ABSTRACT
Our previous research implied mouse skin-derived precursors (mSKPs) possessed the capacity of anti-ultraviolet B (UVB) irradiation damage, and the mechanisms might be associated with transforming growth factor-β (TGF-β) signaling pathway activation. In this study, we investigated and compared the response to UVB irradiation between mSKPs and dermal mesenchymal stem cells (dMSCs), and explored the underlying mechanisms. Irradiation damage such as decreased cell viability, cell senescence, and cell death was observed in both mSKPs and dMSCs at 24 h after UVB exposure. In mSKPs, change in cell morphology, viability, cell senescence and death at the following time points implied the recovery of UVB irradiation damage. Additionally, thrombospondin1 (TSP1) and TGF-β1 increased significantly in mSKPs’ supernatant after UVB irradiation. The gene expression of TSP1, TGF-β1, metalloproteinase 1 (MMP1), and Collagen I elevated shortly after the UVB exposure. The protein expression of TSP1, TGF-β1, MMP1, Collagen I, smad2/3, and p-smad2/3 at multiple time points after the UVB exposure was consistent with the gene expression results. In dMSCs, no obvious recovery was noticed. Together, these results revealed that in mSKPs, one of the mechanisms to attenuate the UVB irradiation damage might be the early activation of TGF-β/Smad pathway by TSP1. Given that mSKPs could differentiate into fibroblast-like SKP-derived fibroblasts (SFBs) in vivo or with the presence of serum, mSKPs might serve as a therapeutic potential for fibroblasts supplement and UVB irradiation damage treatment.
Abbreviations: SKPs: skin-derived precursors; mSKPs: mouse SKPs; UVB: ultraviolet B; TGF-β/Smad: transforming growth factor-β/Smad; TSP1: thrombospondin 1; MMP 13: metalloproteinases 13; TβRII: TGF-β receptor II; SFBs: SKP-derived fibroblasts; KEGG: Kyoto encyclopedia of genes and genomes; DEGs: differentially expressed genes; dMSCs: dermal mesenchymal stem cells; LM: light microscope; CCK-8: cell counting kit 8; ELISA: Enzyme-linked immuno sorbent assay; qRT-PCR: quantitative real-time polymerase chain reaction; TSPs: thrombospondins; ECM: extracellular matrix; R-smads: receptor-regulated smads
KEYWORDS: Skin-derived precursors, dermal mesenchymal stem cells, ultraviolet B, thrombospondin 1, TGF-β/Smad pathway
1. Introduction
Skin-derived precursors (SKPs) have been regarded as superior to the other types of stem cells derived from dermis, in differentiation potential and neurological function recovery therapy [1–3]. According to our previous in vitro and in vivo research, mouse SKPs (mSKPs) also had the capacity of anti-ultraviolet B (UVB) irradiation and anti-photo damage. Zhong et al. reported that after UVB irradiation, mSKPs maintained spherical colonies and outnumbered unirradiated ones, showing high Ki67 expression and low TUNEL, SA-al, and pH2AX expression [4]. Besides, mSKPs were found to have a photoprotective role against UV-induced damage in hairless mice. Lesions such as erythema, edema, scales, and wrinkles observed on the dorsal skin of mice due to UV irradiation were significantly ameliorated by subcutaneous mSKPs injection. Hyperkeratosis, acanthosis, and spongiosis in the epidermis, as well as dermal papillae edema and inflammatory cell infiltration were resolved with mSKPs treatment [5].
In addition, our study implied that one of mSKPs’ anti-UV irradiation mechanisms might be associated with transforming growth factor-β/Smad (TGF-β/Smad) signaling pathway. In nude mice, the increased mRNA expression of matrix metalloproteinases 13 (MMP 13) and decreased mRNA and protein expression of TGF-β receptor II (TβRII) caused by UV were diminished by mSKPs transplantation [6]. When attached onto culture dishes by serum, mSKPs initiated differentiation and developed into a fibroblast-like SKP-derived fibroblasts (SFBs). We compared the transcriptomes of mSKPs and SFBs, and noticed thrombospondin 1 (TSP1) gene up-regulated significantly. Kyoto encyclopedia of genes and genomes (KEGG) analysis indicated that differentially expressed genes (DEGs) were significantly enriched in TGF-β signaling pathway [7]. TSP1 is an endogenous activator of TGF-β pathway [8], which is inhibited by UVB irradiation [9,10]. TSP1 has been reported to increase and decrease in parallel with that of TGF-β1 and collagen III [11]. The up-regulation of the TSP1 gene indicated that it might help in inducing mSKPs to differentiate into SFBs through TGF-β pathway by increasing the proliferation of mSKPs.
