Abstract
Ampligen® [polyI:polyC12U] is a mismatched double-stranded RNA that acts by inducing interferon production (immunomodulator) and by activating an intracellular enzyme (RNase-L) against viral RNA transcripts (antiviral). Ampligen®, currently under development by Hemispherx Biopharma in the US, acts on the immunological system through T-lymphocyte stimulation and is indicated for the treatment of chronic fatigue syndrome and acquired immunodeficiency deficiency syndrome (AIDS), as part of the combined therapy.
Ampligen® is available for licensing worldwide.
In February 2004, Fujisawa Deutschland GmbH, a subsidiary of Fujisawa Pharmaceutical Co., entered into an option agreement with Hemispherx Biopharma with the intent of becoming a distributor for Ampligen® for the potential treatment of chronic fatigue syndrome in Germany, Switzerland and Austria. An option fee of €400 000 was paid pursuant to the terms of the option agreement and upon execution of the Distribution Agreement, Fujisawa will pay Hemispherx fees and milestone payments with a potential worth of several millions of dollars.[1]
In September 2003, Hemispherx Biopharma Inc. entered into an agreement with Guangdong Medicine Group Corporation to organise clinical trials, marketing, sales and distribution for both of its lead compounds, Ampligen® and Alferon N® in the People’s Republic of China. The agreement stipulates that the Guangdong Medicine Group Corporation (GMC) will conduct clinical trials with Ampligen® for the treatment of HIV. All costs related to the trials are to be covered by GMC. Additionally, GMC has to develop and implement marketing and promotional programmes.[2]
In May 2003, Hemispherx Biopharma and the Center for Cell and Gene Therapy entered into a research project agreement that will see Ampligen® implemented in a protocol used in patients with relapsed EBV-positive Hodgkin’s Lymphoma.[3]
In March 2002, Esteve and Hemispherx Biopharma entered into a collaborative agreement under which Esteve will be the sole distributor of Ampligen® in Spain, Portugal and Andorra for the treatment of chronic fatigue syndrome. Under this agreement, in addition to other terms, Esteve will also collaborate in the drug product development by conducting clinical studies in Spain in patients coinfected with HIV/HCV.
In July 2001 Hemispherx Biopharma announced that it had formed a strategic alliance with Empire Health Resources for clinical trials of Ampligen® in the treatment of HIV and hepatitis C virus infections. Empire Health Resources, a healthcare management firm, will be responsible for accrual and retention of patients for HIV trials, and protocols for trials in patients with hepatitis C or both HIV and hepatitis C infections.
Hemispherx has entered into a collaboration with RED Laboratories, and RED Laboratories NV expects that this will facilitate the continued development of Ampligen®. Hemispherx has also entered into an agreement with Schering Plough to use a Schering facility as its principal manufacturing platform in the US. This agreement may be expanded to include other territories.
Hemispherx and AOP Orphan Pharmaceuticals have signed a marketing agreement for Ampligen® for the treatment of chronic fatigue syndrome for Austria, the Czech Republic, Poland and Hungary.
In an arrangement between Hemispherx and Bioclones, Bioclones has certain marketing rights for Ampligen® in the Southern Hemisphere, UK and Ireland.
In the US, Ampligen® has been granted orphan drug status for the treatment of AIDS, renal cell carcinoma (phase II, completed), chronic fatigue syndrome (phase III) and invasive/metastatic malignant melanoma (phase II).
In August 2004, Hemispherx announced that it intends to use the proceeds from the private placement of company stock to complete the clinical work for its immunotherapeutics/ antivirals Ampligen® and Oragens™.
Previously, Hemispherx submitted an application to the EMEA for the approval of Ampligen® for the treatment of chronic fatigue syndrome; the first stage of the regulatory review has been cleared. In 2000, Hemispherx Europe (Hemispherx) obtained orphan drug status for Ampligen® for the treatment of chronic fatigue syndrome in the EU, providing Hemispherx with 10 years of marketing exclusivity following the launch of the drug, as well as potential financial research benefits for the agent.
In February 2000, Crystaal Corporation (now Biovail Pharmaceuticals Canada) acquired exclusive marketing rights to Ampligen® in Canada, where it submitted an NDA for the agent for the treatment of chronic fatigue syndrome. In the meantime, Ampligen® has been available since May 1996 under the Canadian Emergency Drug Release Programme for the treatment of chronic fatigue syndrome and immune dysfunction syndrome by Rivex Pharma (Helix BioPharma).
