Abstract
Background
Spontaneous bacterial peritonitis is a complication of cirrhotic ascites that occurs in the absence of any intra‐abdominal, surgically treatable source of infection. Antibiotic therapy is indicated and should be initiated as soon as possible to avoid severe complications that may lead to death. It has been proposed that empirical treatment should cover gram‐negative enteric bacteria and gram‐positive cocci, responsible for up to 90% of spontaneous bacterial peritonitis cases.
Objectives
This review aims to evaluate the beneficial and harmful effects of different types and modes of antibiotic therapy in the treatment of spontaneous bacterial peritonitis in cirrhotic patients.
Search methods
We performed electronic searches in The Cochrane Hepato‐Biliary Group Controlled Trials Register (July 2008), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 3, 2008), MEDLINE (1950 to July 2008), EMBASE (1980 to July 2008), and Science Citation Index EXPANDED (1945 to July 2008). In addition, we handsearched the references of all identified studies and contacted the first author of each included trial.
Selection criteria
Randomised studies comparing different types of antibiotics for spontaneous bacterial peritonitis in cirrhotic patients.
Data collection and analysis
Data were independently extracted from the trials by at least two authors. Peto odds ratios or average differences, with their 95% confidence intervals, were estimated.
Main results
This systematic review attempted to summarise evidence from randomised clinical trials on the treatment of spontaneous bacterial peritonitis. Thirteen studies were included; each one of them compared different antibiotics in their experimental and control groups. No meta‐analyses could be performed, though data on the main outcomes were collected and analysed separately for each included trial. Currently, the evidence showing that lower dosage or short‐term treatment with third generation cephalosporins is as effective as higher dosage or long‐term treatment is weak. Oral quinolones could be considered an option for those with less severe manifestations of the disease.
Authors' conclusions
This review provides no clear evidence for the treatment of cirrhotic patients with spontaneous bacterial peritonitis. In practice, third generation cephalosporins have already been established as the standard treatment of spontaneous bacterial peritonitis, and it is clear, that empirical antibiotic therapy should be provided in any case. However, until large, well‐conducted trials provide more information, practice will remain based on impression, not evidence.
Keywords: Humans, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Ascites, Ascites/complications, Bacterial Infections, Bacterial Infections/drug therapy, Bacterial Infections/mortality, Liver Cirrhosis, Liver Cirrhosis/complications, Peritonitis, Peritonitis/drug therapy, Peritonitis/mortality, Randomized Controlled Trials as Topic
Plain language summary
Antibiotics for spontaneous bacterial peritonitis in cirrhotic patients
Cirrhosis is a severe end‐stage liver disease marked by irreversible scarring of liver tissue. Ascites (the accumulation of fluid in the abdomen), is one of the many complications associated with cirrhosis. Ascites is associated with poor quality of life, increased risk of infection, and renal failure. The presence of ascites is a sign of poor prognosis. Spontaneous bacterial peritonitis (inflammation and infection of the membrane that is lining the abdominal cavity) is a complication of cirrhotic ascites that occurs in the absence of any intra‐abdominal, surgically treatable source of infection. Antibiotic therapy is indicated and should be initiated as soon as possible to avoid severe complications that may lead to death. This review aimed to evaluate the beneficial and harmful effects of different types and modes of antibiotic therapy in the treatment of spontaneous bacterial peritonitis in cirrhotic patients. Thirteen trials were included; each one of them compared different antibiotics in their experimental and control groups. No meta‐analyses could be performed, though data on the main outcomes were collected and analysed separately for each included trial. Based on the identified evidence, we cannot suggest the most appropriate management to treat spontaneous bacterial peritonitis in regard to the type, dosage, duration, or administration route of the antibiotic therapy. The clinical trials found dealt with different types of antibiotics, and, therefore, could not be combined. This review found no evidence that the effect or safety of one antibiotic is more beneficial than another. Further randomised clinical trials with an adequate design, including a large number of participants and sufficient duration should be carefully planned to provide a more precise estimate of the beneficial and harmful effects of antibiotic treatment for spontaneous bacterial peritonitis.
Background
Ascites is the most common major complication of cirrhosis; it is associated with poor quality of life, increased risk of infection, and renal failure. Ascites is also a poor prognostic sign. Characteristically, it develops during late stages of the disease and indicates disturbances in the water and sodium retention, arterial dysfunction, and portal hypertension (Leiva 2007).
Spontaneous bacterial peritonitis is a frequent complication of ascites in patients with cirrhosis. It is secondary to impaired humoral and cellular immune responses and to the ascitic fluid acting as culture medium for several bacterial agents (Chavez‐Tapia 2007).
The diagnosis of spontaneous bacterial peritonitis is based on the polymorphonuclear (PMN) cell count in ascitic fluid. A PMN count of more than 250/mm3 is highly suspicious of spontaneous bacterial peritonitis and constitutes an indication to initiate empiric antibiotic treatment (in patients with haemorrhagic ascites (ascites red blood cells count > 10 000/mm3), a subtraction of one PMN per 250 red blood cells should be made to adjust for the presence of blood in ascites). A diagnosis of spontaneous bacterial peritonitis established only on the basis of symptoms and signs is less reliable. Bacterascites refers to the colonization of ascitic fluid by bacteria in the absence of an inflammatory reaction in the peritoneal fluid. Therefore, the diagnosis of bacterascites is currently made when 1) there is a positive ascitic fluid culture in the setting of an ascitic fluid PMN count <250/mm3 or 2) there is secondary peritonitis (Rimola 2000).
Several risk factors have been associated with spontaneous bacterial peritonitis. The most important are a) low serum sodium level, b) low ascitic fluid total protein (Kaymakoglu 1997), and the c) Model for End‐Stage Liver Disease score (Obstein 2007).
The incidence varies widely and depends on the clinical setting. For example in cirrhotic outpatients undergoing large‐volume paracentesis, the prevalence varies from 0% to 0.5% (Castellote 2008). On other hand in patients admitted to a liver units, the prevalence reaches 16.3% (Fasolato 2007), with a mortality rate of 10% to 32.6% (Thuluvath 2001; Thanapoulou 2002).
Antibiotic treatment should be started as soon as the diagnosis is made (based on the PMN count in the ascites). The most commonly observed agents are Escherichia coli, Klebsiella, Staphylococcus aureus, Enterococcus faecalis, and Streptococcus pneumonia (Francés 2008).
The recommended and most commonly used antibiotics are third‐generation cephalosporins, the most commonly used agent of this class of antibiotics is cefotaxime, although other agents like ceftriaxone and ceftazidime have similar efficacy. Patients on antibiotic prophylaxis have a higher chance of being infected by a Gram‐positive micro‐organism. An important recent finding is that intravenous administration of albumin to patients with spontaneous bacterial peritonitis reduces the risk of complications, such as hepatorenal syndrome, and may significantly improve survival (Kuiper 2007).
Objectives
To evaluate the beneficial and harmful effects of different types of antibiotic therapy in the treatment of spontaneous bacterial peritonitis in cirrhotic patients.
To estimate the mortality and frequency of spontaneous bacterial peritonitis recurrence.
To assess the frequency of adverse effects associated with different types of antibiotic therapy.
Methods
Criteria for considering studies for this review
Types of studies
We attempted to identify any randomised clinical trial comparing different types of antibiotic therapy, regardless of dose, route of administration or schedule, for the treatment of spontaneous bacterial peritonitis in cirrhotic patients. The studies were included regardless of language and publication status.
Types of participants
Cirrhotic patients, adults, irrespective of sex or nationality, who developed an infection of the ascitic fluid in the absence of another local source of infection, and received antibiotic therapy.
Types of interventions
We considered the interventions given below no matter whether they had been used as a single intervention or in combination. For the control group, we considered placebo or no intervention, or any of the following antibiotics:
Intravenous antibiotic therapy
Aminoglycoside (gentamicin, tobramycin);
Beta‐lactam (ampicillin, cephalotin);
Third generation cephalosporin (cefotaxime, ceftriaxone, cefonicid);
Amoxycillin + clavulanic acid;
Aztreonam.
Oral antibiotic therapy
Quinolones (pefloxacin, ofloxacin);
Amoxycillin;
Trimethoprim/sulphamethoxazole.
Types of outcome measures
Primary outcomes
Death.
Cure.
Recurrence of spontaneous bacterial peritonitis.
Secondary outcomes
Numbers of days of hospitalisation.
Adverse events:
‐ Any serious adverse event that is fatal, life‐threatening, requiring inpatient hospitalisation or prolongation of existing hospitalisation; significant disability or incapacity or any important medical event that may not be immediately life‐threatening or results in death or hospitalisation, but may jeopardise the patient or may require intervention to prevent one of the above outcomes.
‐ Any adverse event that requires discontinuation of medication (WHO 2005).
Search methods for identification of studies
Electronic searches
