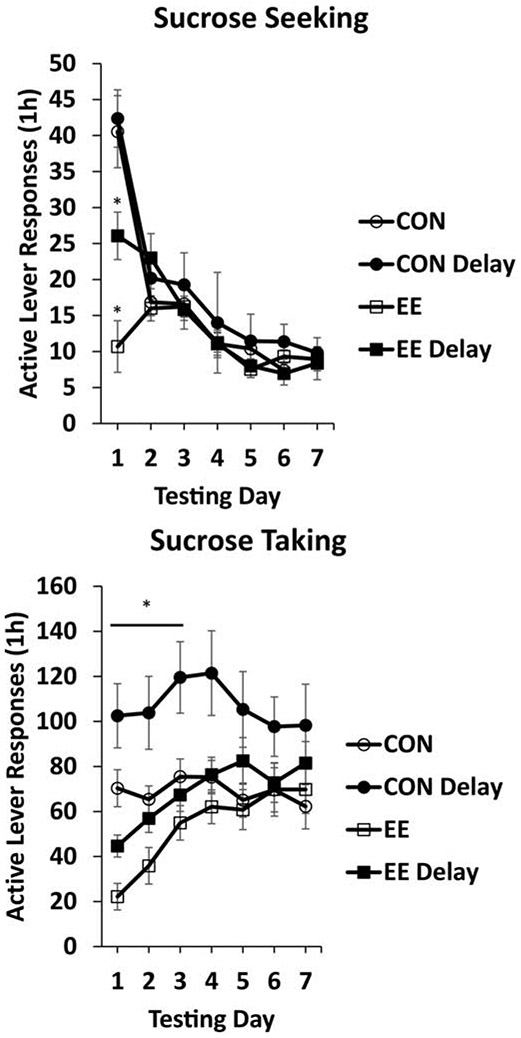
Examining the persistence of EE-mediated decreased seeking and taking could help reveal why EE reduces these behaviors, and also how EE might best translate as a therapeutic approach to reducing addiction behaviors in humans. Our recent evaluation of the persistence of “anti-craving” effects of EE assessed within- and between-session persistence (Grimm et al., 2019). Specifically, we examined how sucrose taking and seeking reductions by acute EE persisted over 12 h of testing and also over seven days of testing. In these studies, EE was acute and followed the final day of sucrose self-administration training. We hypothesized that effects would likely persist for at least 1 day, as in the parametrics study (Grimm et al. 2013) where decreased sucrose taking was observed 24 h post removal Of EE. In the first experiment, EE resulted in a 2+ fold (57%) reduction in response rate for the sucrose-paired cue and 3+ fold (70%) reduction in response rate for sucrose itself over the 12-h test session. Slopes of responding in the 12th h indicated that the EE effect was reduced at this point as slopes no longer differed between Controls and rats that had experienced EE. Nonetheless, a single EE overnight experience resulted in a large absolute difference in both sucrose seeking and taking over 12 hours of testing (Figure 5).
Fig. 5. Acute EE reduces sucrose seeking or taking for at least 12 h in a within-session analysis.
After sucrose self-administration training, rats either remained single-housed (CON), or were placed into EE overnight (EE). Testing was for 12 h the next day. Means ± SEMs indicated on figures. Figure adapted with permission from “Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats” by J.W. Grimm, J. Hyde, E. Glueck, K. North, D. Ginder, K. Jiganti, M. Hopkins, F. Sauter, D. MacDougall, and D. Hovander, 2019, Appetite, 139, p. 53. Copyright 2019 by Elsevier.
We then evaluated the effect of acute EE on persistence of sucrose seeking and taking over seven daily test sessions. Reductions in responding for the sucrose-paired cue lasted for over 24 h (Figure 6). Responding was most effectively reduced in a group that was tested immediately following EE. The reduction was slightly less in a group that had a 24-h delay between EE and testing (EE Delay). Reductions in responding for sucrose itself were more persistent, lasting at least 3 test sessions (Figure 6).
Fig. 6. Acute EE reduces sucrose seeking or taking for up to three test sessions in a between-session analysis.
After sucrose self-administration training, rats either remained single-housed (CON), or were placed into EE overnight (EE). Testing was for 1 h/day for the next seven days. For the Seeking figure, * indicates significant difference from CON for that Delay condition, P < .05. For the Taking figure, post-hoc tests after collapsing across the Delay condition revealed decreased responding by EE subjects across Days 1-3 of testing (indicated with *, P < .05). Means ± SEMs indicated on figures. Figure adapted with permission from “Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats” by J.W. Grimm, J. Hyde, E. Glueck, K. North, D. Ginder, K. Jiganti, M. Hopkins, F. Sauter, D. MacDougall, and D. Hovander, 2019, Appetite, 139, p. 54. Copyright 2019 by Elsevier.
The difference in persistence between seeking and taking is likely due to the fact that each test for seeking is an extinction test. Therefore responding should decrease with each test. A future study could examine the “delay” manipulation in more detail by expanding the delay to several days. That being stated, a potential confound to this approach is that a delay to test is essentially a procedure to examine the incubation of sucrose craving.
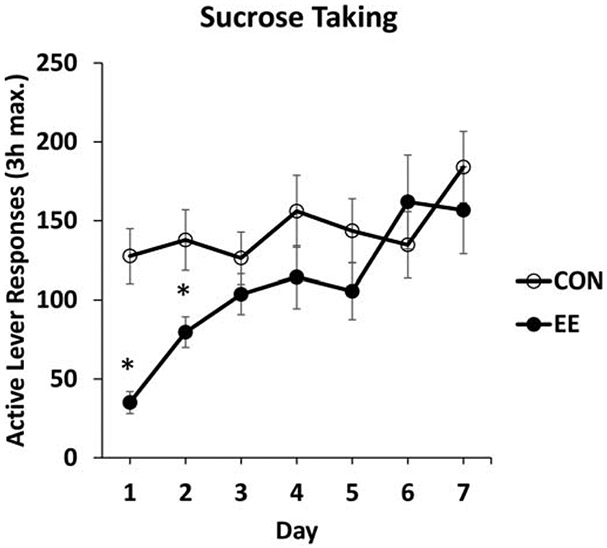
Persistence of the EE effect on sucrose taking was examined in a third experiment where rats responded on a PR instead of a fixed-ratio schedule of reinforcement. This was to establish whether acute EE would reduce responding maintained by motivation to acquire reinforcement (Roberts et al., 1989). Acute EE reduced PR responding and reinforcers earned; this effect persisted for two test sessions (Figure 7).