Another type of stem cells from dermis, dermal mesenchymal stem cells (dMSCs), have been extensively studied in both basic and clinical research. Our investigation revealed mSKPs and dMSCs shared similar stem cells characteristics, whereas demonstrated distinct transcriptome profiles. The majority of enriched DEGs in mSKPs were immune-related, while enriched DEGs in dMSCs were diseases or physical process-related. KEGG analysis also identified up-regulated signaling pathways in mSKPs were predominantly immune-related [12]. We then presumed that mSKPs might be superior to dMSCs in anti-UV irradiation damage capacity, given the fact that UV irradiation present in sunlight is immune suppressive [13,14].
In the present study, we aim to explore the potentials of mSKPs alleviating UVB irradiation damage and to elucidate the underlying mechanisms involving TGF-β/Smad pathway activation by TSP1. dMSCs isolated from the same dermis sample served as the comparison. The results might provide the theoretic basis for novel mSKPs-based therapeutic strategies for UVB irradiation damage and potential usage in the relevant morbidity management.
2. Material and methods
2.1. Ethics statement
This study was approved by the Animal Ethics Committee of West China hospital, Sichuan University (Approval No. 2017064A) and was strictly carried out under the guide for the care and use of laboratory animals.
2.2. Cell culture medium setup
2.2.1. SKP culture medium
DMEM/F12 (3:1, Invitrogen, USA) containing 0.1% penicillin/streptomycin (Invitrogen, USA), 40 ng/mL bFGF (Millipore, USA), 20 ng/mL EGF (Millipore, USA), and 2% B27 supplement (Gibco, USA).
2.2.2. dMSC culture medium
Low glucose DMEM (Invitrogen, USA) containing 10% FBS (Clarks, Australia) and 1% penicillin/streptomycin.
2.3. Animals and cell culture
mSKPs and dMSCs isolation and characterization were performed according to the standard protocols reported previously [15] and conducted routinely in our lab [7,12]. Briefly, we isolated the cell suspension from neonatal Balb/C mice (aged 1–3 day) dermis. At the final step, different protocols were employed for mSKPs and dMSCs culture, respectively. For mSKPs culture, the plating density was 2.5 × 105 cells/mL, and the volume of mSKPs culture medium was 5 mL in a T25 culture flask (Corning, USA). mSKPs cultures were fed every 3 days with an addition of 1 mL fresh medium containing all growth factors and supplements (bFGF, EGF, B27) at a concentration that would replenish the entirety of the culture medium. To passage, mSKPs were collected, mechanically and enzymatically dissociated into single cells and re-plated in SKP culture medium. For dMSCs culture, the plating density was 2.5 × 105 cells/mL, and the volume of dMSCs culture medium was 10 mL in a 10 cm2 dish (Corning, USA). The dMSC medium was replaced with the fresh medium every 3 days, and the cells were passaged when the confluency reached 80%-90%. Cultures were maintained in a humidified incubator at 37°C, perfused with 5% CO2. Cells between passage numbers 1–3 were used for all experiments.
2.4. UVB irradiation
The dose of UVB (280nm-320nm) was determined as 30 mJ/cm2, 60 mJ/cm2, or 90 mJ/cm2 based on our previous study [4–6]. UVB irradiation was performed using a UVB lighter (Sigma, Shanghai, China) and cells were vertically irradiated at a distance of 15 cm from lighter without the lid. The UVB lighter shut off automatically when the accumulated irradiation had reached the required dose. The thickness of the medium during UVB irradiation was approximately 3–4 mm. The cells were then incubated for another 24 h to 96 h until the following measurements were performed.