Relevant randomised trials were identified by searching the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2008), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 3, 2008), MEDLINE (1950 to July 2008), EMBASE (1980 to September 2008), and Science Citation Index EXPANDED (1945 to July 2008) (Royle 2003). The search strategies used are given in Appendix 1 with the time span of the searches.
Searching other resources
The references of all identified studies were inspected for more studies. Additionally, the first or corresponding author of each included study, and the researchers active in the field, were contacted for information regarding unpublished trials or complementary information on their own trial.
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and the Cochrane Hepato‐Biliary Group Module (Gluud 2008).
Selection of studies
Three authors (KSW, MB, and NC) independently inspected the abstract of each reference identified by the search and applied the inclusion criteria. For possible relevant articles, or in cases of disagreement between the two authors, the full article was obtained and inspected independently by the three authors (KSW, MB, and NC). Where resolving disagreement by discussion was not possible, the article was added to those 'Studies awaiting classification' and the authors of the study contacted for clarification. In an event of no reply from the authors within six months, an internal ombudsman was used to solve the disagreements. Quasi‐randomised studies were included only after agreement of at least two reviewers.
Data extraction and management
Three authors (KSW, MB, and NC) independently extracted the data of included trials. In case of disagreement between the three authors, a fourth author (LL) extracted the data. The data extraction was discussed, decisions documented, and, where necessary, the authors of the studies were contacted for clarification. Justification for excluding studies from the review was documented.
Trials were identified by the name of the first author and year in which the trial was first published, and ordered chronologically. The following data were extracted, checked, and recorded:
Characteristics of trials
Publication status.
Case definitions used (clinical, serological, bacteriological).
Sponsor of trial (specified, known or unknown).
Characteristics of participants
Number of participants in each group.
Age, gender, country.
Severity of liver disease and cirrhosis according to the aetiology of liver disease, regardless of the criteria used.
Characteristics of interventions
Type of antibiotic, dose, route of administration, schedule, length of follow‐up (in months).
Characteristics of outcome measures
Number of deaths in the treatment and control group.
Number of cured or recurrences in each group.
Number of days of hospitalisation.
Fatal or life‐threatening adverse events.
Any other adverse or medical event related to the treatment.
Lost of follow‐up (dropouts) after randomisation.
Two authors (KSW, NC) entered data in Review Manager Version 5.0 (RevMan 2008). Continuous outcomes were expressed as mean differences with 95% confidence intervals while dichotomous outcomes were expressed as relative risks with a 95% confidence interval (CI). For each outcome, we extracted the number of participants assigned to each group, and whenever possible we extracted data to allow for an intention‐to‐treat analysis. If the number randomised and the numbers analysed were inconsistent, we reported this as the percentage lost to follow‐up. For binary outcomes, we recorded the number of participants experiencing the event in each group. For continuous outcomes, we extracted the arithmetic means and standard deviations for each group. Any disagreement was resolved by discussion with reference to the trial report and resolution by a co‐author (MB). For outcomes for which data were not reported or were reported in a different format, we contacted the authors for clarification.
Assessment of risk of bias in included studies
Two authors (NC, KSW) assessed bias risk of the trials independently, without masking of the trial names. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and the Cochrane Hepato‐Biliary Group Module (Gluud 2008). Due to the risk of biased overestimation of intervention effects in randomised trials with inadequate methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), we assessed the influence of methodological quality of the trials on the results by evaluating the methodological components described below. If information was not available in the published trial, we contacted the authors in order to assess the trials correctly.
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice were also considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding
Adequate, if the trial was described as double blind and the method of blinding involved identical placebo or active drugs.
Unclear, if the trial was described as double blind, but the method of blinding was not described.
Not performed, if the trial was not double blind.
Incomplete data outcomes
Adequate, if there were no post‐randomisation drop‐outs or withdrawals.
Unclear, if it is not clear whether there are any drop‐outs or withdrawals or if the reasons for these drop‐outs are not clear.
Inadequate, if the reasons for missing data are likely to be related to true outcomes.
Selective outcome reporting
Low risk of bias (considering that most of the included trials were made before of the obligatory registration on randomised controlled trials databases, and the pre‐specified outcomes are not available. The following outcomes were considered fundamental as outcome to avoid selective reporting a) mortality, b) response rate, and c) adverse events).
Uncertain risk of bias (there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting).
High risk of bias (not all of the trial's pre‐specified primary outcomes have been reported or similar).
Other sources of bias
Low risk of bias (the trial appears to be free of other sources of bias, considering a) baseline imbalance, b) source of funding, c) early stopping, and d) interim analysis).
Uncertain risk of bias (there is insufficient information to assess whether other sources of bias are present).
High risk of bias (it is likely that potential sources of bias).
Follow‐up
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
Furthermore, we registered whether or not the randomised clinical trials had used 'intention‐to‐treat' analysis (Gluud 2001) and sample size calculation.
Any disagreement was resolved by discussion and settled by a third author (MB). We contacted the trial author for clarification as necessary.
Assessment of heterogeneity
We planned to check the heterogeneity among trials by visual inspection of the forest plots and by using the chi‐squared and I2 tests for heterogeneity (Higgins 2008). Statistical heterogeneity was defined as P‐value ≤ 0.10 (chi‐squared) or I2 > 25%. When heterogeneity existed, we would have explored the potential sources of heterogeneity according to:
Intervention (type of antibiotic, route of administration, and schedule).
Participants (stage of cirrhosis, history of antibiotic prophylaxis, and presence of bleeding).
Trial quality (risk of bias scores).
Subgroup analyses were planned in order to assess the impact of these possible sources of heterogeneity in the main results.
Assessment of reporting biases
We used a funnel plot to explore bias. It was not possible to perform linear regression approach described by Egger et al to determine the funnel plot asymmetry (Egger 1997).
Data synthesis
For the statistical analyses, we used RevMan Analyses (RevMan 2008). Dichotomous data were analysed by calculating the Peto odds ratios for each trial with the uncertainty in each result being expressed using 95% confidence intervals (fixed‐effect model).
Where possible, comparisons were made between the mean duration of symptoms in the two groups. These continuous data were analysed by using the mean and standard deviation of each trial and calculating the effect size (average mean difference) and the 95% confidence interval (fixed‐effect model).
Sensitivity analysis
We analysed data by both the fixed‐effect model analysis and random‐effects model analysis, but only reported the former in the text if the outcome of both analyses were the same. Outcomes were analysed as reported in the trial, that is, either per protocol or as intention‐to‐treat analysis. In order to examine the influence of drop outs, we performed both worst‐case (assigning bad outcomes to all of the missing experimental arm patients and good outcomes to all of the missing control arm patients) and best‐case (assigning good outcomes to all of the missing experimental arm patients and bad outcomes to all of the missing control arm patients) analyses.
Results
Description of studies
Thirteen studies were included in the review (Rimola 1984; Felisart 1985; Runyon 1991; Gomez‐Jimenez 1993; Rimola 1995; Figueiredo 1996; Navasa 1996; Terg 1997; Ricart 2000; Tuncer 2003; Grange 2004; Chen 2005; Angeli 2006). Six of them were conducted in Spain, one in Brazil, USA, Italy, Taiwan, France, Turkey and Argentina respectively (see 'Characteristics of included studies' for details).
Thirteen studies were excluded from this review because of lack of randomisation procedure (Fong 1989; Mercader 1989; Silvain 1989; Llovet 1993; Fernandez 2002; Taskiran 2004) or quasi‐randomised design (Ariza 1991; Rastegar 1998), or less than 10% of the patients had spontaneous bacterial peritonitis (Sifuentes 1989; McCormick 1997), or because there was no antibiotic comparison (Franca 2002), or because the trial was published only as an abstract and contained no relevant information about the trial design (Pariente 1988). We contacted the authors of the latter in order to obtain further information, but they did not reply.
Most patients in the included trials had diagnostic of spontaneous bacterial peritonitis confirmed by ascitic fluid PMN more than 250/mm3 and positive ascitic culture. Grange 2004 did not report confirmation of spontaneous bacterial peritonitis diagnosis. Seven trials had the stage of cirrhosis determined by the Child‐Pugh score (Runyon 1991; Gomez‐Jimenez 1993; Rimola 1995; Terg 1997; Ricart 2000; Tuncer 2003; Angeli 2006). In another two trials (Felisart 1985; Ricart 2000), 20% and 50% of the patients, respectively, were diagnosed with bacteraemia, urinary tract infection, or pneumonia when included in the trial.