Bioclones has initiated clinical studies with Ampligen® for the treatment of chronic fatigue syndrome in Australia. The active substance for Ampligen® is manufactured by F.H. Faulding Ltd. Clinical treatment programmes for chronic fatigue syndrome in other Pacific Rim countries are planned. Ampligen® is available for severe chronic fatigue syndrome on a named patient, cost-recovery basis in South Africa.
Hemispherx has developed a ‘ready-to-use’ liquid formulation of the drug and has begun treating patients with chronic fatigue syndrome in ongoing clinical trials. Hemispherx has also developed an oral version of the drug (Oragen®), which is undergoing preclinical evaluation.
In February 2001, Hemispherx Biopharma announced that it was initiating phase II/III trials of Ampligen® in the treatment of late-stage, multidrug-resistant strains of HIV in the European Union. Patients treated in these studies will have exhausted all other treatment options.
In July 2001, Hemispherx stated that Ampligen® was being evaluated in a phase IIb trial in patients with HIV in the US. The trial, comprising two studies, REARMI and REARMII (Research/Evaluation of Ampligen® for Retroviral Mutations I and II), will evaluate the ability of Ampligen® to prevent the emergence of mutated, drug-resistant strains of the virus. ‘Several hundred’ patients currently on antiretroviral therapy and at risk of viral relapse will be enrolled at centres in Connecticut, New York, Florida and California. A second phase IIb study evaluating the effect of Ampligen® on structured treatment interruptions (STI) is also underway. Final results from this study were reported in December 2002.
NIH sponsored studies of potential therapies for SARS have identified Ampligen® as having unusually high and consistent antiviral activity against human coronavirus, the pathogen implicated as the causative agent of the disease. Ampligen® demonstrated very high potency at very low concentrations (0.4 μg/mL) and had a favourable safety profile.[4]
In October 2003, Hemispherx announced that, based on these promsing new results, the company will stockpile injectible and/or oral formats of Ampligen® and Alferon N®.[5]
Independent researchers have demonstrated the antiviral activity of Ampligen® against flaviviruses (West Nile virus, Equine Encephalitis virus, Dengue fever virus and Japanese Encephalitis virus) as well as virus classes associated with bioterrorism. In an animal study, Ampligen® was shown to prevent destruction of nerve cells, reduce virus concentrations in the brain and blood stream and increase survival rates.[6]
Researchers at the Rega Institute in Belgium have published results from an animal study demonstrating that Ampligen® was superior at protecting mice against coxsackie B3 virus-induced myocarditis compared with pegylated interferon.[7]
In May 2004 Hemispherx announced that it had filed an expanded US patent application covering the use of Ampligen® for the potential treatment and prevention of severe acute respiratory syndrome (SARS) and dreaded emerging viruses.
Keywords: Zidovudine, West Nile Virus, Chronic Fatigue Syndrome, Severe Acute Respiratory Syndrome, Severe Acute Respiratory Syndrome
1. Profile
1.1 Adverse Events
During clinical trials in which HIV-infected patients received IV infusions of mismatched double-stranded RNA at dose levels of 10–570 mg/m2/dose, the most frequent adverse events were flushing, chills, fever, tight chest, dyspnoea, ’flu-like symptoms and nausea. All reactions were self-limiting and generally subsided after the infusion was completed. Infusions were usually accompanied by transient leucopenia and then leucocytosis. One patient who received mismatched double-stranded RNA 570 mg/m2 discontinued therapy after developing ’flu-like symptoms and fatigue.[8–10]
During combination therapy with zidovudine and Ampligen®, toxicity was similar to that caused by zidovudine alone.[11]
In a study that compared Ampligen® with placebo in zidovudine recipients, the total number of adverse events did not differ significantly between treatment groups.[12]
In HIV-1-positive patients undergoing structured treatment interruptions (STI) of HAART, adverse events associated with Ampligen® were mild and self-limiting. There was no incidence of insulin resistance, hyperlipidaemia, or adverse changes in lactic acid levels.[13]
The most common adverse events in a clinical trial with metastatic melanoma patients included myalgia, headache and fatigue.[14]
Table I.

