Abstract
Background
Acute toxoplasma retinochoroiditis causes transient symptoms of ocular discomfort and may lead to permanent visual loss. Antibiotic treatment aims primarily to reduce the risk of permanent visual loss, recurrent retinochoroiditis, and the severity and duration of acute symptoms. There is uncertainty about the effectiveness of antibiotic treatment.
Objectives
To compare the effects of antibiotic treatment versus placebo or no treatment for toxoplasma retinochoroiditis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision group Trials Register) (2016, Issue 1), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2016), EMBASE (January 1980 to February 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to February 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 22 February 2016. We searched the reference lists of identified articles and contacted pharmaceutical companies for unpublished trials.
Selection criteria
We included randomised controlled trials that compared any antibiotic treatment against placebo or no treatment. We excluded trials that included immunocompromised participants. We considered any antibiotic treatment known to be active against Toxoplasma gondii. Antibiotic treatment could be given in any dose orally, by intramuscular injection, by intravenous infusion, or by intravitreal injection.
Data collection and analysis
The primary outcomes for this review were visual acuity at least three months after treatment and risk of recurrent retinochoroiditis. Secondary outcomes were improvement in symptoms and signs of intraocular inflammation, size of lesion, and adverse events. We used standard methodological procedures expected by Cochrane.
Main results
Four trials that randomised a total of 268 participants met the inclusion criteria. In all four studies antibiotic was administered orally.
One study conducted in Brazil in both adults and children compared trimethoprim‐sulfamexacocol over 20 months to no treatment and was judged to be at high risk of performance, detection, and attrition bias. The other three studies compared antibiotic treatment to placebo. We judged these three studies to be at a mixture of low or unclear risk of bias due to poor reporting. One study conducted in the US in adults studied pyrimethamine‐trisulfapyrimidine for eight weeks; one study conducted in the UK in children and adults evaluated pyrimethamine for four weeks; and one study conducted in Brazil in adults investigated trimethoprim‐sulfamethoxazole for 12 months. In the last study, all participants had active retinochoroiditis and were treated with antibiotics for 45 days prior to randomisation to trimethoprim‐sulfamethoxazole versus placebo.
Only the study in Brazil of trimethoprim‐sulfamethoxazole over 12 months, in participants with healed lesions, reported the effect of treatment on visual acuity. People treated with antibiotics may have a similar change in visual acuity compared with people treated with placebo at one year (mean difference ‐1.00 letters, 95% confidence interval (CI) ‐7.93 to 5.93 letters; 93 participants; low‐quality evidence).
Treatment with antibiotics probably reduces the risk of recurrent retinochoroiditis compared with placebo (risk ratio (RR) 0.26, 95% CI 0.11 to 0.63; 227 participants; 3 studies; I2 = 0%; moderate‐quality evidence); similar results were seen for acute and chronic retinochoroiditis.
The UK study of pyrimethamine for four weeks reported an improvement in intraocular inflammation in treated compared with control participants (RR 1.76, 95% CI 0.98 to 3.19; 29 participants; low‐quality evidence). The study in Brazil of trimethoprim‐sulfamethoxazole for 12 months stated that the severity of inflammation was higher in the comparator group when compared to the antibiotic‐treated group but did not provide further details. In the US study of pyrimethamine‐trisulfapyrimidine for eight weeks intraocular inflammation had almost completely resolved by eight weeks in all participants, however in this study all participants received steroid treatment.
Two studies (UK and US studies) reported an increased risk of adverse events in treated participants. These were a fall in haemoglobin, leucocyte, and platelet count, nausea, loss of appetite, rash, and arthralgia.
Authors' conclusions
Treatment with antibiotics probably reduces the risk of recurrent toxoplasma retinochoroiditis, but there is currently no good evidence that this leads to better visual outcomes. However, absence of evidence of effect is not the same as evidence of no effect. Further trials of people with acute and chronic toxoplasma retinochoroiditis affecting any part of the retina are required to determine the effects of antibiotic treatment on visual outcomes.
Keywords: Adult; Child; Humans; Administration, Oral; Anti‐Bacterial Agents; Anti‐Bacterial Agents/administration & dosage; Anti‐Bacterial Agents/therapeutic use; Chorioretinitis; Chorioretinitis/drug therapy; Chorioretinitis/parasitology; Drug Combinations; Pyrimethamine; Pyrimethamine/therapeutic use; Randomized Controlled Trials as Topic; Recurrence; Secondary Prevention; Sulfadiazine; Sulfadiazine/therapeutic use; Sulfamerazine; Sulfamerazine/therapeutic use; Sulfamethazine; Sulfamethazine/therapeutic use; Toxoplasmosis, Ocular; Toxoplasmosis, Ocular/complications; Toxoplasmosis, Ocular/drug therapy; Trimethoprim, Sulfamethoxazole Drug Combination; Trimethoprim, Sulfamethoxazole Drug Combination/administration & dosage; Trimethoprim, Sulfamethoxazole Drug Combination/therapeutic use; Visual Acuity; Watchful Waiting
Plain language summary
Antibiotics compared with no treatment or placebo for the treatment of toxoplasma retinochoroiditis
Review question Are antibiotics an effective treatment for toxoplasma retinochoroiditis?
Background Toxoplasma retinochoroiditis occurs when a parasite called Toxoplasma gondii gets into the retina (the light‐sensitive layer inside the eye) and the choroid (layer of the eyeball near the retina). This causes inflammation that can scar the retina and reduce vision. Symptoms include a sudden feeling of discomfort in the eye and loss of vision, which usually resolve spontaneously within six to eight weeks. The infection can keep returning, increasing the chances of damage. Antibiotics are sometimes used to try to reduce the inflammation and scarring, or to prevent the infection from re‐emerging, but it is unclear how well they work.
Study characteristics We found four studies with a total of 268 participants. These studies were conducted in Brazil, the UK, and the US. The evidence is current to 22 February 2016.
Key results Only one of the four studies compared the effect of antibiotic treatment for 12 months with placebo on visual acuity and found similar changes in both groups. Three studies examined the effect of antibiotics on reducing the number of recurring episodes of the disease. Two of these three studies were conducted in Brazil in adults infected with the more aggressive South American strains of the parasite, which can cause frequently recurring eye symptoms. The studies from Brazil found that the long‐term antibiotics over 14 and 12 months, respectively, reduced the number of recurrent episodes of retinochoroiditis. The other study did not find that short‐term (eight weeks) treatment with antibiotics made any difference. Two studies reported an improvement in intraocular inflammation in antibiotic‐treated compared with untreated participants, and one study reported no changes. Two studies investigated side effects of giving antibiotics such as decreased white blood cells, loss of appetite, rashes and other allergic reactions and found only weak evidence that antibiotics increase the risk of side effects.
Quality of the evidence
There were problems with the design, conduct, and analyses of all of the studies, which could have biased the results. There was a lack of evidence about whether antibiotics (short or long term) prevent vision loss. More trials are needed, including trials of newer antibiotics.
