Abstract
Background
Xerostomia is the subjective sensation of dry mouth. Common causes of xerostomia include adverse effects of many commonly prescribed medications, disease (e.g. Sjogren's Syndrome) and radiotherapy treatment for head and neck cancers. Non‐pharmacological techniques such as acupuncture or mild electrostimulation may be used to improve symptoms.
Objectives
To assess the effects of non‐pharmacological interventions administered to stimulate saliva production for the relief of dry mouth.
Search methods
We searched the Cochrane Oral Health Group's Trials Register (to 16th April 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3), MEDLINE via OVID (1948 to 16th April 2013), EMBASE via OVID (1980 to 16th April 2013), AMED via OVID (1985 to 16th April 2013), CINAHL via EBSCO (1981 to 16th April 2013), and CANCERLIT via PubMed (1950 to 16th April 2013). The metaRegister of Controlled Clinical Trials (www.controlled‐trials.com) and ClinicalTrials.gov (www.clinicaltrials.gov) were also searched to identify ongoing and completed trials. References lists of included studies and relevant reviews were also searched. There were no restrictions on the language of publication or publication status.
Selection criteria
We included parallel group randomised controlled trials of non‐pharmacological interventions to treat dry mouth, where participants had dry mouth symptoms at baseline.
Data collection and analysis
At least two review authors assessed each of the included studies to confirm eligibility, assess risk of bias and extract data using a piloted data extraction form. We calculated mean difference (MD) and 95% confidence intervals (CI) for continuous outcomes or where different scales were used to assess an outcome, we calculated standardised mean differences (SMD) together with 95% CIs. We attempted to extract data on adverse effects of interventions. Where data were missing or unclear we attempted to contact study authors to obtain further information.
Main results
There were nine studies (total 366 participants randomised) included in this review of non‐pharmacological interventions for dry mouth which were divided into three comparisons. Eight studies were assessed at high risk of bias in at least one domain and the remaining study was at unclear risk of bias.
Five small studies (total 153 participants, with dry mouth following radiotherapy treatment) compared acupuncture with placebo. Four were assessed at high risk and one at unclear risk of bias. Two trials reported outcome data for dry mouth in a form suitable for meta‐analysis. The pooled estimate of these two trials (70 participants, low quality evidence) showed no difference between acupuncture and control in dry mouth symptoms (SMD ‐0.34, 95% CI ‐0.81 to 0.14, P value 0.17, I2 = 39%) with the confidence intervals including both a possible reduction or a possible increase in dry mouth symptoms. Acupuncture was associated with more adverse effects (tiny bruises and tiredness which were mild and temporary). There was a very small increase in unstimulated whole saliva (UWS) at the end of 4 to 6 weeks of treatment (three trials, 71 participants, low quality evidence) (MD 0.02 ml/minute, 95% CI 0 to 0.04, P value 0.04, I2 = 57%), and this benefit persisted at the 12‐month follow‐up evaluation (two trials, 54 participants, low quality evidence) (UWS, MD 0.06 ml/minute, 95% CI 0.01 to 0.11, P value 0.03, I2 = 10%). For the outcome of stimulated whole saliva (SWS, three trials, 71 participants, low quality evidence) there was a benefit favouring acupuncture (MD 0.19 ml/minute, 95% CI 0.07 to 0.31, P value 0.002, I2 = 1%) an effect which also persisted at the 12‐month follow‐up evaluation (SWS MD 0.28 ml/minute, 95% CI 0.09 to 0.47, P value 0.004, I2 = 0%) (two trials, 54 participants, low quality evidence).
Two small studies, both at high risk of bias, compared the use of an electrostimulation device with a placebo device in participants with Sjögren's Syndrome (total 101 participants). A further study, also at high risk of bias, compared acupuncture‐like electrostimulation of different sets of points in participants who had previously been treated with radiotherapy. None of these studies reported the outcome of dry mouth. There was no difference between electrostimulation and placebo in the outcomes of UWS or SWS at the end of the 4‐week treatment period in the one study (very low that provided data for these outcomes. No adverse effects were reported.
A single study at high risk of bias, compared the stimulatory effect of powered versus manual toothbrushing and found no difference for the outcomes of UWS or SWS.
Authors' conclusions
There is low quality evidence that acupuncture is no different from placebo acupuncture with regard to dry mouth symptoms, which is the most important outcome. This may be because there were insufficient participants included in the two trials to show a possible effect or it may be that there was some benefit due to 'placebo' acupuncture which could have biased the effect to the null. There is insufficient evidence to determine the effects of electrostimulation devices on dry mouth symptoms. It is well known that dry mouth symptoms may be problematic even when saliva production is increased, yet only two of the trials that evaluated acupuncture reported dry mouth symptoms, a worrying reporting bias. There is some low quality evidence that acupuncture results in a small increase in saliva production in patients with dry mouth following radiotherapy.
There is insufficient evidence to determine the effects of electrostimulation devices on dry mouth symptoms or saliva production in patients with Sjögren's Syndrome. Reported adverse effects of acupuncture are mild and of short duration, and there were no reported adverse effects from electrostimulation.
Keywords: Humans, Acupuncture Therapy, Acupuncture Therapy/adverse effects, Acupuncture Therapy/methods, Electric Stimulation Therapy, Electric Stimulation Therapy/methods, Electroacupuncture, Electroacupuncture/instrumentation, Electroacupuncture/methods, Randomized Controlled Trials as Topic, Salivation, Sjogren's Syndrome, Sjogren's Syndrome/therapy, Xerostomia, Xerostomia/therapy
Plain language summary
Non‐drug treatments for dry mouth symptoms
Review question
This review, carried out by authors of the Cochrane Oral Health Group, has been produced to assess the effects of non‐drug treatments used to stimulate saliva production for the relief of dry mouth (xerostomia) symptoms.
Background
Dry mouth is a common problem with an estimated incidence of between 10% and 26% in men and between 10% and 33% in women, which may or may not be due to reduced saliva secretion. Common causes of dry mouth include the side effects of many commonly prescribed medications, diseases (such as Sjögren's syndrome where the immune system destroys tissues in the glands which produce saliva) and radiotherapy treatments for head and neck cancers.
Saliva moistens the skin in the mouth and helps to maintain oral health. The presence of saliva facilitates speech, acts to wash away food residue from around the teeth, neutralises potentially damaging food and bacterial acids, enhances a person's ability to taste the food, and generally lubricates the mouth. Saliva also acts to soften food, making it easier to chew and swallow. Enzymes in saliva start the digestion of starch and fats, and other substances in saliva, such as epidermal growth factors, promote tissue growth, differentiation and wound healing. The antibacterial, antifungal and antiviral agents in saliva balance the oral flora and help to prevent oral infections, while the minerals in saliva help to maintain tooth enamel.
Non‐drug treatments such as acupuncture, mild electrical stimulation, lasers, tooth brushing and other stimulation techniques are used to improve dry mouth symptoms.
Study characteristics
The evidence on which this review is based was up‐to‐date as of 16 April 2013.
Nine studies were included in this review. A total of 366 adult participants took part in these trials, with an average of 40 participants per trial, and an age range from 12 to 77 years. The causes of dry mouth were radiotherapy for oral cancers in four trials, Sjögren's syndrome in three trials, medication‐related in one trial, and in the remaining trial participants had a range of causes of dry mouth.
The included studies were divided into three groups, according the interventions evaluated.
1. Five small studies with a total 153 participants evaluated acupuncture.
2. Three studies evaluated electrostimulation devices.
3. One study evaluated a power toothbrush.
Key results
The five studies evaluating the effects of acupuncture in people who had dry mouth were generally of poor quality. There was no evidence of a difference in dry mouth symptoms, but there was some evidence of a small increase in saliva production which persisted for a year after the end of the acupuncture treatment. There may not have been enough people included in the trials to show a difference in dry mouth, or it may have been that both the real acupuncture and the 'placebo' acupuncture had some beneficial effect. Acupuncture was associated with more adverse effects (tiny bruises and tiredness which were mild and temporary).
The studies evaluating the effects of electrostimulation devices were poorly conducted and reported, and provided insufficient evidence to determine the effects of these devices on either dry mouth or saliva production.
The single small study of a powered versus a manual toothbrush also found no difference for either dry mouth or saliva production.
None of the included studies reported the outcomes of duration of effectiveness, quality of life, patient satisfaction, or oral health assessment.
Quality of the evidence
These studies were generally of poor quality (low and very low).
Summary of findings
Summary of findings for the main comparison. Acupuncture versus placebo.
| Acupuncture compared with placebo for dry mouth symptoms | ||||
|
Patient or population: People with dry mouth due to either radiotherapy or Sjögren's Syndrome Settings: Outpatients Intervention: Acupuncture Comparison: Placebo (sham acupuncture) | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Mouth dryness Different scales (at the end of treatment 4‐6 weeks) |
SMD ‐0.34 (‐0.81 to 0.14) | 70 (2 RCTs*) | ⊕⊕⊝⊝ low 1 | *Participants had dry mouth following radiotherapy treatment for head and neck cancers |
|
Unstimulated whole saliva millilitres/minute (end of treatment) |
MD 0.02 (0 to 0.04) | 71 (3 RCTs**) | ⊕⊕⊝⊝ low 2 | **Most of the participants in these trials had dry mouth following radiotherapy treatment |
|
Stimulated whole saliva (end of treatment) millilitres/minute (end of treatment) |
MD 0.19 (0.07 to 0.31) | 71 (3 RCTs**) | ⊕⊕⊝⊝ low 2 | **Most of the participants in these trials had dry mouth following radiotherapy treatment |
|
Unstimulated whole saliva (12 months) millilitres/minute (1 year follow‐up) |
MD 0.06 (0.01 to 0.11) | 54 (2 RCTs**) | ⊕⊕⊝⊝ low 3 | **Most of the participants in these trials had dry mouth following radiotherapy treatment |
|
Stimulated whole saliva (12 months) millilitres/minute (1 year follow‐up) |
MD 0.28 (0.09 to 0.47) | 54 (2 RCTs**) | ⊕⊕⊝⊝ low 3 | **Most of the participants in these trials had dry mouth following radiotherapy treatment |
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||
1Quality of the body of evidence is downgraded due to risk of bias (2 very small RCTs at high risk of bias), and also because three trials which evaluated this comparison did not report the outcome of dry mouth 2Quality of the body of evidence is downgraded due to high risk of bias (2 studies at high risk of bias and 1 unclear), and heterogeneity (I2 = 57%) 3Quality of the body of evidence is downgraded due to risk of bias (1 high, 1 unclear risk of bias) and small number of participants
Summary of findings 2. Electrostimulation versus placebo.
| Electrostimulation compared with placebo for dry mouth symptoms | ||||
|
Patient or population: People with dry mouth due to Sjögren's Syndrome Settings: Outpatients Intervention: Electrostimulation Comparison: Placebo | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Unstimulated whole saliva millilitres/minute (end of treatment) |
MD 0.02 grams/2 minutes (95% CI ‐0.12 to 0.16) |
24 (1 RCT*) | ⊕⊝⊝⊝ very low 1 | *Participants had Sjögren's Syndrome |
|
Stimulated whole saliva millilitres/minute (end of treatment) |
MD 0.16 grams/2 minutes (95% CI ‐0.05 to 0.37) |
24 (1 RCT*) | ⊕⊝⊝ very low 1 | *Participants had Sjögren's Syndrome |
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||
1Quality of the body of evidence downgraded due to high risk of bias, imprecision (confidence intervals include both a potential benefit and a potential negative effect on saliva production), and estimate based on a single small study. This study did not report the primary outcome of this review
Background
Description of the condition
Xerostomia is the subjective sensation of a dry mouth (Napenas 2009; Visvanathan 2010), which can have a number of different causes. Dry mouth may be an objective finding with a reduction in the quantity of saliva produced, or a change in the composition of the saliva (Napenas 2009; Visvanathan 2010), or it may be a subjective sensation of dry mouth, found in patients with normal salivary gland function.