2.5. Cells morphology observation and viability assay
mSKPs or dMSCs were plated in a 24-well plate at the density of 3 × 104 cells/mL, and were grouped as control group and 60 mJ/cm2 UVB irradiated group (5 wells per group). The morphological change was monitored with light microscope (LM) (Olympus, Japan). The cell viability was tested 24 h, 48 h, 72 h, and 96 h after the 60 mJ/cm2 UVB irradiation by use of cell counting kit 8 (CCK-8, Dojindo, Japan) according to the manufacturer’s instructions.
2.6. β-galactosidase staining
mSKPs or dMSCs were plated at the density described above, and were β-galactosidase (Beyotime Biotechnology, Shanghai, China) stained 24 h and 48 h after the 60 mJ/cm2 UVB irradiation. The staining steps were performed according to the manufacturer’s instructions. Positively β-galacntosidase blue-stained cells were counted in five randomly selected fields using LM. Percentage of positively stained cells (%) = count of positively stained cells/count of total cells×100%.
2.7. Calcein-AM/propidium iodide staining
mSKPs or dMSCs were plated at the density described above, and were Calcein-AM/Propidium Iodide (Dojindo, Japan) stained 24 h and 48 h after the 60 mJ/cm2 UVB irradiation. The staining steps were performed according to the manufacturer’s instructions. Calcein-AM stained cells (yellow-green stained, live cells) were observed, counted, and photographed at the wavelength of 490 nm. Propidium Iodide stained cells (red stained, dead cells) were then observed, counted, and photographed at the wavelength of 545 nm. Dead cells were counted in five randomly selected fields under LM. Percentage of dead cells (%) = count of dead cells/count of total cells×100%.
2.8. Enzyme-linked immunosorbent assay (ELISA)
mSKPs or dMSCs were plated at the density described above, and were grouped as control group, 30 mJ/cm2 UVB irradiated group, 60 mJ/cm2 UVB irradiated group, and 90 mJ/cm2 UVB irradiated group (3 wells per group). The supernatant was collected 6 h, 12 h, and 24 h after the irradiation. The concentration of TSP1 and TGF-β1 in the supernatant was determined by ELISA, and the steps were performed according to the manufacturer’s instructions (Cusabio, Wuhan, China).
2.9. Quantitative real-time polymerase chain reaction (qRT-PCR)
The expression of major genes (TGF-β1, Collagen I, TSP1, and MMP1) involved in UVB irradiation and TGF-β/Smad signaling pathway was analyzed by qRT-PCR. Cells were grouped as control group and 60 mJ/cm2 UVB irradiated group. The procedure of cell culture and irradiation was in the same way as described above. Cell samples were collected 3 h, 6 h, 12 h, and 24 h after the irradiation. RNA was extracted by Trizol total RNA extraction kit (Invitrogen, USA). cDNA was synthesized by iScript cDNA synthesis kit (BioRad, USA). qRT-PCR was performed on iCycler iQ instrument (BioRad, USA) and β-actin was used as internal reference gene. The evaluation of relative mRNA levels among groups was performed by using the ΔΔCT method. The primers information could be found in Table 1.
Table 1.
qRT-PCR Primers.
| Gene | Primer | |
|---|---|---|
| β-actin | F | CATGTACGTTGCTATCCAGGC |
| R | CTCCTTAATGTCACGCACGAT | |
| TGF-β1 | F | GAAGGACCTGGGTTGGAAGT |
| R | CCGGGTTGTGTTGGTTGTAG | |
| Collagen 1 | F | TTCACCTACAGCACCCTTGT |
| R | TTGGGGTGGAGGGAGTTTAC | |
| TSP1 | F | CAACCGCATTCCAGAGTCTG |
| R | GCCAGTGTTGTCTTTCCGTT | |
| MMP1 | F | GGACAAGCAGTTCCAAAGGC |
| R | GATGCTTAGGGTTGGGGTCT |
2.10. Western blot
The expression of major proteins (TSP1, TGF-β1, MMP1, Collagen I, smad2/3, and p-smad2/3) involved in UVB irradiation and TGF-β/Smad signaling pathway was analyzed by Western Blot. Cells were grouped as control group and 60 mJ/cm2 UVB irradiated group. Cell samples were collected 6 h, 12 h, and 24 h after the irradiation. Samples were lysed in lysis buffer (Beyotime Biotechnology, Shanghai, China) and protein loading buffer (Beyotime Biotechnology, Shanghai, China) for total protein extraction, respectively. The proteins were then subjected to Western Blot analysis with anti-TSP1 polyclonal antibody (Abcam, England), anti-TGF-β1 monoclonal antibody (Abcam, England), anti-MMP1 polyclonal antibody (Abcam, England), anti-Collagen I polyclonal antibody (Abcam, England), anti-smad2/3 polyclonal antibody (Abcam, England), and anti-p-smad2/3 polyclonal antibody (Cell Signaling Technology, USA). The dilution ratio for the antibodies was according to the manufacturer’s brochure. Immuno-detection on 5% SDS-PAGE revealed protein bands. Mouse β-actin was used as internal reference protein.
