Abstract
Background
The 5-year survival rate of patients with pancreatic ductal adenocarcinoma (PDAC) is around 5% due to the fact that the majority of patients present with advanced disease that is treatment resistant. Familial pancreatic cancer (FPC) is a rare disorder that is defined as a family with at least two affected first degree relatives, with an estimated incidence of 4%–10%. The genetic basis is unknown in the majority of families although around 10%–13% of families carry germline mutations in known genes associated with hereditary cancer and pancreatitis syndromes.
Methods
Panel sequencing was performed of 35 genes associated with hereditary cancer in 43 PDAC cases from families with an apparent hereditary pancreatic cancer syndrome. Findings: Pathogenic variants were identified in 19% (5/26) of PDAC cases from pure FPC families in the genes MLH1, CDKN2A, POLQ and FANCM. Low frequency potentially pathogenic VUS were also identified in 35% (9/26) of PDAC cases from FPC families in the genes FANCC, MLH1, PMS2, CFTR, APC and MUTYH. Furthermore, an important proportion of PDAC cases harboured more than one pathogenic, likely pathogenic or potentially pathogenic VUS, highlighting the multigene phenotype of FPC.
Interpretation
The genetic basis of familial or hereditary pancreatic cancer can be explained in 21% of families by previously described hereditary cancer genes. Low frequency variants in other DNA repair genes are also present in 35% of families which may contribute to the risk of pancreatic cancer development.
Funding
This study was funded by the Instituto de Salud Carlos III (Plan Estatal de I + D + i 2013–2016): ISCIII (PI09/02221, PI12/01635, PI15/02101 and PI18/1034) and co-financed by the European Development Regional Fund ‘‘A way to achieve Europe’’ (ERDF), the Biomedical Research Network in Cancer: CIBERONC (CB16/12/00446), Red Temática de investigación cooperativa en cáncer: RTICC (RD12/0036/0073) and La Asociación Española contra el Cáncer: AECC (Grupos Coordinados Estables 2016).
Keywords: Familial pancreatic cancer, Panel sequencing, DNA repair and hereditary cancer genes, Pathogenic variants
Research in context.
Evidence before this study
Previous studies have identified germline mutations in CDKN2A, TP53, MLH1, BRCA2, ATM and BRCA1 via next-generation sequencing that are associated with pancreatic cancer development in an estimated 5.5% of unselected PDAC cases. Approximately 10%–13% of FPC families carry germline mutations in BRCA2, PALB2, ATM, CHEK2, CDKN2A, Lynch syndrome mismatch repair genes, Fanconi anaemia related genes and PRSS1 and SPINK2 (hereditary pancreatitis), among others. A search of the current literature was performed to identify genes associated with hereditary pancreatic cancer risk and a panel of 35 genes was selected to perform a genetic screening of familial PDAC cases.
Added value of this study
On the whole, this study provides increasing evidence that the genetic basis of hereditary pancreatic cancer can be explained by previously described hereditary cancer associated genes such as MLH1, CDKN2A, POLQ and FANCM. In addition, previously undescribed likely pathogenic variants were found in PTEN, POLQ and TET2 in PDAC cases from 2 different families, which may be novel variants associated with this syndrome. Furthermore, low frequency potentially pathogenic VUS were identified in additional genes such as FANCC, PMS2, CFTR, APC and MUTYH, although their role in pancreatic cancer risk needs to be clarified in other cohorts.
Implications of all the available evidence
The understanding of the genetic basis of hereditary pancreatic cancer has important implications for the identification of true high risk individuals in order to optimise secondary screening strategies.
Alt-text: Unlabelled box
1. Introduction
The prognosis of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) is dismal with a 5-year survival rate of around 5% as the majority of patients present with advanced disease, which is aggressive and treatment resistant. Screening high-risk populations would result in an earlier diagnosis and a higher chance for cure. Very few risk factors have been identified, although there is good evidence to suggest that smoking, obesity, a family history of pancreatic cancer, pancreatitis and diabetes increase pancreatic cancer risk [1]. More recently, it has been shown that alterations in the microbioma may play a role in PC development [2]. There are no effective biomarkers for early detection in the general population. CA19-9 is currently used in the clinic, although the sensitivity and specificity for the diagnosis of symptomatic pancreatic cancer is 79%–81% and 82%–90%, respectively [3]. Several potential biomarkers have been recently described for early detection, such as a three-protein panel in urine [4], Galectin-1 (Gal-1) in serum [5], thrombospondin-2 (THBS2) in plasma [6], circulating tumour DNA (ctDNA) [7] and the IMMray™ PanCan-d 29 biomarker signature in serum [8].
Sporadic PDAC occurs worldwide at an approximate frequency of 7 in 100,000 person-years and its incidence rate increases at 1% per year [9,10]. However, the risk of developing PDAC increases according to the number of affected family members, the standard incidence ratio is 4.6 with one affected family member to 32 with three affected family members [11]. Familial pancreatic cancer (FPC) is defined as a family with at least one pair of affected first degree relatives and an estimated 4%–10% of pancreatic cancers diagnosed have a familial background [12,13]. Next-generation sequencing analysis has identified germline mutations in 6 genes including CDKN2A, TP53, MLH1, BRCA2, ATM and BRCA1 that are associated with pancreatic cancer development in 5.5% of all pancreatic cancer (PC) cases, both sporadic and those with a family history of pancreatic cancer [14,15]. FPC appears to be inherited in an autosomal dominant manner and around 10%–13% of families carry germline mutations in BRCA2, PALB2, ATM, CHEK2, CDKN2A, Lynch syndrome mismatch repair genes, Fanconi anaemia related genes and PRSS1 and SPINK2 (hereditary pancreatitis) as well as other novel genes [16,17,18]. However, the major underlying genetic defect(s) are still unknown in the majority of families. Families present with either a high incidence of only pancreatic cancer or in combination with other cancer syndromes such as Hereditary Breast and Ovarian Cancer (HBOC), Peutz Jeghers Syndrome (PJS) and Familial Atypical Multiple Mole Melanoma (FAMMM).
One of the keys to improving patient prognosis is early diagnosis and FPC is a known high risk population that would benefit from early detection strategies. The Spanish familial pancreatic cancer registry (Pan-Gen-FAM) was established in 2009 in Spain with the principal objective to characterise the phenotypic and genetic background of FPC [19]. The registry currently includes over 200 individuals representing some 88 families presenting with pancreatic cancer alone or in combination with known cancer syndromes. Healthy high risk individuals are offered a secondary screening program for the early detection of PC at a potentially curative stage. This consists of annual magnetic resonance imaging (MRI) and endoscopic ultra-sound (EUS) and blood biomarkers, including CA19-9 analysis in serum [20,21].
