Abstract
Background
Emergency sclerotherapy is still widely used as a first line therapy for variceal bleeding in patients with cirrhosis, particularly when banding ligation is not available or feasible. However, pharmacological treatment may stop bleeding in the majority of these patients.
Objectives
To assess the benefits and harms of emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis.
Search methods
Search of trials was based on The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded through January 2010.
Selection criteria
Randomised clinical trials comparing sclerotherapy with vasoactive drugs (vasopressin (with or without nitroglycerin), terlipressin, somatostatin, or octreotide) for acute variceal bleeding in cirrhotic patients.
Data collection and analysis
Outcome measures were failure to control bleeding, five‐day treatment failure, rebleeding, mortality, number of blood transfusions, and adverse events. Data were analysed by a random‐effects model according to the vasoactive treatment. Sensitivity analyses included combined analysis of all the trials irrespective of the vasoactive drug, type of publication, and risk of bias.
Main results
Seventeen trials including 1817 patients were identified. Vasoactive drugs were vasopressin (one trial), terlipressin (one trial), somatostatin (five trials), and octreotide (ten trials). No significant differences were found comparing sclerotherapy with each vasoactive drug for any outcome. Combining all the trials irrespective of the vasoactive drug, the risk differences (95% confidence intervals) were failure to control bleeding ‐0.02 (‐0.06 to 0.02), five‐day failure rate ‐0.05 (‐0.10 to 0.01), rebleeding 0.01 (‐0.03 to 0.05), mortality (17 randomised trials, 1817 patients) ‐0.02 (‐0.06 to 0.02), and transfused blood units (8 randomised trials, 849 patients) (weighted mean difference) ‐0.24 (‐0.54 to 0.07). Adverse events 0.08 (0.03 to 0.14) and serious adverse events 0.05 (0.02 to 0.08) were significantly more frequent with sclerotherapy.
Authors' conclusions
We found no convincing evidence to support the use of emergency sclerotherapy for variceal bleeding in cirrhosis as the first, single treatment when compared with vasoactive drugs. Vasoactive drugs may be safe and effective whenever endoscopic therapy is not promptly available and seems to be associated with less adverse events than emergency sclerotherapy. Other meta‐analyses and guidelines advocate that combined vasoactive drugs and endoscopic therapy is superior to either intervention alone.
Plain language summary
Emergency sclerotherapy is not better than pharmacological therapy for acute variceal bleeding in cirrhosis
Variceal bleeding in cirrhosis is associated with a high risk of death. Although banding ligation of varices is considered the choice for endoscopic treatment, emergency sclerotherapy is frequently used particularly where ligation is not available or when it is not feasible. However, vasoactive drugs stop bleeding in most patients, and emergency sclerotherapy may carry risks to the patient and is more demanding on the health‐care system. All of the identified randomised clinical trials comparing emergency sclerotherapy with vasopressin (+/‐ intravenous or transdermal nitroglycerin), terlipressin, somatostatin, or octreotide have been reviewed. A total of 17 randomised trials including 1817 patients were included. Sclerotherapy did not appear to be superior to the vasoactive drugs in terms of control of bleeding, number of transfusions, 42‐day rebleeding and mortality, or rebleeding and mortality before other elective treatments. However, adverse events were significantly more frequent and severe with sclerotherapy than with vasoactive drugs.
Background
Variceal rupture is the cause of 60% to 70% of upper digestive bleeding in patients with cirrhosis; the other more frequent causes are portal hypertensive gastropathy (20%) and gastric varices (5%). The mortality per episode of variceal bleeding ranges from 10% to 20% (Chalasani 2003; D'Amico 2003; Sorbi 2003; Carbonel 2004).
Since it is difficult to define the duration of active variceal bleeding and when a new haematemesis or melaena should be considered a rebleeding episode, several consensus conferences stated definitions to design and report of clinical studies with regard to 'time zero', occurrence of bleeding and clinically significant bleeding, control of bleeding, and separating the index bleeding from rebleeding (Burroughs 1987; Grace 1998; De Franchis 2000; De Franchis 2005).
The incidence of early rebleeding ranges between 10% to 20% during the first six weeks following variceal bleeding (D'Amico 2003; Sorbi 2003), becoming thereafter virtually equal to that of before bleeding (Graham 1981). Early rebleeding is significantly related to death within six weeks (Graham 1981; Burroughs 1989; D'Amico 2003), suggesting that its prevention should be a primary objective in the therapeutic approach to variceal bleeding. Endoscopic therapy for bleeding oesophageal varices is widely used and currently recommended as a first‐line treatment in combination with vasoactive drugs (Grace 1997; Jalan 2000; Garcia‐Tsao 2007; Garcia‐Tsao 2008). Moreover, if banding ligation is used, it allows starting a specific treatment for the prevention of long‐term rebleeding, since it is also recommended as the preferred endoscopic prevention of variceal rebleeding (Garcia‐Tsao 2007; Garcia‐Tsao 2008). However, banding ligation is not always feasible and sclerotherapy is still used in 30% to 50% of patients (Sorbi 2003; Bosch 2004; Bosch 2008). Furthermore endoscopic emergency therapy requires a skilled endoscopist to be available and is associated with serious adverse events in 10% to 20% of the patients, also including some lethal adverse events (Williams 1996; Norberto 2007).
Yet, whether to use first vasoactive drugs or endoscopic therapy is still a matter of debate ( Jalan 2000; Sorbi 2003; Garcia‐Tsao 2007), and it has never been assessed whether it is more convenient to use endoscopic therapy only in vasoactive drug failures rather than in all patients.
The efficacy of emergency sclerotherapy has been compared with pharmacological treatments in several randomised clinical trials with inconsistent results (D'Amico 1995; Triantos 2006), while no randomised trials have been reported comparing banding ligation with vasoactive treatments.
The widespread use of emergency endoscopy in cirrhotic patients with upper digestive bleeding is almost entirely based on the widely perceived superiority of emergency sclerotherapy or band ligation over pharmacological treatments for controlling variceal bleeding. Therefore, if the results of the available randomised trials would not support this superiority, a different approach to the emergency treatment of upper digestive bleeding in cirrhosis could be considered.
To investigate the benefits and harms of emergency endoscopic therapy compared with pharmacological therapy, we have previously performed a Cochrane systematic review including all the randomised trials available until April 2001 (D'Amico 2002). The conclusion of that review was that there was no convincing evidence that sclerotherapy is superior to vasoactive drugs for the treatment of acute variceal bleeding. Since then, more randomised trials comparing emergency sclerotherapy with vasoactive drugs have been published. We have, therefore, updated our previous review.
Objectives
To assess the benefits and harms of emergency sclerotherapy versus vasoactive drugs for acute variceal bleeding in cirrhotic patients.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials comparing sclerotherapy with vasopressin +/‐ nitroglycerin, or terlipressin, or somatostatin, or octreotide for acute variceal bleeding in cirrhotic patients. Trials either published as full reports or as abstracts and regardless of the language were included in this review. Studies using quasi‐randomisation methods, eg, day of birth or date of admission, were excluded.
Types of participants
Cirrhotic patients with acute bleeding from oesophageal varices regardless of the aetiology and the severity of cirrhosis.
Types of interventions
Emergency sclerotherapy of oesophageal varices versus one of the following pharmacological treatments (irrespective of dose, duration, and mode of administration): ‐ vasopressin with or without nitroglycerin; ‐ terlipressin; ‐ somatostatin; ‐ octreotide. Additional or collateral interventions were allowed if they were given to both trial groups.
Types of outcome measures
1. Failure to control bleeding: number of patients in which the interventions did not control the acute bleeding (ie, initial or index bleeding). 2. Five‐day treatment failure: number of patients without control of bleeding or with rebleeding or dying within five days from randomisation. 3. Rebleeding: number of patients rebleeding within 42 days or while in hospital. 4. Rebleeding before other elective treatments for prevention of the rebleeding: number of patients with recurrent bleeding before receiving other elective treatments for prevention of the rebleeding. 5. Mortality: number of deaths within 42 days or while in hospital. 6. Mortality before other elective treatments for prevention of the rebleeding: number of patients who died before receiving other elective treatments for prevention of the rebleeding. 7. Transfusions: number of blood units transfused while in hospital. 8. Adverse events: number of adverse events (ICH‐GCP 1997). 9. Serious adverse events: number of serious adverse events (ICH‐GCP 1997).
Search methods for identification of studies
Retrieval of randomised clinical trials was based on The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003) (Appendix 1). A manual search of the reference lists of pertinent studies, articles, reviews, editorials from the English language medical literature was also performed. Proceedings of the most relevant international congresses (AASLD, EASL, UEGW) were handsearched. The last search update was January 2010.
Data collection and analysis
We performed the review following the recommendations of The Cochrane Collaboration (Higgins 2008) and The Cochrane Hepato‐Biliary Group (Gluud 2009).
Inclusion of trials Decisions on which trials to include were taken independently by two of the authors (IT, GP), who were unblinded with regard to the names of the authors, investigators, institution, source, and results of the identified trials. Disagreements were resolved by discussion. Excluded trials were identified with the reason for exclusion. Data extraction Data were extracted by two independent authors (IT, GP). Discrepancies were solved by discussion. The following data were extracted from each trial: a) trial characteristics: time interval between hospital admission and randomisation, definition of each of the outcomes of interest; b) patients' characteristics: number of patients, mean age, sex, prevalence of alcoholic cirrhosis, Child‐Pugh score, per cent of patients in each Child Pugh class, per cent of patients with ascites; c) treatments: sclerosant, modality of sclerotherapy, type and modality of control treatment, interventions other than the trial treatments and their specific indications; d) outcomes: number of patients with each of the outcomes of interest.
Methodological quality Methodological quality was defined as the control of bias (Kjaergard 2001; Gluud 2006; Wood 2008). The randomisation methods (allocation sequence generation and concealment of treatment allocation) were extracted as measures of bias control. The generation of the allocation sequence was classified as adequate if based on a computer, random number table, or similar. Allocation concealment was classified as adequate if based on a central independent unit, serially numbered opaque, sealed envelopes, or on‐site locked computer. Randomisation methods were classified as unclear when not described. Control of bias was considered adequate when both the allocation sequence generation and the allocation concealment were adequate. Blinding of outcome assessments, reporting of number and reasons for losses to the follow‐up, and reporting of the intention‐to‐treat analysis were considered additional measures of methodological quality. The use of the same treatment in both trial groups for failures of the trial treatments and for the prevention of rebleeding were considered also additional measures of internal validity. There was only one quasi‐randomised study among the retrieved trials, and it was excluded from the analysis. Double blinding was not assessed considering the nature of the interventions. Combination of trials according to the intervention Sclerosants and different modalities of performing emergency sclerotherapy are considered to be equivalent since there is no evidence that they may have different clinical efficacy (Goulis 1999).
We decided to perform separate meta‐analyses according to the type of vasoactive drug used as a control treatment because there are no definitive, convincing proofs that the vasoactive drugs we assessed as a control treatment for emergency sclerotherapy are equivalent. This position is supported both by haemodynamic and clinical studies (D'Amico 1999; Garcia‐Pagan 1999) and holds true even when the native hormones vasopressin and somatostatin are compared with their analogues terlipressin and octreotide, respectively (D'Amico 1999; Garcia‐Pagan 1999). Randomised trials and meta‐analyses have previously shown that terlipressin significantly reduces mortality when compared with placebo (Ioannau 2003). A similar effect has never been shown for somatostatin, octreotide, or vasopressin (Gøtzsche 1995; D'Amico 1999; Gøtzsche 2008). Double blind placebo controlled clinical trials have shown that somatostatin significantly reduces the five‐day failure rate in controlling bleeding (Burroughs 1990). A similar beneficial effect was not found for octreotide (Burroughs 1996). Moreover, a meta‐analysis has shown that somatostatin significantly reduces the failure rate of initial control of bleeding when compared with vasopressin (Imperiale 1995). Adverse events are also significantly less frequent and serious with somatostatin and octreotide than with vasopressin or terlipressin (Imperiale 1995; D'Amico 1999; Ioannau 2003).
Statistical methods Analyses were performed by RevMan 5 (RevMan 2008). Data were analysed by a random‐effects model because of the expected clinical heterogeneity. The outcomes were presented as pooled risk differences according to Der Simonian & Laird (Der Simonian 1986) with 95% confidence intervals (95% CI). Chi‐square and I‐square statistics are reported as estimates of intertrial heterogeneity. Analyses were performed using the intention‐to‐treat principle including all patients randomised as they were reported in each single study. For statistically significant risk differences (ie, when the 95% CI of the summary risk difference excludes 0), the number‐needed‐to‐treat (NNT) and 95% CI were calculated by the reciprocal of the risk difference (Laupacis 1988). Likewise, the number‐needed‐to‐harm (NNH) was calculated by the reciprocal of the risk difference of adverse events. All the results are plotted so that a risk difference less than zero favours sclerotherapy. The risk difference is reported as rate difference and not as percentage difference (for example, ‐0.15 instead of ‐15%).
To explore the robustness of results, we performed the following sensitivity analyses:
1. By combining all the included trials irrespective of the pharmacological treatment. We decided to perform this analysis because this is the most powerful sensitivity analysis, including the largest available number of patients, and because other reviews assessed the clinical efficacy of emergency sclerotherapy by combining all the randomised clinical trials independently on the pharmacological control treatment (Goulis 1999; Triantos 2006)
1.1. By removing from the above analysis one trial (Escorsell 1998), which included patients only after the control of the initial bleeding for the assessment of treatment effect on mortality.
2. By including only the trials published as full reports.
3. By including only the trials with adequate control of bias.
4. By including only the trials comparing sclerotherapy versus somatostatin but excluding the Escorsell 1998 trial as in 1.1.
Results
Description of studies
The strategy searches yielded a total of 1092 references ‐ full text publications or abstracts. Among these, 1058 were excluded because of the following reasons: a) non‐randomised trial; b) duplicate report of the same study; c) emergency sclerotherapy compared with non‐pharmacological treatments; d) sclerotherapy for the prevention of the first variceal bleeding; e) sclerotherapy for the prevention of recurrent variceal bleeding; f) review articles. Thirty‐four studies were eligible for the present meta‐analysis according to the above reported criteria: 17 studies were excluded for the reasons reported in the table 'Characteristics of excluded studies' and 17 randomised trials were included. Of the included randomised trials, three are still available only as abstracts (Di Febo 1990; Poo 1996; Lopez 1999) after 19, 13, and 10 years of their reports; the remaining 14 randomised trials are available as full reports.
The characteristics of the included randomised trials are reported in the tables 'Characteristics of included studies' and additional Table 1. Characteristics of the patients are reported in additional Table 10. Methodological characteristics of the included studies are reported in Table 3.
1. Characteristics of participants in the included studies.
| Study ID | Patients No. | Mean age, yr | Males,% | Alcoholic | Child‐Pugh | Ascites (%) | Active bleeding | Interval from |
| First author, year | EVS/Control | EVS/control | EVS/Control | etiology: EVS/Control (%) | Class A/B/C % | EVS/Control | on endoscopy EVS/Control (%) | bleeding to randomisation, hrs |
| Westaby, 1989 | 33/31 | 55/54 | 52/62 | 39/71 | EVS:12/51/36; Control:23/45/32 | Not reported. | 100/100 | Not reported |
| Escorsell, 2000 | 114/105 | 55/56 | 68/76 | 41/39 | EVS:17/52/31; Control 23/45/32 | Not reported. | 43/35 | <24 (mean=6) |
| Di Febo, 1990 | 24/23 | 57/56 | 71/83 | not reported | EVS:62/‐/‐; Control 56/‐/‐ | Not reported. | Not reported. | Not reported. |
| Shields, 1992 | 41/39 | 59/57 | 63/72 | 63/72 | EVS:18/41/41; Control 15/20/64 | Not reported. | 61/69 | <24 (mean = 4.5) |
| Planas, 1994 | 35/35 | 57 | 68/61 | 80/63 | EVS:20/46/34; Control:14/51/35 | 60/66 | 49/51 | 17 |
| Escorsell, 1998 | 79/90 | 59/57 | 73/73 | 46/53 | The mean Pugh score was reported: EVS 8.5; Control 8.2 | Not reported. | 33/32 | 56 |
| Sung, 1993 | 49/49 | 57/55 | 79/90 | 35/43 | EVS:14/43/43; Control:12/43/45 | 59/53 | 37/51 | Not reported. |
| Poo, 1996 | 21722 | Not reported. | Not reported. | 76/77 | Not reported | Not reported. | 38/23 | Not reported. |
| Jenkins, 1997 | 77/73 | 52/57 | 57 | 65/63 | EVS:15/32/53; Control:16/30/53 | Not reported. | 63/49 | 21 |
| Lopez, 1999 | 33/31 | Not reported. | Not reported. | Not reported. | Not reported. | Not reported. | Not reported. | Not reported. |
| Bildozola, 2000 | 37/39 | 52/52 | 73/84 | 73/72 | EVS:54/38/8; Control:41/46/13 | 24/33 | 49/39 | Not reported. |
| Sivri, 2000 | 36/30 | 48/46 | 32/41 | 25/21 | EVS:11/36/53; Control:8/37/55 | 87/91 | 100/100 | Not reported. |
| Ramires, 2000 | 19/21 | 56/47 | 68/57 | 42/43 | EVS:10/68/22; Control 5/57/38 | Not reported | 53/24 | Not reported |
| Shaikh, 2002 | 188/180 | 41/41 | 68/66 | not reported | EVS:76/88/24; Control:60/96/24 | Not reported | Not reported | Not reported |
| Freitas, 2000 | 53/58 | 56/55 | 67/69 | 92/94 | EVS:24/41/35; Control:20/45/35 | Not reported | Stigmata in all | < 12 |
| Silva 2004 | 43/13 | 63/61 | 69/31 | 74/46 | EVS:35/28/37; Control:23/54/23 | Not reported | 93/77 | Not reported |
| Yousuf 2000 | 48/48 | 45/46 | 75/81 | 0/0 | EVS:46/31/23;Control:40/42/18 | Not reported | 100/100 | Not reported |
The median (and range) of the most relevant patient and trial characteristics were: sample size 76 patients (40 to 368 patients), median or mean age 55 years (41 to 61 years), males 68% (32% to 83%), alcoholic aetiology of cirrhosis 64% (22% to 91%), Child‐Pugh class A 18% (0% to 59%), Child‐Pugh class B 42% (31% to 62%), and Child‐Pugh class C 34% (13% to 54%). Information on the time frame from hospital admission to randomisation was reported only in seven trials: the median was 5.5 hours (range 2 to 46 hours).
