Abstract
Introduction
Human parainfluenza virus type 3 (HPIV-3) causes significant morbimortality in immunocompromised patients. Outbreaks of severe pneumonitis have been previously described in this setting.
Materials and methods
Retrospective observational study in children diagnosed with acute leukemia and a documented HPIV-3 infection in the context of a nosocomial outbreak occurred in a single center.
Result
During summer 2012, an HPIV-3 infection was detected in six hospitalized children with acute leukemia. All the patients had respiratory symptoms and one of them suffered from parotitis.
Conclusion
Early diagnoses using multiplex real-time polymerase chain reaction (PCR) let us control this outbreak. A phylogenetic analysis confirmed person-to-person transmission of a single HPIV-3 variant.
Keywords: Acute leukemia, Children, Parainfluenza, Multiplex real-time PCR
Introduction
Human parainfluenza virus (HPIV) has been reported as a significant cause of morbimortality in patients diagnosed with acute leukemia [1]. In fact, mortality reaches 30–75 % during aplasia after hematopoietic stem cell transplantation (HSCT) [2–4]. Human parainfluenza virus type 3 (HPIV-3) causes bronchiolitis and pneumonia in children <6 months old, and mild respiratory tract infections in both immunocompetent children and adults during the spring and summer months. Outbreaks of severe pneumonitis due to HPIV-3 have been previously described in immunocompromised patients, particularly after HSCT [2, 3, 5]. Therefore, a rapid screening of HPIV-3 in symptomatic patients at risk is indicated to investigate and control nosocomial transmission. Multiplex real-time polymerase chain reaction (PCR) is a rapid method that allows the simultaneous identification of several respiratory viruses [6].
We describe an outbreak of HPIV-3 infection in a pediatric hemato-oncology ward, detected through multiplex real-time PCR.
Methods
A retrospective observational study was conducted in children diagnosed with acute leukemia and a documented HPIV-3 infection in the context of a nosocomial (HPIV-3) outbreak which occurred in the University Hospital Sant Joan de Deu during June and July 2012. This hospital is a pediatric reference center with 365 beds, located in Barcelona, Spain.
Screening of viral respiratory infection was routinely performed by a rapid multiplex real-time PCR.
Total nucleic acids were extracted with the Roche MagNA Pure Compact System (Roche Molecular Diagnostics, Mannheim, Germany) from nasopharyngeal aspirates or bronchoalveolar lavage (BAL) of immunocompromised children with fever and respiratory symptoms. The rapid multiplex real-time PCR based on Tagging Oligonucleotide Cleavage Extension technology (Anyplex™ II, RV16 detection, Laboratories Seegene, Korea) was performed for the simultaneous detection of 16 human respiratory viruses and an internal control [adenovirus, influenza A virus, influenza B virus, HPIV-1, HPIV-2, HPIV-3, HPIV-4, rhinovirus A/B/C, respiratory syncytial virus (RSV) A, RSV virus B, bocavirus 1/2/3/4, coronavirus (CoV) 229E, CoV NL63, CoV OC43, metapneumovirus, and enterovirus], with an internal control from clinical samples, including positive and negative controls per run in a CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Spain).
Phylogenetic analysis was performed in order to prove a person-to-person transmission. The hemagglutinin-neuraminidase (HN) gene has a single open reading frame (ORF) of 1,719 nucleotides that encodes a polypeptide of 572 amino acids. Almost complete HN coding sequence (1,703 bp) from laboratory-confirmed HPIV-3 specimens collected during the study period was amplified by one-step RT-PCR and sequenced using primers as previously described [7], with minor modifications. In addition, other HPIV-3-positive samples collected after and before the outbreak occurred were also included in the phylogenetic analysis (collection dates are shown in brackets of taxon names of the phylogenetic tree, Fig. 1). Purified PCR products using ExoSAP-IT (USB, Affymetrix, Inc., Cleveland, OH, USA) were sequenced by the ABI Prism BigDye Terminator Cycle Sequencing Kit v3.1 on an ABI PRISM 3130XL sequencer (Applied Biosystems, Foster City, CA, USA). The nucleotide sequences were assembled and edited using SeqScape v2.5 software (Applied Biosystems, Foster City, CA, USA). Sequences were aligned using the MUSCLE program, implemented in MEGA 5.1 [8]. The molecular evolutionary models of nucleotide substitution were fitted to the multiple sequence alignments using evolutionary analyses conducted in MEGA 5.1. The phylogenetic trees were reconstructed using a neighbor-joining distance method as implemented in MEGA 5.1 with the evolutionary model with the lowest Bayesian information criterion score. All positions containing gaps and missing data were eliminated. Reliability for the internal branch was assessed using the non-parametric bootstrap analysis with 1,000 replicates.