Neither included trial compared similar experimental and control treatments; therefore, no statistical combination could be performed. Angeli 2006 compared IV ciprofloxacin versus IV ceftazidime; Chen 2005 compared IV cefotaxim versus IV amikacin; Felisart 1985 compared IV cefotaxime versus IV ampicillin‐tobramycin; Figueiredo 1996 compared IV versus oral cephalosporins; Gomez‐Jimenez 1993 compared two types of IV cephalosporins; Grange 2004 compared IV/PO moxifloxacin versus IV/PO amoxicillin‐clavulanic acid; Navasa 1996 compared IV cefotaxime versus oral ofloxacin; Ricart 2000 compared IV + oral amoxicillin‐clavulanic acid versus IV cefotaxime; Rimola 1984 compared ampicillin‐tobramycin versus ampicillin‐tobramycin‐neomycin‐colistin‐nistatin; Rimola 1995 compared low versus high dosages of cefotaxime; Runyon 1991 compared short, 5 days‐term treatment versus long, 10 days‐term treatment with cefotaxime; Terg 1997 compared IV ciprofloxacin versus IV + oral ciprofloxacin and Tuncer 2003 compared oral ciprofloxacin versus IV cefotaxime with IV ceftriaxone.
We also contacted all the authors of the included trials in order to obtain missing details in the reports of their trials.
Risk of bias in included studies
The most important bias observed in the trials was lack of a proper blinding. This is intrinsic bias due to the fact that dosages and timing are different among the drugs assessed. However, an important bias element is the incomplete outcome (being the most common, the exclusion of death events during the first 24 to 48 hs), and only three trials describe properly the allocation concealment (Runyon 1991; Navasa 1996; Angeli 2006). The source of outcome bias was limited since the vast majority of the trials described their outcomes completely (Figure 1; Figure 2).
1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
All the included trials had data on the main outcomes, death and spontaneous bacterial peritonitis resolution.
No meta‐analyses could be performed, as each trial compared different antibiotics in their experimental and control group. In an attempt to provide evidence‐based information regarding the treatment of spontaneous bacterial peritonitis in patients with cirrhosis, data for each included trial were analysed. We provide the results bellow:
Rimola 1984 compared ampicillin‐tobramycin‐neomycin‐nystatin‐colistin with ampicillin‐tobramycin in 37 patients. No significant difference was observed in mortality (Peto OR 2.08, 95% CI 0.58 to 7.46) or no resolution of spontaneous bacterial peritonitis (Peto OR 2.53, 95% CI 0.57 to 11.13).
In Felisart 1985, cefotaxime was compared with a combination of ampicillin and tobramycin in 73 patients. In this trial there was no significant difference in the number of people who died (Peto OR 1.57, 95% CI 0.59 to 4.14) or with fatal adverse events (Peto OR 0.13, 95% CI 0.00 to 6.64). There was, however, a significant difference in favour of cefotaxime in the resolution of spontaneous bacterial peritonitis (Peto OR 0.34, 95% CI 0.13 to 0.87).
Runyon 1991 tried to evaluate whether short and long‐term treatment would have had the same effect in the resolution of spontaneous bacterial peritonitis and reduce mortality in 90 patients. The results reported no significant difference neither in mortality (Peto OR 0.66, 95% CI 0.28 to 1.53) nor in no resolution of spontaneous bacterial peritonitis (Peto OR 1.15, 95% CI 0.49 to 2.71) for those receiving cefotaxime for five or ten days. In addition, relapse and/or reinfection were similar in the two groups (Peto OR 0.90, 95% CI 0.26 to 3.16).
Gomez‐Jimenez 1993 also compared two third generation cephalosporins administered intravenously (cefonicid versus ceftriaxone) in 60 patients. No significant difference was found between the two groups for any of the outcomes. The results for the primary outcomes were death (Peto OR 1.34, 95% CI 0.46 to 3.89) and no resolution of spontaneous bacterial peritonitis (Peto OR 7.93, 95% CI 0.79 to 79.26).
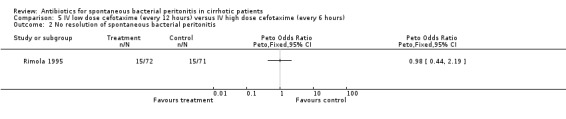
Rimola 1995 appraised whether lower dosages of cefotaxime had the same effectiveness and less adverse events than higher dosages in 143 cirrhotic patients with spontaneous bacterial peritonitis. Again, no significant difference was found for any of the outcomes provided. The results for the primary outcomes were death (Peto OR 0.59, 95% CI 0.28 to 1.25) and no resolution of spontaneous bacterial peritonitis (Peto OR 0.98, 95% CI 0.44 to 2.19).
In Figueiredo 1996, two third generation cephalosporins (oral cefixime versus IV ceftriaxone) were compared in 38 patients. No significant difference was found for death (Peto OR 1.24, 95% CI 0.25 to 6.28) or no resolution of spontaneous bacterial peritonitis (Peto OR 1.81, 95% CI 0.18 to 18.64). Personal communication with the first author provided information for all outcomes. No significant difference could be found between the experimental (cefixime) or control (ceftriaxone) groups.
Navasa 1996 compared oral ofloxacin with IV cefotaxime in 123 patients with uncomplicated spontaneous bacterial peritonitis (patients with hepatic encephalopathy, renal failure, vomiting, ileus, shock or gastrointestinal haemorrhage were excluded from the trial). There was no difference found in the number of deaths between the two groups (Peto OR 1.01, 95% CI 0.41 to 2.49), no resolution of spontaneous bacterial peritonitis (Peto OR 1.03, 95% CI 0.39 to 2.73), or in the presence of adverse events (Peto OR 0.92, 95% CI 0.13 to 6.70).
Terg 1997 compared oral + IV with IV ciprofloxacin in 80 cirrhotic patients with spontaneous bacterial peritonitis. No difference was found between the two groups for the outcomes of death (Peto OR 1.00, 95% CI 0.38 to 2.65) and no resolution of spontaneous bacterial peritonitis (Peto OR 1.16, 95% CI 0.40 to 3.36).
Ricart 2000 compared amoxicillin‐clavulanic acid with cefotaxime in 96 cirrhotic patients with bacterial infections, 50% of them had spontaneous bacterial peritonitis (and only these patients were analysed in this review). No significant difference was found between the two groups for any of the outcomes. Following are the results for the primary outcomes for patients with spontaneous bacterial peritonitis: death (Peto OR 0.56, 95% CI 0.12 to 2.50), and no resolution of spontaneous bacterial peritonitis (Peto OR 0.72, 95% CI 0.15 to 3.52).
Tuncer 2003 compared ciprofloxacin with cefotaxime or ceftriaxone in 53 patients. No significant differences in death between ciprofloxacin and cefotaxime (Peto OR 1.15, 95% CI 0.20 to 6.55), ciprofloxacin and ceftriaxone (Peto OR 0.66, 95% CI 0.14 to 3.14) or ceftriaxone and cefotaxime (Pero OR 3, 95% CI 0.38 to 23.47) were observed. No resolution of spontaneous bacterial peritonitis was not significantly different in all comparisons as well (ciprofloxacin versus cefotaxime: Peto OR 0.87, 95% CI 0.19 to 3.92; ciprofloxacin versus ceftriaxone: Peto OR 0.94, 95% CI 0.21 to 4.19; ceftriaxone versus cefotaxime: Peto OR 0.53, 95% CI 0.11 to 2.53).
In Grange 2004, moxifloxacin and amoxicillin‐clavulanic acid were compared in 35 patients. No significant difference in resolution of spontaneous bacterial peritonitis was observed (Peto OR 6.99, 95% CI 0.14 to 352.83).
In Chen 2005, amikacin was compared to cefotaxime in 37 patients. No significant difference was observed in death (Peto OR 1.43, 95% CI 0.32 to 6.28), and the difference in resolution of spontaneous bacterial peritonitis did not reach statistical significance (Peto OR 2.29, 95% CI 0.57 to 9.23).
Finally, Angeli 2006, intravenous ciprofloxacin was compared to ceftazidime in different doses (according to the creatinine level) in 116 patients. No significant differences were found for death (Peto OR 0.55, 95% CI 0.21 to 1.43) or no resolution of spontaneous bacterial peritonitis (Peto OR 1.25, 95% CI 0.49 to 3.20). Additionally, no difference was found in the presence of adverse events (Peto OR 1.78, 95% CI 0.18 to 17.48).
Data regarding number of days of hospitalisation are shown in Additional Table 1. In addition, neither a test for heterogeneity nor subgroup analysis could be performed, as trials could not be combined, and there was not enough information to assess the impact of the intervention, participants, or trial quality. Furthermore, the number of the included trials was too small to allow a funnel plot estimation or a meta‐regression analysis to examine potential selection bias.
1. Mean number of days of hospitalisation.
| Study | Mean (T) | SD (T) | Mean (C) | SD (C) |
| Terg 1997 | 17 | 6 | 19 | 5 |
| Ricart 2000 | 18.4 | 10 | 17 | 7.1 |
| Chen 2005 | 13 | 9 | 12 | 8 |
Discussion
In this systematic review we attempted to summarize evidence from randomised clinical trials on the treatment of spontaneous bacterial peritonitis. As with all such analyses, it is important to state that the results are totally dependent on obtaining a reasonable number of trials with low bias risk. Currently, all thirteen identified trials dealt with different comparisons, and an attempt was made to use the results of this review, trying to answer clinical relevant questions in the treatment of spontaneous bacterial peritonitis.
First, we wanted to study whether third generation cephalosporins (particularly cefotaxime) are superior to other antibiotic therapies for spontaneous bacterial peritonitis, and whether these drugs should be regarded as the 'gold‐standard' treatment. We found no reliable evidence to place cefotaxime as the 'first choice of treatment' as suggested by many authors in the field (Arroyo 1994; Bhuva 1994; Rimola 1995a; Guarner 1997). This assumption was based on the effectiveness of cefotaxime to treat other infectious diseases and on the results of one single trial (Felisart 1985). This trial randomised 73 patients to receive either ampicillin‐tobramycin or cefotaxime for the treatment of spontaneous bacterial peritonitis. One should bear in mind, however, that this trial presented some flaws, as no sample size was calculated a priori, deaths that occurred in the first 48 hours were excluded from the final analysis, and follow‐up was for only two days after the withdrawal of the antibiotic. This updated review identified four more recent trials comparing third generation cephalosporins with other antibiotics: Chen 2005 compared amikacin versus cefotaxime, Tuncer 2003 compared ciprofloxacin versus cefotaxime or ceftriaxone, and Angeli 2006 compared ciprofloxacin versus ceftazidime, and other comparing moxifloxacin versus amoxicillin‐clavulanic acid Grange 2004. All three trials did not demonstrate any significant advantage for cephalosporins in mortality or resolution of spontaneous bacterial peritonitis. Another two trials compared ceftriaxone with either cefixime (Figueiredo 1996) or cefonicid (Gomez‐Jimenez 1993). Both trials, however, were small and no conclusions could be made. Current evidence does not demonstrate superiority of third generation cephalosporins over other antibiotics, but rather equal efficacy. Despite of the new included trials, no substantial changes from the previous review were observed (Soares‐Weiser 2001).
