Abstract
The mucosal tissues of the gastrointestinal, respiratory, reproductive, and urinary tracts, and the surface of the eye present an enormous surface area to the exterior environment. All of these tissues are covered with resident microbial flora, which vary considerably in composition and complexity. Mucosal tissues represent the site of infection or route of access for the majority of viruses, bacteria, yeast, protozoa, and multicellular parasites that cause human disease. Mucin glycoproteins are secreted in large quantities by mucosal epithelia, and cell surface mucins are a prominent feature of the apical glycocalyx of all mucosal epithelia. In this review, we highlight the central role played by mucins in accommodating the resident commensal flora and limiting infectious disease, interplay between underlying innate and adaptive immunity and mucins, and the strategies used by successful mucosal pathogens to subvert or avoid the mucin barrier, with a particular focus on bacteria.
Supplementary information
The online version of this article (doi:10.1038/mi.2008.5) contains supplementary material, which is available to authorized users.
Mucins—an Integral Part of the Mucosal Barrier
Mucosal epithelial tissues have evolved multiple mechanisms of defense in response to their vulnerability to microbial attack due to their exposure to the external environment. The mucosal epithelial cells form a contiguous lining that acts as a barrier between the moist exterior environment and the remainder of the host. In addition, these cells, both constitutively and in response to microbes, together with underlying leukocytes, secrete many defensive compounds into the mucosal fluid, including mucins, antibodies, defensins, protegrins, collectins, cathlecidins, lysozyme, histatins, and nitric oxide.1, 2 and 3 Together, these different defensive compounds form a physical barrier and have direct antimicrobial activity, and the ability to opsonize microbes to aid clearance. Mucin glycoproteins, however, can fulfill all of these roles individually.
Mucosal pathogens, almost by definition, have evolved mecha-nisms to subvert these mucosal defensive measures. The first barrier the pathogen encounters is the highly hydrated mucus gel that covers the mucosal surface and protects the epithelial cells against chemical, enzymatic, microbial, and mechanical insult. Mucosal surfaces are coated with a layer of viscous mucus ranging in thickness from 10 μm in the eye4 and trachea5 to 300 μm in the stomach and 700 μm in the intestine.6, 7 and 8 This mucus layer is not static but moves to clear trapped material. In the gastrointestinal tract, the outer mucus layer is continually removed by movement of the luminal contents, whereas in the respiratory tract cilia drive its movement. Mucin glycoproteins produced by mucus-producing cells in the epithelium or submucosal glands are the major macromolecular constituent of mucus and are responsible for the viscous properties of the mucus gel. In addition to forming a relatively impervious gel, which acts as a lubricant, a physical barrier, and a trap for microbes, mucus provides a matrix for a rich array of antimicrobial molecules.
Underneath the mucus layer, the cells present a dense forest of highly diverse glycoproteins and glycolipids, which form the glycocalyx. Membrane-anchored cell-surface mucin glyco-proteins are a major constituent of the glycocalyx in all mucosal tissues. The glycocalyx is highly variable from tissue to tissue; for example, the glycocalyx of human intestinal microvilli tips is thick (100–500 nm) in comparison with the glycocalyx of the lateral microvilli surface (30–60 nm).9, 10 The oligosaccharide moieties of the molecules forming the glycocalyx and the mucus layer are highly diverse, and the average turnover time of the human jejunal glycocalyx is 6–12 h.11 Consequently, both the secreted and adherent mucosal barriers are constantly renewed and could potentially be rapidly adjusted to changes in the environment, for example, in response to microbial infection.
Mucin Biosynthesis and Structure
The tremendous energy investment by mucosal tissues in the production of mucins in the basal state, but particularly in response to infection, is testimony to the importance of these glycoproteins. Epithelial mucins are a heterogenous family of large complex glycoproteins containing a dense array of O-linked carbohydrates typically comprising over 70% of their mass. These glycans are concentrated in large peptide domains of repeating amino-acid sequences rich in serine and threonine. The size and number of repeats vary between the mucins, and in many of the genes there are genetic polymorphisms in the number of repeats (variable number of tandem repeats or VNTR polymorphisms), which means the size of individual mucins can differ substantially between individuals. Each mucin is thought to form a filamentous protein carrying typically 100s of complex oligosaccharide structures,12 giving the mucin a “bottle-brush” appearance. To date, at least 16 human mucins have been included in the family, and the expression profile of mucins varies between tissues with the gastrointestinal tract showing the highest and most diverse expression (see Table 1).
Table 1. Tissue distribution of mucins.
| Mucin | Distribution | References |
|---|---|---|
| Secreted gel forming | ||
| MUC2 | Small intestine, colon, respiratory tract, eye, middle ear epithelium | 231, 232, 233, 234 and 235231–235 |
| MUC5AC | Respiratory tract, stomach, cervix, eye, middle ear epithelium | 235, 236, 237, 238 and 239235–239 |
| MUC5B | Respiratory tract, salivary glands, cervix, gallbladder, seminal fluid, middle ear epithelium | 235, 236, 240, 241, 242, 243 and 244235,236,240–244 |
| MUC6 | Stomach, duodenum, gallbladder, pancreas, seminal fluid, cervix, middle ear epithelium | 235, 243, 245, 246 and 247235,243,245–247 |
| MUC19 | Sublingual gland, submandibular gland, respiratory tract, eye, middle ear epithelium | 16, 235 and 24816,235,248 |
| Secreted non-gel forming (monomeric) | ||
| MUC7 | Salivary glands, respiratory tract, middle ear epithelium | 235, 249 and 250235,249,250 |
| Cell surface | ||
| MUC1 | Stomach, breast, gallbladder, cervix, pancreas, respiratory tract, duodenum, colon, kidney, eye, B cells, T cells, dendritic cells, middle ear epithelium | 235, 251, 252, 253, 254, 255 and 256235,251–256 |
| MUC3A/B | Small intestine, colon, gall bladder, duodenum, middle ear epithelium | 235, 243, 257 and 258235,243,257,258 |
| MUC4 | Respiratory tract, colon, stomach, cervix, eye, middle ear epithelium | 235, 255, 259, 260 and 261235,255,259–261 |
| MUC12 | Colon, small intestine, stomach, pancreas, lung, kidney, prostate, uterus | 32, 26232,262 |
| MUC13 | Colon, small intestine, trachea, kidney, appendix, stomach, middle ear epithelium | 35, 235 and 26235,235,262 |
| MUC15 | spleen, thymus, prostate, testis, ovary, small intestine, colon, peripheral blood leukocyte, bone marrow, lymph node, tonsil, breast, fetal liver, lungs, middle ear epithelium | 235, 263235,263 |
| MUC16 | Peritoneal mesothelium, reproductive tract, respiratory tract, eye, middle ear epithelium | 235, 264, 265, 266 and 267235,264–267 |
| MUC17 | Small intestine, colon, duodenum, stomach, middle ear epithelium | 235, 268235,268 |
| MUC20 | Kidney, placenta, colon, lung, prostate, liver, middle ear epithelium | 235, 269235,269 |
Mucins can be divided into three distinct subfamilies: (a) secreted gel-forming mucins, (b) cell-surface mucins, and (c) secreted non-gel-forming mucins (Table 1). Gel-forming mucins, which are the major constituent of mucus and confer its viscoelastic properties, are encoded by a cluster of four highly related genes on chromosome 1113, 14 and 15 and a similar gene on chromosome 12.16 Gel-forming mucins contain N- and C-terminal cysteine-rich domains that are both involved in homo-oligomerization mediated by inter-molecular disulfide bonds.17, 18 The current model for mucin oligomerization is that dimerization occurs rapidly during biosynthesis in the endoplasmic reticulum preceding or concomitant with N-glycosylation but before O-glycosylation in the Golgi apparatus, which in turn is followed by multimerization of dimers.19 Oligomerization is likely to produce either extended filamentous structures or, more probably, web-like molecular structures likely to be critical to the rheological properties of the mucus gel.20, 21, 22, 23 and 24 The extended conformation caused by dense glycosylation enables the molecules to occupy large volumes, with the secreted oligomeric mucins occupying volumes equivalent to those of small bacteria.25 The secreted non-oligomerizing mucins include the MUC7 salivary mucin, which can self-aggregate but is not thought to contribute significantly to mucus properties, and the MUC8 respiratory mucin, which has not been fully characterized to date.
There are 11 known genes encoding cell-surface mucins expressed by a wide diversity of mucosal tissues, with substantial redundancy in many tissues (see Table 1). Cell-surface mucins are present on the apical membrane of all mucosal epithelial cells and contain large extracellular VNTR domains predicted to form rigid elongated structures. Together with their high expression, this indicates that these molecules are likely to be a prominent, probably dominating, constituent of the glycocalyx and may provide a barrier that limits access of other cells and large molecules to the cell surface. During synthesis, most cell-surface mucins appear to be cleaved into two subunits in a region known as an SEA module, which is often flanked by epidermal growth factor-like domains.26 Structural studies of the MUC1 SEA module suggest that the cleavage occurs via autoproteolysis and that the two subunits remain non-covalently associated throughout biosynthesis.27 However, importantly, the extracellular α-subunit can be shed from the cell surface either mediated via a second distinct cleavage event28, 29 or perhaps via physical shear forces separating the two domains at the original cleavage site as suggested by Macao et al.27 Mutation of the cleavage site inhibits MUC1 shedding in transfected mammary and respiratory epithelial cells without affecting cell surface expression, indicating the importance of the initial cleavage in shedding.30 Furthermore, most cell-surface mucin genes appear to undergo alternative splicing and also encode directly secreted isoforms lacking transmembrane and cytoplasmic domains.31, 32, 33 and 34 These isoforms are stored in subapical granules and in goblet cell thecae and are secreted both constitutively and following stimuli. Consequently, due to shedding and direct secretion, cell-surface mucins can also be seen as components of secreted mucus.35
MUC1 is the most extensively studied membrane-associated mucin and is the most ubiquitously expressed across all mucosal tissues. MUC1 has been estimated to be 200–500 nm in length (depending on the number of tandem repeats), suggesting it will tower above other molecules attached to the plasma membrane.36 MUC1 associated with the cell surface is constantly internalized (0.9% of the surface fraction min−1) and recycled.37 Internalization occurs by clathrin-mediated endo-cytosis, and alterations in O-glycan structure stimulate endo-cytosis and intracellular accumulation.38 During recycling, sialic acid is added to the premature form of MUC1.37 Complete sialylation requires several rounds of recycling, one cycle taking approximately 2.5 h.37 Pulse-chase experiments indicate that the half-life of MUC1 in the plasma membrane is 16–24 h,37, 39 suggesting that the average MUC1 molecule recycles up to 10 times before release.37 Recycling rates vary between cell lines and possibly environmental conditions and have not been measured in non-transformed cells, making it very difficult to extrapolate to the real rate of recycling and cell-surface half-life in vivo. The cytoplasmic tail appears to interact with the cytoskeleton and secondary signaling molecules,40, 41 and 42 whereas the extracellular domains of MUC1 and other cell-surface mucins interact with extracellular matrix components and other cells.43, 44, 45, 46 and 47
The cytoplasmic domains of the cell surface mucins are complex, often contain known phosphorylation motifs, and are highly conserved across species, suggesting important intra-cellular functions. The best-studied mucin in this regard is MUC1, which has been explored mainly in terms of its role in cancer cell biology rather than in mucosal defense. We and others have shown phosphorylation of the MUC1 cytoplasmic domain41, 48, 49, 50, 51 and 52 as well as molecular association with β-catenin,41, 51 linking MUC1 with the Wnt pathway, which is involved in epithelial growth, migration, and wound repair. More recently, it has been shown that the cytoplasmic domain can be cleaved and that the cleaved domain translocates to mitochondria and, together with the p53 transcription factor, to the nucleus, where it modulates the cell cycle and protects against the apoptotic response to genotoxic stress.53, 54 Some pathogenic bacteria produce genotoxins, and thus this protective effect, first identified in cancer cells, may have evolved as part of the natural epithelial defense against microbial genotoxins. We have recently shown that in vitro MUC1 protects p53-expressing epithelial cells from the effects of cytolethal distending toxin, a genotoxin produced by Campylobacter jejuni.55, 56In vivo, C. jejuni more densely colonized the stomachs of Muc1−/− mice, but this effect was not seen in isogenic mutants lacking cytolethal distending toxin, indicating that Muc1 lowers gastric colonization at least in part via inhibiting the activity of cytolethal distending toxin.55 Many of the other cell-surface mucins also contain potential phosphorylation sites and cleavage motifs in the immediate intracellular region of their cytoplasmic domains and may be similarly cleaved. Importantly, there is also evidence that interaction with bacteria can induce phosphorylation of MUC1 in vitro.57 Signaling by the cytoplasmic domains of cell-surface mucins is complex and much remains to be elucidated about their mode of action. However, the evidence to date suggests that these domains are involved in cellular programs regulating growth and apoptosis in mucosal cells perhaps in response to microbes and/or their toxins.