The aim of the study was to analyse individuals recruited to the familial pancreatic registry for a pathogenic germline mutation in genes frequently associated with hereditary cancer. Panel sequencing was performed of 35 genes associated with hereditary cancer in 43 PDAC cases with an apparent hereditary pancreatic cancer syndrome, 11 high risk individuals who participate in the early detection program and 9 individuals diagnosed with non-pancreatic tumours or pancreatitis from families within the Pan-Gen-FAM registry.
2. Methods
2.1. Recruitment of cases and high risk individuals
This study was approved by the local ethics committee. High risk families were identified in the clinical oncology or familial cancer unit in 10 hospitals participating throughout Spain. High risk families with the following phenotype or characteristics were included in the study: Familial pancreatic cancer families with at ≥2 affected first degree relatives (FPC), HBOC families with at least one case of PC, FAMMM families, Hereditary Non Polyposis Colorectal Cancer (HNPCC) families with at least one case of PC and PC cases diagnosed at ≤50 years of age. Patients were asked to sign the informed consent before inclusion into the study and an exhaustive family tree consisting of at least 3 generations was prepared for each participating family. The index case reported the occurrence of pancreatic cancer and other relevant cancer types and diseases within the family. Blood samples in EDTA tubes were taken on entry into the study and leucocytes and lymphocytes fractions were isolated from whole blood and stored until DNA extraction and sequencing analysis. All clinical and personal data was stored in a secure database (RedCap).
2.2. Next generation sequencing of PDAC cases, high risk individuals and non-PDAC cases
A power calculation was performed using the online tool ClinCalc.com (https://clincalc.com/stats/samplesize.aspx). A group of 38–96 PDAC cases were required to identify pathogenic variants with an assumed frequency of 1%–2% in the test population (PDAC familial cases) and 0.001%–0.005% in the general population, with a power of 0.8, alpha value of 0.05 and a Beta value of 0.2. All diagnosed PDAC cases with a source of germline DNA (lymphocytes or leucocytes) were included in the sequencing study and finally 43 cases were included. According to the power calculation, this would allow pathogenic mutations to be identified with an assumed 2% frequency in the test population and 0.005% frequency in the general population, with a power of 0.8, alpha value of 0.05 and a Beta value of 0.2. Briefly, genomic DNA was extracted from leucocyte using the Flexigene DNA kit (Qiagen) according to the manufacturer´s instructions and quantified using the Qubit V2.0 Fluorometer (Life Technologies). A search of the literature was performed using the terms “familial pancreatic cancer” and “hereditary pancreatic cancer” in order to identify publications that report germline mutations associated with pancreatic cancer. Furthermore, a search for “pancreatic cancer gene panels” was performed in order to identify additional genes that are included in commercially available sequencing panels. A targeted capture sequencing panel that included 35 genes associated with cancer was created. The genes included in the panel were APC, ATM, BMPR1A, BRCA1, BRCA2, CDH1, CDKN2A, CHEK2, EPCAM, MLH1, MSH2, MSH6, MUTYH, PALB2, PMS2, PTEN, SMAD4, STK11, TP53, VHL, PRSS1, TERT, CFTR, TET2 9, DNMT3A, POLN 6, POLQ 6, ASXL1 5, FANCG 4, BUB1B 3, ESCO2 3, FANCC 3, FANCM 3, MSH4 3 and RAD54L. The panel was designed using the Agilent SureDesign Software (Agilent Technologies Inc.). 50 ng of genomic DNA was fragmented and adaptors were added in a single enzymatic step. The adaptor-tagged DNA library was purified and amplified. Next, 750 ng of each library were hybridised using SureSelectQXT capture library for 90 min. The resulting libraries were recovered using streptavidin magnetics beads, and a post-capture PCR amplification was carried out. Library fragment size distribution was assessed using the 2200 TapeStation Instrument (Agilent Technologies). Sequencing was performed on the MiSeq (Illumina) using paired-end protocol 2 × 150 bp and Micro V2 chemistry. On average, 5.1 million of pass filter reads were produced per sequencing run and 93.9% of reads had an average Phred score quality above Q30 and the read depth was greater than 200. Samples from the trio family CEPH1463 [22] were used to validate the sequencing panel and determine the sensitivity, specificity and overall depth of the sequencing panel. Further information is available in supplementary methods.
2.3. Identification of potentially pathogenic mutations
The Ingenuity variant analysis (IVA) software was used to analyse the sequencing data and identify pathogenic and potentially pathogenic variants. The tertiary analysis strategy is summarised in Supplementary methods and Supplementary Fig. M1. Briefly, “Genetic Disease” was selected as the analysis design and the biological term “familial cancer” (germinal disease) was used as the primary filter. The variants identified were filtered based on the following criteria. (1) An allele fraction ≥ 25 and call quality ≥ 20 was used to exclude false positives, only variants that complied with this criteria in both cases and controls (CEPH trio) were retained. (2) Common variants with a frequency of more than 1% in the general population were excluded. (3) Variants with a predicted pathogenic or likely pathogenic consequence according to the ACMG guidelines were retained, which included nonsense (STOP gain), frameshift, missense with a damaging or possibly damaging effect according to SIFT or PolyPhen-2 function predictions and canonical splice site variants. (4) Variants with an inferred gain or loss of function were selected in the final “genetic analysis” parameter. The reported pathogenic potential of these 64 variants in hereditary cancer syndromes was assessed in various databases including ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), InSiGHT (https://www.insight-group.org/syndromes/lynch-syndrome/) and HGMD: The Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php). The predicted pathogenicity of the 64 selected variants according to the different databases consulted is included in Supplementary Table 1. Variants were finally classified into 3 groups. (1) Pathogenic variants, which were missense or premature stop variants with a demonstrated pathogenic potential in familial pancreatic cancer or other familial cancer syndromes. (2) Likely Pathogenic variants, which were premature stop variants with an unknown role in familial pancreatic cancer. (3) Potentially pathogenic variants with an unknown significance (VUS), which were defined as missense or splice site variants with an unknown significance in familial pancreatic cancer.