Sclerotherapy versus vasopressin We only identified one eligible trial (Westaby 1989). Immediate sclerotherapy was compared with a 12‐hour vasopressin and nitroglycerin infusion. After 12 hours all the patients in the medical treatment group underwent sclerotherapy, which provided the patients a different and more precise assessment of their bleeding than to patients in the sclerotherapy group. The latter group of patients underwent repeated endoscopy only in the case of suspected persistence of bleeding. Sclerotherapy was thereafter continued weekly in both groups until variceal obliteration.
Sclerotherapy versus terlipressin We only identified one eligible trial (Escorsell 2000). This is the largest reported trial of emergency sclerotherapy. Patients were randomised at the time of diagnostic endoscopy, and treatment started immediately. Terlipressin was administered intravenously (iv) 2 mg every 4 hours until control of bleeding up to a maximum of 48 hours, and 1 mg every 4 hours thereafter for five more days. All patients underwent the same long‐term rebleeding prevention program after the sixth to seventh study day.
Sclerotherapy versus somatostatin We identified five trials, one available only as abstract (Di Febo 1990) and four as full reports (Shields 1992; Planas 1994; Escorsell 1998; Ramires 2000). Only patients with proven variceal bleeding were included, and randomisation was carried out at diagnostic endoscopy or immediately after in all the four trials. The Escorsell et al trial assessed the treatment efficacy in preventing early rebleeding and death (Escorsell 1998). Patients consecutively admitted for acute, endoscopically proven variceal bleeding were included in this trial after control of acute bleeding: this was defined as a 24 hour bleeding‐free period. Only patients achieving control of bleeding within 48 hours of hospital admission were included in the trial. We decided to include this trial for the assessment of the treatment effect on rebleeding, as the trial population was very similar to that of all the other included trials. When assessing the treatment effect for mortality, we decided to perform a sensitivity analysis by excluding this trial since patients who died of uncontrollable bleeding had not been included in the trial resulting in an overall 42‐day mortality slightly lower than expected if patients with acute bleeding had been included.
Doses of somatostatin and modality of administration were identical across the five trials (initial bolus of 250 µg followed by continuous iv infusion of 250 µg/h); in only one trial boluses were repeated six‐hourly for 24 hours (Ramires 2000). The duration of therapy was 48 hours in three trials (Di Febo 1990; Planas 1994; Ramires 2000) and 120 hours in the other two. Sclerosant and modality of sclerotherapy were not reported in one study (Di Febo 1990). In the other trials, 1% polydocanol or 5% ethanolamine were used. Polidocanol was injected either intra‐ or para‐variceally and ethanolamine intra‐variceally. A single sclerotherapy session was performed in the four fully reported trials; no details were given in the abstract. Immediate or 48 hour bleeding control, and five and seven day treatment failure were the principal outcomes assessed. After the initial five‐day study period, the same treatment for the prevention of long‐term recurrence of bleeding was given in both groups in three fully published trials; no details were reported in the abstract and in one full report (Ramires 2000). Four trials assessed 30‐ or 42‐day outcome, while in one only 7‐day mortality was reported.
Sclerotherapy versus octreotide We identified ten trials. Two are still available only as abstract (Poo 1996; Lopez 1999), and eight are full reports (Sung 1993; Jenkins 1997; Bildozola 2000; Freitas 2000; Sivri 2000; Yousuf 2000; Shaikh 2002; Silva 2004). Only patients with proven variceal bleeding were included, and treatment was started immediately after randomisation, which was performed during the diagnostic endoscopy in eight trials; randomisation was performed immediately after endoscopy in one trial (Bildozola 2000) while the time of randomisation was unclear in one (Silva 2004). Octreotide was given as a continuous iv infusion of 50 µg/h in all the trials. However, the infusion was continued for 12 hours in one trial (Sivri 2000), 24 hours in one (Lopez 1999), 48 hours in five (Sung 1993; Poo 1996; Jenkins 1997; Bildozola 2000; Freitas 2000), 72 hours in one (Shaikh 2002), and 120 hours in one (Silva 2004). In two trials (Bildozola 2000; Shaikh 2002), octreotide was then administered by subcutaneous 100 µg or 50 µg injections every eight hours for 72 hours after the 48 hour iv infusion, making the total therapy duration 120 hours. Sclerotherapy was done by intra‐paravariceal 1% to 2% polidocanol, absolute ethanol, sodium tetradecyl sulphate, or ethanolamine (details in table Characteristics of included studies). Following the initial study period, patients entered a long‐term elective sclerotherapy program in three trials (Sung 1993; Freitas 2000; Sivri 2000) and a randomised trial for long‐term prevention of recurrent bleeding in one study (Bildozola 2000). The treatment given for the prevention of long‐term rebleeding was not reported in the two abstracts (Poo 1996; Lopez 1999) and in three full reports (Jenkins 1997; Shaikh 2002; Silva 2004). The main outcomes reported were failure to control bleeding and rebleeding during the study treatment period (or up to 50 hours thereafter in the Sivri 2000 et al trial). Mortality was reported at 3‐ to 5‐day in three trials (Bildozola 2000; Freitas 2000; Yousuf 2000) and at 30‐ to 60‐day in the remainder.
Definitions of the outcomes in the included trials The details of the used definitions are reported in Table 11.
2. Definition of outcomes and other treatments.
| Study ID | Definition of | Definition of | Definition of | Definition of | Treatment for | Long‐term |
| First author, year | control of bleeding | five‐day failure of treatment | re‐bleeding | need of blood transfusion | failure to control initial bleeding | prevention of re‐bleeding |
| Westaby, 1989 | Absence of active variceal bleeding either by repeat endoscopy or by nasogastric aspirate. | Not assessed. | Endoscopy proven variceal bleeding after stools had cleared of melaena. | Not reported. | Additional therapy if two or more tx were needed over two hrs. In both groups: vasopressine + NG, balloon tamponade or sclerotherapy. No predefined criteria. | EVS: sclerotherapy. Control: sclerotherapy 12 hrs after admission and then with the same schedule as EVS group. |
| Escorsell, 2000 | 24‐h bleeding free period within 48 hrs after randomisation. Bleeding was haematemesis, blood in gastric aspirate, hypovolaemia with melaena, blood in gastric aspirate, or bleeding at endoscopy. | Failure to control bleeding within 48 h or re‐bleeding within five days after control of bleeding. | New evidence of bleeding after a 24‐h bleeding free period. | To maintain haematocrit between 0.28 and 0.30. | Both groups: vasoactive therapy (except terlipressin), endoscopic therapy, balloon tamponade, surgical shunt, or TIPS. | Both groups, after the initial six to seven days of the study: endoscopic therapy, or pharmacological therapy, or combination or surgical shunt, or TIPS. |
| Di Febo, 1990 | No haematemesis and/or melaena, Hb stable or drop less than 2g/dl, stable vital signs. | The used outcome was seven‐day failure, defined as failure to control bleeding within 48‐h or re‐bleeding between day two and seven. | Haematemesis and/or melaena or Hb drop more than 2g/dl or fall of vital signs in absence of haematemesis or melaena. | Not reported. | Both groups: sclerotherapy. | Not reported. |
| Shields, 1992 | Cessation of bleeding assessed endoscopically 15 to 20 min after starting somatostatin or ten min after EVS. | Failure to control bleeding or re‐bleeding within five days. | Further haematemesis or melaena accompanied by either systemic disturbance or Hb fall. | Not reported. | Both groups: balloon tamponade. | Both groups: sclerotherapy |
| Planas, 1994 | No haematemesis or melaena with stable systolic blood pressure and heart rate during the 48‐h trial period. | The used outcome was seven‐day failure, defined as failure to control bleeding or re‐bleeding within 48 h or re‐bleeding between day two and seven. | Recurrence of haemorrhage within five days after the 48‐h trial period: haematemesis or melaena associated with drop of systolic blood pressure more than 30 mmHg, HRmore than 120 or need of more than 2 blood units to maintain vital signs. | To maintain vital signs. | EVS: Somatostatin Control: EVS, Sengstaken Blackmore (SB) tube. | Both groups: Propranol plus isosorbide5‐mononitrate or long‐term sclerotherapy or shunt surgery in a randomised trial. |
| Escorsell, 1998 | 24‐h bleeding free period within 48 hrs from admission. Bleeding was haematemesis, hypovolaemia (systolic blood pressure less than 80 mmHg and heart rate more than 120), haematocrit drop more than 10 in six‐hour, blood in gastric aspirate. | Haematemesis or fresh blood in six consecutive hourly nasogastric aspirates and hypovolaemia, need of two blood units to maintain haemodynamic stability; need for further treatment or for alternative therapy. | Haematemesis, hypovolaemia (systolic blood pressure less than 80 mmHg and heart rate more than 120), haematocrit drop more than 10 in six‐hour, blood in gastric aspirate. | To maintain haemodynamic stability. | Treatment for re‐bleeding: alternative therapy, using either SB, terlipressin, TIPS, shunt surgery, or a combination of the above. | Both groups, after the five‐day study period: EVS, pharmacological therapy, or shunt surgery. |
| Sung, 1993 | Absence of: recurrent haematemesis or melaena of more than 500 ml, systolic blood pressure less than 90 mmHg, heart rate more than 110, or 6 units of blood or plasma within 12‐h. | 48‐h treatment failure was assessed and was defined as need to use balloon tamponade: this was used for recurrence of haematemesis or melaena of 500 ml or more, blood pressure less than 90 mmHg or heart rate more than 110 for two hrs, or more than 6 units of blood or plasma to sustain blood pressure. | Recurrent haematemesis or melaena of more than 500 ml, systolic blood pressure less than 90 mmHg, heart rate more than 110, or six units of blood or plasma within 12 hrs. | To sustain blood pressure. | Both groups: balloon tamponade (Minnesota tube). | Both groups: elective EVS. |
| Poo, 1996 | Absence of recurrent bleeding within 48 hrs, defined as haematemesis or melaena or more than 3 blood Units to maintain systolic blood pressure more than 90 mmHg or heart rate less than 100. | Not assessed. | Recurrence of haematemesis or melaena or more than 3 blood Units to maintain systolic blood pressure more than 90 mmHg or heart rate less than 100. | To maintain systolic blood pressure more than 90 mmHg or heart rate less than 100 | Both groups: balloon tamponade. | Not reported. |
| Jenkins, 1997 | Haematemesis and/or melaena with instability of vital signs or fall of Hb requiring blood transfusion, over the 48 hrs following randomisation. | Not assessed. | Haematemesis and/or melaena with instability of vital signs or fall of Hb requiring blood transfusion. | Not reported. | Both groups: balloon tamponade. | Not reported. |
| Lopez, 1999 | Based on endoscopy 24 hrs after starting therapy. Criteria were not reported. | Not assessed. | Not assessed. | Not reported. | Both groups: balloon tamponade. | Not reported. |
| Bildozola, 2000 | Absence of fresh blood in the hourly gastric aspiration for 12 consecutive hrs together with stability of haematocrit and vital signs. | Persistence of bleeding more than 6 hrs; recurrence of bleeding (haematemesis or fresh blood in the gastric aspirate) requiring tree blood units in three hrs to maintain stable the haematocrit and vital signs (i.e., blood pressure more than 90 mmHg and heart rate less than 100). | Recurrence of haematemesis or melaena, haemodynamic instability ( blood pressure less than 90 mmHg and heart rate less than 100) and 5% haematocrit fall after initial control of bleeding. | To maintain constant haematocrit and vital signs. | Both groups: endoscopic therapy and/or balloon tamponade or surgery. | Treatment not reported, but all patients surviving the five‐day study period entered a randomised trial of treatment for long‐term prevention of recurrent bleeding. |
| Sivri, 2000 | Stable blood pressure (no reduction more than 20 mmHg after reaching stable values); stable Hb (more than 9g/dl); haematocrit more than 30% and tx requirement less than 2/2h or less than 4/24h. | Not assessed. | Overt haemorrhage or aspiration of more than 100 ml of fresh blood; fresh melaena; fall of Hb more than 4g/72hr; heart rate more than 100 and blood pressure less than 100 mmHg in the presence of continuing melaena. | To maintain haematocrit more than 30%. | Alternative therapy or balloon tamponade. | Both groups: EVS. |
| Ramires, 2000 | Absence of haematemesis or melaena accompanied by systolic blood pressure less than 100 mmHg or heart rate more than 100 within 48 hrs. Absence of bleeding at the 48‐h control endoscopy | 7‐day failure: persistent bleeding, re‐bleeding or death | Haematemesis or melaena accompanied by systolic blood pressure less than 100 mmHg or heart rate more than 100. re‐bleeding was always endoscopically confirmed. | Not reported | Both groups: sclerotherapy | Not reported |
| Freitas, 2000 | Absence of haematemesis or less than 100 cc of bright blood in naso‐gastric aspirate; no bright red blood per rectum; no haemoglobin decrease of more than 4g/dL in 48 hrs; absence of shock and melaena. | 7‐day failure: persistent bleeding, re‐bleeding or death | Haematemesis or more than 100 cc of bright blood in naso‐gastric aspirate; bright red blood per rectum; haemoglobin decrease of more than 4g/dL in 48 hrs; shock and melaena. | Not reported | Both groups: emergency sclerotherapy. When this failed: balloon tamponade, surgery or other. | Both groups: EVS or band ligation at the end of the 48 hrs trial period |
| Yousuf, 2000 | Absence of blood in gastric aspirate for at least 1 hour and stable vital signs after 12 hrs from randomisation | Not assessed | Fresh blood in gastric aspirate at any time following initial control of bleeding and unstable vital signs | Not reported | Not reported | Not reported |
| Silva, 2004 | According to Baveno III | Not reported | New haematemesis, haemodynamic instability, need of transfusions to maintain Ht more than 27% | To maintain haemoglobin more than 8g/dl | Both groups: endoscopic therapy followed by TIPS incase of persistent bleeding | Not reported |
| Shaikh, 2002 | Absence of re‐bleeding at 4 hrs | Not reported | New haematemesis or melaena with haemodynamic instability and/or need for transfusions | Not reported | Not reported | Not reported |
| Abbreviations: tx = blood transfusion;Hb = haemoglobulin; h = hour; hrs = hours. |
Failure to control bleeding In the 17 trials, failure to control bleeding was defined as evidence of haematemesis, melaena, blood in the gastric aspirate, and/or unstable haemodynamic parameters and haemoglobin level. Time to evaluate failure to control bleeding varied markedly across studies, ranging from 10 to 20 minutes to 148 hours. Seven trials did not state a minimum bleeding‐free interval to consider the bleeding as having been controlled. Five‐day treatment failure Five‐day treatment failure was reported in ten trials, and in eight it was defined as failure to control bleeding or recurrence of bleeding or death within five days of treatment, while no definition was reported in two. Recurrence of bleeding The evidence for recurrence of bleeding was defined by the same criteria as for failure to control bleeding. We used the 42 days rebleeding rate. When a study reported rebleeding at a different time, the figure at the longest available time interval, up to 42‐day, was used. Recurrence of bleeding before other elective treatments for the prevention of rebleeding This was not specifically defined in any of the included trials. The rebleeding rate recorded at the end of the experimental treatment period was, however, reported in 13/17 trials. Mortality The overall 42‐day mortality was evaluated in this review regardless of the cause of death. This was decided in agreement with several consensus conferences (Burroughs 1987; De Franchis 1992; De Franchis 1996; Grace 1998; De Franchis 2000), stating that any death occurring within 42 days after a variceal bleeding should be assumed to be caused by the bleeding per se, independent of the ultimate cause of death. When a study reported mortality at a different time, the figure at the longest available time interval, up to 42 days, was used. Mortality before other elective treatments for the prevention of re‐bleeding As for the recurrence of bleeding, this was not specifically defined in any of the included trials. The mortality recorded at the end of the experimental treatment period was, however, reported in 7/17 trials. Number of blood transfusions Only eight trials reported criteria to give blood transfusions. These were based on the target of maintaining the haematocrit or haemoglobin within a certain range or to maintain vital parameters stable. Adverse events No specific definitions were a priori reported. Serious adverse events No specific definitions were a priori reported. Only three trials stated that adverse events were considered serious when they required specific treatment or withdrawal of the experimental treatment.
Risk of bias in included studies
Details on the quality of each trial are reported in the Table 12 'Methodological quality of included studies'.