Fig. 7. Acute EE reduces motivation to take sucrose for two test sessions in a between-session analysis.
Between-session time course of sucrose taking on the progressive ratio (PR) schedule of reinforcement following acute EE. After sucrose PR self-administration training, rats either remained single-housed (CON), or were placed into EE overnight (EE). Testing was for up to 3 h/day for the next seven days. * indicates significant difference from CON, P < .05. Figure adapted with permission from “Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats” by J.W. Grimm, J. Hyde, E. Glueck, K. North, D. Ginder, K. Jiganti, M. Hopkins, F. Sauter, D. MacDougall, and D. Hovander, 2019, Appetite, 139, p. 55. Copyright 2019 by Elsevier.
The results of these three experiments indicate that a relatively brief EE experience decreases responding maintained by either sucrose-paired cues or sucrose. The PR results further identify that the reductions may be due to a decrease in motivation to acquire sucrose. The delay manipulation in the second experiment allowed us to determine if the effect of EE depends on an immediate contrast between the EE and self-administration contexts. As shown in the Figures above, this immediate contrast was not necessary. In the EE delay condition the rats move from home cage (“Control condition”) to operant conditioning chamber as do Control subjects, yet their responding is reduced compared to Controls. In addition, rats provided access to 10% sucrose during acute EE drink substantially less sucrose than rats provided sucrose in the home cage. Specifically, rats in EE just barely prefer sucrose over water (preference ratio of 1.2) but Controls strongly prefer sucrose over water (preference ratio of 2.9) (unpublished observations). Overall, these data support a conclusion that acute EE results in a diminished interest in sucrose that begins during EE and persists for over 24 hours after leaving the EE environment. We recently completed a follow-up study on the persistence of EE effects on sucrose taking on the PR schedule of reinforcement. This study included a chronic EE condition, and both males and females. The persistence of chronic EE at reducing motivation to respond for sucrose was slightly less than that of acute EE for males. For females, acute, and especially chronic EE were overall less effective at reducing responding for sucrose on a PR schedule of reinforcement than for males (Grimm et al., 2019).
3.4. Incentive relativism due to EE
Future studies are required to identify sex-related variables that might explain this sex difference, and further research is required to uncover what behavioral mechanisms underlie the decreased interest in sucrose with EE. Thus far, we are fairly confident that EE does not simply make rats more or less “hungry”. At least in our studies, chronic EE does not result in a body weight gain trajectory different from Control-housed rats. Instead of a general hunger-suppressing effect of EE, we hypothesize that EE decreases motivation for sucrose by reducing the relative incentive value of sucrose seeking or taking. We speculated previously (Grimm et al., 2019) that at least some amount of the anti-seeking/taking effect of EE is due to incentive downshift. Following EE, the subject is in a temporary state where a sucrose-paired cue and sucrose itself is not as reinforcing. There is incentive relativism between Control housing, the sucrose self-administration context (operant conditioning chamber), and the EE cage. This interpretation is similar to that of other investigators (Gill and Cain, 2011; Yates et al., 2019).
The closest phenomenon described in the literature is consummatory successive negative contrast (cSNC). The procedures to induce cSNC vary from runway- to operant-based. For example, DiLollo and Beez (1966) baited a runway with food pellets and measured running time to the goal area containing the pellets. If a rat had previously been reinforced for running with a large number pellets and now was reinforced with a small number, subsequent running time increased. The increase in running time was interpreted to be due to negative contrast. Most studies now use an operant-based procedure with rats reinforced with a sweet solution such as saccharin or sucrose. In a typical procedure “incentive downshift”, observed as decreased self-administration, is created when the reinforcer is switched from the training sucrose concentration of 32 to 4%. This shift has been observed to result in reduced sucrose taking for at least 4 days (Papini et al., 2014) and up to 5 days for a subgroup of animals especially sensitive to contrast (Annicchiarico and Cuenya, 2018). Some have hypothesized cSNC is due to frustration and anxiogenesis (Flaherty et al., 1992) and decreased sucrose intake has been argued to be an index of “depression” (Scheggi et al., 2018). If EE-induced decreased sucrose taking indicates negative affect, this counters proposed anti-stress effects of EE supported by some reports (Solinas et al., 2010; but see Thiel et al., 2012 and Grimm et al., 2016). It could, however, fit with a hypothesis of EE providing a mild stress “inoculation” against subsequent stressors (Crofton et al. 2015).
There are also many procedural differences between EE and cSNC. Most salient is that the contrast for EE is between the EE context, Control housing, and the operant conditioning chamber while cSNC is due to a change in the concentration of sucrose available for self-administration. It could be that the procedures (EE, cSNC) have several effects on behavior, some overlapping. Overlap is illustrated by results where EE and cSNC were combined in one study. EE reduced cSNC and this, along with results from a second experiment with contrast assessed in the 5-choice serial reaction time task, led the authors to conclude that EE rats find the operant conditioning chamber itself to be less reinforcing than the EE cage; the isolated rats find the operant conditioning chamber to be more reinforcing than their home cages (Mitchell et al., 2012). Again, incentive relativism provides a parsimonious explanation of EE effects on sucrose-directed behaviors.
As with the persistence of cSNC for sucrose (Galatzer-Levy and Papini, 2014; Annicchiarico and Cuenya, 2018), previous results from studies with EE in cocaine-experienced rats (Thiel et al., 2011) and mice (Nader et al., 2012) indicate that the duration of persistence of reduced cocaine seeking is less than 1 week. In addition, both laboratories reported a rebound effect: one week after removal from EE, rats responded at a higher rate compared to Controls in subsequent extinction sessions (Thiel et al.), and mice demonstrated enhanced conditioned place preference (CPP) one week following removal of EE (Nader et al.). We observed a rebound similar to Thiel et al., but with rats that had self-administered sucrose (unpublished observations). Thus, the effect of EE at shifting the incentive value of sucrose, and possibly other reinforcers, is time-dependent in terms of time passing post-EE. In addition, because this time away from EE is also time away from the reinforcer, incubation of craving (Grimm, 2020) may partly explain the rebound effect of “loss of EE”. In summary, it appears that initially the relative reinforcing value of EE is greater than that of the testing environment. But over many days, perhaps due to fading memory of the EE experience and incubation of craving, the value of the testing environment rebounds. This may occur in tandem with a shift in stress levels, as proposed by others (Solinas et al., 2010; Hammami-Abrand Abadi et al., 2016).