2.4. Validation of pathogenic and likely pathogenic variants
The corresponding genomic sequence of the pathogenic and likely pathogenic variants was retrieved from the ensemble Human GRCh38.p12 data base (https://www.ensembl.org/index.html). Pre-designed PCR/Sanger Sequencing primers (ThermoFisher) were used for validation when available. If not, primers were designed using the primer blast option in the NCBI database (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and PCR amplicons from 400 to 800 bp were selected. DNA was isolated from an independent leucocyte or lymphocyte sample from the same patient using the DNA and Blood DNA isolation kit (Qiagen). DNA was quantified by nanodrop and 10–30 ng of genomic DNA was used for each PCR reaction. PCR was performed using the Amplitaq gold reagent (Applied Biosystems) according to the manufacturer's instructions. The PCR conditions of each primer pair was optimised on an individual basis and the list of primers used and corresponding PCR conditions are shown in Supplementary Table M1. Sanger sequencing was performed using the Big Dye Terminator kit (Applied Biosystems) and fragments were analysed using the ABI prism system at the Complutense University of Madrid. Sequences were analysed manually by visualization of the chromatograms and using the Blast suite of programs in the NCBI database (https://www.ncbi.nlm.nih.gov/).
2.5. Statistical analysis
The Chi-square or Fishers exact test were used to analyse the differences in the frequency of the different variant types between the 3 patient groups (PDAC cases, non-PDAC cases and high risk individuals) and the different family phenotypes (FPC vs. HBOC-PC).
3. Results
3.1. Validation of the sequencing panel
Sequencing was performed in a CEPH trio reference panel (two parents and their child) of European origin (www.hapmap.org) [22], the sensitivity of the panel was 0.995475 and the specificity was 0.996492. With regard to the panel design used in this study, 98.53% of the panel had a depth of greater than 200X and the coverage at this depth was always greater than 98%. Panel sequencing was also performed in 3 previously identified BRCA2 mutation carriers, one individual with a BRCA2 and BRCA1 germline mutation one CDKN2 mutation carrier from the Pan-Gen-FAM registry in order to validate the panel for the ability to detect pathogenic mutations. All mutations were successfully identified with the panel (Supplementary Tables 1 and 2).
3.2. Identification of pathogenic, likely pathogenic variants and potentially pathogenic VUS in PDAC cases
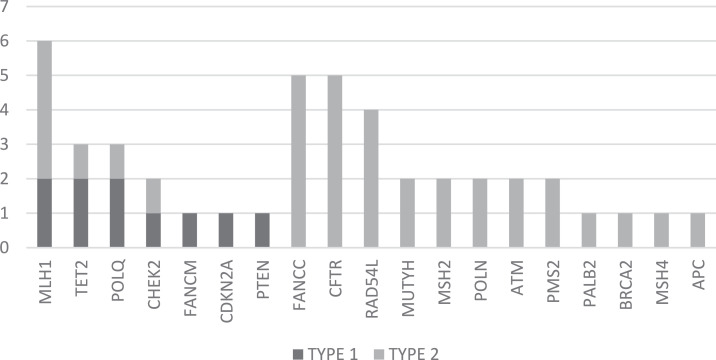
Panel sequencing was performed in 43 PDAC cases from 43 different families, one case was also previously diagnosed with breast cancer. The characteristics of these cases are shown in Table 1 and Supplementary Table 1. Pathogenic, likely pathogenic or potentially pathogenic VUS were identified in 32/43 (74%) of PDAC cases and 11/43 (26%) cases were negative for variants. These were most frequently found in the genes MLH1, CFTR, FANCC and RAD54L and the frequency of these variants is shown in Supplementary Table 1 and Fig. 1. 9/43 (21%) cases had pathogenic or likely pathogenic variants and 3 of these cases also had potentially pathogenic VUS. Pathogenic and likely pathogenic variants were validated by Sanger sequencing in the original DNA sample used for panel sequencing as well as an independent sample of lymphocytes from the same patient and the results were consistent.
Table 1.
Characteristics of the 43 PDAC cases included in the study.
| Demographic data | |
|---|---|
| Sex | 19 males 24 females |
| Median age (range) | 59.5 (29–84) |
| Median age males (range) | 57 (31–80) |
| Median age female (range) | 62 (29–84) |
| Family phenotype | |
| FPC | 26 |
| HBOC + PC | 8 |
| CYSTIC FIBROSIS + PC | 1 |
| PC<=50 years | 8 |
FPC: at least 2 affected 1st degree relatives; HBOC + PC: hereditary breast and ovarian cancer with at least one case of PC; HBOC + PC <=50 Y: hereditary breast and ovarian cancer with at least one case of PC less than 50 years of age; PC<=50 years: 1 affected case less than 50 years of age; FAMMM-PC: FAMMM syndrome with at least one case of PC. CYSTIC FIBROSIS + PC: case of cystic fibrosis and pancreatic cancer.
Fig. 1.
The frequency of pathogenic and potentially pathogenic VUS in 43 sequenced cases.
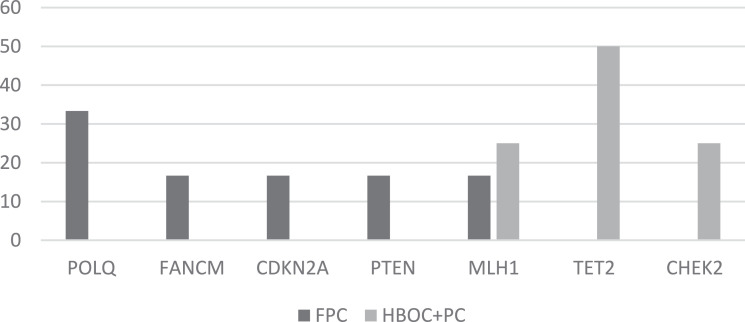
Pathogenic variants were found in 3/43 (7%) cases, which involved the genes MLH1 and CDKN2A. The pathogenic MLH1 variant c.1852A>G (p.Lys618Glu) was found in 2 cases from 2 different families and the CDKN2A c.176T>G (p.Val59Gly) variant was found in one case (Table 2). Likely pathogenic variants (premature stop codon) with an unknown role in FPC were also identified in 6/43 (14%) cases involving the POLQ, CHEK2, PTEN, TET2 and FANCM genes (Table 2). The variants in the genes FANCM, POLQ and CHEK2 were identified in 3 individuals from different families and have been previously described in public databases. Previously undescribed variants in PTEN, and POLQ were also identified in 2 cases from 2 different families. Two likely pathogenic variants were identified in TET2 in the same individual, one of which had been previously described. Pathogenic and likely pathogenic variants were most frequently found in the genes PTEN, CDKN2A, FANCMandPOLQ in FPC families. Whereas, pathogenic and likely pathogenic variants in CHEK2 and TET2 were most frequently found in HBOC+PC families (Fig. 2a). Variants in MLH1 were found in both FPC and HBOC- PC families (Fig. 2a). The difference in the frequency of variants between the different family phenotypes was not statistically significant according to the Fishers exact test.
Table 2.
