Abstract
This report describes a case of heart failure with preserved ejection fraction in a post-menopausal woman with obesity. The case highlights a metabolic phenotype of heart failure with preserved ejection fraction that commonly manifests in women, with the discussion focusing on pathophysiology, mechanistic pathways, and potential targeted therapeutic strategies. (Level of Difficulty: Intermediate.)
Key Words: heart failure with preserved ejection fraction, HFpEF, obesity, women
Abbreviations and Acronyms: BMI, body mass index; BNP, B-type natriuretic peptide; cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; GC, guanylate cyclase; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LV, left ventricular; NO, nitric oxide; pGC, particulate guanylate cyclase; PKG, protein kinase G; sGC, soluble guanylate cyclase
Graphical abstract
This report describes a case of heart failure with preserved ejection fraction in a post-menopausal woman with obesity. The case highlights a metabolic…
History of Presentation
A 53-year-old woman presented to the emergency department with dyspnea, lower extremity edema, and chest pressure for 1 week. Initial vital signs were blood pressure of 184/92 mm Hg, heart rate of 80 beats/min, respiratory rate of 30 breaths/min, and body mass index (BMI) of 55 kg/m2. Physical examination was notable for a jugular venous pressure of 15 cm H2O, clear lungs, distant cardiac sounds without murmurs, and 2+ bilateral lower extremity edema.
Learning Objectives
-
•
Identify distinct pathways that contribute to HFpEF in post-menopausal women with obesity.
-
•
Understand the role of the cGMP-PKG pathway in HFpEF pathophysiology.
-
•
Discuss novel treatments that up-regulate the cGMP-PKG pathway and may specifically benefit post-menopausal women with concomitant obesity and HFpEF.
Past Medical History
Apart from obesity, she had no medical history. She was post-menopausal (last menstrual period >1 year ago). She previously had regular menses. She had 3 previous normal vaginal deliveries and 1 spontaneous abortion. She was taking no medications. She had never used tobacco or alcohol. Her father and mother had histories of fatal premature myocardial infarctions (age <65 years).
Differential Diagnosis
The differential diagnosis included myocardial ischemia, pulmonary embolus, and parenchymal lung disease.
Investigations
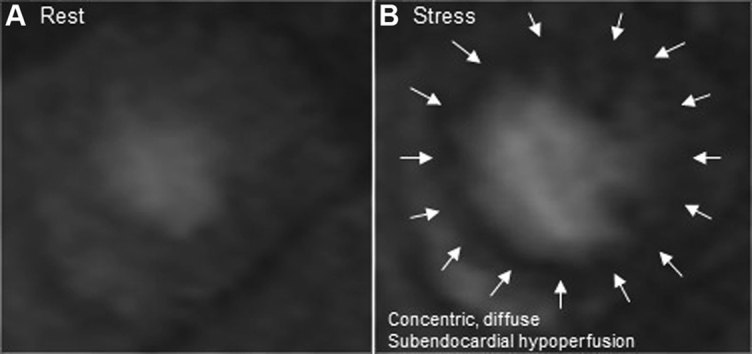
Laboratory test results were notable for the following: B-type natriuretic peptide (BNP), 190 pg/ml; creatinine, 1.13 mg/dl; troponin I, 0.02 ng/ml (reference range <0.04 ng/ml); hemoglobin A1c, 6.0%; total cholesterol, 200 mg/dl; low-density lipoprotein cholesterol, 117 mg/dl; high-density lipoprotein cholesterol, 47 mg/dl; and triglycerides 181 mg/dl. Electrocardiography showed normal sinus rhythm with right bundle branch block. Transthoracic echocardiography showed normal left ventricular (LV) size, mild LV hypertrophy, ejection fraction of 55%, and normal right ventricular size and function. Left atrial volume (67 ml) was enlarged but normalized when indexed to body surface area (27.6 ml/m2). She had an E/A ratio of 0.8, a septal eʹ velocity of 0.03 m/s, a lateral eʹ velocity of 0.04 m/s, an average E/eʹ ratio of 20, and pulmonary artery systolic pressure of 36 mm Hg. These findings were consistent with severely impaired LV relaxation, elevated LV filling pressures, and grade 2 (moderate) diastolic dysfunction. Cardiac catheterization showed normal coronary arteries with a right atrial pressure of 16 mm Hg, pulmonary artery pressure of 51/23 (36) mm Hg, pulmonary capillary wedge pressure of 19 mm Hg, cardiac index of 2.36 l/min/m2, and pulmonary vascular resistance of 3.33 WU. Adenosine stress cardiac magnetic resonance imaging revealed significant subendocardial ischemia consistent with coronary microvascular dysfunction (Figures 1A and 1B). Computed tomography of the chest showed no lung disease or pulmonary embolus but was notable for significant chest wall adiposity (Figure 2).