Mucin Glycosylation
The carbohydrate structures present on mucosal surfaces vary according to cell lineage, tissue location, and developmental stage. Evidence is emerging that mucin glycosylation can alter in response to mucosal infection and inflammation, and this may be an important mechanism for unfavorably changing the niche occupied by mucosal pathogens. The extensive O-glycosylation of the mucins protects them from proteolytic enzymes and induces a relatively extended conformation.25 The oligosaccharides on secreted mucins are clustered into heavily glycosylated domains (typically 600–1,200 amino acids long) separated by shorter nonglycosylated regions.25 The O-linked glycans contain 1–20 residues, which occur both as linear and branched structures (see Table 2). The carbohydrate chain is initiated with an N-acetylgalactosamine (GalNAc) linked to serine or threonine and is elongated by the formation of the so-called core structures followed by the backbone region (type-1 and type-2 chains). The chains are typically terminated by fucose (Fuc), galactose (Gal), GalNAc, or sialic acid residues in the peripheral region, forming histo-blood-group antigens such as A, B, H, Lewis-a (Lea), Lewis-b (Leb), Lewis-x (Lex), Lewis-y (Ley), as well as sialyl-Lea and sialyl-Lex structures. Sulfation of Gal and N-acetylglucosamine (GlcNAc) residues causes further diversification. In addition to the O-linked glycans, mucins contain a smaller number of N-linked oligosaccharides, which have been implicated in folding, oligomerization (MUC2), or surface localization (MUC17).58, 59 and 60
Table 2. Common O-linked oligosaccharide structures on mucins.
| Nomenclature | Structure |
|---|---|
| Core type | |
| Core 1 | -Galβ1-3GalNAcα1-Ser/Thr |
| Core 2 | -Galβ1-3(-GlcNAcβ1-6)GalNAcα1-Ser/Thr |
| Core 3 | -GlcNAcβ1-3GalNAcα1-Ser/Thr |
| Core 4 | -GlcNAcβ1-3(GlcNAcβ1-6)GalNAcα1-Ser/Thr |
| N-Acetyllactosamine elongation type | |
| Type 1 | -Galβ1-3GlcNAcβ1- |
| Type 2 | -Galβ1-4GlcNAcβ1- |
| Branching | |
| i-antigen | -Galβ1-4GlcNAcβ1-3Galβ1- (unbranched) |
| I-antigen | -Galβ1-4GlcNAcβ1-3(-Galβ1-4GlcNAcβ1-6)Galβ1- (branched) |
| Terminal structures | |
| Blood group H | Fucα1-2Galβ1- |
| Blood group A | Fucα1-2(GalNAcα1-3)Galβ1- |
| Blood group B | Fucα1-2(Galα1-3)Galβ1- |
| Terminal structures (Type 1 based) | |
| Lewis a (Lea) | Galβ1-3(Fucα1-4)GlcNAcβ1- |
| Lewis b (Leb) | Fucα1-2Galβ1-3(Fucα1-4)GlcNAcβ1-(includes H) |
| Sialyl-Lea | NeuAc(α2-3)Galβ1-3(Fucα1-4)GlcNAcβ1- |
| Terminal structures (Type 2 based) | |
| Lewis x (Lex) | Galβ1-4(Fucα1-3)GlcNAcβ1- |
| Lewis y (Ley) | Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ1-(includes H) |
| Sialyl-Lex | NeuAcα2-3 Galβ1-4(Fucα1-3)GlcNAcβ1- |
| Sulfation | |
| 3 Sulfation | HSO3-3Galβ1- |
| 6 Sulfation | HSO3-6GlcNAcβ1- |
| Examples of combined epitopes | |
| H-type 1 | Fucα1-2Galβ1-3GlcNAcβ1- |
| Sialylated type 2 | NeuAcα2-3Galβ1-4GlcNAcβ1- |
The carbohydrate structures present on mucins are determined by the expression of specific glycosyl transferases. Thus, mucin glycosylation is governed by genetics (due to polymorphisms in these enzymes), tissue-specific enzyme expression, and host and environmental factors influencing transferase expression. As an example of the impact of host genotype, the H type-1 structure is made by the secretor (Se) gene product, and the majority (80% of Caucasians, all South American Indians and Orientals) carry this structure and are thus referred to as “secretors”.61 Individuals may also express the Lewis (Le) gene (90% of the Caucasian population) and, provided that they are also secretors, will modify H type-1 into the Leb structure.61, 62 If they are nonsecretors, type-1 chains without its blood group antigen H will be turned into Lea structures.61, 62 The third Se phenotype with simultaneous expression of Lea and Leb antigens has been described as the “weak-Secretor” (Sew) phenotype.63, 64 The dual expression of Lea/Leb is a consequence of an enzymatically weak Se-transferase in combination with an intact Le-transferase.63, 64 The terminal structures of mucin oligosaccharides are highly heterogeneous and vary between/within species and between and even within tissues. The array of oligosaccharide structures on individual mucin molecules is also somewhat determined by stochastic events as the mucin protein moves through the Golgi apparatus.65 This structural diversity may allow the mammalian host to cope with diverse and rapidly changing pathogens, as reflected by the observation that susceptibility to specific pathogens differs between people with different histo-blood groups,66 as exemplified by the observations that individual Se phenotype may determine the ratio of infection as well as the course and severity of urinary tract infections, Norwalk virus induced acute gastroenteritis and Helicobacter pylori-induced gastric diseases.67, 68 and 69 There is also a strong correlation between distinct adhesive properties of H. pylori endemic in specific human populations and the mucin blood group carbohydrate structures expressed by these populations.70 These differences in the external barrier to infection can be equated with the diversity in underlying innate and adaptive immunity (e.g., polymorphisms in MHC, cytokines), which is thought to have evolved for the same reasons.
Regulation and Modulation of the Mucin Barrier
The gel-forming mucins are produced by cells in the epithelial surface and/or by glands located in the submucosal connective tissues, and secretion occurs via both constitutive and regulated pathways.71 Both gel-forming and cell-surface mucins show constitutive and inducible gene expression in mucosal epithelial cells. The promoters of the MUC genes have generally not been fully characterized, although partial promoter characterization is available for human MUC1,72, 73 and 74MUC2,75, 76, 77, 78, 79, 80 and 81MUC3,82MUC4,83, 84 and 85MUC5AC,79, 86 and MUC5B.87 Differential regulation of individual mucin genes is evident between different mucosal tissues and throughout differing regions of the larger epithelial tracts. For example, differing promoter regions are involved in the differential regulation of constitutive MUC2 expression in the small and large intestines.78 Expression of cell-surface and gel-forming mucins can be upregulated by inflammatory cytokines such as interleukin (IL)-1β, IL-4, IL-6, IL-9, IL-13, interferons, tumor necrosis factor-α, nitric oxide, and other uncharacterized inflammatory factors.82, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109 and 110 Responsiveness to these cytokines provides a link between mucins, innate mucosal immunity, and mucosal inflammatory responses. Neutrophils can also stimulate increases in production of both gel-forming and cell-surface mucins by mucosal epithelial cells via neutrophil elastase.111, 112, 113, 114, 115 and 116 Microbial products can stimulate increased production of mucins by mucosal epithelial cells.103, 117, 118, 119 and 120 In fact, there is evidence that adherence of probiotic bacteria upregulates cell-surface mucin expression in vitro,121, 122 perhaps representing an important part of the mechanism by which probiotic bacteria limit infection by pathogens. In contrast, the lipopolysaccharide of the pathogen H. pylori decreases mucin synthesis in gastric epithelial cells in vitro via activation of cPLA-2,123 representing a mechanism by which a pathogen can favorably modulate the mucus barrier.
The constitutive pathway continuously secretes sufficient mucin to maintain the mucus layer, whereas the regulated pathway affords a massive discharge as a response to environmental and/or (patho)physiological stimuli, including cholinergic stimuli, inflammatory cytokines, prostaglandins, lipopoly-saccharide, bile salts, nucleotides, nitric oxide, vasoactive intestinal peptide, and neutrophil elastase.93, 95, 103, 113, 124, 125, 126, 127, 128, 129, 130 and 131 Stimulated mucin release can occur immediately and is accompanied by hydration, resulting in approximately a hundredfold expansion in volume of the secretory granule contents.132, 133 Shedding of the large extracellular α-subunits of cell-surface mucins from the cell surface and release of secreted splice variants of cell-surface mucins are less well understood. The protease ADAM17 (also known as TACE) has been shown to trigger shedding of MUC1 in endometrial cells in response to tumor necrosis factor-α,28, 134 and the matrix metalloproteinase-1 also appears to be an effective MUC1 sheddase.29
In addition to regulation of their synthesis and release, mucins are regulated in terms of their glycosylation. Altering mucin carbo-hydrates may block mechanisms that pathogens use to subvert the mucin barrier. Tumor necrosis factor-α alters sialylation of mucins produced by a tracheal cell line135 and expression of both fucosyltransferases and α-2,3-sialyltransferases by normal bronchial mucosal explants.136 In respiratory epithelial cells, the Th2 cytokines IL-4 and IL-13 increase expression of core 2 β-1,6-N-acetylglucosaminyltransferase, which forms β-1,6-branched structures, including core 2, core 4, and blood group I antigen.137 In addition, glycosylation changes occur during infection/inflammation, for example, in individuals with cystic fibrosis or chronic bronchitis,138 as well as H. pylori-infected individuals.69, 139 The inflammation-associated mucin sialylation shown in patients with H. pylori infection returns to the normal pattern following successful bacterial clearance with anti-biotics.140 In rhesus monkeys that share strong similarities in mucin glycosylation and the natural history of H. pylori infection with humans,141H. pylori infection induces time-dependent changes of mucosal glycosylation that alter the H. pylori adhesion targets.69 Such fine-tuned kinetics of host glycosylation dynamically modulate host–bacterial interactions, appearing to balance the impact of infection and thereby may determine the severity of disease.69 Another example of dynamic changes in mucins occurs following infection of rats with the intestinal parasite Nippostrongylus brasiliensis; infection induces increased production and several alterations in the glycosylation of intestinal Muc2 gel-forming mucin, one of which coincides with expulsion of the parasite.142, 143, 144, 145, 146 and 147 These alterations in glycosylation appear to be driven partly by CD4+ T cells, as CD4 but not CD8 depletion blocks the increase in mucin production, change in glycosylation and worm expulsion,148 and also by T-cell-independent mechanisms.149 Such changes in mucin glycosylation need to be considered as a component of innate and adaptive immune responses to mucosal infection.
Microbial Adherence to the Epithelium
To colonize mucosal surfaces and invade the host, microbes typically must first penetrate the secreted mucus barrier and then either attach to the apical surface of epithelial cells decorated with the cell-surface mucins or release toxins that disrupt epithelial integrity. Bacterial adhesion to host cells can be mediated by hydrophobic interactions, cation bridging (i.e., divalent cations counteracting the repulsion of the negatively charged surfaces of bacteria and host) and receptor ligand binding. One of the most extensively studied mechanisms of bacterial adhesion is via lectins and their corresponding glycosylated receptors. Binding is usually of low affinity, but clustering of adhesins and receptors results in multivalent binding. Fimbriae (or pili), outer membrane proteins, and cell wall components (e.g., lipopoly-saccharide) may all function as adhesins. Adhesion can affect the bacteria by stimulation/inhibition of growth as well as induction of other adhesive structures and proteins required for invasion such as secretion systems. On the other hand, effects of adhesion on host cells can include altered morphology, fluid loss, induction of cytokine release, upregulation of adhesion molecules, and apoptosis.150
Many bacterial adhesins bind oligosaccharides present on mucins. Whether bacterial–mucin binding events favor the bacteria or the host is a key question. In reality, for some organisms, this may be a truly commensal relationship with benefits for both the bacteria (by facilitating retention in a favorable niche and even by providing mucin oligosaccharides for meta-bolism) and the host (by retaining bacteria in the outer areas of the mucus barrier where they cannot harm the underlying epithelium and also limiting the niche available for pathogenic bacteria). Numerous interactions between microorganisms and mucins and/or mucin-type carbohydrates have been demonstrated (see Table 3). Bacteria may have multiple adhesins with different carbohydrate specificities, and modulation of surface receptor density, kinetic parameters, or topographical distributions of these receptors on cell membranes regulate adhesion. As an example, H. pylori binds to mucin oligosaccharides via at least four adhesins, which differ substantially with anatomical site along the oro-gastric infection route, mucin type, pH, and gastric disease status.139, 151, 152 and 153 Thus, for H. pylori, binding to mucins can have differing consequences during colonization of the oral-to-gastric niches and during long-term infection.