In a healthy individual, saliva production ranges from 0.5 to 1.5 litres per day (Mese 2007; Porter 2004). Approximately 90% of this saliva is produced by three pairs of major glands (the parotid, submandibular and sublingual salivary glands), with the remaining 10% of saliva produced by the minor salivary glands which are distributed around the mouth (in the labial, buccal, lingual and palatal mucosa) (Mese 2007; Napenas 2009). Secretion of saliva from the salivary glands is controlled by the brain via the saliva reflex arch. The taste, smell and/or chewing of food stimulates the salivary centre in the medulla of the brain, which then stimulates the nerves to the salivary glands which produce saliva (Proctor 2007). Saliva moistens the oral mucosa and helps to maintain oral health. The presence of saliva facilitates speech, acts to wash away food residue from around the teeth, neutralises potentially damaging food and bacterial acids, enhances a person's ability to taste the food, and generally lubricates the mouth (Hopcraft 2010; Mese 2007). Saliva also acts to soften food, making it easier to chew and swallow. Enzymes in saliva start the digestion of starch and fats, and other substances in saliva, such as epidermal growth factors, promote tissue growth, differentiation and wound healing. The antibacterial, antifungal and antiviral agents in saliva balance the oral flora and help to prevent oral infections, while the minerals in saliva help to maintain tooth enamel.
Dry mouth is a very common symptom, with an estimated incidence of between 10% and 26% in men and between 10% and 33% in women (Hopcraft 2010). Sufferers may complain of their mouth feeling dry or sticky in texture, report difficulty with chewing and swallowing food, and describe a decreased sensation of taste. Irritation when wearing dentures has also been reported (Visvanathan 2010).
The causes of xerostomia can be separated into two groups: salivary and non‐salivary (Napenas 2009). Non‐salivary causes of dry mouth include: mouth breathing, anxiety, neurological dysfunction and dehydration (Napenas 2009). Salivary causes of dry mouth symptoms can be further subdivided into those associated with salivary gland pathology such as Sjögren's Syndrome, sarcoidosis, diabetes mellitus and hepatitis C virus . There is some potential overlap between these groups as Sjögren's Syndrome is not only associated with direct salivary gland damage but is also associated with neuropathy (Tobón 2012) which could result in neurologically mediated salivary dysfunction. In addition, there are over 500 medications reported to cause oral dryness through various proposed mechanisms (Femiano 2008; Porter 2004). It is reported that drugs can inhibit salivation via effects on central and peripheral receptors (Proctor 2007; Scully 2004). The medications known to cause oral dryness are wide ranging and often very commonly prescribed preparations such as those used to treat depression, epilepsy and hypertension. Moreover, xerostomia is a recognised side effect of both radiotherapy (Shiboski 2007) and chemotherapy (Porter 2004) used to treat cancer.
Xerostomia is especially common among the elderly. While salivary glands certainly undergo changes due to age, the impact of changes due to aging on salivary gland function is contentious (Mese 2007). It has been suggested that subjective complaints of dry mouth in the elderly population can mostly be attributed to causes other than age‐related changes to the tissues in the salivary glands (Mese 2007); notably the increased prevalence of chronic conditions in this population, and resultant 'polypharmacy' (Femiano 2008; Porter 2004).
The experience of having a dry mouth can have a detrimental effect on a sufferer's quality of life, and can force them to modify their daily behaviour in order to cope with their symptoms (Hopcraft 2010). Dry mouth can be associated with a number of negative consequences which include: difficulty with speaking, chewing, swallowing and tasting food; soreness of the gums and oral mucosa, making the wearing of dentures uncomfortable or impossible; impaired sleep; psychological and social disability; increased risk of caries; oral candidiasis and salivary gland infections (Enger 2011; Fedele 2008; Hackett 2012; Porter 2010; Visvanathan 2010; Wolff 2012). Effective management of this condition is therefore important to improve the quality of life of sufferers. In addition, from both a public health and patient perspective it is important to manage dry mouth symptoms in order to minimise possible sequelae such as dental decay and oral infections.
Because dry mouth is a subjective symptom it is assessed by questioning individuals. A series of questions targeting different aspects of dry mouth may provide more information regarding the aspects which have greatest impact on an individual's quality of life. There are numerous such tools used to assess dry mouth symptoms, including various quality of life scales and some specific tools such as the Xerostomia Questionnaire (XQ) and the Xerostomia Inventory (XI). The Xerostomia Inventory is an 11‐item summated rating scale which has been validated as both a discriminative measure of the severity of dry mouth symptoms, and as a responsive measure of the effects of interventions for dry mouth (Thomson 2007). The range of possible XI scores is from 11 to 55 (Appendix 1) and a change in XI score of six points is likely to be clinically meaningful (Thomson 2007).
In some patients it may be possible to manage the problems associated with a dry mouth through optimal management of the underlying condition(s); for example through better management of diabetes. Smoking cessation and a reduction in alcohol consumption may also be of some benefit, as both these factors may exacerbate symptoms of dry mouth (Mese 2007). For individuals with mild symptoms, sucking ice chips or frequent sips of cold water may provide sufficient relief (Hopcraft 2010).
Topical application of salivary substitutes may provide short‐term relief during waking hours (Femiano 2008). Salivary stimulation by means of either systemic or topical medications, or chewing gum, may be appropriate for use by patients with some degree of salivary gland function (Porter 2004). However, while the use of some systemic pharmacotherapies, such as pilocarpine, to stimulate saliva production are effective (Davies 2007), these drugs have associated adverse effects and may be contraindicated in patients with existing chronic respiratory, cardiovascular and renal disease (Fedele 2008).
Description of the intervention
Non‐pharmacological interventions, such as electrostimulation of the salivary glands, acupuncture or the application of low level laser therapy, have the potential to increase saliva production. In electrostimulation, a hand‐held battery‐operated device may be used to administer an electrical stimulus to the tongue or hard palate. Alternatively, a transcutaneous electrical nerve stimulation (TENS) machine may be used and electrodes connected to the skin. Electrostimulation may be administered in the patient's home or at a medical facility. In acupuncture, needles are inserted by a professional into pre‐determined acupuncture points on the body. In low level laser therapy, a laser beam is applied by a professional to the salivary glands of patients with xerostomia. For patients with some residual salivary gland function, and co‐morbidities or contraindications to pharmacological therapies, identifying effective alternative means for stimulating saliva production, could provide a useful management strategy.
How the intervention might work
Acupuncture and electrostimulation have been reported to have both biological and clinical plausibility with regards to the treatment of dry mouth (O'Sullivan 2010; Wolff 2012; Zhuang 2013). It is proposed that application of electrical impulses to one or more arms of the salivary reflex arch may increase salivation (Fedele 2008). Electrostimulation of the efferent trigeminal fibres of the lingual nerve may promote the submandibular and sublingual glands to increase saliva secretion (Wolff 2012). Acupuncture is suggested to produce physiological effects such as stimulation of the autonomic nervous system and increased peripheral blood flow which may in turn stimulate saliva production (O'Sullivan 2010). The mechanism of action for low level laser therapy is complex and is also poorly understood, however laser therapy is thought to increase salivary secretion through the stimulation of mitotic activity in salivary gland epithelial tissue (Lončar 2011).
Why it is important to do this review
The number of people living with dry mouth symptoms is expected to rise as life expectancy increases, and treatment for chronic diseases becomes more effective (Mese 2007; Porter 2004). Dry mouth conditions can have considerable negative impact on the quality of life of patients (Enger 2011; Hackett 2012; Porter 2010). Effective treatments for patients unable to use systemic pharmacotherapies would not only improve the quality of life for these patients, but would also help maintain oral health, avoiding further potentially painful, debilitating and costly oral disease and tooth loss.
This review complements other existing Cochrane reviews of treatments for dry mouth.
Pharmacological interventions for preventing salivary gland dysfunction following radiotherapy (Tavender 2004).
Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy (Davies 2007).
Amifostine for salivary glands in high dose radioactive iodine treated differentiated thyroid cancer (Ma 2009).
Interventions for the management of dry mouth: topical therapies (Furness 2011) which includes the use of saliva substitutes and saliva stimulants such as pastilles and chewing gum.
Objectives
To assess the effects of non‐pharmacological interventions administered to stimulate saliva production for the relief of dry mouth.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing techniques designed or used to stimulate saliva production (such as acupuncture, laser or electrostimulation) with either placebo or with another treatment. Trials were included irrespective of language or publication status.
We excluded cross‐over studies from this review due to the potential for non‐pharmacological therapies, such as acupuncture, laser therapy and electrostimulation, to exert a prolonged effect that could carry‐over to subsequent periods of the trial (Higgins 2011).
Types of participants
Trials where participants were seeking treatment for the symptoms of dry mouth (xerostomia) due to any cause. Participants must have had xerostomia at baseline. Causes of xerostomia may have included haemodialysis, hormonal disorders (diabetes), autoimmune conditions (Sjögren's Syndrome, systemic lupus erythematosus and rheumatoid arthritis) and immune disorders (such as AIDS and graft versus host disease). This review also included patients currently undergoing, or who have previously received, radiotherapy; and patients seeking treatment for xerostomia due to current use of medications to control chronic or neoplastic conditions.
Types of interventions
Non‐pharmacological interventions, such as acupuncture, electrostimulation or low level laser therapy, for the management of xerostomia. Active interventions were compared with either placebo, no treatment or another active non‐systemic treatment, such as topical salivary stimulants. Trials which compared a non‐pharmacological intervention with systemic treatments such as oral pilocarpine or oral cevimeline were excluded. Systemic pharmacological treatments are effective, but may be contraindicated in some patients with co‐morbidities or concomitant medications and it is this group who may benefit from non‐pharmacological interventions. Trials which compared different frequencies of treatment were included.
Types of outcome measures
Primary outcomes
Xerostomia both short term (4 weeks after start of treatment) and longer term (3 months after end of treatment). Dry mouth may have been measured using a visual analogue scale (VAS) or been subjectively assessed as improved, no change or worse compared to baseline. Dry mouth symptoms may also have been measured using a validated questionnaire such as the Xerostomia Questionnaire (XQ).
Secondary outcomes
Duration of effectiveness.
Quality of life: assessed using a standard quality of life instrument, or a specific instrument such as head and neck quality of life (HNQOL), or similar.
Patient satisfaction with the treatment(s).
Adverse events.
Salivary flow: a clinically measured objective outcome such as unstimulated whole saliva (UWS) or stimulated whole saliva (SWS).
Oral health assessment.
Search methods for identification of studies
For the identification of studies included or considered for this review, detailed search strategies were developed for each database searched. These were based on the search strategy developed for MEDLINE (Appendix 2) but appropriately revised for each database to take account of differences in syntax rules and controlled vocabulary. This subject strategy was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (as published in box 6.4.c in the Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011).
Electronic searches
We searched the following databases:
The Cochrane Oral Health Group's Trials Register (to 16th April 2013) (Appendix 3)
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3) (Appendix 4)
MEDLINE via OVID (1948 to 16th April 2013) (Appendix 2)
EMBASE via OVID (1980 to 16th April 2013) (Appendix 5)
AMED via OVID (1985 to 16th April 2013) (Appendix 6)
CINAHL via EBSCO (1981 to 16th April 2013) (Appendix 7)
CANCERLIT via PubMed (1950 to 16th April 2013) (Appendix 8).
Only handsearching carried out as part of The Cochrane Collaboration's handsearching programme was included in the search, where these references have been incorporated into the CENTRAL database (see the Cochrane Master List of journals which have been handsearched).
The metaRegister of Controlled Clinical Trials (www.controlled‐trials.com) and ClinicalTrials.gov (www.clinicaltrials.gov) were also searched to identify ongoing and completed trials and to contact trialists for further information. There were no restrictions on the language of publication or publication status.
Searching other resources
The reference lists of related review articles and all articles obtained were checked for further trials.
Data collection and analysis
Selection of studies
At least two review authors screened the results of the searches to identify possible included studies. Paper copies were obtained of all trials which appeared to meet the inclusion criteria or where there was insufficient information in the title or abstract or both to make a clear decision about eligibility. At least two review authors assessed each of these papers to determine which met the inclusion criteria for this review. Any disagreements were resolved by discussion. Papers not in English were translated by members of The Cochrane Collaboration as required.
Data extraction and management
All randomised controlled trials which appeared to meet the inclusion criteria for this review were assessed by at least two review authors to confirm eligibility, assess risk of bias and extract data using a piloted data extraction form. Disagreements were resolved by discussion. The following data were recorded.
Study design, location, funding, number of centres.
Inclusion and exclusion criteria, number of patients recruited, number of patients randomised to each group, number of patients withdrawn, numbers evaluated.
Intervention(s), comparator, dose, frequency, duration of treatment, concomitant medications.