3.5. EE: other mechanisms
Future studies will require examining behaviors (e.g. open field assessment, anxiety measures, social interaction) during EE and for the days after, to provide a more complete description of how EE affects sucrose seeking and taking. In this direction, a small number of studies evaluating behavioral changes related to EE have reported effects that could contribute to the anti-seeking and/or taking effects of EE. For instance, EE-housed rat were found to be less impulsive than Controls (Wood et al., 2006; Perry et al., 2008). EE-housed rats also have an altered sensitivity to reward value. For example, van der Harst et al. (2003) reported standard-housed rats to be more sensitive to rewards than enriched housed rats. In addition, EE-housed rats responded less for the novelty of a stimulus light presentation (Cain et al., 2006). In addition, Beckmann and Bardo (2012) reported that EE-raised rats were more goal-directed towards a food reinforcer and exhibited less sign-tracking than Controls. The authors concluded that EE results in rats attributing less incentive value to reward-paired cues (Beckmann and Bardo). Finally, rats with a history of enrichment learned more quickly to drive a “car” and found the experience more reinforcing than Control-housed rats; the rats were also described to be more stress-resilient based on biochemical measures (Crawford et al., 2019). Continued, multi-dimensional examination of EE effects (e.g. anticipatory behavior, impulsivity, goal vs. sign tracking, stress resilience) may reveal clues to behavioral mechanisms underlying EE effects on sucrose- (and likely drug-) directed behaviors.
The characterization of the anti-seeking and anti-taking effects of EE, summarized in Table 1, includes the observation that acute and chronic EE reduces sucrose seeking and taking both early and late into abstinence from sucrose self-administration training. Furthermore, the effect is somewhat persistent but not permanent. The anti-seeking and anti-taking effects of EE are likely mediated by more than one mechanism. For example, a subject may contrast the EE context with the Control housing and/or operant conditioning chamber creating a shift in incentive value of sucrose. This may be accompanied by an increase or decrease in arousal and possibly, especially for rats that have been in chronic EE, an enhanced ability to learn (accelerated extinction learning and decreased impulsivity). More behavioral studies, including both males and females, are required to parse out the relative contributions of these mechanisms, and to reveal other factors involved in the anti-seeking and anti-taking effects of EE. In addition, behavioral pharmacology and studies evaluating molecular changes in brain regions accompanying the anti-seeking and anti-taking effects of EE could provide insight into how the effects are mediated in the brain. In the following two sections, results of preliminary behavioral pharmacology studies and molecular mapping studies are described.
Table 1.
Environmental/Learning Manipulations Summary: Effects on Sucrose-Directed Behaviors (operant)
| Citation | EE manipulation | Effects |
|---|---|---|
| Stairs et al., (2006) | Rats reared in EE | EE resulted in faster extinction of responding previously reinforced with sucrose |
| Grimm et al., (2008) | Rats reared in EE | EE increased non-reinforced responding during training in late adolescence; no effect on subsequent sucrose seeking |
| Grimm et al., (2008) | 29 day EE as intervention post-training | EE decreased sucrose cue-reactivity and attenuated incubation of sucrose craving |
| Green et al., (2010) | Rats reared in EE | EE decreased sucrose self-administration |
| Gill and Cain, (2011) | Rats reared in EE | EE decreased acquisition of sucrose self-administration |
| Grimm et al., (2013) | Overnight (acute) or 29 day (chronic) EE as intervention post-training | Parametric evaluation: EE including toys and conspecifics most effective at reducing sucrose seeking; acute EE more effective than chronic EE; EE reduced both sucrose seeking and taking |
| Grimm et al., (2019) | Overnight (acute) or 29 day (chronic) EE as intervention post-training; testing for 7 days post-EE | Persisting effect on sucrose seeking and taking post-EE: 12 hour reduction within-session; up to 3 days between-session |
| Yates et al., (2019) | Rats reared in EE | EE increased elasticity of demand for sucrose |
Note. Unless otherwise specified, studies tested sucrose seeking and taking by adult, male rats.
4. Behavioral pharmacology
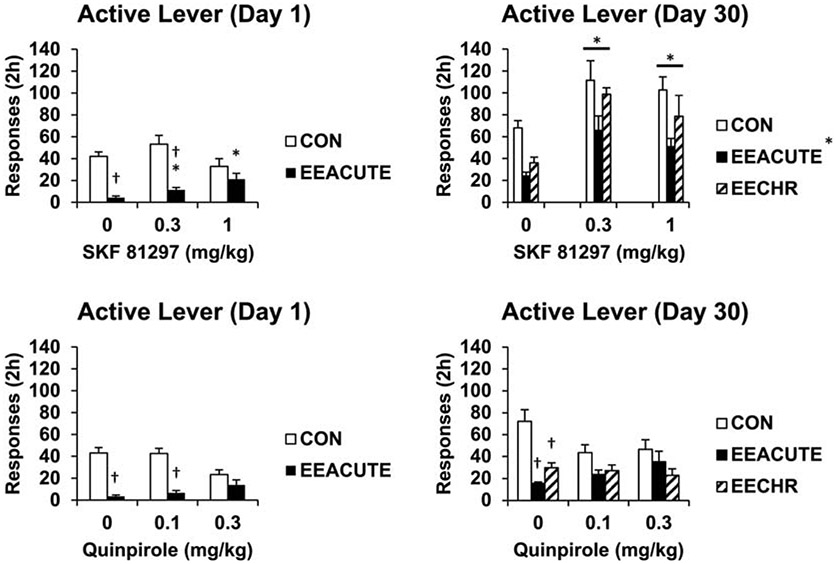
Pharmacological manipulation provides an additional means to elucidate how EE reduces behavior directed at sucrose. So far we are aware of only one study (from our laboratory) that has examined the anti-sucrose seeking effect of EE using behavioral pharmacology (Glueck et al., 2017). As described for our previous studies, rats self-administered sucrose along with a tone+light cue in 2-h daily sessions for 10 days. Rats remained in Control housing or were placed into EE either with testing the next day (acute EE followed by testing on Day one of abstinence) or 30 days later (Control housing with acute EE on Day 29 of abstinence or chronic EE for the 30 days). Testing was immediately preceded by systemic injection of either SKF81297 (dopamine D1 agonist) or quinpirole (dopamine D2 agonist). Meso-cortico-limbic dopamine is a key signal of reinforcement (Wise, 2013). The aim of the experiment was to see if dopamine receptor agonism would attenuate the anti-seeking effect of either acute or chronic EE. Our rationale was based on previous reports of changes in brain dopamine D1 receptor protein and mRNA levels (Del Arco et al. 2007; Gill et al. 2013; Ferland et al. 2014) and D1-mediated behavioral and brain cFos-expression level changes following chronic EE (Del Arco et al.; Mazarakis et al. 2014). We hypothesized that D1 receptor agonism, at least, would restore some responding reduced by EE. In fact, D1 agonism was more effective at “restoring” sucrose seeking compared to D2 agonism. This was following either acute or chronic EE, with the caveat that Control rats 30 days in abstinence had a sensitized response to D1 agonism compared to Control rats 1 day into abstinence (Figure 8).