Table 3. Characterized interactions between mucins and microbes.
| Tissue derived mucins | Mucin | Carbohydrate | Microbe | References |
|---|---|---|---|---|
| Respiratory mucins | MUC1 | Sialic acids | P. aeruginosa, Haemophilus influenzae, S. aureus, influenza viruses | 163, 181, 270, 271 and 272163,181,270–272 |
| Salivary mucins | MUC5B MUC7 (DMBT1-Muclin) | Sulfated Lea Sialic acids, Sialyl Lex, Leb | P. aeruginosa, H. pylori, Streptococcus sanguis, Streptococcus gordonii, Actinobacillus actinomycetemcomitans, Streptococcus spp., Candida albicans | 273, 274, 275, 276, 277, 278, 279 and 280273–280 |
| Gastric mucins | MUC5AC MUC1 | A, B, H, Leb | H. pylori | 139, 151, 176, 281 and 282139,151,176,281,282 |
| Intestinal mucins | MUC2 | Enterotoxigenic Escherichia coli, Enteropathogenic E. coli, Salmonella typhimurium, Shigella boydii, Shigella sonnei, Campylobacter upsaliensis, Yersinia enterolitica, C. albicans, reoviruses | 162, 283, 284, 285, 286, 287, 288, 289 and 290162,283–290 |
In most studies, only the tissue origin of the mucin has been determined. Which mucins and carbohydrates are responsible for the binding was only determined for a small proportion of the interactions. The mucin and carbohydrate columns thus do not indicate that all microbes listed interact via these specific structures, but merely that these have been shown to bind to some of the bacteria.
Mucins as Decoys for Microbial Adhesins
Mucus hypersecretion ensuing from infection is testament to the role of mucus as a component of host defense. Although mucins are the major macromolecular constituent of mucus and are largely responsible for formation of the mucus gel, the precise nature of their role in host defense has not been well demons-trated empirically. Formation of the mucus gel is important in itself, as it provides a biophysical barrier as well as a matrix supporting the retention of a host of antimicrobial molecules. However, the secreted mucins themselves are likely to function as decoys for adhesins that have been evolved by pathogens to engage the cell surface, as the mucins express many of the oligosaccharide structures found on the cell surface and are constitutively produced in large amounts, constantly washing the mucosal surfaces (Figure 1). Some mucins are effective viral agglutinating agents and exogenously applied mucins are effective inhibitors of viral infection in in vitro-cultured cells.154, 155Streptococcus pyogenes, Tritrichomonas foetus, Tritrichomonas mobilensis, influenza viruses, reoviruses, adenoviruses, enteroviruses, and coronaviruses bind to sialic acids, which are present both at the epithelial surface and on mucins.156, 157, 158, 159, 160, 161, 162, 163, 164 and 165
Figure 1.
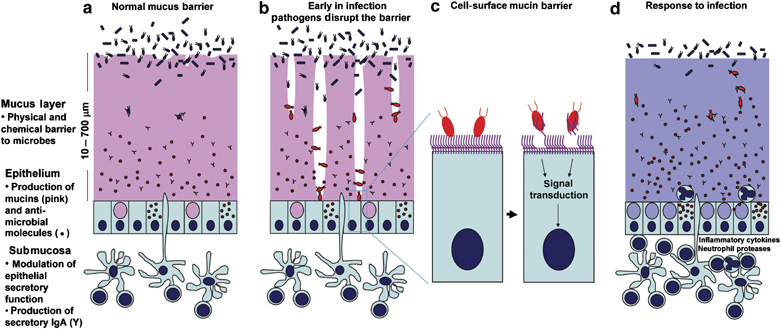
Diagrammatic representation of mucins in the mucosal barrier to infection. (a) The normal mucosa is covered with a continuously replenished thick mucus layer retaining host-defensive molecules. Commensal and environmental microbes may live in the outer mucus layer but the layer ensures that contact of microbes with epithelial cells is rare. (b) Early in infection, many pathogens actively disrupt the mucus layer and thereby gain access to the epithelial cell surface. In addition, this alters the environment for commensal and environmental microbes and opportunistic pathogenesis may occur. (c) Pathogens that break the secreted mucus barrier reach the apical membrane surface, which is decorated with a dense network of large cell-surface mucins. Pathogens bind the cell-surface mucins via lectin interactions and the mucin extracellular domains are shed as releasable decoy molecules. Consequent to contact with microbes and shedding of the extracellular domain, signal transduction by the cytoplasmic domains of the cell-surface mucins modulates cellular response to the presence of microbes. (d) In response to infection, there are alterations in mucins that are driven directly by epithelial cells and in response to signals from underlying innate and adaptive immunity. These alterations include goblet cell hyperplasia and increased mucin secretion and altered mucin glycosylation (depicted by the color change) affecting microbial adhesion and the ability of microbes to degrade mucus. These changes in mucins work in concert with other arms of immunity to clear the infection.
Despite the accepted dogma that secreted mucins limit infection, there are few empirical in vivo data demonstrating their importance. The only secreted mucin for which genetically deficient animals are available is the intestinal mucin, Muc2. Muc2−/− mice develop spontaneous inflammation, presumably due to the absence of the major component of intestinal mucus, leading to increased exposure to the normal intestinal microbial flora.166, 167 As yet, there are no reports of controlled infection experiments in these mice. Further models of secreted mucin deficiency are required to comprehensively determine the importance of secreted mucins in preventing and clearing mucosal infection.
Many pathogens require direct binding to, or penetration of, mucosal epithelial cells to cause pathology. The widest diversity of cell-surface mucin expression is in the mucosal tissues most at risk of infection, such as the gastrointestinal tract, respiratory tract, and eye; notably, nine of the ten cell-surface mucins are expressed in the large intestine, which is the most microbe-rich mucosal environment. Importantly, their ability to be shed from the cell surface has led us to hypothesize that one of the main functions of cell-surface mucins is to act as releasable decoy ligands for microbes attempting to anchor themselves to the glycocalyx. Cell-surface mucins initiate intracellular signaling in response to bacteria, suggesting that they have both a barrier and reporting function on the apical surface of all mucosal epithelial cells.57 However, until recently, much of the evidence had been circumstantial or restricted to in vitro analysis. For example, upregulation of MUC3 expression in colonic cells has been correlated with decreased binding of enteropathogenic E. coli.121, 122In vitro studies have shown that expression of MUC1 by transfection inhibits reovirus and adenovirus infection of MDCK cells by up to tenfold.168, 169 Milk can limit bacterial and viral infections of the gastrointestinal tract and this has been attributed in part to the presence of large amounts of cell-surface mucins, chiefly MUC1 and MUC15, in the milk-fat globule membrane.170, 171 and 172Muc1−/− mice were reported to display chronic infection and inflammation of the reproductive tract, reducing fertility rates. In this latter study, only normal endogenous bacteria were isolated, suggesting that these species become opportunistic pathogens in the absence of Muc1.173 In addition, Muc1−/− mice were reported to have a high frequency of eye inflammation/infection involving Corynebacteria, Staphylococci and Streptococci,174 although this could not be duplicated in a different mouse background held in alternative housing conditions.175
We recently demonstrated that the intestinal pathogen C. jejuni binds to fucosylated mucin oligosaccharides. Controlled infection experiments demonstrated rapid transit of C. jejuni across the gastrointestinal barrier and greater intestinal patho-logy in Muc1−/− mice.55 Bone marrow transplantation studies demonstrated that the increased susceptibility was due to loss of Muc1 on epithelium rather than on leukocytes (which can also express Muc1). Loss of Muc1 had no discernable effects on the abundance or constituency of the intestinal microbial flora. Muc1 appears to prevent C. jejuni infection both by protecting cells from the effects of the cytolethal distending toxin (see above) and by acting as a releasable decoy.55 We have also demonstrated that even though H. pylori can bind Muc1, that primary murine gastric epithelial cells expressing Muc1 bind fewer H. pylori than Muc1−/− cells.176 This paradoxical result is explained by Muc1 acting as a releasable decoy, i.e., the bacteria bind Muc1 expressed on epithelial cells, which is then shed by the host. Due to the absence of this decoy molecule, Muc1−/− mice develop an approximately fivefold greater colonization density of H. pylori from the first days following infection that is maintained for at least 2 months. Consequently, Muc1−/− mice develop severe gastritis not found in wild-type mice.176 Heterozygous mice that have a lower level of gastric Muc1 protein expression show intermediate colonization densities, which suggests that polymorphisms in MUC1 or genes that regulate its expression could underlie susceptibility to H. pylori-induced pathology in human populations. In fact, in humans, individuals with short MUC1 alleles (encoding smaller extra-cellular mucin domains) have a higher propensity to develop gastritis following H. pylori infection.177, 178 and 179 This may be indicative of lower efficacy of smaller MUC1 extracellular mucin domains allowing increased access of bacteria to the epithelial surface, or these alleles may be surrogate markers of polymorphisms influencing the level of gastric MUC1 expression. Recently, a similar protective role has been demonstrated for the extremely large MUC16 cell-surface mucin in human corneal epithelial cells. Greater binding of Staphlylococcus aureus occurs to in vitro-cultured corneal cells when MUC16 is depleted by RNAi.180
Paradoxically, our demonstrations of Muc1-limiting infection in the gastrointestinal tract are opposite to that found in a model of respiratory Pseudomonas aeruginosa infection in the lung in which Muc1−/− mice have an increased clearance of bacteria and a reduced inflammatory response to infection.181 MUC1 binds the P. aeruginosa flagellin,182, 183 but intriguingly appears to inhibit flagellin-stimulated TLR5-mediated activation of NF-κB by an as yet unclear mechanism requiring the MUC1 cytoplasmic domain.181, 184 Whereas an infection-promoting role of a molecule highly expressed on the apical surface of a broad array of mucosal epithelia appears counterintuitive, an anti-inflammatory role for MUC1 in some tissues is consistent with evolutionary adaptations to clear infection by local defense without potentially damaging inflammation, where possible. Further investigations are required with a broad array of pathogens in multiple tissues to clearly delineate the participation of the family of cell-surface mucins in mucosal defense.
Other Protective Roles of Mucins
Mucins have direct and indirect roles in defense from infection distinct from their ability to form a physical barrier and act as adhesion decoys. Not only do mucin oligosaccharides bind microbes, but also, in some cases, they either have direct antimicrobial activity or carry other antimicrobial molecules. A mucin oligosaccharide, α-1-4-linked N-acetyl-glucosamine, which is expressed by some gastric mucins, has been shown to directly interfere with synthesis of H. pylori cell wall components.185 H. pylori must live within gastric mucus to remain protected from luminal gastric pH and prevent expulsion into the intestine. The antimicrobial mucin oligosaccharide probably acts to limit H. pylori expansion within gastric mucus. The non-oligomerizing secreted salivary mucin MUC7 has inherent direct candidacidal activity via a histatin domain at its N-terminus.186, 187 In addition, there is evidence for direct binding of antimicrobial molecules such as histatins and statherin by mucins that would help retain the antimicrobial molecules in the correct mucosal microenvironment where they can best protect the host. For example, MUC7 binds statherin and histatin-1,188 and the other major mucin in saliva, MUC5B, binds histatin-1, -3, and -5 and statherin.189 Secretory IgA (sIgA) is secreted via mucosal epithelial cells and needs to be retained in the immediate mucosal environment to maximize exclusion of pathogens. sIgA is retained at high concentrations in mucus where it can efficiently trap the progress of pathogens, although the mechanism(s) for retaining sIgA in mucus are not well understood. Interestingly, secretory component, which is tightly bound to the Fc region of dimeric-IgA to form sIgA, carries oligosaccharide structures similar to those on mucins.190 In the absence of secretory component carbohydrates, sIgA fails to associate with mucus and fails to prevent infection in a murine respiratory bacterial infection model, substantiating both the physiological importance of sIgA–mucin interactions and the importance of secretory component carbohydrates in maintaining this interaction.191 It is also tempting to speculate that the poly-anionic mucins bind the poly-cationic antimicrobial defensin peptides that are co-secreted into mucus. Interactions of mucins with other secreted antimicrobial molecules has not been fully explored largely due to difficulties in extracting and purifying mucins in the absence of denaturing agents likely to disrupt such interactions. The cell-surface mucins are an integral component of the glycocalyx where they are likely to interact with proteoglycans and other molecules that could retain host defense molecules in a molecular complex covering the apical cell surface.192, 193 Therefore, other mucins and mucin oligosaccharides may yet prove to have direct and indirect antimicrobial activity. Regardless of whether antimicrobial molecules are retained in mucus by direct binding with mucins or by the biophysical properties of mucus, if mucin synthesis is aberrant or secreted mucins are degraded, the antimicrobial molecules will have impaired efficacy.