Second, we wanted to study whether oral antibiotic is as effective as intravenous antibiotic to reduce mortality, resolve symptoms of spontaneous bacterial peritonitis, and reduce adverse events. Intravenous third generation cephalosporins were compared with ofloxacin (Navasa 1996), amoxicillin‐clavulanic acid (Ricart 2000), or oral third generation cephalosporin (Figueiredo 1996) in an attempt to evaluate the cost‐effectiveness of oral treatment for spontaneous bacterial peritonitis. An additional trial (Terg 1997) compared the effectiveness of intravenous with intravenous + oral ciprofloxacin in the treatment of spontaneous bacterial peritonitis. Additionally, this trial attempted to identify which patients could be treated outside the hospital after a short course of intravenous antibiotic therapy. Once more, each comparison was tested in a single, small trial and should be considered inconclusive. However, it is important to mention that in both trials that tested the use of oral quinolones (Navasa 1996; Terg 1997), there was no difference in the effectiveness and mortality for the experimental (oral ofloxacin or IV + oral ciprofloxacin) or control groups (Navasa 1996: IV cefotaxime; Terg 1997: IV ciprofloxacin). Although it has been suggested that patients, who present with moderate symptoms or who show a relevant improvement of the symptoms after a short course of intravenous antibiotics, could benefit from this form of treatment (Arroyo 1994; Rimola 1995a; Navasa 1996; Guarner 1997; Terg 1997): Further research should be planned to compare the effectiveness of intravenous and oral antibiotic in the treatment of spontaneous bacterial peritonitis. Third, we wanted to study whether a reduction in the daily dose of intravenous cephalosporin would result in the same effectiveness described with higher dosages. The rationale for this question is based on the fact that cephalosporins are partly metabolised in the liver and acquire high concentration in the ascitic liquid. Therefore, one would expect that the same dose given to cirrhotic patients with ascites would have a prolonged half‐life than in a patient with normal liver function. Furthermore, if the decreased dosage of cephalosporins could be achieved without compromising the therapeutic effectiveness, this would result in a reduction of the treatment costs and also have an important impact in resistance to cephalosporins (Angeli 2006). In the single trial that evaluated this question (Rimola 1995) there was a tendency for lower dosages of cefotaxime to cause less death and to resolve the spontaneous bacterial peritonitis symptoms. Although these results go in line with the results described in the literature (Rimola 1995a), no conclusions can be made, and further studies should be planned in order to confirm these initial findings.
Fourth, we wanted to study whether a reduction in the length of treatment would not compromise the effectiveness of antibiotic therapy. Only one study comparing five and ten days of treatment was found (Runyon 1991). It is also important to point out that in all the other trials included in this review, length of treatment was based on the disappearance of signs and symptoms of the disease, and not on a predetermined interval. It is clear that a reduced length of treatment would reduce the cost of spontaneous bacterial peritonitis therapy, but this reduction would only be meaningful if it could be demonstrated that a short‐term treatment would not be counterbalanced by a decrease in antibiotic effectiveness.
Finally, whether antibiotic treatment for spontaneous bacterial peritonitis should also cover gram‐positive cocci (particularly enterococcus), as up to 10% of infections are caused by these bacteria. This seems to be relevant, particularly as the antibiotic prophylaxis of spontaneous bacterial peritonitis with quinolones has been suggested to increase the number of gram‐positive spontaneous bacterial peritonitis in patients resistant to cephalosporins or quinolones (Guarner 1997; Ricart 2000). Both ampicillin‐tobramycin and amoxicillin‐clavulanic acid were used with the assumption that gram‐positive spontaneous bacterial peritonitis would be covered; however, no conclusive results are available at this time.
Authors' conclusions
Implications for practice.
Currently, this review provides no clear evidence derived from randomised clinical trials for the treatment of cirrhotic patients with spontaneous bacterial peritonitis. In practice, third generation cephalosporins have already been established as the standard treatment of spontaneous bacterial peritonitis, and it is clear that empirical antibiotic therapy should be provided in any case. However, until large, well‐conducted trials provide more information, practice will remain based on impression, not evidence.
Implications for research.
This review has identified several important gaps in our evidence base that warrant future research. For example, the most frequently recommended first‐option treatment for spontaneous bacterial peritonitis, cefotaxime and other third generation cephalosporins, lack a robust evidence base. Furthermore, we were unable to answer even one of the main relevant questions that clinicians may face in the day‐by‐day, when dealing with patients with spontaneous bacterial peritonitis. Currently, it is not possible to decide whether lower dosages or short‐term treatment is as effective as higher dosages and long‐term treatment, whether oral quinolones should be considered an option for those with less severe manifestations of the disease, and whether antibiotic therapy should also aim to cover enterococci.
Further randomised clinical trials with adequate design, involving a large number of participants and sufficient duration should be carefully planned to provide a more precise estimate of the effects of antibiotic treatment for spontaneous bacterial peritonitis. For example, to answer questions about clinical effectiveness 20% greater than that with the standard treatment, a sample size of at least 268 participants would be needed (90% of power (1‐beta), 95% confidence). To assess the impact of different variants of spontaneous bacterial peritonitis in the treatment, much greater numbers would be needed. Any planned trial should also follow up patients for larger period of time, as it has been suggested that spontaneous bacterial peritonitis recurrence and/or liver failure decreases long‐term survival in these patients. In addition, this study should take into consideration the possible development of antibiotic resistance, particularly if dealing with quinolones, as these drugs have been widely used in the prophylaxis of spontaneous bacterial peritonitis.
We also suggest that future trials follow recommended guidelines for reporting their results. Begg 1996 presented guidelines that have been adopted by several leading journals (CONSORT ‐ Consolidated Standards of Reporting Trials). This group developed a checklist of 21 items that include descriptions of the randomisation procedure (allocation concealment), number of people lost during the follow‐up and some details about the analysis made. Such descriptions would help evaluating the quality of trials and would benefit when collecting information for systematic reviews.
What's new
| Date | Event | Description |
|---|---|---|
| 24 July 2008 | New citation required but conclusions have not changed | The conclusions remain as in the previous published review version. New lead author. |
| 24 July 2008 | New search has been performed | Updated. |
| 17 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Figueiredo (Brazil), Grange (France), Terg (Argentina), Runyon (USA), and Angeli (Italy) for the additional information on their trials that they have given to us.
We thank Dimitrinka Nikolova and Christian Gluud of The Cochrane Hepato‐Biliary Group for ongoing support for this review.
We thank R.L. Koretz, USA for all the helpful comments on our review.
Contact Editor: C Gluud, Denmark.
Appendices
Appendix 1. Search Strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | July 2008 | (antibiotic* OR antibacteri*) AND periton* AND cirrho* |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 3, 2008 | #1 MeSH descriptor Anti‐Bacterial Agents explode all trees in MeSH products #2 antibiotic* in All Fields in all products #3 antibacteri* NEAR agent in All Fields in all products #4 (#1 OR #2 OR #3) #5 MeSH descriptor Peritoneal Diseases explode all trees in MeSH products #6 periton* in All Fields in all products #7 (#5 OR #6) #8 MeSH descriptor Liver Cirrhosis explode all trees in MeSH products #9 cirrho* in All Fields in all products #10 (#8 OR #9) #11 (#4 AND #7 AND #10) |
| MEDLINE (WinSPIRS 5.0) | 1950 to July 2008 | #1 explode "Anti‐Bacterial‐Agents"/ all subheadings #2 antibiotic* #3 antibacteri* agent #4 #1 or #2 or #3 #5 explode "Peritoneal‐Diseases"/ all subheadings #6 periton* #7 #5 or #6 #8 explode "Liver‐Cirrhosis"/ all subheadings #9 cirrho* #10 #8 or #9 #11 #4 and #7 and #10 #12 random* or blind* or placebo* or meta‐analysis #13 #11 and #12 |
| EMBASE (WinSPIRS 5.0) | 1980 to July 2008 | #1 explode "antibiotic‐agent"/ all subheadings #2 antibiotic* #3 antibacteri* agent #4 #1 or #2 or #3 #5 explode "peritoneal‐disease"/ all subheadings #6 periton* #7 #5 or #6 #8 explode "liver‐cirrhosis"/ all subheadings #9 cirrho* #10 #8 or #9 #11 #4 and #7 and #10 #12 random* or blind* or placebo* or meta‐analysis #13 #11 and #12 |
| Science Citation Index EXPANDED (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1945 to July 2008 | #1 TS=(antibiotic* OR antibacteri* agent) #2 TS=(periton*) #3 TS=(cirrho*) #4 #3 AND #2 AND #1 #5 TS=(random* or blind* or placebo* or meta‐analysis) #6 #5 AND #4 |
Data and analyses
Comparison 1. Ampicillin‐tobramycin‐neomycin‐nystatin‐colistin versus ampicillin‐tobramycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1 Ampicillin‐tobramycin‐neomycin‐nystatin‐colistin versus ampicillin‐tobramycin, Outcome 1 Death.
1.2. Analysis.

