Abstract
Genetics are a major etiological contributor to autism spectrum disorder (ASD). Environmental factors, however, also appear to contribute. ASD pathophysiology due to gene x environment is also beginning to be explored. One reason to focus on environmental factors is that they may allow opportunities for intervention or prevention. Two such factors that have been associated with a significant proportion of ASD risk are prenatal stress exposure and maternal immune dysregulation. Maternal stress susceptibility appears to interact with prenatal stress exposure to affect offspring neurodevelopment. Additionally, understanding of the impact of maternal immune dysfunction on ASD has recently been advanced by recognition of specific fetal brain proteins targeted by maternal autoantibodies, and identification of unique mid-gestational maternal immune profiles. Animal models have been developed to explore pathophysiology targeting both of these factors. We are beginning to understand the behavioral, pharmacopathological, and epigenetic effects related to these interactions, as well as potential mitigating factors. Continued growth in understanding of these mechanisms may ultimately allow for the identification of multiple potential points for prevention or intervention for this subset of environmental-associated ASD cases.
Keywords: Autism spectrum disorder, prenatal stress, immune dysregulation, maternal antibodies, microbiome
INTRODUCTION
While genetics is well-established as a leading contributor to the etiology of autism spectrum disorder (ASD) [1], the importance of non-genetic risk factors is increasingly recognized [2], with heritability estimated at 0.83 by the latest, more conservative, analysis [3], and 0.808 in a recent five country cohort [4]. While considerable research has explored the mechanism of action underlying specific genetic etiologies of ASD, environmental risk factors are less understood. The developmental origins of health and disease (DOHaD) theory proposes that the environment experienced during development in utero influences health after birth [5], including effects salient to ASD. Furthermore, genetics can critically interact with these influences, yielding gene x environment interactions (GxE) of importance for pathophysiology. Understanding the mechanisms of environmental risk may allow for possible prevention or reduced outcome severity, at least in a subset of ASD cases with known genetic risk factors, where the biological effects of environmental exposure can be enhanced due to genetic risk [6].
Several environmental factors that may occur during the prenatal period are associated with increased incidence of ASD. Two broad categories of factors with links to ASD risk are environmental exposures and maternal physiological states.
Maternal exposure to pollutants has consistently been associated with an increased risk, particularly for maternal exposure to air pollutants [7–10], with evidence of an interaction between air pollutant exposure and polymorphisms of the tyrosine kinase MET receptor gene [7]. Increased risk of ASD has also been associated with exposure to medication use in pregnancy, most notably for valproic acid [11]. Previous research has also reported an increased risk of ASD in association with prenatal exposure to β2-adrenergic agonists, commonly used to arrest premature labor, with an interaction between drug exposure and maternal polymorphisms in the β2-adrenergic receptor [12]. Other risk factors are being explored and identified including pesticides, endocrine disrupting chemicals, such as phthalates and bisphenol A, and maternal dietary factors, including a lack of folate supplementation during early pregnancy [13–15]. Increased parental age and short intervals between pregnancies have also been associated with increased risk of ASD [16,17]. Other studies have found no specific association with ASD, such as exposure to heavy metals [18].
Two prominent maternal psychophysiological risk factors repeatedly shown to be associated with the development of ASD are maternal stress exposure during gestation [19–21] and maternal immune dysfunction [22,23]. Elucidating the mechanism of action of these factors may allow multiple points for intervention or prevention.
PRENATAL STRESS
Psychological stress during pregnancy is known to affect behavioral and developmental outcomes in humans in general [24]. Early personality development in children, schizophrenia risk, and emotional disturbances are all impacted by maternal stress [25–28]. Relationships between maternal stress and offspring abnormal fear and anxiety-relevant responses as well as abnormal physiological stress reactivity have been observed persisting into adulthood in animal models [29,30]. The extent of this risk for ASD has been explored in a number of settings [6]. Initial evidence stemmed from surveys completed by mothers of children with ASD, Down syndrome, and neurotypical controls regarding the history and timing of prenatal psychosocial stressors that revealed a higher overall incidence of stressors among mothers of children with ASD compared to other groups. A peak was observed in reported stressors among mothers of children with ASD specifically at 25–28 weeks of gestation, which was not observed in the other groups [20]. Another independent group subsequently found a relationship between the occurrence and severity of tropical storms in Louisiana with specificity to a similar gestational timeframe, during the 5th-6th months of gestation, in association with the incidence of ASD births [21]. Further, prenatal maternal stress after a major Quebec ice storm was also found to be predictive of ASD traits in offspring [31]. Finally, a recent study reported that children with ASD exposed to prenatal stress may in general represent a more severe group than those with no history of prenatal stress exposure [32].
Larger epidemiological studies also support the relationship between prenatal stress and ASD [6]. Although a large Danish national registry study reported no association between maternal bereavement and ASD [33], an association was observed between maternal bereavement and ASD in this study prior to accounting for covariates such as maternal psychiatric conditions [33]. Other reports examining data from a Danish national registry study found that maternal psychiatric conditions were one of the strongest prenatal risk factors for ASD [34]. Reports examining data from a Swedish registry study also revealed a relationship between 3rd trimester maternal stress exposure and risk of ASD [35]. Furthermore, reports examining data from the Nurses’ Health Study revealed that maternal exposure to partner abuse during pregnancy is strongly associated with ASD, although the timing of exposure with the strongest association with ASD was found to be earlier in gestation [19]. A recent meta-analysis has supported the association between prenatal maternal stress and risk of ASD [36].
Stress susceptibility
In each of these studies, a significant proportion of stress-exposed mothers had unaffected children. To understand why prenatal stressors might contribute to ASD only in some cases, GxE models are of particular interest. In GxE models, the effect of stress exposure is more salient in a subset of the population due to genetic risk.
For maternal stress exposure and its relationship to ASD, the serotonin transporter (SERT) gene is of particular interest because of its well-studied role in stress reactivity. The SERT gene encodes for the SERT protein, which transports extracellular serotonin back into the neuron [37]. Genetic variations in this gene can alter aspects of its function, such as protein expression and serotonin uptake [38–40]. The most widely studied variation is an insertion or deletion of a 43 base-pair segment of DNA within the promoter region of the SERT gene, SLC6A4, resulting in a long (L), rather than a short (S) allele [37–39]. Presence of the S-allele has also been linked to suicidality [41], greater cognitive flexibility impairment after stress exposure [42], increased susceptibility to anxiety [39], as well as greater activation of the amygdala, the brain region critical for fear reactions [43] and altered in its function in ASD [44].
This gene also has relevance to ASD outside the setting of stress. Rigid-compulsive behaviors in ASD patients have also been associated with the region of the genome containing SERT in linkage studies [45]. A variation in a single nucleotide on the SERT gene, Gly56Ala, is additionally linked to increased risk of ASD [40]. An additional variant, the Ile425Leu variant, displayed a segregation pattern suggestive of male-biased linkage [46]. Furthermore, the S-allele of the SERT gene has been linked to ASD in some, but not all, studies [47–50].
In a mouse model, offspring of the dams that lacked a SERT gene and were exposed to prenatal stress had decreased social interaction as assessed by social approach and social novelty seeking with the 3-chamber social approach test [51]. The potential clinical salience of this model has been explored. Two independent sample populations of mothers were examined for both the presence of the S-allele as a genetic marker and for the presence of prenatal stress on stress surveys [52]. In both of the independent samples, it was found that the presence of the S-allele and the history of prenatal stress significantly co-segregated in mothers of children with ASD when stress occurred in the later critical period of pregnancy suggested in previous work [20,21,35]. Furthermore, there was no increased report of prenatal stress exposure, regardless of genotype, from these same mothers when queried about pregnancies of unaffected siblings, suggesting that the risk of the S-allele is not an overall increase in maternal recall of stress during pregnancy.
While observation of a specific GxE interaction for one candidate gene provides some insight, a wide variety of genes can interact with the stress response, including a range of other variants that affect SERT function [53]. Understanding general mechanisms by which the physiological effects of stress might act on the developing brain are necessary to move towards potential intervention efforts, and analysis of stress-associated or other genes may reveal molecular mechanisms contributing to an increased risk of ASD in association with prenatal stress. G x E interactions for prenatal stress exposure has received significant recent interest for neuropsychiatric conditions in general [54]. Examining the effect of these broader pathways on downstream neurobiological systems is important for understanding potential avenues of intervention.