Summary of findings
Summary of findings for the main comparison. Antibiotic versus no treatment for toxoplasma retinochoroiditis.
| Antibiotic versus no treatment for toxoplasma retinochoroiditis | ||||||
| Patient or population: People with toxoplasma retinochoroiditis Settings: Primary care Intervention: Antibiotic1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic | |||||
|
Change in visual acuity
Follow‐up: 12 months Measured as number of letters read on an ETDRS chart; higher number of letters = better vision |
People in the control group gained on average 22 letters over 12 months | People in the antibiotic group gained on average 1 fewer letters (95% CI 8 less to 6 more) | ‐ | 93 (1 study) | ⊕⊕⊕⊝ low2,3 | ‐ |
|
1 or more recurrences of retinochoroiditis Follow‐up: 8 weeks to 24 months |
Low risk (e.g. US/UK) | RR 0.26 (0.11 to 0.63) | 227 (3 studies) | ⊕⊕⊕⊝ moderate4 | ‐ | |
| 100 per 1000 | 26 per 1000 (11 to 63) | |||||
| High risk (e.g. Brazil) | ||||||
| 250 per 1000 | 65 per 1000 (28 to 158) | |||||
|
Improvement in intraocular inflammation Follow‐up: 4 weeks |
Study population | RR 1.76 (0.98 to 3.19) | 29 (1 study) | ⊕⊕⊝⊝ low2 | Study conducted in the UK (pyrimethamine for 4 weeks) | |
| 500 per 1000 | 880 per 1000 (490 to 1000) | |||||
|
Size of lesion Follow‐up: 4 weeks to 24 months |
See comment | None of the included studies reported this outcome | ||||
|
Adverse events Follow‐up: 8 weeks to 24 months |
Study population | RR 3 (0.37 to 24.17) | 20 (1 study) | ⊕⊕⊝⊝ low5 | Study conducted in the US (pyrimethamine‐trisulfapyrimidine for 8 weeks). Adverse events included nausea, loss of appetite, rash, arthralgia, and low platelet count |
|
| 100 per 1000 | 300 per 1000 (37 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Different combinations were used. One RCT used pyrimethamine (200 mg/day 1, 100 mg/day 2, 50 mg/day on days 3 to 15, 25 mg/day on days 16 to 56); trisulfapyrimidine 2 g/day for 8 weeks; prednisolone (40 mg/day on days 1 to 7, 20 mg/day on days 8 to 56). Other RCT used trimethoprim‐sulfamethoxazole tablet (800 mg/160 mg) every 2 days for 12 months, while remaining RCTs used pyrimethamine (Daraprim) 25 mg daily for 4 weeks versus inert tablet and trimethoprim 160 mg and sulfamexacocol 800 mg, both orally every 3 days for 20 months, respectively. 2Downgraded for imprecision (‐1): the confidence interval was compatible with both benefit and harm and included clinically meaningful effects. 3Downgraded for indirectness (‐1): trial was a different design to others and evaluated antibiotic treatment after lesions had been successfully treated. 4Downgraded for risk of bias (‐1): in one RCT, medication was administered in an unmasked fashion, participants were followed by the clinician responsible for the trial. 5Downgraded for imprecision (‐2): there is very little information owing to 20 participants, and the confidence interval was very wide.
ETDRS: Early Treatment Diabetic Retinopathy Study RCT: randomised controlled trial
Background
Description of the condition
Toxoplasma gondii (T.gondii) is a ubiquitous human parasite. Infection of the retina results in acute intraocular inflammation (retinochoroiditis) and the formation of a retinochoroidal scar. Retinochoroiditis can recur at any time, often years after first infection, and may be due to the local release of T.gondii parasites from cysts in the retina (Holland 2003). Alternatively, retinal antigens normally hidden from immune surveillance may be released due to cyst reactivation and stimulate an inflammatory response (Roberts 1999).
Acute toxoplasma retinochoroiditis may cause sudden onset of visual loss and pain, sometimes associated with floaters or photophobia. Symptoms often resolve spontaneously within six to eight weeks, leaving a healed retinochoroidal scar (Rothova 1993a). Scars involving the posterior pole (within the macular arcade or near the optic nerve head) or very large lesions leading to vitreous opacifications can permanently impair visual acuity. Peripheral lesions may cause field defects but are unlikely to affect visual acuity.
The lifetime risk of symptoms due to acute toxoplasma retinochoroiditis ranges from 18 out of 100,000 (95% confidence interval (CI) 11 to 25) for people born in the UK to 382 out of 100,000 (95% CI 99 to 664) for people born in West Africa (Gilbert 1999). The presentation of retinochoroiditis following prenatal or postnatal infection is similar and can only be distinguished in people with systemic signs of toxoplasmosis (Gilbert 1999). The risk of retinochoroiditis after prenatal toxoplasmosis is 20% in the early childhood years (Dunn 1999; Guerina 1994; Lebech 1999), and may be as high as 80% by adolescence (Koppe 1986). Less information is available on the risk of retinochoroiditis after postnatal infection; reports range from 0.3% to 0.7% one year after infection, in Burnett 1998, to 3% (duration of follow‐up unknown), in Perkins 1973. However, as prenatal toxoplasmosis is approximately 1000 times less common than postnatally acquired infection, the vast majority of toxoplasma retinochoroiditis seen by ophthalmologists is due to postnatal infection (Gilbert 2000a). Much higher rates of toxoplasma infection and ocular disease are seen in South America than in North America and Europe, and ocular manifestations are more severe in South America (Gilbert 2008).
Description of the intervention
The aim of treatment for toxoplasma retinochoroiditis is to reduce the risk of permanent visual impairment (by reducing the size of the retinochoroidal scar), the risk of recurrence (Rothova 1993b), and the severity and duration of acute symptoms. Antibiotics are usually given for six to eight weeks. Adjunctive steroid therapy may sometimes be used to reduce the duration and severity of acute symptoms due to intraocular inflammation. Infants with prenatal toxoplasmosis but without retinochoroiditis are often given prophylactic treatment for a year or more to reduce the risk of retinochoroiditis (Dunn 1999).
How the intervention might work
Antibiotic treatment is thought to eradicate the tachyzoite form of the parasite during the acute inflammatory phase. Antibiotics are not effective against the latent bradyzoite cyst form of the parasite.
Why it is important to do this review
There is uncertainty about the effectiveness of antibiotic treatment for toxoplasma retinochoroiditis (Holland 2004). As a result, the type of antibiotics prescribed varies (Engstrom 1991), and some clinicians do not treat patients with acute retinochoroiditis in the periphery of the retina (Rothova 1993b). In addition, the antibiotics used have adverse effects. We systematically reviewed the evidence for the effectiveness of antibiotic treatment for toxoplasma retinochoroiditis.
The protocol for this review was published on the Cochrane Library (Gilbert 2000b).
Objectives
To compare the effects of antibiotic treatment compared with placebo or no treatment for toxoplasma retinochoroiditis.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs).
Types of participants
We included studies involving participants of any age with retinochoroiditis likely to be due to toxoplasmosis. We included people treated for acute retinochoroiditis or those with healed scars who were treated prophylactically to prevent new lesions. We excluded any studies involving a majority of immunocompromised participants, as the presentation and clinical course of the disease differs to that in immunocompetent people.
Types of interventions
We included any antibiotic treatment known to be active against Toxoplasma gondii that was compared against placebo or no treatment. Antibiotic treatment could be given in any dose orally, by intramuscular injection, by intravenous infusion, or by intravitreal injection.
Types of outcome measures
Primary outcomes
We considered that treatment is given to prevent long‐term visual impairment, which can only be assessed after the acute inflammation has subsided. Our primary outcomes were therefore:
visual acuity or change in visual acuity (using any measure) at least three months after the start of treatment;
risk of one or more recurrences of retinochoroiditis at the end of follow‐up (of any duration).
Secondary outcomes
A secondary aim of treatment is to reduce the severity and duration of pain and visual loss due to acute inflammation. Our secondary outcomes were:
duration and severity of symptoms of visual impairment and ocular discomfort due to acute retinochoroiditis (any measure);
size of lesion at the end of follow‐up (any measure), as this affects the degree of long‐term visual impairment;
adverse events (any mentioned). Possible adverse events included decreased platelet or white blood cell count, gastrointestinal symptoms, rashes and other allergic phenomena.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision group Trials Register) (2016, Issue 1), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2016), EMBASE (January 1980 to February 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to February 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 22 February 2016. We searched the reference lists of identified articles and contacted pharmaceutical companies for unpublished trials.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6), and the ICTRP (Appendix 7).
Searching other resources
We also contacted the pharmaceutical companies that produce drugs licensed for the treatment of toxoplasmosis for any unpublished trials, and we scrutinised reference lists from review articles and published trials. We searched the abstracts from the conference proceedings of ARVO (Association for Research in Vision and Ophthalmology) (available from 1980 to 2001, with keywords from 1988) and reports of international symposia on uveitis using keywords 'antibiotic', 'choroiditis', 'retinochoroiditis', 'toxoplasma', 'toxoplasmic' and 'toxoplasmosis'.