Pathogenic and likely pathogenic variants identified in PDAC cases from familial pancreatic cancer families.
| Family Phenotype | Genbank accession and transcript | Variant classification |
|---|---|---|
| FPC |
NM_000077.4 (CDKN2A): c.176T>G (p.Val59Gly) |
Pathogenic variants with a determined pathogenic role in familial cancer |
| FPC |
NM_000249.3(MLH1): c.1852A>G (p.Lys618Glu) |
|
| HBOC + PC |
NM_000249.3(MLH1): c.1852A>G (p.Lys618Glu) |
|
| FPC | NM_199,420.3(POLQ): c.4541_4544delCTTC (p.P1514fs*3) |
Likely pathogenic premature stop variants with an unknown consequence in familial pancreatic cancer |
| HBOC + PC |
NM_007194.3(CHEK2): c.409C>T (p.Arg137Ter) |
|
| PC<=50 years |
NM_000314.6(PTEN): c.156delG (p.H53fs*9) |
|
| HBOC + PC | NM_001127208.2 (TET2): c.1954C>T (p.Q652*) and c.3368delC (p.P1123fs*14) | |
| FPC | NM_199,420.3(POLQ): c.7486dupC (p.H2496fs*17) |
|
| FPC |
NM_020937.3(FANCM): c.5791C>T (p.Arg1931Ter) |
FPC: at least 2 affected 1st degree relatives; FPC + <=50 years: 2 affected 1st degree relatives and one diagnosed at less than age 50; HBOC + PC: hereditary breast and ovarian cancer with at least one case of PC; HBOC + PC <=50 Y: hereditary breast and ovarian cancer with at least one case of PC less than 50 years of age; PC<=50 years: 1 affected case less than 50 years of age; FAMMM-PC: FAMMM syndrome with at least one case of PC.
Fig. 2a.
The frequency of pathogenic and likely pathogenic variants in PDAC cases from FFC and HBOC+PC families.
Potentially pathogenic VUS missense or splice site variants were found in 26/43 (60%) PDAC cases. 23/43 (53%) had only potentially pathogenic VUS and 8/43 (19%) had 2 or more potentially pathogenic VUS. 8 potentially pathogenic VUS were identifie d in 2 or more cases from different families (Supplementary Table 1). These variants all have a MAF of ≤ 0.01. The FANCC variant c.584A>T (p.Asp195Val) was found in 4 PDAC cases from 4 different families and theRAD54L variant c.1487G>C (p.G496A) was found in 2 PDAC and one breast case from 3 different families. Potentially pathogenic VUS in PMS2 (c.52A>G (p.Ile18Val)), MLH1 (c.1853A>C (p.Lys618Trp)) and MUTYH (c.1187G>A (p.Gly396Asp)) were found in 2 PDAC cases from 2 different families and the c.3949G>C (p.Glu1317Gln) variant in APC was found in one PDAC case and 1 breast case from 2 different families (Table 3).
Table 3.
Potentially pathogenic VUS identified in 2 or more individuals/PDAC cases from different families.
| Genbank accession an transcript | rs code | MAF Frequency (dbSNP) | Number of cases/individuals and family phenotype |
|---|---|---|---|
| NM_000136.2(FANCC):c.584A>T (p.Asp195Val)* | 1800365 | 0.011 | 4 PC cases (3 from FPC family and 1 from HBOC + PC family) |
| NM_001142548.1(RAD54L):c.1487G>C (p.G496A)* | 138546115 | 0.002 | 2 PC cases and 1 PC/breast case (PC case from HBOC + PC and PC/breast case from FPC and PC case from PC<=50 years family) |
| NM_000535.6(PMS2):c.52A>G (p.Ile18Val)* | 63750123 | 0.01 | 2 PC cases (1 from FPC and 1 from FPC>=3 PC family) |
| 0NM_000249.3(MLH1):c.1853A>C (p.Lys618Trp)* | 63750449 | 0.001 | 2 PC cases (1 from PC<=50 years and 1 from FPC>=3 PC family) |
| NM_001128425.1 (MUTYH): c.1187G>A (p.Gly396Asp)* | 36053993 | 0.009 | 2 PC cases (1 from FPC and1 from HBOC + PC family) |
| NM_000038.5(APC):c.3949G>C (p.Glu1317Gln) | 1801166 | 0.006 | 1 PC case and 1 breast case (PC case from FPC and breast case from HBOC + PC family) |
| NM_000492.3(CFTR):c.2758G>T (p.Val920Leu) | 373885282 | 0.00004 | 1 PC cases and 2 high risk (1 PC case from FPC and 2 high risk from the same FPC>=3 PC family) |
| NM_001211.5(BUB1):c.341G>A (p.Arg114Gln) | 1,372115983 | 0.000008 | 1 high risk and 1 BRCA2 carrier from HBOC + PC <=50 Y families |
variants found in at least 2 PC cases from different families.
FPC: at least 2 affected 1st degree relatives; FPC>=3 PC: 3 or more affected 1st degree relatives; FPC + <=50 years: 2 affected 1st degree relatives and one diagnosed at less than age 50; HBOC + PC: hereditary breast and ovarian cancer with at least one case of PC; HBOC + PC <=50 Y: hereditary breast and ovarian cancer with at least one case of PC less than 50 years of age; PC<=50 years: 1 affected case less than 50 years of age.
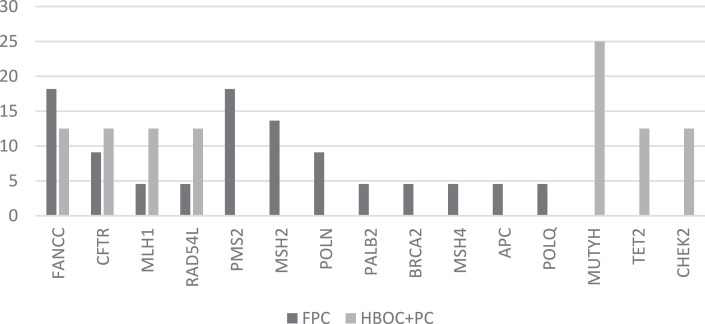
Potentially pathogenic VUS were most frequently found in the genes CFTR, MSH2, FANCC and PMS2 in FPC families. Whereas, variants in MUTYH, TET2 and CHEK2 were most frequently found in HBOC+PC families (Fig. 2b). The difference in the frequency of variants between the different family phenotypes was not statistically significant according to the Fishers exact test.
Fig. 2b.
The frequency of potentially pathogenic VUS in PDAC cases from FFC and HBOC+PC families.