2.11. Statistical analysis
All data were expressed as means ± SE. Statistical significance was evaluated using Student’s t-tests for comparisons between two groups, or by ANOVA for multiple comparisons. Chi-square test was employed for comparisons among fourfold table data. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. mSKPs maintained spherical morphology after 60 mJ/cm2 UVB irradiation
mSKPs were morphologically invariant 24 h and 48 h after the 60 mJ/cm2 UVB irradiation (Figure 1(a)). dMSCs became round and afloat 24 h after the irradiation, with the adherent dMSCs decreasing. The round and afloat dMSCs increased 48 h after the irradiation (Figure 1(b)).
Figure 1.
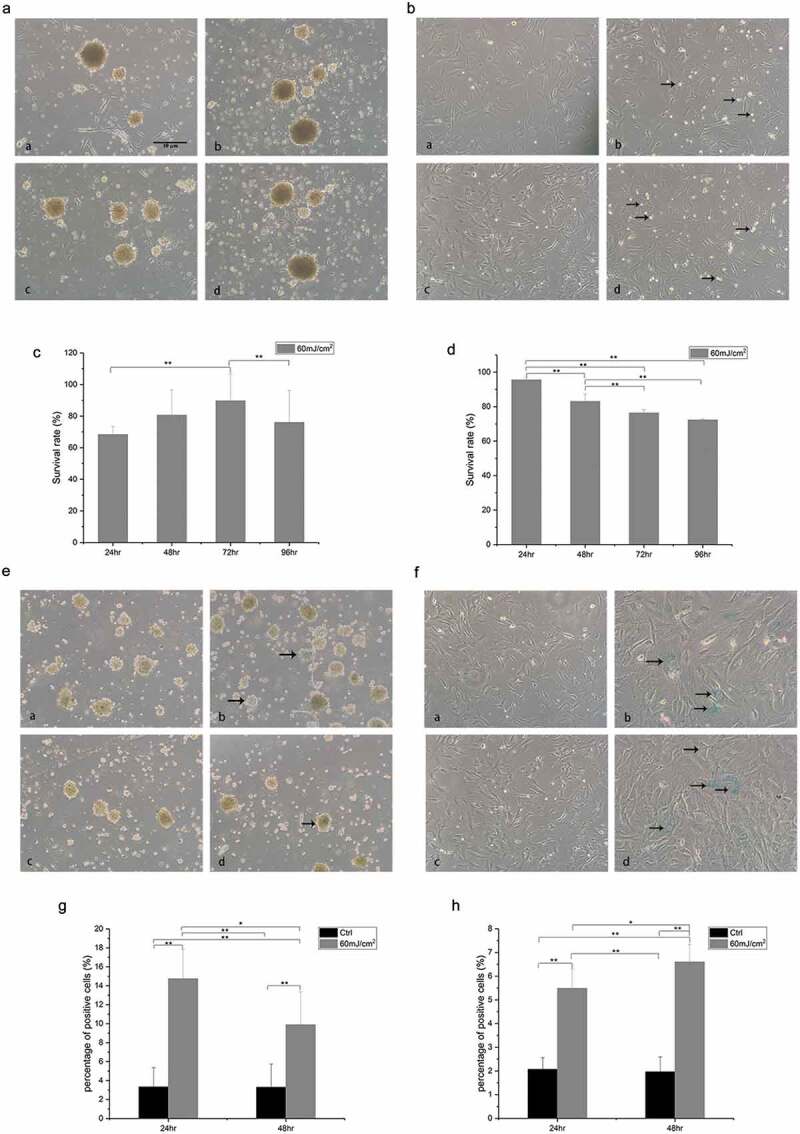
Cell morphology, cell viability, and cell senescence results. (a) mSKPs maintained spherical morphology after 60 mJ/cm2 UVB irradiation. a/c: 24 h/48 h, control group; b/d: 24 h/48 h after 60 mJ/cm2 UVB irradiation. (b) dMSCs became round and afloat (indicated by arrows) after 60 mJ/cm2 UVB irradiation. a/c: 24 h/48 h, control group; b/d: 24 h/48 h after 60 mJ/cm2 UVB irradiation. (c) mSKPs viability at different time points after 60 mJ/cm2 UVB irradiation. (d) dMSCs viability at different time points after 60 mJ/cm2 irradiation. (e) Senescent mSKPs (β-galactosidase blue-stained cells, indicated by arrows) reduced 48 h after 60 mJ/cm2 UVB irradiation. a/c: 24 h/48 h, control group; b/d: 24 h/48 h after 60 mJ/cm2 UVB irradiation. (f) Senescent dMSCs (β-galactosidase blue-stained cells, indicated by arrows) did not reduce 48 h after 60 mJ/cm2 UVB irradiation. a/c: 24 h/48 h, control group; b/d: 24 h/48 h after 60 mJ/cm2 UVB irradiation. (g) The percentage of β-galactosidase