Subversion of the Mucin Barrier by Mucosal Pathogens
Perhaps the best evidence for the importance of the mucin barrier to infection is the wide variety of strategies used by microbes to subvert or avoid this barrier. Mucin barrier subversion strategies used by microbes include the production of enzymes capable of degrading mucin core proteins and mucin carbohydrates, and effective motility through mucus gels. Motility is important for bacterial mucosal pathogens to facilitate breaking through the physical mucus barrier. In fact, a vast proportion of mucosal bacterial pathogens are flagellated.194, 195H. pylori that have dysfunctional flagella have a greatly reduced ability to infect.196H. pylori uses motility for initial colonization and to attain robust infection. In conjunction with motility, degradative enzymes such as glycosulfatases, sialidases, sialate O-acetylesterases, N-acetyl neuraminate lyases and mucinases are produced by a broad range of bacterial pathogens to destabilize the mucus gel and remove mucin decoy carbohydrates for adhesins.197, 198, 199, 200 and 201 The protozoan parasite Entamoeba histolytica cleaves the MUC2 mucin, which is the major structural component of the intestinal mucus, and this cleavage is predicted to depolymerize the MUC2 polymers.202 The size of the polymer is important for the formation of entangled gels and the viscous properties of mucus; consequently, cleavage of the mucin polymer will effectively result in a local disintegration of mucus.203 There is evidence that these degradative enzymes are critical for microbial pathogenesis. For example, the Vibrio cholerae Hap A, which has both mucinolytic and cytotoxic activity, is induced by mucin and required for translocation through mucin-containing gels.204 The widespread and critically required expression of neuraminidases by a wide variety of sialic acid-binding mucosal viruses underlines the importance of elimination of mucin carbohydrates for their pathogenicity.160 Lipopolysaccharide from H. pylori decreases mucin synthesis,123 and the mucin carbohydrate-binding adhesins BabA and SabA undergo phase variation and change expression during infection,153, 205 which may allow them to evade this host defense mechanism.
Avoidance of the Mucin Barrier by Mucosal Pathogens
Another strategy commonly used by mucosal pathogens is to avoid the mucin barrier. Intestinal M cells, specifically designed to capture and present microbes to the underlying lymphoid tissue, can be regarded as a hole in the mucin barrier. The dome epithelium in which they lie lacks goblet cells, and therefore does not produce gel-forming mucins, and their apical cell surface has only sparse microvilli and an apparently thin glycocalyx.206, 207 Although no studies have measured the expression of individual cell-surface mucins in M cells, there appear to be differences in the glycocalyx mucins between M cells and adjacent intestinal mucosal epithelial cells. In some species, M cells can be identified by their pattern of lectin binding to specific cell-surface carbohydrates that differ with other mucosal epithelial cells.208, 209 Consequently, even though M cells constitute only a very small percentage of mucosal epithelial cells, they are the major point of attachment and/or entry used by a large number of mucosal pathogens including bacteria (e.g., S. typhimurium, Shigella flexneri, Yersinia enterocolitica, and V. cholerae), viruses (e.g., reovirus, HIV-1, and polio virus) and parasites (e.g., Cryptosporidia).206, 210 and 211 Another strategy used by pathogens to avoid the cell-surface mucin barrier, once mucus is penetrated or M cells are invaded, is to disrupt the tight junctions between adjacent mucosal epithelial cells thereby exposing the vulnerable lateral membranes not protected by the glycocalyx. Such examples include S. flexneri,212 enteropathogenic E. coli,213Porphyromonas gingivalis214 and H. pylori.215
Models to Investigate Interactions between Microbes and Mucins
Numerous models, including cancer cell-lines, organ cultures of gastric biopsies and whole animals have been used to investigate mucin–microbe interactions. Although they express orthologs to most human mucins, the most commonly used laboratory animals such as rats and mice have differing glycosylation of some of their mucins. In fact, it is tempting to speculate that differences in mucin glycosylation between mammalian species may underlie some of the differences in infectivity/pathogenicity for individual microbial pathogens. Murine knockout models are only available for Muc1,216Muc2,166 and Muc13 (M.A. McGuckin, unpublished data), and there are also mutants with aberrant Muc2 assembly.217 Thus, there is a need for more models, as mouse knockouts, although limited by the slightly different glycosylation, still represent an important way to collect information of the in vivo function of mucins in infection. Because human pathogens commonly have adhesins for human carbohydrate structures, it is important to select appropriate models for individual pathogens. For example, the effects of H. pylori infection on the mouse are mild, and gastric cancer is not induced even after long-term exposure without other stimuli or genetic defects, although the mouse may develop chronic atrophic gastritis.218, 219H. pylori can colonize the guinea pig and the Mongolian gerbil and cause a severe inflammatory response but does not induce cancer in the absence of exogenous chemical carcinogens.220 These small animal models are useful to study some aspects of H. pylori infection and have the advantage of being relatively cheap. In contrast, rhesus monkeys naturally have persistent H. pylori infection leading to loss of mucus, gastritis, gastric ulcers and even cancer.221, 222, 223 and 224 In addition, the anatomy and physiology of the GI tract of the rhesus monkey, as well as the expression of mucins and mucin glycosylation are very similar to that in human.141 However, this model is expensive, the monkeys can have preexisting natural infection, and primate research has a higher level of ethical considerations.
In vitro microbial–mammalian cocultures are used extensively to elucidate the mechanisms by which microbes adhere, invade, and signal to the host, and to examine ensuing mammalian cell responses. These complex interactions are reliant on appropriate gene expression and cellular functioning of both the microbial and mammalian cells. It is therefore critical that appropriate microbial and mammalian cells are used and that the environment created experimentally is as similar to the human mucosal environment as possible. Human cell lines commonly used for in vitro infection studies have a highly variable expression of mucins and mucin glycosylation, and generally have very low production and secretion of gel-forming mucins.225 Investigators using these models need to be aware of these limitations and consider them in interpreting their data. Additional important issues to consider are choice of cell line and, depending on the type of bacteria, oxygen tension.225, 226 With respect to appropriate mucin production, primary human tracheobronchial epithelial cells cultured in an air–liquid interface represent the most physiological cell cultures in which infection studies can currently be undertaken.227Ex vivo-cultured tissue explants provide another potential avenue for exploring microbial mucin interactions in vitro.228, 229 and 230
Conclusions
The personal repertoire of expression of mucin core proteins and their glycans, mucin allele length, and transient changes in mucin expression and glycosylation in response to infection or stress, as well as variations in environmental conditions may all affect microbial interaction with host mucins and the pathogenic consequences of microbial colonization. Rather than a static barrier, mucins should be considered as a dynamic responsive component of the mucosal barrier that interacts with and responds to other elements of innate and adaptive immunity. Difficulties in working with these complex glycoproteins and the paucity of physiological experimental systems need to be overcome if we are to fully understand the roles of mucins in host defense from infection.
Disclosure
The authors declare no conflict of interest.
PowerPoint slides
References
- 1.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 1997;100,:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Teh C, Kishore U, Reid KB. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim. Biophys. Acta. 2002;1572,:387–400. doi: 10.1016/S0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 3.Raj PA, Dentino AR. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 2002;206,:9–18. doi: 10.1111/j.1574-6968.2002.tb10979.x. [DOI] [PubMed] [Google Scholar]
- 4.Prydal JI, Muir MG, Dilly PN. Comparison of tear film thickness in three species determined by the glass fibre method and confocal microscopy. Eye. 1993;7,:472–475. doi: 10.1038/eye.1993.96. [DOI] [PubMed] [Google Scholar]
- 5.Mercer RR ML R, Crapo JD. Mucous lining layers in human and rat airways. Ann. Rev. Resp. Dis. 1992;145,:355. doi: 10.1164/ajrccm/145.2_Pt_1.355. [DOI] [Google Scholar]
- 6.Strugala V, Allen A, Dettmar PW, Pearson JP. Colonic mucin: methods of measuring mucus thickness. Proc. Nutr. Soc. 2003;62,:237–243. doi: 10.1079/PNS2002205. [DOI] [PubMed] [Google Scholar]
- 7.Jordan N, Newton J, Pearson J, Allen A. A novel method for the visualization of the in situ mucus layer in rat and man. Clin. Sci. (London) 1998;95,:97–106. doi: 10.1042/cs0950097. [DOI] [PubMed] [Google Scholar]
- 8.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280,:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 9.Soler M. Adhesion-related glycocalyx study: quantitative approach with imaging-spectrum in the energy filtering transmission electron microscope (EFTEM) FEBS Lett. 1998;429,:89–94. doi: 10.1016/S0014-5793(98)00570-5. [DOI] [PubMed] [Google Scholar]
- 10.Ito S. Structure and function of the glycocalyx. Fed. Proc. 1969;28,:12–25. [PubMed] [Google Scholar]
- 11.Madara J, Trier J. Physiology of the Gastrointestinal Tract. Raven press; 1987. Functional morphology of the mucosa of the small intestine. [Google Scholar]
- 12.Klein A. Isolation and structural characterization of novel sialylated oligosaccharide-alditols from respiratory-mucus glycoproteins of a patient suffering from bronchiectasis. Eur. J. Biochem. 1993;211,:491–500. doi: 10.1111/j.1432-1033.1993.tb17575.x. [DOI] [PubMed] [Google Scholar]
- 13.Pigny P. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38,:340–352. doi: 10.1006/geno.1996.0637. [DOI] [PubMed] [Google Scholar]
- 14.Desseyn JL, Aubert JP, Porchet N, Laine A. Evolution of the large secreted gel-forming mucins. Mol. Biol. Evol. 2000;17,:1175–1184. doi: 10.1093/oxfordjournals.molbev.a026400. [DOI] [PubMed] [Google Scholar]
- 15.Desseyn JL. Evolutionary history of the 11p15 human mucin gene family. J. Mol. Evol. 1998;46,:102–106. doi: 10.1007/PL00006276. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y. Genome-wide search and identification of a novel gel-forming Mucin MUC19/Muc19 in glandular tissues. Am. J. Respir. Cell Mol. Biol. 2003;30,:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- 17.Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994;269,:2440–2446. [PubMed] [Google Scholar]
- 18.Perez-Vilar J, Eckhardt AE, DeLuca A, Hill RL. Porcine submaxillary mucin forms disulfide-linked multimers through its amino-terminal d-domains. J. Biol. Chem. 1998;273,:14442–14449. doi: 10.1074/jbc.273.23.14442. [DOI] [PubMed] [Google Scholar]