Fig. 1.
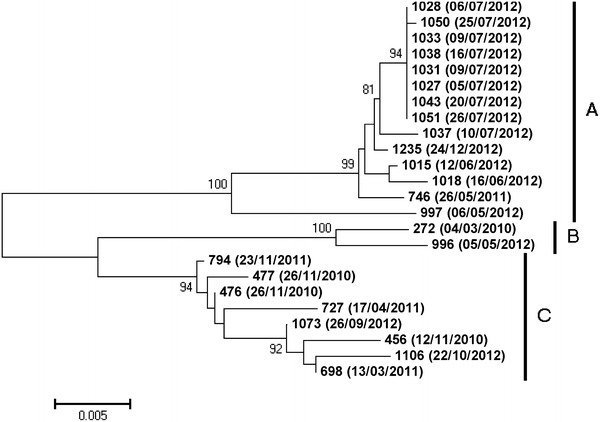
Phylogenetic analysis based on the partial coding sequence of the HN gene of the 13 HPIV-3 strains of the suspected nosocomial outbreak and the other 13 strains collected out of the outbreak period. The tree was constructed by the neighbor-joining method. The evolutionary distances were computed using the Tamura–Nei method and are in the units of the number of base substitutions per site. The rate variation among sites was modeled with a gamma distribution (shape parameter = 0.12). Reliability for the internal branch was assessed using the non-parametric bootstrap analysis with 1,000 replicates. Only bootstrap value higher than 80 are shown
Demographic information, clinical characteristics, laboratory data, diagnostic procedures, therapy, and outcome were collected from immunocompromised children with fever and respiratory symptoms, or in immunocompetent patients with severe pneumonia and no other etiologic diagnosis. Finally, infection control measures and follow-up of the outbreak were revised.
Results
From January 2010 to August 2012, a total of 717 respiratory samples were studied with a multiplex real-time PCR. Twenty-four were laboratory-confirmed for a single HPIV-3 infection, 11 out 24 were detected during June–July 2012, and six of them were affected by acute leukemia.
Phylogenetic analysis constructed from the partial sequences of the HN gene showed that all 25 HPIV-3 strains were divided into three distinct clusters (A, B, and C) with bootstrap values close to 100 %, as shown in Fig. 1 (collection dates are shown in brackets of taxon names of the phylogenetic tree). In the most represented genetic clade (A), eight HPIV-3 contemporary strains, collected during July 2012, clustered together (bootstrap value = 94) and were accompanied by other genetically close HPIV-3 strains, which were collected within or out of the 3-month study period.
The median age of the six patients was 7 years (range 20 months to 16 years). Three patients were diagnosed with acute lymphoblastic leukemia (ALL), two with acute myeloid leukemia (AML), and one with relapsed ALL. Five patients had been discharged after receiving intensive chemotherapy in the hematology ward during the week previous to the symptoms onset, whereas one patient, under reverse isolation for neutropenic fever, had been admitted a month before.
All these patients had fever and upper respiratory tract infection (URTI) symptoms and received empirical wide-spectrum antimicrobial therapy according to our unit’s protocol for fever in neutropenic patients after (collecting) obtaining blood and urine samples for bacteriological studies.
Three patients had self-limited fever and URTI episodes (5–10 days). A 10-year-old boy developed bronchospasm without radiographic evidence of lung condensation, and a 5-year-old girl had evidence of pneumonia. In a 22-month-old boy with persistent fever, a thoracic computed tomography (CT) scan was performed, which showed unspecific peribronchial inflammation. In these three patients, symptoms disappeared when hematologic recovery took place.
BAL specimens from two patients who were admitted to the intensive care unit (ICU) were positive for HPIV-3. A 20-month-old male diagnosed with AML had to be admitted to the ICU for severe Streptococcus mitis sepsis. He was in complete remission and presented this complication during aplasia after consolidation chemotherapy treatment. He had prolonged fever and infant respiratory distress syndrome. He was treated with wide-spectrum antibiotics and invasive mechanical ventilation with a favorable outcome after hematologic recovery. A 16-year-old male with early relapsed ALL was admitted to the ICU for respiratory failure. He had been treated for a probable invasive fungal disease (lung aspergillosis) 2 months before this admission. He had clinical and radiological improvement with voriconazole treatment and has received secondary prophylactic treatment since then. Prior to the ICU admission, he had suffered from respiratory symptoms and fever for 10 days in the context of severe and prolonged aplasia after a clofarabine-containing regimen. Cytomegalovirus (CMV) and HPIV-3 were detected by PCR in the BAL specimen. A thoracic high-resolution CT showed new multiple small nodules suggestive of viral pneumonitis (Fig. 2e). In spite of antibiotics, combined antifungal treatment, and foscarnet, the patient died due to respiratory worsening, diffuse alveolar hemorrhage, and multiorgan failure.