blue-stained mSKPs at different time points after 60 mJ/cm2 UVB irradiation. (h) The percentage of β-galactosidase blue-stained dMSCs at different time points after 60 mJ/cm2 UVB irradiation. (*p < 0.05, **p < 0.01) Color should be used for Figure 1 in print.
3.2. mSKPs viability recovered after 60 mJ/cm2 UVB irradiation
In mSKPs, the cell viability assay revealed cell death and a decreased viability 24 h after 60 mJ/cm2 UVB irradiation, but an increase in the viability 48 h and 72 h after the irradiation. In radiated group, the mSKPs viability at 72 h was 89.7% ± 17%, compared with 68.4% ± 5% at 24 h and 76.1% ± 20.2% at 96 h (p < 0.01) (Figure 1(c)). The viability of dMSCs kept decreasing at all the time points after 60 mJ/cm2 UVB irradiation (Figure 1(d)).
3.3. mSKPs senescence recovered after 60 mJ/cm2 UVB irradiation
Senescent mSKPs (β-galactosidase blue-stained cells) were noticed 24 h after the irradiation. The percentage of SA-β-Gal blue-stained mSKPs at 24 h after 60 mJ/cm2 UVB irradiation was 14.7% ± 3.1%, compared with 9.9% ± 3.5% at 48 h after UVB irradiation (p < 0.05) (Figure 1(e,g)).
Senescent dMSCs were also noticed 24 h after the irradiation. The percentage of SA-β-Gal blue-stained dMSCs at 24 h after 60 mJ/cm2 UVB irradiation was 5.5% ± 0.8%, compared with 6.6% ± 0.7% at 48 h after UVB irradiation (p < 0.05) (Figure 1(f,h)).
3.4. mSKPs death recovered after 60 mJ/cm2 UVB irradiation
Dead mSKPs (Propidium Iodide red-stained cells) were noticed 24 h after the irradiation, but the amount of dead cells reduced 48 h after the irradiation (Figure 2(a)). The percentage of dead cells was 15.9% and 10.3% 24 h and 48 h after the irradiation, respectively (Figure 2(b)).
Figure 2.