Primary and secondary outcomes, times measured, numbers of patients included in the outcome evaluation.
Whether a sample size calculation was performed.
Information was entered into the table of characteristics of included studies and additionally into an Excel spreadsheet from which a summary of the characteristics of the studies was made. Where the published paper was unclear concerning aspects of trial design, attempts were made to contact the study authors for clarification or more information or both.
Assessment of risk of bias in included studies
This was conducted using the recommended approach for assessing the risk of bias in studies included in Cochrane reviews (Higgins 2011). We used the two‐part tool, addressing the six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other bias'). Each domain included one or more specific entries in a 'Risk of bias' table. Within each study, the first part of the tool involved describing what was reported to have happened in the study. The second part of the tool involved assigning a judgement relating to the risk of bias for that entry. This was achieved by answering a pre‐specified question about the adequacy of the study in relation to the entry, such that a judgement of 'low' indicated low risk of bias, 'high' indicated high risk of bias, and 'unclear' indicated unclear or unknown risk of bias.
The domains of sequence generation, allocation concealment, incomplete outcome data and selective outcome reporting are each addressed in the tool by a single entry for each study. For blinding two entries were used because assessments needed to be made separately for a) patients and b) outcome assessor. Where the patients self assessed the outcomes to the trial, this was noted. The final domain ('other sources of bias') was assessed as a single entry for studies as a whole.
The risk of bias assessment was undertaken independently and in duplicate by two review authors as part of the data extraction process.
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories.
Low risk of bias (plausible bias unlikely to seriously alter the results) for all key domains.
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more key domains were assessed as unclear.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more key domains were assessed to be at high risk of bias.
A 'Risk of bias' table was completed for each included study. The results were also presented graphically.
Measures of treatment effect
For dichotomous outcomes (e.g. xerostomia improved or not), the estimate of treatment effect of an intervention would have been expressed as risk ratios (RR) (xerostomia improved/not) together with 95% confidence intervals (CIs). For continuous outcomes (such as mean VAS scores), mean differences and standard deviation were used to summarise the data for each trial.
Dealing with missing data
Where data were missing from the published report of a trial, we attempted to contact the author(s) to obtain the data and clarify any uncertainty. The analysis generally included only the available data (ignoring missing data), however, methods for estimating missing standard deviations in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) would have been used if appropriate. Otherwise we did not undertake any imputations or use any statistical methods to impute missing data.
Assessment of heterogeneity
Heterogeneity was assessed by inspection of the point estimates and confidence intervals on the forest plots. The variation in treatment effects was assessed by means of Cochran's test for heterogeneity and quantified by the I2 statistic. Heterogeneity was considered statistically significant if P value was < 0.1. A rough guide to the interpretation of the I2 statistic given in the Cochrane Handbook for Systematic Reviews of Interventions is: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis, publication bias would have been assessed according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry were identified we would have examined possible causes.
Data synthesis
A meta‐analysis was only conducted if there were studies of similar comparisons reporting the same outcome measures. We would have combined risk ratios for dichotomous data, and we combined mean differences for continuous data, using fixed‐effect models. If there were more than three studies included in any meta‐analysis, we would have used random‐effects models.
Subgroup analysis and investigation of heterogeneity
We planned to investigate clinical heterogeneity by examining the different causes of xerostomia. If there had been sufficient studies of each intervention and outcome, we planned, a priori, to conduct subgroup analyses for different causes of xerostomia (type of drug or type of condition causing xerostomia).
Sensitivity analysis
If there had been sufficient studies for each outcome and intervention, we would have undertaken sensitivity analysis based on the overall risk of bias.
Presentation of main results
A summary of findings table was developed for the main outcomes of this review using GRADEPro software. The quality of the body of evidence was assessed with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias and the magnitude of the effect. The quality of the body of evidence of each of the main outcomes was categorised as high, moderate, low or very low.
Results
Description of studies
Results of the search
After de‐duplication a total of 1133 references were identified from the electronic searches. Titles and abstracts were screened by two review authors and 1101 were discarded as being not relevant to this systematic review. Thirty‐two references were retrieved in full text and of these, 11 references to nine RCTs met the inclusion criteria for this review and 21 references to 18 studies were listed as excluded (Figure 1).
1.
Study flow diagram.
Included studies
Characteristics of the trial designs and settings
Nine RCTs met the inclusion criteria and were included in this review (Characteristics of included studies). All were parallel group trials and all except one (Wong 2003) had two arms comparing an experimental arm with a control arm. Four trials were conducted in the USA (Papas 2006; Pfister 2010; Steller 1988; Talal 1992), three in Sweden (Blom 1992; Blom 1996; List 1998) and one in each of Canada (Wong 2003) and Korea (Cho 2008). Two studies were described as either a pilot study (Cho 2008) or a Phase I/II study (Wong 2003), and there were no sample size calculations reported for any of the included studies. Eight of the included studies took place in a single centre but Talal 1992 was a three‐centre study. Three studies had some support from companies who supplied the products being evaluated (Papas 2006; Talal 1992; Wong 2003), four were funded solely by research grants from publicly funded bodies (Blom 1996; Cho 2008; List 1998; Pfister 2010), and the remaining two (Blom 1992; Steller 1988) did not state the sources of funding for the studies.
Characteristics of the participants
A total of 366 participants took part in these trials with a mean of 40 participants per trial and a range of 12 to 77. All participants were adults. The causes of xerostomia were radiotherapy for oral cancers in four trials (Blom 1996; Cho 2008; Pfister 2010; Wong 2003), Sjögren's Syndrome in three trials (List 1998; Steller 1988; Talal 1992), medication‐related in one trial (Papas 2006) and in the remaining trial (Blom 1992) participants had a range of causes of xerostomia.
Characteristics of the interventions
We divided the included studies into three groups, according to the interventions evaluated. Five trials evaluated acupuncture (Blom 1992; Blom 1996; Cho 2008; List 1998; Pfister 2010), three evaluated electrostimulation devices (Steller 1988; Talal 1992; Wong 2003) and one evaluated a powered toothbrush in participants with dry mouth (Papas 2006).
1. Acupuncture
Three trials (Blom 1992; Blom 1996; Cho 2008) compared acupuncture to a sham acupuncture (placebo) control group, one trial (List 1998) compared acupuncture with both manual and electrical stimulation of the needles to acupuncture with manual stimulation only, and Pfister 2010 compared acupuncture to 'usual care'. The duration of treatment varied between these studies. The study by Cho 2008 used a 6‐week treatment period, in Pfister 2010 treatment was over 4 weeks and in the study by List 1998 participants had a 10‐week course of treatment. In both the other trials (Blom 1992; Blom 1996) treatment was two courses of 6 weeks separated by a 2‐week rest period.
2. Electrostimulation
Two trials (Steller 1988; Talal 1992) of electrostimulation devices compared a device with a placebo (sham device). Participants received training in the use of the devices, initially under the supervision of the researchers, and then used the devices at home three times daily. In the three‐arm trial (Wong 2003) a comparison was made between transcutaneous electrostimulation of three different sets of acupuncture points.
3. Powered toothbrush
The final trial in this review (Papas 2006) compared the salivary stimulation achieved by brushing teeth, gums and tongue with a powered toothbrush, with that resulting from a similar brushing pattern with a manual toothbrush.
Characteristics of the outcomes
The primary outcome of our review was mouth dryness and three of the five included trials which evaluated acupuncture treatments stated that they collected data on this outcome. Cho 2008 used a Xerostomia Questionnaire to assess dry mouth and List 1998 stated that they used a VAS scale to measure mouth dryness. Pfister 2010 evaluated the effect of acupuncture primarily on pain due to radiotherapy but this trial reported mouth dryness as measured by Xerostomia Inventory as a secondary outcome. Neither Blom 1992 nor Blom 1996 reported the outcome of dry mouth symptoms.
In the electrostimulation group, both Wong 2003 and Talal 1992 described collecting data on dry mouth symptoms but did not report outcome data for each randomised group. The trial by Steller 1988 did not report dry mouth symptoms.
The powered versus manual toothbrush study (Papas 2006) stated that they used a VAS scale to measure mouth dryness as an outcome but this trial did not report dry mouth outcome data.
None of the included studies reported the outcomes of duration of effectiveness, quality of life, patient satisfaction, or oral health assessment.
Measures of stimulated salivary flow (SWS) were reported by eight trials (Blom 1992; Blom 1996; Cho 2008; List 1998; Papas 2006; Steller 1988; Talal 1992; Wong 2003) and seven of these also reported the outcome of unstimulated salivary flow (UWS) (Blom 1992; Blom 1996; Cho 2008; List 1998; Papas 2006; Steller 1988; Wong 2003).
Excluded studies
After at least two review authors had assessed the full‐text study report, 21 references to a total of 18 studies were excluded from this review. Ten studies were found not to be randomised controlled trials (Blom 2000; Cheville 2006; Fontanesi 1991; Garcia 2009; Loncar 2011; Niemtzow 2007; Schiff 2009; Simcock 2009; Weiss 1986; Wong 2012), five studies were of interventions to prevent dry mouth where participants did not have dry mouth symptoms at baseline (Braga 2011; Deng 2008; Meng 2012; Simoes 2010; Wong 2010) and three studies used a cross‐over design which was an explicit exclusion criteria for this review (Simcock 2013; Strietzel 2007; Strietzel 2011). See Characteristics of excluded studies for further details.
Risk of bias in included studies
Allocation
We judged three studies (33.3%) to have adequate sequence generation (Pfister 2010; Steller 1988; Talal 1992) and two of these (Pfister 2010; Talal 1992) also described adequate allocation concealment, and therefore these trials were assessed as at low risk of selection bias.
The trial by Steller 1988 did not report sufficient information to determine whether allocation concealment was done. The remaining six trials (Blom 1992; Blom 1996; Cho 2008; List 1998; Papas 2006; Wong 2003) reported insufficient information for us to make a judgement. These seven trials were assessed at unclear risk of selection bias.
Blinding
Blinding of participants to the allocated treatment by use of a placebo was done in five of the included studies (Blom 1992; Blom 1996; Cho 2008; Steller 1988; Talal 1992) and these trials were assessed at low risk of performance bias. The other four trials did not blind participants to the allocated treatment and were therefore assessed at high risk of performance bias.
Outcome assessors were blinded to allocated treatment in four trials (Blom 1992; Blom 1996; Steller 1988; Talal 1992) and these trials were assessed at low risk of detection bias. One trial (Wong 2003) did not use blinded outcome assessment and was judged at high risk of detection bias. The remaining four trials did not report sufficient information concerning outcome evaluation and were assessed at unclear risk of detection bias.
Incomplete outcome data
Incomplete outcome data were a problem in two trials (Steller 1988; Wong 2003) where 17% and 20% of trial participants respectively, were excluded from the outcome evaluation, and the numbers lost were not similar in each arm of these trials. These two trials were assessed at high risk of attrition bias.
Two trials (Blom 1996; Talal 1992) did not report the number of participants included in the outcome evaluation and these trials were assessed at unclear risk of attrition bias. The remaining five trials were assessed at low risk of attrition bias.
Selective reporting
We found selective reporting in three trials in this review (Papas 2006; Talal 1992; Wong 2003) and these trials were assessed at high risk of reporting bias. Talal 1992 and Wong 2003 stated in the methods that they were collecting data on dry mouth symptoms but neither of these trials reported these data for the allocated treatment groups. Papas 2006 described in the methods that VAS scores for mouth dryness would be collected but these were not reported, and participant satisfaction was only reported for the powered toothbrush.
Reporting bias was unclear in Blom 1992; Blom 1996 as neither of these trials reported a dry mouth outcome.
In the remaining four trials there was a low risk of reporting bias.
Other potential sources of bias
Three of the included trials were assessed at high risk of other bias (Blom 1996; Cho 2008; Papas 2006). The authors of Blom 1996 acknowledged in the discussion of this trial report that the placebo intervention of superficial needling appears to have some effect on dry mouth and so use of this placebo may have reduced the relative benefit of acupuncture treatment. In Cho 2008 at baseline, the two trial arms were not balanced with regard to saliva flow rates, although the groups were so small that differences were not statistically significant. In the Papas 2006 trial powered toothbrushes were given to all participants and this may have introduced some bias into the assessment of participant satisfaction.
The remaining six trials were assessed at low risk of other bias.