- 19.Asker N, Baeckstrom D, Axelsson MAB, Carlstedt I, Hansson GC. The human MUC2 mucin apoprotein appears to dimerize before O-glycosylation and shares epitopes with the insoluble mucin of rat small intestine. Biochem. J. 1995;308,:873–880. doi: 10.1042/bj3080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J. Biol. Chem. 1999;274,:31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 21.Lidell ME. The recombinant C-terminus of the human MUC2 mucin forms dimers in Chinese-hamster ovary cells and heterodimers with full-length MUC2 in LS 174 T cells. Biochem. J. 2003;372,:335–345. doi: 10.1042/bj20030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godl K. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J. Biol. Chem. 2002;277,:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan JK. Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem. J. 2000;347(Part 1):37–44. doi: 10.1042/bj3470037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehan JK, Howard M, Richardson PS, Longwill T, Thornton DJ. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem. J. 1999;338,:507–513. doi: 10.1042/bj3380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jentoft N. Why are proteins O-glycosylated? Trends Biochem. Sci. 1990;15,:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 26.Wreschner DH. Generation of ligand-receptor alliances by “SEA” module-mediated cleavage of membrane-associated mucin proteins. Protein Sci. 2002;11,:698–706. doi: 10.1110/ps.16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 2006;13,:71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 28.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J. Biol. Chem. 2003;278,:3386–3394. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 29.Thathiah A, Carson DD. MT1-MMP mediates MUC1 shedding independently of TACE/ADAM17. Biochem. J. 2004;382(Part 1):363–373. doi: 10.1042/BJ20040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lillehoj EP, Han F, Kim KC. Mutagenesis of a Gly-Ser cleavage site in MUC1 inhibits ectodomain shedding. Biochem. Biophys. Res. Commun. 2003;307,:743–749. doi: 10.1016/S0006-291X(03)01260-9. [DOI] [PubMed] [Google Scholar]
- 31.Zrihan-Licht S. Characterization and molecular cloning of a novel MUC1 protein devoid of tandem repeats expressed in human breast cancer tissue. Eur. J. Biochem. 1994;224,:787–795. doi: 10.1111/j.1432-1033.1994.00787.x. [DOI] [PubMed] [Google Scholar]
- 32.Williams SJ. Two novel mucin genes downregulated in colorectal cancer identified by differential display. Cancer Res. 1999;59,:4083–4089. [PubMed] [Google Scholar]
- 33.Williams SJ, Munster DJ, Quin RJ, Gotley DC, McGuckin MA. The MUC3 gene encodes a transmembrane mucin and is alternatively spliced. Biochem. Biophys. Res. Commun. 1999;261,:83–89. doi: 10.1006/bbrc.1999.1001. [DOI] [PubMed] [Google Scholar]
- 34.Choudhury A. Alternate splicing at the 3′-end of the human pancreatic tumor-associated mucin MUC4 cDNA. Teratog. Carcinog. Mutagen. 2001;21,:83–96. doi: 10.1002/1520-6866(2001)21:1<83::AID-TCM8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Williams SJ. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 2001;276,:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 36.Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem. Sci. 1992;17,:359–363. doi: 10.1016/0968-0004(92)90315-Z. [DOI] [PubMed] [Google Scholar]
- 37.Litvinov SV, Hilkens J. The epithelial sialomucin, episialin, is sialylated during recycling. J. Biol. Chem. 1993;268,:21364–21371. [PubMed] [Google Scholar]
- 38.Altschuler Y. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol. Biol. Cell. 2000;11,:819–831. doi: 10.1091/mbc.11.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thingstad T, Vos HL, Hilkens J. Biosynthesis and shedding of epiglycanin: a mucin-type glycoprotein of the mouse TA3Ha mammary carcinoma cell. Biochem. J. 2001;353,:33–40. doi: 10.1042/bj3530033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55,:4000–4003. [PubMed] [Google Scholar]
- 41.Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J. Biol. Chem. 2001;276,:6061–6064. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Ren J, Kufe D. Interaction of human MUC1 and beta-catenin is regulated by Lck and ZAP-70 in activated Jurkat T cells. Biochem. Biophys. Res. Commun. 2004;315,:471–476. doi: 10.1016/j.bbrc.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 43.Regimbald LH. The breast mucin MUC1 as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56,:4244–4249. [PubMed] [Google Scholar]
- 44.Nath D. Macrophage-tumour cell interactions: identification of muc1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology. 1999;98,:213–219. doi: 10.1046/j.1365-2567.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gubbels JA. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5,:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin. Exp. Metastasis. 2002;19,:339–345. doi: 10.1023/A:1015590515957. [DOI] [PubMed] [Google Scholar]
- 47.Komatsu M, Carraway CAC, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell–cell interactions by overexpression of sialomucin complex. J. Biol. Chem. 1997;272,:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- 48.Li YQ, Bharti A, Chen DS, Gong JL, Kufe D. Interaction of glycogen synthase kinase 3β with the DF3/MUC1 carcinoma-associated antigen and β-catenin. Mol. Cell Biol. 1998;18,:7216–7224. doi: 10.1128/MCB.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quin RJ, McGuckin MA. Phosphorylation of MUC1 correlates with changes in cell–cell adhesion. Int. J. Cancer. 2000;87,:499–506. doi: 10.1002/1097-0215(20000815)87:4<499::AID-IJC6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 50.Li Y. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J. Biol. Chem. 2001;276,:35239–35242. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 51.Ren J, Li Y, Kufe D. Protein kinase C delta regulates function of the DF3/MUC1 carcinoma antigen in beta-catenin signaling. J. Biol. Chem. 2002;277,:17616–17622. doi: 10.1074/jbc.M200436200. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22,:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren J. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5,:163–175. doi: 10.1016/S1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7,:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 55.McAuley JL. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 2007;117,:2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lara-Tejero M, Galan JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290,:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 57.Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287,:L809–L815. doi: 10.1152/ajplung.00385.2003. [DOI] [PubMed] [Google Scholar]
- 58.McCool DJ, Okada Y, Forstner JF, Forstner GG. Roles of calreticulin and calnexin during mucin synthesis in LS180 and HT29/A1 human colonic adenocarcinoma cells. Biochem. J. 1999;341(Part 3):593–600. doi: 10.1042/bj3410593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho JJ. N-glycosylation is required for the surface localization of MUC17 mucin. Int. J. Oncol. 2003;23,:585–592. [PubMed] [Google Scholar]
- 60.Kui Wong N. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J. Biol. Chem. 2003;278,:28619–28634. doi: 10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- 61.Oriol R. Blood Cell Biochemistry. Plenum press, New York; 1995. ABO, Hh, Lewis, and secretion serology, genetics, and tissue distribution. [Google Scholar]
- 62.Oriol R, Le Pendu J, Mollicone R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986;51,:161–171. doi: 10.1111/j.1423-0410.1986.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 63.Henry S, Oriol R, Samuelsson B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 1995;69,:166–182. doi: 10.1111/j.1423-0410.1995.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 64.Yu LC. Polymorphism and distribution of the secretor alpha(1,2)-fucosyltransferase gene in various Taiwanese populations. Transfusion. 2001;41,:1279–1284. doi: 10.1046/j.1537-2995.2001.41101279.x. [DOI] [PubMed] [Google Scholar]
- 65.Gerken TA. Kinetic modeling confirms the biosynthesis of mucin core 1 (beta-Gal(1-3) alpha-GalNAc-O-Ser/Thr) O-glycan structures are modulated by neighboring glycosylation effects. Biochemistry. 2004;43,:4137–4142. doi: 10.1021/bi036306a. [DOI] [PubMed] [Google Scholar]
- 66.Marionneau S. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83,:565–573. doi: 10.1016/S0300-9084(01)01321-9. [DOI] [PubMed] [Google Scholar]
- 67.Marionneau S, Airaud F, Bovin NV, Le Pendu J, Ruvoen-Clouet N. Influence of the combined ABO FUT2, and FUT3 polymorphism on susceptibility to Norwalk virus attachment. J. Infect. Dis. 2005;192,:1071–1077. doi: 10.1086/432546. [DOI] [PubMed] [Google Scholar]
- 68.Lomberg H, Jodal U, Leffler H, De Man P, Svanborg C. Blood group non-secretors have an increased inflammatory response to urinary tract infection. Scand. J. Infect. Dis. 1992;24,:77–83. doi: 10.3109/00365549209048404. [DOI] [PubMed] [Google Scholar]
- 69.Linden SK. Glycan secretor phenotypes modulate mucosal innate immunity and affect H. pylori infection. PLoS Pathog. 2008;4,:e2. doi: 10.1371/journal.ppat.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aspholm-Hurtig M. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305,:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 71.Rogers DF. Airway goblet cells: responsive and adaptable front-line defenders. Eur. Respir. J. 1994;7,:1690–1706. doi: 10.1183/09031936.94.07091678. [DOI] [PubMed] [Google Scholar]
- 72.Kovarik A, Peat N, Wilson D, Gendler SJ, Taylor-Papadimitriou J. Analysis of the tissue-specific promoter of the MUC1 gene. J. Biol. Chem. 1993;268,:9917–9926. [PubMed] [Google Scholar]
- 73.Abe M, Kufe D. Transcriptional regulation of DF3 gene expression in human MCF-7 breast carcinoma cells. J. Cell Physiol. 1990;143,:226–231. doi: 10.1002/jcp.1041430205. [DOI] [PubMed] [Google Scholar]
- 74.Zaretsky JZ. Analysis of the promoter of the MUC1 gene overexpressed in breast cancer. FEBS Lett. 1999;461,:189–195. doi: 10.1016/S0014-5793(99)01452-0. [DOI] [PubMed] [Google Scholar]
- 75.Velcich A, Palumbo L, Selleri L, Evans G, Augenlicht L. Organization and regulatory aspects of the human intestinal mucin gene (MUC2) locus. J. Biol. Chem. 1997;272,:7968–7976. doi: 10.1074/jbc.272.12.7968. [DOI] [PubMed] [Google Scholar]
- 76.Gum JR, Hicks JW, Kim YS. Identification and characterization of the MUC2 (human intestinal mucin) gene 5′-flanking region: promoter activity in cultured cells. Biochem. J. 1997;325,:259–267. doi: 10.1042/bj3250259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nogami H. Sp1 protein contributes to airway specific rat Muc2 mucin gene transcription. Gene. 1997;198,:191–201. doi: 10.1016/S0378-1119(97)00314-4. [DOI] [PubMed] [Google Scholar]
- 78.Gum JR., Jr Goblet cell-specific expression mediated by the MUC2 mucin gene promoter in the intestine of transgenic mice. Am. J. Physiol. 1999;276,:G666–G676. doi: 10.1152/ajpgi.1999.276.3.G666. [DOI] [PubMed] [Google Scholar]
- 79.Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I. Induction of MUC2 and MUC5AC mucin by factors of the epidermal growth factor family is mediated by EGF-R/Ras/Raf/MAPK signaling cascade and Sp1. J. Biol. Chem. 2002;19,:19. doi: 10.1074/jbc.M204862200. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem. Biophys. Res. Commun. 2003;300,:813–818. doi: 10.1016/S0006-291X(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 81.van der Sluis M. The murine Muc2 mucin gene is transcriptionally regulated by the zinc-finger GATA-4 transcription factor in intestinal cells. Biochem. Biophys. Res. Commun. 2004;325,:952–960. doi: 10.1016/j.bbrc.2004.10.108. [DOI] [PubMed] [Google Scholar]
- 82.Shekels LL, Ho SB. Characterization of the mouse Muc3 membrane bound intestinal mucin 5′ coding and promoter regions: regulation by inflammatory cytokines. Biochim. Biophys. Acta. 2003;1627,:90–100. doi: 10.1016/S0167-4781(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 83.Perez A, Barco R, Fernandez I, Price-Schiavi SA, Carraway KL. PEA3 transactivates the Muc4/Sialomucin complex promoter in mammary epithelial and tumor cells. J. Biol. Chem. 2003;278,:36942–36952. doi: 10.1074/jbc.M300264200. [DOI] [PubMed] [Google Scholar]
- 84.Fauquette V. Transcription factor AP-2alpha represses both the Mucin MUC4 expression and pancreatic cancer cell proliferation. Carcinogenesis. 2007;28,:2305–2312. doi: 10.1093/carcin/bgm158. [DOI] [PubMed] [Google Scholar]
- 85.Perrais M. Characterization of human mucin gene MUC4 promoter: importance of growth factors and proinflammatory cytokines for its regulation in pancreatic cancer cells. J. Biol. Chem. 2001;276,:30923–30933. doi: 10.1074/jbc.M104204200. [DOI] [PubMed] [Google Scholar]
- 86.Kim SW. Regulation of mucin gene expression by CREB via a nonclassical RA signaling pathway. Mol. Cell Biol. 2007;27,:6933–6947. doi: 10.1128/MCB.02385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Seuningen I, Perrais M, Pigny P, Porchet N, Aubert JP. Sequence of the 5′-flanking region and promoter activity of the human mucin gene MUC5B in different phenotypes of colon cancer cells. Biochem. J. 2000;348,:675–686. doi: 10.1042/bj3480675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shirotani K, Taylor-Papadimitriou J, Gendler SJ, Irimura T. Transcriptional regulation of the MUC1 mucin gene in colon carcinoma cells by a soluble factor—identification of a regulatory element. J. Biol. Chem. 1994;269,:15030–15035. [PubMed] [Google Scholar]
- 89.Vos, H.L., van der Valk, S.W., Prinsenberg, T., Mooi, W.J. & Hilkens, J. IL6-induction of the MUC1/episialin promoter in T47D cells. In Proceedings of 4th International Workshop on Carcinoma-Associated Mucins30 (1996).