Fig. 8. Dopamine D1 agonist is more effective than D2 agonist at restoring sucrose seeking following acute or chronic EE.
Following sucrose self-administration training, subjects were tested for sucrose seeking in a 2-h session either the next day (Day 1) or after 29 days of abstinence (Day 30). Some rats were placed into EE overnight before testing (EEACUTE) or for the 29 days of abstinence (EECHR). Immediately before testing, subjects were injected with a dose of D1 (SKF 81297) or D2 (quinpirole) agonist. * indicates significant difference from 0 dose; † indicates significant difference from CON housing condition at that dose, P < .05. Means ± SEMs indicated on figures. Figures adapted with permission from “Effects of dopamine D1 and D2 receptor agonists on environmental enrichment attenuated sucrose cue reactivity in rats” by E. Glueck, D. Ginder, J. Hyde, K. North, and J.W. Grimm, 2017, Psychopharmacology, 234, pp. 819-820. Copyright 2017 by Springer Nature.
These results indicate that dopamine, especially at the D1 receptor subtype, is involved in the anti-sucrose seeking effect of EE. There are some findings with cSNC that may inform future behavioral pharmacology studies of EE. In the cSNC model, dopamine agonism (amphetamine) attenuated negative contrast while dopamine antagonism (alpha-flupenthixol) enhanced contrast (Phelps et al., 2015). In addition, rats downshifted from a high to low concentration of sucrose had a reduced spike in extracellular nucleus accumbens dopamine (meso-limbic terminal) when consuming sucrose compared to rats not experiencing a downshift; decreased dopamine overflow correlated with decreased sucrose intake (Genn et al., 2004). These findings with dopamine in the cSNC model align well with our findings with EE. Other pharmacological targets are of interest for study. For example, a benzodiazepine (GABA agonist) attenuated contrast (Flaherty et al., 1992; but see Phelps et al., 2015), as did the opiate agonist morphine (Rowan and Flaherty, 1987). The opiate antagonist naloxone enhanced contrast (Pellegrini et al., 2005). Pharmacological evaluation in non-operant based studies also informs future studies of EE effects on sucrose seeking and taking. In a binge model (sucrose bottle choice/intake) EE-exposed mice were not sensitive to a dose of orexin 1 receptor antagonist SB334867 that reduced sucrose intake by Control-housed mice (Rodríguez-Ortega et al., 2019b). Further research could then examine how compounds targeting these receptors either reduce or exaggerate the anti-seeking and anti-taking effects of EE.
In summary, we still have much to learn from behavioral pharmacology about how the anti-seeking and anti-taking effects of EE are mediated. Roles for dopamine D1 and D2, and perhaps orexin 1 receptors have been established (above). Other receptor candidates include opiate and glutamate receptors as antagonism of opiate and glutamate neurotransmission decreases sucrose seeking (Grimm et al., 2007; Uejima et al., 2007). Also, serotonin receptors are of interest as agonists (1B and 1C in particular) decrease appetite (Voigt and Fink, 2015). Perhaps systemic or brain site-directed (see next section) behavioral pharmacology will reveal more detail of receptor systems involved in the anti-seeking and anti-taking effects of EE.
5. Molecular mapping
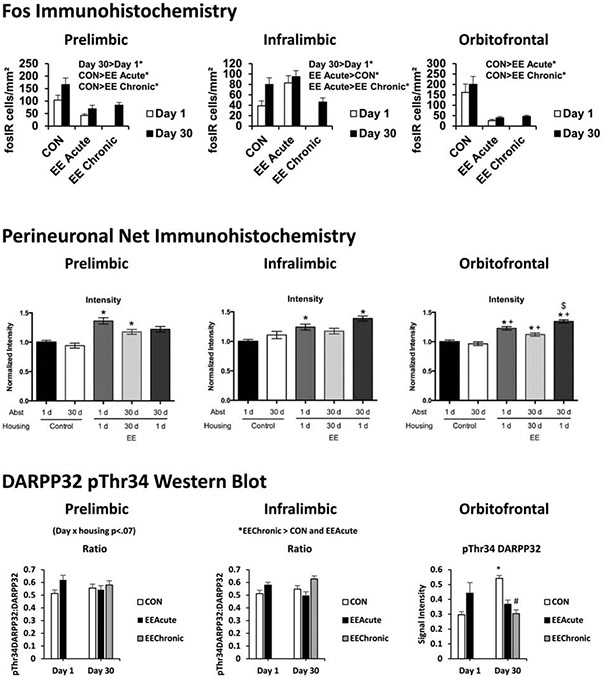
Identification of brain regions affected by EE should provide a starting point for subsequent functional analyses of the role of regions in mediating anti-seeking and anti-taking effects of EE. While the effects of EE on various aspects of brain physiology have been reported over decades (Simpson and Kelly, 2011), there are very few studies examining effects in a model incorporating food-directed behaviors. In an initial study designed to “map” brain regions affected by EE in rats with a history of sucrose self-administration and subsequent sucrose seeking, we labeled brain slices to visualize cFos immunoreactivity (IR) following EE and a sucrose cue-reactivity test. cFos protein expression increases in recently-activated neurons (Herrera and Robertson, 1996). Our cFos study was also conducted with the role of the dopamine D1 receptor in mind, as cFos IR is indirectly coupled to D1 agonism (Hu et al., 2008) and connects with our previous examination of effects of D1 antagonism on sucrose seeking (Grimm et al., 2011) and more recent examination of D1 receptor agonism effectiveness at reversing EE-reduced sucrose seeking (Glueck et al., 2017). We hypothesized that our results would be similar to findings with another reinforcer, cocaine. Indeed, similar to findings with rats that had self-administered cocaine and were tested for cocaine seeking (Thiel et al. 2010), chronic EE resulted in reduced cFos IR in meso-cortico-limbic terminals (Grimm et al., 2016). These brain regions are involved in a variety of reward-related processes including primary and secondary reinforcement (nucleus accumbens; Wise, 2013), risk/reward decision-making (prelimbic cortex; van Holstein and Floresco, 2019), response inhibition (infralimbic cortex; Hardung et al., 2017), and reward valuation (orbitofrontal cortex; Stalnaker et al., 2015). As we additionally examined effects of acute EE, we were able to establish that acute EE also reduced cFos IR in these regions (Figure 9). There was an exception to a general rule of EE reducing cFos IR in the infralimbic cortex; infralimbic cFos IR was elevated by EE (Grimm et al., 2016).