Overall risk of bias
All of the trials included in this review had at least one domain where risk of bias was either unclear or high. Consequently eight trials were assessed at high risk of bias and the remaining one (Blom 1992) at unclear risk of bias (Figure 2; Figure 3). For further details see Characteristics of included studies.
2.
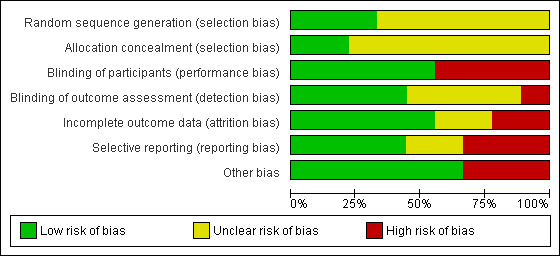
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Acupuncture versus placebo
Dry mouth
The primary outcome of this review was the effect of the interventions on dry mouth. According to their methods sections, this outcome was planned to be recorded in three of the six (Cho 2008; List 1998; Pfister 2010) included acupuncture trials, but only Cho 2008 and Pfister 2010 reported dry mouth outcome data suitable for inclusion in a meta‐analysis. Each of these trials compared acupuncture (either a 4‐week or a 6‐week course of treatment) with placebo and each measured dry mouth using a different scale, so these data were combined using a standardised mean difference (SMD). The pooled estimate (SMD ‐0.34, 95% confidence interval (CI) ‐0.81 to 0.14, P value 0.17, I2 = 39%) (Analysis 1.1) showed no evidence of a difference between acupuncture and placebo, but the combined trials included only 70 participants. An SMD of 0.3 is generally considered to be a small to moderate effect (Cohen 1988), but the confidence interval ranges between a large decrease in mouth dryness and a very small increase. The heterogeneity in this estimate (I2 = 39%) is likely to have been due to the different causes of dry mouth in the participants in these trials. There may have been insufficient participants and statistical power to show a difference (Type 1 error) if indeed a difference exists, or as the authors of the trials suggest, the 'placebo' acupuncture may have had some benefit, thus biasing the effect towards the null.
1.1. Analysis.
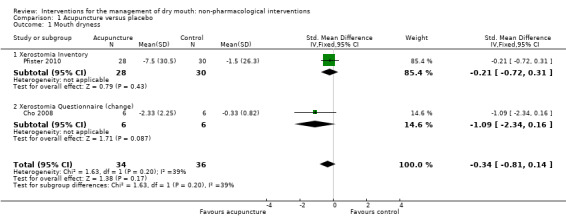
Comparison 1 Acupuncture versus placebo, Outcome 1 Mouth dryness.
A third study (List 1998) compared acupuncture with both electrical and manual stimulation to acupuncture with manual stimulation (placebo) in patients with Sjögren's Syndrome and reported a statistically significant reduction in the outcome of dry mouth (P < 0.05). However, data from this study (medians and ranges Additional Table 3) could not be used in the meta‐analysis.
1. Additional outcome data from included studies.
| Study ID | Outcome | Intervention group | N | Control group | N |
|
List 1998 Acupuncture versus placebo |
Mouth dryness 10‐point VAS |
Median 5.5 (Range 3.2 to 10) | 10 | Median 6.8 (Range 0 to 9.5) | 11 |
| UWS (ml/15 minutes) |
Median 0.0 (Range 0.0 to 0.6) | 10 | Median 0.0 (Range 0 to 0.2) | 11 | |
| SWS (ml/5 minutes) |
Median 1.2 (Range 0.05 to 2.6) | 10 | Median 0.6 (Range 0.1 to 2.5) | 11 | |
|
Talal 1992 Electrostimulation versus placebo |
SWS (ml/min) | Mean 0.385 | 40 | Mean 0.196 | 37 |
SWS = stimulated whole saliva; UWS = unstimulated whole saliva; VAS = visual analogue scale
Adverse effects
In the trials evaluating acupuncture, both Blom 1992 and Blom 1996 noted that tiny bruises appeared at acupuncture sites in some participants and there was tiredness after treatments and List 1998 noted that "significant discomfort in the eyes was registered in the acupuncture group". Pfister 2010 reported that no serious adverse effects were noted but "participants reported temporary increases in pain, minor bruising or bleeding and constitutional symptoms" associated with acupuncture. The study by Cho 2008 did not mention adverse effects.
Unstimulated whole saliva
Three trials, two at high risk of bias and one unclear, reported data for this outcome at the end of treatment. In two studies (Blom 1992; Blom 1996) participants had 6 weeks of either acupuncture or sham acupuncture treatment, followed by a 2‐week 'rest' and then a second course of 6 weeks of treatment. The study by Cho 2008 reported outcomes at the end of a 6‐week course of treatment. A fourth study (List 1998) reported this outcome and found no difference, but data from this study (medians and ranges in each group, Additional Table 3) could not be included in the meta‐analysis.
Meta‐analysis of unstimulated whole saliva data from these three trials shows a very small effect favouring acupuncture which is unlikely to be clinically important (mean difference (MD) 0.02 ml/minute, 95% CI 0 to 0.04, P value 0.04, I2 = 57%) (Analysis 1.2). Heterogeneity is likely to be due to the different reasons for dry mouth in participants in these trials.
1.2. Analysis.
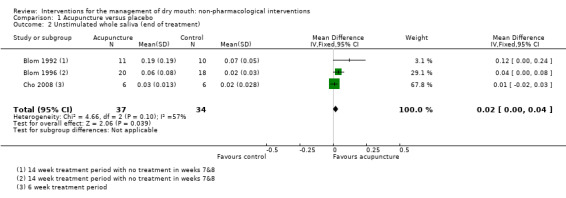
Comparison 1 Acupuncture versus placebo, Outcome 2 Unstimulated whole saliva (end of treatment).
Likewise after 12 months follow‐up there is a very small benefit associated with acupuncture (MD 0.06 ml/minute, 95% CI 0.01 to 0.11, P value 0.03, I2 = 10%) which may or may not be associated with a clinically meaningful improvement (Analysis 1.4).
1.4. Analysis.
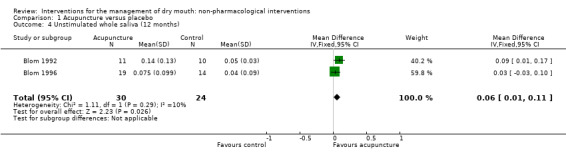
Comparison 1 Acupuncture versus placebo, Outcome 4 Unstimulated whole saliva (12 months).
Stimulated whole saliva
At the end of the treatment period the meta‐analysis of three trials of acupuncture reported data which showed an increase in stimulated whole saliva production favouring the acupuncture groups (MD 0.19 ml/minute, 95% CI 0.07 to 0.31, P value 0.002, I2 = 1%) (Analysis 1.3). The difference favouring acupuncture persisted at 12 months of follow‐up (MD 0.28 ml/minute, 95% CI 0.09 to 0.47, P value 0.004, I2 = 0%) (Analysis 1.5). A fourth study (List 1998) reported this outcome and found no difference but data from this study (medians and ranges in each group) could not be included in the meta‐analysis. The clinical importance of this finding is unclear. It may indicate a small increase in saliva production, but the effect of increased saliva production on the symptom of dry mouth is not evaluated.
1.3. Analysis.
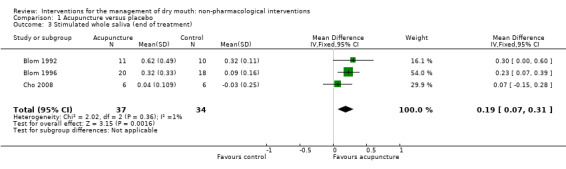
Comparison 1 Acupuncture versus placebo, Outcome 3 Stimulated whole saliva (end of treatment).
1.5. Analysis.
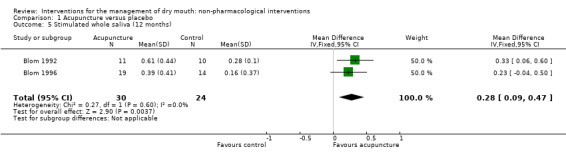
Comparison 1 Acupuncture versus placebo, Outcome 5 Stimulated whole saliva (12 months).
Electrostimulation
Dry mouth
Two small studies compared the use of an electrostimulation device with a placebo device in patients with Sjögren's Syndrome. Neither of these trials reported outcome data for dry mouth. One trial (Talal 1992) reported that data on dry mouth were collected, but these data were not reported and we have not been able to obtain them from the trial authors. The only information available from this study is that "patients using the active device experienced relief from six of the most common symptoms of xerostomia secondary to Sjögren's Syndrome, with two of these symptoms being statistically significantly improved compared to patient's using the placebo device". No other data were reported to support this statement (Additional Table 3).
The outcome of dry mouth was not mentioned by Steller 1988. A further study (Wong 2003) compared acupuncture‐like electrostimulation of different sets of points in patients who had been treated with radiotherapy. There was no control group in this study which did not report outcomes by randomised group.
Adverse effects
Wong 2003 stated that "no adverse effects were caused by Codetron" in their Phase I‐II study of different treatment points. Neither of the other two reports (Steller 1988; Talal 1992) mentioned whether or not adverse effects occurred during the trial.
Unstimulated whole saliva
Steller 1988 reported no difference in unstimulated whole saliva between electrostimulation and control at the end of 4 weeks of treatment (MD 0.02 grams/2 minutes, 95% CI ‐0.12 to 0.16) (Analysis 2.1).
2.1. Analysis.
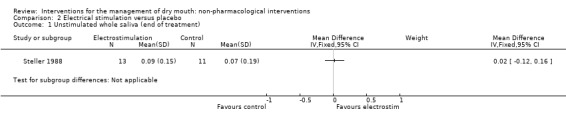
Comparison 2 Electrical stimulation versus placebo, Outcome 1 Unstimulated whole saliva (end of treatment).
Stimulated whole saliva
Two included trials of electrostimulation (Steller 1988; Talal 1992) reported the outcome of stimulated whole saliva, but Talal 1992 reported no estimate of variance in each group so we were unable to use the data from this trial (Additional Table 3).
Steller 1988 found no difference between the active and placebo groups (MD 0.16 grams/2 minutes, 95% CI ‐0.05 to 0.37) (Analysis 2.2). The study by Talal 1992 reported that saliva secretion increased more in the active electrostimulation group compared to those receiving placebo at each of the weekly treatment sessions, but this increase in saliva production did not appear to persist as the pre‐treatment mean saliva secretion was similar prior to each treatment in both groups.
2.2. Analysis.

Comparison 2 Electrical stimulation versus placebo, Outcome 2 Stimulated whole saliva (end of treatment).
Powered versus manual toothbrush
Unstimulated whole saliva
The single trial by Papas 2006 which compared powered and manual toothbrushes in participants with dry mouth found no difference between the groups in the volume of saliva produced 45 minutes after brushing (MD ‐0.07, 95% CI ‐0.74 to 0.60) (Analysis 3.1).
3.1. Analysis.

Comparison 3 Powered versus manual toothbrush, Outcome 1 Unstimulated whole saliva (45 minutes after brushing).
Stimulated whole saliva
There was no difference between the groups in the volume of saliva produced 5 minutes after brushing, assuming that brushing may have stimulated saliva production (MD ‐0.13, 95% CI ‐1.92 to 1.66) (Analysis 3.2).
3.2. Analysis.

Comparison 3 Powered versus manual toothbrush, Outcome 2 Stimulated whole saliva (5 minutes after brushing).
Duration of effectiveness, quality of life, participant satisfaction, oral health assessment
These outcomes were not reported by any of the trials included in this review.
Sensitivity analyses
Planned sensitivity analyses were not conducted because there were insufficient trials included in this review.
Discussion
Summary of main results
The nine studies included in this review of non‐pharmacological interventions for dry mouth were divided into three comparisons.