Data collection and analysis
Selection of studies
Two review authors (Sarah See and Leanne Jones) independently reviewed titles and abstracts of all studies and retrieved potentially relevant studies in hard copy. Another two review authors (RG and MS) reviewed the hard copies against the inclusion criteria. Disagreements were resolved by consensus. We contacted the authors of trials that appeared to meet the inclusion criteria but did not report sufficient data.
For the update in 2016, EP and SB screened search results.
Data extraction and management
Two review authors (MS and RG) independently extracted relevant details about the design (randomised or quasi‐randomised, concealment of allocation, and masking of outcome assessment) and the results of each study.
For the update in 2016, EP and SB independently extracted data.
Assessment of risk of bias in included studies
We assessed risk of bias in five domains: selection bias, performance bias, detection bias, attrition bias, and selective reporting bias. We also commented on any other sources of bias. We classified the risk of bias according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions as low risk, high risk, or unclear (Higgins 2011).
Measures of treatment effect
We planned to compare the risk of long‐term visual impairment based on the most clinically relevant measure reported. For the other outcomes we summarised results in terms of the risk ratio of:
one or more recurrences in the treatment compared with no‐treatment group;
improvement compared with the same or worsening acute symptoms of visual impairment and ocular discomfort; and
adverse events.
Unit of analysis issues
All four included studies measured outcomes at the participant level, that is a single observation per person.
Dealing with missing data
We planned to conduct an intention‐to‐treat analysis using imputed data if computed by the trial investigators using an appropriate method. However, the included studies did not report intention‐to‐treat data, and so we have done an available‐case analysis, which assumes that data are missing at random. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up and reasons for loss to follow‐up by treatment group.
Assessment of reporting biases
We used the 'Risk of bias' assessment tool to look for selective or incomplete reporting. See Assessment of risk of bias in included studies.
If in future updates of this review 10 or more trials are included in a meta‐analysis, we will construct funnel plots and consider tests for asymmetry for assessment of publication bias, according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We used a random‐effects model to calculate summary measures, as eligible studies represented clinically varied populations of participants.
Subgroup analysis and investigation of heterogeneity
When data were available, we considered acute and recurrent retinochoroiditis separately.
Sensitivity analysis
We did not have enough data to do sensitivity analyses. In future updates of this review we will do a sensitivity analysis excluding studies at high risk of bias in one or more domains.
Summary of findings table
We prepared a 'Summary of findings' table presenting relative and absolute risks. We graded independently the overall quality of the evidence for each outcome using the GRADE classification (GRADEpro 2015). We included the following outcomes in the 'Summary of findings' table. This list of outcomes was not prespecified because the 'Summary of findings' table has only been prepared in this (2016) update.
Visual acuity
Recurrence of retinochoroiditis
Intraocular inflammation
Size of lesion
Adverse effects
Results
Description of studies
Results of the search
In the initial electronic search in 2000, we screened 152 studies and identified 10 that were potentially relevant. Three of these studies were not randomised controlled trials (Crespo 1993; Rothova 1993b; Theodossiadis 1989), a further three trials involved comparisons of different antibiotic regimens (Abreu 1988; Colin 1989; Jeddi 1997), and one trial compared steroids versus no steroids (Chodos 1961). Three studies met the inclusion criteria (Acers 1964; Perkins 1956; Silveira 2002).
Update searches
We updated the searches in December 2005 and screened 512 reports of trials, but did not find any new studies. We performed further updates in January 2008 and February 2011, identifying 211 and 289 study reports, respectively. The Cochrane Information Specialist (CIS) scanned the search results and removed any references that were not relevant to the scope of the review. Both search updates did not identify any references that met the inclusion criteria for the review.
Update searches run in February 2016 identified 834 new records (Figure 1). The CIS removed 128 duplicate records, screened the remaining 706 records, and removed 661 records that were not relevant to the scope of the review. We screened the remaining 45 records and discarded 44 reports as not relevant. We obtained one full‐text report (Felix 2014), which met the inclusion criteria for the review; see Characteristics of included studies for details.
1.
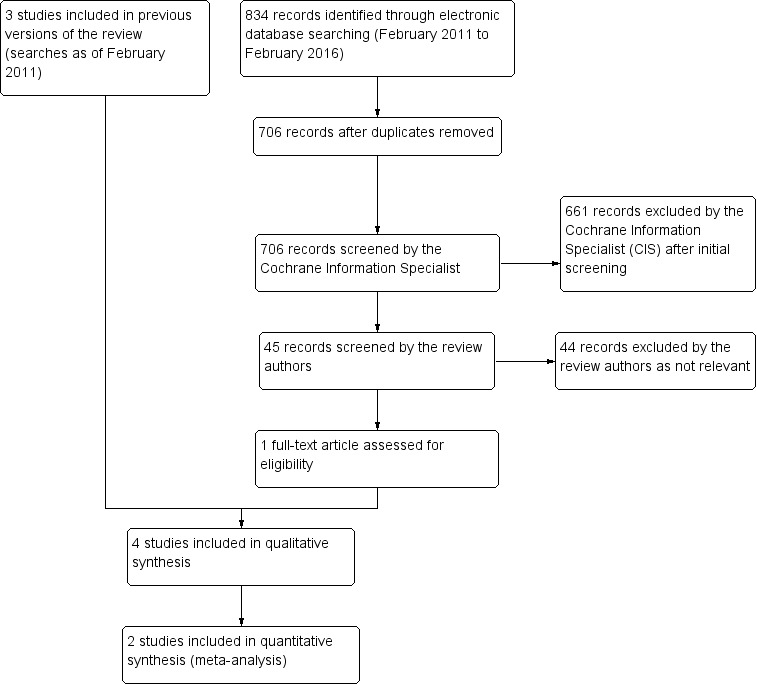
Study flow diagram.
Included studies
See the Characteristics of included studies table.
The type of participant and type and duration of treatment varied. Acers 1964 compared the effect of eight weeks of pyrimethamine‐trisulfapyrimidine versus lactose capsules in participants with acute toxoplasma retinochoroiditis. In contrast, Silveira 2002 determined the effect of long‐term (20 months) of prophylactic trimethoprim‐sulfamexacocol treatment compared with no treatment in participants with chronic relapsing toxoplasma retinochoroiditis. Felix 2014 evaluated the effect of 12 months of prophylactic treatment with trimethoprim‐sulphamethoxazole compared with placebo in participants with healed lesions of active recurrent toxoplasma retinochoroiditis. Perkins 1956 studied the effects of four weeks of pyrimethamine (Daraprim) compared with inert tablets in participants with acute uveitis due to any cause. He presented results for the subgroup with posterior uveitis that were toxoplasma antibody positive.
Felix 2014 reported on assessment of change in best corrected visual acuity over the 12‐month period as one of the primary outcomes. None of the other three studies reported the effect of treatment on vision. Three studies reported data on the primary outcome of recurrent retinochoroiditis, at 12 months (Felix 2014), at 14 and 17 months (Silveira 2002), and at two years (Acers 1964). Three studies, Acers 1964, Perkins 1956, and Silveira 2002, reported on changes in intraocular inflammation as assessed by the ophthalmologist. This outcome was regarded as a measure of the duration and severity of symptoms of acute inflammation, defined as a secondary outcome for this review. Two studies reported on adverse events (Acers 1964; Perkins 1956).
Excluded studies
See the Characteristics of excluded studies table for study details.
Risk of bias in included studies
2.
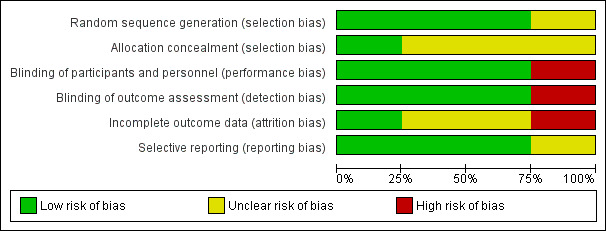
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
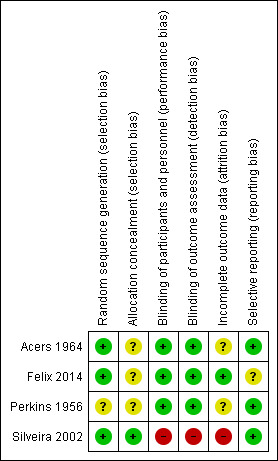
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
None of the four included studies made statements about allocation concealment. We assessed concealment of treatment allocation to be low risk of bias in the study by Felix 2014, as a nurse enrolled and assigned participants in the interventions in a masked fashion, and in Silveira 2002 'closed' envelopes were opened after eligibility criteria had been confirmed (unpublished data). Perkins 1956 allocated participants to tablets labelled A or B but did not mention how this allocation was concealed. Acers 1964 used a random listing but did not describe allocation concealment.