Fig. 2.
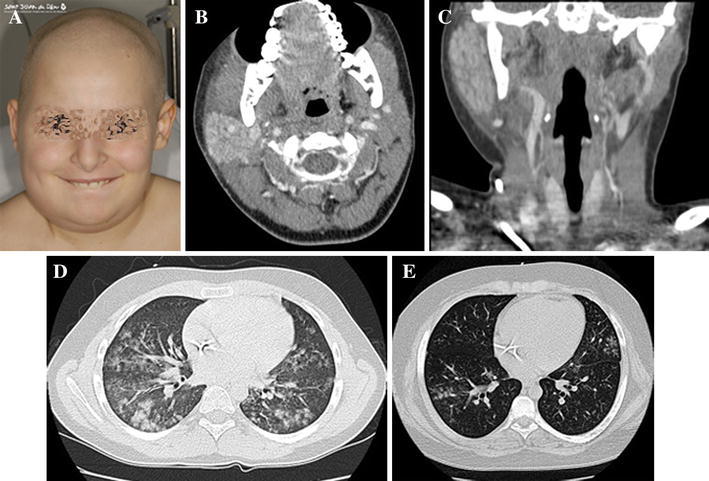
a Patient with asymmetric enlargement of the parotid gland. b, c Computed tomography (CT) scan of the neck: inflammation of the right parotid gland. d High-resolution CT scan of the lungs, showing multiple and bilateral small peribronchial nodules, with a tendency to consolidation. e High-resolution CT scan of the lungs, which shows small peribronchial nodules
A 9-year-old girl, diagnosed with AML, had prolonged fever (>30 days) associated to URTI, a clinical and radiological pneumonia without respiratory insufficiency (Fig. 2d), and parotitis lasting 4 days treated with non-steroidal anti-inflammatory drugs (Fig. 2a–c). HPIV-3 PCR was positive in both BAL and saliva. The fever disappeared after hematologic recovery.
Regarding HPIV-3, patients received symptomatic treatment but no specific therapy, except for the administration of intravenous immunoglobulin (IVIG) when IgG levels were lower than 3,000 mg/dL.
The first case of HPIV-3 infection was diagnosed in the last week of June, but only general infection control measures were adopted. After 2 weeks, a particular emphasis on hand washing and barrier nursing were adopted, adding to advice given to patients’ carers to avoid contact with other people admitted in the ward. No more cases were diagnosed after that.
The nucleotide sequences of the present study were submitted to the GenBank database with accession numbers from KF217146 to KF217170.
Discussion
We describe a series of six pediatric patients with acute leukemia and HPIV-3 infection. Prompt diagnoses through multiplex PCR for respiratory viruses allowed us to take measures to fight against infection and to reduce the risk of viral transmission. A retrospective phylogenetic analysis of the coding sequence of the HN gene confirmed a nosocomial outbreak.
HPIV-3 causes a variety of seasonal respiratory syndrome infections. Symptoms usually are unspecific, but secondary immunosuppression due to chemotherapy and glucocorticoids has been related to the risk of progression from upper to lower tract disease [5]. In fact, we found 5 of 6 patients to have clinical and radiographic evidence of lower tract infection after URTI and fever.
Extra-respiratory manifestations of HPIV-3 infection can also occur. Lange et al. [9] described a 23-year-old male who received HSCT and had parotitis due to HPIV-3. As in our case, positive PCR for HPIV-3 in saliva, in spite of no histologic confirmation, led the authors to think that it was very likely the cause of this inflammation.
Regarding mortality, it is difficult to establish HPIV-3 as the direct cause of death in our patients because they had serious comorbidities. In fact, some authors did not associate any deaths to HPIV-3 infection in their series [2], whereas others have described high mortality rates in hematologic patients infected with HPIV-3 alone [3]. We think that coinfection of HPIV-3 with other pathogens in our patients may have contributed to worsen the outcome, as lung inflammation can lead to a more severe respiratory insufficiency.
Of note, HPIV-3 infection can cause prolonged fever, as happened in one of our patients. Demonstration of HPIV-3 infection in a patient with persistent fever may help to orientate its origin. Although coinfection with other pathogens needs to be ruled out, extra diagnostic procedures and treatment to find the origin of the persistent fever must be conducted keeping it in mind. In regard to HPIV-3 diagnosis, multiplex real-time PCR has demonstrated to be a rapid, sensitive, and specific technique for the detection of several respiratory viruses in a single assay [6]. This is especially useful in immunocompromised patients, as it permits a prompt diagnosis during the early phase of the infection, when only slight symptoms have appeared. On the other hand, when this test cannot be performed, other radiological findings could help to suspect this infection, such as the presence of multiple small and non-cavitating nodules with irregular margins, which has been specifically related with HPIV-3 infection, although consolidation has been described too [10].