Fig. 9. EE alters synaptic plasticity and dopamine-related signaling in meso-cortico-limbic terminals in rats with a history of sucrose self-administration.
Selected molecular mapping results: A comparison of effects in the prelimbic, infralimbic, and orbitofrontal cortices. Top: cFos expression assessed using immunohistochemistry. Middle: PNN intensity assessed using immunohistochemistry. Bottom: DARPP32 phosphorylation at Thr34 assessed using Western blot. Top: * indicates significant difference (comparisons depicted above each panel), Middle: * indicates significant difference from Day 1 Controls; + indicates significant difference from Day 30 Controls; $ indicates significant difference from Day 30 Chronic EE, Bottom: * indicates significant difference from Day 1 for that housing condition or as otherwise depicted above each panel; # indicates significant difference from CON on that day of abstinence; P < .05. Means ± SEMs indicated on figures. Top figure reproduced from “Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats,” by J.W. Grimm, J.L. Barnes, J. Koerber, E. Glueck, D. Ginder, J. Hyde, and L. Eaton, 2016, Brain Structure and Function, 221, p. 2825. Copyright 2015 by Springer-Verlag Berlin Heidelberg. Figure adapted with permission. Middle figure reproduced from “Impact of environmental enrichment on perineuronal nets in the prefrontal cortex following early and late abstinence from sucrose self-administration in rats” by M. Slaker, J. Barnes, B.A. Sorg, and J.W. Grimm, 2016, PLoS One, 11:e0168256, Copyright 2016 by the authors. Bottom figure reproduced from “Sucrose abstinence and environmental enrichment effects on mesocorticolimbic DARPP32 in rats” by J.W. Grimm, E. Glueck, D. Ginder, J. Hyde, K. North, and K. Jiganti, 2018, Scientific Reports, 8:13174. Copyright 2018 by Springer Nature under the terms of the Creative Commons, creativecommons.org/licenses/by/4.0/
These results provide a scaffold for more detailed study of brain regions involved in the anti-seeking and anti-taking effects of EE. As many of the EE-related changes in cFos were in meso-cortico-limbic terminals, we have since conducted studies focusing on these brain regions. In the first, we identified staining intensity of perineuronal nets (PNNs) following EE and sucrose seeking testing. Also included was a comparison condition of subjects that had only experienced EE. PNNs are a collection of trans-synaptic extracellular matrix proteins that organize around inhibitory neurons, visualized using Wisteria floribunda agglutinin (WFA) IR in brain slices (Slaker et al., 2016a). PNNs have been shown to regulate signaling across a synapse and to be related to learning (Shen, 2018). Overall, PNNs have a “stabilizing” effect on synapses (Christensen et al. 2019). PNNs can regulate excitability of synapses and this excitability was associated with cells in the hippocampus reacting to environmental stimuli. Specifically, dissolution of PNNs in medial entorhinal cortex (hippocampal subregion) resulted in decreased inhibitory spiking and impaired coding of place cells when the subject was introduced to a novel environment (Christensen et al.).
In our study (Slaker et al., 2016b), PNNs were examined in rats without or with a history of sucrose self-administration and subsequent sucrose seeking testing. We hypothesized that, as PNNs appear to have a role in synaptic plasticity and learning, changes in PNNs might relate to the acute and/or chronic EE experience. Acute EE resulted in changes in PNN intensities across three cortical regions for rats with no history of sucrose self-administration or seeking testing. PNN intensities were decreased in the prelimbic, but increased in the orbitofrontal cortices in these animals (data not shown). For rats that had a history of sucrose self-administration training and seeking testing, PNN intensities were increased in prelimbic, infralimbic, and orbitofrontal cortices (Slaker et al.) (Figure 9). These results indicate that EE can rapidly change PNN intensity in these meso-cortical terminals, and that the history of sucrose self-administration (taking) and/or activity of responding for a sucrose-paired cue (seeking) interacts with these changes. Determining whether these changes reflect EE-mediated neuroadaptations related to incentive relativism will require further research.
In a subsequent study, we examined an intracellular marker of dopamine/glutamate integration in meso-cortico-limbic terminals. The protein, dopamine-regulated neuronal phosphoprotein 32 kDa (DARPP32), has several phosphorylation sites. The threonine 34 (Thr34) site, when phosphorylated (pThr34), indirectly promotes neuronal plasticity by activating cyclic AMP response element-binding protein (CREB) and cFos. Thr34 phosphorylation is enhanced by stimulation of dopamine D1 receptors and reduced by stimulation of glutamate NMDA receptors (Fernandez et al., 2006). DARPP32 therefore integrates dopamine and glutamate input. D1-mediated regulation of DARPP32 was of interest to us as we previously found that a dopamine D1 agonist reversed the anti-seeking effects of EE (section 4. Behavioral pharmacology). We therefore hypothesized that EE would alter phosphorylation state of DARPP32 in meso-cortico-limbic terminals. Using Western blot protein chemistry, we found that EE resulted in changes in DARPP32 levels (total and/or DARPP32 phosphorylated at Thr34) in several brain regions including infralimbic and orbitofrontal cortices, dorsomedial and dorsolateral striatum, and nucleus accumbens core and shell (Grimm et al., 2018; selected results presented in Figure 9). As defined above, these cortical regions participate in response inhibition and reward valuation, and accumbal regions in primary and conditioned reinforcement. The striatal regions participate in habit formation (Wise, 2013). Changes in DARPP32 phosphorylation at Thr34 alone, or relative to the total DARPP32 protein, may be indicative of a shift in dopamine D1 vs. glutamate NMDA receptor stimulation (increased pThr34 with more D1, decreased pThr34 with more NMDA, stimulation) (Fernandez et al.). Further research is required to better understand both the contribution of dopamine and glutamate in these brain regions to the anti-seeking effect of EE, as well as how changes in DARPP32 amount and/or activity affects downstream signaling (including gene expression) that results in changes in sucrose seeking.