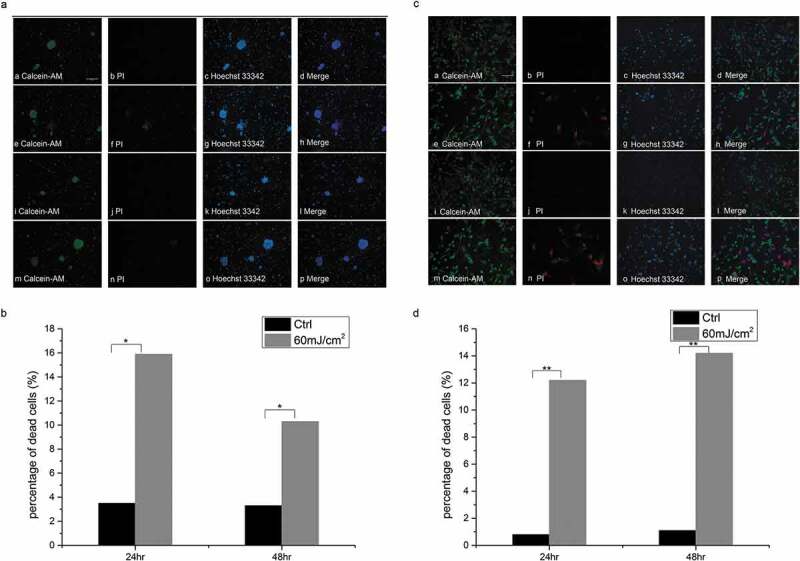
Cell death results (Calcein-AM/Propidium Iodide staining). (a) Dead mSKPs (Propidium Iodide red-stained cells, indicated by arrows) reduced 48 h after 60 mJ/cm2 UVB irradiation. a/b/c/d: 24 h, control group; e/f/g/h: 24 h after 60 mJ/cm2 UVB irradiation; i/j/k/l: 48 h, control group; m/n/o/p: 48 h after 60 mJ/cm2 UVB irradiation. (b) The percentage of dead mSKPs at different time points after 60 mJ/cm2 UVB irradiation. (c) Dead dMSCs (Propidium Iodide red-stained cells, indicated by arrows) did not reduce 48 h after 60 mJ/cm2 UVB irradiation. a/b/c/d: 24 h, control group; e/f/g/h: 24 h after 60 mJ/cm2 UVB irradiation; i/j/k/l: 48 h, control group; m/n/o/p: 48 h after 60 mJ/cm2 UVB irradiation. (d) The percentage of dead dMSCs at different time points after 60 mJ/cm2 UVB irradiation. (*p < 0.05, **p < 0.01) Color should be used for Figure 2 in print.
Cell death was also noticed in dMSCs, but the recovery was not as obvious (Figure 2(c)). The percentage of dead cells was 12.2% and 14.2% 24 h and 48 h after the irradiation, respectively (Figure 2(d)).
3.5. TSP1 and TGF-β1 in mSKPs’ supernatant increased significantly after UVB irradiation
In mSKPs, compared with the control groups, TSP1 in the supernatant increased significantly 6 h after 30 mJ/cm2, 60 mJ/cm2, 90 mJ/cm2 UVB irradiation (p < 0.05), 12 h after 90 mJ/cm2 UVB irradiation (p < 0.01), and 24 h after 30 mJ/cm2, 60 mJ/cm2, 90 mJ/cm2 UVB irradiation (p < 0.01). Compared with the control groups, TGF-β1 in the supernatant increased significantly 6 h and 12 h after 30 mJ/cm2, 60 mJ/cm2, 90 mJ/cm2 UVB irradiation (p < 0.01), and decreased significantly 24 h after 30 mJ/cm2, 60 mJ/cm2, 90 mJ/cm2 UVB irradiation (p < 0.05) (Figure 3(a)).
Figure 3.