Five small studies (total 153 participants, with dry mouth following radiotherapy treatment) compared acupuncture with placebo and two trials reported outcome data for dry mouth in a form suitable for meta‐analysis. The pooled estimate for dry mouth showed no difference between acupuncture and control in these two trials (SMD ‐0.34, 95% CI ‐0.81 to 0.14, P value 0.17, I2 = 39%) with the confidence intervals including both a possible reduction or a possible increase in dry mouth symptoms. Acupuncture was associated with more adverse effects (tiny bruises and tiredness which were mild and temporary). There was a very small increase in unstimulated whole saliva (UWS) at the end of treatment (three trials) (MD 0.02 ml/minute, 95% CI 0 to 0.04, P value 0.04, I2 = 57%), and this benefit persisted at the 12‐month follow‐up evaluation (UWS, MD 0.06 ml/minute, 95% CI 0.01 to 0.11, P value 0.03, I2 = 10%). For stimulated whole saliva (SWS) there was a benefit favouring acupuncture (MD 0.19 ml/minute, 95% CI 0.07 to 0.31, P value 0.002, I2 = 1%) an effect which also remained at the 12‐month follow‐up evaluation (SWS MD 0.28 ml/minute, 95% CI 0.09 to 0.47, P value 0.004, I2 = 0%) (Table 1). It is unclear whether this small increase in saliva production is associated with a clinically important benefit because dry mouth symptoms were not assessed in all of the studies that measured saliva production.
Two small studies compared the use of an electrostimulation device with a placebo device (total 101 patients with Sjögren's Syndrome). A further study compared acupuncture‐like electrostimulation of different sets of points in participants who had previously been treated with radiotherapy. None of these studies reported the outcome of dry mouth. There was no difference between electrostimulation and placebo in the outcomes of UWS or SWS at the end of the 4‐week treatment period in the one study that provided data for these outcomes. No adverse effects were reported (Table 2).
A single study compared the stimulatory effect of powered versus manual toothbrushing and found no difference for the outcomes of UWS or SWS.
Overall completeness and applicability of evidence
We have included nine trials which randomised a total of 366 participants. However, the total number included in the outcome assessment is unclear as this information was not stated in one study (Talal 1992) and our attempts to contact the authors were not successful. Only two of the nine trials included in this review reported the primary outcome of this review: dry mouth. We pooled the data using standardised mean difference because each of the trials used a different scale to measure dry mouth. The confidence intervals of the pooled estimate cross the line of no effect, suggesting that there is no difference between acupuncture and placebo, although the point estimate SMD 0.34 could be interpreted as showing a small benefit in favour of acupuncture. It could be that the number of participants included in this meta‐analysis is too small to detect a statistically significant effect, and further RCTs evaluating acupuncture are required to determine whether this is the case. There is also a suggestion from the authors of these trials that the placebo ('sham' acupuncture) may actually have a beneficial effect on dry mouth. If there is a beneficial effect from the placebo treatment this would make it less likely to show a difference between the two arms of these trials. Consideration should be given to designing a different control intervention.
In the published protocol, we decided a priori to exclude trials with cross‐over designs from this review. The reason for this decision is that there is some suggestion that acupuncture may have a sustained effect on dry mouth. With regard to electrostimulation and laser therapies, there is little information available concerning the duration of effects.
Electrostimulation devices have undergone development over recent years and it is likely that the trials included in this review which were published between 1988 and 2003 evaluate 'first‐generation' devices which may be considered obsolete. Newer 'second generation' devices which employ electrodes embedded within a removable oral splint are now available (Fedele 2008). A recent trial of a 'second generation' electrostimulation device (Strietzel 2011) has been excluded from this review due to the use of a cross‐over design in this study. This trial reported a "cumulative positive effect" from the electrostimulation device. It is hoped that further research with this or similar electrostimulation devices will be undertaken using a double‐blind parallel‐group design.
Another consideration is the variation between the participants in these trials. Three trials included participants with Sjögren's Syndrome, four included patients who had previously undergone radiotherapy for head and neck cancer and two trials included participants with a range of cause of dry mouth symptoms. The nature and extent of salivary gland disease is likely to vary between these participants with resultant variations in residual gland function, disease natural history and prognosis amongst participants. Meta‐analysis of the acupuncture trials showed some evidence of a benefit due to acupuncture on the outcomes of both unstimulated and stimulated saliva production. It is not known whether or not this small average benefit, translated to an improvement in the symptoms of dry mouth or the (oral health related) quality of life for these trial participants because this information was not reported. From the literature we know that increased saliva production may or may not reduce dry mouth symptoms.
However, while there is little effect as measured by the mean differences between the randomised groups in these four trials, in both trials some individuals did appear to benefit from the active intervention.
Quality of the evidence
None of the trials included in this review are at low risk of bias. All are small (range of 12 to 77 participants per trial), none reported sample size calculations and all are likely to lack statistical power to detect a difference between the arms of the trial should such a difference exist. Few reported important outcomes such as dry mouth symptoms, quantifiable adverse effects or (oral health related) quality of life. There is evidence of reporting bias, whereby important patient‐centred outcomes are either not measured and/or not reported, and this is a major limitation on the findings of this review. The quality of the body of evidence for all the main outcomes of this review has been assessed as low or very low.
Potential biases in the review process
We conducted a broad search of several databases and placed no restrictions on the language of publication when searching the electronic databases or reviewing reference lists of included studies. However, it is likely that there are other studies, which may or may not be RCTs, published in the Chinese language literature which we have not identified for this review. However a similar published review prepared by a team of authors based in China (Zhuang 2013) identified the same three RCTs for treatment of radiotherapy‐induced xerostomia that we included in this review.
We decided to exclude cross‐over studies from this review because we were unable to determine empirically the duration of any potential effect of either acupuncture or electrostimulation techniques on dry mouth symptoms. It seemed likely that the potential effects of these interventions could extend for weeks or months after the end of the treatment phase. In this case the use of a cross‐over study design to evaluate these interventions would be inappropriate.
Agreements and disagreements with other studies or reviews
Our findings are broadly in agreement with those of other published systematic reviews (Garcia 2013; O'Sullivan 2010; Zhuang 2013) which focused on participants with post‐radiotherapy xerostomia and found evidence of some increase in saliva production but no difference in dry mouth symptoms. A review of treatment approaches for patients with xerostomia due to Sjögren's Syndrome (Wolff 2012) refers to one of the studies included in our Cochrane review (Talal 1992) and reports that electrostimulation is effective in stimulating saliva secretion. This review goes on to describe initial testing in a cross‐over trial of a small intraoral electrostimulation device, which is activated by a remote control. Early results appear promising for patients and further research is ongoing and may be included in future updates of this Cochrane review.
Authors' conclusions
Implications for practice.
There is insufficient evidence to determine the effects of any of the interventions included in this review on dry mouth symptoms. There is some evidence that acupuncture increases saliva production in patients with dry mouth following radiotherapy. There is insufficient evidence to determine the effects of electrostimulation devices on dry mouth symptoms or saliva production in patients with Sjögren's Syndrome. Reported adverse effects of both acupuncture and electrostimulation are mild and transient. The use of relatively non‐invasive techniques with favourable side‐effect profiles, such as electrostimulation and acupuncture are certainly desirable in dry mouth patients. Some patients with dry mouth symptoms may benefit from one of these treatments, but in the absence of good evidence of their effectiveness, neither acupuncture nor electrostimulation treatments are likely to be funded by healthcare providers, and therefore any cost is likely to be borne by the patient. However, due to the paucity of data in relation to quality of life outcomes, patient satisfaction and longevity of clinical benefit, the clinical effectiveness of such treatments remains obscured. The use of such treatments outside of the clinical trial setting remains difficult to justify at present.
Implications for research.
Both acupuncture and electrostimulation show some indications of possible benefit in some patients with dry mouth symptoms. Further well‐designed and conducted double‐blind trials with sufficient numbers of participants are required to determine the benefits of these interventions. Trials should be designed and conducted according to SPIRIT 2013 guidelines and reported according to CONSORT 2010 guidelines. Trials should include outcomes which are important to people with dry mouth such as dry mouth symptoms, quality of life, together with duration of effectiveness and satisfaction with the intervention.
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2013 | Amended | Changes to external sources of support |
| 4 September 2013 | New citation required but conclusions have not changed | Authorship change |
Acknowledgements
Our thanks to Phil Riley (Deputy Managing Editor of the Cochrane Oral Health Group) for his help with the administration of this review, and to Anne Littlewood for undertaking the searches for this review. Our thanks to the referees who provided comments on this review.
Appendices
Appendix 1. Xerostomia Inventory
Individuals are asked to choose a response to the following 11 questions. Each response is assigned a score between 1 and 5 and the combined total score (a number between 11 and 55) is calculated, which represents the severity of the underlying xerostomia (score of 11 represents very mild xerostomia and 55 represents severe xerostomia) (Thomson 2005).
My mouth feels dry
I have difficulty in eating dry foods
I get up at night to drink
My mouth feels dry when eating a meal
I sip liquids to aid in swallowing food
I suck sweets or cough lollies to relieve dry mouth
I have difficulties swallowing certain foods
The skin of my face feels dry
My eyes feel dry
My lips feel dry
The inside of my nose feels dry
| Response to each question | Score |
| Never | 1 |
| Hardly ever | 2 |
| Occasionally | 3 |
| Fairly often | 4 |
| Very often | 5 |
Appendix 2. MEDLINE via OVID search strategy
1. Xerostomia/ 2. xerostomia.mp. 3. (dry$ adj2 (oral or mouth$)).mp. 4. (asialia or "salivary gland hypofunction" or hyposalivat$).mp. 5. (radioxerostomia or radio‐xerostomia).mp. 6. or/1‐5 7. Electrical Stimulation/ 8. ((electric$ adj3 stimulat$) or neuroelectrostimulation or "masticatory stimulation").mp. 9. Lasers/ 10. laser$.mp. 11. "intra‐oral device$".mp. 12. Acupuncture/ 13. acupuncture.mp. 14. Hypnosis/ 15. (hypnosis or hypnotism or "autogenic train$" or autosuggestion or auto‐suggestion).mp. 16. or/7‐15 17. 6 and 16
Appendix 3. Cochrane Oral Health Group's Trials Register search strategy
((xerostomia or "dry mouth*" or asialia or "salivary gland hypofunction" or hyposaliva*) AND (neuroelectrostimulation or "masticatory stimulation" or (electro and stimulat*) or "intra‐oral device*" or acupuncture* or hypnosis or hypnotism or "autogenic* train*" or autosuggest* or auto‐suggest* or laser*))
Appendix 4. CENTRAL search strategy
#1 MeSH descriptor Xerostomia this term only #2 xerostomia in All Text #3 ((dry* in All Text near/2 oral in All Text) or (dry in All Text near/2 mouth* in All Text)) #4 (asialia in All Text or "salivary gland hypofunction" in All Text or hyposalivat* in All Text) #5 (radioxerostomia in All Text or radio‐xerostomia in All Text) #6 (#1 or #2 or #3 or #4 or #5) #7 MeSH descriptor Electric Stimulation this term only #8 ((electric* in All Text near/3 stimulat* in All Text) or neuroelectrostimulation in All Text or "masticatory stimulation" in All Text) #9 MeSH descriptor Lasers this term only #10 laser* in All Text #11 "intra‐oral device*" in All Text #12 MeSH descriptor Acupuncture this term only #13 acupuncture in All Text #14 MeSH descriptor hypnosis this term only #15 (hypnosis in All Text or hypnotism in All Text or "autogenic train*" in All Text or autosuggestion in All Text or auto‐suggestion in All Text) #16 (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15) #17 (#6 and #16)
Appendix 5. EMBASE via OVID search strategy
1. Xerostomia/ 2. xerostomia.mp. 3. (dry$ adj2 (oral or mouth$)).mp. 4. (asialia or "salivary gland hypofunction" or hyposalivat$).mp. 5. (radioxerostomia or radio‐xerostomia).mp. 6. or/1‐5 7. Electrostimulation/ 8. ((electric$ adj3 stimulat$) or neuroelectrostimulation or "masticatory stimulation").mp. 9. Lasers/ 10. laser$.mp. 11. "intra‐oral device$".mp. 12. Acupuncture/ 13. acupuncture.mp. 14. Hypnosis/ 15. (hypnosis or hypnotism or "autogenic train$" or autosuggestion or auto‐suggestion).mp. 16. or/7‐15 17. 6 and 16
The above subject search was linked to the following Filter for EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 6. AMED via OVID search strategy
1. xerostomia.mp. 2. (dry$ adj2 (oral or mouth$)).mp. 3. (asialia or "salivary gland hypofunction" or hyposalivat$).mp. 4. (radioxerostomia or radio‐xerostomia).mp. 5. or/1‐4 6. laser$.mp. 7. ((electric$ adj3 stimulat$) or neuroelectrostimulation or "masticatory stimulation").mp. 8. "intra‐oral device$".mp. 9. acupuncture.mp. 10.(hypnosis or hypnotism or "autogenic train$" or autosuggestion or auto‐suggestion).mp. 11. or/6‐10 12. 5 and 11
Appendix 7. CINAHL via EBSCO search strategy
S1 MH Xerostomia S2 xerostomia S3 ((dry* N2 oral) or (dry* N2 mouth*)) S4 (asialia or "salivary gland hypofunction" or hyposalivat*) S5 (radioxerostomia or radio‐xerostomia) S6 S1 or S2 or S3 or S4 or S5 S7 MH "Electric Stimulation" S8 ((electric* N3 stimulat*) or neuroelectrostimulation or "masticatory stimulation") S9 "intra‐oral device*" S10 MH Acupuncture S11 acupuncture S12 MH Hypnosis S13 (hypnosis or hypnotism or "autogenic train*" or autosuggestion or auto‐suggestion) S14 MH Lasers S15 laser* S16 S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 S17 S6 and S16
Appendix 8. CANCERLIT via PubMed search strategy
#1 Search Xerostomia [mh:noexp] #2 Search xerostomia #3 Search (dry* and (oral or mouth*)) #4 Search (asialia or "salivary gland hypofunction" or hyposalivat*) #5 Search radioxerostomia or radio‐xerostomia #6 Search #1 or #2 or #3 or #4 or #5 #7 Search Electrical Stimulation [mh:noexp] #8 Search ((electric* and stimulat*) or neuroelectrostimulation or "masticatory stimulation") #9 Search "intra‐oral device*" #10 Search Acupuncture [mh:noexp] #11 Search acupuncture #12 Search Hypnosis [mh:noexp] #13 Search hypnosis or hypnotism or "autogenic train*" or autosuggestion or auto‐suggestion #14 Laser [mh:noexp] #15 laser* #16 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 #6 and #16
Appendix 9. Search strategy for Clinicaltrials.gov
1. xerostomia and laser 2. xerostomia and acupuncture 3. xerostomia and electrostimulation 4. or/1‐3
Data and analyses
Comparison 1. Acupuncture versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mouth dryness | 2 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.81, 0.14] |
| 1.1 Xerostomia Inventory | 1 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.72, 0.31] |
| 1.2 Xerostomia Questionnaire (change) | 1 | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐2.34, 0.16] |
| 2 Unstimulated whole saliva (end of treatment) | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [0.00, 0.04] |
| 3 Stimulated whole saliva (end of treatment) | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.07, 0.31] |
| 4 Unstimulated whole saliva (12 months) | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.01, 0.11] |
| 5 Stimulated whole saliva (12 months) | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.09, 0.47] |
Comparison 2. Electrical stimulation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Unstimulated whole saliva (end of treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Stimulated whole saliva (end of treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 3. Powered versus manual toothbrush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Unstimulated whole saliva (45 minutes after brushing) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Stimulated whole saliva (5 minutes after brushing) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blom 1992.