One such system downstream of stress pathways is GABAergic inhibitory neuronal circuitry [54]. Significant abnormalities in the GABAergic system have been found in ASD [55,56], including expression of the GABA-producing enzyme, GAD67, which is reduced in post-mortem brains of individuals with ASD [57,58]. Magnetic resonance spectroscopy has shown reduced concentrations of GABA in auditory and motor brain regions and in the frontal cortex in vivo in patients with ASD [59–61]. Prenatal stress has additionally been shown to alter GABAergic neuronal migration and later GABAergic development [56,62,63]. Recent evidence also suggests that prenatal stress effects on other GABAergic progenitor processes, most significantly in striatum, and are most prominent in male offspring [64]. Thus, effects of prenatal stress on the GABAergic system are of interest for ASD.
Prenatal stress and epigenetics
Another potential set of mechanisms by which prenatal stress exposure might impact offspring development includes epigenetic mechanisms. MicroRNAs (miRNAs), small RNA molecules that do not code for proteins, are gene regulatory mechanisms that can be influenced by environmental factors such as stress. Identifying alterations in miRNA after prenatal stress associated with ASD may help narrow the existing gap in our understanding of the mechanisms of GxE in ASD. For example, miRNAs in neonatal offspring brains can be modified by hemizygous deletion of a key placental gene that responds to stress in males only, O-GlcNAc transferase (OGT). OGT is one of a number of placental factors that respond to maternal stress in a sex-specific way, including peroxisome proliferator-activated receptors α (PPARα), insulin-like growth factor-binding protein 1 (IGFBP-1), GLUT4, and HIF3α [65,66]. These prenatal sex differences that influence developmental miRNA in the brain are of particular interest given the higher percentage of males with ASD.
miRNAs play a significant regulatory role in serotonergic pathways [67,68] and immune regulation [69], and are affected by prenatal stress in general [70–72]. In the G x E mouse model encompassing the heterozygous SERT knockout and prenatal stress, gene expression and miRNA changes induced by prenatal stress in offspring brains were greatly attenuated by maternal heterozygous SERT knockout genotype, in contrast to the robust changes observed in offspring brains after prenatal stress exposure in wild type pregnant dams [73]. This attenuation of gene expression and miRNA changes was associated with genome hypermethylation [73] (increased methyl groups bound to specific sites on the gene that affect the expression of that portion of DNA) which appeared to occur only through combined gene and environmental effects. Dysregulation of miR-103, miR-145, miR-219, miR-323, and miR-98 in offspring brain was found due to maternal stress exposure in rats [74], factors that may regulate neuroinflammatory responses and developmental pathways in brain. Additionally, other miRNAs are found to be associated with the stress response. For example, miR-135 regulates response to chronic stress through interaction with serotonergic activity [75], which may relate to the mechanisms contributing to ASD risk. MiR-155 is critical in immunity and inflammation [69]. Lastly, the role of specific miRNAs has been reported in regulating serotonergic genes (Let-7a) [67] and SERT (miR-16 & miR-15a) [76,77] as well as SLC6A4 (miR-325) [78].
Numerous epigenetic markers are also differentially expressed in ASD [79,80], with several involving critical components of the immune system detectable in blood [81]. As diffusible factors in blood are a central route by which maternal stress effects are communicated to offspring, it will be important to determine whether prenatal stress induced expression and methylation changes reported in animals [73] are also observed in maternal blood in prenatal stress-associated ASD cases. Furthermore, GABAergic changes observed in ASD [55,56], and the effects of prenatal stress on GABA systems as well as striatal dopamine [62,82], emphasize the importance of epigenetic changes on genes, such as changes on GABAergic genes related to behavioral deficits [83], which may be correctable by psychopharmacology after prenatal stress in mouse models. Such work may be fruitful for establishing biomarkers to monitor responsiveness, as novel therapeutic approaches are developed targeting these mechanisms. Lastly, Bale and colleagues have shown that epigenetic changes with prenatal stress may be transgenerational [84]. Clinical implications of this transgenerational effect for ASD are unknown, as are the intriguing findings from animal models demonstrating effects of paternal stress exposure mediated by miRNA [85,86]. Additionally, animal model data suggests that preconceptual stress might impact a range of autism-associated behaviors in animal models, particularly in males [87], and in human populations, maternal exposure to childhood abuse has been associated with ASD [88], effects which also are likely mediated epigenetically [89].
Prenatal stress and the microbiome
Another critical factor that may play a role in the mechanism of prenatal stress is the microbiome. Significant attention has recently been drawn to the potential roles of the microbiome in neurodevelopment in general. The central nervous system (CNS) and the enteric nervous system (ENS) have long been known to have a bidirectional interaction, with implications for neurological disorders [90–92]. Recent evidence has suggested the importance of the gut microbiome in these relationships, and its specific importance in behavior [93–95], ENS and CNS neurodevelopment [94–96], CNS disorders [97], and cognitive development [98]. In animal models, the gut microbiome has been demonstrated to affect myelination [99,100] and associations between specific microbial profiles and CNS myelination have also been observed in human samples [101]. Gut microbiome composition has also been associated with differences in temperament in human infants, with some of the relationships observed to be stronger in males [102].
Maternal stress and microbial transmission together play an important role in early life programming and neurodevelopment [103]. Maternal prenatal stress is also known to affect infant intestinal micribiome as well as infant gastrointestinal problems in humans [104], and maternal anxiety also affects maternal microbial composition during pregnancy in humans [105]. In addition, maternal gut microbiota anomalies and/or maternal immune activation induce similar neurodevelopmental abnormalities in mouse offspring [106]. The microbiome has also been shown to be involved in immune regulation of the maternal immune activation (MIA) model of ASD [107]. The MIA model, in which mice are exposed to administration of polyinosinic:polycytidylic acid (poly I:C) models viral infection and results in progeny with ASD-associated abnormalities in behavior and CNS neurodevelopment as well as differences in the intestinal microbiome and intestinal permeability [93]. In one such model, investigators noted a dysbiosis in the stool of MIA progeny mice, particularly in the classes Clostridia and Bacteroides. Interestingly, treating the dysbiosis in these mice with Bacteroides fragilis corrected both the gastrointestinal (GI) permeability defects and ASD-related behavioral abnormalities [93]. These findings converge with human studies that demonstrate that in utero exposure to fever or infection, particularly of viral origin, are prominent risk factors for ASD [108–111]. As described below, immune system impairment has also been noted in both mothers of ASD patients and in ASD patients themselves [112].
The microbiome may be impacted by environmental risk factors other than in utero fever or infection. One recent study explored the mechanism underlying the connection between maternal obesity and increased ASD risk [113]. The investigators in this study demonstrated that the pups derived from a maternal obesity diet-induced model had a microbial dysbiosis that was reversed with microbial reconstitution with Lactobacillus reuteri. Interestingly, these microbial changes were also correlated with normalized oxytocin levels in the CNS and reversal of abnormalities in synaptic potentiation in the ventral tegmental area [114], suggesting that the microbiome may have direct effects on brain signaling in this model.
The microbiome may also play a role in influencing the development or enhancement of comorbidities associated with ASD. For example, there may be a relationship between the microbiome and GI function in these individuals. GI dysfunction is over four-fold more common in children with ASD compared to neurotypical controls [115]. In the first multi-omic study of its kind in ASD, specific microbiome and immune profiles were found to be associated with comorbid gastrointestinal problems and abdominal pain [116]. Anxiety and stress reactivity also commonly co-occur in individuals with ASD, which may influence the microbiome [117] and stress reactivity is associated with GI disturbances in ASD [118]. Further, targeting the microbiome can reverse the impact of chronic stress in mice [119] and has anti-inflammatory effects in the CNS by attenuating stress-induced microglial changes and anxiety-like behavior [120]. In this way, the microbiome may be both a biomarker for and a route for therapeutic targeting of anxiety and stress-reactivity for individuals with ASD. The higher rates of ASD in males may also relate to sex-specific effects of the microbiome on the hippocampal serotonergic system [121], which is critically involved in stress responses.