3.3. Potentially pathogenic VUS in cases with other cancer types and high risk individuals
Panel sequencing was also performed in 9 individuals diagnosed with other tumour types or pancreatitis from 9 additional families, this included 3 breast cancer cases, 1 pancreatic neuroendocrine tumour, 1 colon cancer, 1 liver cancer, 1 thyroid cancer, 1 ampuloma and 1 pancreatitis case. Sequencing was also performed in 11 high risk individuals from 9 different families, who participate in the early detection program. 8 of these high risk individuals were diagnosed with a relevant lesions during follow-up, this included 2 suspected PDAC cases and 1 pancreatic neuroendocrine tumour (pNET) case that underwent a surgical resection, 2 IPMN and 3 pancreatic cysts. The characteristics of the cases/high risk individuals and their family phenotype are shown in Supplementary Table 1.
The potentially pathogenic VUS found in FANCM (c.5501G>A (p.Arg1834His)) and APC (c.3949G>C (p.Glu1317Gln)) were identified in 2 breast cancer cases from HBOC+PC families. The same APCvariant was also found in a PDAC case from a different family. The remaining cases with breast, liver, colon and thyroid tumours, ampuloma and pancreatitis were negative for pathogenic, likely pathogenic or potentially pathogenic VUS. Potentially pathogenic VUS in MUTYH (c.1187G>A (p.Gly396Asp)), CFTR(c.3154T>G (p.Phe1052Val)) and the splice site variant in CHEK2 (c.592+50A>T (SPLICE SITE) were identified in an individual diagnosed with a pancreatic neuroendocrine tumour from a family with multiple cases of colon cancer and also pancreatic cancer (Supplementary Table 1).
One high risk individual with an IPMN lesion identified during secondary screening had a potentially pathogenic VUS splice site variant in ATM (c.7630–3C>T) and another high risk individual with a pancreatic cyst had a potentially pathogenic VUS variant in BUB1 (c.341G>A (p.Arg114Gln). Two high risk individuals with no relevant pancreatic lesions identified during follow-up had the potentially pathogenic VUS variant in FANCM (c.1642G>C (p.Glu548Gln)) and ATM (c.8560C>T (p.Arg2854Cys)), respectively. 2 high risk individuals from the same FPC family (with 8 cases of PC) had 2 potentially pathogenic VUS variants in MSH6 (c.1857A>C (p.Glu619Asp)) and CFTR ((c.2758G>T (p.Val920Leu)). Three individuals with pancreatic tumours diagnosed during secondary screening were negative for pathogenic, likely pathogenic or potentially pathogenic VUS (Supplementary Tables 1 and 3).
3 potentially pathogenic VUS were found in an unaffected individual with a pathogenic BRCA2 germline mutation (c.6959T>A (p.Leu2320Ter)) in ATM (c.998C>T (p.Ser333Phe)), BUB1 (c.341G>A (p.Arg114Gln)) and BRCA2 (c.8850G>T (p.Lys2950Asn)). The same BRCA2 potentially pathogenic VUS was also found in the sibling who also carried the same pathogenic BRCA2 pathogenic variant and also had a cystic pancreatic lesion that was identified during screening (Supplementary Table 1).
According to the chi-square analysis, the frequency of potentially pathogenic VUS variants was higher in PDAC versus non-PDAC cases, which was statistically significant (chi-square statistic: 4.3797, p = 0.036). There was no significant difference in potentially pathogenic VUS variant frequency between PDAC cases and high risk individuals.
4. Discussion
This study shows that an important number (21%) of hereditary or familial pancreatic cancer cases harbour pathogenic and likely pathogenic variants in previously described genes associated with hereditary cancer. In this study, 7% of PDAC cases from FPC families had a pathogenic mutation in the genes CDKN2A and MLH1 that are traditionally associated with Lynch syndrome and hereditary melanoma. The CDKN2A variant (c.176T>G; p.Val59Gly) was identified in a case from an FPC family with more than 3 cases of PC and was previously described in association with head and neck cancers. CDKN2A germline mutations are rare in FPC families. However, members of familial atypical multiple mole melanoma (FAMMM) syndrome families are at a higher risk of developing PC and should therefore be offered screening for early detection [14,23,24]. A predisposition to PC is observed in individuals with Lynch syndrome (LS), which is characterised by pathogenic variants in the mismatch repair genes MLH1, MSH2, MSH6 and PMS2. The pathogenic MLH1 variant c.1852A>G (p.Lys618Glu) was found in 2 cases from 2 different families and the potentially pathogenic VUS c.52A>G (p.Ile18Val) in PMS2 was found in 2 cases from 2 different families. LS individuals have an 8.6-fold increased risk of PC compared with the general population and upper gastrointestinal tract tumours predominantly occur in MLH1 pathogenic variant carriers [25,26].
Pathogenic, likely pathogenic and potentially pathogenic VUS) were found in MLH1, TET2, POLQ and CHEK2, whereas only pathogenic and likely pathogenic variants were found in FANCM, CDKN2A and PTEN. Interestingly, potentially pathogenic VUS were commonly found in FANCC, CFTR and RAD54L. CFTR has been previously associated with pancreatic cancer risk, whereas the association with FANCC and RAD54 variants is unclear. Patients with cystic fibrosis have an increased lifetime risk of around 6 fold of developing tumours of the digestive tract, including pancreatic cancer. This is thought to be a consequence of the damage caused to the pancreas by chronic pancreatitis and inflammation that commonly manifest in these patients [27]. CFTR pathogenic variants are more common among PC cases compared to controls and a previous study has shown that common CFTR mutations were detected in 5.3% of cases vs. 3.8% of controls [28]. There is a modest increased PC risk associated with CFTR mutations (odds ratios 1.4), particularly among smokers and those diagnosed at a younger age, with an odds ratio of 1.82 [28,29].
Germline mutations in the DNA repair genes such as BRCA, RAD51 and POLQ are associated with hereditary breast cancer [30]. Interestingly in this study, pathogenic BRCA2 mutations were generally found in the context of HBOC syndrome with at least of one case of PDAC and were not detected in any of the pure pancreatic cancer families. The Fanconi anaemia (FA) pathway genes are related with DNA damage and repair and mono-allelic mutations in these genes increases the risk of several types of cancer in a sporadic fashion. In fact, the FANCD1 gene is actually BRCA2 [31]. FANCM and FANCC form part of the FA nuclear core protein complex, which consists of eight proteins, with ubiquitin ligase activity that is required for the monoubiquitination of FANCD2 in response to DNA damage. The FANCM likely pathogenic variant c.5791C>T (p.Arg1931Ter) found in one case in this study is associated with non-BRCA1/BRCA2 related breast cancer and has also been recently described in an individual from a FPC family who was diagnosed with breast cancer at age 45 and PC some 20 years later (32). It is also of interest that the FANCC potentially pathogenic VUS c.584A>T (p.Asp195Val) was also found in 4 cases.