| Methods | Location: Sweden Number of centres: 1 Recruitment period: Not stated Funding source: Not stated Trial design: Parallel group RCT |
|
| Participants | Inclusion criteria: Severe xerostomia "mostly associated with systemic diseases". 12 (57%) had either primary or secondary Sjögren's Syndrome, 9 (43%) hypothyroidism, and remainder xerostomia following radiation therapy or of unknown aetiology Exclusion criteria: Not stated Age range: 33‐72 Number randomised: 21 Number evaluated: 20 |
|
| Interventions |
Comparison: Acupuncture versus placebo (superficial acupuncture) Group A (n = 11): Acupuncture twice weekly for 6 weeks then 2 week break and further 6 weeks treatment Group B (n = 10): Superficial acupuncture (designed to act as placebo) Duration of follow‐up: 12 months |
|
| Outcomes | Stimulated and unstimulated salivary flow rates (median and range reported), mean and SD calculable at 7 weeks, minor adverse effects noted | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: No method of sequence generation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to determine |
| Blinding of participants (performance bias) | Low risk | Quote: "Neither the person who evaluated salivary flow nor the patients themselves knew whether they received acupuncture or superficial needling" Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the person who evaluated salivary flow nor the patients themselves knew whether they received acupuncture or superficial needling" Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1/21 lost to follow‐up after 3 months. This was judged unlikely to have introduced a bias |
| Selective reporting (reporting bias) | Unclear risk | Comment: No patient‐reported symptoms included. Outcome measure is objective salivary flow |
| Other bias | Low risk | Comment: No other sources of bias identified |
Blom 1996.
| Methods | Location: Sweden Number of centres: 1 Recruitment period: Not stated Funding source: 3 grants. Swedish Patent Revenue Research Fund, Swedish Dental Society and King Gustav V Research Fund Trial design: Parallel group RCT |
|
| Participants | Inclusion criteria: Xerostomia following radiation therapy Exclusion criteria: None stated Number randomised: 41 Number evaluated: Between 32 and 38 depending on outcome |
|
| Interventions |
Comparison: Acupuncture versus placebo (superficial acupuncture) Group A (n = 21): Classical acupuncture, 12 x 20‐minute treatments during which 5‐8 points were stimulated manually until the appearance of a needling reaction (Q1). 2 sessions per week for 6 weeks, then 2‐week break then further 6 weeks of treatment Group B (n = 20): Superficial acupuncture delivered to 5‐8 points on same schedule as above Duration of follow‐up: 12 months |
|
| Outcomes | Stimulated and unstimulated salivary flow rates (median and range reported), mean and SD calculable at 8 weeks, minor adverse effects noted | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Comment: No method of sequence generation described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to determine |
| Blinding of participants (performance bias) | Low risk | Quote: "double blind" Comment: Patients and outcome assessors. Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: Patients and outcome assessors. Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 41 patients randomised 38 patients evaluated and 1/20 and 4/18 patients lost to follow‐up in acupuncture and placebo groups respectively. No reasons for drop‐out/withdrawal described |
| Selective reporting (reporting bias) | Unclear risk | Comment: No patient‐evaluated outcomes of xerostomia reported |
| Other bias | High risk | Comment: In the discussion section study authors suggest that superficial needling is not reliable as placebo because it has some activity in some people |
Cho 2008.
| Methods | Location: South Korea Number of centres: Unclear. Konyang University Hospital, Daejon and Dunsan Oriental Hospital of Daejon University both approved study Recruitment period: Not stated Funding source: Acupuncture, Moxibustion and Meridian Research Project (K06070) of Korean Institute of Oriental medicine R&D Project (B050018) Ministry of Health and Welfare, Republic of Korea Trial design: Parallel group pilot study |
|
| Participants | Inclusion criteria: Patients with xerostomia with history of radiation therapy (minimum dose 38 Gy and at least 50% of parotid glad exposed to radiation) Exclusion criteria: Patients with distant metastases, and inflammatory disease or ECOG scores > 2 Number randomised: 12 Number evaluated: 12 |
|
| Interventions |
Comparison: Acupuncture versus placebo (sham acupuncture) Group A (n = 6): Acupuncture delivered to 4 points in 2 sessions per week for 6 weeks Group B (n = 6): Sham acupuncture delivered to 4 points at least 2 cm away from 'real' acupoints, in 2 sessions per week for 6 weeks Duration of follow‐up: 6 weeks |
|
| Outcomes | Xerostomia Questionnaire, stimulated and unstimulated salivary flow rates at end of treatment | |
| Notes | Sample size calculation: Pilot study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated using block randomization" Comment: Random component not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to determine |
| Blinding of participants (performance bias) | Low risk | Comment: Patients likely to be blinded because placebo acupuncture used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information to determine 'yes' or 'no' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All randomised participants included in outcome evaluations |
| Selective reporting (reporting bias) | Low risk | Comment: Xerostomia questionnaire (patient‐evaluated measure) and UWS, SWS reported |
| Other bias | High risk | Groups very unbalanced at baseline |
List 1998.
| Methods | Location: Sweden Number of centres: 1 Recruitment period: Not stated Funding source: Swedish National Social Insurance Board Trial design: Parallel group RCT |
|
| Participants | Inclusion criteria: Patients diagnosed with primary Sjögren's Syndrome Exclusion criteria: None stated Age range: 44‐78 years (mean 65 years) Number randomised: 21 Number evaluated: 20 |
|
| Interventions |
Comparison: Acupuncture versus placebo (manual stimulation only) Group A (n = 10): 30‐minute session twice weekly with both electrical and manual stimulation for a total of 10 weeks Group B (n = 10): No treatment during the first 10 weeks and then acupuncture as above weeks 11‐20 Duration of follow‐up: 20 weeks |
|
| Outcomes | UWS, SWS, median mouth dryness (10‐point VAS scale), adverse effects | |
| Notes | Sample size calculation: Not reported Email sent to author 29 April 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized into 2 groups" Comment: Random component not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to determine |
| Blinding of participants (performance bias) | High risk | Comment: Blinding not possible. Manual versus electrical stimulation. Electrical stimulation evoked visible muscle contractions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The evaluation was performed by one person, treatment by another" Comment: Unclear if person evaluating patients was blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1/21 patients excluded from evaluation |
| Selective reporting (reporting bias) | Low risk | Comment: UWS, SWS, VAS scores (patient‐evaluated outcome) reported |
| Other bias | Low risk | Comment: No other sources of bias identified |
Papas 2006.
| Methods | Location: Boston, USA Number of centres: 1 Recruitment period: Not stated Funding source: Philips Oral Healthcare provided the Sonicare Advance Toothbrushes Trial design: Parallel group with control group crossing over to powered toothbrush after 9 weeks |
|
| Participants | Inclusion criteria: Participants aged 40‐80 years, on a medication (list reported) of medications likely to result in dry mouth, > 10 natural teeth, UWS < 0.3 ml/min Exclusion criteria: Advanced periodontitis, infection, wasting diseases requiring premedication, participation in another clinical trial, on a chronic antibiotic regimen Number randomised: 61 Number evaluated: 58 |
|
| Interventions |
Comparison: Powered versus manual toothbrush Group A (n = 29): Sonicare Advance Toothbrush used according to directions given by investigators. Daily diaries to record toothbrushing, flossing and type of dentifrice used Group B (n = 29): Manual toothbrush (Oral B) used according to directions given by investigators. Daily diaries to record toothbrushing, flossing and type of dentifrice used |
|
| Outcomes | UWS, SWS, patient's preference, microbiology of oral cavity, changes in VAS for responders and non‐responders | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized to each of the treatment groups" Comment: Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to determine 'yes' or 'no' |
| Blinding of participants (performance bias) | High risk | Comment: Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3 participants lost to follow‐up |
| Selective reporting (reporting bias) | High risk | VAS for responders and non‐responders planned as an outcome but not reported |
| Other bias | High risk | Study funded by Philips who provided the toothbrushes. Unclear whether participants were aware that they would all be given a Sonicare electric toothbrush at the end of the research |
Pfister 2010.
| Methods | Location: New York, USA Number of centres: 1 Recruitment period: 2004‐2007 Funding source: NIH grant CA098792 Trial design: Parallel group RCT |
|
| Participants | Inclusion criteria: Patients who had undergone neck dissection for cancer, with pain and dysfunction likely to be attributable to neck dissection, at least 3 months post neck dissection and radiation, with moderate to severe pain on Constant‐Murley score </= 70 Exclusion criteria: Those who had received acupuncture in previous 6 weeks Cause of xerostomia: Radiation treatment for cancer Number randomised: 58 Number evaluated: 58 |
|
| Interventions |
Comparison: Acupuncture versus usual care Group A (n = 28): Acupuncture once a week for 4 weeks. Needles were placed at both standard and customised points to "optimise efficacy while facilitating reproducibility". Additional 5th treatment was added to improve compliance with assessment visit after 4th treatment Group B (n = 30): Usual care ‐ antiinflammatory and analgesic medication Duration of follow‐up: 4 weeks |
|
| Outcomes | Pain on constant Murley scale, pain (numerical rating scale), Xerostomia Inventory, adverse effects | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...random assignment was stratified by neck (surgical) procedure type (selective, modified or radical), and baseline Constant‐Murley score, using blocks of random length" |
| Allocation concealment (selection bias) | Low risk | "..random assignment was implemented via a secure computerised database, ensuring full allocation concealment" |
| Blinding of participants (performance bias) | High risk | Open trial, no placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Patients self assessed main outcomes but attempts were made to use blinded clinical assessment of objective components of Constant‐Murley scale |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 58 randomised participants are included in the outcome assessment |
| Selective reporting (reporting bias) | Low risk | Planned primary and secondary outcomes are reported |
| Other bias | Low risk | No other sources of bias identified |
Steller 1988.