Initial steps will be to incorporate behavioral pharmacology with the microinjection technique. We are interested in the orbitofrontal cortex as EE altered both cFos and DARPP32 pThr34 in this region (Figure 9) and the orbitofrontal cortex is involved in reward valuation (Stalnaker et al., 2015). A possible study would be to determine if the anti-seeking effect of EE is reversed following stimulation of the orbitofrontal cortex with a glutamate agonist; if so, a role for orbitofrontal cortex glutamate receptors in the anti-seeking effect of EE would be established. Other molecular markers could be examined and perhaps manipulated directly. Microinjection of specific agonists or antagonists is one approach, but there are also chemogenetic (e.g. DREADDS) or optogenetic approaches to consider. Table 2 provides a summary of pharmacological and molecular mapping studies of the effects of EE on sucrose seeking.
Table 2.
Pharmacology/Molecular Summary: Relationship to EE and/or Sucrose-Directed Behaviors (operant)
| Citation | Manipulation/Technique | Effects |
|---|---|---|
| Grimm et al., (2016) | Overnight (acute) or 29 day (chronic) EE as intervention post-training cFos immunohistochemistry immediately after sucrose seeking test |
(selected results) Prelimbic cortex: acute or chronic EE ↓ cFos Infralimbic cortex: acute EE ↑ cFos Orbitofrontal cortex: acute or chronic EE ↓ cFos Nucleus Accumbens Core: late abstinence acute or chronic EE ↓ cFos Nucleus Accumbens Shell: late abstinence acute or chronic EE ↓ cFos Dorsolateral Striatum: acute or chronic EE ↓ cFos |
| Slaker et al., (2016) | Overnight (acute) or 29 day (chronic) EE as intervention post-training Wisteria agglutinin immunohistochemistry; intensity of immunoreactivity |
Immediately after acute EE (no sucrose history): Prelimbic cortex: ↓ PNN intensity Infralimbic cortex: no change Orbitofrontal cortex: ↑ PNN intensity Or Immediately after sucrose-seeking test; acute or chronic EE (Figure 9): Prelimbic cortex: acute or chronic EE ↑ PNN intensity Infralimbic cortex: acute EE ↑ PNN intensity Orbitofrontal cortex: acute or chronic EE ↑ PNN intensity |
| Glueck et al., (2017) | Overnight (acute) or 29 day (chronic) EE as intervention post-training Dopamine D1 or D2 agonist administered after EE but prior to sucrose seeking |
D1 agonist (SKF81297) reversed EE anti-seeking effect at both early and late abstinence; D2 agonist (quinpirole) reversed EE anti-seeking effect only at early abstinence |
| Grimm et al., (2018) | Overnight (acute) or 29 day (chronic) EE as intervention post-training DARPP32 and DARPP32 pThr34 immunoreactivity using Western blot following sucrose seeking or no test control |
(selected results of changes in pThr34) Prelimbic cortex: no change Infralimbic cortex: chronic EE ↑ Orbitofrontal cortex: chronic EE ↓, acute EE early abstinence ↑ (trend), acute EE late abstinence ↓ (trend) Nucleus accumbens core: acute EE ↑ Nucleus accumbens shell: no change Dorsolateral striatum: no change |
Note. All studies used adult, male rats.
6. Summary
Both overnight (22 h acute EE) and one month (chronic EE) reduce sucrose seeking and taking by rats assessed using operant conditioning procedures. Chronic EE has also been demonstrated to reduce sucrose choice/intake using non-operant based procedures. The effects of EE examined using operant conditioning procedures are robust, and persist for 12 h within-session and up to three days across test sessions. Parametric evaluation of the contributing aspects of EE revealed that the large cage environment with toys plus conspecifics is most effective at reducing sucrose seeking and taking. Further research is required to better understand how EE so profoundly alters behavior both when EE is present and when it is removed. This will require a nuanced assessment of the potential relative contribution of changes in incentive motivation, affect, learning, and likely other factors related to the EE experience.
Initial evaluation of the neurobiology of the anti-seeking and anti-taking effects of EE reveals a role for dopamine D1 and D2 receptors and orexin 1 receptor, and the potential contribution of several meso-cortico-limbic dopamine terminals. More detail is required to develop a comprehensive picture of how EE alters activity in systems rather than in single brain regions, including delving into the many likely neurotransmitter and signaling cascade molecules affected by EE. Studies will need to incorporate sex differences and compare effects of EE on various reward classes.
7. Conclusions
Addiction behaviors associated with food likely contribute to epidemic rates of overweight and obesity (WHO, 2018). The anti-sucrose seeking and taking effects of EE therefore have obvious translational implications. These include evidence that exposure to certain activities and environments can improve affect and, in some cases, decrease food-directed behaviors. For example, some obese children are more successful at losing weight over 16 weeks when living in an enriched environment (Best et al., 2012) and adult BMI was found to be negatively correlated with participation in physical, social, and cognitive activities (Carr and Epstein, 2018). Shorter interventions also appear to be effective. Three minutes playing the video game Tetris® resulted in a slight reduction in food craving (Skorka-Brown et al., 2014) and 15 minutes of moderate treadmill access (brisk walking) reduced craving for high-calorie sugary snacks (Ledochowski et al., 2015). Future research into the anti-sucrose seeking and taking effects of EE should yield not only more detail of how EE down-shifts the incentive value of food, but also insight into new translational approaches for reducing food-directed behaviors that contribute to overweight and obesity. Finally, inasmuch as the neurobiology of food and drug craving overlap (Volkow et al., 2013), a better understanding of the anti-sucrose seeking and taking effects of EE could inform treatments for both drug and food addiction behaviors.
Highlights.
Environmental enrichment (EE) reduces sucrose seeking and taking by rats
Both overnight (acute) and one month (chronic) EE reduce sucrose seeking and taking
Effects persist for over 8 h within-session and over 48 h between-sessions
EE may alter dopamine signaling in meso-cortico-limbic terminals
EE may affect processing of incentive valence
Acknowledgements:
The authors wish to thank the undergraduate and graduate student researchers whose insight, enthusiasm, and effort allowed completion of many of the studies described in this review.