Comparison 1 Ampicillin‐tobramycin‐neomycin‐nystatin‐colistin versus ampicillin‐tobramycin, Outcome 2 No resolution of spontaneous bacterial peritonitis.
Comparison 2. Cefotaxime versus ampicillin‐tobramycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Fatal adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Any adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Lost of follow‐up before end of study | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2 Cefotaxime versus ampicillin‐tobramycin, Outcome 1 Death.
2.2. Analysis.

Comparison 2 Cefotaxime versus ampicillin‐tobramycin, Outcome 2 No resolution of spontaneous bacterial peritonitis.
2.3. Analysis.

Comparison 2 Cefotaxime versus ampicillin‐tobramycin, Outcome 3 Fatal adverse events.
2.4. Analysis.

Comparison 2 Cefotaxime versus ampicillin‐tobramycin, Outcome 4 Any adverse events.
2.5. Analysis.

Comparison 2 Cefotaxime versus ampicillin‐tobramycin, Outcome 5 Lost of follow‐up before end of study.
Comparison 3. Short‐term cefotaxime versus long‐term cefotaxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Any adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3 Short‐term cefotaxime versus long‐term cefotaxime, Outcome 1 Death.
3.2. Analysis.

Comparison 3 Short‐term cefotaxime versus long‐term cefotaxime, Outcome 2 No resolution of spontaneous bacterial peritonitis.
3.3. Analysis.

Comparison 3 Short‐term cefotaxime versus long‐term cefotaxime, Outcome 3 Recurrence of spontaneous bacterial peritonitis.
3.4. Analysis.

Comparison 3 Short‐term cefotaxime versus long‐term cefotaxime, Outcome 4 Any adverse events.
Comparison 4. IV Cefonicid versus IV ceftriaxone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Fatal adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Any adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Lost of follow‐up before end of study | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.

Comparison 4 IV Cefonicid versus IV ceftriaxone, Outcome 1 Death.
4.2. Analysis.

Comparison 4 IV Cefonicid versus IV ceftriaxone, Outcome 2 No resolution of spontaneous bacterial peritonitis.
4.3. Analysis.

Comparison 4 IV Cefonicid versus IV ceftriaxone, Outcome 3 Fatal adverse events.
4.4. Analysis.

Comparison 4 IV Cefonicid versus IV ceftriaxone, Outcome 4 Any adverse events.
4.5. Analysis.

Comparison 4 IV Cefonicid versus IV ceftriaxone, Outcome 5 Lost of follow‐up before end of study.
Comparison 5. IV low dose cefotaxime (every 12 hours) versus IV high dose cefotaxime (every 6 hours).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Lost of follow‐up before end of study | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.

Comparison 5 IV low dose cefotaxime (every 12 hours) versus IV high dose cefotaxime (every 6 hours), Outcome 1 Death.
5.2. Analysis.
Comparison 5 IV low dose cefotaxime (every 12 hours) versus IV high dose cefotaxime (every 6 hours), Outcome 2 No resolution of spontaneous bacterial peritonitis.
5.3. Analysis.

Comparison 5 IV low dose cefotaxime (every 12 hours) versus IV high dose cefotaxime (every 6 hours), Outcome 3 Lost of follow‐up before end of study.
Comparison 6. Oral Cefixime versus IV ceftriaxone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Any adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.

Comparison 6 Oral Cefixime versus IV ceftriaxone, Outcome 1 Death.
6.2. Analysis.

Comparison 6 Oral Cefixime versus IV ceftriaxone, Outcome 2 No resolution of spontaneous bacterial peritonitis.
6.3. Analysis.

Comparison 6 Oral Cefixime versus IV ceftriaxone, Outcome 3 Any adverse events.
Comparison 7. Ofloxacin versus cefotaxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Any adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
7.1. Analysis.

Comparison 7 Ofloxacin versus cefotaxime, Outcome 1 Death.
7.2. Analysis.

Comparison 7 Ofloxacin versus cefotaxime, Outcome 2 No resolution of spontaneous bacterial peritonitis.
7.3. Analysis.

Comparison 7 Ofloxacin versus cefotaxime, Outcome 3 Any adverse events.
Comparison 8. IV + oral ciprofloxacin versus IV ciprofloxacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
8.1. Analysis.

Comparison 8 IV + oral ciprofloxacin versus IV ciprofloxacin, Outcome 1 Death.
8.2. Analysis.

Comparison 8 IV + oral ciprofloxacin versus IV ciprofloxacin, Outcome 2 No resolution of spontaneous bacterial peritonitis.
Comparison 9. Amoxicillin‐clavulinic acid versus cefotaxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Any adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Average number of days of hospitalisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
9.1. Analysis.

Comparison 9 Amoxicillin‐clavulinic acid versus cefotaxime, Outcome 1 Death.
9.2. Analysis.

Comparison 9 Amoxicillin‐clavulinic acid versus cefotaxime, Outcome 2 No resolution of spontaneous bacterial peritonitis.
9.3. Analysis.

Comparison 9 Amoxicillin‐clavulinic acid versus cefotaxime, Outcome 3 Any adverse events.
9.4. Analysis.

Comparison 9 Amoxicillin‐clavulinic acid versus cefotaxime, Outcome 4 Average number of days of hospitalisation.
Comparison 10. Ciprofloxacin versus cefotaxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.

Comparison 10 Ciprofloxacin versus cefotaxime, Outcome 1 Death.
10.2. Analysis.

Comparison 10 Ciprofloxacin versus cefotaxime, Outcome 2 No resolution of spontaneous bacterial peritonitis.
Comparison 11. Ciprofloxacin versus ceftriaxone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
11.1. Analysis.

Comparison 11 Ciprofloxacin versus ceftriaxone, Outcome 1 Death.
11.2. Analysis.

Comparison 11 Ciprofloxacin versus ceftriaxone, Outcome 2 No resolution of spontaneous bacterial peritonitis.
Comparison 12. Cefotaxime versus ceftriaxone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
12.1. Analysis.

Comparison 12 Cefotaxime versus ceftriaxone, Outcome 1 Death.
12.2. Analysis.

Comparison 12 Cefotaxime versus ceftriaxone, Outcome 2 No resolution of spontaneous bacterial peritonitis.
Comparison 13. Moxifloxacin versus amoxicillin‐clavulanic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
13.1. Analysis.

Comparison 13 Moxifloxacin versus amoxicillin‐clavulanic acid, Outcome 1 No resolution of spontaneous bacterial peritonitis.
Comparison 14. Amikacin versus cefotaxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
14.1. Analysis.

Comparison 14 Amikacin versus cefotaxime, Outcome 1 Death.
14.2. Analysis.

Comparison 14 Amikacin versus cefotaxime, Outcome 2 No resolution of spontaneous bacterial peritonitis.
14.3. Analysis.

Comparison 14 Amikacin versus cefotaxime, Outcome 3 Recurrence of spontaneous bacterial peritonitis.
Comparison 15. Ciprofloxacin versus ceftazidime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 No resolution of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of spontaneous bacterial peritonitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Any adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
15.1. Analysis.

Comparison 15 Ciprofloxacin versus ceftazidime, Outcome 1 Death.
15.2. Analysis.

Comparison 15 Ciprofloxacin versus ceftazidime, Outcome 2 No resolution of spontaneous bacterial peritonitis.
15.3. Analysis.

Comparison 15 Ciprofloxacin versus ceftazidime, Outcome 3 Recurrence of spontaneous bacterial peritonitis.
15.4. Analysis.