POLQ at 3q13.31 codes for, pol θ, a translesional DNA polymerase involved in double strand break repair [30]. Therefore, the p.P1514fs*3 POLQ likely pathogenic variant found in this study is a strong causative gene candidate in the described case. The RAD genes play an important role in homologous recombination and defects in these genes are associated with chromosomal instability and also lymphoma predisposition [33]. However, pathogenic variants in these genes have not yet been associated with FPC. Therefore, it is of interest that the potentially pathogenic VUS c.1487G>C (p.G496A) was identified in 2 PDAC cases and a breast cancer case from 3 different families considering that the frequency of this variant in the general population is 0.002 (https://www.genome.gov/10001688/international-hapmap-project/). CHEK2 (Checkpoint kinase 2) is a serine/threonine kinase that forms part of the DNA damage response pathway. Germline mutations in CHEK2 have been associated with prostate and breast cancer [34]. The likely pathogenic variant c.409C>T (p.Arg137Ter) found in this study is in the FHA domain of CHEK2. The variant rs1801166 in APC (c.3949G>C (p.Glu1317Gln)) is classified as conflicting interpretation of pathogenicity in ClinVar, although it may have pathogenic potential and was found in one PC case and one breast case from different families [35].
Germline mutations in PTEN have been rarely described in PC. Recently, exome sequencing identified the missense mutation, p.Arg234Gln in PTEN in a female diagnosed with a solid pancreatic tumour but without characteristics of PTEN hamartoma tumour syndromes (PHTS). Loss of PTEN expression and overexpression of TP53 was also confirmed by IHC in the primary tumour and the somatic KRAS p.Gly12Val mutation was also identified, which is characteristic of a PDAC [36]. However, the 2 truncating variants found in the PDAC cases in this study have not been previously described in familial cancer cases. TET proteins (Ten-Eleven Translocation proteins) are enzymes involved in DNA demethylation and inactivation can lead to promoter hypermethylation and somatic and germline alterations in these genes have been implicated in myeloid and lymphoid tumours [37]. Interestingly, 2 likely pathogenic variants were found in this gene in one case from a HBOC+ PC family.
The potentially pathogenic VUS c.1187G>A (p.Gly396Asp) in MUTYH was found in 2 cases from 2 different families, one pure FPC family and one family with HBOC with PC. Pathogenic variants have been previously described in this gene in PC cases, although not in association with FPC [16]. In this study, we also identified high risk individuals with potentially pathogenic VUS in this gene. BUB1 forms part of the components of the spindle assembly checkpoint (SAC) and controls chromosome segregation and germline mutations have been reported in hereditary colorectal cancer (CRC). BUB1 germline mutations have been previously described in pancreatic cancer cases, although the potentially pathogenic VUS found in a high risk individual in this study has not previously described in familial cancer [38,39].
The majority of germline truncating variants found in this study have not been described previously in the context of pancreatic cancer [14,16,17,18,40,41]. Around 20% of cases included in this study had more than one pathogenic, likely pathogenic or potentially pathogenic VUS in the genes studied. The presence of more than one deleterious variant also been previously described in FPC [42]. The genes associated with familial PDAC in this study included CDKN2A, MLH1, BRCA2, POLQ, CHEK2, PTEN, TET2, FANCM, FANCC, RAD54L, PMS2 and MUTYH, which is consistent with previous reports of panel sequencing in these individuals [14].
With regard to the distribution of pathogenic, likely pathogenic and potentially pathogenic VUS in different cancer syndromes, our data show that some genes are implicated in both FPC and HBOC syndrome cases, whereas, other genes are associated with either FPC or HBOC cases. POLQ, FANCM, CDKN2A and PTEN pathogenic and likely pathogenic variants were only found in FPC cases, whereas TET2 and CHEK2 variants were only found in HBOC cases. However, pathogenic, likely pathogenic and potentially pathogenic VUS in MLH1 were found in both FPC and HBOC cases. With regard to potentially pathogenic VUS, these were more frequently found in the genes MUTYH, TET2 and CHEK2 in HBOC cases, whereas potentially pathogenic VUS were most frequently found in PMS2, POLN and MSH2 in FPC cases.
There are some limitations of the study. Firstly, only 43 PDAC familial cases were included due to the rare nature of this syndrome. Therefore it would be ideal to validate these findings in an independent cohort from another international registry. Furthermore, it would be interesting to perform the same study in sporadic PDAC cases as there is increasing evidence that these cases may also harbour germline risk variants. In fact, an estimated 4%–10% of patients with pancreatic cancer harbour mutations in hereditary pancreatic cancer susceptibility genes [43]. Although, due to the frequency of sporadic cases with germline mutations, it is possible that these are actually familial cases that have not been adequately identified as such.
This study was restricted to a panel of the most relevant 35 genes in familial cancer. Even though the frequency of pathogenic, likely pathogenic and potentially pathogenic VUS was quite high, it is possible that these cases harbour pathogenic variants in other genes that have not been previously described. Therefore, these cases should also be studied by whole genome or whole exome sequencing, even those with identified pathogenic mutations due to likelihood that this syndrome has a multi-gene basis with a variable penetrance.
This and other studies have shown that the genetic basis of hereditary pancreatic cancer can be explained by previously described hereditary cancer associated genes such as MLH1, CDKN2A, POLQ and FANCM. According to our data, the genetic basis of hereditary pancreatic cancer can be explained in 21% of families by these previously described genes that includes 19% of pure FPC families. Furthermore, low frequency variants in other DNA repair genes such as FANCC, PMS2, CFTR, APC and MUTYH were also present in 35% of families which may contribute to the risk of pancreatic cancer development. However, their role in pancreatic cancer risk needs to be clarified in other cohorts.
5. Conclusion
-
•
Pathogenic and likely pathogenic variants were identified in 19% of pure FPC families (MLH1, CDKN2A, POLQ, TET2, FANCM). Potentially pathogenic VUS were identified in 31% of pure FPC families (FANCC, MLH1, PMS2, CFTR, MUTYH)
-
•
The genes associated with familial PDAC in this study included CDKN2A, MLH1, BRCA2, POLQ, CHEK2, PTEN, TET2, FANCM, FANCC, RAD54L, PMS2 and MUTYH.
-
•
These results could contribute to the design of a potential useful genetic signature for screening programs in a high-risk population.
Declaration of Competing Interest
None.
Acknowledgments
We would like to thank the collaboration of the clinical and nursing staff that have contributed to this project, Carmen Guillen Ponce, María Teresa Salazar López, Andrea Santos Gil, Maria del Carmen Perez Ruiz, Manuela Hernando, Maria Jesus Casado Cespedosa and Clara Pacheco Torres. Furthermore, we would also like to thanks Francisco Del Castillo for help with the sequencing analysis and Alfonso Muriel for the statistical analysis.