Figure 1.
Stress Perfusion Cardiac Magnetic Resonance Imaging
(A) Normal perfusion at rest. (B) Concentric subendocardial perfusion defect (arrows) seen with stress.
Figure 2.

Computed Tomography of the Chest
Severe chest wall adiposity up to 10 inches.
Management
She was diagnosed with heart failure (HF) with preserved ejection fraction (HFpEF). Her symptoms improved with intravenous diuretic agents. Her blood pressure was controlled with carvedilol, amlodipine, and enalapril. She was also started on spironolactone and torsemide and subsequently discharged.
Discussion
This case highlights the common clinical scenario of obesity and HFpEF in post-menopausal women. Given that no therapy has improved overall mortality in all-comers with HFpEF, better understanding of mechanisms that lead to distinct HFpEF phenotypes is needed. Here we discuss 1) mechanisms related to HFpEF pathophysiology that may be more prevalent in post-menopausal women with obesity and 2) potential targeted treatment options.
HFpEF is more prevalent in older women compared with men and is the most common subtype of HF in women (1,2). Clustering analyses have consistently identified older women with obesity, hypertension, and diabetes as comprising a prominent phenotype within HFpEF (3).
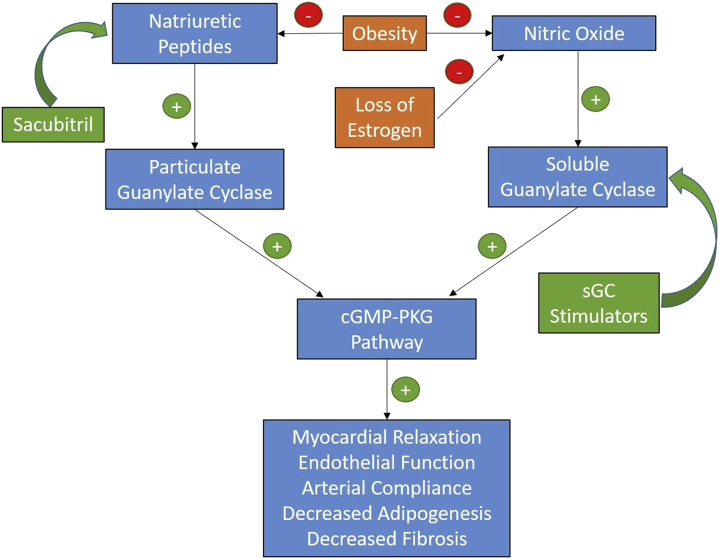
The pathophysiology underlying this phenotype features down-regulation of the cyclic guanosine monophosphate (cGMP)–protein kinase G (PKG) pathway. The cGMP is produced by cytosolic soluble guanylate cyclase (sGC) and transmembrane particulate guanylate cyclase (pGC), which in turn are activated by nitric oxide (NO) and natriuretic peptides, respectively. PKG is a downstream effector of cGMP that affects the myocardium, adipose tissue, kidneys, and pulmonary vasculature. Effects include LV relaxation, attenuation of ischemia-reperfusion injury, decreased adipogenesis, antifibrotic effects, and antihypertrophic properties (4). Low myocardial PKG activity has been demonstrated in patients with HFpEF (5).