Based on these interactions between the microbiome, immunity, neurodevelopment and social and affective behaviors [122], the role of the microbiome has received considerable attention for its potential importance in ASD [123–127]. Although there is currently no distinct microbial profile characteristic of ASD or a specific profile that is associated with its neurobehavioral manifestations, clinical trials examining the effects of probiotics and/or fecal microbial transplant on ASD have ensued. Mice administered Lactobacillus reuteri demonstrate improvements in GI and behavioral manifestations in ASD-associated behavioral models associated with environmental exposures including infection and maternal obesity [114,128]. Additionally, administration of Lactobacillus reuteri was found to rescue social deficits in other genetic, environmental, and idiopathic ASD models, which, interestingly, did not appear to be mediated by restoration of the host’s gut microbiome, which remained altered in these models, and instead appearing to interact in a vagal nerve-dependent manner [129]. Further, pilot studies in humans indicated that a probiotic mixture of Lactobacilli and Bifidobacteria can improve GI symptoms and the quality of life in ASD patients [130]. The first fecal transplant study in individuals with ASD had excellent GI and behavioral outcomes. The study, however, was small and open-label [131] and did not address additional questions about immune profiles in ASD that might be secondarily impacted. It has recently been reported, however, that the benefits for gastrointestinal symptoms and autism-related symptoms, and increases in microbial diversity, appeared to be retained two years after the completion of treatment [132], again limited by the possibility of placebo affects in the open-label setting, with the lack of a control group to monitor change over time in untreated individuals, and the small sample.
Gut microbiome studies thus far have been small, utilized very different methodological analyses, and evaluated distinct patient populations [133]. Although these may be reasons why microbial biomarkers have not yet been identified, another possibility is the large heterogeneity of ASD phenotypes [134] including the contributions of prenatal exposures across phenotypes. Highlighting the underlying mechanisms by which ASD phenotypes are guided by the developmental impact of the enteric microbiome on the central and enteric nervous systems may thus further of our understanding of ASD, and may bring us closer to the development of novel therapeutics.
Potential points of intervention for prenatal stress exposure
Certainly, efforts to mitigate the psychological effects of stress during pregnancy would seem prudent to minimize the impact on the developing offspring (see Figure 1). Of course, the risk vs benefit of pharmacological interventions for stress mitigation during pregnancy are unclear. Interventions targeting the microbiome and epigenetic mechanisms would also be of significant interest in this setting. However, intriguing evidence does suggest one low risk intervention.
Figure 1.
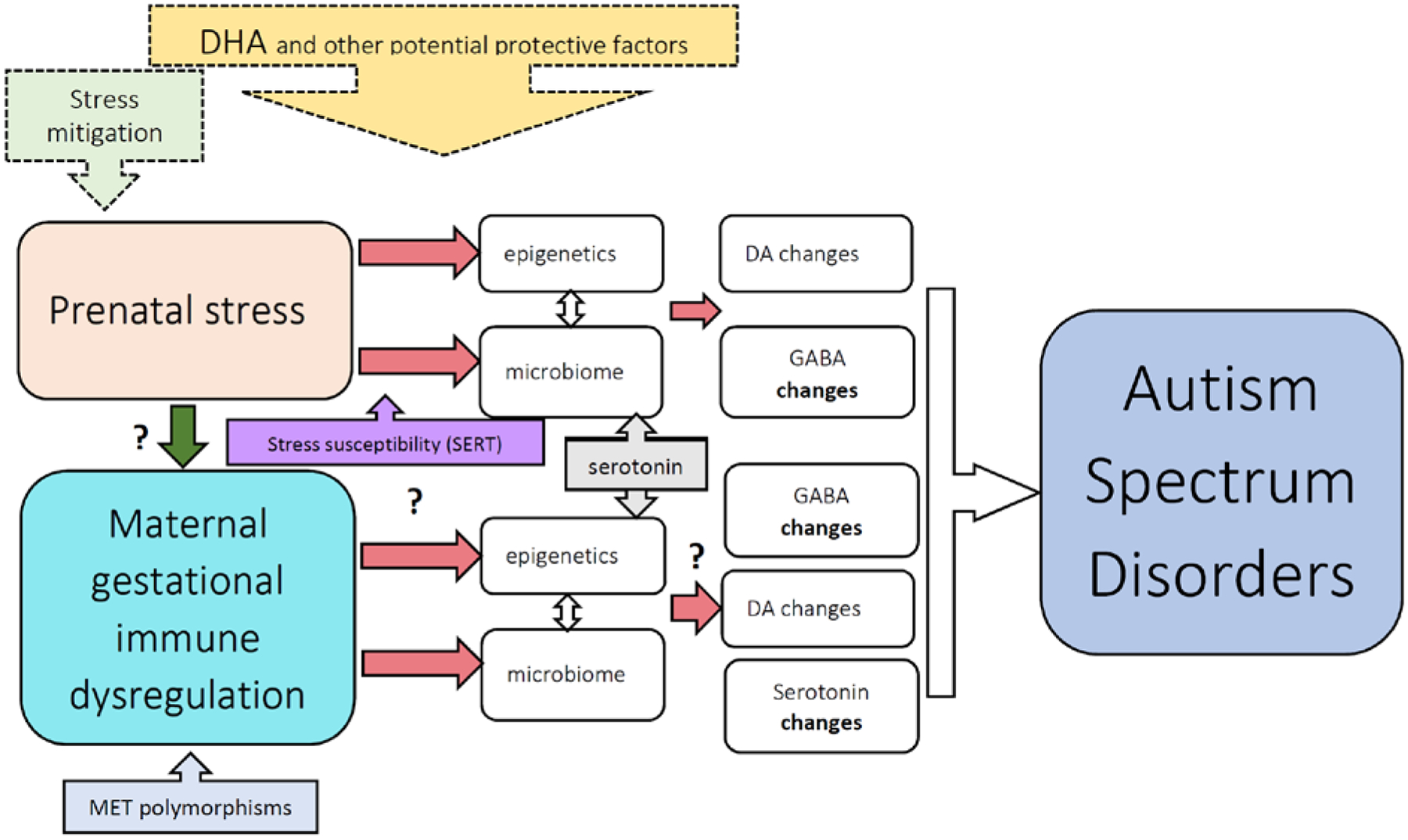
Schematic of potential pathways of effects of prenatal stress exposure and maternal immune dysregulation on neurodevelopment relevant to ASD. Evidence suggests that the microbiome and epigenetic changes as described herein are involved with the mechanism of prenatal stress, with suspected downstream changes on neurotransmitter systems. The effects of prenatal stress can be enhanced by variations in maternal genetics impacting other neurotransmitter systems. The specific mechanisms are less established for maternal gestational immune dysregulation. While we know some of the effects of maternal autoantibodies on developmental pathways, we are less clear on the effects of maternal inflammation during gestation. Maternal immune dysregulation can also be impacted by genetics. Further, how to mitigate these effects is still under investigation. This figure designed more to be illustrative than comprehensive, suggests several potential points for intervention along these pathways. Efforts at stress mitigation would clearly act on the prenatal stress level, and DHA and other potential protective factors may act at a level afferent to the neurotransmitter effects.
In addition to the aforementioned GABAergic changes observed with exposure to prenatal stress, recent evidence has also revealed that prenatal stress-exposed mice from dams with heterozygous KO of SERT have significantly increased striatal dopamine [82]. It will be of future interest to determine whether ASD patients exposed to prenatal stress also represent a subgroup with significant striatal changes. Notably, administration of 1% docosahexaenoic acid (DHA) omega-3 fatty acid throughout pregnancy in dams, continuing the diet given to pups, reversed repetitive grooming behaviors, social interaction abnormalities, and altered striatal dopamine in mice exposed to prenatal stress born from dams with only one copy of the SERT gene, as compared to offspring that were untreated with DHA or only given DHA after birth [82]. While the clinical implications of this are as of yet unknown, DHA has been of particular interest due to its effects on a range of other neurological conditions due to its effects on a range of anti-oxidant pathways [135], and some evidence suggests synergistic effects with other anti-oxidant agents [136]. Additionally, redox dysregulation and maternal antioxidants may significantly impact offspring GABAergic system development [137]. Effects of DHA on redox regulation, the GABAergic system and on epigenetics in this setting are as of this time unknown. However, DHA has the advantage of being widely used, safely, during pregnancy. Furthermore, recent evidence suggests that combination with other biobotanicals, such as quercetin, can significantly lower the dose of DHA required to have a given level of antioxidant effect [136]. Finally, changes in the western diet across time to include a greater pro-inflammatory diet and a lower proportion of omega-3 fatty acids [138] might be relevant, and research might be warranted to determine whether a diet characterized by greater anti-inflammatory effects might serve to be protective against the impact of prenatal stress on offspring.