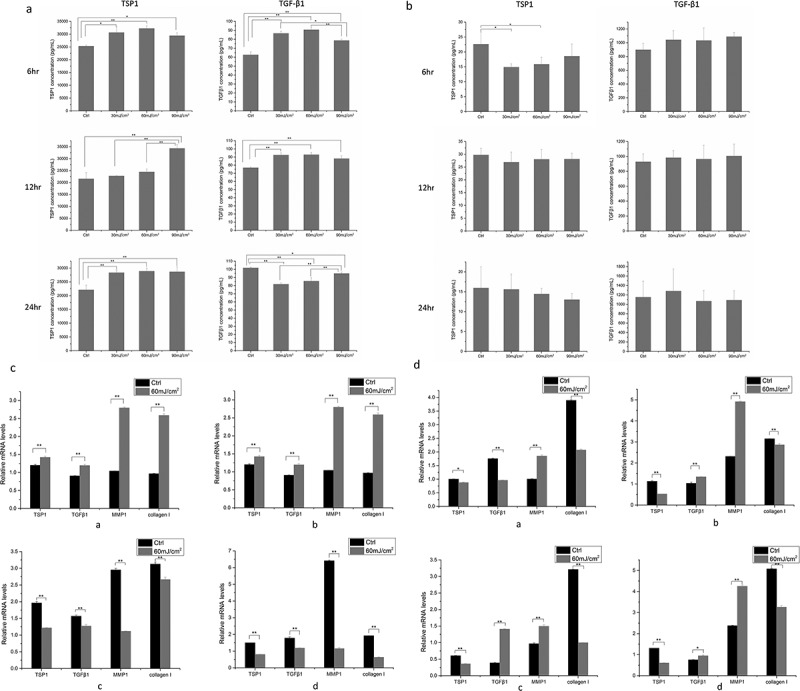
TSP1 and TGF-β1 in supernatant, gene expression of TSP1, TGF-β1, MMP1, and Collagen I. (a) TSP1 and TGF-β1 at different time points after 30 mJ/cm2 UVB, 60 mJ/cm2 UVB, 90 mJ/cm2 UVB irradiation in mSKPs supernatant. (b) TSP1 and TGF-β1 at different time points after 30 mJ/cm2 UVB, 60 mJ/cm2 UVB, 90 mJ/cm2 UVB irradiation in dMSCs supernatant. (c) Gene expression of TSP1, TGF-β1, MMP1, and Collagen I at different time points after 60 mJ/cm2 UVB irradiation in mSKPs. a: 3 h after 60 mJ/cm2 UVB irradiation; b: 6 hr after 60 mJ/cm2 UVB irradiation; c: 12 h after 60 mJ/cm2 UVB irradiation; d: 24 h after 60 mJ/cm2 UVB irradiation. (d) Gene expression of TSP1, TGF-β1, MMP1, and Collagen I at different time points after 60 mJ/cm2 UVB irradiation in dMSCs. a: 3 h after 60 mJ/cm2 UVB irradiation; b: 6 h after 60 mJ/cm2 UVB irradiation; c: 12 h after 60 mJ/cm2 UVB irradiation; d: 24 h after 60 mJ/cm2 UVB irradiation. (All data are presented as the mean±SD across three-independent experiments. *p < 0.05, **p < 0.01).
In dMSCs, compared with the control groups, TSP1 in the supernatant decreased significantly 6 h after 30 mJ/cm2, 60 mJ/cm2 UVB irradiation (p < 0.05), and no significant change was present in the 90 mJ/cm2 irradiated group. No significant change in TSP1 was observed 12 h and 24 h after the UVB irradiation. No significant change in TGF-β1 was observed in dMSCs’ supernatant at all time points after all dose UVB irradiation (Figure 3(b)).
3.6. Gene expression of TSP1, TGF-β1, MMP1, and Collagen I implied an early activation of TGF-β pathway by TSP1 in mSKPs
In mSKPs, compared with the control groups, the gene expression of TSP1, TGF-β1, MMP1, and Collagen I increased significantly 3 h and 6 h after the 60 mJ/cm2 UVB irradiation (p < 0.01), but decreased significantly 12 h and 24 h after the irradiation (p < 0.01) (Figure 3(c)).
In dMSCs, compared with the control groups, the gene expression of TSP1 and Collagen I decreased significantly at all time points after 60 mJ/cm2 UVB irradiation (p < 0.01), the gene expression of MMP1 increased significantly at all time points after the irradiation (p < 0.01), the gene expression of TGF-β1 decreased significantly 3 h after the irradiation, and increased significantly at the other time points (p < 0.05) (Figure 3(d)).