Funding: J.W. Grimm has been supported by NIH NIDA DA016285(1-5) and the State of Washington.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander BK, 2008. The Globalization of Addiction: A Study in Poverty of the Spirit. Oxford University Press, New York. [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF, 1978. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology (Berl) 58(2), 175–179. [DOI] [PubMed] [Google Scholar]
- Annicchiarico I, Cuenya L, 2018. Two profiles in the recovery of reward devaluation in rats: Latent class growth analysis. Neurosci Lett 684, 104–108. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C, 2001. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 155(3), 278–284. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Bardo MT, 2012. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behav Brain Res 226(1), 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, Perri MG, Schechtman KB, Epstein LH, Wilfley DE, 2012. Behavioral economic predictors of overweight children's weight loss. J Consult Clin Psychol 80(6), 1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch AM, Kelly AM, 2019. Lifelong environmental enrichment in the absence of exercise protects the brain from age-related cognitive decline. Neuropharmacology 145(Pt A), 59–74. [DOI] [PubMed] [Google Scholar]
- Boswell RG, Kober H, 2016. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev 17(2), 159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J, 2008. Effects of environmental enrichment and social isolation on sucrose consumption and preference: associations with depressive-like behavior and ventral striatum dopamine. Neurosci Lett 436(2), 278–282. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT, 2006. Environmental enrichment decreases responding for visual novelty. Behav Processes 73(3), 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KA, Epstein LH, 2018. Influence of sedentary, social, and physical alternatives on food reinforcement. Health Psychol 37(2), 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AC, Lensjo KK, Lepperod MW, Dragly S, Sutterud H, Blackstad JS, Fyhn M, Hafting T, 2019. Perineuronal nets stabilize the grid cell network. bioRxiv. https://www.biorxiv.org/content/10.1101/796342v1 Accessed November 25, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LE, Knouse LE, Kent M, Vavra D, Harding O, LeServe D, Fox N, Hu X, Li P, Glory C, Lambert KG, 2019. Enriched environment exposure accelerates rodent driving skills. Behav Brain Res, 112309. [DOI] [PubMed] [Google Scholar]
- Crofton EJ, Zhang Y, Green TA, 2015. Inoculation stress hypothesis of environmental enrichment. Neurosci Biobehav Rev, 49, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F, 2007. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm (Vienna) 114(1), 43–48. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Beez V, 1966. Negative contrast effect as a function of magnitude of reward decrement. Psychonomic Science 5, 99–100. [Google Scholar]
- Ferland JM, Zeeb FD, Yu K, Kaur S, Taves MD, Winstanley CA, 2014. Greater sensitivity to novelty in rats is associated with increased motor impulsivity following repeated exposure to a stimulating environment: implications for the etiology of impulse control deficits. Eur J Neurosci 40(12), 3746–3756. [DOI] [PubMed] [Google Scholar]
- Fernandez É, Schiappa R, Girault JA, Le Novère N, 2006. DARPP-32 is a robust integrator of dopamine and glutamate signals. PLoS computational biology 2(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty CF, Becker HC, Checke S, Rowan GA, Grigson PS, 1992. Effect of chlorpromazine and haloperidol on negative contrast. Pharmacol Biochem Behav 42(1), 111–117. [DOI] [PubMed] [Google Scholar]
- Genn RF, Ahn S, Phillips AG, 2004. Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav Neurosci 118(4), 869–873. [DOI] [PubMed] [Google Scholar]
- Gill KE, Beveridge TJ, Smith HR, Porrino LJ, 2013. The effects of rearing environment and chronic methylphenidate administration on behavior and dopamine receptors in adolescent rats. Brain Res 1527, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MJ, Cain ME, 2011. Effects of satiety on operant responding in rats raised in enrichment. Behav Pharmacol 22(1), 40–48. [DOI] [PubMed] [Google Scholar]
- Glueck E, Ginder D, Hyde J, North K, Grimm JW, 2017. Effects of dopamine D1 and D2 receptor agonists on environmental enrichment attenuated sucrose cue reactivity in rats. Psychopharmacology (Berl) 234(5), 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, ... Nestler EJ, 2010. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry 67(1), 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, 2011. Craving, in: Olmstead C, Walz W (Eds.), Animal Models of Drug Addiction. Humana Press, New York, pp. 311–336. [Google Scholar]
- Grimm JW, Barnes JL, Koerber J, Glueck E, Ginder D, Hyde J, Eaton L, 2016. Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats. Brain Struct Funct 221(5), 2817–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP, 2005. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav 84(1), 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S, 2011. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology (Berl) 216(2), 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, 2020. Incubation of food craving in rats: A review. Journal of the experimental analysis of behavior 113(1), 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hyde J, Glueck E, North K, Ginder D, Jiganti K, Hopkins M, Sauter F, MacDougall D, Hovander D, 2019. Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats. Appetite 139, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Manaois M, Osincup D, Wells B, Buse C, 2007. Naloxone attenuates incubated sucrose craving in rats. Psychopharmacology (Berl) 194(4), 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH, 2008. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol 19(8), 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Weber R, Barnes J, Koerber J, Dorsey K, Glueck E, 2013. Brief exposure to novel or enriched environments reduces sucrose cue-reactivity and consumption in rats after 1 or 30 days of forced abstinence from self-administration. PLoS One 8(1), e54164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami-Abrand Abadi A, Miladi-Gorji H, Bigdeli I, 2016. Effect of environmental enrichment on physical and psychological dependence signs and voluntary morphine consumption in morphine-dependent and morphine-withdrawn rats. Behav Pharmacol 27(2-3 Spec Issue), 270–278. [DOI] [PubMed] [Google Scholar]
- Hardung S, Epple R, Jackel Z, Eriksson D, Uran C, Senn V, Gibor L, Yizhar O, Diester I, 2017. A Functional Gradient in the Rodent Prefrontal Cortex Supports Behavioral Inhibition. Curr Biol 27(4), 549–555. [DOI] [PubMed] [Google Scholar]
- Harkness JH, Webb S, Grimm JW, 2010. Abstinence-dependent transfer of lithium chloride-induced sucrose aversion to a sucrose-paired cue in rats. Psychopharmacology (Berl) 208(4), 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO, 1947. The effects of early experience on problem-solving at maturity. American Psychologist 2, 306–307. [Google Scholar]
- Herrera DG, Robertson HA, 1996. Activation of c-fos in the brain. Prog Neurobiol 50(2-3), 83–107. [DOI] [PubMed] [Google Scholar]
- Holgate JY, Shariff M, Mu EW, Bartlett S, 2017. A Rat Drinking in the Dark Model for Studying Ethanol and Sucrose Consumption. Front Behav Neurosci 11, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Nasif FJ, Zhang J, Xu M, 2008. Fos regulates neuronal activity in the nucleus accumbens. Neurosci Lett 448(1), 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB, 2003. Environmental influence on recovery after brain lesions--experimental and clinical data. J Rehabil Med(41 Suppl), 11–16. [DOI] [PubMed] [Google Scholar]