3. Methodological quality of included trials.
| Trial ID | Randomisation | Concealment | Control of bias | Blinding | Intention‐to‐treat analysis | Losses to follow‐up | treatment for failure | Prevention of re‐bleeding |
| Bildozola 2000 | Adequate | Unclear | Unclear | None | No | None | Same in both groups | Same in both groups |
| Di Febo 1990 | Unclear | Unclear | Unclear | None | No | None | Same in both groups | Same in both groups |
| Escorsell 1998 | Adequate | Adequate | Adequate | None | Yes | None | Same in both groups | Same in both groups |
| Escorsell 2000 | Adequate | Adequate | Adequate | None | No | None | Same in both groups | Same in both groups |
| Freitas 2000 | Unclear | Unclear | Unclear | None | No | Not reported | Same in both groups | Same in both groups |
| Jenkins 1997 | Adequate | Adequate | Adequate | None | Yes | None | Same in both groups | Same in both groups |
| Lopez 1999 | Unclear | Unclear | Unclear | None | No | Not reported | Same in both groups | Same in both groups |
| Planas 1994 | Adequate | Adequate | Adequate | None | Yes | None | Same in both groups | Same in both groups |
| Poo 1996 | Unclear | Unclear | Unclear | None | No | Not reported | Same in both groups | Same in both groups |
| Ramires 2000 | Unclear | Adequate | Unclear | None | No | None | Same in both groups | Not reported |
| Shaikh 2002 | Unclear | Unclear | Unclear | None | Yes | Not reported | Not reported | Not reported |
| Shields 1992 | Unclear | Adequate | Unclear | None | Yes | None | Same in both groups | Same in both groups |
| Silva 2004 | Unclear | Unclear | Unclear | None | Yes | Not reported | Not reported | Not reported |
| Sivri 2000 | Adequate | Adequate | Adequate | None | Yes | None | Same in both groups | Same in both groups |
| Sung 1993 | Adequate | Adequate | Adequate | None | No | None | Same in both groups | Same in both groups |
| Westaby 1989 | Adequate | Unclear | Unclear | None | Yes | None | Same in both groups | Same in both groups |
| Yousuf 2000 | Unclear | Unclear | Unclear | None | Yes | None | Not reported | Not reported |
Control of bias was adequate only in six trials: one using terlipressin (Escorsell 2000), two somatostatin (Planas 1994; Escorsell 1998), and three octreotide (Sung 1993; Jenkins 1997; Sivri 2000) as control treatment. None of the trials reported blinded outcome assessment. Information on patients lost to the follow‐up was not reported in five trials, and in eight trials, an intention‐to‐treat analysis was not reported or the information was unclear. Treatments for failures of the trial interventions and for prevention of rebleeding were not reported in three and four trials, respectively. In one trial (Bildozola 2000), eight patients were excluded after randomisation because of important protocol violations, which were reported in detail: eligibility criteria were not fulfilled in six and adjunctive sclerotherapy sessions were done in two. The randomised treatment and the outcome of these patients were not reported, and therefore, a complete intention‐to‐treat analysis was not possible. In general, a major problem in the majority of trials, particularly those of octreotide, was the insufficient methodology for observing and recording adverse events, which were reported without any attempt to graduate their severity.
Effects of interventions
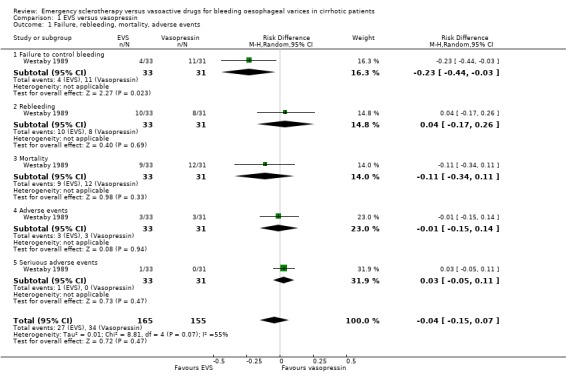
Sclerotherapy versus vasopressin Sixty‐four patients were included in the trial comparing sclerotherapy with vasopressin (Westaby 1989). Randomisation was performed at the time of the first endoscopy when a diagnosis of variceal bleeding was made. Sclerotherapy was performed immediately in patients randomised to this treatment. Failure to control bleeding was significantly reduced by sclerotherapy (RD ‐0.23; 95% CI ‐0.44 to ‐0.03): the potential assessment bias above reported (see description of studies) was not accounted for nor commented upon. Rebleeding and mortality were recorded at the end of the stay in hospital. It is also worth to note that all the patients in both groups were given weekly sessions of elective sclerotherapy until variceal eradication. With these limitations, the risk difference for re‐bleeding was ‐0.04 (95% CI ‐0.17 to 0.26) and for mortality ‐0.11 (95% CI ‐0.34 to 0.11). No significant differences were found between the two intervention groups in the number of transfusions and adverse events.
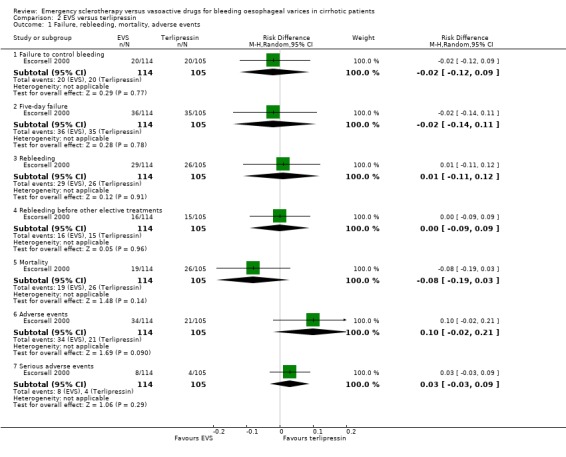
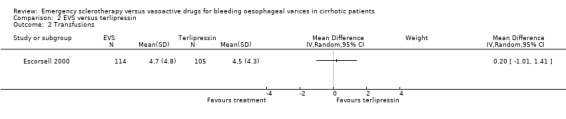
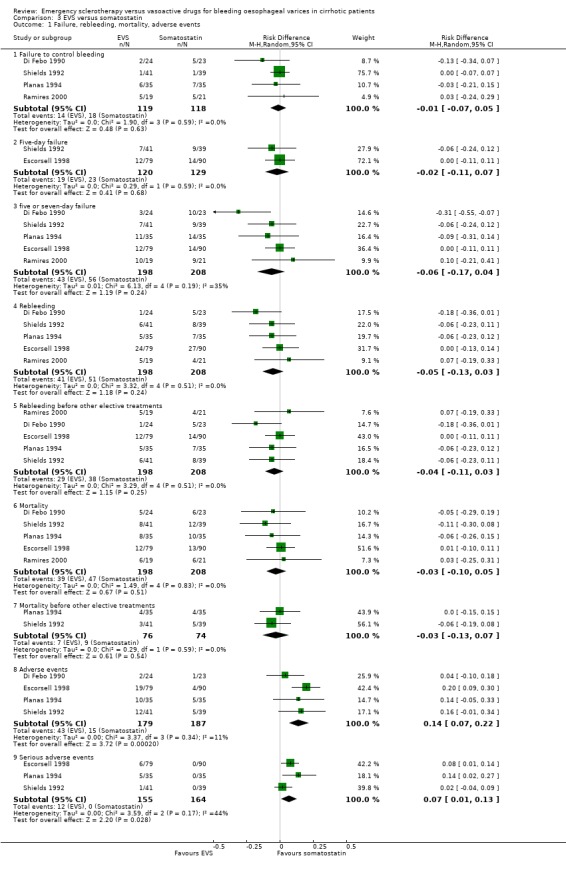
Sclerotherapy versus terlipressin The only trial comparing sclerotherapy with terlipressin (Escorsell 2000) included 219 patients and showed no significant difference in failure to control bleeding (RD ‐0.02; 95% CI ‐0.12 to 0.09) and five day treatment failure (RD ‐0.02; 95% CI ‐0.14 to 0.11). The 42‐day re‐bleeding risk difference was 0.01 (95% CI ‐0.11 to 0.12). However, after the six to seven days study period, the patients were given various types of elective therapy to prevent recurrent bleeding, according to the protocol prevailing at each participating centre. Whether these treatments were comparable between the two study groups was not shown in the trial report, although it is conceivable that they were. The re‐bleeding before these treatments was 14% in both groups. Also the 42‐day mortality was similar for the two treatment groups (RD ‐0.08; 95% CI ‐0.19 to 0.03). However, mortality before other elective treatments was not reported. The mean difference (MD) for the number of blood transfusions was 0.20 (‐1.01 to 1.41), and also adverse events were not significantly different in the two intervention groups (RD 0.10; 95% CI ‐0.02 to 0.21). Eight patients in the sclerotherapy group experienced serious adverse events (three got aspiration pneumonia, four got bleeding oesophageal ulcer, and one got sepsis from pleural empyema resulting in death). In the terlipressin group the patients who experienced serious adverse events were four (two got lower limb ischaemia, one got severe hyponatraemia, and one got seizures). The RD between the two treatments was 0.03 (95% CI ‐0.03 to 0.09). Sclerotherapy versus somatostatin Four trials (Di Febo 1990; Shields 1992; Planas 1994; Ramires 2000), including 237 patients, assessed failure to control initial bleeding and did not observe a significant difference between the two treatments (RD ‐0.01, 95% CI ‐0.07 to 0.05). Five‐day failure was assessed in two trials (Shields 1992; Escorsell 1998) including overall 249 patients, and no significant difference was found between the two treatments (RD ‐0.02, 95% CI ‐0.11 to 0.07). Since the other three trials reported the seven‐day treatment failure rate (Di Febo 1990; Planas 1994; Ramires 2000), we also performed a post‐hoc analysis by including all the five trials (406 patients): the overall five to seven day treatment failure rate was ‐0.07 (95%CI ‐0.17 to 0.03).
In the Escorsell et al trial (Escorsell 1998), the re‐bleeding rate was reported both at the end of the five‐day somatostatin infusion and at 42 days. In the other trials, re‐bleeding was assessed before other elective treatments were started: at five days in Shields et al trial and at seven days in the three remaining trials. No significant difference was found on the overall re‐bleeding rate (4 randomised trials, 406 patients: RD ‐0.05; 95% CI ‐0.13 to 0.03) or on the re‐bleeding before other elective treatments (5 randomised trials, 406 patients: RD ‐0.04; 95% CI ‐0.11 to 0.03).
The mortality did not differ significantly between the two interventions (5 randomised trials, 406 patients: RD ‐0.03; 95% CI ‐0.10 to 0.05). This was also the case regarding mortality before other elective treatments (reported only by Shields 1992 and Planas 1994 and including overall 150 patients: RD ‐0.03; 95% CI ‐0.13 to 0.07). Also after exclusion of the Escorsell et al trial, no significant difference in mortality was found (RD ‐0.06; 95% CI ‐0.17 to 0.05). When the only trial with adequate control of bias and reporting mortality before other treatments was considered (Planas 1994), the RD between the two treatments was 0.00 (95% CI ‐0.15 to 0.15).
The number of blood transfusions was reported in the three fully published trials. No statistically significant differences were found between the treatment groups in any of them. The mean difference was assessable only in two trials (Planas 1994; Escorsell 1998) including 239 patients: 0.48 (95% CI ‐1.79 to 2.75). There was an important heterogeneity between the two trials (Chi‐square = 3.73, P = 0.05; I2 = 73%), probably accounted for by the different definitions used for the need of the transfusion of one blood unit.
Adverse events were reported in four trials (366 patients) and were significantly more frequent in the sclerotherapy group than in the somatostatin group (RD 0.14, 95% CI 0.07 to 0.22; NNH = 7 patients x 2; 95% CI 4 to 14). Severe adverse events were reported only in three trials (Shields 1992; Planas 1994; Escorsell 1998), including 319 patients, and were observed only in sclerotherapy treated patients: the pooled risk difference was 0.07 (95% CI 0.01 to 0.13) without significant heterogeneity (Chi‐square = 3.51; P = 0.17) (NNH = 14, 95% CI 8 to 100). Severe adverse events were observed in 12/155 patients treated by sclerotherapy and included four deaths (two oesophageal perforation, one sepsis, one pleural empyema), five clinically significant bleeding from oesophageal ulcers, one cerebrovascular accident and pulmonary embolism, one pulmonary embolism, and one bacterial peritonitis.
Sclerotherapy versus octreotide A total of 1128 patients were included in the ten randomised trials comparing sclerotherapy with octreotide. Failure to control bleeding was not significantly different between the two treatments (RD ‐0.01; 95% CI ‐0.07 to 0.04).
Five‐day treatment failure was reported only in two trials (Bildozola 2000; Silva 2004): RD ‐0.14 (95%CI ‐0.30 to 0.03). In two other trials (Sung 1993; Freitas 2000), a similar definition of treatment failure was given, although the outcome was assessed at 48 hours. We, therefore, performed a post‐hoc analysis by pooling the four trials (341 patients), which confirmed the lack of significant difference between the two treatments (RD ‐0.05; 95% CI ‐0.14 to 0.04).
Re‐bleeding was assessed at 48 hours in two trials (Sung 1993; Jenkins 1997), 72 hours in two (Sivri 2000; Yousuf 2000), 120 hours in two (Bildozola 2000; Silva 2004), 168 hours in one (Freitas 2000), and 30 days in one (Poo 1996). In one trial (Shaikh 2002), early re‐bleeding was reported without specifying the time frame, and in one, re‐bleeding was not reported (Lopez 1999). A total of 1064 patients were included in the nine trials. The pooled RD was 0.02 (95% CI ‐0.03 to 0.08). The re‐bleeding rate before other elective treatments was reported in five trials (486 patients) and the RD was ‐0.01 (95% CI ‐0.07 to 0.05).
Mortality was assessed at 72 hours in one trial (Yousuf 2000), 120 hours days in one trial (Bildozola 2000), 7 days in one (Freitas 2000), 30 days or while in hospital in five (Sung 1993; Poo 1996; Lopez 1999; Sivri 2000; Shaikh 2002), and 42 days in two (Jenkins 1997; Silva 2004). The pooled RD (10 randomised trials, 1128 patients) was ‐0.01 (95% CI ‐0.06 to 0.04). Mortality before other elective treatments was reported in five trials (Sung 1993; Jenkins 1997; Bildozola 2000) including 521 patients, and the RD was ‐0.00 (95% CI ‐0.05 to 0.04).
Transfusion requirement was not reported in four trials (Lopez 1999; Freitas 2000; Yousuf 2000; Shaikh 2002); it was reported as the median number of blood units transfused (3.0 in the sclerotherapy group and 3.5 in the octreotide group, P = 0.34, Mann‐Whitney U test) in one (Sung 1993). In five trials, the mean number of blood units transfused and standard deviation was reported (Jenkins 1997; Poo 1996; Bildozola 2000; Sivri 2000; Silva 2004): the mean difference in these four trials, which included 391 patients, was ‐0.20 (95% CI ‐0.60 to 0.20), without significant heterogeneity (Chi‐square = 3.36, P = 0.36).
Adverse events were reported in six trials (Sung 1993; Poo 1996; Jenkins 1997; Bildozola 2000; Sivri 2000; Yousuf 2000). Lopez et al reported that thoracic pain was observed in nine patients and oesophageal ulcers in 12 patients in the sclerotherapy group, whereas in the octreotide group only transient hyperglycaemia was observed (Lopez 1999). However, the number of patients with hyperglycaemia was not reported. In the other trials, all the observed adverse events were reported without qualification of their severity. Only the Bildozola 2000 et al trial stated that sclerotherapy produced four major complications (aspiration pneumonia in two patients, perforation in one, and pericardial effusion in one) whereas no major complications were observed in the octreotide group. When the six trials reporting complete data are combined (529 patients), the pooled RD was 0.06 (95% CI ‐0.03 to 0.15). However, there was significant heterogeneity (Chi‐square = 13.81, P = 0.02; I2 = 63.8%).
Sensitivity analyses Combining all the included trials irrespective of pharmacological intervention The overall estimates confirmed that adverse events were significantly more frequent with sclerotherapy than with pharmacological therapy (12 trials, 1178 patients: RD 0.08, 95% CI 0.03 to 0.14; NNH 12.5 patients, 95% CI 7 to 33) as well as were serious adverse events (5 trials, 602 patients: RD 0.05; 95% CI 0.02 to 0.08; NNH 20 patients, 95% CI 12.5 to 50). There were no statistically significant differences for any of the other outcomes including 42‐day mortality (17 trials, 1817 patients : RD ‐0.02; 95% CI ‐0.06 to 0.02).
Assessment of treatment effect on mortality by excluding the Escorsell et al trial This analysis confirmed that there was no significant difference between the two treatments: RD ‐0.03; 95% CI ‐0.07 to 0.02.
Full reports This analysis included 14 trials. The lack of any significant difference was confirmed for all the assessed outcomes except for the number of transfused blood units which number was significantly reduced with sclerotherapy (MD ‐0.35, 95% CI from ‐0.56 to ‐0.13). The significant excess of adverse events and serious adverse events was also confirmed.
Randomised trials with adequate control of bias This analysis included six trials and 772 patients. The lack of any difference for control of bleeding, re‐bleeding, death and number of blood units transfused as well as the significant excess of adverse events and serious adverse events with sclerotherapy were all confirmed.
Effect on mortality of sclerotherapy compared with somatostatin by excluding the Escorsell et al 1998 trial This analysis confirmed that there was not a significant difference between the two treatments.
Discussion
This systematic review included 17 randomised trials and a total of 1817 patients. Although, in general, the combinability of the trials was acceptable and no significant heterogeneity was found, there was appreciable diversity of the methodological quality, and study conduct important to be aware of when translating the overall estimates here provided into clinical practice.
The methodological quality of the included trials was in general unsatisfactory, with only five trials reporting adequate control of selection bias as assessed by the method of generation of the randomisation list and of treatment allocation concealment. In none of the included trials a blinded design was adopted. However, it is unlikely that unblinded assessment of bleeding or death, the major outcomes assessed in the included trials and in this systematic review, may lead to biased conclusions (Wood 2008). Other criteria of internal validity, like completeness of follow‐up, other treatments used for failure of control of bleeding and for prevention of re‐bleeding were also unsatisfactorily reported; therefore, any potential bias deriving from them was not adequately evaluable.
There were appreciable differences across trials in the definitions of the major outcomes. This was particularly evident for the definition of failure to control bleeding. The most important difference concerned the time of assessment of the initial control of bleeding, which varied from 10 to 20 minutes in one trial (Shields 1992) to 48 hours in most of the other trials. However, a bleeding‐free interval was usually required to consider a bleeding as controlled and to separate a re‐bleeding from the index bleeding, making the definitions of these outcomes satisfactorily reproducible in clinical practice. Five‐day treatment failure was assessed in a few trials, and most of them included in their definition failure to control bleeding, recurrence of bleeding, and death. In some trials these criteria were used to define treatment failure 48 hours or seven days after randomisation.