PRENATAL IMMUNE DYSREGULATION
Under normal conditions, the maternal immune system maintains a pathogen-free and non-inflammatory environment for the developing fetus [139,140]. Disruption of immune factors including cytokines, chemokines, and autoimmune sequelae including the production of autoantibodies during gestation can have adverse developmental consequences for the fetus. Epidemiological research has consistently found maternal fever or infection (viral, bacterial, and parasitic) during the first and/or second trimester are associated with increased risk of neurodevelopmental disorders (NDD), including ASD [141–146]. Further, using data from a large multi-site study, it was noted that women who had an infection during the second trimester of pregnancy accompanied by a fever were more likely to have children with ASD [147]. Thus, the above findings suggest that only more severe infections accompanied by a robust inflammatory response increase the risk of ASD in the child, and this phenomenon is not dependent on the pathogen itself, but rather the maternal response to the pathogen.
The maternal immune response to pathogens is largely driven by cytokines, which are signaling molecules produced by immune and other cells in response to infectious stimuli. In addition to directing the expansion or suppression of immune cells, the production of cytotoxic factors, and the production of antibodies, cytokines also mediate signals between the immune and nervous systems. This interaction can impact neuronal development and pruning, thereby shaping the developing brain. Cytokines and chemokines are involved in numerous aspects of typical neurodevelopment, including proliferation and differentiation of neural and glial cells, neuronal migration, dendritic branching, and synapse formation [148,149]. Some maternal cytokines may cross the placenta during gestation, as in the case of IL-6 [150–152], or act on placental cells to stimulate the downstream production of immune and other mediators in the fetal compartment [153]. The placenta of males has shown greater fluctuations in cytokine profiles and other changes after prenatal stress [84].
Fluctuations in the maternal levels of cytokines and chemokines can alter normal neurodevelopmental trajectories, potentially resulting in altered brain morphology and behavior in the offspring. Results derived from epidemiological studies utilizing mid-gestational maternal samples have further strengthened the notion that cytokine and chemokine dysregulation contributes to altered neurodevelopment relevant to ASD in offspring. Archived maternal serum from routine prenatal testing at 15–19 weeks of pregnancy was assessed in the Early Markers for Autism (EMA) Study, (a nested case-control prospective study) in order to investigate the relationship between mid-gestational maternal cytokines and chemokines and the risk of having a child with ASD or DD. In the pilot EMA study, increased maternal levels of circulating IFNγ, IL-4, and IL-5 at 15 to 19 weeks of gestation have been reported in mothers of children with ASD as compared to mothers of general population (GP) control children [154]. In addition, increased mid-gestational levels of IL-2, IL-4, and IL-6 were found in mothers of children with developmental delay (DD) compared to the mothers of GP controls [154], suggesting that unique mid-gestational cytokine profiles might influence specific neurodevelopmental processes and result in different types of NDDs. In the subsequent larger EMA study, levels of 22 cytokines and chemokines were measured on an independent sample of 1,031 mid-gestational maternal specimens. Significant elevation in the mid-gestational levels of several pro-inflammatory cytokines and chemokines was found to be distinctly associated with an increased risk of having a child with ASD with intellectual disability (ASD+ID) compared to both GP and DD groups [155]. This larger study enabled the differentiation between ASD sub-phenotypes and suggests that mothers of children with ASD and ID have significantly elevated mid-gestational levels of inflammatory cytokines/chemokines compared to all other groups examined. The immunologic distinction between mothers of children with ASD and ID and those with ASD without ID or DD without ASD suggests that the ID associated with ASD might be etiologically distinct from DD without ASD [155]. Studies thus far have primarily been cross-sectional, with limited assessment of the relative importance of different stages of pregnancy or of differential effects in males and females. Based on studies of gestational cytokine profiles during a typical pregnancy [156,157], greater differences could be induced in cytokines between the 1st and 2nd trimesters, prior to the development of the maximal cellular immune regulation needed to avoid fetal rejection.
Animal models have provided additional evidence that specific maternal immune factor changes, including those observed in maternal immune activation, can affect behavior and neuromorphology in offspring [158–162]. These immune-mediated effects have been employed to model several neurodevelopmental disorders, including schizophrenia [163–165], cerebral palsy [166], and ASD [167,168]. Additionally, the effects of maternal immune activation have been demonstrated to be long-lasting on offspring brain development and behavior [159,169,170]. Such studies also provide the opportunity to explore the mechanisms by which maternal immune factors alter the neurodevelopmental trajectory. A striking example of these principles involves maternal autoantibodies reactive towards fetal brain proteins which have been observed in a significant number of mothers of children with ASD while only rarely being detected in mothers of unaffected children [171–173]. Maternal IgG antibodies readily cross the placenta during pregnancy in humans to equip the immunologically naïve fetus with antibodies to protect against infectious agents. These maternal IgG antibodies persist up to six months postnatally [174]. Along with IgG antibodies that are immunoprotective, however, autoantibodies that react to fetal ‘self’-proteins are also able to cross the placenta. Several neonatal autoimmune diseases are known to result from this transfer of pathogenic maternal IgG [175–177] and reports are suggestive of a similar role of these autoantibodies in ASD [178–181]. Additional studies have confirmed that these autoantibodies are present in mothers for up to 18 years following the birth of an affected child [182]. Polymorphisms of the MET gene appears to relate to the maternal production of these autoantibodies [183]. Furthermore, these IgG maternal autoantibodies, when derived from human samples and incorporated into animal models, have been demonstrated to produce ASD-relevant behaviors [184]. In a non-human primate study, IgG isolated from mothers of children with ASD was administered intravenously to rhesus monkey dams during gestation, resulting in notable whole-body stereotypies and increased motor activity at 15 months [185]. The specific autoantibodies involved have been recently identified as the fetal brain proteins lactate dehydrogenase A and B (LDH-A, LDH-B), collapsin response mediator proteins 1 and 2 (CRMP1, CRMP2), Y-box binding protein 1 (YBX1), stress-induced phosphoprotein 1 (STIP1), and guanine deaminase (GDA) [172]. In this initial discovery study, maternal reactivity to particular combinations of these autoantigens is significantly associated with an outcome of ASD in the child. When all autoantigen reactivity patterns were combined, a total of ~20% of mothers of children with ASD had one of the autoantibody patterns containing two or more of the target autoantigenic proteins relative to <1% of control mothers. Non-human primate studies using passive transfer of human IgG reactive to the LDH, CRMP1, STIP1 ASD-specific pattern have also demonstrated that antibody profiles in ASD may be related to early neurodevelopmental alterations, as well as brain overgrowth in exposed offspring, a finding that was specific to males [186]. Further, an LDH, CRMP1, STIP1 antigen-driven endogenous mouse model of maternal autoantibody related (MAR) ASD has recently demonstrated that prenatal exposure to these ASD-specific maternal autoantibodies resulted in ASD-relevant alterations to behavior and neuroanatomical measures in prenatally exposed offspring for both males and females [23]. Immune alterations in the brain, including neuroglial (i.e. microglial) activation and CNS inflammation [187,188] have been noted in individuals with ASD. Furthermore, circulating antibodies reactive to neuronal tissue have been observed in children with ASD [171,181,189–193]. These studies suggest that immune dysregulation in the ASD child is also of potential significance. At this time, we do not have recognized treatment options for either the prenatal inflammatory sequelae or for the mitigation of the maternal autoantibody response.
As stress can have a notable impact on immune function [138], and maternal immune challenges such as infection during pregnancy increase ASD risk [145], the roles of brain-reactive autoantibodies and other immune molecules are of significant interest for understanding prenatal exposure more broadly. In many settings, chronic stress is considered an immunosuppressant. However, the immunosuppressant effects of stress can cause paradoxical pro-inflammatory reactions by reactivation of latent viruses [194] and acute stress responses may be pro-inflammatory [195]. Prenatal stress effects on microglia in mouse offspring have been shown to involve maternal IL-6 signaling [196]. Prenatal stress has been demonstrated to increase cord-blood IgE levels [197] and, in a non-human primate model, may influence maternal transfer of IgG to offspring in a sex-dependent manner [198]. Furthermore, recent evidence revealed strong relationships between SERT function, previously discussed as important in stress regulation, and the immune response, including SERT expression in B-cells responsible for antibody production [199]. With these known interactions between stress, serotonin, and immunity, it will be of interest to see how these and other stress-related factors interact in clinical populations at risk for ASD.