Blinding
We assessed performance and detection bias as low risk in the three studies that used inert tablets (Acers 1964; Felix 2014; Perkins 1956), although the similarity of these to the active treatment was not specified. Consequently, clinicians may have been able to deduce treatment allocation through scrutiny of the participant's treatment or by monitoring the results of blood counts (leukocyte and platelet depression is common with pyrimethamine treatment). Felix 2014 stated that participants were randomly assigned to group 1 or group 2 and received interventions in a masked fashion, while medical events were recorded monthly on a standardised form by a member of the medical staff in a masked fashion. Acers 1964 commented that the ophthalmologists who assessed intraocular inflammation at eight weeks were masked to treatment but not for the assessment of recurrence during the two years of the study. Perkins 1956 mentioned that the ophthalmic assessor was unaware of treatment allocation and usually unaware of the result for toxoplasma antibodies, but differences in the proportions of participants receiving pyrimethamine (Daraprim) in the dye test positive and negative groups raise the possibility of a lack of masked outcome measures and breaches in allocation concealment. We judged the study by Silveira 2002 to be at high risk of bias as participants were randomised to treatment or no treatment, and the clinician who entered them into the study carried out the follow‐up assessments.
Incomplete outcome data
We rated attrition bias in the study by Felix 2014 as low as outcomes were assessed over a 12‐month period during which there was a lost to follow‐up of one in each group. Completeness of follow‐up was unclear for two studies (Acers 1964; Perkins 1956). Acers 1964 assessed all participants at eight weeks' post‐treatment, but the length of follow‐up and number assessed for recurrence during the two years of the study were not stated. Perkins 1956 reported results for a subgroup of 29 participants with posterior uveitis and positive toxoplasma antibodies but did not state the numbers of participants randomised to each group. A further concern is that an unknown number of participants were excluded, but it is not stated at what point in the study these exclusions occurred. The study by Silveira 2002 provided unpublished data showing that 10% of participants were lost to follow‐up in both arms at 14 months, but this rose to 31% for treated and 21% for untreated participants by 17 months. We have therefore reported results for 14 months.
Selective reporting
We rated the risk in Felix 2014 as unclear as the study protocol was not available. We rated the other three studies as low risk for selective reporting bias (Acers 1964; Perkins 1956; Silveira 2002).
Other potential sources of bias
One further concern about the study by Perkins 1956 is that it is not clear whether the subgroup analyses according to site of uveitis and presence of toxoplasma antibodies were predefined. In addition, the four‐week follow‐up period was insufficient to detect recurrence, hence there was high risk of bias toward no effect (Perkins 1956).
Effects of interventions
See: Table 1
Primary outcomes
Visual acuity or change in visual acuity at least three months after the start of treatment
In Felix 2014, there were similar changes in best corrected visual acuity in the treatment and the placebo group at 12 months' follow‐up (mean difference ‐1.00 letters, 95% confidence interval (CI) ‐7.93 to 5.93; 93 participants) (Analysis 1.1).
1.1. Analysis.
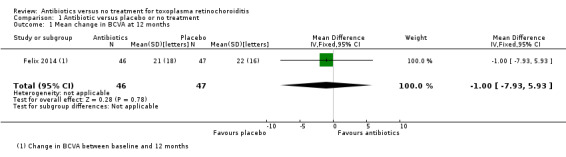
Comparison 1 Antibiotic versus placebo or no treatment, Outcome 1 Mean change in BCVA at 12 months.
None of the other studies provided results for long‐term visual outcomes.
One or more recurrences of retinochoroiditis at the end of follow‐up (of any duration)
The effect of treatment on recurrent retinochoroiditis was strongly influenced by Silveira 2002, who reported results for participants with relapsing retinochoroiditis treated for 14 months (risk ratio (RR) of recurrence 0.29, 95% CI 0.10 to 0.81). Silveira 2002 took place in Brazil, where the strain of Toxoplasma gondii is known to cause more frequent and more severe lesions than that seen in Europe or North America. In another study from Brazil, Felix 2014 also reported a lower risk of recurrences of retinochoroiditis with antibiotic treatment. In this study, treatment for 12 months showed RR of 0.08, 95% CI 0.00 to 1.3. Acers 1964 reported recurrent lesions in 1 out of 10 treated and 1 out of 10 untreated participants after two years' follow‐up (RR 1.00, 95% CI 0.07 to 13.87).
The results of pooling these three studies was a pooled RR of 0.26, 95% CI 0.11 to 0.63; 227 participants; 3 studies; I2 = 0% (Analysis 1.2). We considered acute and chronic retinochoroiditis separately, but there was little evidence for any difference in effect, however with only three studies the power of this analysis was low.
1.2. Analysis.
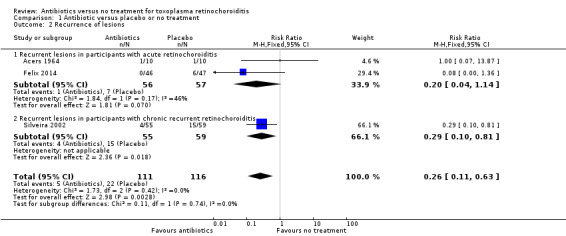
Comparison 1 Antibiotic versus placebo or no treatment, Outcome 2 Recurrence of lesions.
Secondary outcomes
Duration and severity of symptoms of visual impairment and ocular discomfort due to acute retinochoroiditis (any measure)
Two studies reported an improvement in intraocular inflammation in treated compared with untreated participants (Perkins 1956; Silveira 2002), and one study reported no difference (Acers 1964). Perkins 1956 reported improvement in 76% of treated and 50% of untreated participants (RR 1.76, 95% CI 0.98 to 3.19; Analysis 1.3). Silveira 2002 stated that the severity of inflammation was higher in the control group but did not provide further details. Acers 1964 stated that intraocular inflammation had almost completely resolved by eight weeks in all participants, and there was no difference in the time to quiescence of lesions between treatment groups. This was the only study where all participants received steroid treatment.
1.3. Analysis.
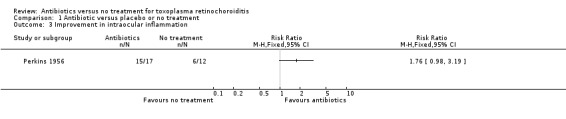
Comparison 1 Antibiotic versus placebo or no treatment, Outcome 3 Improvement in intraocular inflammation.
Size of lesion at the end of follow‐up (any measure)
This outcome was not reported.
Adverse events
Perkins 1956 reported adverse events for a group of uveitis participants in addition to those with toxoplasma retinochoroiditis. It was not clear whether this group included only randomised participants, and so we did not pool results. Of those participants treated with pyrimethamine (Daraprim), 53 out of 113 had a fall in haemoglobin of more than 5%, and 3 out of 113 had a fall in leukocyte count, compared with none of the 70 untreated participants. Acers 1964 reported adverse events (nausea, loss of appetite, rash, arthralgia, and stopping treatment due to low platelet count) in 3 out of 10 treated compared with 1 out of 10 untreated participants (RR 3.00, 95% CI 0.37 to 24.17).
Discussion
Summary of main results
See Table 1.