In most trials the 42‐day, or in‐hospital re‐bleeding, or mortality was reported as a major treatment outcome measure, even when other elective treatments were performed between the end of the experimental treatment and the time when the outcome was recorded. Moreover, these adjunctive elective treatments were not always reported. Although there is consensus that any death, occurring within six weeks after an upper digestive bleeding in cirrhosis, should be considered as a bleeding‐related death (Burroughs 1987; Grace 1998; De Franchis 2000), it is hard to relate this relatively late mortality to a treatment ended 30 or 40 days earlier. Many factors have been reported as being significantly associated with six‐week mortality after variceal bleeding (D'Amico 1997). All of them should be properly accounted for when assessing the relation of a treatment with mortality in this time interval. Of course, a major factor to be accounted for is the treatment given for the long‐term prevention of recurrent bleeding, since several treatments used to this aim are given as early as possible following the initial control of bleeding and are also associated with significant improvement of survival (Jalan 2000; Garcia‐Tsao 2007). To overcome this problem, we abstracted also the re‐bleeding and mortality before other elective treatments were given. For re‐bleeding, this information was reported in 10/17 trials, including 1071 patients, and for mortality in seven trials, including 681 patients. The analysis of these outcome measures confirmed that emergency sclerotherapy is not significantly superior to vasoactive drugs for acute variceal bleeding.
It is of interest that most of the included trials did not report the criteria to give a patient a blood transfusion although a formal statistical analysis of the difference of blood transfusions between the treatment groups was provided. When significant, this difference was also reported as a proof of treatment efficacy. However, it is hard to draw conclusions using this outcome measure, considering the many variables included in the decision to give a transfusion. Furthermore, as these trials were unblinded, such an outcome measure may be significantly biased. The inconsistency of sensitivity analyses with respect to this outcome may probably be explained at least in part by such considerations.
Problems similar to those encountered with blood transfusions may have affected the assessment of adverse events. The adverse events were never a priori defined and the criteria to assess an event as an adverse event were never reported. Blind assessment of adverse events was never attempted or at least was never reported. Only very few trials reported a distinction between non‐serious or serious adverse events. With these limitations, adverse events were more frequent with sclerotherapy than with terlipressin, somatostatin, or octreotide (significantly so when compared with somatostatin), whereas no significant difference was found in the trial comparing sclerotherapy with vasopressin. In the sensitivity analysis including all the trials both adverse events and serious adverse events were significantly more frequent with sclerotherapy.
This systematic review showed that emergency sclerotherapy is not significantly superior to any of the vasoactive drugs ‐ vasopressin (with or without nitroglycerin), terlipressin, somatostatin, or octreotide ‐ with regard to any of the assessed efficacy outcomes and may even carry a higher burden of adverse events.
Only the trial comparing sclerotherapy with vasopressin showed a significant reduction of failure to control bleeding with sclerotherapy (Westaby 1989). In this trial, however, there was an important potential bias in the assessment of this outcome because all the patients in the vasopressin group underwent sclerotherapy at the end of the 12‐hour study period. Thus, endoscopy provided a more precise method of evaluating the persistence of bleeding in this group as compared with the sclerotherapy group, in which endoscopy was repeated only if bleeding was clinically suspected. It is hard to evaluate how this potential bias did actually affect the conclusions drawn in the trial. However, it is important to note that among all the included trials comparing sclerotherapy with vasoactive drugs, this trial was the only one showing superiority of sclerotherapy in controlling initial bleeding.
The similar effect of sclerotherapy and pharmacological therapy on re‐bleeding and death was also confirmed when the corresponding outcome rates before any other elective treatment were considered. The absence of any significant difference between sclerotherapy and vasoactive drugs was even more evident when all the trials were combined, independently of the control treatment used: in this analysis no significant differences were found with regard to failure to control bleeding, five‐day treatment failure, re‐bleeding, and mortality, while a significant increase of adverse events and serious adverse events was associated with sclerotherapy. These results were consistently confirmed in the analyses including only the full reports and only the trials with adequate control of bias.
The results of the present meta‐analysis are in contrast with a recently reported review of randomised trials comparing sclerotherapy with vasoactive drugs (Triantos 2006). However, that review included a total of 15 randomised trials and 1322 patients compared with 17 randomised trials and 1817 patients in the present. Moreover, one of the 15 trials (El‐Jackie 1998) included by Triantos 2006 was excluded from our meta‐analysis because it enrolled only patients with schistosomal portal hypertension, while we included only cirrhotic patients. In fact, the trial by El‐Jackie 1998 behaved as an outlier in the Triantos' review with the 95% CI for failure to control bleeding completely outside of the CI of the overall estimate. Besides this, that trial is still in abstract 10 years after its initial report. With that trial among the 15 included, Triantos 2006 et al found a RD of ‐0.059 (95% CI from ‐0.103 to ‐0.015) for failure to control bleeding and ‐0.043 (95% CI from ‐0.081 to ‐0.006) for mortality. It is important to note that the overall estimates are quite similar to those we found, with small differences between the two treatments. However, the inclusion of the trial in non‐cirrhotic patients, whose mortality at variceal bleeding is generally lower than in cirrhotic patients, makes the differences to be statistically significant, although the difference for mortality is very close to non significance.
Moreover, we based our meta‐analysis on separate analyses according to the different pharmacological interventions, for the reasons reported in the methods, and performed the analysis including all the trials as a sensitivity analysis to identify even small effects by increasing the power of the analysis. We did not find any beneficial effect of sclerotherapy neither when compared with each single vasoactive drug nor when all the trials were included, independently of the pharmacological control therapy.
Authors' conclusions
Implications for practice.
Limitations
There are several major limitations for application of this meta‐analysis in clinical practice. First, current guidelines and recent consensus conferences have recommended the use of combined pharmacological and endoscopic therapy as a first line treatment for acute variceal bleeding (De Franchis 2005; Garcia‐Tsao 2007; Garcia‐Tsao 2008). This recommendation is based on the evidence that the combination therapy is more effective than either therapy alone (Banares 2002; D'Amico G 2002) in the control of bleeding. Second, band ligation is considered to be superior to sclerotherapy for acute variceal bleeding (Lo 1997; Avgerinos 2004; Villanueva 2006), and this has been confirmed also in patients receiving somatostatin prior to endoscopic therapy (Villanueva 2006). Moreover, the administration of somatostatin before sclerotherapy is associated with better control of bleeding and makes sclerotherapy easier (Avgerinos 1997).
Perspectives
The evidence shows that emergency sclerotherapy does not significantly improve the outcome of cirrhotic patients with variceal bleeding when compared with vasopressin, terlipressin, somatostatin, or octreotide. It is, however, associated with more frequent and serious adverse events, and it puts the treatment of acute variceal bleeding in a new perspective. In fact, sclerotherapy, which is still largely used (Sorbi 2003; Bosch 2004; Bosch 2008), may be safely delayed in places where emergency endoscopy is not available on a 24 hours basis. Moreover, endoscopic therapy may be easier and safer if performed after starting vasoactive therapy (Avgerinos 1997; Villanueva 1999). In fact, vasoactive treatment is recommended as soon as an underlying cirrhosis is suspected in a patient with upper digestive bleeding (Garcia‐Tsao 2007). Since there are no randomised trials comparing emergency band ligation of varices with vasoactive therapy, these conceptions might also be extended to band ligation. Furthermore, it has never been assessed whether the combination of vasoactive and endoscopic therapy in all patients is superior to the association of endoscopic therapy to vasoactive drugs only in pharmacological failures. This is important because the association of endoscopic therapy significantly increases the risk of adverse reactions (Villanueva 1999; D'Amico 2009). Yet, the assumption that emergency band ligation may start the elective treatment for prevention of long‐term re‐bleeding has been challenged by a recently reported randomised trial, showing that the combination of ligation with nadolol and 5‐isosorbide‐nononitrate (ISMN) is not significantly superior to nadolol and ISMN (Garcia‐Pagan 2009). Therefore, patients with variceal bleeding controlled by vasoactive therapy might not need to be treated also with ligation or sclerotherapy.
Implications for research.
Current guidelines for the treatment of variceal bleeding recommend the association of endoscopic and pharmacological therapy (Garcia‐Tsao 2007). However, whether immediate combination of endoscopic therapy with vasoactive drugs is superior to the combination therapy only when pharmacological therapy fails (vasoactive drug plus eventual banding or sclerotherapy), should be assessed in well‐planned randomised trials.
In future randomised trials on acute bleeding varices in cirrhosis, the guidelines set at the previous consensus conferences should be followed, including the reporting of results in prognostically important subgroups, criteria for blood transfusions, and the methodology for assessing adverse treatment effects. Such trials ought to be reported according to the CONSORT guidelines (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 11 January 2010 | New search has been performed | New search was performed, and no new trials were found. |
| 14 May 2009 | New citation required but conclusions have not changed | Conclusions did not change. |
Acknowledgements
The authors are most grateful to Lise Lotte Gluud for help in the protocol phase; Bodil Als‐Nielsen, Lise Lotte Gluud, and Christian Gluud for their criticism and suggestions and for revising the manuscript; the entire Cochrane Hepato‐Biliary Group for discussing this review with the authors during a meeting held at the Cochrane Hepato‐Biliary Group office in Copenhagen in January 2001, and to Kate Whitfield, Dimitrinka Nikolova, and Sarah Klingenberg for expert assistance in the identification of trials.
Peer Reviewers of the Review: Paul Calè, France; Robert Sutton, UK, Roberto De Franchis, France; Ronald L. Koretz, USA. Contact Editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy | |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | January 2010. | ('emergency sclerotherap*' or vasopressin* or terlipressin* or somtostatin* or octreotide) AND (((bleed* or hemorrhage* OR haemorrhage*) and *esophag* and varice*) or haematemesis OR hematemesis or maelena OR melena) | |
| The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 4, 2009. | #1 MeSH descriptor Sclerotherapy explode all trees in MeSH products #2 MeSH descriptor Vasopressins explode all trees in MeSH products #3 MeSH descriptor Somatostatinexplode all trees in MeSH products #4 MeSH descriptor Octreotide explode all trees in MeSH products #5 emergency sclerotherap* or vasopressin* or terlipressin* or somtostatin* or octreotide in All Fields in all products #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Esophageal and Gastric Varices explode all trees in MeSH products #8 MeSH descriptor Hematemesisexplode all trees in MeSH products #9 MeSH descriptor Melena explode all trees in MeSH products #10 ((bleed* or h*emorrhage*) and (esophag* or oesophag*) and varice*) or h*ematemesis or m*elena in All Fieldsin all products #11 (#7 OR #8 OR #9 OR #10) #12 (#6 AND #11) | |
| MEDLINE (WinSPIRS 5.0) | 1950 to January 2010. | 1. exp Sclerotherapy/ 2. exp Vasopressins/ 3. exp Somatostatin/ 4. exp Octreotide/ 5. (emergency sclerotherap* or vasopressin* or terlipressin* or somtostatin* or octreotide).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 6. 1 or 2 or 3 or 4 or 5 7. exp "Esophageal and Gastric Varices"/ 8. exp Hematemesis/ 9. exp Melena/ 10. (((bleed* or h*emorrhage*) and (esophag* or oesophag*) and varice*) or h*ematemesis or m*elena).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 11. 7 or 8 or 9 or 10 12. 6 and 11 13. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 14. 12 and 13 | |
| EMBASE (WinSPIRS 5.0) | 1980 to January 2010. | 1. exp sclerotherapy/ 2. exp vasopressin/ 3. exp terlipressin/ 4. exp somatostatin/ 5. exp octreotide/ 6. (emergency sclerotherap* or vasopressin* or terlipressin* or somtostatin* or octreotide).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp esophagus varices/ 9. exp esophagus hemorrhage/ 10. exp hematemesis/ 11. exp melena/ 12. (((bleed* or h*emorrhage*) and (esophag* or oesophag*) and varice*) or h*ematemesis or m*elena).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 13. 8 or 9 or 10 or 11 or 12 14. 7 and 13 15. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 16. 14 and 15 | |
| Science Citation Index Expanded (http://apps.isiknowledge.com) | 1900 to January 2010. | # 1 TS=(emergency sclerotherap* or vasopressin* or terlipressin* or somtostatin* or octreotide) # 2 TS=(((bleed* or h*emorrhage*) and (esophag* or oesophag*) and varice*) or h*ematemesis or m*elena) # 3 #2 AND #1 # 4 TS=(random* OR blind* OR placebo* OR meta‐analysis) # 5 #4 AND #3 |
Data and analyses
Comparison 1. EVS versus vasopressin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure, rebleeding, mortality, adverse events | 1 | 320 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.15, 0.07] |
| 1.1 Failure to control bleeding | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.23 [‐0.44, ‐0.03] |
| 1.2 Rebleeding | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.17, 0.26] |
| 1.3 Mortality | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.34, 0.11] |
| 1.4 Adverse events | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.15, 0.14] |
| 1.5 Seriuous adverse events | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
1.1. Analysis.
Comparison 1 EVS versus vasopressin, Outcome 1 Failure, rebleeding, mortality, adverse events.
Comparison 2. EVS versus terlipressin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure, rebleeding, mortality, adverse events | 1 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Failure to control bleeding | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.09] |
| 1.2 Five‐day failure | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.14, 0.11] |
| 1.3 Rebleeding | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.11, 0.12] |
| 1.4 Rebleeding before other elective treatments | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 1.5 Mortality | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 1.6 Adverse events | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.02, 0.21] |
| 1.7 Serious adverse events | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.03, 0.09] |
| 2 Transfusions | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
2.1. Analysis.
Comparison 2 EVS versus terlipressin, Outcome 1 Failure, rebleeding, mortality, adverse events.
2.2. Analysis.
Comparison 2 EVS versus terlipressin, Outcome 2 Transfusions.
Comparison 3. EVS versus somatostatin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure, rebleeding, mortality, adverse events | 5 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Failure to control bleeding | 4 | 237 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 1.2 Five‐day failure | 2 | 249 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 1.3 five or seven‐day failure | 5 | 406 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.17, 0.04] |
| 1.4 Rebleeding | 5 | 406 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| 1.5 Rebleeding before other elective treatments | 5 | 406 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 1.6 Mortality | 5 | 406 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.05] |
| 1.7 Mortality before other elective treatments | 2 | 150 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 1.8 Adverse events | 4 | 366 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.22] |
| 1.9 Serious adverse events | 3 | 319 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.13] |
| 2 Transfusions | 2 | 239 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.79, 2.75] |
3.1. Analysis.
Comparison 3 EVS versus somatostatin, Outcome 1 Failure, rebleeding, mortality, adverse events.
3.2. Analysis.
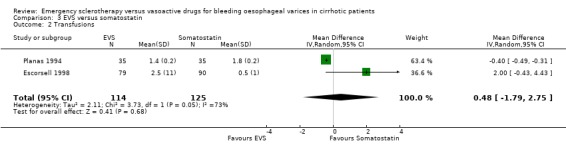
Comparison 3 EVS versus somatostatin, Outcome 2 Transfusions.
Comparison 4. EVS versus octreotide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure, rebleeding, mortality, adverse events | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Failure to control bleeding | 10 | 1128 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.04] |
| 1.2 Five‐day failure rate | 2 | 132 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.30, 0.03] |
| 1.3 two to five‐day failure | 4 | 341 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.14, 0.04] |
| 1.4 Rebleeding | 9 | 1064 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.03, 0.08] |
| 1.5 Rebleeding before other elective treatments | 5 | 486 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 1.6 Mortality | 10 | 1128 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 1.7 Mortality before other elective treatments | 5 | 531 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 1.8 Adverse events | 6 | 529 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.03, 0.15] |
| 2 Transfusions | 5 | 391 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
4.1. Analysis.
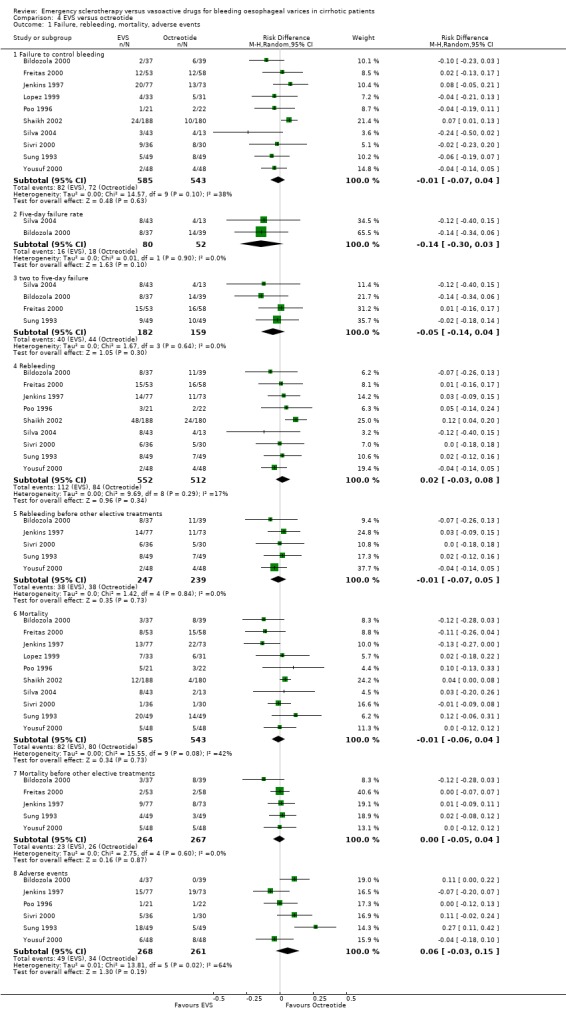
Comparison 4 EVS versus octreotide, Outcome 1 Failure, rebleeding, mortality, adverse events.
4.2. Analysis.
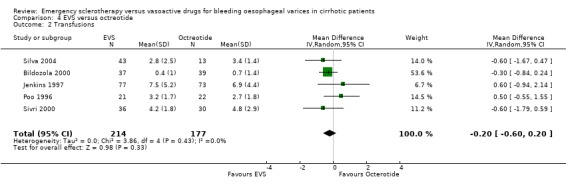
Comparison 4 EVS versus octreotide, Outcome 2 Transfusions.