- 90.Temann UA. A novel role for murine IL-4 in vivo: induction of MUC5ac gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 1997;16,:471–478. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- 91.Dabbagh K. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 1999;162,:6233–6237. [PubMed] [Google Scholar]
- 92.Longphre M. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J. Clin. Invest. 1999;104,:1375–1382. doi: 10.1172/JCI6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smirnova MG, Birchall JP, Pearson JP. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine. 2000;12,:1732–1736. doi: 10.1006/cyto.2000.0763. [DOI] [PubMed] [Google Scholar]
- 94.Kim YD. Regulation of IL-1beta-mediated MUC2 gene in NCI-H292 human airway epithelial cells. Biochem. Biophys. Res. Commun. 2000;274,:112–116. doi: 10.1006/bbrc.2000.3107. [DOI] [PubMed] [Google Scholar]
- 95.Enss ML. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm. Res. 2000;49,:162–169. doi: 10.1007/s000110050576. [DOI] [PubMed] [Google Scholar]
- 96.Louahed J. Interleukin-9 upregulates mucus expression in the airways. Am. J. Respir. Cell Mol. Biol. 2000;22,:649–656. doi: 10.1165/ajrcmb.22.6.3927. [DOI] [PubMed] [Google Scholar]
- 97.Shim JJ. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280,:L134–L140. doi: 10.1152/ajplung.2001.280.1.L134. [DOI] [PubMed] [Google Scholar]
- 98.Gaemers IC, Vos HL, Volders HH, van der Valk SW, Hilkens J. A stat-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J. Biol. Chem. 2001;276,:6191–6199. doi: 10.1074/jbc.M009449200. [DOI] [PubMed] [Google Scholar]
- 99.Smirnova MG, Kiselev SL, Birchall JP, Pearson JP. Up-regulation of mucin secretion in HT29-MTX cells by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-6. Eur. Cytokine Netw. 2001;12,:119–125. [PubMed] [Google Scholar]
- 100.Whittaker L. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am. J. Respir. Cell Mol. Biol. 2002;27,:593–602. doi: 10.1165/rcmb.4838. [DOI] [PubMed] [Google Scholar]
- 101.Seong JK. Upregulation of MUC8 and downregulation of MUC5AC by inflammatory mediators in human nasal polyps and cultured nasal epithelium. Acta Otolaryngol. 2002;122,:401–407. doi: 10.1080/00016480260000094. [DOI] [PubMed] [Google Scholar]
- 102.Kim YD. Interleukin-1beta induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J. Korean Med. Sci. 2002;17,:765–771. doi: 10.3346/jkms.2002.17.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003;221,:42–49. doi: 10.1016/S0008-8749(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 104.Iwashita J. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol. Cell Biol. 2003;81,:275–282. doi: 10.1046/j.1440-1711.2003.t01-1-01163.x. [DOI] [PubMed] [Google Scholar]
- 105.Song KS. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J. Biol. Chem. 2003;278,:23243–23250. doi: 10.1074/jbc.M300096200. [DOI] [PubMed] [Google Scholar]
- 106.Li S, Intini G, Bobek LA. Modulation of MUC7 mucin expression by exogenous factors in airway cells in vitro and in vivo. Am. J. Respir. Cell Mol. Biol. 2006;35,:95–102. doi: 10.1165/rcmb.2005-0305OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Damera G, Xia B, Sachdev GP. IL-4 induced MUC4 enhancement in respiratory epithelial cells in vitro is mediated through JAK-3 selective signaling. Respir. Res. 2006;7,:39. doi: 10.1186/1465-9921-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koga T. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: Its signaling pathway and biological implication. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293,:L693–L701. doi: 10.1152/ajplung.00491.2006. [DOI] [PubMed] [Google Scholar]
- 109.Andrianifahanana M. IFN-gamma-induced expression of MUC4 in pancreatic cancer cells is mediated by STAT-1 upregulation: a novel mechanism for IFN-gamma response. Oncogene. 2007;26,:7251–7261. doi: 10.1038/sj.onc.1210532. [DOI] [PubMed] [Google Scholar]
- 110.Song JS. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respir. Res. 2007;8,:28. doi: 10.1186/1465-9921-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am. J. Respir. Cell Mol. Biol. 2002;26,:447–452. doi: 10.1165/ajrcmb.26.4.4473. [DOI] [PubMed] [Google Scholar]
- 112.Fischer BM. Neutrophil elastase increases MUC4 expression in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284,:L671–L679. doi: 10.1152/ajplung.00220.2002. [DOI] [PubMed] [Google Scholar]
- 113.Voynow JA. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 1999;20,:835–843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 114.Lundgren JD, Rieves RD, Mullol J, Logun C, Shelhamer JH. The effect of neutrophil protenase enzymes on the release of mucus from feline and human airway cultures. Resp. Med. 1994;88,:511–518. doi: 10.1016/S0954-6111(05)80333-6. [DOI] [PubMed] [Google Scholar]
- 115.Dwyer TM, Farley JM. Human neutrophil elastase releases two pools of mucinlike glycoconjugate from tracheal submucosal gland cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278,:L675–L682. doi: 10.1152/ajplung.2000.278.4.L675. [DOI] [PubMed] [Google Scholar]
- 116.Kuwahara I. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289,:L355–L362. doi: 10.1152/ajplung.00040.2005. [DOI] [PubMed] [Google Scholar]
- 117.Yanagihara K, Seki M, Cheng PW. Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am. J. Respir. Cell Mol. Biol. 2001;24,:66–73. doi: 10.1165/ajrcmb.24.1.4122. [DOI] [PubMed] [Google Scholar]
- 118.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 2002;8,:41–46. doi: 10.1038/nm0102-41. [DOI] [PubMed] [Google Scholar]
- 119.Dohrman A. Mucin gene (MUC2 and MUC5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta. 1998;1406,:251–259. doi: 10.1016/S0925-4439(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 120.Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292,:G315–G322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 121.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999;276,:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 122.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52,:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slomiany BL, Slomiany A. Cytosolic phospholipase A2 activation in Helicobacter pylori lipopolysaccharide-induced interference with gastric mucin synthesis. IUBMB Life. 2006;58,:217–223. doi: 10.1080/15216540600732021. [DOI] [PubMed] [Google Scholar]
- 124.Kim KC, Lee BC, Pou S, Ciccolella D. Effects of activation of polymorphonuclear leukocytes on airway goblet cell mucin release in a co-culture system. Inflamm. Res. 2003;52,:258–262. doi: 10.1007/s00011-003-1171-y. [DOI] [PubMed] [Google Scholar]
- 125.Fischer BM, Krunkosky TM, Wright DT, Dolanokeefe M, Adler KB. Tumor necrosis factor-alpha (TNF-alpha) stimulates mucin secretion and gene expression in airway epithelium in vitro. Chest. 1995;107(3 Suppl):133S–135S. doi: 10.1378/chest.107.3_Supplement.133S. [DOI] [PubMed] [Google Scholar]
- 126.Hollande E, Fanjul M, Claret S, Forguelafitte ME, Bara J. Effects of VIP on the regulation of mucin secretion in cultured human pancreatic cancer cells (CAPAN-1) In vitro Cell Dev. Biol. 1995;31,:227–233. doi: 10.1007/BF02639438. [DOI] [PubMed] [Google Scholar]
- 127.Kishioka C, Okamoto K, Kim J, Rubin BK. Regulation of secretion from mucous and serous cells in the excised ferret trachea. Respir. Physiol. 2001;126,:163–171. doi: 10.1016/S0034-5687(01)00214-6. [DOI] [PubMed] [Google Scholar]
- 128.Klinkspoor JH. Mucin secretion by the human colon cell line LS174T is regulated by bile salts. Glycobiology. 1999;9,:13–19. doi: 10.1093/glycob/9.1.13. [DOI] [PubMed] [Google Scholar]
- 129.Jarry A, Vallette G, Branka JE, Laboisse C. Direct secretory effect of interleukin-1 via type I receptors in human colonic mucous epithelial cells (HT29-C1.16E) Gut. 1996;38,:240–242. doi: 10.1136/gut.38.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim KC, Park HR, Shin CY, Akiyama T, Ko KH. Nucleotide-induced mucin release from primary hamster tracheal surface epithelial cells involves the p-2u purinoceptor. Eur. Respir. J. 1996;9,:542–548. doi: 10.1183/09031936.96.09030542. [DOI] [PubMed] [Google Scholar]
- 131.Gottke M, Chadee K. Exogenous nitric oxide stimulates mucin secretion from LS174T colonic adenocarcinoma cells. Inflamm. Res. 1996;45,:209–212. doi: 10.1007/BF02285163. [DOI] [PubMed] [Google Scholar]
- 132.Tam PY, Verdugo P. Control of mucus hydration as a Donnan equilibrium process. Nature. 1981;292,:340–342. doi: 10.1038/292340a0. [DOI] [PubMed] [Google Scholar]
- 133.Verdugo P. Mucin exocytosis. Am. Rev. Respir. Dis. 1991;144,:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 134.Thathiah A. TNF-alpha stimulates MUC1 synthesis and Ectodomain release in a human uterine epithelial cell line. Endocrinology. 2004;145,:4192–4203. doi: 10.1210/en.2004-0399. [DOI] [PubMed] [Google Scholar]
- 135.Delmotte P. Tumor necrosis factor alpha increases the expression of glycosyltransferases and sulfotransferases responsible for the biosynthesis of sialylated and/or sulfated Lewis x epitopes in the human bronchial mucosa. J. Biol. Chem. 2002;277,:424–431. doi: 10.1074/jbc.M109958200. [DOI] [PubMed] [Google Scholar]
- 136.Delmotte P. Influence of TNFalpha on the sialylation of mucins produced by a transformed cell line MM-39 derived from human tracheal gland cells. Glycoconj. J. 2001;18,:487–497. doi: 10.1023/A:1016038219183. [DOI] [PubMed] [Google Scholar]
- 137.Beum PV, Basma H, Bastola DR, Cheng PW. Mucin biosynthesis: upregulation of core 2 beta 1,6 N-acetylglucosaminyltransferase by retinoic acid and Th2 cytokines in a human airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288,:L116–L124. doi: 10.1152/ajplung.00370.2003. [DOI] [PubMed] [Google Scholar]
- 138.Schulz BL. Glycosylation of sputum mucins is altered in cystic fibrosis patients. Glycobiology. 2007;17,:698–712. doi: 10.1093/glycob/cwm036. [DOI] [PubMed] [Google Scholar]
- 139.Linden, S.K., Wickstrom, C., Lindell, G. & Carlstedt, I. Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter (in press) (2008). [DOI] [PubMed]
- 140.Ota H. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1998;433,:419–426. doi: 10.1007/s004280050269. [DOI] [PubMed] [Google Scholar]
- 141.Lindén S, Borén T, Dubois A, Carlstedt I. Rhesus monkey gastric mucins and their Helicobacter pylori binding properties. Biochem. J. 2004;379,:1–11. doi: 10.1042/bj20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koninkx JF, Mirck MH, Hendriks HG, Mouwen JM, van Dijk JE. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp. Parasitol. 1988;65,:84–90. doi: 10.1016/0014-4894(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 143.Yamauchi J. Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. APMIS. 2006;114,:270–278. doi: 10.1111/j.1600-0463.2006.apm_353.x. [DOI] [PubMed] [Google Scholar]
- 144.Oinuma T, Abe T, Nawa Y, Kawano J, Suganuma T. Glycoconjugates in rat small intestinal mucosa during infection with the intestinal nematode Nippostrongylus brasiliensis. Adv. Exp. Med. Biol. 1995;371B,:975–978. [PubMed] [Google Scholar]
- 145.Holmen JM, Olson FJ, Karlsson H, Hansson GC. Two glycosylation alterations of mouse intestinal mucins due to infection caused by the parasite Nippostrongylus brasiliensis. Glycoconj. J. 2002;19,:67–75. doi: 10.1023/A:1022589015687. [DOI] [PubMed] [Google Scholar]
- 146.Karlsson NG. Identification of transient glycosylation alterations of sialylated mucin oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem. J. 2000;350,:805–814. doi: 10.1042/bj3500805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Olson FJ. Blood group A glycosyltransferase occurring as alleles with high sequence difference is transiently induced during a Nippostrongylus brasiliensis parasite infection. J. Biol. Chem. 2002;277,:15044–15052. doi: 10.1074/jbc.M112287200. [DOI] [PubMed] [Google Scholar]
- 148.Khan WI, Abe T, Ishikawa N, Nawa Y, Yoshimura K. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis-infection in mice. Parasite Immunol. 1995;17,:485–491. doi: 10.1111/j.1365-3024.1995.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 149.Kawai Y. T cell-dependent and -independent expression of intestinal epithelial cell-related molecules in rats infected with the nematode Nippostrongylus brasiliensis. APMIS. 2007;115,:210–217. doi: 10.1111/j.1600-0463.2007.apm_510.x. [DOI] [PubMed] [Google Scholar]
- 150.Salyers A, Whitt D. Bacterial Pathogenesis: A Molecular Approach. ASM Press, Washington, DC; 1994. [Google Scholar]
- 151.Linden S, Mahdavi J, Hedenbro J, Boren T, Carlstedt I. Effects of pH on Helicobacter pylori binding to human gastric mucins: identification of binding to non-MUC5AC mucins. Biochem. J. 2004;384,:263–270. doi: 10.1042/BJ20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Teneberg S. Carbohydrate binding specificity of the neutrophil-activating protein of Helicobacter pylori. J. Biol. Chem. 1997;272,:19067–19071. doi: 10.1074/jbc.272.30.19067. [DOI] [PubMed] [Google Scholar]