Features and properties
1.2 Drug Interactions
Mismatched double-stranded RNA is synergistic with multiple anti-HIV drugs. Combination indices were determined for 14 anti-HIV agents alone and in combination with mismatched double-stranded RNA using dose-effect analyses and the CalcuSyn for Windows software. Mismatched double-stranded RNA was synergistic in combination with abacavir, zidovudine, zalcitabine, didanosine, sta-vudine, efavirenz, indinavir, ritonavir, nelfinavir and amprenavir. It was synergistic to antagonistic with lamivudine, delavirdine, nevirapine and saquinavir.[15]
1.3 Pharmacodynamics
1.3.1 Viral Infections
Preclinical studies: Ampligen® reduced serum and liver viral DNA levels in ducks congenitally infected with duck hepatitis B virus, and had at least additive effects with ganciclovir. Ampligen® had no effect on circulating duck HBsAg and viral replication returned to baseline levels after treatment discontinuation.[16]
Clinical studies: The effects of mismatched double-stranded RNA on immune function were compared with those of placebo in a randomised, double-blind, crossover study (ACTG 056) in volunteers. Overall, there were no significant differences between the two treatments in terms of production of interferon and biological markers of interferon, T lymphocytic function, lymphocyte proliferative responses and natural killer cell activity. Symptom scores were greater in mismatched double-stranded RNA recipients than in placebo recipients, but symptoms were mild in nature and the between-group difference was only significant on the day after dosing.[10]
In 15 patients with chronic fatigue syndrome, Ampligen® normalised the upregulated 2-5A synthetase/RNase L system and cleared human herpesvirus 6 antigen-positive cells from peripheral blood mononuclear cell cultures.[17]
1.4 Antimicrobial Activity
1.4.1 Viral Infections
HIV-infections: Mismatched double-stranded RNA induces expression of the physiologically functioning antiviral protection system (2-5A synthetase/RNase L system) in cells. In vitro, Ampligen® 9.7 µg/mL inhibited the HIV-related reduction of Molt-3 cell growth rate by 50%. It acted synergistically with a reverse transcriptase inhibitor and with an antisense oligonucleotide directed against the tat gene.[18]
Ampligen® did not display any synergism against HIV in vitro when used in combination with didanosine. In contrast, synergism was demonstrated between Ampligen® and zidovudine at combination ratios ranging from 100 : 1 to 1 : 50.[19] Ampligen® also acted synergistically with zidovudine against zidovudine-resistant strains.[11]
The compound has been shown to inhibit human herpes virus-6 (HHV-6) infection in T lymphocytes at concentrations of 100 and 200 µg/mL,[20,21] and is also active against rotavirus.[22]
Table II.
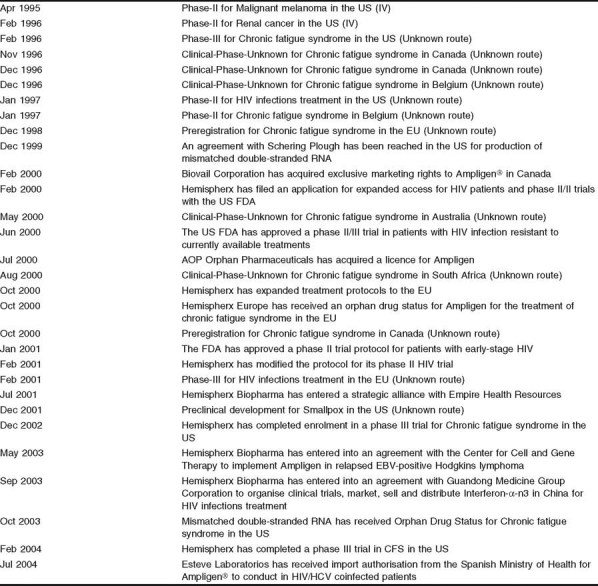
Drug development history
Mismatched double-stranded RNA was equally active against wild-type HIV and HIV resistant to nevirapine, protease inhibitors and nucleoside reverse trancriptase inhibitors.[15]
1.5 Therapeutic Trials
1.5.1 Cancer
Melanoma: Ten patients with metastatic melanoma were treated with mismatched double-stranded RNA in an open-label phase I/II trial. The drug was administered IV at low dose (80mg) to one patient, and at high dose (200–1000mg) to nine patients, over 30–60 minutes twice weekly for 3–111 weeks. Two of the patients receiving high-dose therapy achieved a complete response. Both received maintenance therapy for at least 12 months following this and have remained in complete response without further therapy at 21 and 91 months. Mismatched double-stranded RNA was well tolerated.[14]
1.5.2 Neurological Disorders
Chronic fatigue syndrome: Ampligen® significantly improved patients’ physical performance as assessed by Treadmill Exercise Tolerance Testing (ETT) in a 40-week placebo-controlled phase III study in 234 patients with severely debilitating chronic fatigue syndrome. Ampligen 400mg twice weekly was superior to placebo for improved ETT (19.4 vs 5.1%) for the patient population that completed 40 weeks’ treatment and also for the intent-to-treat population (17.7 vs 4.3%) that completed <40 weeks treatment.[23,24]