Four trials that randomised a total of 268 participants met the inclusion criteria. Two studies were conducted in Brazil, one in the UK, and one in the US. One study compared antibiotic to no treatment and was judged to be at high risk of performance, detection, and attrition bias. The other studies compared antibiotic treatment to placebo and were generally judged to be at a mixture of low or unclear risk of bias due to poor reporting. Only one study reported the effect of treatment on visual acuity and found a similar change in visual acuity between antibiotic‐ and placebo‐treated groups at one year. Three studies reported results for recurrent retinochoroiditis. There was a lower risk of recurrent retinochoroiditis with antibiotic treatment; similar results were seen for acute and chronic retinochoroiditis. Two studies reported an improvement in intraocular inflammation in treated compared with untreated participants, and one study reported no difference; it was not possible to pool these data. Two studies found an increased risk of adverse events in treated participants.
Overall completeness and applicability of evidence
Overall, with only four relatively small studies, we cannot consider the evidence adequate to inform policy; two of these studies were conducted nearly 40 years ago, and one study was available in abstract form only.
Two of the studies are from Brazil where the strain of Toxoplasma gondii is known to cause more frequent and more severe lesions, and so the results of this study may not apply to other parts of the world.
None of the studies considered newer antibiotics such as clindamycin or azithromycin (which have a lower risk of adverse events) with no treatment.
Quality of the evidence
We graded the quality of the evidence as moderate to low, downgrading for imprecision and risk of bias, depending on the outcome.
Agreements and disagreements with other studies or reviews
Other reviews have also found a lack of evidence to support routine antibiotic treatment for toxoplasma retinochoroiditis, but evidence that treatment reduces recurrence (Harrell 2014; Kim 2013).
We found weak evidence to suggest that treatment involving pyrimethamine increases the risk of adverse events. However, adverse effects of pyrimethamine are well established (BNF 2001).
Our review did not include any trials of intravitreal antibiotics because we did not find any RCTs comparing these with a control. We identified two studies that compared intravitreal antibiotics with oral antibiotics and therefore did not fall under the scope of this review (Baharivand 2013; Soheilian 2011).
Authors' conclusions
Implications for practice.
We found a lack of evidence to support routine antibiotic treatment for toxoplasma retinochoroiditis to prevent visual impairment. We found weak evidence that the risk of recurrence of retinochoroiditis is reduced after long‐term treatment with systemic antibiotics.
Implications for research.
Randomised placebo‐controlled trials are required to determine the effectiveness of antibiotic treatment for people with acute or chronic toxoplasma retinochoroiditis affecting any part of the retina. Studies should ensure masked assessment of long‐term visual impairment, recurrent retinochoroiditis and duration of symptoms and signs of acute inflammation. Low risk of adverse events should be an important factor in the choice of antibiotic for evaluation.
What's new
| Date | Event | Description |
|---|---|---|
| 22 February 2016 | New search has been performed | Electronic searches were updated |
| 22 February 2016 | New citation required but conclusions have not changed | One new trial met the inclusion criteria (Felix 2014) |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 27 June 2011 | New search has been performed | Issue 8, 2011: Updated searches yielded no new trials. 'Risk of bias' tables completed for included studies and new subheadings activated |
| 16 October 2008 | Amended | Converted to new review format |
| 23 January 2008 | New search has been performed | Updated search in January 2008 found no new trials |
| 14 November 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Cochrane Eyes and Vision created and ran the electronic searches. We thank Jennifer Evans and Anupa Shah from Cochrane Eyes and Vision for their support on this review update and the peer reviewers for helpful comments on a previous version of this review. We thank Leanne Jones, Sarah See, and Melissa Harden for their work on earlier versions of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 toxoplasm* and (uveitis or ocular or gondii or gondi or infection or congenital or retinitis or retinochoroiditis or retino‐choroiditis) #2 retinochoroiditis or choroidoretinitis or choroiditis or T gondi or T gondii or Retino‐choroiditis or Chorioretinal toxoplasmosis or Neuroretinitis or Chorioretinitis or pars‐planitis or scleritis or papillitis or uveitis #3 MeSH descriptor Toxoplasmosis, Ocular #4 MeSH descriptor Toxoplasmosis, Congenital #5 MeSH descriptor Uveitis #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 clindamycin or pyrimethamine or sulphadiazine or sulphadoxine or tetracycline or septrin or azithromycin or atovaquone or fluorocortolone or hydroxynaphthoquinone or lincomycin or trisulfapyrimidine or dapsone‐pyrimethamine or trimethoprim‐sulfamethoxazole or daraprim or trimetrexate or piritrexim or leucovorin or co‐trimoxazole or malocid‐sulfadiazine or malocid‐sulphadiazine or sulfadiazine or sulfadoxine or trisulphapyrimidine or trimethoprim‐sulphamethoxazole or Sulfisoxazole or Sulphisoxazole or ciprofloxacin or spiramycin or lincosaminide or roxithromycin or doxycycline or ribabutin or rovamycin or miocamycin or dirithromycin or erythromycin or erythromycin or macrolides or piritrexin or sulfonamides or sulphonamides or sulfamerazine or sulphamerazine or cotrimoxazole or minocycline or clarithromycin or nifurtimox‐pyrimethamine or aerosolized pentamidine or fansidar or clindamycin hydrochloride or cleocin #8 MeSH descriptor Clindamycin #9 MeSH descriptor Pyrimethamine #10 MeSH descriptor Tetracycline #11 MeSH descriptor Trimethoprim‐Sulfamethoxazole Combination #12 MeSH descriptor Lincomycin #13 MeSH descriptor Dapsone #14 MeSH descriptor Sulfadiazine #15 MeSH descriptor Trimetrexate #16 MeSH descriptor Leucovorin #17 MeSH descriptor Sulfadoxine #18 MeSH descriptor Sulfisoxazole #19 MeSH descriptor Ciprofloxacin #20 MeSH descriptor Spiramycin #21 MeSH descriptor Doxycycline #22 MeSH descriptor Miocamycin #23 MeSH descriptor Erythromycin #24 MeSH descriptor Macrolides #25 MeSH descriptor Sulfonamides #26 MeSH descriptor Sulfamerazine #27 MeSH descriptor Minocycline #28 MeSH descriptor Nifurtimox #29 MeSH descriptor Methotrexate #30 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29) #31 (#6 AND #30)
Appendix 2. MEDLINE search strategy
1 randomized controlled trial.pt. 2 (randomized or randomised).ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 exp animals/ 10 exp humans/ 11 9 not (9 and 10) 12 8 not 11 13 ((uveitis or ocular or gondii or gondi or infection or congenital or retinitis or retinochoroiditis or retino‐choroiditis) adj4 toxoplasm$).tw. 14 (retinochoroiditis or choroidoretinitis or choroiditis or T gondi or T gondii or Retino‐choroiditis or Chorioretinal toxoplasmosis or Neuroretinitis or Chorioretinitis or pars‐planitis or scleritis or papillitis or uveitis).tw. 15 exp Toxoplasmosis, Ocular/ 16 exp Toxoplasmosis, Congenital/ 17 exp uveitis/ 18 or/13‐17 19 (clindamycin or pyrimethamine or sulphadiazine or sulphadoxine or tetracycline or septrin or azithromycin or atovaquone or fluorocortolone or hydroxynaphthoquinone or lincomycin or trisulfapyrimidine or dapsone‐pyrimethamine or trimethoprim‐sulfamethoxazole or daraprim or trimetrexate or piritrexim or leucovorin or co‐trimoxazole or malocid‐sulfadiazine or malocid‐sulphadiazine or sulfadiazine or sulfadoxine or trisulphapyrimidine or trimethoprim‐sulphamethoxazole or Sulfisoxazole or Sulphisoxazole or ciprofloxacin or spiramycin or lincosaminide or roxithromycin or doxycycline or ribabutin or rovamycin or miocamycin or dirithromycin or erythromycin or erythromycin or macrolides or piritrexin or sulfonamides or sulphonamides or sulfamerazine or sulphamerazine or cotrimoxazole or minocycline or clarithromycin or nifurtimox‐pyrimethamine or aerosolized pentamidine or fansidar or clindamycin hydrochloride or cleocin).tw. 20 exp Clindamycin/ 21 exp Pyrimethamine/ 22 exp Tetracycline/ 23 exp Trimethoprim‐Sulfamethoxazole‐Combination/ 24 exp Lincomycin/ 25 exp Dapsone/ 26 exp Sulfadiazine/ 27 exp Trimetrexate/ 28 exp Leucovorin/ 29 exp Sulfadoxine/ 30 exp Sulfisoxazole/ 31 exp Ciprofloxacin/ 32 exp Spiramycin/ 33 exp Doxycycline/ 34 exp Miocamycin/ 35 exp Erythromycin/ 36 exp Macrolides/ 37 exp Sulfonamides/ 38 exp Sulfamerazine/ 39 exp Minocycline/ 40 exp Nifurtimox/ 41 exp Methotrexate/ 42 or/19‐41 43 18 and 41 44 12 and 43