- 153.Mahdavi J. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297,:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen CC, Baylor M, Bass DM. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105,:84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 155.Yolken RH, Ojeh C, Khatri IA, Sajjan U, Forstner JF. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J. Infect. Dis. 1994;169,:1002–1006. doi: 10.1093/infdis/169.5.1002. [DOI] [PubMed] [Google Scholar]
- 156.Hytonen J, Haataja S, Finne J. Streptococcus pyogenes glycoprotein-binding strepadhesin activity is mediated by a surface-associated carbohydrate-degrading enzyme, Pullulanase. Infect. Immun. 2003;71,:784–793. doi: 10.1128/IAI.71.2.784-793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schwegmann C, Zimmer G, Yoshino T, Enss M, Herrler G. Comparison of the sialic acid binding activity of transmissible gastroenteritis coronavirus and E. coli K99. Virus Res. 2001;75,:69–73. doi: 10.1016/S0168-1702(01)00228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bisaillon M, Senechal S, Bernier L, Lemay G. A glycosyl hydrolase activity of mammalian reovirus sigma1 protein can contribute to viral infection through a mucus layer. J. Mol. Biol. 1999;286,:759–773. doi: 10.1006/jmbi.1998.2495. [DOI] [PubMed] [Google Scholar]
- 159.Helander A. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J. Virol. 2003;77,:7964–7977. doi: 10.1128/JVI.77.14.7964-7977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matrosovich M, Klenk HD. Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev. Med. Virol. 2003;13,:85–97. doi: 10.1002/rmv.372. [DOI] [PubMed] [Google Scholar]
- 161.Alexander DA, Dimock K. Sialic acid functions in enterovirus 70 binding and infection. J. Virol. 2002;76,:11265–11272. doi: 10.1128/JVI.76.22.11265-11272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Willoughby RE. Rotaviruses preferentially bind O-linked sialylglycoconjugates and sialomucins. Glycobiology. 1993;3,:437–445. doi: 10.1093/glycob/3.5.437. [DOI] [PubMed] [Google Scholar]
- 163.Couceiro JN, Paulson JC, Baum LG. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29,:155–165. doi: 10.1016/0168-1702(93)90056-S. [DOI] [PubMed] [Google Scholar]
- 164.Babal P, Russell LC. Sialic acid-specific lectin-mediated adhesion of Tritrichomonas foetus and Tritrichomonas mobilensis. J. Parasitol. 1999;85,:33–40. doi: 10.2307/3285696. [DOI] [PubMed] [Google Scholar]
- 165.Ryan PA, Pancholi V, Fischetti VA. Group A streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors. Infect. Immun. 2001;69,:7402–7412. doi: 10.1128/IAI.69.12.7402-7412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Velcich A. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295,:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 167.Van der Sluis M. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131,:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 168.Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J. Biol. Chem. 2002;277,:23709–23713. doi: 10.1074/jbc.M200292200. [DOI] [PubMed] [Google Scholar]
- 169.Arcasoy SM. MUC1 and other sialoglycoconjugates inhibit adenovirus mediated gene transfer to epithelial cells. Am. J. Respir. Cell Mol. Biol. 1997;17,:422–435. doi: 10.1165/ajrcmb.17.4.2714. [DOI] [PubMed] [Google Scholar]
- 170.Peterson JA, Patton S, Hamosh M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol. Neonate. 1998;74,:143–162. doi: 10.1159/000014020. [DOI] [PubMed] [Google Scholar]
- 171.Yolken RH. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Invest. 1992;90,:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dekker J, Tytgat KM. Binding of rotavirus by a 46 kD milk-glycoprotein may prevent gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1993;17,:228–230. doi: 10.1097/00005176-199308000-00019. [DOI] [PubMed] [Google Scholar]
- 173.DeSouza MM. MUC1/episialin: a critical barrier in the female reproductive tract. J. Reprod. Immunol. 1999;45,:127–158. doi: 10.1016/S0165-0378(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 174.Kardon R. Bacterial conjunctivitis in Muc1 null mice. Invest. Ophthalmol. Vis. Sci. 1999;40,:1328–1335. [PubMed] [Google Scholar]
- 175.Danjo Y, Hazlett LD, Gipson IK. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Invest. Ophthalmol. Vis. Sci. 2000;41,:4080–4084. [PubMed] [Google Scholar]
- 176.McGuckin MA. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology. 2007;133,:1210–1218. doi: 10.1053/j.gastro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 177.Vinall LE. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology. 2002;123,:41–49. doi: 10.1053/gast.2002.34157. [DOI] [PubMed] [Google Scholar]
- 178.Carvalho F. MUC1 gene polymorphism and gastric cancer—an epidemiological study. Glycoconj. J. 1997;14,:107–111. doi: 10.1023/A:1018573201347. [DOI] [PubMed] [Google Scholar]
- 179.Silva F. MUC1 polymorphism confers increased risk for intestinal metaplasia in a Colombian population with chronic gastritis. Eur. J. Hum. Genet. 2003;11,:380–384. doi: 10.1038/sj.ejhg.5200978. [DOI] [PubMed] [Google Scholar]
- 180.Blalock TD. Functions of MUC16 in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2007;48,:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 181.Lu W. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J. Immunol. 2006;176,:3890–3894. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- 182.Lillehoj EP. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280,:L181–L187. doi: 10.1152/ajplung.2001.280.1.L181. [DOI] [PubMed] [Google Scholar]
- 183.Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282,:L751–L756. doi: 10.1152/ajplung.00383.2001. [DOI] [PubMed] [Google Scholar]
- 184.Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of TLR5 signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293,:L686–L692. doi: 10.1152/ajplung.00423.2006. [DOI] [PubMed] [Google Scholar]
- 185.Kawakubo M. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305,:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- 186.Gururaja TL. Candidacidal activity prompted by N-terminus histatin-like domain of human salivary mucin (MUC7) Biochim. Biophys. Acta Prot. Struct. Mol. Enzymol. 1999;1431,:107–119. doi: 10.1016/S0167-4838(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 187.Bobek LA, Situ H. MUC7 20-Mer: investigation of antimicrobial activity, secondary structure, and possible mechanism of antifungal action. Antimicrob. Agents Chemother. 2003;47,:643–652. doi: 10.1128/AAC.47.2.643-652.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bruno LS. Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim. Biophys. Acta. 2005;1746,:65–72. doi: 10.1016/j.bbamcr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 189.Iontcheva I, Oppenheim FG, Offner GD, Troxler RF. Molecular mapping of statherin- and histatin-binding domains in human salivary mucin MG1 (MUC5B) by the yeast two-hybrid system. J. Dent. Res. 2000;79,:732–739. doi: 10.1177/00220345000790020601. [DOI] [PubMed] [Google Scholar]
- 190.Royle L. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 2003;278,:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 191.Phalipon A. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17,:107–115. doi: 10.1016/S1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 192.Salathe M, Forteza R, Conner GE. Post-secretory fate of host defence components in mucus. Novartis Found Symp. 2002;248,:20–26. [PubMed] [Google Scholar]
- 193.Forteza R. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 2001;15,:2179–2186. doi: 10.1096/fj.01-0036com. [DOI] [PubMed] [Google Scholar]
- 194.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002;291,:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 195.Moens S, Vanderleyden J. Functions of bacterial flagella. Crit. Rev. Microbiol. 1996;22,:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- 196.Ottemann KM, Lowenthal AC. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 2002;70,:1984–1990. doi: 10.1128/IAI.70.4.1984-1990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 1992;60,:3971–3978. doi: 10.1128/iai.60.10.3971-3978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haider K. Production of mucinase and neuraminidase and binding of Shigella to intestinal mucin. J. Diarrhoeal Dis. Res. 1993;11,:88–92. [PubMed] [Google Scholar]
- 199.Corfield AP. The roles of enteric bacterial sialidase, sialate O-acetyl esterase and glycosulfatase in the degradation of human colonic mucin. Glycoconj. J. 1993;10,:72–81. doi: 10.1007/BF00731190. [DOI] [PubMed] [Google Scholar]
- 200.Homer KA, Whiley RA, Beighton D. Production of specific glycosidase activities by Streptococcus intermedius strain UNS35 grown in the presence of mucin. J. Med. Microbiol. 1994;41,:184–190. doi: 10.1099/00222615-41-3-184. [DOI] [PubMed] [Google Scholar]
- 201.Roberton AM, Wright DP. Bacterial glycosulphatases and sulphomucin degradation. Canad. J. Gastroenterol. 1997;11,:361–366. doi: 10.1155/1997/642360. [DOI] [PubMed] [Google Scholar]
- 202.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl. Acad. Sci. USA. 2006;103,:9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol. Rev. 1993;73,:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 204.Silva AJ, Pham K, Benitez JA. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology. 2003;149,:1883–1891. doi: 10.1099/mic.0.26086-0. [DOI] [PubMed] [Google Scholar]
- 205.Solnick JV, Hansen LM, Salama NR, Boonjakuakul JK, Syvanen M. Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proc. Natl. Acad. Sci. USA. 2004;101,:2106–2111. doi: 10.1073/pnas.0308573100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Siebers A, Finlay BB. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4,:22–29. doi: 10.1016/0966-842X(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 207.Neutra MR, Mantis NJ, Frey A, Giannasca PJ. The composition and function of M cell apical membranes: implications for microbial pathogenesis. Semin. Immunol. 1999;11,:171–181. doi: 10.1006/smim.1999.0173. [DOI] [PubMed] [Google Scholar]
- 208.Lelouard H, Reggio H, Mangeat P, Neutra M, Montcourrier P. Mucin-related epitopes distinguish M cells and enterocytes in rabbit appendix and Peyer's patches. Infect. Immun. 1999;67,:357–367. doi: 10.1128/iai.67.1.357-367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lelouard H. Glycocalyx on rabbit intestinal M cells displays carbohydrate epitopes from Muc2. Infect. Immun. 2001;69,:1061–1071. doi: 10.1128/IAI.69.2.1061-1071.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vazquez-Torres A, Fang FC. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 2000;3,:54–59. doi: 10.1016/S1369-5274(99)00051-X. [DOI] [PubMed] [Google Scholar]
- 211.Jones B, Pascopella L, Falkow S. Entry of microbes into the host: using M cells to break the mucosal barrier. Curr. Opin. Immunol. 1995;7,:474–478. doi: 10.1016/0952-7915(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 212.Sakaguchi T, Kohler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol. 2002;4,:367–381. doi: 10.1046/j.1462-5822.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 213.Goosney DL, Gruenheid S, Finlay BB. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 2000;16,:173–189. doi: 10.1146/annurev.cellbio.16.1.173. [DOI] [PubMed] [Google Scholar]
- 214.Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect. Immun. 2000;68,:1441–1449. doi: 10.1128/IAI.68.3.1441-1449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Amieva MR. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300,:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J. Biol. Chem. 1995;270,:30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 217.Heazlewood CK. Aberrant mucin assembly in mice causes ER stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sjunnesson H, Sturegard E, Grubb A, Willen R, Wadstrom T. Comparative study of Helicobacter pylori infection in guinea pigs and mice—elevation of acute-phase protein C3 in infected guinea pigs. FEMS Immunol. Med. Microbiol. 2001;30,:167–172. doi: 10.1111/j.1574-695X.2001.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 219.Kim DH. Long-term evaluation of mice model infected with Helicobacter pylori: focus on gastric pathology including gastric cancer. Aliment Pharmacol. Ther. 2003;18(Suppl 1):14–23. doi: 10.1046/j.1365-2036.18.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 220.Sturegard E. Severe gastritis in guinea-pigs infected with Helicobacter pylori. J. Med. Microbiol. 1998;47,:1123–1129. doi: 10.1099/00222615-47-12-1123. [DOI] [PubMed] [Google Scholar]
- 221.Dubois A. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect. Immun. 1996;64,:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dubois A. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology. 1999;116,:90–96. doi: 10.1016/S0016-5085(99)70232-5. [DOI] [PubMed] [Google Scholar]