Comparison 15 Ciprofloxacin versus ceftazidime, Outcome 4 Any adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angeli 2006.
| Methods | Randomisation: Sealed envelopes prepared with random numbers generated by software. Blinding: none. Intention‐to‐treat: yes. Interim analysis: no. Exclusions from analysis: none. Follow‐up period: until death, liver transplantation or 3 months following inclusion. | |
| Participants | Italy. Spontaneous bacterial peritonitis confirmed by ascitic fluid PMN count >250 cells/mm3. Stage of cirrhosis determined by Chil‐Pugh and MELD score. | |
| Interventions | Experimental: IV ceftazidime (2 grams bid, 1 gram bid or 1 gram once daily according to level of creatinine). Control: IV ciprofloxacin 200 mg bid or once daily if creatinine >2.5 mg/dL. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolutions. Number of superinfections. Number of recurrences. Severe adverse events. | |
| Notes | Patients with type 1 hepatorenal syndrome were treated with terlipressin and albumin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Sequence generated by computer software. |
| Allocation concealment? | Low risk | Using sealed envelopes. |
| Blinding? All outcomes | High risk | The trial was not blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | There were no post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Follow‐up? | Low risk | All patients randomised were followed until death, liver transplantation, or until 3 months after inclusion. |
Chen 2005.
| Methods | Randomisation: Methods not described. Blinding: none. Intention‐to‐treat: no. Interim analysis: no. Exclusions from analysis: 8/45 randomised patients, 2 in the amikacin group due to secondary peritonitis, 3 in each group due to death or refusal within 48 hours. Follow‐up period: 4 weeks after antibiotic discontinuation. | |
| Participants | Taiwan spontaneous bacterial peritonitis confirmed by ascitic fluid PMN count > 500 cells/mm3. Stage of cirrhosis not determined. |
|
| Interventions | Experimental: IV cefotaxime (1 g four times a day). Control: IV amikacin 500 mg once daily or 8 mg/kg once daily if weight < 60 kg. |
|
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis cures. Number of afebrile patients. Number of superinfections. Number of recurrences. Days of hospitalisation. Nephrotoxicity. | |
| Notes | The dosage of amikacin was adjusted according to plasma levels. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | High risk | There were 8 post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Follow‐up? | Low risk | All patients discharged alive were followed up to 4 weeks after completion of treatment. |
Felisart 1985.
| Methods | Randomisation: table of random number, no further information. Blinding: none. Intention‐to‐treat: no. Interim analysis: yes, abstract with data from 69 patients with similar results. Exclusions from analysis: 8/73 randomised patients. Follow‐up period: up to 2 days after antibiotic withdrawal. | |
| Participants | Spain.
Spontaneous bacterial peritonitis, UTI, and bacteraemia confirmed by positive culture. Stage of cirrhosis: not determined. |
|
| Interventions | Experimental: IV cefotaxime 2g every 6 or 8 hs (according to creatinine clearance). Control: IV tobramycin, 1.75 mg/kg every 8 hs + ampicillin, 2g every 6 or 8 hs (according to creatinine clearance). | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolutions. Number of therapeutic failures. Number of superinfections. Nephrotoxicity. Number of adverse events. | |
| Notes | The dosages of tobramycin and ampicillin were adjusted according to renal function. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | High risk | There were 8 post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | High risk | The trial perform interim analysis. |
| Follow‐up? | Low risk | All survivors, up to 2 days after antibiotic withdrawal. |
Figueiredo 1996.
| Methods | Randomisation: Random numbers generated by computer software. Blinding: not blinded trial. Intention‐to‐treat: no information. Interim analysis: no information. Exclusion from analysis: no information. Follow‐up period: up to 2 days after antibiotic withdrawal. | |
| Participants | Brazil. Spontaneous bacterial peritonitis confirmed by ascitic fluid PMN > 250cells/mm3. Stage of cirrhosis determined by Child‐Pugh score. | |
| Interventions | Experimental: oral cefixime, 400 mg every 24 hs. Control: IV ceftriaxone, 1g every 12 hs. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Treatment duration. Costs. | |
| Notes | Data and details of randomisation provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers generated by computer software. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The trial was not blinded. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No information provided. |
| Free of selective reporting? | Unclear risk | No information provided. |
| Free of other bias? | Unclear risk | No information provided. |
| Follow‐up? | Unclear risk | No information provided. |
Gomez‐Jimenez 1993.
| Methods | Randomisation: method not described. Blinding: none. Intention‐to‐treat: no. Interim analysis: no information. Exclusion from analysis: 7/60 randomised patients who died in the first 48 hs were considered therapeutic failure and excluded. Follow‐up period: 10 days or 4 days after becoming afebrile. | |
| Participants | Spain. Spontaneous bacterial peritonitis confirmed by positive ascitic culture and ascitic fluid PMN > 250cells/mm3. Stage of cirrhosis determined by Child‐Pugh score. | |
| Interventions | Experimental: IV cefonicid, 2 g every 12 hs. Control: IV ceftriaxone, 2 g once a day. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of therapeutic failure. Number of superinfection. Number of adverse events. | |
| Notes | One patient died with anaphylactic shock after the first dose of cefonicid. This trial was supported by a government grant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The trials was not blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | There were 7 post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | Unclear risk | Is unclear if an interim analysis was done. |
| Follow‐up? | Unclear risk | No information provided. |
Grange 2004.
| Methods | Randomisation: methods not described.
Blinding: none.
Intention‐to‐treat: yes.
Interim analysis: no.
Exclusions from analysis: none. Follow‐up period: last follow‐up visit (21 to 31 days after end of therapy). |
|
| Participants | France. Spontaneous bacterial peritonitis and UTI confirmation not reported. Stage of cirrhosis not determined. | |
| Interventions | Experimental: IV/PO moxifloxacin (400 mg once daily). Control: PO/IV amoxicillin‐clavulanate 1200 mg IV or 625 mg PO three times a day. | |
| Outcomes | Number of spontaneous bacterial peritonitis resolution. Hepatic failure or damage. QT interval prolongation. | |
| Notes | Patients with spontaneous bacterial peritonitis received IV albumin. Some data provided by the author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not provided. |
| Allocation concealment? | Unclear risk | Information not provided. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | Low risk | There were no post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | High risk | Some relevant outcomes were not reported. |
| Free of other bias? | Unclear risk | No information provided. |
| Follow‐up? | Unclear risk | No information provided. |
Navasa 1996.
| Methods | Randomisation: multicenter, central telephone randomisation, sealed envelopes with random numbers. Blinding: none. Intention‐to‐treat: yes. Interim analysis: no information. Exclusion from analysis: no. Follow‐up period: no information, duration of treatment from 4 to 14 days. | |
| Participants | Spain. Spontaneous bacterial peritonitis confirmed by positive ascitic culture and ascitic fluid PMN > 250cells/mm3. Stage of cirrhosis: not determined. | |
| Interventions | Experimental: oral ofloxacin, 400 mg every 12 hs. Control: IV cefotaxime, 2g every 6 hs. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of therapeutic failure. Number of superinfections. Number of adverse events. | |
| Notes | The dosages of ofloxacin and cefotaxime were adjusted according to renal function. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Central telephone randomisation. |
| Allocation concealment? | Low risk | Sealed envelopes with random numbers. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | Low risk | There were no post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | Unclear risk | Is unclear if an interim analysis was done. |
| Follow‐up? | Unclear risk | No information provided. |
Ricart 2000.
| Methods | Randomisation: method not described. Blinding: none. Intention‐to‐treat: yes. Interim analysis: performed after inclusion of 1/3 of the patients. Exclusion from analysis: 48 patients were excluded because other than spontaneous bacterial peritonitis was the source infection. Follow‐up period: until resolution of infection (5 to 12 days). | |
| Participants | Spain. Spontaneous bacterial peritonitis confirmed by positive ascitic culture and ascitic fluid PMN > 250cells/mm3. Stage of cirrhosis determined by Child‐Pugh score. Other bacterial infections: UTI, bacteraemia and pneumonia. | |
| Interventions | Experimental: amoxicillin‐clavulanic acid, IV: 1g‐0.2g every 8hs; Oral: 500mg‐125mg every 8hs. Control: IV cefotaxime, 1g every 6 hs. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of bacterial infection resolution. Number of therapeutic failure. Average length of hospitalisation. Number of adverse events. | |
| Notes | The dosages of amoxicillin‐clavulanic acid and cefotaxime were adjusted according to renal function.
Patients were stratified according to previous prophylaxis with norfloxacin.
Bacteria resistance was stated to happen in those previously treated with norfloxacin. The analysis was made with data of patients with spontaneous bacterial peritonitis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | Low risk | There were no post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | Unclear risk | Interim analysis was performed. |
| Follow‐up? | Unclear risk | No information provided. |
Rimola 1984.
| Methods | Randomisation: method not described. Blinding: none. Intention‐to‐treat: no information. Interim analysis: no information. Exclusions from analysis: 4/37 randomised patients, all died after randomisation. Follow‐up period: up to 2 days after antibiotic withdrawal. | |
| Participants | Spain. Spontaneous bacterial peritonitis confirmed by positive ascitic culture and ascitic fluid PMN > 1000cells/mm3. Stage of cirrhosis: not determined. | |
| Interventions | Experimental: IV tobramycin, 2 mg/kg every 8 hs + ampicillin, 2g every 4 hs + neomicin, 1g + colistin, 1.5 x 10 6 U + nistatin, 10 6 U. Control: IV tobramycin, 2 mg/kg every 8 hs + ampicillin, 2g every 4 hs. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of adverse events. | |