Comparison 5. Sensitivity analysis 1: all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to control bleeding | 16 | 1648 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 1.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.23 [‐0.44, ‐0.03] |
| 1.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.09] |
| 1.3 EVS compared with somatostatin | 4 | 237 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 1.4 EVS compared with octreotide | 10 | 1128 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.04] |
| 2 Five‐day failure rate | 9 | 926 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.10, 0.01] |
| 2.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.14, 0.11] |
| 2.3 EVS compared with somatostatin | 4 | 366 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.20, 0.03] |
| 2.4 EVS compared with octreotide | 4 | 341 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.14, 0.04] |
| 3 Rebleeding | 16 | 1753 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 3.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.17, 0.26] |
| 3.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.11, 0.12] |
| 3.3 EVS compared with somatostatin | 5 | 406 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| 3.4 EVS compared with octreotide | 9 | 1064 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.03, 0.08] |
| 4 Rebleeding before other elective treatments | 10 | 1071 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 4.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 4.3 EVS compared with somatostatin | 4 | 366 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.13, 0.02] |
| 4.4 EVS compared with octreotide | 5 | 486 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 5 Mortality | 17 | 1817 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 5.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.34, 0.11] |
| 5.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 5.3 EVS compared with somatostatin | 5 | 406 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.05] |
| 5.4 EVS compared with octreotide | 10 | 1128 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 6 Mortality before other elective treatments | 7 | 681 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 6.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 EVS compared with terlipressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 EVS compared with somatostatin | 2 | 150 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 6.4 EVS compared with octreotide | 5 | 531 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 7 Transfusions | 8 | 849 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.54, 0.07] |
| 7.1 EVS compared with vasopressin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 EVS compared with terlipressin | 1 | 219 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.01, 1.41] |
| 7.3 EVS compared with somatostatin | 2 | 239 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.79, 2.75] |
| 7.4 EVS compared with octreotide | 5 | 391 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 8 Adverse events | 12 | 1178 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.03, 0.14] |
| 8.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.15, 0.14] |
| 8.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.02, 0.21] |
| 8.3 EVS compared with somatostatin | 4 | 366 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.22] |
| 8.4 EVS compared with octreotide | 6 | 529 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.03, 0.15] |
| 9 Serious adverse events | 5 | 602 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.02, 0.08] |
| 9.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
| 9.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.03, 0.09] |
| 9.3 EVS compared with somatostatin | 3 | 319 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.13] |
| 9.4 EVS compared with octreotide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
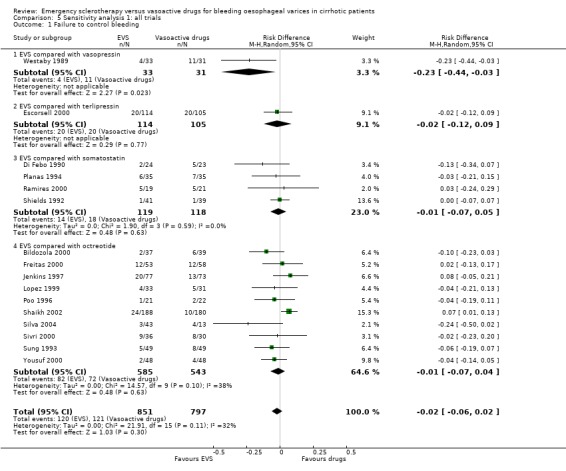
Comparison 5 Sensitivity analysis 1: all trials, Outcome 1 Failure to control bleeding.
5.2. Analysis.
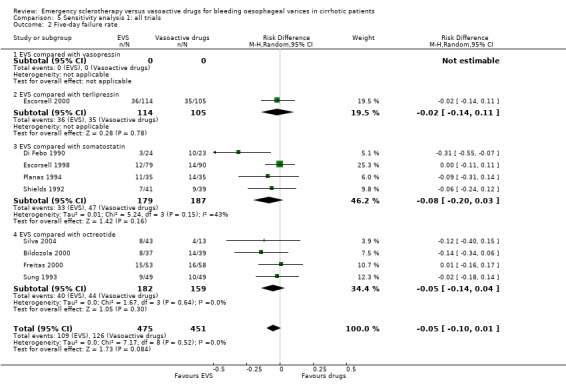
Comparison 5 Sensitivity analysis 1: all trials, Outcome 2 Five‐day failure rate.
5.3. Analysis.
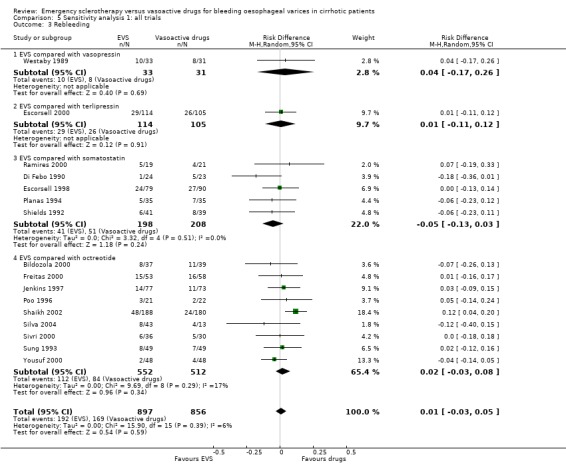
Comparison 5 Sensitivity analysis 1: all trials, Outcome 3 Rebleeding.
5.4. Analysis.
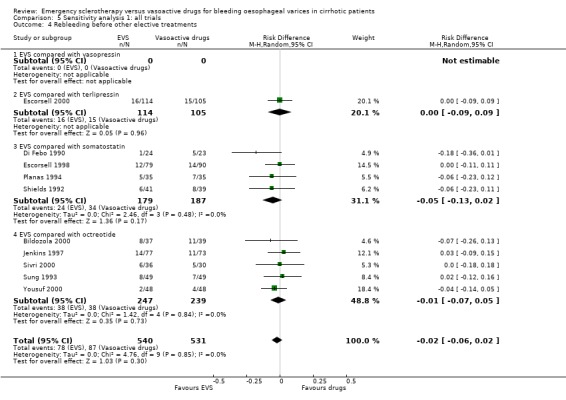
Comparison 5 Sensitivity analysis 1: all trials, Outcome 4 Rebleeding before other elective treatments.
5.5. Analysis.
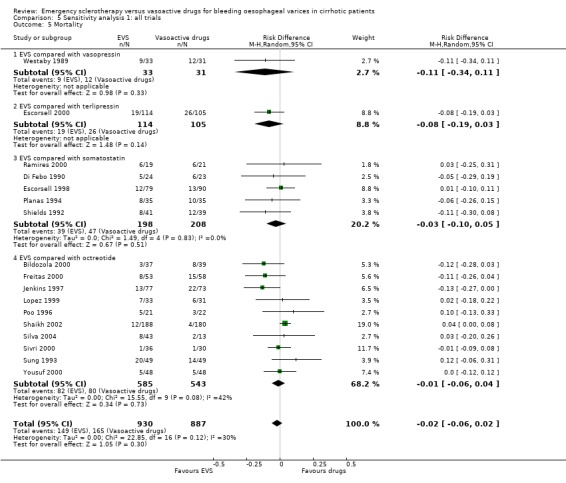
Comparison 5 Sensitivity analysis 1: all trials, Outcome 5 Mortality.
5.6. Analysis.
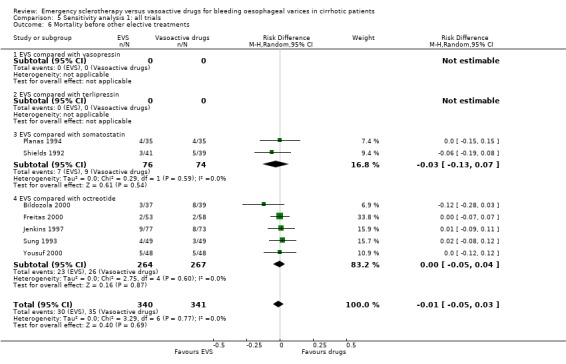
Comparison 5 Sensitivity analysis 1: all trials, Outcome 6 Mortality before other elective treatments.
5.7. Analysis.
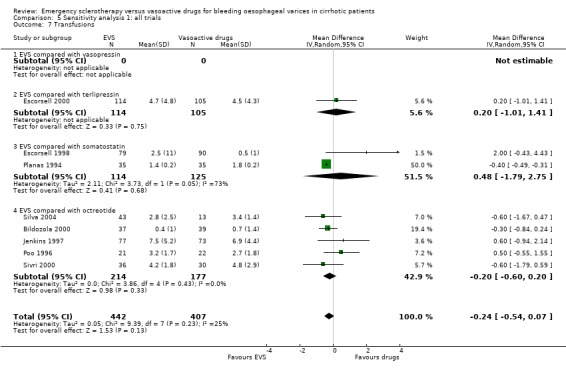
Comparison 5 Sensitivity analysis 1: all trials, Outcome 7 Transfusions.
5.8. Analysis.
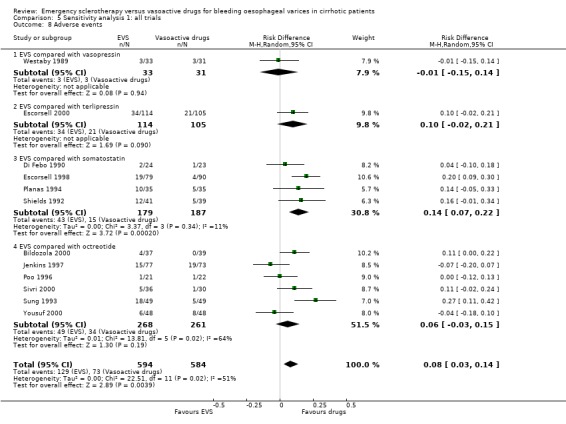
Comparison 5 Sensitivity analysis 1: all trials, Outcome 8 Adverse events.
5.9. Analysis.
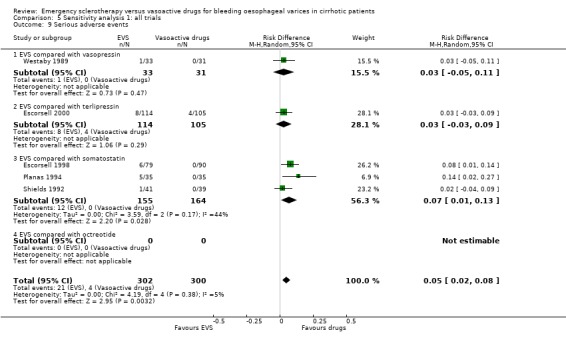
Comparison 5 Sensitivity analysis 1: all trials, Outcome 9 Serious adverse events.
Comparison 6. Sensitivity analysis 2: full reports.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to control bleeding | 13 | 1551 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.02] |
| 1.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.23 [‐0.44, ‐0.03] |
| 1.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.09] |
| 1.3 EVS compared with somatostatin | 4 | 247 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 1.4 EVS compared with octreotide | 8 | 1021 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 2 Five‐day failure rate | 8 | 879 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.02] |
| 2.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.14, 0.11] |
| 2.3 EVS compared with somatostatin | 3 | 319 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 2.4 EVS compared with octreotide | 4 | 341 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.14, 0.04] |
| 3 Rebleeding | 14 | 1663 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 3.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.17, 0.26] |
| 3.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.11, 0.12] |
| 3.3 EVS compared with somatostatin | 4 | 359 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 3.4 EVS compared with octreotide | 8 | 1021 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 4 Rebleeding before other elective treatments | 9 | 1024 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 4.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 4.3 EVS compared with somatostatin | 3 | 319 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.11, 0.05] |
| 4.4 EVS compared with octreotide | 5 | 486 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 5 Mortality | 14 | 1663 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 5.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.34, 0.11] |
| 5.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 5.3 EVS compared with somatostatin | 4 | 359 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 5.4 EVS compared with octreotide | 8 | 1021 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 6 Mortality before other elective treatments | 7 | 681 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 6.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 EVS compared with terlipressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 EVS compared with somatostatin | 2 | 150 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 6.4 EVS compared with octreotide | 5 | 531 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 7 Transfusions | 7 | 806 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.56, ‐0.13] |
| 7.1 EVS compared with vasopressin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 EVS compared with terlipressin | 1 | 219 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.01, 1.41] |
| 7.3 EVS compared with somatostatin | 2 | 239 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.79, 2.75] |
| 7.4 EVS compared with octreotide | 4 | 348 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.75, 0.11] |
| 8 Adverse events | 9 | 1024 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.04, 0.17] |
| 8.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.02, 0.21] |
| 8.3 EVS compared with somatostatin | 3 | 319 | Risk Difference (M‐H, Random, 95% CI) | 0.18 [0.10, 0.26] |
| 8.4 EVS compared with octreotide | 5 | 486 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.04, 0.18] |
| 9 Serious adverse events | 4 | 538 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 9.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.03, 0.09] |
| 9.3 EVS compared with somatostatin | 3 | 319 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.13] |
| 9.4 EVS compared with octreotide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
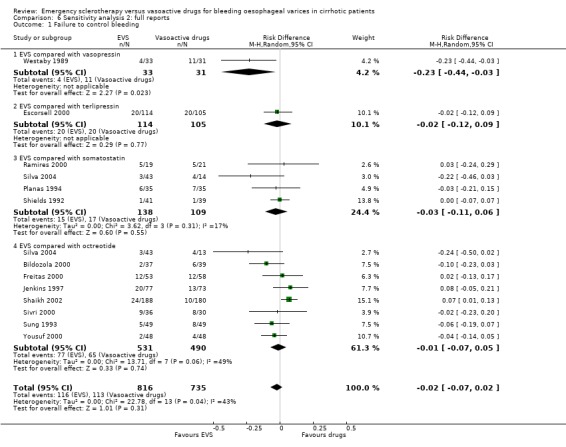
Comparison 6 Sensitivity analysis 2: full reports, Outcome 1 Failure to control bleeding.
6.2. Analysis.
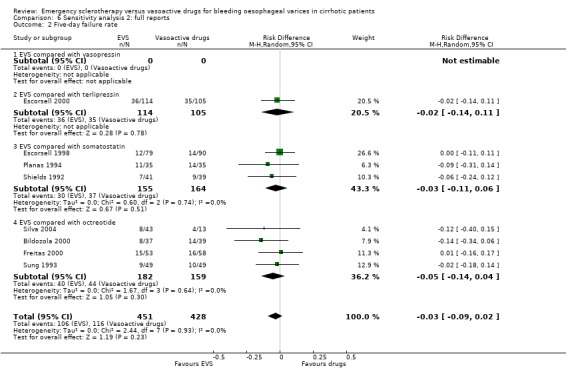
Comparison 6 Sensitivity analysis 2: full reports, Outcome 2 Five‐day failure rate.
6.3. Analysis.
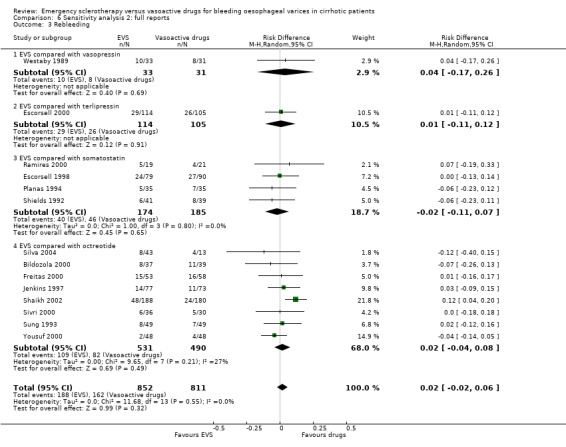
Comparison 6 Sensitivity analysis 2: full reports, Outcome 3 Rebleeding.
6.4. Analysis.
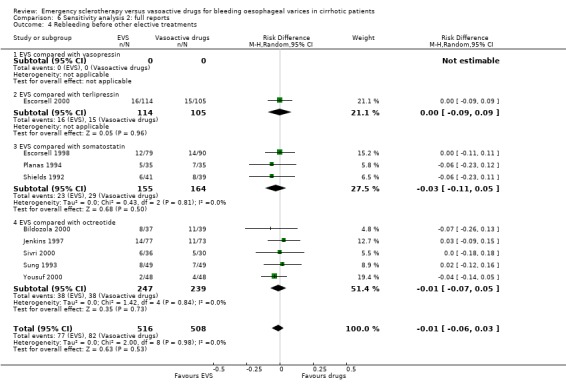
Comparison 6 Sensitivity analysis 2: full reports, Outcome 4 Rebleeding before other elective treatments.
6.5. Analysis.
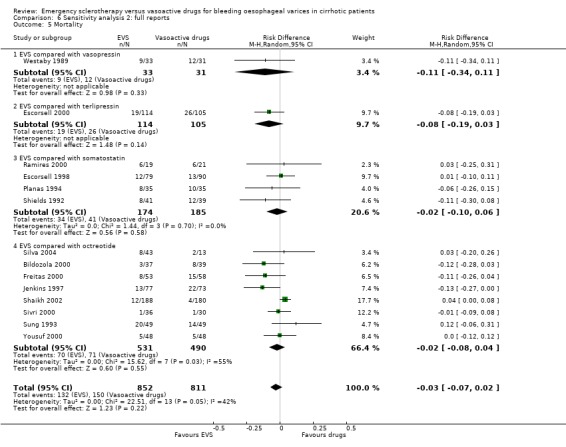
Comparison 6 Sensitivity analysis 2: full reports, Outcome 5 Mortality.
6.6. Analysis.
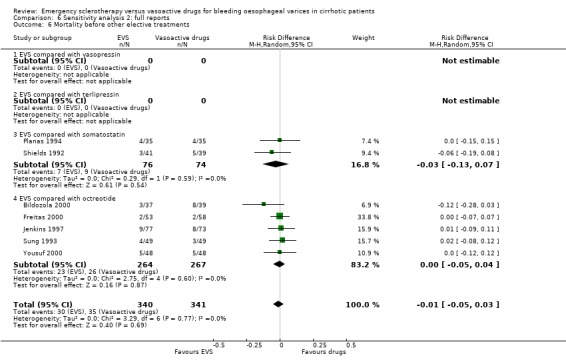
Comparison 6 Sensitivity analysis 2: full reports, Outcome 6 Mortality before other elective treatments.