Potential points of intervention for prenatal immune dysregulation
If it is indeed revealed that prenatal stress and maternal immunity are related, interventions targeting prenatal stress exposure might also have an impact on maternal immunity (see Figure 1). Furthermore, mitigation of inflammation during pregnancy may also affect systems related to the stress response. As we come to better understand the relationship between stress, immune dysregulation and neurodevelopment, we will be better able to construct targeted interventions to reduce the impact on neurodevelopmental outcome. In general, given that downstream disruptions arising from prenatal stress and maternal immunity in the offspring may be the same, interventions to correct previously induced changes at the level of the offspring or through maternal intervention during the critical lactation period may be effective. Recent evidence has revealed that exercise reverses the behavioral and synaptic abnormalities that arise after maternal inflammation [200], raising the possibility of exercise interventions for other types of maternal immune dysregulation, and perhaps the possibility from other interventions that might target similar mechanisms. Finally, as with prenatal stress, and given the relationship between pro-inflammatory diets and microbiome [201], and the increase in the pro-inflammatory effects of the western diet across recent history [138], research might be warranted to determine whether a diet with greater anti-inflammatory content might also serve to be protective against the effects of maternal immune dysregulation on offspring.
CONCLUSIONS
While genetic factors are a major contributor to the etiology of ASD, mounting evidence supports a role for environmental factors, allowing possibilities for prevention or early intervention. Prenatal stress and maternal immune dysfunction appear to contribute in some way to a significant proportion of these ASD cases. Efforts towards gaining a better understanding of how these factors interact with genetic susceptibility, particularly in mothers, will result in an increased ability to identify those individuals at greatest risk of developing ASD with such exposures. Furthermore, research aimed at gaining an understanding of downstream mechanisms will allow for the identification of multiple potential points for novel preventative measures and/or interventions (Figure 1). It will also be important to see if these factors result in common downstream mechanistic pathways with some of the genetic contributors to ASD, allowing for a more comprehensive approach for intervention in ASD based on personalized therapeutic approaches [202]. While interventions that target these mechanisms are as of yet unknown, evidence is beginning to suggest several intriguing possible approaches.
ACKNOWLEDGMENTS
Dr. Beversdorf receives funding from the Department of Defense, Autism Treatment Network, and the National Institutes of Health.
ABBREVIATIONS
- ASD
autism spectrum disorder
- CNS
central nervous system
- CRMP
collapsin response mediator proteins
- DD
developmental delay
- DHA
docosahexaenoic acid
- DNA
deoxyribonucleic acid
- DOHaD
developmental origins of health and disease
- EMA
Early Markers for Autism
- ENS
enteric nervous system
- GABA
gamma amino butyric acid
- GDA
guanine deaminase
- GP
general population
- GI
gastrointestinal
- GxE
gene x environment interactions
- ID
intellectual disability
- Ig
immunoglobulin
- IGFBP-1
insulin-like growth factor-binding protein 1
- KO
knockout
- LDH
lactate dehydrogenase
- L-allele
long allele
- MAR
maternal autoantibody related
- MIA
maternal immune activation
- miRNA
MicroRNAs
- NDD
neurodevelopmental disorders
- OGT
O-GlcNAc transferase
- poly I:C
polyinosinic:polycytidylic acid
- PPARα
peroxisome proliferator-activated receptors α
- RNA
ribonucleic acid
- S-allele
short allele
- SERT
serotonin transporter
- STIP
stress-induced phosphoprotein
- YBX
Y-box binding protein
Footnotes
CONFLICTS OF INTEREST
Dr. Beversdorf serves in an advisory capacity for Yamo Pharmaceuticals, Quadrant Biosciences, and Stalicla, unrelated to this work. Drs Stevens, Margolis, and Van de Water have nothing to disclose.
REFERENCES
- 1.The Autism Genome Project Consortium. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nature Genet 2007; 39: 319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychaitry 2011; 68: 1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. Heritability of autism spectrum disorder. J Am Med Assoc 2017; 318: 1182–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai D, Yip BHK, Windham GC, Sourander A, Francis R, Yoffe R, Glasson E, Mahjani B, Suominen A, Leonard H, Gissler M, Buxbaum JD, Wong K, Schendel D, Kodesh A, Breshnahan M, Levine SZ, Parner ET, Hansen SN, Hultman C, Reichenberg A, Sandin S. Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry 2019; Epub ahead of print. doi: 10.1001/jamapsychiatry.2019.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gage SH, Munafò MR, Smith GD. Causal inference in developmental origins of health and disease (DOHaD) research. Annu Rev Psychol 2016; 67: 567–85. [DOI] [PubMed] [Google Scholar]
- 6.Beversdorf DQ, Stevens HE, Jones KL. Prenatal stress, maternal immune dysregulation, and their association with autism spectrum disorders. Curr Psychiatry Rep 2018; 20(9): 76. doi: https://doi.org/10/1007/s11920-018-0945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volk HE, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, Campbell DB. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology 2014; 25: 44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology 2014; 25:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, Weisskopf MG. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect 2015; 123: 264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, Thayer BP, Daniels JL. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 2015; 26:30–42. [DOI] [PubMed] [Google Scholar]
- 11.Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol 2009; 28: 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Connors SL, Crowell DE, Eberhart CG, Copeland J, Newschaffer CJ, Spence SJ, Zimmerman AW. β2-adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol 2005; 20: 876–84. [DOI] [PubMed] [Google Scholar]
- 13.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol 2014; 43: 443–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry 2014; 4: e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, Lie K, Lipkin I, Magnus P, Reichborn-Kjennerud T, Schjølberg S, Davey Smith G, Øyen A- S, Susser E, Stoltenberg C. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. J Am Med Assoc 2013; 309: 570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, Sjölander A, Larsson H, Lichtenstein P. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry 2014; 71: 432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheslack-Postava K, Suominen A, Jokiranta E, Lehti V, McKeague IW, Sourander A, Brown AS. Increased risk of autism spectrum disorders at short and long interpregnancy intervals. Child Adolesc Psychiatry 2014; 53:1074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdullah MM, Ly AR, Goldberg WA, Clarke-Stewart KA, Dudgeon JV, Mull CG, Chan TJ, Kent KE, Mason AZ, Ericson JE. Heavy metal in children’s tooth enamel: related to autism and disruptive behaviors? J Autism Dev Disord 2012; 42: 929–36. [DOI] [PubMed] [Google Scholar]
- 19.Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Maternal exposure to intimate partner abuse before birth is associated with risk of autism spectrum disorder in offspring. Autism 2016; 20: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev Disord 2005; 35: 471–8. [DOI] [PubMed] [Google Scholar]
- 21.Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Devel Disord 2008; 28: 481–8. [DOI] [PubMed] [Google Scholar]
- 22.Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacol 2017; 42: 284–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones KL, Pride MC, Edmiston E, Yang M, Silverman JL, Crawley JN, Van de Water J. Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Mol Psychiatry 2019; [Epub ahead of print] doi: 10.1038/s41380-018-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson G, Ashman SB, Carver LJ. 2000. The role of early experience in shaping behavioral and brain development and its implications for social policy. Devel Psychopathol 2000; 12: 695–712. [DOI] [PubMed] [Google Scholar]
- 25.Niederhofer H, Reiter A. Maternal stress during pregnancy, its objectivation by ultrasound observation of fetal intrauterine movements and child’s temperament at 6 months and 6 years of age: A pilot study. Psychol Rep 2000; 86: 526–8. [DOI] [PubMed] [Google Scholar]