| Methods | Location: USA Number of centres: 1 Recruitment period: Not stated Funding source: Not stated Trial design: Parallel group RCT |
|
| Participants | Inclusion criteria: Participants aged > 18 years with dry mouth, focal sialoadenitis in labial salivary gland biopsy specimen Exclusion criteria: On medication known to affect saliva production, presence of pacemaker, pregnancy, UWS < 0.2 gram/minute Cause of xerostomia: Sjögren's Syndrome Number randomised: 29 Number evaluated: 24 |
|
| Interventions |
Comparison: Electrical stimulation versus placebo Group A (n = 14): Electrical stimulation used for 3 minutes under supervision, then at home 3 times daily for 4 weeks Group B (n = 15): Placebo electrical stimulation used under supervision for 3 minutes then at home 3 times daily for 4 weeks The electronic stimulation device consisted of a hand held probe, tipped with 2 electrodes, and a control console box which housed the battery and controls Duration of follow‐up: 4 weeks |
|
| Outcomes | Mean UWS and SWS | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a table of random numbers" Comment: Random number table. Probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to determine |
| Blinding of participants (performance bias) | Low risk | Quote: "double blind" Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 5/29 patients withdrew (1 per intervention arm, 4 per control arm) (17%) |
| Selective reporting (reporting bias) | Low risk | Comment: SWS, visual examination, patient evaluation of any increase in saliva |
| Other bias | Low risk | Comment: No other sources of bias identified |
Talal 1992.
| Methods | Location: USA Number of centres: 3 Recruitment period: Not stated Funding source: Biosonics Trial design: Parallel group RCT |
|
| Participants | Inclusion criteria: Participants aged > 18 years with Sjögren's Syndrome plus 1 other rheumatic condition (from specified list) and maximum saliva production of 0.4 g saliva/2 minutes Exclusion criteria: None stated Cause of xerostomia: Sjögren's Syndrome Number randomised: 77 Number evaluated: Unclear |
|
| Interventions |
Comparison: Active electrostimulation device versus sham device Group A (n = 40): Active electrostimulation device Group B (n = 37): Sham electrostimulation device System comprised a control module plus a hand held stimulus probe with 2 electrodes, to be placed between the tongue and the roof of the mouth. Patients were instructed on the use of the system, supervised and then instructed to use it at home 3 times daily for 4 weeks Duration of follow‐up: 4 weeks |
|
| Outcomes | SWS, symptom relief | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..assignment was performed according to a statistical table of random numbers" Comment: Random number table |
| Allocation concealment (selection bias) | Low risk | Quote: "...neither the technician nor the physician/investigator were provided with the code for active and placebo devices" Comment: Probably done |
| Blinding of participants (performance bias) | Low risk | Quote: "double blind" Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Numbers of participants included in outcome evaluations unclear |
| Selective reporting (reporting bias) | High risk | Comment: Salivary output data reported per group but no estimates of variance and data for symptom improvement not provided |
| Other bias | Low risk | Groups similar at baseline with regard to salivary output |
Wong 2003.
| Methods | Location: Canada Number of centres: 1 Recruitment period: Not stated Funding source: Hamilton Health Sciences Corporation Foundation and equipment provision by EHM Rehabilitation Technologies Inc Trial design: Parallel (3 groups) RCT |
|
| Participants | Inclusion criteria: Patients treated with radiotherapy for head and neck cancer who had symptoms of xerostomia. Those who had no response to pilocarpine were also included after a 1 month washout period Exclusion criteria: Patients taking medications that may induce xerostomia, those with unstable cardiac disease, with a pacemaker, those taking pilocarpine Cause of xerostomia: Radiotherapy Number randomised: 46 Number evaluated: 37 |
|
| Interventions |
Comparison: Transcutaneous nerve stimulation to different acupuncture points Group A (n = 13): Sp6, St36, L14 (active electrode) and CV 24 (indifferent electrode) Group B (n = 10): Sp6, St36, P6 (active electrode) and CV 24 (indifferent electrode) Group C (n = 14): Sp6, St 5 and 6, P6 (active electrode) and CV 24 (indifferent electrode) All 3 groups had twice weekly stimulation sessions to the prespecified points for 6 weeks, followed by a 2‐week break and then a second 6‐week phase of treatment Duration of treatment: 14 weeks Follow‐up: At 3, 6 and 12 months after the end of treatment |
|
| Outcomes | Total xerostomia symptom score, patient‐reported improvement in tongue dryness, speech, swallowing and overall mouth comfort. UWS, SWS, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized into 3 groups" Comment: Random component not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to determine |
| Blinding of participants (performance bias) | High risk | Comment: Not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 9 (9/46) of those randomised did not complete the trial. No information as to which groups they were from. 13, 10 and 14 described in groups A, B and C respectively |
| Selective reporting (reporting bias) | High risk | Comment: Results reported as means and SDs for the whole cohort only, or on graphs for VAS scores ‐ unclear whether the randomisation to different groups had any effect |
| Other bias | Low risk | No other sources of bias identified |
ECOG = Eastern Cooperative Oncology Group; Gy = gray; RCT = randomised controlled trial; SD = standard deviation; SWS = stimulated whole saliva; UWS = unstimulated whole saliva; VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blom 2000 | Retrospective study |
| Braga 2011 | Participants did not have xerostomia at baseline |
| Cheville 2006 | Not randomised. No control group |
| Deng 2008 | Participants did not have xerostomia at baseline |
| Fontanesi 1991 | Not randomised. No control group. 6 patients received hyperbaric oxygen therapy in a non‐randomised, non‐controlled pilot study. The authors also report the results of a retrospective uncontrolled study in 5 patients |
| Garcia 2009 | Not RCT |
| Loncar 2011 | Not RCT |
| Meng 2012 | Participants did not have xerostomia at baseline |
| Niemtzow 2007 | Not RCT |
| Schiff 2009 | Not RCT |
| Simcock 2009 | Not RCT |
| Simcock 2013 | Cross‐over study |
| Simoes 2010 | Participants did not have xerostomia at baseline |
| Strietzel 2007 | Cross‐over study with 158 different treatment sessions tested on 20 patients with only 90 minutes between treatments |
| Strietzel 2011 | Cross‐over study where participants experienced both active (mechanical and electrical stimulation) and sham (mechanical only) device effects for 1 month in a random order. A carry‐over effect cannot be discounted |
| Weiss 1986 | Not RCT |
| Wong 2010 | Participants did not have xerostomia at baseline |
| Wong 2012 | Non‐randomised phase II feasibility trial of acupuncture‐like transcutaneous electrical nerve stimulation (ALTENS) |
RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01141231.
| Trial name or title | Acupuncture in treating dry mouth caused by radiation therapy in patients with head and neck cancer |
| Methods | Randomised controlled trial |
| Participants | Patients with grade 2 or 3 xerostomia following radiotherapy for head and neck cancer. No prior use of acupuncture and at least 9 months after last radiotherapy treatment |
| Interventions | Acupuncture twice weekly for 4 weeks (2 regimens) versus standard oral hygiene care |
| Outcomes | Xerostomia, saliva production |
| Starting date | November 2012 |
| Contact information | Dr Lorenzo Cohen (lcohen@mdanderson.org) |
| Notes |
Differences between protocol and review
The review was amended to clarify that participants in included studies must have dry mouth symptoms at baseline.
Contributions of authors
Gemma Bryan (GB), Sue Furness (SF) and Helen Worthington (HW) wrote the protocol. Roddy McMillan (RM) and Sarah Birchenough (SB) provided a clinical perspective on xerostomia and treatments for dry mouth.
The search results were screened against the inclusion criteria for this review by GB and SF. Full‐text copies of papers appearing to meet the inclusion criteria were then evaluated by at least two authors (GB, SF or HW) independently, and any disagreements were resolved by discussion, following clinical input as required from RM.
Risk of bias assessment and data extraction were conducted by GB, SF and HW, with at least two authors independently evaluating each included study. Data entry and analysis were conducted by SF.
The text of the review was drafted by SF and reviewed by all the other authors.
Sources of support
Internal sources
The University of Manchester, UK.
-
Manchester Academic Health Sciences Centre (MAHSC), UK.
The Cochrane Oral Health Group is supported by MAHSC and the NIHR Manchester Biomedical Research Centre
External sources
-
Cochrane Oral Health Group Global Alliance, UK.
All reviews in the Cochrane Oral Health Group are supported by Global Alliance member organisations (British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK) providing funding for the editorial process (http://ohg.cochrane.org/)
-
National Institute for Health Research (NIHR), UK.