- 223.Dubois A. Seroepizootiology of Helicobacter pylori gastric infection in nonhuman primates housed in social environments. J. Clin. Microbiol. 1995;33,:1492–1495. doi: 10.1128/jcm.33.6.1492-1495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dubois A. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology. 1994;106,:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 225.Linden SK, Driessen KM, McGuckin MA. Improved in vitro model systems for gastrointestinal infection by choice of cell line, pH, microaerobic conditions and optimization of culture conditions. Helicobacter. 2007;12,:341–353. doi: 10.1111/j.1523-5378.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 226.Cottet S, Corthesy-Theulaz I, Spertini F, Corthesy B. Microaerophilic conditions permit to mimic in vitro events occurring during in vivoHelicobacter pylori infection and to identify Rho/Ras-associated proteins in cellular signaling. J. Biol. Chem. 2002;277,:33978–33986. doi: 10.1074/jbc.M201726200. [DOI] [PubMed] [Google Scholar]
- 227.Thornton DJ. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278,:L1118–L1128. doi: 10.1152/ajplung.2000.278.6.L1118. [DOI] [PubMed] [Google Scholar]
- 228.Smoot DT. Development of a human stomach explant organ culture system to study the pathogenesis of Helicobacter pylori. Digestion. 1990;46,:46–54. doi: 10.1159/000200277. [DOI] [PubMed] [Google Scholar]
- 229.Olfat FO, Naslund E, Freedman J, Boren T, Engstrand L. Cultured human gastric explants: a model for studies of bacteria-host interaction during conditions of experimental Helicobacter pylori infection. J. Infect. Dis. 2002;186,:423–427. doi: 10.1086/341459. [DOI] [PubMed] [Google Scholar]
- 230.Haque A. Early interactions of Salmonella enterica serovar with human small intestinal epithelial explants. Gut. 2004;53,:1424–1430. doi: 10.1136/gut.2003.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gum JR. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphisms. J. Biol. Chem. 1989;264,:6480–6487. [PubMed] [Google Scholar]
- 232.Ogata S, Uehara H, Chen A, Itzkowitz SH. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 1992;52,:5971–5978. [PubMed] [Google Scholar]
- 233.Dohrman A. Distribution of lysozyme and mucin (MUC2 and MUC3) mRNA in human bronchus. Exp. Lung Res. 1994;20,:367–380. doi: 10.3109/01902149409064393. [DOI] [PubMed] [Google Scholar]
- 234.Berry M, Ellingham RB, Corfield AP. Human preocular mucins reflect changes in surface physiology. Br. J. Ophthalmol. 2004;88,:377–383. doi: 10.1136/bjo.2003.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007;117,:1666–1676. doi: 10.1097/MLG.0b013e31806db531. [DOI] [PubMed] [Google Scholar]
- 236.Porchet N. Human mucin genes: genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem. Soc. Trans. 1995;23,:800–805. doi: 10.1042/bst0230800. [DOI] [PubMed] [Google Scholar]
- 237.Inatomi T. Expression of secretory mucin genes by human conjunctival epithelia. Invest. Ophthalmol. Vis. Sci. 1996;37,:1684–1692. [PubMed] [Google Scholar]
- 238.Gipson IK. Mucin genes expressed by human female reproductive tract epithelia. Biol. Reprod. 1997;56,:999–1011. doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- 239.Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Exp. Eye Res. 2007;84,:939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gipson IK. The Amount of MUC5B mucin in cervical mucus peaks at midcycle. J. Clin. Endocrinol. Metab. 2001;86,:594–600. doi: 10.1210/jcem.86.2.7174. [DOI] [PubMed] [Google Scholar]
- 241.Escande F, Porchet N, Aubert JP, Buisine MP. The mouse Muc5b mucin gene: cDNA and genomic structures, chromosomal localization and expression. Biochem. J. 2002;363,:589–598. doi: 10.1042/bj3630589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Davies JR. Respiratory tract mucins: structure and expression patterns. Novartis Found Symp. 2002;248,:76–88. [PubMed] [Google Scholar]
- 243.Vandenhaute B. Mucin gene expression in biliary epithelial cells. J. Hepatol. 1997;27,:1057–1066. doi: 10.1016/S0168-8278(97)80150-X. [DOI] [PubMed] [Google Scholar]
- 244.Russo CL. Mucin gene expression in human male urogenital tract epithelia. Hum. Reprod. 2006;21,:2783–2793. doi: 10.1093/humrep/del164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Toribara NW. Human gastric mucin. Identification of a unique species by expression cloning. J. Biol. Chem. 1993;268,:5879–5885. [PubMed] [Google Scholar]
- 246.Debolos C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with lewis antigen expression in the human stomach. Gastroenterology. 1995;109,:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 247.Bartman AE. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J. Pathol. 1998;186,:398–405. doi: 10.1002/(SICI)1096-9896(199812)186:4<398::AID-PATH192>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 248.Yu DF. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjogren syndrome patients. Exp. Eye Res. 2007;86,:403–411. doi: 10.1016/j.exer.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 249.Bobek LA, Tsai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7) J. Biol. Chem. 1993;268,:20563–20569. [PubMed] [Google Scholar]
- 250.Sharma P. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am. J. Respir. Cell Mol. Biol. 1998;19,:30–37. doi: 10.1165/ajrcmb.19.1.3054. [DOI] [PubMed] [Google Scholar]
- 251.Pinkus GS, Kurtin PJ. Epithelial membrane antigen—a diagnostic discriminant in surgical pathology: immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum. Pathol. 1985;16,:929–940. doi: 10.1016/S0046-8177(85)80132-5. [DOI] [PubMed] [Google Scholar]
- 252.Swallow DM. The human tumour-associated epithelial mucins are coded by an expressed hypervariable gene locus PUM. Nature. 1987;328,:82–84. doi: 10.1038/328082a0. [DOI] [PubMed] [Google Scholar]
- 253.Wreschner DH. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur. J. Biochem. 1990;189,:463–473. doi: 10.1111/j.1432-1033.1990.tb15511.x. [DOI] [PubMed] [Google Scholar]
- 254.Ho SB. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55,:2681–2690. [PubMed] [Google Scholar]
- 255.Gipson IK, Inatomi T. Mucin genes expressed by the ocular surface epithelium. Prog. Retinal Eye Res. 1997;16,:81–98. doi: 10.1016/S1350-9462(96)00022-5. [DOI] [Google Scholar]
- 256.Wykes M. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. J. Leukoc. Biol. 2002;72,:692–701. [PubMed] [Google Scholar]
- 257.Ho SB. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53,:641–651. [PubMed] [Google Scholar]
- 258.Pratt WS. Multiple transcripts of MUC3: evidence for two genes, MUC3A and MUC3B. Biochem. Biophys. Res. Commun. 2000;275,:916–923. doi: 10.1006/bbrc.2000.3406. [DOI] [PubMed] [Google Scholar]
- 259.Porchet N. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem. Biophys. Res. Commun. 1991;175,:414–422. doi: 10.1016/0006-291X(91)91580-6. [DOI] [PubMed] [Google Scholar]
- 260.Moniaux N. Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur. J. Biochem. 2000;267,:4536–4544. doi: 10.1046/j.1432-1327.2000.01504.x. [DOI] [PubMed] [Google Scholar]
- 261.Gipson IK. MUC4 and MUC5b transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol. Reprod. 1999;60,:58–64. doi: 10.1095/biolreprod60.1.58. [DOI] [PubMed] [Google Scholar]
- 262.Packer LM, Williams SJ, Callaghan S, Gotley DC, McGuckin MA. Expression of the cell surface mucin gene family in adenocarcinomas. Int. J. Oncol. 2004;25,:1119–1126. [PubMed] [Google Scholar]
- 263.Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur. J. Biochem. 2002;269,:2755–2763. doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- 264.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J. Biol. Chem. 2001;276,:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 265.Hori Y, Spurr-Michaud S, Russo CL, Argueso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest. Ophthalmol. Vis. Sci. 2004;45,:114–122. doi: 10.1167/iovs.03-0903. [DOI] [PubMed] [Google Scholar]
- 266.Andersch-Bjorkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol. Cell Proteomics. 2007;6,:708–716. doi: 10.1074/mcp.M600439-MCP200. [DOI] [PubMed] [Google Scholar]
- 267.Gipson IK. MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol. Reprod. 2008;78,:134–142. doi: 10.1095/biolreprod.106.058347. [DOI] [PubMed] [Google Scholar]
- 268.Gum JR, Jr, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 2002;291,:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- 269.Higuchi T. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J. Biol. Chem. 2004;279,:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 270.Kubiet M, Ramphal R, Weber A, Smith A. Pilus-mediated adherence of Haemophilus influenzae to human respiratory mucins. Infect. Immun. 2000;68,:3362–3367. doi: 10.1128/IAI.68.6.3362-3367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Scharfman A. Adhesion of Pseudomonas aeruginosa to respiratory mucins and expression of mucin-binding proteins are increased by limiting iron during growth. Infect. Immun. 1996;64,:5417–5420. doi: 10.1128/iai.64.12.5417-5420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shuter J, Hatcher VB, Lowy FD. Staphylococcus aureus binding to human nasal mucin. Infect. Immun. 1996;64,:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Reddy MS. Binding of the pili of Pseudomonas aeruginosa to a low-molecular-weight mucin and neutral cystatin of human submandibular-sublingual saliva. Curr. Microbiol. 1998;37,:395–402. doi: 10.1007/s002849900399. [DOI] [PubMed] [Google Scholar]
- 274.Hoffman MP, Haidaris CG. Analysis of Candida albicans adhesion to salivary mucin. Infect. Immun. 1993;61,:1940–1949. doi: 10.1128/iai.61.5.1940-1949.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Veerman EC. Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology. 1997;7,:737–743. doi: 10.1093/glycob/7.6.737. [DOI] [PubMed] [Google Scholar]
- 276.Murray PA, Prakobphol A, Lee T, Hoover CI, Fisher SJ. Adherence of oral streptococci to salivary glycoproteins. Infect. Immun. 1992;60,:31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Prakobphol A. Separate oligosaccharide determinants mediate interactions of the low-molecular-weight salivary mucin with neutrophils and bacteria. Biochem. 1999;38,:6817–6825. doi: 10.1021/bi990145m. [DOI] [PubMed] [Google Scholar]
- 278.Liu B. The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral Streptococci and exhibits candidacidal activity. Biochem. J. 2000;345,:557–564. doi: 10.1042/bj3450557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liu B. Interaction of human salivary mucin MG2, its recombinant N-terminal region and a synthetic peptide with Actinobacillus actinomycetemcomitans. J. Periodontal. Res. 2002;37,:416–424. doi: 10.1034/j.1600-0765.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- 280.Bosch JA. Stress as a determinant of saliva-mediated adherence and coadherence of oral and nonoral microorganisms. Psychosom. Med. 2003;65,:604–612. doi: 10.1097/01.PSY.0000074759.71084.AB. [DOI] [PubMed] [Google Scholar]
- 281.Hirmo S, Artursson E, Puu G, Wadstrom T, Nilsson B. Helicobacter pylori interactions with human gastric mucin studied with a resonant mirror biosensor. J. Microbiol. Methods. 1999;37,:177–182. doi: 10.1016/S0167-7012(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 282.Linden S. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology. 2002;123,:1923–1930. doi: 10.1053/gast.2002.37076. [DOI] [PubMed] [Google Scholar]
- 283.Sun R, Anderson TJ, Erickson AK, Nelson EA, Francis DH. Inhibition of adhesion of Escherichia coli k88ac fimbria to its receptor, intestinal mucin-type glycoproteins, by a monoclonal antibody directed against a variable domain of the fimbria. Infect. Immun. 2000;68,:3509–3515. doi: 10.1128/IAI.68.6.3509-3515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mack DR, Blainnelson PL. Disparate in vitro inhibition of adhesion of enteropathogenic Escherichia coli rdec-1 by mucins isolated from various regions of the intestinal tract. Pediatr. Res. 1995;37,:75–80. doi: 10.1203/00006450-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 285.Rajkumar R, Devaraj H, Niranjali S. Binding of Shigella to rat and human intestinal mucin. Mol. Cell Biochem. 1998;178,:261–268. doi: 10.1023/A:1006844125976. [DOI] [PubMed] [Google Scholar]
- 286.Vimal DB, Khullar M, Gupta S, Ganguly NK. Intestinal mucins: the binding sites for Salmonella typhimurium. Mol. Cell Biochem. 2000;204,:107–117. doi: 10.1023/A:1007015312036. [DOI] [PubMed] [Google Scholar]
- 287.Jin LZ, Zhao X. Intestinal receptors for adhesive fimbriae of enterotoxigenic Escherichia coli (ETEC) K88 in swine—a review. Appl. Microbiol. Biotechnol. 2000;54,:311–318. doi: 10.1007/s002530000404. [DOI] [PubMed] [Google Scholar]
- 288.Sylvester FA, Philpott D, Gold B, Lastovica A, Forstner JF. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, Campylobacter upsaliensis. Infect. Immun. 1996;64,:4060–4066. doi: 10.1128/iai.64.10.4060-4066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mantle M, Husar SD. Binding of Yersinia enterocolitica to purified, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect. Immun. 1994;62,:1219–1227. doi: 10.1128/iai.62.4.1219-1227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.de Repentigny L, Aumont F, Bernard K, Belhumeur P. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect. Immun. 2000;68,:3172–3179. doi: 10.1128/IAI.68.6.3172-3179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