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE search strategy
1 exp randomized controlled trial/ 2 exp randomization/ 3 exp double blind procedure/ 4 exp single blind procedure/ 5 random$.tw. 6 or/1‐5 7 (animal or animal experiment).sh. 8 human.sh. 9 7 and 8 10 7 not 9 11 6 not 10 12 exp clinical trial/ 13 (clin$ adj3 trial$).tw. 14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15 exp placebo/ 16 placebo$.tw. 17 random$.tw. 18 exp experimental design/ 19 exp crossover procedure/ 20 exp control group/ 21 exp latin square design/ 22 or/12‐21 23 22 not 10 24 23 not 11 25 exp comparative study/ 26 exp evaluation/ 27 exp prospective study/ 28 (control$ or prospectiv$ or volunteer$).tw. 29 or/25‐28 30 29 not 10 31 30 not (11 or 23) 32 11 or 24 or 31 33 ((uveitis or ocular or gondii or gondi or infection or congenital or retinitis or retinochoroiditis or retino‐choroiditis) adj4 toxoplasm$).tw. 34 (retinochoroiditis or choroidoretinitis or choroiditis or T gondi or T gondii or Retino‐choroiditis or Chorioretinal toxoplasmosis or Neuroretinitis or Chorioretinitis or pars‐planitis or scleritis or papillitis or uveitis).tw. 35 exp Toxoplasmosis/ 36 exp Congenital Toxoplasmosis/ 37 exp uveitis/ 38 or/33‐37 39 (clindamycin or pyrimethamine or sulphadiazine or sulphadoxine or tetracycline or septrin or azithromycin or atovaquone or fluorocortolone or hydroxynaphthoquinone or lincomycin or trisulfapyrimidine or dapsone‐pyrimethamine or trimethoprim‐sulfamethoxazole or daraprim or trimetrexate or piritrexim or leucovorin or co‐trimoxazole or malocid‐sulfadiazine or malocid‐sulphadiazine or sulfadiazine or sulfadoxine or trisulphapyrimidine or trimethoprim‐sulphamethoxazole or Sulfisoxazole or Sulphisoxazole or ciprofloxacin or spiramycin or lincosaminide or roxithromycin or doxycycline or ribabutin or rovamycin or miocamycin or dirithromycin or erythromycin or erythromycin or macrolides or piritrexin or sulfonamides or sulphonamides or sulfamerazine or sulphamerazine or cotrimoxazole or minocycline or clarithromycin or nifurtimox‐pyrimethamine or aerosolized pentamidine or fansidar or clindamycin hydrochloride or cleocin).tw. 40 exp Clindamycin/ 41 exp Pyrimethamine/ 42 exp Tetracycline/ 43 exp Sulfadoxine Trimethoprim/ 44 exp Lincomycin/ 45 exp Dapsone/ 46 exp Sulfadiazine/ 47 exp Trimetrexate/ 48 exp folinic acid/ 49 exp Sulfadoxine/ 50 exp Sulfisoxazole/ 51 exp Ciprofloxacin/ 52 exp Spiramycin/ 53 exp Doxycycline/ 54 exp Miokamycin/ 55 exp Erythromycin/ 56 exp Macrolide/ 57 exp Sulfonamide/ 58 exp Sulfamerazine/ 59 exp Minocycline/ 60 exp Nifurtimox/ 61 exp Methotrexate/ 62 or/39‐61 63 38 and 61 64 32 and 63
Appendix 4. LILACS search strategy
Toxoplasm$ or Coroidoretinit$ or Coroidit$ or Choroiditis or Retinitis or Retinite or Retinites or T‐gondi or T‐gondii or Gondi or gondii or Neuroretinit$ or Neuro or retinit$ or Pars‐planitis or Parsplanitis or Planitis or Scleritis or Esclerit$ or Papillitis or Papilit$ or Uveitis or Uveit$ and antibiotic$ or clindamycin or pyrimethamine or sulphadiazine or sulphadoxine or tetracycline or septrin or azithromycin or atovaquone or fluorocortolone or hydroxynaphthoquinone or lincomycin or trisulfapyrimidine or dapsone‐pyrimethamine or trimethoprim‐sulfamethoxazole or daraprim or trimetrexate or piritrexim or leucovorin or co‐trimoxazole or malocid‐sulfadiazine or malocid‐sulphadiazine or sulfadiazine or sulfadoxine or trisulphapyrimidine or trimethoprim‐sulphamethoxazole or Sulfisoxazole or Sulphisoxazole or ciprofloxacin or spiramycin or lincosaminide or roxithromycin or doxycycline or ribabutin or rovamycin or miocamycin or dirithromycin or erythromycin or erythromycin or macrolides or piritrexin or sulfonamides or sulphonamides or sulfamerazine or sulphamerazine or cotrimoxazole or minocycline or clarithromycin or nifurtimox‐pyrimethamine or aerosolized pentamidine or fansidar or clindamycin hydrochloride or cleocin
Appendix 5. ISRCTN search strategy
toxoplasmosis or retinochoroiditis
Appendix 6. ClinicalTrials.gov search strategy
Toxoplasmosis OR retinochoroiditis
Appendix 7. ICTRP search strategy
toxoplasmosis or retinochoroiditis
Data and analyses
Comparison 1. Antibiotic versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA at 12 months | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.93, 5.93] |
| 2 Recurrence of lesions | 3 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.63] |
| 2.1 Recurrent lesions in participants with acute retinochoroiditis | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 1.14] |
| 2.2 Recurrent lesions in participants with chronic recurrent retinochoroiditis | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.81] |
| 3 Improvement in intraocular inflammation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.4. Analysis.

Comparison 1 Antibiotic versus placebo or no treatment, Outcome 4 Adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acers 1964.
| Methods | Participants allocated by a "random listing" It was not clear whether 1 or both eyes were included |
|
| Participants | Participants (n = 20) residing in Baltimore, USA with active retinitis, positive toxoplasma skin test and antibody test (dye test), and no other cause of retinitis. Mean age 32.9 years (treatment), 30.9 years (controls) (age range 18 to 49 years) | |
| Interventions | Pyrimethamine (200 mg/day 1, 100 mg/day 2, 50 mg/day on days 3 to 15, 25 mg/day on days 16 to 56); trisulfapyrimidine 2 g/day for 8 weeks; prednisolone (40 mg/day on days 1 to 7, 20 mg/day on days 8 to 56) versus lactose capsules and prednisolone as above | |
| Outcomes |
Outcomes assessed masked to treatment allocation at 8 weeks but not thereafter. Follow‐up complete at 8 weeks but not stated for the 2 years of the study |
|
| Notes | Date study conducted: Not reported Funding source: Training Grant No. 2B‐5217, from the National Institute of Neurological Diseases and Blindness, US Public Health Service Declaration of interest: not reported Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random listing |
| Allocation concealment (selection bias) | Unclear risk | No relevant statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A lactose capsule was used as a placebo: “placed in identical bottles.” Masking of ocular outcomes from clinical data on side effects |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Separate, independent assessors were used for clinical signs and for ophthalmic examination. The ophthalmic assessor “did not inquire about, or obtain, any subjective information or laboratory data.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up for 8 weeks, but this would not be adequate for detecting recurrence. Completeness of follow‐up over the entire 2‐year study period unclear |
| Selective reporting (reporting bias) | Low risk | Recurrence was a secondary outcome, although no details are given about the completeness of follow‐up for this outcome |
Felix 2014.