- Ledochowski L, Ruedl G, Taylor AH, Kopp M, 2015. Acute effects of brisk walking on sugary snack cravings in overweight people, affect and responses to a manipulated stress situation and to a sugary snack cue: a crossover study. PLoS One 10(3), e0119278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarakis NK, Mo C, Renoir T, van Dellen A, Deacon R, Blakemore C, Hannan AJ, 2014. 'Super-Enrichment' Reveals Dose-Dependent Therapeutic Effects of Environmental Stimulation in a Transgenic Mouse Model of Huntington's Disease. J Huntingtons Dis 3(3), 299–309. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE, 1996. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol 7(8), 754–763. [PubMed] [Google Scholar]
- Mitchell EN, Marston HM, Nutt DJ, Robinson ES, 2012. Evaluation of an operant successive negative contrast task as a method to study affective state in rodents. Behav Brain Res 234(2), 155–160. [DOI] [PubMed] [Google Scholar]
- Morano R, Hoskins O, Smith BL, Herman JP, 2018. Loss of Environmental Enrichment Elicits Behavioral and Physiological Dysregulation in Female Rats. Front Behav Neurosci 12, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader J, Chauvet C, Rawas RE, Favot L, Jaber M, Thiriet N, Solinas M, 2012. Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology 37(7), 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini S, I RG-L, Papini MR, 2014. Identifying profiles of recovery from reward devaluation in rats. Behav Brain Res 275, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini S, Wood M, Daniel AM, Papini MR, 2005. Opioid receptors modulate recovery from consummatory successive negative contrast. Behav Brain Res 164(2), 239–249. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT, 2008. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res 193(1), 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CE, Mitchell EN, Nutt DJ, Marston HM, Robinson ES, 2015. Psychopharmacological characterisation of the successive negative contrast effect in rats. Psychopharmacology (Berl) 232(15), 2697–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHS, 2015. PHS Policy on Humane Care and Use of Laboratory Animals. NIH; https://olaw.nih.gov/policies-laws/phs-policy.htm Accessed November 25, 2019. [Google Scholar]
- Preston KE, Corwin RL, Bader JO, Crimmins SL, 2018. Relatively enriched housing conditions delay binge onset but do not attenuate binge size. Physiol Behav 184, 196–204. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, 1998. Cocaine use is associated with increased craving in outpatient cocaine abusers. Exp Clin Psychopharmacol 6(2), 217–224. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G, 1989. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 97(4), 535–538. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ortega E, Alcaraz-Iborra M, de la Fuente L, Cubero I, 2019. Protective and therapeutic benefits of environmental enrichment on binge-like sucrose intake in C57BL/6J mice. Appetite 138, 184–189. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ortega E, Alcaraz-Iborra M, de la Fuente L, de Amo E, Cubero I, 2019. Environmental Enrichment During Adulthood Reduces Sucrose Binge-Like Intake in a High Drinking in the Dark Phenotype (HD) in C57BL/6J Mice. Front Behav Neurosci 13, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan GA, Flaherty CF, 1987. The effects of morphine in the consummatory contrast paradigm. Psychopharmacology (Berl) 93(1), 51–58. [DOI] [PubMed] [Google Scholar]
- Scheggi S, Pelliccia T, Gambarana C, De Montis MG, 2018. Aripiprazole relieves motivational anhedonia in rats. J Affect Disord 227, 192–197. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y, 2002. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54(1), 1–42. [DOI] [PubMed] [Google Scholar]
- Shen HH, 2018. Core Concept: Perineuronal nets gain prominence for their role in learning, memory, and plasticity. Proc Natl Acad Sci U S A 115(40), 9813–9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Kelly JP, 2011. The impact of environmental enrichment in laboratory rats--behavioural and neurochemical aspects. Behav Brain Res 222(1), 246–264. [DOI] [PubMed] [Google Scholar]
- Skillings EA, Wood NI, Morton AJ, 2014. Beneficial effects of environmental enrichment and food entrainment in the R6/2 mouse model of Huntington's disease. Brain Behav 4(5), 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorka-Brown J, Andrade J, Whalley B, May J, 2015. Playing Tetris decreases drug and other cravings in real world settings. Addict Behav 51, 165–170. [DOI] [PubMed] [Google Scholar]
- Slaker M, Barnes J, Sorg BA, Grimm JW, 2016. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats. PLoS One 11(12), e0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker ML, Harkness JH, Sorg BA, 2016. A standardized and automated method of perineuronal net analysis using Wisteria floribunda agglutinin staining intensity. IBRO Rep 1, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BL, Lyons CE, Correa FG, Benoit SC, Myers B, Solomon MB, Herman JP, 2017. Behavioral and physiological consequences of enrichment loss in rats. Psychoneuroendocrinology 77, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M, 2008. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A 105(44), 17145–17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M, 2010. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol 92(4), 572–592. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M, 2010. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol 92(4), 572–592. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT, 2006. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol 17(7), 597–604. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G, 2015. What the orbitofrontal cortex does not do. Nat Neurosci 18(5), 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL, 2011. The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats. Pharmacol Biochem Behav 97(3), 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL, 2012. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol 17(2), 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL, 2010. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience 171(4), 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL, 2009. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol 12(9), 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, Lu L, 2007. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res 181(2), 292–296. [DOI] [PubMed] [Google Scholar]
- van der Harst JE, Baars AM, Spruijt BM, 2003. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res 142(1-2), 151–156. [DOI] [PubMed] [Google Scholar]
- van Holstein M, Floresco SB, 2019. Dissociable roles for the ventral and dorsal medial prefrontal cortex in cue-guided risk/reward decision making. Neuropsychopharmacology [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y, 2016. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224, 25–52. [DOI] [PubMed] [Google Scholar]
- Voigt JP, Fink H, 2015. Serotonin controlling feeding and satiety. Behav Brain Res 277, 14–31. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD, 2013. Obesity and addiction: neurobiological overlaps. Obes Rev 14(1), 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight Accessed November 25, 2019.
- Wise RA, 2013. Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biol Psychiatry 73(9), 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DA, Siegel AK, Rebec GV, 2006. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav 88(1-2), 132–137. [DOI] [PubMed] [Google Scholar]
- Yates JR, Bardo MT, Beckmann JS, 2019. Environmental enrichment and drug value: a behavioral economic analysis in male rats. Addict Biol 24(1), 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]