A prompt identification of these patients with real-time PCR or both clinical and radiological findings leads not only to an accurate diagnosis, but an adequate isolation of these patients to avoid a nosocomial outbreak. In fact, as HPIV-3 is stable for hours in the environment and spread by direct contact, asymptomatic or prolonged viral shedding can contribute to viral transmission [3].
Similarly to our case, other authors have revised infection control measures in their wards, as standard were not apparently effective to control an HPIV-3 nosocomial outbreak [3]. Emphasis on hand washing, barrier nursing, as well as avoiding contact between patients’ carers seem to be necessary in this kind of virus infection.
A retrospective phylogenetic analysis helped us to determine that our cases were genetically related, including two other patients that shared contiguous spaces, suggesting a nosocomial outbreak of HPIV-3. Moreover, we discovered that at least four of the patients had had a direct contact during the last time they had been at the hospital. A limitation of the present study is that screening of health carers and workers was not performed.
Regarding treatment, although its efficacy has not yet been demonstrated, intravenous and inhaled ribavirin have been used by some authors, suggesting a slight benefit if the drug is given at an early stage of the infection [5]. In fact, the administration of ribavirin with or without IVIG had no effect on the 30-day mortality in a series of 253 HSCT patients [11]. For this reason, we decided to administer symptomatic treatment and substitutive IVIG to our patients.
In summary, HPIV-3 infection causes lower respiratory tract infections and prolonged fever in children with acute leukemia. Other extra-pulmonary manifestations, like parotitis, could appear too. The early diagnosis of HPIV-3 infection using multiplex real-time PCR in symptomatic patients is important in order to insist on infection control measurements to avoid nosocomial transmission in this immunocompromised population.
Acknowledgments
This research was supported by Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, co-financed by the European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015), and AGAUR (Agència de Gestió de Ajuts Universitaris I de Recerca) 2009/SGR 00136.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Chemaly RF, Hanmod SS, Rathod DB, Ghantoji SS, Jiang Y, Doshi A, Vigil K, Adachi JA, Khoury AM, Tarrand J, Hosing C, Champlin R. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012;119:2738–2745. doi: 10.1182/blood-2011-08-371112. [DOI] [PubMed] [Google Scholar]
- 2.Harvala H, Gaunt E, McIntyre C, Roddie H, Labonte S, Curran E, Othieno R, Simmonds P, Bremner J. Epidemiology and clinical characteristics of parainfluenza virus 3 outbreak in a Haemato-oncology unit. J Infect. 2012;65:246–254. doi: 10.1016/j.jinf.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Jalal H, Bibby DF, Bennett J, Sampson RE, Brink NS, MacKinnon S, Tedder RS, Ward KN. Molecular investigations of an outbreak of parainfluenza virus type 3 and respiratory syncytial virus infections in a hematology unit. J Clin Microbiol. 2007;45:1690–1696. doi: 10.1128/JCM.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sydnor ER, Greer A, Budd AP, Pehar M, Munshaw S, Neofytos D, Perl TM, Valsamakis A. An outbreak of human parainfluenza virus 3 infection in an outpatient hematopoietic stem cell transplantation clinic. Am J Infect Control. 2012;40:601–605. doi: 10.1016/j.ajic.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Falsey AR. Current management of parainfluenza pneumonitis in immunocompromised patients: a review. Infect Drug Resist. 2012;5:121–127. doi: 10.2147/IDR.S25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almajhdi FN, Alshaman MS, Amer HM. Molecular characterization and phylogenetic analysis of human parainfluenza virus type 3 isolated from Saudi Arabia. J Med Virol. 2012;84:1304–1311. doi: 10.1002/jmv.23326. [DOI] [PubMed] [Google Scholar]
- 8.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange T, Franke G, Niederwieser D. Parotitis associated with a parainfluenza virus type 3 infection during aplasia after unrelated allogeneic stem cell transplantation. Leuk Lymphoma. 2006;47:1714–1715. doi: 10.1080/10428190600648606. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson PE, Sorrell TC, Bradstock KF, Carr P, Gilroy NM. Parainfluenza virus type 3 pneumonia in bone marrow transplant recipients: multiple small nodules in high-resolution lung computed tomography scans provide a radiological clue to diagnosis. Clin Infect Dis. 2009;48:905–909. doi: 10.1086/597297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7:11S–15S. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]