- 26.Van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia – the May 1940 invasion of The Netherlands. Br J Psychiatry 1998; 172: 324–6. [DOI] [PubMed] [Google Scholar]
- 27.Ward AJ. A comparison and analysis of the presence of family problems during pregnancy of mothers of “autistic” children and mothers of typically developing children. Child Psychiatry Hum Devel 1990; 20: 279–88. [DOI] [PubMed] [Google Scholar]
- 28.Ward AJ. Prenatal stress and child psychopathology. Child Psychiatry Hum Devel 1991; 22: 97–110. [DOI] [PubMed] [Google Scholar]
- 29.Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav 2000; 70: 359–66. [DOI] [PubMed] [Google Scholar]
- 30.Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neurosci Biobehav Rev 1997; 21: 1–10. [DOI] [PubMed] [Google Scholar]
- 31.Walder DJ, Laplante DP, Sousa-Pires A, Veru F, Brunet A, King S. Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res 2014; 219: 353–60. [DOI] [PubMed] [Google Scholar]
- 32.Vacrin KJ, Alvares GA, Uljarević M, Whitehouse AJO. Prenatal maternal stress and phenotypic outcomes in autism spectrum disorder. Autism Res 2017; 10: 1866–77. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Vestergaard M, Obel C, Christensen J, Precht DH, Lu M, Olsen J. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics 2009; 123: 1102–7. [DOI] [PubMed] [Google Scholar]
- 34.Larsson JH, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, Schendel D, Thorsen P, Mortensen PB. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiology 2005; 161: 916–25. [DOI] [PubMed] [Google Scholar]
- 35.Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, Hultman CM, Långström N, Lichtenstein P, D’Onofrio BM. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med 2014; 44: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzari N, Matvienko-Sikar K, Baldoni F, O’Keefe GW, Khashan AS. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol 2019; doi: 10.1007/s00127-019-01745-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: Gene, genetic disorders, and pharmacogenomics. Mol Interv 2004; 4: 109–123. [DOI] [PubMed] [Google Scholar]
- 38.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66: 2621–4. [DOI] [PubMed] [Google Scholar]
- 39.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274: 1527–31. [DOI] [PubMed] [Google Scholar]
- 40.Prasad HC, Zhu CB, McCauley J, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A 2005; 102: 11545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bondy B, Buettner A, Zill P. Genetics of suicide. Mol Psychiatry 2006; 11: 336–51. [DOI] [PubMed] [Google Scholar]
- 42.Beversdorf DQ, Carpenter AL, Alexander JK, Jenkins NT, Tilley MR, White CA, Hillier AJ, Smith RA, Gu HH. Influence of serotonin transporter SLC6A4 genotype on the effect of psychosocial stress on cognitive performance: an exploratory pilot study. Cogn Behav Neurol 2018; 31: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297: 400–3. [DOI] [PubMed] [Google Scholar]
- 44.Hennessey T, Andari E, Rainnie DG. RDoC-based categorization of amygdala functions and its implications in autism. Neurosci Biobehav Rev 2018; 90: 115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, Folstein SE, Haines JL, Sutcliffe JS. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid compulsive subset of autism. Am J Med Genet B Neuropsychiatr Genet 2004; 127B:104–12. [DOI] [PubMed] [Google Scholar]
- 46.Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 2016; 432: 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brune C, Kim S, Salt J, Leventhal B, Lord C, Cook E. 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry 2006. 163: 2148–56. [DOI] [PubMed] [Google Scholar]
- 48.Cook E, Courchesne R, Lord C, Cox N, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal B. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry 1997; 2: 247–50. [DOI] [PubMed] [Google Scholar]
- 49.Losh M, Sullivan P, Trembath D, Piven J. Current developments in the Genetics of Autism: From Phenome to Genome. J Neuropathol Exp Neurol 2008; 67: 829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong N, Ye L, Ju W, Brown W, Tsiouris J, Cohen I. 5-HTTLPR variants not associated with autistic spectrum disorders. Neurogenetics 1999; 2: 129–31. [DOI] [PubMed] [Google Scholar]
- 51.Jones KL, Smith RM, Edwards KS, Givens B, Tilley MR, Beversdorf DQ. Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction. Int J Devel Neurosci 2010; 28: 529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hecht PM, Hudson M, Connors SL, Tilley MR, Liu X, Beversdorf DQ. Maternal serotonin transporter genotype affects risk for ASD with exposure to prenatal stress. Autism Res 2016; 9: 1151–60. [DOI] [PubMed] [Google Scholar]
- 53.Murphy DL, Maile MS, Vogt NM. 2013. 5HTTLPR: white knight or dark blight? ACS Chem Neurosci 2013; 4: 13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbott PW, Gumusoglu SB, Bittle J, Beversdorf DQ, Stevens HE. Prenatal stress and genetic risk: how prenatal stress interacts with genetics to alter risk for psychiatric illness. Psychoneuroendocrinol 2018; 90: 9–21. [DOI] [PubMed] [Google Scholar]
- 55.Smith-Hicks CL. GABAergic dysfunction in pediatric neuro-developmental disorders. Front Cell Neurosci 2013; 7: 00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry 2014; 19: 641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry 2002; 52: 805–10. [DOI] [PubMed] [Google Scholar]
- 58.Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol 2007; 113: 559–68. [DOI] [PubMed] [Google Scholar]
- 59.Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TP. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage 2014; 86: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage 2014; 86: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord 2011; 41: 447–54. [DOI] [PubMed] [Google Scholar]
- 62.Lussier SJ, Stevens HE. Delays in GABAergic Interneuron Development and Behavioral Inhibition after Prenatal Stress. Devel Neurobiol 2016; 76: 1078–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens HE, Su T, Yanagawa Y, Vaccarino FM. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinol 2013; 38: 509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevens H, Lussier S, Michaelson J, Radhakrishna S, Elser B. Embryonic GABAergic proliferation as a contributing mechanism of sex differences in prenatal stress effects on brain and behavior. Neuropsychopharmacology 2017; 43: S497 [Google Scholar]
- 65.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress 2011; 14: 348–56. [DOI] [PubMed] [Google Scholar]
- 66.Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci U S A 2013; 110: 5169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Millan MJ. MicroRNA in the regulation and expression of serotonergic transmission in the brain and other tissues. Curr Opin Pharmacol 2011; 11: 11–22 [DOI] [PubMed] [Google Scholar]
- 68.Bai M, Zhu XZ, Zhang Y, Zhang S, Zhang L, Xue L, Zhong M, Zhang X. Anhedonia was associated with the dysregulation of hippocampal HTR4 and microRNA Let-7a in rats. Physiol Behav 2014; 129: 135–41. [DOI] [PubMed] [Google Scholar]
- 69.Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013; 532: 1–12. [DOI] [PubMed] [Google Scholar]
- 70.Monteleone MC, Adrover E, Pallarés ME, Antonelli MC, Frasch AC, Brocco MA. Prenatal stress changes the glycoprotein GPM6A gene expression and induces epigenetic changes in rat offspring brain. Epigenetics 2014; 9: 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh NP, Singh UP, Guan H, Nagarkatti P, Nagarkatti M. Prenatal exposure to TCDD triggers significant modulation of microRNA expression profile in the thymus that affects consequent gene expression. PLoS One 2012; 7: e45054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 2015; 48: 70–91. [DOI] [PubMed] [Google Scholar]
- 73.Sjaarda CP, Hecht P, McNaughton AJM, Zhou A, Hudson ML, Will MJ, Smith G, Ayub M, Liang P, Chen N, Beversdorf D, Liu X. Interplay between maternal Slc6a4 mutation and prenatal stress: a possible mechanism for autistic behavior development. Scientific Rep 2017; 7: 8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, Kovalchuk I, Kovalchuk O, Metz GA. Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS One 2013; 8: e56967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, Gil S, Mayberg HS, Dunlop BW, Menke A, Awatramani R, Binder EB, Deneris ES, Lowry CA, Chen A. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 2014; 83: 344–60. [DOI] [PubMed] [Google Scholar]
- 76.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 2010; 329: 1537–41. [DOI] [PubMed] [Google Scholar]
- 77.Moya PR, Wendland JR, Salemme J, Fried RL, Murphy DL. miR-15a and miR-16 regulate serotonin transporter expression in human placental and rat brain raphe cells. Int J Neuropsychopharmacol 2013; 16: 621–9. [DOI] [PubMed] [Google Scholar]