6.7. Analysis.
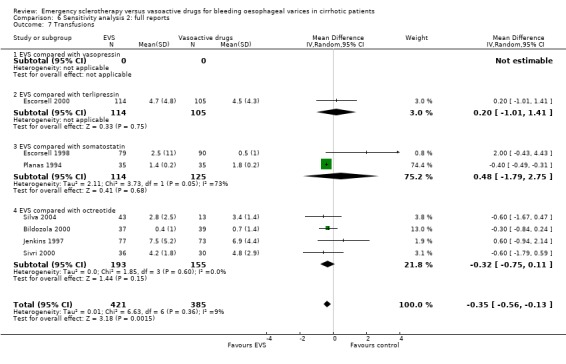
Comparison 6 Sensitivity analysis 2: full reports, Outcome 7 Transfusions.
6.8. Analysis.
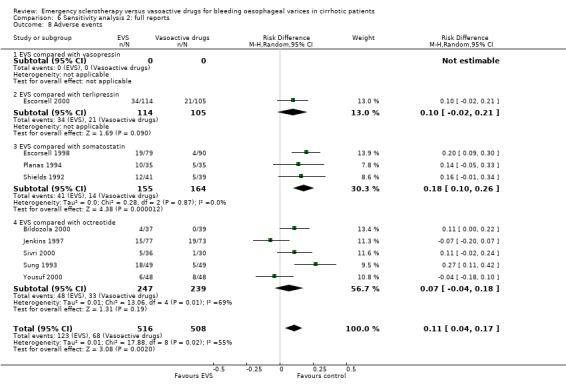
Comparison 6 Sensitivity analysis 2: full reports, Outcome 8 Adverse events.
6.9. Analysis.
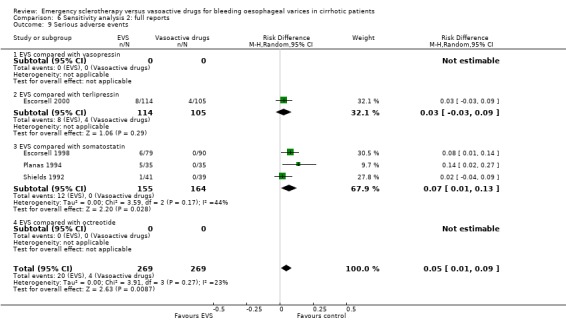
Comparison 6 Sensitivity analysis 2: full reports, Outcome 9 Serious adverse events.
Comparison 7. Sensitivity analysis 3: randomised trials with adequate control of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to control bleeding | 5 | 603 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.06] |
| 1.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.09] |
| 1.3 EVS compared with somatostatin | 1 | 70 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.21, 0.15] |
| 1.4 EVS compared with octreotide | 3 | 314 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.09, 0.10] |
| 2 Five‐day failure rate | 4 | 556 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 2.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.14, 0.11] |
| 2.3 EVS compared with somatostatin | 2 | 239 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 2.4 EVS compared with octreotide | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 3 Rebleeding | 6 | 772 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.05, 0.06] |
| 3.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.11, 0.12] |
| 3.3 EVS compared with somatostatin | 2 | 239 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 3.4 EVS compared with octreotide | 3 | 314 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.06, 0.10] |
| 4 Rebleeding before other elective treatments | 6 | 772 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 4.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 4.3 EVS compared with somatostatin | 2 | 239 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 4.4 EVS compared with octreotide | 3 | 314 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.06, 0.10] |
| 5 Mortality | 6 | 772 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 5.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 5.3 EVS compared with somatostatin | 2 | 239 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 5.4 EVS compared with octreotide | 3 | 314 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.14, 0.10] |
| 6 Mortality before other elective treatments | 5 | 474 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.04] |
| 6.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 EVS compared with terlipressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 EVS compared with somatostatin | 2 | 150 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 6.4 EVS compared with octreotide | 3 | 324 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.09, 0.06] |
| 7 Transfusions | 5 | 674 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.68, 0.46] |
| 7.1 EVS compared with vasopressin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 EVS compared with terlipressin | 1 | 219 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.01, 1.41] |
| 7.3 EVS compared with somatostatin | 2 | 239 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.79, 2.75] |
| 7.4 EVS compared with octreotide | 2 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.26, 1.06] |
| 8 Adverse events | 6 | 772 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [0.03, 0.21] |
| 8.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.02, 0.21] |
| 8.3 EVS compared with somatostatin | 2 | 239 | Risk Difference (M‐H, Random, 95% CI) | 0.18 [0.09, 0.27] |
| 8.4 EVS compared with octreotide | 3 | 314 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |
| 9 Serious adverse events | 3 | 458 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.02, 0.12] |
| 9.1 EVS compared with vasopressin | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.03, 0.09] |
| 9.3 EVS compared with somatostatin | 2 | 239 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.03, 0.14] |
| 9.4 EVS compared with octreotide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
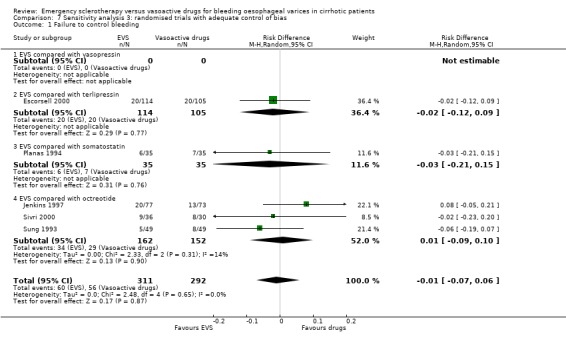
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 1 Failure to control bleeding.
7.2. Analysis.
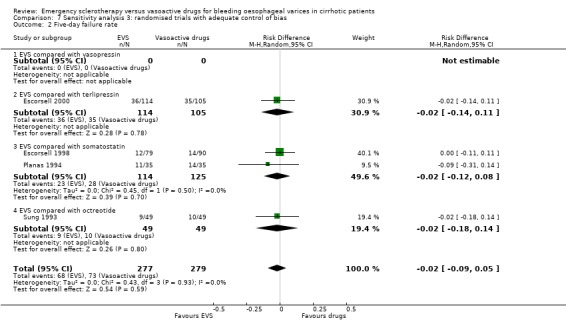
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 2 Five‐day failure rate.
7.3. Analysis.
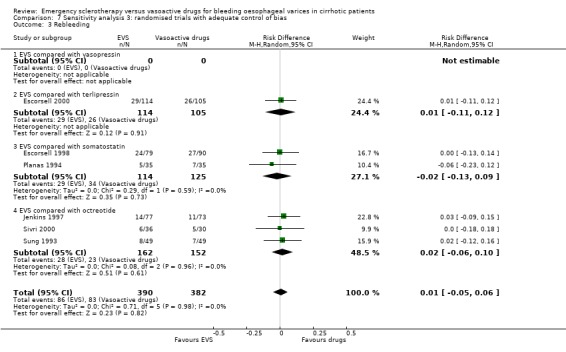
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 3 Rebleeding.
7.4. Analysis.
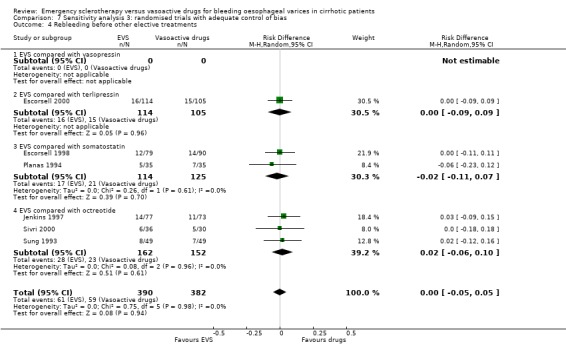
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 4 Rebleeding before other elective treatments.
7.5. Analysis.
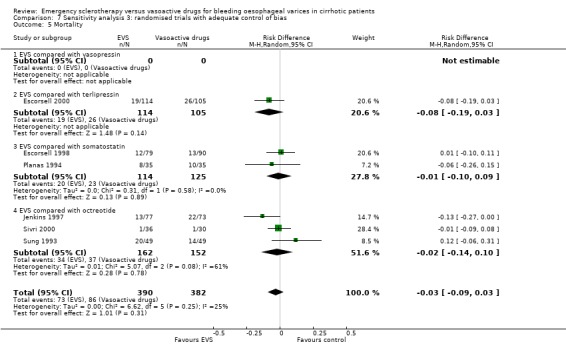
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 5 Mortality.
7.6. Analysis.
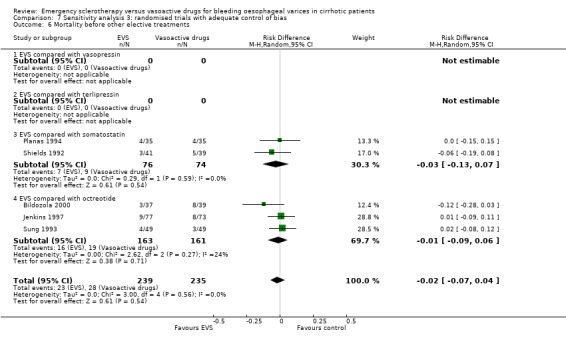
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 6 Mortality before other elective treatments.
7.7. Analysis.
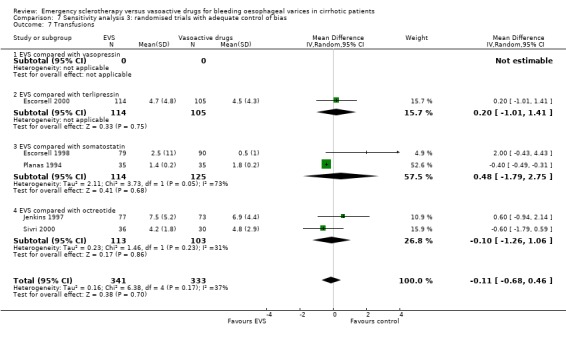
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 7 Transfusions.
7.8. Analysis.
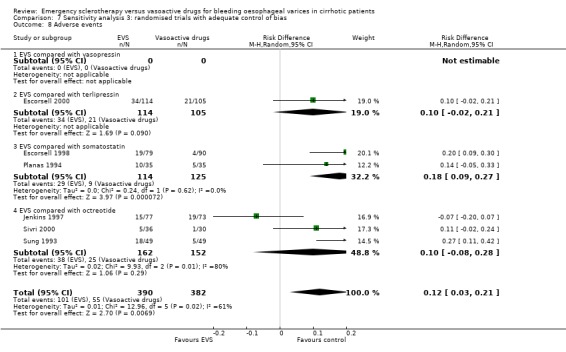
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 8 Adverse events.
7.9. Analysis.
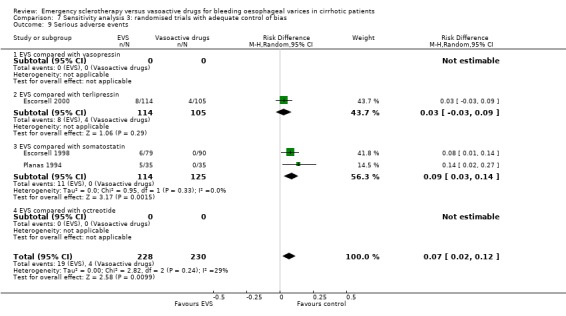
Comparison 7 Sensitivity analysis 3: randomised trials with adequate control of bias, Outcome 9 Serious adverse events.
Comparison 8. Sensitivity analysis 4: EVS versus somatostatin, excluding the Escorsell's study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 237 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.17, 0.05] |
| 1.1 Mortality | 4 | 237 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.17, 0.05] |
8.1. Analysis.
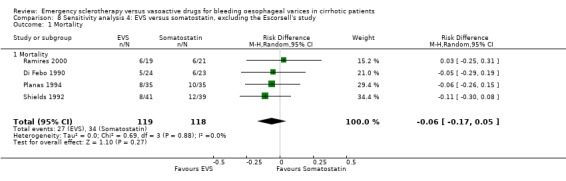
Comparison 8 Sensitivity analysis 4: EVS versus somatostatin, excluding the Escorsell's study, Outcome 1 Mortality.
Comparison 9. Sensitivity analysis 1.1: all trials excluding the Escorsell's study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 16 | 1648 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 1.1 EVS compared with vasopressin | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.34, 0.11] |
| 1.2 EVS compared with terlipressin | 1 | 219 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 1.3 EVS compared with somatostatin | 4 | 237 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.17, 0.05] |
| 1.4 EVS compared with octreotide | 10 | 1128 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
9.1. Analysis.
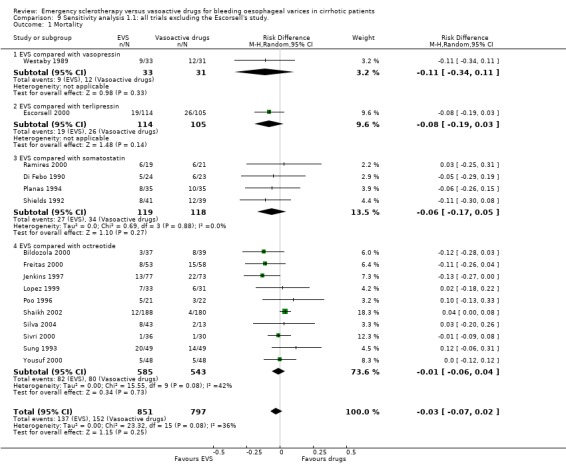
Comparison 9 Sensitivity analysis 1.1: all trials excluding the Escorsell's study., Outcome 1 Mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bildozola 2000.
| Methods | Randomisation sequence: by random number table. Concealment: method not reported. Eight patients excluded after randomisation because of protocol violations did not complete the assigned treatment. 76 patients completed the treatment as per protocol. Follow‐up: five days. Lost to follow‐up: none. Intention‐to‐treat analysis: no. | |
| Participants | Country: Argentina. Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusion: referred patients already treated with vasoactive drugs or SB; EVS in the last two months. Aetiology of cirrhosis: alcohol 72%. | |
| Interventions | EVS: intra‐ para‐ variceal injections of 2% polidocanol up to max 25 ml. Control: after an initial 100 µg iv bolus, 48‐hour continuous iv infusion of octreotide 50 µg/h followed by subcutaneous injections of 100 µg every eight hours for 72 hours. | |
| Outcomes | Failure to control bleeding. Five‐day re‐bleeding. and treatment failure. Five‐day mortality. | |
| Notes | Time frame for assessing control of bleeding unclear. After the five‐day study period, all patients were entered in a randomised trial of long‐term treatment for prevention of recurrent bleeding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Unclear risk | Unclear |
Di Febo 1990.
| Methods | Randomisation sequence: method not reported. Concealment: not reported. Number of patients completing the assigned treatment ‐ not reported. Follow‐up: 30 days. Lost to the follow‐up: none. Intention‐to‐treat analysis: yes. | |
| Participants | Country: Italy. Patients with endoscopy‐proven variceal bleeding. No other in/exclusion criteria reported. Aetiology of varices not reported. | |
| Interventions | EVS: sclerosant and modality of sclerotherapy not reported. Control: 48‐hour continuous iv infusion of somatostatin 250 µg/h after an initial 250 µg bolus. | |
| Outcomes | 48‐hour failure to control bleeding. Re‐bleeding within day two and seven days. 30‐day mortality. | |
| Notes | Only abstract available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | B ‐ Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Escorsell 1998.
| Methods | Randomisation: computer‐generated list. Concealment: opaque, sealed envelopes. Treatment performed as per protocol in all patients. Follow‐up: 42 days. No patient was lost to follow‐up or withdrawn from treatment. Intention‐to‐treat analysis: yes. | |
| Participants | 50%. | |
| Interventions | EVS: intra‐paravariceal injections of 5% ethanolamine or 1% polidocanol (maximally 20 ml). Control: five‐day continuous iv infusion of somatostatin 250 µg/h after an initial bolus of 250 µg. | |
| Outcomes | Five‐day re‐bleeding. 42‐day re‐bleeding and death. | |
| Notes | Randomised trial aimed at the prevention of early re‐bleeding and was based on the Baveno consensus conferences guidelines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
Escorsell 2000.
| Methods | Randomisation sequence: blocked computer‐generated list. Concealment: sealed, opaque envelopes. Treatment completed as per protocol in all patients. Follow‐up: 42 days, no patients lost. Intention‐to‐trat analysis: yes. Two patients excluded because of serious protocol violation. | |
| Participants | Country: Spain. Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusion: recent sclerotherapy or band ligation; bleeding from fundal varices; other concomitant bleeding sources. | |
| Interventions | EVS: intra‐paravariceal injection of either 5% ethanolamine or 1% polidocanol, single session. Terlipressin: iv injections of 2mg/4h until control of bleeding (48 hours max) and 1 mg/4h thereafter for 5 more days. | |
| Outcomes | 48‐hour failure to control bleeding. Re‐bleeding within five days after initial control. 42‐day re‐bleeding and death. | |
| Notes | Randomised trial based on the Baveno consensus conferences guidelines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
Freitas 2000.
| Methods | Generation of the randomisation list and allocation concealment: method not reported. Lost to the follow‐up period: not reported. Complete results reported only for 104/111 patients. Intention‐to‐ treat‐analysis: no. | |
| Participants | Country: Portugal. Cirrhotic patients with endoscopy‐proven non spurting variceal bleeding. | |
| Interventions | EVS: intra‐para variceal 0.5 to 1 ml of absolute ethanol (max 12 ml). Octreotide: 48‐h continuous iv infusion of 25 µg/h. | |
| Outcomes | 48‐h failure to control bleeding. 7‐day re‐bleeding. 30‐day mortality. | |
| Notes | Patients with spurting variceal bleeding were included in a parallel trial of sclerotherapy plus octreotide vs sclerotherapy alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
Jenkins 1997.