This study was funded by the Instituto de Salud Carlos III (Plan Estatal de I+D+i 2013-2016): ISCIII (PI09/02221, PI12/01635, PI15/02101 and PI18/1034) and co-financed by the European Development Regional Fund ‘‘A way to achieve Europe’’ (ERDF), the Biomedical Research Network in Cancer: CIBERONC (CB16/12/00446), Red Temática de investigación cooperativa en cáncer: RTICC (RD12/0036/0073) and La Asociación Española contra el Cáncer: AECC (Grupos Coordinados Estables 2016).
The funding sources of this study did play any role in the study design, the collection, analysis and interpretation of the data, in the writing of the manuscript and in the decision to submit the paper for publication.
The corresponding author has full access to all the data in the study and assumes the responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102675.
Contributor Information
Julie Earl, Email: julie.earl@salud.madrid.org.
Teresa Ramon y Cajal, Email: TRamon@santpau.cat.
Luis Robles Diaz, Email: luis.robles@salud.madrid.org.
Isabel Chirivella-Gonzalez, Email: chirivella_isa@gva.es.
Montse Rodriguez, Email: montse.rodriguez.pedreira@sergas.es.
Eva Martínez de Castro, Email: emartinez@humv.es.
Nuría Malats, Email: nmalats@cnio.es.
Alfredo Carrato, Email: acarrato@telefonica.net.
Appendix. Supplementary materials
References
- 1.Becker A.E., Hernandez Y.G., Frucht H., Lucas A.L. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20(32):11182–11198. doi: 10.3748/wjg.v20.i32.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ertz-Archambault N., Keim P., Von Hoff D. Microbiome and pancreatic cancer: a comprehensive topic review of literature. World J Gastroenterol. 2017;23(10):1899–1908. doi: 10.3748/wjg.v23.i10.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballehaninna U.K., Chamberlain R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3(2):105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radon T.P., Massat N.J., Jones R., Alrawashdeh W., Dumartin L., Ennis D. Identification of a three-biomarker panel in urine for early detection of pancreatic adenocarcinoma. Clin Cancer Res. 2015;21(15):3512–3521. doi: 10.1158/1078-0432.CCR-14-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Bosch N., Barranco L.E., Orozco C.A., Moreno M., Visa L., Iglesias M. Increased plasma levels of galectin-1 in pancreatic cancer: potential use as biomarker. Oncotarget. 2018;9(68):32984–32996. doi: 10.18632/oncotarget.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J., Bamlet W.R., Oberg A.L., Chaffee K.G., Donahue G., Cao X.-J. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 2017;9(398) doi: 10.1126/scitranslmed.aah5583. http://stm.sciencemag.org/content/9/398/eaah5583.abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J.D., Javed A.A., Thoburn C., Wong F., Tie J., Gibbs P. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci. 2017;114(38) doi: 10.1073/pnas.1704961114. http://www.pnas.org/lookup/doi/10.1073/pnas.1704961114 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellby L.D., Nyberg A.P., Johansen J.S., Wingren C., Nordestgaard B.G., Bojesen S.E. Serum biomarker signature-based liquid biopsy for diagnosis of early-stage pancreatic cancer. J Clin Oncol. 2018 doi: 10.1200/JCO.2017.77.6658. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saad A.M., Turk T., Al-Husseini M.J., Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018;18(1):688. doi: 10.1186/s12885-018-4610-4. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon-Dseagu V.L., Devesa S.S., Goggins M., Stolzenberg-Solomon R. Pancreatic cancer incidence trends: evidence from the surveillance, epidemiology and end results (SEER) population-based data. Int J Epidemiol. 2017;47(2):427–439. doi: 10.1093/ije/dyx232. http://academic.oup.com/ije/article/doi/10.1093/ije/dyx232/4628151 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein A.P. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64(7):2634–2638. doi: 10.1158/0008-5472.can-03-3823. http://cancerres.aacrjournals.org/content/64/7/2634.long Available from: [DOI] [PubMed] [Google Scholar]
- 12.Matsubayashi H., Takaori K., Morizane C., Maguchi H., Mizuma M., Takahashi H. Familial pancreatic cancer: concept, management and issues. World J Gastroenterol. 2017;23(6):935–948. doi: 10.3748/wjg.v23.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen G.M. Familial pancreatic cancer. Semin Oncol. 2016;43(5):548–553. doi: 10.1053/j.seminoncol.2016.09.002. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5234085/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu C., Hart S.N., Polley E.C., Gnanaolivu R., Shimelis H., Lee K.Y. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA. 2018;319(23):2401–2409. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K.L., Mashl R.J., Wu Y., Ritter D.I., Wang J., Oh C. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173(2):355–370. doi: 10.1016/j.cell.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaffee K.G., Oberg A.L., McWilliams R.R., Majithia N., Allen B.A., Kidd J. Prevalence of germline mutations in cancer genes among pancreatic cancer patients with positive family history. Genet Med. 2018;20(1):119–127. doi: 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhen D.B., Rabe K.G., Gallinger S., Syngal S., Schwartz A.G., Goggins M.G. BRCA1, BRCA2, PALB2, and cdkn2a mutations in familial pancreatic cancer (FPC): a PACGENE study. Genet Med. 2015;17(7):569–577. doi: 10.1038/gim.2014.153. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4439391/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts N.J., Norris A.L., Petersen G.M., Bondy M.L., Brand R., Gallinger S. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov. 2016;6(2):166–175. doi: 10.1158/2159-8290.CD-15-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocci E., Guillen-Ponce C., Earl J., Marquez M., Solera J., Salazar-López M.-T. PanGen-Fam: spanish registry of hereditary pancreatic cancer. Eur J Cancer. 2015;51(14):1911–1917. doi: 10.1016/j.ejca.2015.07.004. http://www.ncbi.nlm.nih.gov/pubmed/26212471 Available from: [DOI] [PubMed] [Google Scholar]
- 20.Bartsch D.K., Slater E.P., Carrato A., Ibrahim I.S., Guillen-Ponce C., Vasen H.F.A. Refinement of screening for familial pancreatic cancer. Gut. 2016;65(8):1314. doi: 10.1136/gutjnl-2015-311098. http://gut.bmj.com/content/65/8/1314.abstract Available from: [DOI] [PubMed] [Google Scholar]