CRG funding acknowledgement: The NIHR is the largest single funder of the Cochrane Oral Health Group
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health
Declarations of interest
Gemma Bryan: no interests to declare. Sue Furness: no interests to declare. Helen Worthington: no interests to declare. Roddy McMillan: no interests to declare. Sarah Birchenough: no interests to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Blom 1992 {published data only}
- Blom M, Dawidson I, Angmar‐Månsson B. The effect of acupuncture on salivary flow rates in patients with xerostomia. Oral Surgery, Oral Medicine, and Oral Pathology 1992;73(3):293‐8. [DOI] [PubMed] [Google Scholar]
Blom 1996 {published data only}
- Blom M, Dawidson I, Angmar‐Månsson B, Johnson G. Effect of acupuncture on salivary flow in subjects with radiation‐induced xerostomia. Caries Research 1993;27:225. [Google Scholar]
- Blom M, Dawidson I, Fernberg JO, Johnson G, Angmar‐Månsson B. Acupuncture treatment of patients with radiation‐induced xerostomia. Oral Oncology, European Journal of Cancer 1996;32B(3):182‐90. [DOI] [PubMed] [Google Scholar]
Cho 2008 {published data only}
- Cho JH, Chung WK, Kang W, Choi SM, Cho CK, Son CG. Manual acupuncture improved quality of life in cancer patients with radiation‐induced xerostomia. The Journal of Alternative and Complementary Medicine 2008;14(5):523‐6. [DOI] [PubMed] [Google Scholar]
List 1998 {published data only}
- List T, Lundeberg T, Lundström I, Lindström F, Ravald N. The effect of acupuncture in the treatment of patients with primary Sjögren's syndrome. Acta Odontologica Scandinavica 1998;56:95‐9. [DOI] [PubMed] [Google Scholar]
Papas 2006 {published data only}
- Papas A, Singh M, Harrington D, Rodriguez S, Ortblad K, Jager M, et al. Stimulation of salivary flow with powered toothbrush in a xerostomic population. Special Care Dentistry 2006;26(6):241‐6. [DOI] [PubMed] [Google Scholar]
Pfister 2010 {published data only}
- Pfister DG, Cassileth BR, Deng GE, Yeung KS, Lee JS, Garrity D, et al. Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. Journal of Clinical Oncology 2010;28(15):2565‐70. [PUBMED: 20406930] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steller 1988 {published data only}
- Steller M, Chou L, Daniels TE. Electrical stimulation of salivary flow in patients with Sjögren's Syndrome. Journal of Dental Research 1988;67:1334‐7. [DOI] [PubMed] [Google Scholar]
Talal 1992 {published data only}
- Talal N, Quinn JH, Daniels TE. The clinical effects of electrostimulation on salivary function of Sjögren's syndrome patients: a placebo controlled trial. Rheumatology International 1992;12:43‐5. [DOI] [PubMed] [Google Scholar]
Wong 2003 {published data only}
- Wong R, Sagar S, Whelan T, Foster G, Farges‐Babjak A, Willan A, et al. The use of acupuncture‐like transcutaneous nerve stimulation (CODETRON) in the treatment of radiation‐induced xerostomia in head and neck cancer patients treated with radical radiotherapy. International Journal of Radiation, Oncology, Biology, and Physics 2011;51:2351. [DOI] [PubMed] [Google Scholar]
- Wong RKW, Jones GW, Sagar SM, Babjak A‐F, Whelan T. A phase I‐II study in the use of acupuncture‐like transcutaneous nerve stimulation in the treatment of radiation‐induced xerostomia in head‐and‐neck cancer patients treated with radical radiotherapy. International Journal of Radiation, Oncology, Biology and Physics 2003;57(2):472‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blom 2000 {published data only}
- Blom M, Lundeberg T. Long‐term follow‐up of patients treated with acupuncture for xerostomia and the influence of additional treatment. Oral Diseases 2000;6(1):15‐24. [PUBMED: 10673783] [DOI] [PubMed] [Google Scholar]
Braga 2011 {published data only}
- Braga FP, Lemos Junior CA, Alves FA, Migliari DA. Acupuncture for the prevention of radiation‐induced xerostomia in patients with head and neck cancer. Brazilian Oral Research 2011;25(2):180‐5. [PUBMED: 21537645] [DOI] [PubMed] [Google Scholar]
- Braga FPF, Migliari DA. Acupuncture for prevention of radiation‐induced xerostomia. Oral Diseases 2010;16(6):539 (Abs No 104). [Google Scholar]
Cheville 2006 {published data only}
- Cheville AM, Basford JR. Home based acupuncture: a study in xerostomia. Focus on Alternative and Complementary Therapies 2006;11(Suppl 1):10‐1. [Google Scholar]
Deng 2008 {published data only}
- Deng G, Hou BL, Holodny AI, Cassileth BR. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at LI‐2 acupuncture point: a randomized controlled study. BMC Complementary and Alternative Medicine 2008;8:37. [PUBMED: 18606019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontanesi 1991 {published data only}
- Fontanesi J, Golden EB, Ciani P. Hyperbaric oxygen therapy can reverse radiation‐Induced xerostomia. Journal of Hyperbaric Medicine 1991;6(3):215‐21. [Google Scholar]
Garcia 2009 {published data only}
- Garcia MK, Chiang JS, Cohen L, Liu M, Palmer JL, Rosenthal DI, et al. Acupuncture for radiation‐induced xerostomia in patients with cancer: a pilot study. Head & Neck 2009;31(10):1360‐8. [PUBMED: 19378325] [DOI] [PubMed] [Google Scholar]
Loncar 2011 {published data only}
- Loncar B, Stipetic MM, Baricevic M, Risovic D. The effect of low‐level laser therapy on salivary glands in patients with xerostomia. Photomedicine and Laser Surgery 2011;29(3):171‐5. [PUBMED: 21054200] [DOI] [PubMed] [Google Scholar]
Meng 2012 {published data only}
- Meng Z, Garcia MK, Hu C, Chiang J, Chambers M, Rosenthal DI, et al. Randomized controlled trial of acupuncture for prevention of radiation‐induced xerostomia among patients with nasopharyngeal carcinoma. Cancer 2012;118(13):3337‐44. [PUBMED: 22072272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Niemtzow 2007 {published data only}
- Niemtzow vonR. Rapid acupuncture treatment for severe dry mouth secondary to radiotherapy, chemotherapy and surgery for head and neck cancer patients [Schnell wirksame akupunktur bei schwerer mundtrockenheit nach bestrahlung, chemotherapie und operation bei kopf und hals carcinomen]. Schmerz und Akupunktur 2007;3:118‐22. [Google Scholar]
Schiff 2009 {published data only}
- Schiff E, Mogilner JG, Sella E, Doweck I, Hershko O, Ben‐Arye E, et al. Hypnosis for postradiation xerostomia in head and neck cancer patients: a pilot study. Journal of Pain and Symptom Management 2009;37(6):1086‐92.e1. [PUBMED: 19186028] [DOI] [PubMed] [Google Scholar]
Simcock 2009 {published data only}
- Simcock R, Fallowfield L, Jenkins V. Group acupuncture to relieve radiation induced xerostomia: a feasibility study. Acupuncture in Medicine: Journal of the British Medical Acupuncture Society 2009;27(3):109‐13. [PUBMED: 19734380] [DOI] [PubMed] [Google Scholar]
Simcock 2013 {published data only}
- Simcock R, Fallowfield L, Monson K, Ivonne ST, Parlour L, Langridge CI, et al. ARIX: A randomized trial of acupuncture versus oral care sessions in patients with chronic radiation‐induced xerostomia following treatment for head and neck cancer. Journal of Clinical Oncology 2012;30(Suppl):Abstract No 5527. [Google Scholar]
- Simcock R, Fallowfield L, Monson K, Solis‐Trapala I, Parlour L, Langridge C, et al. ARIX: a randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Annals of Oncology 2013;24(3):776‐83. [DOI] [PubMed] [Google Scholar]
Simoes 2010 {published data only}
- Simoes A, Campos L, Souza DN, Matos JA, Freitas PM, Nicolau J. Laser phototherapy as topical prophylaxis against radiation induced xerostomia. Photomedicine and Laser Surgery 2010;28(3):357‐63. [DOI] [PubMed] [Google Scholar]
Strietzel 2007 {published data only}
- Strietzel FP, Martin‐Granizo R, Fedele S, Lo Russo L, Mignogna M, Reichart PA, et al. Electrostimulating device in the management of xerostomia. Oral Diseases 2007;13(2):206‐13. [PUBMED: 17305624] [DOI] [PubMed] [Google Scholar]
Strietzel 2011 {published data only}
- Alajbeg I, Falcao DP, Tran SD, Martin‐Granizo R, Lafaurie GI, Matranga D, et al. Intraoral electrostimulator for xerostomia relief: a long‐term, multicenter, open‐label, uncontrolled, clinical trial. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2012;113(6):773‐81. [PUBMED: 22668705] [DOI] [PubMed] [Google Scholar]
- Strietzel FP, Lafaurie GI, Bautista Mendoza GR, Alajbeg I, Pejda S, Vuletić Mantilla R, et al. Efficacy and safety of an intraoral electrostimulation device for xerostomia relief: a multicenter, randomized trial. Arthritis and Rheumatism 2011;63(1):180‐90. [DOI] [PubMed] [Google Scholar]
Weiss 1986 {published data only}
- Weiss WW Jr, Brenman HS, Katz P, Bennett JA. Use of an electronic stimulator for the treatment of dry mouth. Journal of Oral and Maxillofacial Surgery 1986;44(11):845‐50. [PUBMED: 3490558] [DOI] [PubMed] [Google Scholar]
Wong 2010 {published data only}
- Wong RK, Sagar SM, Chen BJ, Yi GY, Cook R. Phase II randomized trial of acupuncture‐like transcutaneous electrical nerve stimulation to prevent radiation‐induced xerostomia in head and neck cancer patients. Journal of the Society for Integrative Oncology 2010;8(2):35‐42. [PUBMED: 20388444] [PubMed] [Google Scholar]
Wong 2012 {published data only}
- Wong RK, James JL, Sagar S, Wyatt G, Nguyen‐Tan PF, Singh AK, et al. Phase 2 results from Radiation Therapy Oncology Group Study 0537: a phase 2/3 study comparing acupuncture‐like transcutaneous electrical nerve stimulation versus pilocarpine in treating early radiation‐induced xerostomia. Cancer 2012;118(17):4244‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01141231 {published data only}
- MD Anderson Cancer Center. Acupuncture in treating dry mouth caused by radiation therapy in patients with head and neck cancer. Available from ClinicalTrials.gov.
Additional references
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd Edition. Hillsdale, New Jersey: Lawrence Erlbaum Associates, 1988. [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2007
- Davies AN, Shorthose K. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003782.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enger 2011
- Enger TB, Palm Ø, Garen T, Sandvik L, Jensen JL. Oral distress in primary Sjögren's syndrome: implications for health‐related quality of life. European Journal of Oral Sciences 2011;119(6):474‐80. [DOI] [PubMed] [Google Scholar]
Fedele 2008
- Fedele S, Wolff A, Strietzel F, Lopez RM, Porter SR, Konttinen YT. Neuroelectrostimulation in treatment of hyposalivation and xerostomia in Sjogren's syndrome: a salivary pacemaker. Journal of Rheumatology 2008;35(8):1489‐94. [PubMed] [Google Scholar]
Femiano 2008
- Femiano F, Lanza A, Buonaiuto C, Gombos F, Rullo R, Festa V, et al. Oral manifestations of adverse drug reactions: guidelines. Journal of the European Academy of Dermatology and Venereology 2008;22:681‐91. [DOI] [PubMed] [Google Scholar]
Furness 2011
- Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008934.pub2] [DOI] [PubMed] [Google Scholar]
Garcia 2013
- Garcia MK, McQuade J, Haddad R, Patel S, Lee R, Yang P, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. Journal of Clinical Oncology 2013;31(7):952‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hackett 2012
- Hackett KL, Newton JL, Frith J, Elliott C, Lendrem D, Foggo H, et al. Impaired functional status in primary Sjögren's syndrome. Arthritis Care & Research 2012;64(11):1760‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopcraft 2010
- Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Australian Dental Journal 2010;55(3):238‐44. [DOI] [PubMed] [Google Scholar]
Lončar 2011
- Lončar B, Stipetić MM, Baričević M, Risović D. The effect of low‐level laser therapy on salivary glands in patients with xerostomia. Photomedicine and Laser Surgery 2011;29(3):171‐5. [DOI] [PubMed] [Google Scholar]
Ma 2009
- Ma C, Xie J, Chen Q, Wang G, Zuo S. Amifostine for salivary glands in high‐dose radioactive iodine treated differentiated thyroid cancer. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007956.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mese 2007
- Mese H, Matsuo R. Salivary secretion, taste and hyposalivation. Journal of Oral Rehabilitation 2007;34(10):711‐23. [DOI] [PubMed] [Google Scholar]
Napenas 2009
- Napenas JJ, Brennan MT, Fox PC. Diagnosis and treatment of xerostomia (dry mouth). Odontology 2009;97(2):76‐83. [DOI] [PubMed] [Google Scholar]
O'Sullivan 2010
- O'Sullivan EM, Higginson IJ. Clincial effectiveness and safety of acupuncture in the treatment of irradiation‐induced xerostomia in patients with head and neck cancer: a systematic review. Acupuncture in Medicine 2010;28(4):191‐9. [DOI] [PubMed] [Google Scholar]
Porter 2004
- Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2004;97(1):28‐46. [DOI] [PubMed] [Google Scholar]
Porter 2010
- Porter SR, Fedele S, Habbab KM. Xerostomia in head and neck malignancy. Oral Oncology 2010;46(6):460‐3. [DOI] [PubMed] [Google Scholar]
Proctor 2007
- Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Autonomic Neuroscience: Basic and Clinical 2007;133:3‐18. [DOI] [PubMed] [Google Scholar]
Scully 2004
- Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Critical Reviews in Oral Biology and Medicine 2004;15(4):221‐39. [DOI] [PubMed] [Google Scholar]
Shiboski 2007
- Shiboski CH, Hodgson TA, Ship JA, Schiødt M. Management of salivary hypofunction during and after radiotherapy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2007;103(Supp 66):e1‐19. [DOI] [PubMed] [Google Scholar]
SPIRIT 2013
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tavender 2004
- Tavender E, Davies AN Glenny AM. Pharmacological interventions for preventing salivary gland dysfunction following radiotherapy. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 2005
- Thomson WM. Issues in the epidemiological investigation of dry mouth. Gerodontology 2005;22(2):65‐76. [DOI] [PubMed] [Google Scholar]
Thomson 2007
- Thomson WM. Measuring change in dry‐mouth symptoms over time using the Xerostomia Inventory. Gerodontology 2007;24(1):30‐5. [DOI] [PubMed] [Google Scholar]
Tobón 2012
- Tobón GJ, Pers JO, Devauchelle‐Pensec V, Youinou P. Neurological disorders in primary Sjögren's Syndrome. Autoimmune Diseases 2012;2012:645967. [DOI: 10.1155/2012/645967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Visvanathan 2010
- Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. International Journal of Clinical Practice 2010;64(3):404‐7. [DOI] [PubMed] [Google Scholar]
Wolff 2012
- Wolff A, Fox PC, Porter S, Konttinen YT. Established and novel approaches for the management of hyposalivation and xerostomia. Current Pharmaceutical Design 2012;18(34):5515‐21. [PUBMED: 22632391] [DOI] [PubMed] [Google Scholar]
Zhuang 2013
- Zhuang L, Yang Z, Zeng X, Zhua X, Chen Z, Liu L, et al. The preventive and therapeutic effect of acupuncture for radiation‐induced xerostomia in patients with head and neck cancer: a systematic review. Integrative Cancer Therapies 2013;12(3):197‐205. [DOI] [PubMed] [Google Scholar]