In a randomised, placebo-controlled trial, Ampligen® therapy improved physical and cognitive performance in patients with chronic fatigue syndrome, a condition with a possible viral aetiology.[25]
In a Belgium study, patients with chronic fatigue syndrome were randomised to receive mismatched double-stranded RNA [Ampligen®] (200–400mg twice weekly) or placebo for 24 weeks. Mismatched double-stranded RNA significantly improved cognitive and physical performance. Physical performance for activities of daily living, measured using the Karnofsky Performance Score, showed an improvement of 43% (from 53 at baseline to 76). Exercise performance was measured by bicycle testing; oxygen uptake improved from 1.16 L/min at baseline to 1.48 L/min after treatment with mismatched double-stranded RNA. The cognitive improvement seen in patients receiving mismatched double-stranded RNA was measured using the cognitive subscale of the SCL 90-R or neuropsychological function tests. Significantly greater improvements were seen in patients receiving mismatched double-stranded RNA compared with placebo. Mismatched double-stranded RNA was generally well tolerated according to a media release (15/10/1996). Of the patients treated with mismatched double-stranded RNA, about 80% experienced an apparent ‘complete clinical recovery’ after 6 months’ treatment; spontaneous improvement in untreated patients with chronic fatigue syndrome is about 2%.[26,27]
1.5.3 Viral Infections
Hepatitis B: Results of a phase I/II study of mismatched double-stranded RNA 400–600mg two to three times/week in eight patients with chronic hepatitis B indicated that the drug may have an antiviral effect. Four patients lost HBV DNA and three patients became HBeAg seronegative during 24 weeks’ treatment. None of the patients have relapsed.[28]
Results from a phase I study (ACTG 038) were unable to confirm previous beneficial effects of mismatched double-stranded RNA in HIV infection. Thirty-nine HIV-infected patients with asymptomatic disease or early ARC received one of six doses of mismatched double-stranded RNA for 9 or 25 weeks. Mismatched double-stranded RNA had no significant effects on p24 antigenaemia, HIV viraemia or the number of HIV-infected cells. However, the rate of CD4+ cell depletion was reduced; this effect was dose-dependent. In addition, no patient developed an opportunistic infection or any other new HIV-associated symptoms while receiving mismatched double-stranded RNA.[9]
In patients with at least 6 months of previous zidovudine treatment, Ampligen® therapy administered prior to the development of advanced stage symptoms was associated with higher CD4 counts and increased immune function compared with untreated patients. Thus, Ampligen® appears to have activity against zidovudine-resistant HIV strains.
In a study conducted in 36 zidovudine-treated patients, mismatched double-stranded RNA therapy was most effective when initiated earlier in the course of HIV disease. Over a 48-week study period, patients treated with Ampligen® (400 or 700mg twice weekly) had a mean decrease in CD4+ count of 52 cells/µL compared with 89 cells/µL in placebo recipients. Ampligen®-treated patients with baseline CD4+ counts ≥300 cells/µL showed a mean increase in CD4+ count of 26 cells/µL, which peaked at week 24. Placebo recipients had a consistent decrease from baseline. A change to Ampligen® therapy after 48 weeks of placebo treatment reversed the CD4+ decline in seven patients. Ampligen® recipients were more likely to exhibit a delayed-type hypersensitivity response and less likely to progress to AIDS than placebo recipients.[12]