| Notes | The dosages of tobramycin and ampicillin were adjusted according to creatinine clearance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | High risk | Four patients were excluded after randomisation. |
| Free of selective reporting? | Unclear risk | No information provided. |
| Free of other bias? | Unclear risk | No information provided. |
| Follow‐up? | Unclear risk | No information provided. |
Rimola 1995.
| Methods | Randomisation: multicenter, randomisation made separately in each hospital, method not described. Blinding: none. Intention‐to‐treat: yes. Interim analysis: yes, abstract with data from 93 patients with similar results. Exclusions from analysis: 7/143 randomised patients, all died after randomisation. Follow‐up period: no information. | |
| Participants | Spain. Spontaneous bacterial peritonitis confirmed by positive ascitic culture and ascitic fluid PMN > 250cells/mm3. Stage of cirrhosis determined by Child‐Pugh score. | |
| Interventions | Experimental: IV cefotaxime, 2 g every 12 hs. Control: IV cefotaxime, 2 g every 6 hs. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of therapeutic failure. Number of superinfections. Number of adverse events. | |
| Notes | An early abstract published in 1992 states that the number of patients to be randomised would be 452 (only 143 patients are described in the trial). The dosages of cefotaxime in both groups were adjusted according to renal function. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | High risk | There were reported 7 post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | High risk | Interim analysis was performed. |
| Follow‐up? | Unclear risk | No information provided. |
Runyon 1991.
| Methods | Randomisation: multicenter, sealed opaque envelopes. Blinding: none. Intention‐to‐treat: no. Interim analysis: no information. Exclusion from analysis: 10/100 randomised patients. Follow‐up period: until death or last known encounter. Sample size calculation: yes. | |
| Participants | USA. Spontaneous bacterial peritonitis confirmed by positive ascitic culture and ascitic fluid PMN > 250cells/mm3 (61 patients); culture‐negative neutrocytic ascites (29 patients). Stage of cirrhosis determined by Child‐Pugh score. | |
| Interventions | Experimental: IV cefotaxime, 2 g every 8 hs for 5 days (15 doses). Control: IV cefotaxime, 2 g every 8 hs for 10 days (30 doses). | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of therapeutic failure. Number of superinfections. Number of re‐infections. Number of adverse events. | |
| Notes | 18 patients (7 in short‐course and 11 in long course) required at least another antibiotic for the treatment. The trial was supported in part by a grant from Hoechst‐Roussel Pharmaceuticals, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Central. |
| Allocation concealment? | Low risk | Sealed opaque envelopes. |
| Blinding? All outcomes | High risk | Not blinded trial. |
| Incomplete outcome data addressed? All outcomes | High risk | There were reported 10 post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | High risk | Is unclear if an interim analysis was done, and the trial was sponsored by industry. |
| Follow‐up? | High risk | Not all patients were followed. |
Terg 1997.
| Methods | Randomisation: multicenter, table of random numbers, independently done by each centre. Blinding: none. Intention‐to‐treat: yes. Interim analysis: no information. Exclusion from analysis: only for efficacy analysis 5/80 patients were excluded because of death in the first 48 hs. Follow‐up period: 7 days. | |
| Participants | Argentina.
Spontaneous bacterial peritonitis confirmed by positive ascitic culture and ascitic fluid PMN > 250cells/mm3. Stage of cirrhosis determined by Child‐Pugh score. |
|
| Interventions | Experimental: IV + oral ciprofloxacin, 200 mg every 12 hs for two days + 500 mg every 12 hs for 5 days. Control: IV ciprofloxacin, 200 mg every 12 hs for 7 days. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of therapeutic failure. | |
| Notes | Some data provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | The protocol dosages impede the blinding for the investigators. |
| Incomplete outcome data addressed? All outcomes | High risk | There were reported 5 post‐randomisation drop‐outs or withdrawals. |
| Free of selective reporting? | High risk | Information about adverse events was not reported. |
| Free of other bias? | Low risk | Is unclear if an interim analysis was done. |
| Follow‐up? | High risk | No information provided. |
Tuncer 2003.
| Methods | Randomisation: method not described. Blinding: none. Intention‐to‐treat: no. Interim analysis: no information. Exclusion from analysis: 4/53 patients were excluded because of death during therapy. Follow‐up period: no information, duration of treatment 5 days. | |
| Participants | Turkey.
Spontaneous bacterial peritonitis confirmed by ascitic fluid PMN > 250cells/mm3. Stage of cirrhosis determined by Child‐Pugh score. |
|
| Interventions | Experimental: Group A ‐ oral ciprofloxacin, 500 mg every 12 hs for 5 days, Group B ‐ IV cefotaxime 2 g every 8 hs for 5 days. Control: Group C ‐ IV Ceftriaxone 2 g daily for 5 days. | |
| Outcomes | Mortality. Number of spontaneous bacterial peritonitis resolution. Number of therapeutic failure. Complication. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | No blinded trial. |
| Incomplete outcome data addressed? All outcomes | High risk | There is exclusion of dead patients from the analysis. |
| Free of selective reporting? | Low risk | All the important outcomes were reported. |
| Free of other bias? | Unclear risk | Is unclear if an interim analysis was done. |
| Follow‐up? | Unclear risk | No information provided. |
IV ‐ intravenous. UTI ‐ urinary tract infection. hs ‐ hours.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ariza 1991 | Spain. Quasi‐RCT. The original protocol is a randomised controlled trial, but in the group of patients treated with aztreonam (26 patients), 17 received concomitantly penicillin, and 4 received vancomicin. |
| Fernandez 2002 | Spain. Prospective, not randomised. |
| Fong 1989 | USA. Prospective (follow‐up) study, not randomised. |
| Franca 2002 | Brazil. Prospective, not randomised, not comparative trial. |
| Llovet 1993 | Spain. Prospective, not randomised. |
| McCormick 1997 | UK. RCT on sepsis and cirrhosis 11/128 patients had infection of the ascitic fluid, and no specific information was provided for this sub‐group of patients. |
| Mercader 1989 | Spain. Prospective (follow up) study, not randomised. |
| Pariente 1988 | France. Prospective, no information on the abstract about methodological design. Authors contact, but did not reply within one year. |
| Rastegar 1998 | Iran. Quasi‐RCT. Inadequate allocation concealment, 2/9 patients were transferred from one group to another after randomisation. |
| Sifuentes 1989 | Mexico. Quasi‐RCT on serious infections and cirrhosis. Treatment of severe bacterial infections, only 5/59 patients had spontaneous bacterial peritonitis. No information about the primary diagnosis of the 11 patients who dropped out before end of study. |
| Silvain 1989 | France. Prospective (follow‐up) study, not randomised. |
| Taskiran 2004 | Turkey. Prospective study, not randomised. |
RCT ‐ randomised controlled trial IV ‐ intravenous
Contributions of authors
Norberto C. Chavez‐Tapia: searching, trial selection, data extraction, report writing, and review updating.
Karla Soares‐Weiser: protocol writing, searching, trial selection, data extraction and assimilation, report writing, and review updating.
Mayer Brezis: protocol writing, trial selection, data extraction and assimilation, report writing (collaborator during the first version published of this review Soares‐Weiser 2001).
Leonard Leibovici: protocol writing, trial selection, data extraction and assimilation, and report writing (collaborator during the first version published of this review Soares‐Weiser 2001).
Sources of support
Internal sources
Tel Aviv University, Israel.
Hadassah University Hospital ‐ Mount Scopus, Israel.
Rabin Medical Center ‐ Beilison Campus, Israel.
Fellowship in Translational Hepatology by the CSF, Italy.
External sources
Danish Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Angeli 2006 {published data only}
- Angeli P, Guarda S, Fasolato S, Miola E, Craighero R, Piccolo F, et al. Switch therapy with ciprofloxacin vs. intravenous ceftazidime in the treatment of spontaneous bacterial peritonitis in patients with cirrhosis: similar efficacy at lower cost. Alimentary Pharmacology & Therapeutics 2006;23(1):75‐84. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen TA, Lo GH, Lai KH, Lin WJ. Single daily amikacin versus cefotaxime in the short‐course treatment of spontaneous bacterial peritonitis in cirrhotics. World Journal of Gastroenterology 2005;11(43):6823‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felisart 1985 {published data only}
- Felisart J, Rimola A, Arroyo V, Perez‐Ayuso RM, Quintero E, Gines P, et al. Cefotaxime is more effective than is ampicillin‐tobramycin in cirrhotics with severe infections. Hepatology 1985;5(3):457‐62. [DOI] [PubMed] [Google Scholar]
- Felisart S, Rimola A, Arroyo V, Rodes J. Randomized comparative study of efficacy and nefrotoxicity of ampicilin plus tobromycin versus cefotaxime in cirrhosis with severe infections. Hepatology 1983;3(5):856. [Google Scholar]
Figueiredo 1996 {published data only}
- Figueiredo FAF, Coelho HSM, Alvariz RG, Silva FD, Godinho F, Salgueiro E. Randomized trial comparing intravenous ceftriaxone with oral cefixime for treatment of spontaneous bacterial peritonitis (SBO) in cirrhotic patients: pilot study. United European Gastroenterology Week, Paris. 1996:P4 0103.
- Figueiredo FAF, Coelho HSM, Soares JAS, Salgueiro E, Pinto CA. Oral cephalosporin for the treatment of non‐severe spontaneous bacterial peritonitis in liver disease: a prospective study of 38 cases [Cefalosporina oral para o tratamento da peritonite bacteriana espontanea nao complicada na cirrose hepatica: um estudo prospectivo de 38 casos]. GED ‐ Gastrenterologia‐Endoscopia‐Digestiva 1997;16(6):231‐6. [Google Scholar]
Gomez‐Jimenez 1993 {published data only}
- Gomez‐Jimenez J, Ribera E, Gasser I, Antaza MA, Del‐Valle O, Pahissa A, et al. Randomized trial comparing ceftriaxone with cefonicid for treatment of spontaneous bacterial peritonitis in cirrhotic patients. Antimicrobial Agents and Chemotherapy 1993;37(8):1587‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grange 2004 {published data only}
- Grange J. Randomized, comparative study of moxifloxacin versus amoxicillin‐clavulanate in the treatment of bacterial infections in cirrhotic patients. Hepatology 2004;40(4 (Suppl 1)):631A. [Google Scholar]
Navasa 1996 {published data only}