| Methods | Participants randomly assigned. Randomisation 1:1, stratified by sex and block sizes of 4. It was not clear whether 1 or both eyes were included |
|
| Participants | Participants (n = 95) > 18 years of age, from public hospital in Campinas, Brazil who had healed lesions on completion of treatment with a tablet of trimethoprim‐sulfamethoxazole (800 mg/160 mg) 2 times daily for 45 days for active recurrent Toxoplasma gondii retinochoroiditis (defined as a new focus of necrotising retinochoroiditis with active inflammation either adjacent to or remote from pre‐existing retinochoroidal scars, with positive immunoglobulin G for toxoplasmosis). Mean age, male/female ratio, and baseline BCVA: 34 years, 20/27, 20/80 (treatment); 33 years, 22/26, 20/100 (controls) | |
| Interventions | 1 trimethoprim‐sulfamethoxazole tablet (800 mg/160 mg) every 2 days (treatment) and 1 identical placebo tablet containing starch every 2 days (control) | |
| Outcomes |
Outcomes assessed for 12‐month period only. Recurrences beyond 12 months not assessed |
|
| Notes | Date study conducted: 24 August 2011 to 28 August 2012 Funding source: Fundac¸a˜o de Amparo a Pesquisa do Estado de Sa˜o Paulo, protocol 2010/15980‐2. Declaration of interest: not reported Trial registration: Influence of trimethoprim‐sulfamethoxazole for the recurrence of ocular toxoplasmosis; clinicaltrials.gov identifier: NCT01449877; http://clinicaltrials.gov/show/NCT01449877 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was 1:1 and was stratified by sex, and block sizes of 4 were used |
| Allocation concealment (selection bias) | Unclear risk | Nurse enrolled and assigned participants in the interventions in a masked fashion |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were randomly assigned to group 1 or group 2 and received interventions in a masked fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medical events were recorded monthly on a standardised form by a member of the medical staff in a masked fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 lost to follow‐up in each group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. Primary outcome, incidence of recurrences of retinochoroiditis, and secondary outcome, changes in BCVA, were well explained in report |
Perkins 1956.
| Methods | Allocation of tablets labelled A or B according to random list. It was not clear whether 1 or both eyes were included |
|
| Participants | Uveitis participants (n = 164) enrolled at first attendance at uveitis clinic, London, UK. Subgroup analyses presented for participants with a positive toxoplasma antibody dye test and posterior uveitis (n = 29). Varying age group from (1 to 19) years to 60+ | |
| Interventions | Pyrimethamine (Daraprim) 25 mg daily for 4 weeks versus inert tablet | |
| Outcomes | No improvement versus improvement in signs of intraocular inflammation at 4 weeks (in 29 people with posterior uveitis and toxoplasma antibodies). Adverse events: depressed leucocyte count (for all uveitis participants only; n = 113 treated and 70 untreated). Ophthalmic assessor unaware of treatment allocation and usually unaware of dye test result. Proportion receiving pyrimethamine (Daraprim) in dye test positive versus negative groups differs, raising the possibility of breaches of allocation concealment |
|
| Notes | Date study conducted: Not reported. Funding source: Not reported. Declaration of interest: Not reported. Trial registration: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...distributed according to a random list.” |
| Allocation concealment (selection bias) | Unclear risk | No statement relevant to allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The clinician in charge of the patient does not know which of the two tablets the patient has received.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The person making the assessment did not know whether the patient had received Daraprim or the inert tablets.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “Some patients had to be excluded because of difficulty in follow‐up or interruption of treatment by intercurrent illness.” These participants do not appear to have been counted in the results |
| Selective reporting (reporting bias) | Low risk | The only outcome report was improvement in symptoms |
Silveira 2002.
| Methods | Randomised using sealed envelopes (information from authors). Participant could have retinochoroidal lesion in 1 or both eyes |
|
| Participants | Participants with chronic recurrent toxoplasma retinochoroiditis, south Brazil (treated n = 61 (28 unilateral and 33 bilateral disease) (age range 8 to 50 years); untreated n = 63 (35 unilateral and 28 bilateral disease) (age range 7 to 53 years)) | |
| Interventions | Trimethoprim 160 mg and sulfamexacocol 800 mg, both orally every 3 days for 20 months versus no treatment | |
| Outcomes |
Published paper does not report losses to follow‐up; we obtained this information from authors. At 14 months, 6 lost in treatment group, 4 lost in control group. At 17 months, 19 lost in treatment group and 13 in control group. No data given on visual acuity |
|
| Notes | Date study conducted: April 1998 to not reported Funding source: Conselho Nacional de Desenvolvimento Cientı´fı´co e Tecnolo´gico (CNPq), Coordenac¸a˜o de Aperfeic¸oamento de Pessoal de Nı´vel Superior (CAPES), Clı´nica Silveira, and Fundac¸a˜o de Amparo a` Pesquisa do Estado de Sa˜o Paulo (FAPESP) Declaration of interest: Not reported. Trial registration: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer generated randomisation list.” |
| Allocation concealment (selection bias) | Low risk | No relevant statement in published study, but the authors stated that closed envelopes were opened only after eligibility criteria were confirmed (unpublished data) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Medication was administered in an unmasked fashion.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masking, and participants followed up only by the clinician responsible for the trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10% lost to follow‐up by 14 months. Slightly higher losses in treatment group (6/61 versus 4/63), possibly reflecting a need to keep attending in the hope of eventually being given antibiotics. No prespecified duration of trial, as lesion recurrence was the criterion for ceasing follow‐up |
| Selective reporting (reporting bias) | Low risk | Recurrence was primary outcome and endpoint for follow‐up |
BCVA: best corrected visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abreu 1988 | Prospective randomised double‐masked study of clindamycin versus spiramycin in people with ocular toxoplasmosis (total studied = 24). Abstract only |
| Chodos 1961 | Compares participants with acute, active posterior retinochoroidal lesions who were given corticosteroids (n = 45) with a control group given no corticosteroids (n = 22). All participants were treated with spiramycin. Participants do not appear to have been randomised |
| Colin 1989 | Participants with toxoplasma retinochoroiditis were randomised to oral pyrimethamine and sulfadiazine (n = 15) versus subconjunctival clindamycin (n = 14) |
| Crespo 1993 | Not a randomised controlled trial. Participants with ocular toxoplasmosis were divided into 2 groups and treated with pyrimethamine and sulfadiazine (n = 36) or clindamycin and sulfadiazine (n = 34) |
| Jeddi 1997 | Participants with unilateral toxoplasma retinochoroiditis were randomised to clindamycin administered subconjunctivally (n = 26) or to oral malocid‐sulfadiazine (n = 17) |
| Rothova 1993b | Not a randomised controlled trial. The study compares cohorts of participants with posterior pole lesions treated according to the antibiotic regimens used in six centres (n = 108). A total of 41 participants in all 6 centres had peripheral lesions and received no treatment |
| Theodossiadis 1989 | Unclear whether participants were randomised. Participants treated with a variety of medications, including pyrimethamine, sulfadiazine, clindamycin, and cortisone (n = 15) were compared with those given laser treatment (n = 18) |
Differences between protocol and review
We have included a 'Summary of findings' table in the current (2016) update of this review.
We included 'intravitreal' in the routes of administration of the antibiotic, but did not identify any trials versus placebo or no treatment for this route of administration.