- 78.Arisawa T, Tahara T, Fukuyama T, Hayashi R, Matsunaga K, Hayashi N, Nakamura M, Toshikuni N, Shiroeda H, Shibata T. Genetic polymorphism of pri-microRNA 325, targeting SLC6A4 3’-UTR, is closely associated with the risk of functional dyspepsia in Japan. J Gastroenterol 2012; 47: 1091–8. [DOI] [PubMed] [Google Scholar]
- 79.Hu VW, Frank BC, Heine S, Lee NH, Quackenbush J. Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genomics 2006; 7: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen AT, Rauch RA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J 2010; 24: 3036–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience 2014; 275: 420–35. [DOI] [PubMed] [Google Scholar]
- 82.Matsui F, Hecht P, Yoshimoto K, Watanabe Y, Morimoto M, Fritsche K, Will M, Beversdorf D. DHA mitigates autistic behaviors accompanied by dopaminergic change on a gene/prenatal stress mouse model. Neuroscience 2017; 371: 407–19. [DOI] [PubMed] [Google Scholar]
- 83.Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology 2013; 68: 184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 2015; 16: 332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013; 33: 9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA 2005; 112: 13699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pisu MG, Boero G, Garau A, Casula C, Cisci S, Biggio F, Concas A, Follesa P, Maciocco E, Porcu P, Serra M. Are preconceptual stressful experiences crucial elements of the aetiology of autism spectrum disorder? Insights from an animal model. Neuropharmacol 2019; 157:107686. doi: 10.1016/j.neuropharm.2019.107686. Epub 2019 Jun 25. [DOI] [PubMed] [Google Scholar]
- 88.Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. 2013. Maternal exposure to childhood abuse is associated with elevated risk of autism. JAMA Psychiatry 2013; 70: 508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan JC, Nugent BM, Bale TL. Parental advisory: maternal and paternal stress can impact offspring neurodevelopment. Biol Psychiatry 2018; 83:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 2016; 13: 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Israelyan N, Margolis KG. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol Res 2018; 132:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Margolis KG. A role for the serotonin reuptake transporter in the brain and intestinal features of autism spectrum disorders and developmental antidepressant exposure. J Chem Neuroanat 2017; 83–84: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013; 155:1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015; 17:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelly JR, Minuto C, Cryan JF, et al. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front Neurosci 2017; 11: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kabouridis PS, Pachnis V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J Clin Invest 2015; 125: 956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun 2014; 38: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, Thompson AL, Geng X, Gilmore JH, Knickmeyer RC. Infant gut microbiome associated with cognitive development. Biol Psychiatry 2018; 83:148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayer EA, Knight R, Mazmanian SK, et al. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 2014. 34: 15490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016; 6: e774; doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ong IM, Gonzalez JG, McIlwain SJ, Sawin EA, Schoen AJ, Adluru N, Alexander AL, Yu JJ. Gut microbiome populations are associated with structure-specific changes in white matter architecture. Transl Psychiatry 2018; 8: 6; doi: 10.1038/s41398-017-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aatsinki A- K, Lahti L, Uusitupa H- M, Munukka E, Keskitalo A, Nolvi S, O’Mahony S, Pietilä S, Elo LL, Eerola E, Karlsson H, Karlsson L. Gut microbiota composition is associated with temperament traits in infants. Brain Behav Immun 2019; 80: 849–58. [DOI] [PubMed] [Google Scholar]
- 103.Jašarević E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol Stress 2015; 1: 81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinol 2015; 53: 233–45. [DOI] [PubMed] [Google Scholar]
- 105.Hechler C, Borewicz K, Beijers R, Saccenti E, Riksen-Walraven M, Smidt H, de Weerth C. Association between psychosocial stress and fecal micobiota in pregnant women. Scientific Rep 2019; 9: 4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim S, Kim H, Yim YS, Ha S, Atarshi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017; 549: 528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lammert CR, Frost EL, Bolte AC, Paysour MJ, Shaw ME, Bellinger CE, Weigel TK, Zunder ER, Lukens JR. Cutting edge: critical roles for microbiota-mediated regulation of the immune system in a prenatal immune activation model of autism. J Immunol 2018; 201: 845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Isaksson J, Pettersson E, Kostrzewa E, et al. Brief Report: Association Between Autism Spectrum Disorder, Gastrointestinal Problems and Perinatal Risk Factors Within Sibling Pairs. J Autism Dev Disord 2017; 47: 2621–7. [DOI] [PubMed] [Google Scholar]
- 109.Hornig M, Bresnahan MA, Che X, et al. Prenatal fever and autism risk. Mol Psychiatry 2018; 23: 759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahic M, Mjaaland S, Bovelstad HM, et al. Maternal Immunoreactivity to Herpes Simplex Virus 2 and Risk of Autism Spectrum Disorder in Male Offspring. mSphere 2017; 2: e00016–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang HY, Xu LL, Shao L, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav Immun 2016; 58: 165–72. [DOI] [PubMed] [Google Scholar]
- 112.Goines P, Van de Water J. The immune system’s role in the biology of autism. Curr Opin Neurol 2010; 23: 111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Connolly N, Anixt J, Manning P, Ping-I Lin D, Marsolo KA, Bowers K. Maternal metabolic risk factors for autism spectrum disorder – An analysis of electronic medical records and linked birth data. Autism Res 2016; 9: 829–37. [DOI] [PubMed] [Google Scholar]
- 114.Buffington SA, Di Prisco GV, Auchtung TA, et al. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016; 165: 1762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McElhanon BO, McCracken C, Karpen S, et al. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 2014; 133: 872–83. [DOI] [PubMed] [Google Scholar]
- 116.Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC. Distinct microbiome-neuroimmune signatures correlated with functional abdominal pain in children with autism spectrum disorder. Cell Mol Gastroenterol Hepatol 2017; 3: 218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 2017; 7:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ferguson BJ, Marler S, Altstein LL, Lee EB, Mazurek M, McLaughlin A, Macklin EA, McDonnell E, Davis DJ, Belenchia AM, Gillespie CH, Peterson CA, Bauman ML, Margolis KG, Veenstra-VanderWeele J, Beversdorf DQ. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brain Behav Immun 2016; 58: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 2017; 82: 472–87. [DOI] [PubMed] [Google Scholar]
- 120.Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Schmidt D, Watkins LR, Maier SF, Lowry CA. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav Immun 2018; [Epub ahead of print] doi: 10.1016/j.bbi.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 2013; 18: 666–73. [DOI] [PubMed] [Google Scholar]
- 122.Sylvia KE, Demas GE. A gut feeling: microbiome-brain-immune interactions modulate social and affective behaviors. Horm Behav 2018; 99:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding HT, Taur Y, and Walkup JT. Gut microbiota and autism: key concepts and findings. J Autism Dev Disord 2017; 47: 480–9. [DOI] [PubMed] [Google Scholar]
- 124.Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci 2017; 11: 120. doi: 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Q, Zhou J-M. The microbiota-gut-brain axis asn its potential therapeutic role in autism spectrum disorder. Neurosci 2016; 324: 131–9. [DOI] [PubMed] [Google Scholar]
- 126.Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep 2013; 15: 337. doi. 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychaitry 2017; 81: 411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Y, Fatheree NY, Mangalat N, et al. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 2010; 299: G1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattioli M. Mechanisms underlying microbial-mediated changes in socail behavior in mouse modesl of autism spectrum disorder. Neuron 2019; 101: 256–59.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arnold LE. Probiotics for Quality of Life in Autism Spectrum Disorders. https://clinicaltrials.gov/ct2/show/NCT02903030. Accessed October 4, 2019.