| Methods | Randomisation sequence: computer‐generated list. Concealment: opaque, sealed envelopes. Treatment completed as per protocol in all patients. Follow‐up: 60 days. No patient was lost to follow‐up. Intention‐to‐treat analysis: yes. | |
| Participants | Country: UK. Patients with endoscopy‐proven variceal bleeding, heart rate more than 100, systolic blood pressure less than 100 mmHg, or requiring 2 blood units to restore the Hb concentration. Cirrhosis was the cause of portal hypertension in 173 out of 180 included patients. Exclusion: source of bleeding other than oesophageal varices; EVS or vasoactive drugs in the previous seven days. Aetiology of portal hypertension: alcohol 64%. | |
| Interventions | EVS: intravariceal 2 to 3 ml of ethanolamine oleate. Total volume injected not reported. Control: 48‐h continuous iv infusion of octreotide 50 µg/h [sclerotherapy after the 48‐h trial period]. | |
| Outcomes | 48‐hour failure to control bleeding. 48‐hour re‐bleeding and death. 60‐day mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
Lopez 1999.
| Methods | Randomisation sequence: method not reported. Concealment: not reported. Number of patients completing the treatment ‐ not known. Length of follow‐up not reported: 24‐hour results available. Intention‐to‐treat analysis: yes. | |
| Participants | Country: Mexico. Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusion criteria not reported. Aetiology of cirrhosis not reported. | |
| Interventions | EVS: polidocanol injections. Details not reported. Control: 24‐hour continuous iv infusion of octreotide 50 µg/h. | |
| Outcomes | 24‐hour failure to control bleeding. Mortality. | |
| Notes | Only abstract available. Only Child‐Pugh class B or C patients were included. It is not clear at what time point mortality was recorded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
Planas 1994.
| Methods | Randomisation sequence: computer‐generated list. Concealment: sealed, opaque envelopes. Treatment completed as per protocol in all patients. Follow‐up: 42 days. Lost to follow‐up: not reported. Intention‐to‐treat analysis: not assessable. | |
| Participants | Country: Spain. Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusion: bleeding from gastric varices. Alcoholic cirrhosis 71%. | |
| Interventions | EVS: intra‐paravariceal injection of 1% polidocanol (mean max volume 48 ml), single session. Control: 48 hour continuous iv infusion of 250 µg/h of somatostatin after an initial iv bolus of 250 µg. | |
| Outcomes | 48‐hour failure to control bleeding or re‐bleeding. re‐bleeding within day two to seven. 42‐day mortality. | |
| Notes | Within a mean of nine days from admission, all surviving patients entered a randomised trial of elective pharmacological therapy versus EVS or shunt surgery. This study was based on the Baveno consensus conferences guidelines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
Poo 1996.
| Methods | Randomisation sequence: method not reported. Concealment: not reported. Number of patients completing the trial treatment: not reported. Follow‐up: 30 days. Number of patients lost to follow‐up or withdrawn from therapy: not reported. Intention‐to‐treat analysis: not assessable. | |
| Participants | Country: Mexico. Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusion: other sources of bleeding, previous vasoactive therapy or EVS. | |
| Interventions | EVS: intravariceal injection of 1% polidocanol. No other details available. Control: 48‐h continuous iv infusion of octreotide 50 ug/h. | |
| Outcomes | 48‐hour re‐bleeding. 30‐day re‐bleeding and mortality. | |
| Notes | Only abstract available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
Ramires 2000.
| Methods | Randomisation sequence: method of generation unclear. Allocation concealment by opaque, sealed envelopes. Two patients were excluded after randomisation because of incomplete treatment. None lost to the follow‐up. Intention‐to‐treat analysis: no. | |
| Participants | Country: Portugal. Cirrhotic patients with endoscopy‐proven variceal bleeding. Two out of 40 included patients had an extrahepatic portal hypertension (portal vein thrombosis). Exclusion: HCC, decompensated diabetes mellitus, serum creatinine more than 2mg/dl. | |
| Interventions | EVS: intravariceal injections of 5% ethanolamine (maximally 10 ml per varix).Somatostatin: 48‐h continuous iv infusion of 250 µg/h, plus 6‐hourly 250µg i.v. boluses during the first 24‐h infusion. | |
| Outcomes | 48‐h failure to control bleeding; 7‐day re‐bleeding; 7‐day mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
Shaikh 2002.
| Methods | Randomisation sequence: method of generation not reported. Allocation concealment: method not reported. No information reported on completion of treatment as per protocol in all patients neither on the number of patients lost to the follow‐up. Follow‐up 30 days. Intention‐to‐treat analysis not assessable. | |
| Participants | Country: Pakistan. Cirrhotic patients with endoscopy‐proven variceal bleeding. Aetiology mostly HBV and HCV. Exclusion: bleeding from causes different from oesophageal varices;HCC; age more than 75; non cirrhotic portal hypertension. | |
| Interventions | EVS: intra injection 4 to 6 ml of 50% ethanolamine on day 1,8,15,29;max volume per session not reported. Control: 48‐hour continuous iv infusion of octreotide 50 µg/h followed by subcutaneous 50 µg injections every eight hours for 3 days. | |
| Outcomes | 4‐hour control of bleeding; early re‐bleeding; 30‐day re‐bleeding; 30‐day mortality. | |
| Notes | This is a three group randomised trial: EVS, EVS plus octreotide, octreotide alone. Only the two groups, ie, EVS vs octreotide are of interest to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
Shields 1992.
| Methods | Randomisation sequence: method not described. A blocked list was generated. Concealment: sealed, opaque envelopes. Treatment completed as per protocol in all patients. Follow‐up: 28 days. Lost to follow‐up: none. Intention‐to‐treat analysis: yes. | |
| Participants | : alcohol 67%. Exclusion: bleeding not from oesophageal varices; patients already under vasoactive therapy or with EVS in the previous seven days. | |
| Interventions | EVS: 2 to 3 ml intravariceal injections of ethanolamine oleate, in a single session. Control: continuous iv infusion of somatostatin 250 µg/h for five days, after initial iv bolus of 250 µg. | |
| Outcomes | Immediate (10 to 20 min) control of bleeding on endoscopy. Five‐day re‐bleeding. 28‐day mortality. | |
| Notes | Need of transfusions was an entry criterion, but criteria to define the need of transfusions were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Low risk | Adequate |
Silva 2004.
| Methods | Randomisation sequence: method not described. Concealment: method not described. Treatment completed as per protocol in all the patients. Follow‐up: 42 days. Number of patients lost at follow‐up not reported. Intention‐to‐treat analysis: not assessable. | |
| Participants | Country: Chile. Crrhotic patients with endoscopic proven bleeding from oesophageal varices. Exclusion criteria: age more than 80; portal hypertension from cause other than cirrhosis; EVS in the last 15 days; hepatocarcinoma; hepato‐renal syndrome or terminal liver failure; non active bleeding; variceal source of bleeding not confirmed on endoscopy. | |
| Interventions | EVS: intravariceal ethanolamine oleate, up to maximum 5 ml per session. Control: 5‐day continuous iv infusion of octreotide 50 µg/h after an initial iv bolus of 50 µg | |
| Outcomes | 24‐hrs and 5‐day failure to control bleeding; 42‐day mortality. | |
| Notes | Remarkable difference in treatment groups size. Multicenter study with randomisation performed according to the local expertise with endoscopic therapy. Some patients received banding ligation: number not reported. This is a three arm randomised trial: EVS, EVS plus octreotide, octreotide alone. Only the two trial groups of interest (EVS vs octreotide) are considered for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
Sivri 2000.
| Methods | Randomisation sequence: Computer‐generated random list. Concealment: sealed, opaque envelopes. Treatment completed as per protocol in all patients. Follow‐up until discharge from hospital. No patient was lost to the follow‐up. Intention‐to‐treat analysis: yes. | |
| Participants | Country: Turkey. Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusion: HCC, source of bleeding other than oesophageal varices. Aetiology: hepatitis B and C viruses 73%. | |
| Interventions | EVS: intra‐ para‐variceal 2‐3 ml of 1% polidocanol up to max 20 ml. Control: 12‐hour continuous iv infusion of octreotide 50 µg/h after an initial 50 µg bolus. | |
| Outcomes | 6‐hour failure to control bleeding. 72‐hour re‐bleeding. Inhospital mortality. | |
| Notes | All patients surviving the initial bleeding episode entered a weekly elective EVS program. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
Sung 1993.
| Methods | Randomisation: computer‐generated list. Concealment: opaque, sealed envelopes. Treatment completed as per protocol: 98 out of 100 randomised. Follow‐up: 30 days. Lost to follow‐up: none. Intention‐to‐treat analysis: no (2 patients excluded). | |
| Participants | Country: China Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusion: bleeding source other than oesophageal varices. Aetiology of portal hypertension: HBsAg, alcohol, HCC, PBC, Wilson's disease. | |
| Interventions | EVS: intravariceal injections of 2 ml 3% sodium tetradecyl sulphate (max volume 20 ml). Control: 48‐hour continuous iv infusion of octreotide 50 µg/h after an initial iv bolus of 50 µg. | |
| Outcomes | 48‐hour failure to control bleeding. 48‐hour re‐bleeding. 48‐hour, in‐hospital and 30‐day mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Low risk | Adequate |
Westaby 1989.
| Methods | Randomisation sequence: by random number table. Concealment: method not described. Treatment completed as per protocol in all patients. Follow‐up: hospital admission. Lost to follow‐up: none. Intention‐to‐treat analysis: yes. | |
| Participants | Country: UK. Cirrhotic patients (patients) with active variceal bleeding on endoscopy. Exclusion criteria not reported. Aetiology: alcohol 55%. | |
| Interventions | EVS: up to 5 ml intravariceal injections of 5% ethanolamine oleate, maximally 20 ml. Control: 12‐hour continuous iv infusion of vasopressin 0.4 IU/min after initial 20 IU bolus and 40‐400 ug/min nitroglycerin. Both groups, after 12‐hours: EVS repeated weekly in a long‐term therapy program. | |
| Outcomes | 12‐hour failure to control bleeding. Inhospital re‐bleeding and death. | |
| Notes | Possible difference in the assessment of 12‐hour failure between the two study groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate |
| Allocation concealment? | Unclear risk | Unclear |
Yousuf 2000.
| Methods | Randomisation and allocation concealment: method not reported. Treatment completed as per protocol in all patients. Lost to follow‐up: none. Itention to treat analysis: yes | |
| Participants | Country: Pakistan. Cirrhotic patients with endoscopy‐proven variceal bleeding. Exclusions: coronary artery disease, cerebrovascular accident, pregnancy, respiratory failure. | |
| Interventions | EVS: intravariceal injections of sodium tetradecyl sulphate (volume not reported).Octreotide: 50 µg sub cutaneous injections every 6 hrs. Treatment duration not reported. | |
| Outcomes | 12‐h failure to control bleeding.36‐h treatment failure.72‐h re‐bleeding72‐h mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
EVS = endoscopic variceal sclerotherapy; SB = Sengstaken Blakemore balloon tamponade; PBC = primary biliary cirrhosis; HCC = hepatocellular carcinoma; HRS = hepatorenal syndrome; OCT = octreotide; SMT = somatostatin; iv = intravenous; h = hour.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexandrino 1990 | Sclerotherapy was added to a conventional therapy, which included vasopressin. In the control group only the conventional therapy was given. The study lacks a comparison between sclerotherapy and a pharmacological therapy. Only abstract available. Randomisation: method not described. |
| Arcidiacono 1992 | Sclerotherapy plus terlipressin was used in both groups. The study compared two different durations of terlipressin therapy. |
| Avguerinos 1997 | This study compared sclerotherapy plus somatostatin with sclerotherapy alone. There was not a control group given pharmacological therapy alone. |
| Besson 1995 | This study compared sclerotherapy plus octreotide with sclerotherapy alone. There was not a control group treated with pharmacological therapy alone. |
| Brunati 1996 | Sclerotherapy was compared with sclerotherapy plus terlipressin and with sclerotherapy plus octreotide. |
| Cales 2001 | Sclerotherapy plus vapreotide (a somatostatin analogue) was compared to sclerotherapy alone. There was not a control group treated by pharmacological therapy alone. |
| El‐Jackie 1998 | Only patients with schistosomal portal hypertension were included. |
| El‐Zayadi 1988 | Study of 'early' and long‐term sclerotherapy after control of initial bleeding. Vasopressin used only in patients with bleeding uncontrolled after the first resuscitative measures. The number of patients given vasopressin was not reported. The dosage of vasopressin was not reported. Only 39/123 patients had cirrhosis. |
| Hartigan 1997 | The study compared sclerotherapy with sham endoscopy. No active pharmacological treatment was used in the sham group. Randomisation and allocation concealment: method not reported. Blinding: a sham endoscopic procedure was performed in the control group. |
| Larson 1986 | The study lacks a comparison between sclerotherapy and a pharmacological therapy. Patients were randomised to standard medical therapy or standard medical therapy plus sclerotherapy (supplementary EVS every three to seven days). Standard medical therapy included intravenous vasopressin infusion and/or balloon tamponade as needed, but indications to these therapies were not reported. The randomisation method was not reported. Follow‐up was two weeks. |
| Morales 2007 | Sclerotherapy plus octreotide was compared to sclerotherapy plus placebo. There was not a control group treated with sclerotherapy alone. |
| Novella 1996 | Sclerotherapy plus octreotide was compared to octreotide alone. There was not a control group treated with sclerotherapy alone. |
| Primignani 1995 | This was a study of the prevention of early re‐bleeding in which sclerotherapy plus subcutaneous octreotide was compared to sclerotherapy alone. There was not a control group treated by pharmacological therapy alone. |
| Signorelli 1999 | Sclerotherapy plus octreotide was compared with sclerotherapy alone. There was not a control group treated with pharmacological therapy alone. |
| Söderlund 1985 | Sclerotherapy was added to a conventional therapy including vasopressin for patients with continuing bleeding or re‐bleeding. In the control group only the conventional therapy was given. The study lacks a comparison between sclerotherapy and a pharmacological therapy. Treatment was assigned by quasi‐randomisation, according to birth date. |
| Villanueva 1999 | Sclerotherapy plus somatostatin was compared with somatostatin alone. There was not a control group treated with sclerotherapy alone. |
| Zuberi 2000 | Sclerotherapy plus octreotide was compared with sclerotherapy alone. There was not a control group treated with pharmacological therapy alone. |
Contributions of authors
Gennaro D'Amico: protocol, analysis, interpretation of results, text. Giata Pietrosi: protocol, trials search, data abstraction, interpretation of results. Ilaria Tarantiono: protocol, trials search, data abstraction, interpretation of results. Luigi Pagliaro: scientific methodology and assessment of clinical relevance.