- Navasa M, Follo A, Llovet JM, Clemente G, Vargas V, Rimola A, et al. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology 1996;111(4):1011‐7. [DOI] [PubMed] [Google Scholar]
- Navasa M, Planas R, Clemente G, Vargas V, Guarner C, Follo A, et al. Oral ofloxacin versus intravenous cefotaxime in the treatment of non‐complicated spontaneous bacterial peritonitis (SBP) in cirrhosis. Results of a multicenter, prospective, randomized trial. Journal of Hepatology 1994;21(Suppl 1):S11. [Google Scholar]
Ricart 2000 {published data only}
- Ricart E, Soriano G, Novella MT, Ortiz J, Sabat M, Kolle L, et al. Amoxicillin‐clavulinic acid versus cefotaxime in the therapy of bacterial infections in cirrhotic patients. Journal of Hepatology 2000;32:596‐602. [DOI] [PubMed] [Google Scholar]
Rimola 1984 {published data only}
- Grupo Colaborativo para el Estudio de la Peritonitis Bacteriana Espontanea. Eficacia de ofloxacino por via oral frente a cefotaxima endovenosa en la peritonitis bacteriana espontanea no complicada. Resultados preliminares de un estudio multicentrico, prospectivo y aleatorizado. Gastroenterologia y Hepatologia 1994;17:215. [Google Scholar]
- Rimola A, Felisart J, Teres J, et al. Estudio controlado de la eficacia terapeutica de dos pautas antibioticas en la peritonitis bacteriana espontanea de la cirrosis: valor prognostico de los datos bacteriologicos. Gastroenterologia y Hepatologia 1984;7(5):235‐41. [Google Scholar]
Rimola 1995 {published data only}
- Rimola A, Salmeron JM, Clemente G, Rodrigo L, Obrador A, Miranda ML, et al. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology 1995;21(3):674‐9. [PubMed] [Google Scholar]
- Spanish Collaborative Group for Study of Spontaneous Bacterial Peritonitis. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis. Preliminary results of a multicenter, randomized, comparative study. Hepatology 1992;16(2):594. [Google Scholar]
- Xiol X, Ariza J. Cefotaxime in the treatment of spontaneous bacterial peritonitis [letter; comment]. Hepatology 1996;23(4):941‐2. [DOI] [PubMed] [Google Scholar]
Runyon 1991 {published data only}
- Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short‐course versus long‐course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology 1991;100(6):1737‐42. [DOI] [PubMed] [Google Scholar]
Terg 1997 {published data only}
- Terg R, Cobas S, Fassio E, Landeira G, Rios B, Vason W, et al. Oral ciprofloxacin after a short course of intravenous ciprofloxacin in the treatment of spontaneous bacterial peritonitis: results of a multicenter randomized study. Journal of Hepatology 2000;33:564‐9. [DOI] [PubMed] [Google Scholar]
- Terg R, Cobas S, Fassio E, Rios B, Vasen W, Abecasis R, et al. Oral ciprofloxacin after short‐course of intravenous ciprofloxacin in the treatment of spontaneous bacterial peritonitis: Results of a randomized, multicenter, controlled study. Hepatology 1998;28(Suppl 4):557A. [DOI] [PubMed] [Google Scholar]
- Terg R, Dobas S, Fassio E, Rios B, Vasen W, Abecasis R. Oral ciprofloxacin after short‐course of intravenous cirpofloxacin versus intravenous ciprofloxacin in the treatment of spontaneous bacterial peritonitis. Preliminary results of a randomized controlled study. Hepatology 1997;26(4 Pt 2):288A. [DOI] [PubMed] [Google Scholar]
Tuncer 2003 {published data only}
- Tuncer I, Topcu N, Durmus A, Turkdogan MK. Oral ciprofloxacin versus intravenous cefotaxime and ceftriaxone in the treatment of spontaneous bacterial peritonitis. Hepato‐Gastroenterology 2003;50(53):1426‐30. [PubMed] [Google Scholar]
References to studies excluded from this review
Ariza 1991 {published data only}
- Ariza J, Xiol X, Esteve M, Fernandez Baneres F, Linares J, Alonso T, et al. Aztreonam vs. cefotaxime in the treatment of gram‐negative spontaneous peritonitis in cirrhotic patients. Hepatology 1991;14(1):91‐8. [DOI] [PubMed] [Google Scholar]
Fernandez 2002 {published data only}
- Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002;35(1):140‐8. [DOI] [PubMed] [Google Scholar]
Fong 1989 {published data only}
- Fong TL, Akriviadis EA, Runyon BA, Reynolds TB. Polymorphonuclear cell count response and duration of antibiotic therapy in spontaneous bacterial peritonitis. Hepatology 1989;9(3):423‐6. [DOI] [PubMed] [Google Scholar]
Franca 2002 {published data only}
- França A, Giordano HM, Sevá‐Pereira T, Soares EC. Five days of ceftriaxone to treat spontaneous bacterial peritonitis in cirrhotic patients. Journal of Gastroenterology 2002;37(2):119‐22. [DOI] [PubMed] [Google Scholar]
Llovet 1993 {published data only}
- Llovet JM, Planas R, Morillas R, Quer JC, Cabre E, Boix J, et al. Short‐term prognosis of cirrhotics with spontaneous bacterial peritonitis: multivariate study. The American Journal of Gastroenterology 1993;88(3):388‐92. [PubMed] [Google Scholar]
McCormick 1997 {published data only}
- McCormick PA, Greenslade L, Kibbler CC, Chin JKT, Burroughs AK, McIntyre N. A prospective randomized trial of ceftazidime versus netilmicin plus mezlocillin in the empirical therapy of presumed sepsis in cirrhotic patients. Hepatology 1997;25:833‐6. [DOI] [PubMed] [Google Scholar]
Mercader 1989 {published data only}
- Mercader J, Gomez J, Ruiz J, Garre MC, Valdes M. Use of ceftriaxone in the treatment of bacterial infections in cirrhotic patients. Chemotherapy 1989;35(Suppl 2):23‐6. [DOI] [PubMed] [Google Scholar]
Pariente 1988 {published data only}
- Pariente EA, Eid G, Borderon E, Legoux JL. Initial antibiotic therapy of spontaneous bacterial peritonitis in cirrhosis: cefotaxime alone vs cefotaxime plus amoxicillin. Journal of Hepatology 1988;7(Suppl 1):S159. [Google Scholar]
Rastegar 1998 {published data only}
- Rastegar LA, Umrani G, Dehbashi N, Malec F. Evaluation of the therapeutic effect of pefloxacin in comparison with ampicillin and gentamicin in cirrhotic patients with spontaneous bacterial peritonitis. Hepato‐Gastroenterology 1998;45(21):783‐5. [PubMed] [Google Scholar]
Sifuentes 1989 {published data only}
- Sifuentes‐Osornio J, Macias A, Amieva RI, Ramos A, Ruiz‐Palacios GM. Intravenous ciprofloxacin and ceftazidime in serious infections. A prospective, controlled clinical trial with third‐party blinding. The American Journal of Medicine 1989;87(5A):202S‐205S. [DOI] [PubMed] [Google Scholar]
Silvain 1989 {published data only}
- Silvain C, Breux JP, Grollier G, Rouffineau J, Becq‐Giraudon B, Beauchant M. Les septicemies et les infections du liquide d'ascite du cirrotique peuvent‐elles etre traitees exclusivement par voie orale?. Gastroentérologie Clinique et Biologique 1989;13:335‐9. [PubMed] [Google Scholar]
Taskiran 2004 {published data only}
- Taskiran B, Colakoglu O, Sozmen B, Unsal B, Aslan SL, Buyrac Z. Comparison of cefotaxime and ofloxacin in treatment of spontaneous bacterial peritonitis. The Turkish Journal of Gastroenterology 2004;15(1):34‐8. [PubMed] [Google Scholar]
Additional references
Arroyo 1994
- Arroyo V, Navasa M, Rimola A. Spontaneous bacterial peritonitis in liver cirrhosis: treatment and prophylaxis. Infection 1994;22(Suppl 3):S167‐S175. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Bhuva 1994
- Bhuva M, Ganger D, Jensen D. Spontaneous bacterial peritonitis: an update on evolution, management and prevention. The American Journal of Medicine 1994;97:169‐75. [DOI] [PubMed] [Google Scholar]
Castellote 2008
- Castellote J, Girbau A, Maisterra S, Charhi N, Ballester R, Xiol X. Spontaneous bacterial peritonitis and bacterascites prevalence in asymptomatic cirrhotic outpatients undergoing large‐volume paracentesis. Journal of Gastroenterology and Hepatology 2008;23(2):256‐9. [DOI] [PubMed] [Google Scholar]
Chavez‐Tapia 2007
- Chavez‐Tapia NC, Torre‐Delgadillo A, Tellez‐Avila FI, Uribe M. The molecular basis of susceptibility to infection in liver cirrhosis. Current Medicinal Chemistry 2007;14(28):2954‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fasolato 2007
- Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology 2007;45(1):223‐9. [DOI] [PubMed] [Google Scholar]
Francés 2008
- Francés R, Zapater P, González‐Navajas JM, Muñoz C, Caño R, Moreu R, et al. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology 2008;47(3):978‐85. [DOI] [PubMed] [Google Scholar]
Gluud 2001
- Gluud C. Alcoholic hepatitis: no glucocorticosteroids?. FALK Symposium 121. Steatohepatitis (NASH and ASH) 2001:322‐42.
Gluud 2008
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2008, Issue 4. Art. No.: LIVER.
Guarner 1997
- Guarner C, Soriano G. Spontaneous bacterial peritonitis. Seminars in Liver Disease 1997;17(3):203‐17. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Colloboration, 2008. Available from www.cochrane‐handbook.org.
Kaymakoglu 1997
- Kaymakoglu S, Eraksoy H, Okten A, Demir K, Calangu S, Cakaloglu Y, et al. Spontaneous ascitic infection in different cirrhotic groups: prevalence, risk factors and the efficacy of cefotaxime therapy. European Journal of Gastroenterology & Hepatology 1997;9(1):71‐6. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kuiper 2007
- Kuiper JJ, Buuren HR, Man RA. Review article: Management of ascites and associated complications in patients with cirrhosis. Alimentary Pharmacology & Therapeutics 2007;26(Suppl 2):183‐93. [DOI] [PubMed] [Google Scholar]
Leiva 2007
- Leiva JG, Salgado JM, Estradas J, Torre A, Uribe M. Pathophysiology of ascites and dilutional hyponatremia: contemporary use of aquaretic agents. Annals of Hepatology 2007;6(4):214‐21. [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Obstein 2007
- Obstein KL, Campbell MS, Reddy KR, Yang YX. Association between model for end‐stage liver disease and spontaneous bacterial peritonitis. American Journal of Gastroenterology 2007;102(12):2732‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rimola 1995a
- Rimola A, Navasa M, Arroyo V. Experience with cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis. Diagnostic Microbiology and Infectious Disease 1995;22:141‐5. [DOI] [PubMed] [Google Scholar]
Rimola 2000
- Rimola A, García‐Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. Journal of Hepatology 2000;32(1):142‐53. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Thanapoulou 2002
- Thanopoulou AC, Koskinas JS, Hadziyannis SJ. Spontaneous bacterial peritonitis (SBP): clinical, laboratory, and prognostic features. A single‐center experience. European Journal of Internal Medicine 2002;13(3):194‐8. [DOI] [PubMed] [Google Scholar]
Thuluvath 2001
- Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis ‐ in‐hospital mortality, predictors of survival, and health care costs from 1988 to 1998. American Journal of Gastroenterology 2001;96(4):1232‐6. [DOI] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. WHO Draft Guidelines for adverse event reporting and learning systems. Geneva: World Health Organization, 2005. [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Soares‐Weiser 2001
- Soares‐Weiser K, Brezis M, Leibovici L. Antibiotics for spontaneous bacterial peritonitis in cirrhotics. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002232] [DOI] [PubMed] [Google Scholar]