- 131.Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017; 5: 10. doi: 10.1186/s40168-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kang D-W, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown R. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Scientific Rep 2019; 9: 5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Navarro F, Liu Y, Rhoads JM. Can probiotics benefit children with autism spectrum disorders? World J Gastroenterol 2016; 22: 10093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol 2014; 10: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannick M, Gu Z, Greenlief CM, Yao JK, Lee JC, Beversdorf D. Docosahexaenoic acid (DHA): an essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2018; 136: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun GY, Li R, Yang B, Fritsche KL, Beversdorf DQ, Lubahn DB, Geng X, Lee JC, Greenlief CM. Quercetin potentiates docosahexaenoic acid to suppress lipopolysaccharide-induced oxidative/inflammatory responses, alter lipid peroxidation products, and enhance the adaptive stress pathways in BV-2 microglial cells. Int J Mol Sci 2019; 20: 932. doi: 10.3390/ijms20040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bittle J, Menezes EC, McCormick ML, Spitz DR, Dailey M, Stevens HE. The role of redox regulation in the effects of prenatal stress on embryonic interneuron migration. Cereb Cortex 2019; (epub ahead of print 10.1093/cercor/bhz052). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kiecolt-Glaser JK, Belury MAbhz052, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, omega-6: omega-3 fatty acids, and inflammation in older adults. Psychosom Med 2007; 69: 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chaouat G. The Th1-Th2 paradigm: still important in pregnancy? Semin Immunopathol 2007; 29: 95–113. [DOI] [PubMed] [Google Scholar]
- 140.Wegman TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993; 14: 353–6. [DOI] [PubMed] [Google Scholar]
- 141.Ataldóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 2012; 130: e1447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ataldóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 143.Chess S. Autism in children with congenital rubella. J Autism Child Schizophr 1971; 1: 33–47. [DOI] [PubMed] [Google Scholar]
- 144.Deykin EY, MacMahon B. Viral exposure and autism. Am J Epidemiol 1979; 109: 628–38. [DOI] [PubMed] [Google Scholar]
- 145.Lee BK, Magnusson C, Gardner RM, Blomström Å, Newschaffer CJ, Burstyn I, Karlsson H, Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun 2015; 44:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is Maternal Influenza or Fever During Pregnancy Associated with Autism or Developmental Delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) Study. J Autism Dev Disord 2013; 43: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Croen LA, Qian Y, Ashwood P, Zerbo O, Schendel D, Pinto-Martin J, Daniele Fallin M, Levy S, Schieve LA, Yeargin-Allsopp M, Sabourin KR, Ames JL. Infection and Fever in Pregnancy and Autism Spectrum Disorders: Findings from the Study to Explore Early Development. Autism Res 2019; [Epub ahead of print] doi: 10.1002/aur.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deverman BE, Patterson PH. Cytokines and CNS Development. Neuron 2009; 64: 61–78. [DOI] [PubMed] [Google Scholar]
- 149.Mehler MF, Kessler JA. Cytokines in brain development and function. Adv Protein Chem 1998; 52: 223–51. [DOI] [PubMed] [Google Scholar]
- 150.Samuelsson AM, Jennische E, Hansson HA, Holmäng A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol 2006; 290: R1345–56. [DOI] [PubMed] [Google Scholar]
- 151.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 2004; 103: 546–50. [DOI] [PubMed] [Google Scholar]
- 152.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry 2006; 11: 47–55. [DOI] [PubMed] [Google Scholar]
- 153.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta 2006; 27: 794–8. [DOI] [PubMed] [Google Scholar]
- 154.Goines PE, Crown LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism 2011; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, DeLorenze GN, Kharrazi M, Yolken R, Ashwood P, Van de Water J. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry 2017; 22: 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shimaoka Y, Hidaka Y, Tada H, Amino N, Nakamura T, Murata Y, Mitsuda N, Morimoto Y. Changes in cytokine production during and after normal pregnancy. Am J Reprod Immunol 2000; 44: 143–7. [DOI] [PubMed] [Google Scholar]
- 157.Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, Goldenberg RL, Culhane JF. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine 2011; 53: 170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol 2011; 93: 23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hsiao EY. Immune dysregulation in autism spectrum disorder. Int Rev Neurobiol 2013; 113: 269–302. [DOI] [PubMed] [Google Scholar]
- 160.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 2012; 26: 607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol 2002; 12: 115–8. [DOI] [PubMed] [Google Scholar]
- 162.Gumusoglu SB, Stevens HE. Preclinical evidence for Inflammation/Immunology in prenatal programming of psychiatric disorders. Biol Psychiatry 2019; 85(2): 107–121. doi: 10.1016/j.biopsych.2018.08.008. Epub 2018 Aug 27 [DOI] [PubMed] [Google Scholar]
- 163.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev 2006; 29: 913–47. [DOI] [PubMed] [Google Scholar]
- 164.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull 2009; 35: 959–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res 2005; 39: 311–23. [DOI] [PubMed] [Google Scholar]
- 166.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun 2010; 24: 881–97. [DOI] [PubMed] [Google Scholar]
- 167.Brown AS, Surcel H-M, Hinkka-Yli-Salomäki S, Cheslack-Postava K, Bao Y, Sourander A. Maternal thyroid autoantibody and elevated risk of autism in a national birth cohort. Prog Neuropsychopharmacol Biol Psychiatry 2015; 57: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Edmiston E, Ashwood P, and Van de Water J. 2017. Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biol Psychiatry 81:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore JH, Coe CL. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry 2010; 67: 965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophrenia Res 2012; 140: 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun 2009; 23: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, Hertz-Picciotto I, Pessah IN, Van de Water J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry 2013; 3: e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry 2013; 18: 1171–7. [DOI] [PubMed] [Google Scholar]
- 174.Simister NE. Placental transport of immunoglobulin G. Vaccine 2003; 21: 3365–9. [DOI] [PubMed] [Google Scholar]
- 175.Tincani A, Bompane D, Danieli E, Doria A. Pregnancy, lupus and antiphospholipid syndrome (Hughes syndrome). Lupus 2006; 15: 156–60. [DOI] [PubMed] [Google Scholar]
- 176.Soares Rolim AM, Castro M, Santiago MB. Neonatal antiphospholipid syndrome. Lupus 2006; 15: 301–3. [DOI] [PubMed] [Google Scholar]
- 177.Fu J, Jiang Y, Liang L, Zhu H. Risk factors of primary thyroid dysfunction in early infants born to mothers with autoimmune thyroid disease. Acta Paediatr 2006; 94: 1043–8. [DOI] [PubMed] [Google Scholar]
- 178.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicol 2008; 29: 226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharrazi M, Hansen RL, Ashwood P, Van de Water J. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry 2008; 64: 583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral Correlates of Maternal Antibody Status Among Children with Autism. J Autism Dev Disord 2012; 42: 1435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol 2008; 194: 165–72. [DOI] [PubMed] [Google Scholar]
- 182.Zimmerman AW, Connors SL, Matteson KJ, Li L-C, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun 2007; 21: 351–7. [DOI] [PubMed] [Google Scholar]
- 183.Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to featl brain proteins and cytokine expression. Transl Psychiatry 2011; 1: e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Brimberg L, Mader S, Jeganathan V, Berlin R, Coleman TR, Gregersen PK, Huerta PT, Volpe BT, Diamond B. Caspr2-reactive antibody cloned from a mother of an ASD child mediates an ASD-like phenotype in mice. Mol Psychiatry 2016; 21: 1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun 2008; 22: 806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bauman MD, Iosif A- M, Ashwood P, Braunschweig D, Lee A, Schumann CM, Van de Water J, Amaral DG. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry 2013; 3: e278. doi: 10.1038/tp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005; 57: 67–81. [DOI] [PubMed] [Google Scholar]
- 188.Campbell DB, D’Oronzio R, Garbett K, Ebert PJ, Mimics K, Levitt P, Persico AM. 2007. Disruption of cerebral cortex MET signaling in autism spectrum disorder. Ann Neurol 2007; 62: 243–50. [DOI] [PubMed] [Google Scholar]
- 189.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific Autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci 2007; 1107: 92–103. [DOI] [PubMed] [Google Scholar]
- 190.Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, Hansen R, Hertz-Picciotto I, Ashwood P, Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun 2011; 25: 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rossi CC, Van de Water J, Rogers SJ, Amaral DG. Detection of plasma autoantibodies to brain tissue in young children with and without autism spectrum disorders. Brain Behav Immun 2011; 25:1123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wills S, Rossi CC, Bennett J, Cerdeño VM, Ashwood P, Amaral DG, Van de Water J. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Mol Autism 2011; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rossi CC, Fuentes J, Van de Water J, Amaral DG. Brief Report: Antibodies Reacting to Brain Tissue in Basque Spanish Children with Autism Spectrum Disorder and Their Mothers. J Autism Dev Disord 2013; 44: 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bennett JM, Glaser R, Malarkey WB, Beversdorf DQ, Peng J, Kiecolt-Glaser JK. Inflammation and reactivation of latent herpesvirus in older adults. Brain Behav Immun 2012; 26:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Okada S, Hori N, Kimoto K, Onuzuka M, Sato S, Sasaguri K. Effects of biting on elevation of blood pressure and other physiological responses to stress in rats: biting may reduce allostatic load. Brain Res 2007; 1185: 189–94. [DOI] [PubMed] [Google Scholar]
- 196.Gumusoglu SB, Fine RS, Murray SJ, Bittle JL, Stevens HE. The role of IL-6 in neurodevelopment after prenatal stress. Brain Behav Immun 2017; 65: 274–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts-Mills TA, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy 2012; 67:454–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Coe CL, Crispen HR. Social stress in pregnant squirrel monkeys (Saimiri boliviensis peruviensis) differentially affects placental transfer of maternal antibody to male and female infants. Health Psychol 2000; 19: 554–9. [PubMed] [Google Scholar]
- 199.Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci 2013; 4: 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Andoh M, Shibata K, Okamoto K, Onodera J, Morishita K, Miura Y, Ikegaya Y, Koyama R. Exercise reverses behavioral and synaptic abnormalitites after maternal inflammation. Cell Rep 2019; 27: 2817–25.e5. [DOI] [PubMed] [Google Scholar]
- 201.Zinöcker MK, Lindseth IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients 2018; 10: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Beversdorf DQ, Wang P, Barnes G, Weisskopf M, Hardan A, Hu V, Mazurek MO, Talebedizah Z, Goldberg W, Jones KL, Campbell DB, Feliciano P, Spence S, Muller RA, Brown RMA, Kanne SM, Sohl K, Smith DG, London E, Bauman ML, Amaral DG. 2016. Phenotyping, etiological factors, and biomarkers: toward precision medicine in autism spectrum disorder. J Dev Behav Pediatr 2016; 37: 659–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