Significantly fewer patients relapsed within the first 30 days of structured treatment interruption (STI) when treated with Ampligen®. In this phase IIb study, the primary endpoint was the mean total time of HAART-free intervals before rebound of plasma HIV-1 RNA. There were two groups of patients: the ‘Harvard cohort’ of eight newly/acutely HIV-infected patients and the ‘study group’, designed to be similar to the referenced Harvard cohort. The similarities being (a) HAART was given for ≥9 months; (b) CD4 ≥400 cells/mL; and (c) HIV RNA levels below 50 copies/mL. The main difference between the groups was that the Harvard cohort were studied within weeks or months of initial infection whereas the study group had been infected with HIV for several years. Interim data analysis were performed with a control (non-Ampligen® treated) group obtained from scientific literature meta-analysis and a second with a concurrent control (non-Ampligen®-treated) group obtained from identical, participating medical institutions. In the meta-analysis, none of the patients relapsed while on Ampligen® within the first 30 days compared with 86% of a control group of patients (p = 0.012). In the second analysis, the median duration of STI in the Ampligen® group was >18 weeks, compared with 7 weeks in the concurrent control, non-Ampligen® group.[29]
In a phase IIb study (AMP 720) of patients with HIV undergoing up to three structured treatment interruptions (STI) of HAART, Ampligen® (400mg IV twice weekly) therapy resulted in significant prolongation of controlled HIV viraemia, compared with untreated controls. Patients taken off HAART but given Ampligen® (n = 7) continued to show virus levels <5000 copies/mL for a mean of 25 weeks and counting. In the control group, patients (n = 9) taken off HAART and not given Ampligen®, developed an HIV rebound within a mean of 13 weeks. In addition, the Ampligen® group demonstrated an increase in CD8+ cells, suggesting induction of immunogenicity.[13,30,31]
References
- 1.Hemispherx Biopharma Inc. Hemispherx Biopharma Signs Option Agreement for Ampligen with Fujisawa Pharmaceutical Company. 2004. [Google Scholar]
- 2.Hemispherx Biopharma Inc. Hemispherx Biopharma Signs Agreement with Guangdong Medicine Group Corporation. 2003. [Google Scholar]
- 3.Hemispherx Biopharma Inc. Hemispherx Biopharma Enters Into Therapeutic Agreement for Ampligen® With The Center for Cell and Gene Therapy at Baylor College of Medicine. 2003. [Google Scholar]
- 4.Hemispherx Biopharma Inc. SARS: Newly NIH Sponsored Studies of Potential Therapies for SARS Now Finds Hemispherx’ Ampligen among the Most Active from a Large Pool of 70 — Seventy — Drug Candidates. 2003. [Google Scholar]
- 5.Hemispherx Biopharma Inc. Hemispherx Biopharma Expands SARS Prevention/Treatment Initiative; Prior to Anticipated Fall/Winter Outbreak also widens Distribution and Clinical Networks. 2003. [Google Scholar]
- 6.Hemispherx Biopharma Inc. Hemispherx Reports Ampligen Prevents Destruction of Nerve Cells and Increases Survival Rates in Flavivirus Infection. 2003. [Google Scholar]
- 7.Hemispherx Biopharma Inc. Hemispherx Biopharma Reports Publication Suggesting Efficacy of Ampligen in Treating Myocarditis Virus. 2004. [Google Scholar]
- 8.McMahon D, Winkelstein A, Huang X-L, et al. Acute reactions associated with the infusion of ampligen. AIDS. 1992;6:235–236. [PubMed] [Google Scholar]
- 9.Armstrong JA, McMahon D, Huang X-L, et al. A phase I study of ampligen in human immunodeficience virus-infected subjects. Journal of Infectious Diseases. 1992;166:717–722. doi: 10.1093/infdis/166.4.717. [DOI] [PubMed] [Google Scholar]
- 10.Hendrix CW, Margolick JB, Petty BG, et al. Biologic effects after a single dose of poly(I):poly(C12U) in healthy volunteers. Antimicrobial Agents and Chemotherapy. 1993;37:429–435. doi: 10.1128/AAC.37.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie D, Hubbell HR, Carter WA, et al. Synergistic inhibition of AZT-resistant HIV by AZT combined with poly(I):poly(C12U), without synergistic toxicity to bone marrow progenitor cell elements. In Vivo. 1994;8:375–381. [PubMed] [Google Scholar]
- 12.Thompson KA, Strayer DR, Salvato PD, et al. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:polyC(12)U in the treatment of HIV infection. European Journal of Clinical Microbiology and Infectious Diseases. 1996;15:580–587. doi: 10.1007/BF01709367. [DOI] [PubMed] [Google Scholar]
- 13.Strayer D, Blick G, Mitchell W, et al. A phase IIB prospective, randomized, controlled study evaluating the immunomodulatory role of poly I:poly C12U against HIV; 2002. p. 37. [Google Scholar]