- 21.Vasen HFA The importance of a well-structured pancreatic screening program for familial and hereditary pancreatic cancer. Fam Cancer; 17(17):1–3. Available from: 10.1007/s10689-017-0066-y. [DOI] [PubMed]
- 22.Eberle M.A., Fritzilas E., Krusche P., Källberg M., Moore B.L., Bekritsky M.A. A reference data set of 5.4 million phased human variants validated by genetic inheritance from sequencing a three-generation 17-member pedigree. Genome Res. 2017;27(1):157–164. doi: 10.1101/gr.210500.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremin C., Howard S., Le L., Karsan A., Schaeffer D.F., Renouf D. CDKN2A founder mutation in pancreatic ductal adenocarcinoma patients without cutaneous features of familial atypical multiple mole melanoma (FAMMM) syndrome. Hered Cancer Clin Pract. 2018;16:7. doi: 10.1186/s13053-018-0088-y. https://www.ncbi.nlm.nih.gov/pubmed/29541281 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWilliams R.R., Wieben E.D., Chaffee K.G., Antwi S.O., Raskin L., Olopade O.I. CDKN2A germline rare coding variants and risk of pancreatic cancer in minority populations. Cancer Epidemiol Biomark Prev. 2018;27(11):1364–1370. doi: 10.1158/1055-9965.EPI-17-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bujanda L., Herreros-Villanueva M. Pancreatic cancer in lynch syndrome patients. J Cancer. 2017;8(18):3667–3674. doi: 10.7150/jca.20750. https://www.ncbi.nlm.nih.gov/pubmed/29151953 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Møller P., Seppälä T.T., Bernstein I., Holinski-Feder E., Sala P., Gareth Evans D. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the prospective lynch syndrome database. Gut. 2018;67(7):1306–1316. doi: 10.1136/gutjnl-2017-314057. https://www.ncbi.nlm.nih.gov/pubmed/28754778 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsubayashi H., Fukushima N., Sato N., Brune K., Canto M., Yeo C.J. Polymorphisms of SPINK1 N34S and CFTR in patients with sporadic and familial pancreatic cancer. Cancer Biol Ther. 2019;2(6):652–655. http://www.ncbi.nlm.nih.gov/pubmed/14688470 Available from: [PubMed] [Google Scholar]
- 28.McWilliams R.R., Petersen G.M., Rabe K.G., Holtegaard L.M., Lynch P.J., Bishop M.D. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations and risk for pancreatic adenocarcinoma. Cancer. 2010;116(1):203–209. doi: 10.1002/cncr.24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cazacu I.M., Farkas N., Garami A., Balaskó M., Mosdósi B., Alizadeh H. Pancreatitis-associated genes and pancreatic cancer risk: a systematic review and meta-analysis. Pancreas. 2018;47(9):1078–1086. doi: 10.1097/MPA.0000000000001145. http://www.ncbi.nlm.nih.gov/pubmed/30134356 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandalize A.P.C., Schüler-Faccini L., Hoffmann J.-.S., Caleffi M., Cazaux C., Ashton-Prolla P. A DNA repair variant in POLQ (c.-1060A >G) is associated to hereditary breast cancer patients: a case-control study. BMC Cancer. 2014;14:850. doi: 10.1186/1471-2407-14-850. https://www.ncbi.nlm.nih.gov/pubmed/25409685 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zhang S., Wu Z.Fanconi anemia pathway defects in inherited and sporadic cancers. 2014;3(4):300–4. [DOI] [PMC free article] [PubMed]
- 32.Slavin T.P., Neuhausen S.L., Nehoray B., Niell M., Ilana S., Rybak C. The spectrum of genetic variants in hereditary pancreatic cancer includes Fanconi anemia genes. Fam Cancer. 2018;17(2):235–245. doi: 10.1007/s10689-017-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellick G., Fielding S., Qureshi M., Catovsky D. Consortium on behalf of the IFC. Germline mutations in RAD51, RAD51AP1, RAD51B, RAD51C,RAD51D, RAD52 and RAD54L do not contribute to familial chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49(1):130–133. doi: 10.1080/10428190701606800. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y., Yu H., Zheng S.L., Na R., Mamawala M., Landis T. A comprehensive evaluation of CHEK2 germline mutations in men with prostate cancer. Prostate. 2018;78(8):607–615. doi: 10.1002/pros.23505. [DOI] [PubMed] [Google Scholar]
- 35.Freudenberg-Hua Y., Freudenberg J., Vacic V., Abhyankar A., Emde A.-K., Ben-Avraham D. Disease variants in genomes of 44 centenarians. Mol Genet genomic Med. 2014;2(5):438–450. doi: 10.1002/mgg3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uemura S., Matsubayashi H., Kiyozumi Y., Uesaka K., Yamamoto Y., Sasaki K. Pancreatic adenocarcinoma with a germline PTEN p.Arg234Gln mutation. Fam Cancer. 2018;17(2):255–259. doi: 10.1007/s10689-017-0025-7. [DOI] [PubMed] [Google Scholar]
- 37.Asmar F., Punj V., Christensen J., Pedersen M.T., Pedersen A., Nielsen A.B. Genome-wide profiling identifies a DNA methylation signature that associates with TET2 mutations in diffuse large B-cell lymphoma. Haematologica. 2013;98(12):1912–1920. doi: 10.3324/haematol.2013.088740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mur P., De Voer R.M., Olivera-Salguero R., Rodriguez-Perales S., Pons T., Setien F. Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are infrequent in familial colorectal cancer and polyposis. Mol Cancer. 2018;17:23. doi: 10.1186/s12943-018-0762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Voer R.M., Geurts van Kessel A., Weren R.D.A., Ligtenberg M.J.L., Smeets D., Fu L. Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are risk factors for colorectal cancer. Gastroenterology. 2013;145(3):544–547. doi: 10.1053/j.gastro.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Catts Z.A., Baig M.K., Milewski B., Keywan C., Guarino M., Petrelli N. Statewide Retrospective Review of Familial Pancreatic Cancer in Delaware, and Frequency of Genetic Mutations in Pancreatic Cancer Kindreds. Ann Surg Oncol. 2016;99:1729–1735. doi: 10.1245/s10434-015-5026-x. [DOI] [PubMed] [Google Scholar]
- 41.Grant R.C., Selander I., Connor A.A., Selvarajah S., Borgida A., Briollais L. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2015;148(3):556–564. doi: 10.1053/j.gastro.2014.11.042. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts N.J., Norris A.L., Petersen G.M., Bondy M.L., Brand R., Gallinger S. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov. 2016;6(2):166–175. doi: 10.1158/2159-8290.CD-15-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F., Childs E.J., Mocci E., Bracci P., Gallinger S., Li D. Analysis of heritability and genetic architecture of pancreatic cancer: a PANC4 study. Cancer Epidemiol Biomark Prev. 2019;28(7):1238–1245. doi: 10.1158/1055-9965.EPI-18-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.