Sources of support
Internal sources
No sources of support supplied
External sources
Copenhagen Hospital Corporation's Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
Danish Medical Research Council's Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bildozola 2000 {published data only}
- Bildozola M, Kravetz, Argonz J, Romero G, Suarez A, Jmelnitzky A, et al. Efficacy of octreotide and sclerotherapy in the treatment of acute variceal bleeding in cirrhotic patients. A prospective, multicenter, and randomized clinical trial. Scandinavian Journal of Gastroenterology 2000;35:419‐25. [DOI] [PubMed] [Google Scholar]
Di Febo 1990 {published data only}
- Febo G, Siringo S, Vacirca M, Santoro P, Merighi S, Mustafà A, et al. Somatostatin and urgent sclerotherapy in active esophageal variceal bleeding. Gastroenterology 1990;98:A583. [Google Scholar]
Escorsell 1998 {published data only}
- Escorsell A, Bordas JM, Arbol LR, Jaramillo JL, Planas R, Banares R, et al. Randomized controlled trial of sclerotherapy versus somatostatin infusion in the prevention of early rebleeding following acute variceal hemorrhage in patients with cirrhosis. Journal of Hepatology 1998;29:779‐88. [DOI] [PubMed] [Google Scholar]
Escorsell 2000 {published data only}
- Escorsell A, Ruiz del Arbol L, Planas R, Albillos A, Banares R, Cales P, et al. Multicenter randomized controlled trial of terlipressin versus sclerotherapy in the treatment of acute variceal bleeding: the TEST study. Hepatology 2000;32:471‐6. [DOI] [PubMed] [Google Scholar]
Freitas 2000 {published data only}
- Freitas SD, Sofia C, Pontes JM, Gregorio C, Cabral PJ, Andrade P, et al. Octreotide in acute bleeding esophageal varices: a prospective randomized study. Hepato‐gastroenterology 2000;47:1310‐4. [PubMed] [Google Scholar]
Jenkins 1997 {published data only}
- Jenkins SA, Shields R, Davies M, Elias E, Turnbull AJ, Bassendine MF, et al. A multicentre randomised trial comparing octreotide and injection sclerotherapy in the management and outcome of acute variceal hemorrhage. Gut 1997;41:526‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 1999 {published data only}
- Lopez F, Vargas R, Margarita G, Rizo T, Arguelles D. Octreotide vs sclerotherapy in the treatment of acute variceal bleeding. Hepatology 1999;30:574A. [Google Scholar]
Planas 1994 {published data only}
- Planas R, Quer JQ, Boix J, Canet J, Armengol M, Cabrè E, et al. A prospective randomized trial comparing somatostatin and sclerotherapy in the treatment of acute variceal bleeding. Hepatology 1994;20:370‐5. [PubMed] [Google Scholar]
Poo 1996 {published data only}
- Poo JL, Bosques S, Guarduno R, Marin E, Moràn MA, Bobadilla J, et al. Octreotide versus emergency sclerotherapy in acute variceal hemorrhage in liver cirrhosis. A randomized multicenter study. Gastroenterology 1996;110:A1297. [Google Scholar]
Ramires 2000 {published data only}
- Ramires R, Zils C, Mattos A. Sclerotherapy versus somatostatin in the treatment of upper digestive hemorrhage caused by rupture of esophageal varices [Escleroterapia versus somatostatina na hemorragia digestiva alta por ruptura de varizes esofagicas]. Arquivos de Gastroenterologia 2000;37:148‐54. [DOI] [PubMed] [Google Scholar]
Shaikh 2002 {published data only}
- Shaikh WM, Shaikh SM, Shaikh MA, Solangi GA, Zuberi BF. A comparative study of efficacy of octreotide and sclerotherapy in variceal bleeding. Journal of the College of Physicians and Surgeons ‐ Pakistan 2002;12(11):677‐81. [Google Scholar]
Shields 1992 {published data only}
- Shields R, Jenkins SA, Baxter JN, Kingsnorth AN, Ellenbogen S, Makin CA, et al. A prospective randomised controlled trial comparing the efficacy of somatostatin with injection sclerotherapy in the control of oesophageal varices. Journal of Hepatology 1992;16:128‐37. [DOI] [PubMed] [Google Scholar]
Silva 2004 {published data only}
- Silva G, Quera RP, Fluxà F, Sanhueza E, Segovia R, Braham J, et al. Octreotide administration and/or endoscopic treatment in cirrhotic patients with acute variceal bleeding: a multicentric study. Revista Medica de Chile 2004;132(3):285‐94. [DOI] [PubMed] [Google Scholar]
Sivri 2000 {published data only}
- Sivri B, Oksuzoglu G, Bayraktar Y, Kayhan B. A prospective randomized trial from Turkey comparing octreotide versus injection sclerotherapy in acute variceal bleeding. Hepato‐Gastroenterology 2000;47:166‐73. [PubMed] [Google Scholar]
Sung 1993 {published data only}
- Sung JJ, Chung SCS, Lai CW, Chan FKL, Leung JWC, Yung MY. Octreotide infusion or emergency sclerotherapy for variceal hemorrhage. Lancet 1993;342:637‐41. [DOI] [PubMed] [Google Scholar]
Westaby 1989 {published data only}
- Westaby D, Hayes P, Gimson AES, Polson R, Williams R. Controlled clinical trial of injection sclerotherapy for active variceal bleeding. Hepatology 1989;9:274‐7. [DOI] [PubMed] [Google Scholar]
Yousuf 2000 {published data only}
- Yousuf MH, Rauf AH, Baig IM, Akram M, Rizwan Z. Initial management of acute variceal haemorrhage. Comparison of octreotide and sclerotherapy. Journal of the College of Physicians and Surgeons ‐ Pakistan 2000;10:95‐7. [Google Scholar]
References to studies excluded from this review
Alexandrino 1990 {published data only}
- Alexandrino P, Alves MM, Fidalgo P, Matos L, Esteves AV, Veloso J, et al. Is sclerotherapy the first choice treatment for active oesophageal variceal bleeding in cirrhotic patients? Final report of a randomized clinical trial. Journal of Hepatology 1990;11(Suppl):S1. [Google Scholar]
Arcidiacono 1992 {published data only}
- Arcidiacono R, Biraghi M, Bonomo M, Fiaccadori F. Randomized controlled trial with terlipressin in cirrhotic patients with bleeding esophageal varices: effects on precocious rebleeding and mortality rate. Current Therapeutic Research, Clinical and Experimental 1992;52:185‐95. [Google Scholar]
Avguerinos 1997 {published data only}
- Avguerinos A, Nevens F, Raptis S, Fevery J, and the ABOVE Study Group. Early administration of somatostatin and efficacy of sclerotherapy in acute oesophageal variceal bleeds: the European Acute Bleeding Oesophageal Variceal Episodes (ABOVE) randomised trial. Lancet 1997;350:1495‐9. [DOI] [PubMed] [Google Scholar]
Besson 1995 {published data only}
- Besson I, Ingrand P, Person B, Boutroux D, Heresbach D, Bernard P, et al. Sclerotherapy with or without octreotide for acute variceal bleeding. New England Journal of Medicine 1995;333:555‐60. [DOI] [PubMed] [Google Scholar]
Brunati 1996 {published data only}
- Brunati X, Ceriani R, Curioni R, Brunelli L, Repaci G, Morini L. Sclerotherapy alone vs sclerotherapy plus terlipressin vs sclerotherapy plus octreotide in the treatment of acute variceal hemorrhage. Hepatology 1996;24(4 Pt 2):207A. [Google Scholar]
Cales 2001 {published data only}
- Cales P, Masliah C, Bernard B, Garnier PP, Silvain C, Szostak‐Talbodec N, et al. Early administration of vapreotide for variceal bleeding in patients with cirrhosis. New England Journal of Medicine 2001;344:23‐8. [DOI] [PubMed] [Google Scholar]
El‐Jackie 1998 {published data only}
- El‐Jackie A, Rowaisha I, Waked I, Saleh S, Ghaffar YA. Octreotide vs sclerotherapy in the control of acute variceal bleeding in schistosomal portal hypertension: A randomized trial. Hepatology 1998;28(4 Suppl1):553. [Google Scholar]
El‐Zayadi 1988 {published data only}
- El‐Zayadi A, El‐Din S, Kabil M. Endoscopic sclerotherapy versus medical treatment for bleeding esophageal varices in patients with schistosomal liver disease. Gastrointestinal Endoscopy 1988;34:314‐7. [DOI] [PubMed] [Google Scholar]
Hartigan 1997 {published data only}
- Hartigan PM, Gebhard RL, Gregory PB, Veterans Affairs Cooperative Variceal Sclerotherapy Group. Sclerotherapy for actively bleeding esophageal varices in male alcoholics with cirrhosis. Gastrointestinal Endoscopy 1997;46:1‐7. [DOI] [PubMed] [Google Scholar]
Larson 1986 {published data only}
- Larson AW, Cohen H, Zwieiban B, Chapman D, Gourdji M, Korula J, et al. Acute esophageal variceal sclerotherapy. JAMA 1986;255:497‐500. [PubMed] [Google Scholar]
Morales 2007 {published data only}
- Morales GF, Pereira Lima JC, Hornos AP, Marques DL, Costa CS, Lima Pereira L, et al. Octreotide for esophageal variceal bleeding treated with endoscopic sclerotherapy: a randomized, placebo‐controlled trial. Hepato‐Gastroenterology 2007;54(73):195‐200. [PubMed] [Google Scholar]
Novella 1996 {published data only}
- Novella MT, Villanueva C, Ortiz J, Pamplona J, Sàinz S, Soriano G, et al. Octreotide vs sclerotherapy and octreotide for acute variceal bleeding. A pilot study. Hepatology 1996;24:207A. [Google Scholar]
Primignani 1995 {published data only}
- Primignani M, Andreoni B, Carpinelli L, Capria A, Rocchi G, Lorenzini I, The New Italian Endoscopic Club. Sclerotherapy plus octreotide versus sclerotherapy alone in the prevention of early rebleeding from esophageal varices: a randomized, double‐blind, placebo‐controlled, multicenter trial. Hepatology 1995;21(5):1322‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Signorelli 1999 {published data only}
- Signorelli S, Paris B, Negrin F, Cesareni P, Minola E, Auriemma L. Octreotide and endoscopic variceal sclerotherapy in the management of acute variceal haemorrhage. Endoscopy 1999;31:E66. [Google Scholar]
Söderlund 1985 {published data only}
- Söderlund C, Ihre T. Endoscopic sclerotherapy v. conservative management of bleeding esophageal varices. Acta Chirurgica Scandinavica 1985;151:449‐56. [PubMed] [Google Scholar]
Villanueva 1999 {published data only}
- Villanueva C, Ortiz J, Sabat M, Gallego A, Torras X, Soriano G, et al. Somatostatin alone or combined with emergency sclerotherapy in the treatment of acute variceal bleeding: a prospective randomized trial. Hepatology 1999;30:384‐9. [DOI] [PubMed] [Google Scholar]
Zuberi 2000 {published data only}
- Zuberi BF, Baloch Q. Comparison of endoscopic variceal sclerotherapy alone and in combination with octreotide in controlling acute variceal hemorrhage and early rebleeding in patients with low‐risk cirrhosis. American Journal of Gastroenterology 2000;95:768‐71. [DOI] [PubMed] [Google Scholar]
Additional references
Avgerinos 1997
- Avgerinos A, Nevens F, Raptis S, Fevery J, and the ABOVE Study Group. Early administration of somatostatin and efficacy of sclerotherapyin acute oesophageal variceal bleeds: the European Acute BleedingOesophageal Variceal Episodes (ABOVE) randomised trial. Lancet 1997;350:1495‐9. [DOI] [PubMed] [Google Scholar]
Avgerinos 2004
- Avgerinos A, Armonis A, Stefanidis G, Mathou N, Vlachogiannakos J, Kougioumtzian A, et al. Sustained rise of portal pressure after sclerotherapy, but not band ligation, in acute variceal bleeding in cirrhosis. Hepatology 2004;39:1623‐30. [DOI] [PubMed] [Google Scholar]
Banares 2002
- Banares R, Albillos A, Rincòn D, Gonzàlez M, Ruiz‐del‐Arbol L, Salcedo M, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta‐analysis. Hepatology 2002;35(3):609‐15. [DOI] [PubMed] [Google Scholar]
Bosch 2004
- Bosch J, Tabuth D, Bendtsen F, D'Amico G, Albillos A, Abraldes J, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double‐blind trial. Gastroenterology 2004;127(4):1123‐30. [DOI] [PubMed] [Google Scholar]
Bosch 2008
- Bosch J, Tabuth D, Albillos A, Carbonel N, Spicak J, Massard J, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized controlled trial. Hepatology 2008;47(5):1604‐14. [DOI] [PubMed] [Google Scholar]
Burroughs 1987
- Burroughs AK ed. Methodology and review of clinical trials in portal hypertension. Excerpta Medica. Amsterdam, 1987.
Burroughs 1989
- Burroughs AK, Mezzanotte G, Phillips A, McCormick A, McIntire N. Cirrhotics with variceal hemorrhage: The importance of the time interval between admission and the start of analysis for survival and rebleeding rates. Hepatology 1989;9:801‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burroughs 1990
- Burroughs AK, McCormick A, Hughes MD, Sprengers D, D'Heygere F, McIntyre N. Randomized, double‐blind, placebo‐controlled trial of somatostatin for variceal bleeding. Emergency control and prevention of early variceal rebleeding. Gastroenterology 1990;99:1388‐95. [DOI] [PubMed] [Google Scholar]
Burroughs 1996
- Burroughs AK. Double blind randomised trial of 5 day octreotide versus placebo, associated with sclerotherapy for trial failures. [Abstract]. Hepatology 1996;24(suppl):352A. [Google Scholar]
Carbonel 2004
- Carbonell N, Pauwels A, Serfaty L, Fourdan O, George Lévy V, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 2004;40:652‐9. [DOI] [PubMed] [Google Scholar]
Chalasani 2003
- Chalasani, N, Kahi C, Francois F, Pinto A, Marathe A, Bini AJ, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. American Journal of Gastroenterology 2003;98:653‐9. [DOI] [PubMed] [Google Scholar]
D'Amico 1995
- D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension. A meta‐analytic review. Hepatology 1995;22:332‐54. [DOI] [PubMed] [Google Scholar]
D'Amico 1997
- D'Amico G, Luca A. Portal hypertension. Natural history. Clinical‐hemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clinical Gastroenterology 1997;11(2):243‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
D'Amico 1999
- D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence based approach. Seminars in Liver Disease 1999;19:475‐506. [DOI] [PubMed] [Google Scholar]
D'Amico 2003
- D'Amico G, Franchis R, and a cooperative group. Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators. Hepatology 2003;38:599‐612. [DOI] [PubMed] [Google Scholar]
D'Amico 2009
- D'Amico G, Pagliaro L, Pietrosi G, Tarantino I. Emergency sclerotherapy versus vasoactive drugs for bleeding oesophageal varices in cirrhotic patients. Cochrane Database of Systematic Reviews 2009, Issue 4 IS THIS REFERENCE CORRECT??? (DOI NUMBER NOT CORRECT!). [DOI: 10.1002/14651858.CD00223] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico G 2002
De Franchis 1992
- Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A consensus development workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. Journal of Hepatology 1992;15:256‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Franchis 1996
- Franchis R. In: Franchis R editor(s). Portal hypertension II. Proceedings of the second Baveno International Consensus Workshop on definitions, methodology, and therapeutic strategies. Oxford: Blackwell Science, 1996. [Google Scholar]
De Franchis 2000
- Franchis R. Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. Journal of Hepatology 2000;33:846‐52. [DOI] [PubMed] [Google Scholar]
De Franchis 2005
- Franchis R. Evolving consensus in portal hypertension report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. Journal of Hepatology 2005;43:167‐76. [DOI] [PubMed] [Google Scholar]
Der Simonian 1986
- Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Garcia‐Pagan 1999
- Garcia‐Pagan JC, Escorsell A, Moitinho E, Bosch J. Influence of pharmacological agents on portal hemodynamics: basis for its use in the treatment of portal hypertension. Seminars in Liver Disease 1999;19:427‐37. [DOI] [PubMed] [Google Scholar]
Garcia‐Pagan 2009
- Garcia‐Pagan JC, Villanueva C, Albillos A, Bañares R, Morillas R, Abraldes JG, et al. Nadolol plus isosorbide mononitrate alone or associated with band ligation in the prevention of recurrent bleeding: A multicenter randomized controlled trial. Gut 2009;12 Feb 2009:[Epub ahead of print]. [DOI: 10.1136/gut.2008.171207] [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2007
- Garcia‐Tsao G, Sanyal A J, Grace N D, Carey W, and the Practice Guidelines Committee of the American Association for the Study of Liver Diseases, the Practice Parameters committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46(3):922‐38. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2008
- Garcia Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding ‐ unresolved issues. Summary of an American Association for the Study of Liver Diseases and European Association for the Study of the Liver ‐ single topic conference. Hepatology 2008;47(5):1764‐72. [DOI] [PubMed] [Google Scholar]
Gluud 2006
- Gluud LL. Bias in clinical intervention research. Americal Journal of Epidemiology 2006;163:493‐501. [DOI] [PubMed] [Google Scholar]
Gluud 2009
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2009, Issue 4. Art. No.: LIVER.
Goulis 1999
- Goulis J, Burroughs AK. Portal hypertensive bleeding: prevention and treatment. In: McDonald J, Burroughs AK, Feagan B editor(s). Evidence based Gastroenterology and Hepatology. London: BMJ Books, 1999:389‐426. [Google Scholar]
Grace 1997
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American Journal of Gastroenterology 1997;92:1081‐91. [PubMed] [Google Scholar]
Grace 1998
- Grace N, Groszman RJ, Garcia‐Tsao G, Burroughs AK, Pagliaro L, Makuch RW, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998;28:868‐80. [DOI] [PubMed] [Google Scholar]
Graham 1981
- Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology 1981;80:800‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Gøtzsche 1995
- Gøtzsche PC, Gjørup I, Bonnen H, Brahe NEB, Becker U, Burcharth F. Somatostatin v placebo in bleeding oesophageal varices: randomized trials and meta‐analysis. BMJ (Clinical Research Ed.) 1995;310:1495‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gøtzsche 2008
- Gøtzsche PC, Hróbjartsson A. Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Colloboration, 2008. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International conference on harmonisation expert working group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice//1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Imperiale 1995
- Imperiale T, Teran C, McCullough AJ. A meta‐analysis of somatostatin versus vasopressin in the management of acute esophageal variceal hemorrhage. Gastroenterology 1995;109:1289‐94. [DOI] [PubMed] [Google Scholar]
Ioannau 2003
- Ioannou GN, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002147] [DOI] [PubMed] [Google Scholar]
Jalan 2000
- Jalan R, Hayes P C. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2000;46(Suppl iii):iii1‐iii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Laupacis 1988
- Laupacis A, Sackett DL, Roberts AS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1988;318:1728‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lo 1997
- Lo GH, Lay KH, Cheng JS, Lin CK, Huang JS, Hsu PI, et al. Emergency banding ligation versus sclerotherapy for the control of active bleeding from esophageal varices. Hepatology 1997;25:1101‐4. [DOI] [PubMed] [Google Scholar]
Norberto 2007
- Norberto L, Polese L, Cillo U, Grigoletto F, Burroughs AK, Neri D, et al. A randomized study comparing ligation with propranolol for primary prophylaxis of variceal bleeding in candidates for liver transplantation. Liver Transplantation 2007;13:1272‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0.. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration.. The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Sorbi 2003
- Sorbi D, Gostout CJ, Peura D, Johnson D, Lanza F, Foutch PG, et al. Acute bleeding varices: a multicenter prospective member‐based study. American Journal of Gastroenterology 2003;98(11):2424‐34. [DOI] [PubMed] [Google Scholar]
Triantos 2006
- Triantos‐CK, Goulis‐J, Patch‐D, Papatheodoridis‐GV, Leandro‐G, Samonakis‐D, et al. An evaluation of emergency sclerotherapy of varices in randomized trials: Looking the needle in the eye. Endoscopy 2006;38(8):797‐808. [DOI] [PubMed] [Google Scholar]
Villanueva 1999
- Villanueva C, Ortiz J, Sabat M, Callego A, Torras X, Soriano G, et al. Somatostatin alone or combined with emergency sclerotherapyin the treatment of acute esophageal variceal bleeding: A prospective randomized trial. Hepatology 1999;30:384‐9. [DOI] [PubMed] [Google Scholar]
Villanueva 2006
- Villanueva C, Piqueras M, Aracil C, Gomez C, Lopez‐Balaguer JM, Gonzalez B, et al. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. Journal of Hepatology 2006;45:560‐7. [DOI] [PubMed] [Google Scholar]
Williams 1996
- Williams JG, Westaby D. Recent advances in the endoscopic management of variceal bleeding. In: Franchis R editor(s). Portal hypertension II. Proceedings of the second Baveno International Consensus Workshop on definitions, methodology, and therapeutic strategies. Oxford: Blackwell Science, 1996:114‐25. [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
D'Amico 2002
- D'Amico G, Pagliaro L, Pietrosi G, Tarantino I. Emergency sclerotherapy versus medical interventions for bleeding oesophageal varices in cirrhotic patients. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002233] [DOI] [PubMed] [Google Scholar]