- 14.Buzaid AC, Strayer DR, Witman PA. Phase I/II study of poly I:polyC(12)U (Ampligen®) shows activity against metastatic melanoma. 1994;13:399. [Google Scholar]
- 15.Essey RJ, McDougall BR, Robinson WE. Mismatched double-stranded RNA (PolyI-polyC(12)U) is synergistic with multiple anti-HIV drugs and is active against drug-sensitive and drug-resistant HIV-1 in vitro. Antiviral Research. 2001;51:189–202. doi: 10.1016/S0166-3542(01)00150-4. [DOI] [PubMed] [Google Scholar]
- 16.Niu J, Wang Y, Dixon R. The use of ampligen alone and in combination with ganciclovir and coumermycin A1 for the treatment of ducks congenitally-infected with duck hepatitis B virus. Antiviral Research. 1993;21:155–171. doi: 10.1016/0166-3542(93)90051-J. [DOI] [PubMed] [Google Scholar]
- 17.Suhadolnik RJ, Reichenbach NL, Hitzges P, et al. Upregulation of the 2-5A synthetase/RNase L antiviral pathway associated with chronic fatigue syndrome. Clinical Infectious Diseases. 1994;18(Suppl.1):96–104. doi: 10.1093/clinids/18.Supplement_1.S96. [DOI] [PubMed] [Google Scholar]
- 18.Ushijima H, Tsiapalis CM, Daum T, et al. Synergistic anti-human immunodeficiency viral (HIV-1) effect of the immu-nomodulator ampligen (mismatched double-stranded RNA) with inhibitors of reverse transcriptase and HIV-1 regulatory proteins. Antiviral Chemistry and Chemotherapy. 1993;4(6):315–321. [Google Scholar]
- 19.O’Marro SD, Armstrong JA, Asuncion C, et al. The effect of combinations of ampligen and zidovudine or dideoxyinosine against human immunodeficiency viruses in vitro. Antiviral Research. 1992;17:169–177. doi: 10.1016/0166-3542(92)90050-F. [DOI] [PubMed] [Google Scholar]
- 20.Ablashi DV, Berneman Z, Strayer DR. Poly(I):poly(C12U) inhibits in vitro replication of human herpesvirus type 6. Clinical Infectious Diseases. 1994;18(Suppl.1):113. [Google Scholar]
- 21.Ablashi DV, Berneman ZN, Williams M, et al. Ampligen inhibits human herpesvirus-6 in vitro. In Vivo. 1994;8:587–591. [PubMed] [Google Scholar]
- 22.Konishi K, Mukoyama A, Muller WEG. Effect of poly(I). poly(C12U) (Ampligen) on enteric virus (rotavirus, poliovirus and Coxsackie B3 virus) infections. Letters in Applied Microbiology. 1994;19:386–390. doi: 10.1111/j.1472-765X.1994.tb00483.x. [DOI] [Google Scholar]
- 23.Hemispherx Biopharma Inc. Hemispherx Biopharma Announces Phase 3 Chronic Fatigue Syndrome Trial Meets Primary Endpoint. 2004. [Google Scholar]
- 24.Carter W, Strayer D, Mitchell W, et al. Phase III, randomized, double-blinded clinical trial in chronic fatigue syndrome shows significant improvement in exercise treadmill performance with ampligen compared to placebo; 2004. p. 13. [Google Scholar]
- 25.Strayer DR, Carter WA, Brodsky I. A controlled clinical trial with a specifically configured RNA drug, poly(I).poly(C12U), in chronic fatigue syndrome. Clinical Infectious Diseases. 1994;18(Suppl.1):88–95. doi: 10.1093/clinids/18.Supplement_1.S88. [DOI] [PubMed] [Google Scholar]
- 26.Hemispherx Biopharma Inc. Belgian study reports good results, trial is expanded. 1996. [Google Scholar]
- 27.Helix BioPharma Corporation. Belgium Clinical Trial Demonstrates that Ampligen® is Effective in the Treatment of Chronic Fatigue Syndrome. 1996. [Google Scholar]
- 28.O’Brien CB, Garcia G, Henzel B. Ampligen treatment of chronic hepatitis B. Gastroenterology. 1994;106(Suppl.):952. [Google Scholar]
- 29.Hemispherx Biopharma Inc. Hemispherx announces poly I: poly C12U increases the control of HIV during strategic treatment interruption in chronically HIV infected patients. 2002. [Google Scholar]
- 30.Mitchell W, Blick G, Strayer D, et al. A phase IIB prospective, randomized, controlled study evaluating AMPLIGEN during structured treatment interruption of HAART in HIV infection. Antiviral Research. 2003;57:41. doi: 10.1016/S0166-3542(02)00199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemispherx Biopharma Inc. Hemispherx Presents New Data from Phase IIB Clinical Trial of RNA-based Ampligen® for Structured Treatment Interruption in HIV at DART Conference. 2002. [Google Scholar]