Patients with HFpEF and obesity have lower BNP levels despite higher biventricular filling pressures (6). This finding could reflect the bidirectional link between high central adiposity and low natriuretic peptides (7). Significant obesity can also cause external constraint on the myocardium from epicardial, pericardial, or chest wall adipose tissue, thereby limiting myocardial stretch during diastole. As seen in our patient, this constraint can simulate constrictive physiology leading to significant ventricular interdependence, especially during exercise. BNP deficiency may be particularly pathogenic in post-menopausal women because estrogen is known to stimulate release of NO from the vascular endothelium through activation of endothelial NO synthase (eNOS). Post-menopausal women have reduced vascular eNOS expression compared with matched men (8). Thus, decreased synthesis of both sGC, through decreased NO, and pGC, through decreased natriuretic peptides, may result in significant PKG deficiency (Figure 3). The combination of sacubitril and valsartan, which increases natriuretic peptide levels, was evaluated in patients with HFpEF in the PARAGON-HF (The Prospective Comparison of ARNI [angiotensin receptor-neprilysin inhibitor] with ARB [angiotensin-receptor blocker] Global Outcomes in HF with Preserved Ejection Fraction) trial (9). Although the trial narrowly missed statistical significance in the primary composite outcome (total hospitalization for HF and cardiovascular death; p = 0.059); in a pre-specified subgroup analysis, there was a significant reduction in the primary composite outcome in women (incidence rate ratio: 0.73; 95% confidence interval: 0.59 to 0.90). Unfortunately, our patient, who may possibly have derived significant benefit from sacubitril-valsartan, would not have been eligible for the PARAGON-HF trial because of her high BMI and low BNP levels. BNP deficiency also contributes to underdiagnosis. Therefore, a diagnostic score such as H2FPEF, which uses BMI and echocardiographic parameters instead of BNP, will be key in preventing incident hospitalizations (10). On the basis of this score, our patient’s probability of having HFpEF was 98.2%.
Figure 3.
Effect of Obesity and Estrogen on the cGMP-PKG Pathway
Pathways and potential therapies for heart failure with preserved ejection fraction in post-menopausal women with obesity. cGMP = cyclic guanosine monophosphate; PKG = protein kinase G; sGC = soluble guanylate cyclase.
Microvascular ischemia and hypertension were 2 additional features of our patient’s HFpEF pathophysiology, both of which can also be linked to deficiency in the NO-sGC-cGMP-PKG pathway. Given that endothelial function is dependent on NO availability, post-menopausal women are susceptible to microvascular ischemia. Therefore, therapies that act downstream of NO such as sGC stimulators may hold significant promise.
Follow-Up
Her course was complicated by recurrent hospitalizations for decompensated HF despite BNP levels below the diagnostic threshold (<100 pg/ml). For her angina, she had sequential trials of isosorbide mononitrate, ranolazine, and rosuvastatin without improvement. Weight loss attempts were unsuccessful, and she declined referral for bariatric surgery.
Conclusions
We present a common, but difficult to manage, case of HFpEF in a post-menopausal woman with morbid obesity and relative BNP deficiency. Focused trials in post-menopausal women, directed at GC-cGMP-PKG deficiency secondary to decreased NO and natriuretic peptides, are needed. Trials are ongoing for therapies that up-regulate this pathway through stimulation of GC. Evidence in favor of targeting this pathway was seen in the PARAGON-HF trial, which showed a marked benefit with a combination of sacubitril and valsartan in women, most of whom were obese and post-menopausal. However, this finding was based on a subgroup analysis; thus, further studies on the potential benefit of neprilysin inhibition in women with HFpEF are necessary.
Footnotes
Dr. Khan has received grant support for this work from the National Heart, Lung, and Blood Institute (KL2TR001424) of the National Institutes of Health and from the American Heart Association (#19TPA34890060). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
References
- 1.Steinberg B.A., Zhao X., Heidenreich P.A. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 2.Eaton C.B., Pettinger M., Rossouw J. risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah S.J., Katz D.H., Selvaraj S. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah S.J., Kitzman D.W., Borlaug B.A. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Heerebeek L., Hamdani N., Falcao-Pires I. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 6.Obokata M., Reddy Y.N.V., Pislaru S.V., Melenovsky V., Borlaug B.A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruden G., Landi A., Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care. 2014;37:2899–2908. doi: 10.2337/dc14-0669. [DOI] [PubMed] [Google Scholar]
- 8.Mannacio V., Di Tommaso L., Antignano A. Endothelial nitric oxide synthase expression in postmenopausal women: a sex-specific risk factor in coronary surgery. Ann Thorac Surg. 2012;94:1934–1939. doi: 10.1016/j.athoracsur.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 9.Solomon S.D., McMurray J.J.V., Anand I.S. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 10.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]