Contributions of authors
2016 update EP: evaluated studies for inclusion and commented on drafts of the report SB: assisted in collating the search results, evaluated studies for inclusion, worked on the 'Summary of findings' table, wrote the Plain language summary and draft of the update report RG: reviewed the edited draft and is the guarantor for the review MS: commented on the update draft of the report
Sources of support
Internal sources
Centre for Paediatric Epidemiology and Biostatistics, Institute of Child Health, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
EP: None known. SB: None known. RG: None known. MS: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Acers 1964 {published data only}
- Acers TE. Toxoplasmic retinochoroiditis: a double‐blind therapeutic study. Archives of Ophthalmology 1964;71:58‐62. [DOI] [PubMed] [Google Scholar]
Felix 2014 {published data only}
- Felix JP, Lira RP, Zacchia RS, Toribio JM, Nascimento MA, Arieta CE. Trimethoprim‐sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: randomized controlled clinical trial. American Journal of Ophthalmology 2014;157(4):762‐6. [DOI] [PubMed] [Google Scholar]
Perkins 1956 {published data only}
- Perkins ES, Smith CH, Schofield PB. Treatment of uveitis with pyrimethamine (Daraprim). British Journal of Ophthalmology 1956;40:577‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silveira 2002 {published and unpublished data}
- Silveira C, Belfort R, Muccioli C, Holland GN, Victora CG, Horta BL, et al. The effect of long‐term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. American Journal of Ophthalmology 2002;134:41‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abreu 1988 {published data only}
- Abreu MT, Martins MC, Belfort R. Clindamycin versus spiramycin in ocular toxoplasmosis: a randomised double masked study. Arquivos Brasileiros de Oftalmologia 1988;51(2):26. [Google Scholar]
Chodos 1961 {published data only}
- Chodos JB, Habegger‐Chodos HE. The treatment of ocular toxoplasmosis with spiramycin. Archives of Ophthalmology 1961;65:109‐17. [DOI] [PubMed] [Google Scholar]
Colin 1989 {published data only}
- Colin J, Harie JC. Presumed toxoplasmic retinochoroiditis: therapeutic comparative study of the association between pyrimethamine with sulfadiazine and clindamycin [Chorioretinites presumees toxoplasmiques: etude comparative des traitements par pyrimethamine et sulfadiazine ou clindamycine]. Journal Francais d' Ophtalmologie 1989;12(3):161‐5. [PubMed] [Google Scholar]
Crespo 1993 {published data only}
- Crespo EJM, Bonfante‐Garrido R, Rodriguez RR. Comparative study of 2 therapeutic schernes in 70 patients with ocular toxoplasmosis [Estudio comparativo de dos esquemas terapeuticos en 70 pacientes con toxoplasmosis ocular]. Gaceta Medica de Caracas 1993;101(3):238‐44. [Google Scholar]
Jeddi 1997 {published data only}
- Jeddi A, Azaiez A, Bouguila H, Kaoueche S, Malouche S, Daghfous F, et al. Advantage of clindamycine in the treatment of ocular toxoplasmosis [Interet de la clindamycine dans le traitement de la toxoplasmose oculaire]. Journal Francais d' Ophtalmologie 1997;20(6):418‐22. [PubMed] [Google Scholar]
Rothova 1993b {published data only}
- Rothova A, Buitenhuis HJ, Meenken C, Baarsma GS, Boen‐Tan TN, Jong PT, et al. Therapy of ocular toxoplasmosis. International Ophthalmology 1989;13(6):415‐9. [DOI] [PubMed] [Google Scholar]
- Rothova A, Meenken C, Buitenhuis HJ, Brinkman CJ, Baarsma GS, Boen‐Tan TN, et al. Therapy for ocular toxoplasmosis. American Journal of Ophthalmology 1993;115(4):517‐23. [DOI] [PubMed] [Google Scholar]
Theodossiadis 1989 {published data only}
- Theodossiadis GP, Koutsandrea C, Tzonou A. A comparative study concerning the treatment of active toxoplasmic retinochoroiditis with argon laser and medication (follow‐up 2‐9 years). Ophthalmologica 1989;199(2‐3):77‐83. [DOI] [PubMed] [Google Scholar]
Additional references
Baharivand 2013
- Baharivand N, Mahdavifard A, Fouladi RF. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. International Ophthalmology 2013;33(1):39‐46. [DOI] [PubMed] [Google Scholar]
BNF 2001
- Mehta D. British National Formulary. March. Vol. 41, London: British Medical Association and the Royal Pharmaceutical Society of Great Britain, 2001. [Google Scholar]
Burnett 1998
- Burnett AJ, Shortt SG, Isaac‐Renton J, King A, Werker D, Bowie WR. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology 1998;105(6):1032‐7. [DOI] [PubMed] [Google Scholar]
Dunn 1999
- Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. Mother‐to‐child transmission of toxoplasmosis: risk estimates for clinical counselling. The Lancet 1999;353(9167):1829‐33. [DOI] [PubMed] [Google Scholar]
Engstrom 1991
- Engstrom RE, Holland GN. Current practices in the management of ocular toxoplasmosis. American Journal of Ophthalmology 1991;111(5):601‐10. [DOI] [PubMed] [Google Scholar]
Gilbert 1999
- Gilbert RE, Dunn D, Lightman S, Murray PI, Pavesio C, Gormley P, et al. Incidence of symptomatic toxoplasma eye disease: aetiology and public health implications. Epidemiology and Infection 1999;123(283):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2000a
- Gilbert RE, Stanford MS. Is ocular toxoplasmosis caused by prenatal or postnatal infection. British Journal of Ophthalmology 2000;84(2):224‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2008
- Gilbert RE, Freeman K, Lago EG, Bahia‐Oliveira LMG, Tan HK, Wallon M, et al. Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Neglected Tropical Diseases 2008;2(8):e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015
- GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime Inc). Available from www.gradepro.org.
Guerina 1994
- Guerina NG, Hsu HW, Meissner HC, Maguire JH, Lynfield R, Stechenberg B, et al. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. The New England Journal of Medicine 1994;330(26):1858‐63. [DOI] [PubMed] [Google Scholar]
Harrell 2014
- Harrell M, Carvounis PE. Current treatment of toxoplasma retinochoroiditis: an evidence‐based review. Journal of Ophthalmology 2014;2014:Article ID 273506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). In: Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holland 2003
- Holland GN. LX Edward Jackson Memorial Lecture: Ocular toxoplasmosis: a global reassessment. Part 1: epidemiology and course of disease. American Journal of Ophthalmology 2003;136(6):973‐88. [DOI] [PubMed] [Google Scholar]
Holland 2004
- Holland GN. LX Edward Jackson Memorial Lecture: Ocular toxoplasmosis: a global reassessment. Part 2: disease manifestations and management. American Journal of Ophthalmology 2004;137(1):1‐17. [PubMed] [Google Scholar]
Kim 2013
- Kim SJ, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS, et al. Interventions for toxoplasma retinochoroiditis: a report by the American Academy of Ophthalmology. Ophthalmology 2013;120(2):371‐8. [DOI] [PubMed] [Google Scholar]
Koppe 1986
- Koppe JG, Loewer Sieger DH, Roever Bonnet H. Results of 20‐year follow‐up of congenital toxoplasmosis. The Lancet 1986;1(8475):254‐6. [DOI] [PubMed] [Google Scholar]
Lebech 1999
- Lebech M, Andersen O, Christensen NC, Hertel J, Nielsen HE, Peitersen B, et al. Feasibility of neonatal screening for toxoplasma infection in the absence of prenatal treatment. Danish Congenital Toxoplasmosis Study Group. The Lancet 1999;353(9167):1834‐7. [DOI] [PubMed] [Google Scholar]
Perkins 1973
- Perkins ES. Ocular toxoplasmosis. British Journal of Ophthalmology 1973;57(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1999
- Roberts F, McLeod R. Pathogenesis of toxoplasmic retinochoroiditis. Parasitology Today 1999;15(2):51‐7. [DOI] [PubMed] [Google Scholar]
Rothova 1993a
- Rothova A. Ocular involvement in toxoplasmosis. British Journal of Ophthalmology 1993;77(6):371‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soheilian 2011
- Soheilian M, Ramezani A, Azimzadeh A, Sadoughi MM, Dehghan MH, Shahghadami R, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 2011;118(1):134‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gilbert 2000b
- Gilbert RE, See SE, Jones LV, Stanford MS, Tufail A. Antibiotics for treating and preventing toxoplasma retinochoroiditis. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002218] [DOI] [PubMed] [Google Scholar]
Gilbert 2002
- Gilbert RE, Harden M, Stanford M. Antibiotics versus control for toxoplasma retinochoroiditis. Cochrane Database of Systematic Reviews 1, Issue 2002. [DOI: 10.1002/14651858.CD002218] [DOI] [PubMed] [Google Scholar]