3.7. Protein expression of TSP1, TGF-β1, MMP1, smad2/3, p-smad2/3, and collagen i was consistent with gene expression in mSKPs
In mSKPs, compared with the control groups, the protein expression of TSP1, TGF-β1, MMP1, and p-smad2/3 increased significantly 6 h, 12 h, and 24 h after 60 mJ/cm2 UVB irradiation (p < 0.01). The protein expression of smad2/3 decreased significantly at all time points after the irradiation (p < 0.01). The protein expression of Collagen I increased significantly 6 h, 12 h after the irradiation (p < 0.01), and decreased significantly 24 h after the irradiation (p < 0.05) (Figure 4).
Figure 4.
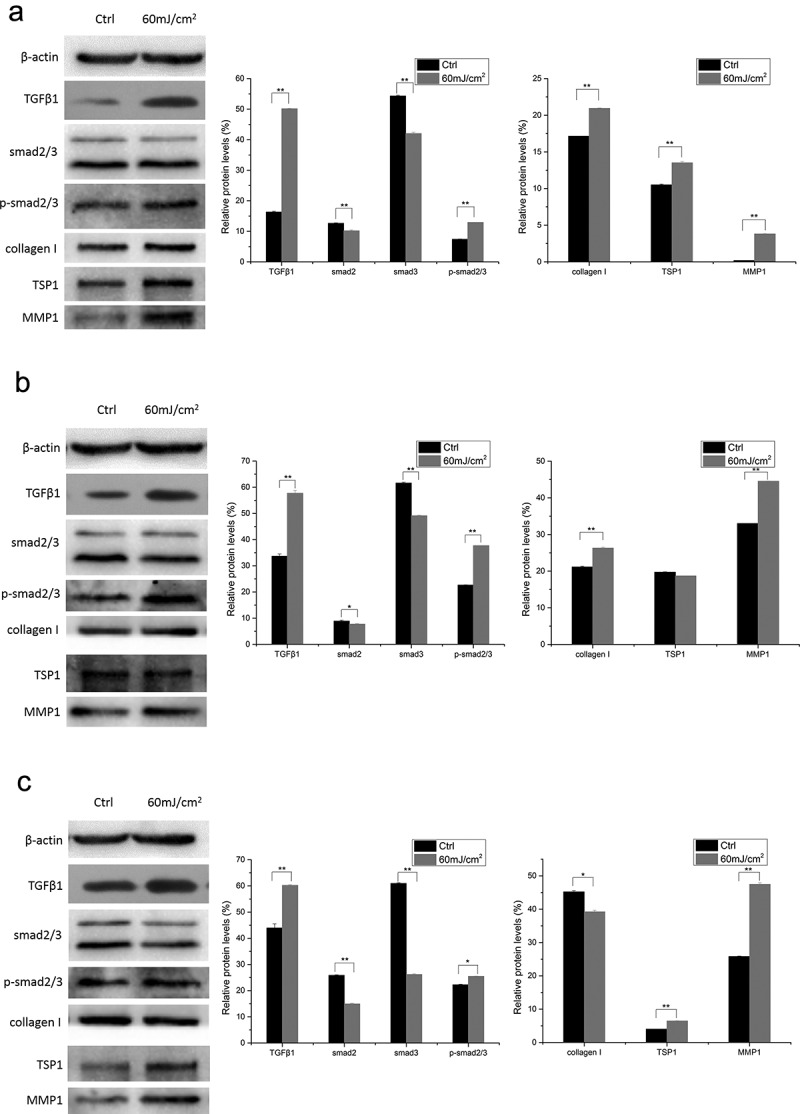
Protein expression of TSP1, TGF-β1, MMP1, smad2/3, p-smad2/3, and Collagen I at different time points after 60 mJ/cm2 UVB irradiation in mSKPs. (a) 6 h after 60 mJ/cm2 UVB irradiation; (b) 12 h after 60 mJ/cm2 UVB irradiation; (c) 24 h after 60 mJ/cm2 UVB irradiation. (All data are presented as the mean±SD across three-independent experiments. *p < 0.05, **p < 0.01).
In dMSCs, compared with the control groups, the protein expression of TGF-β1 and MMP1 increased significantly at all time points after 60 mJ/cm2 UVB irradiation (p < 0.01). The expression of other proteins decreased significantly at all time points after the irradiation (p < 0.01) (Figure 5).
Figure 5.
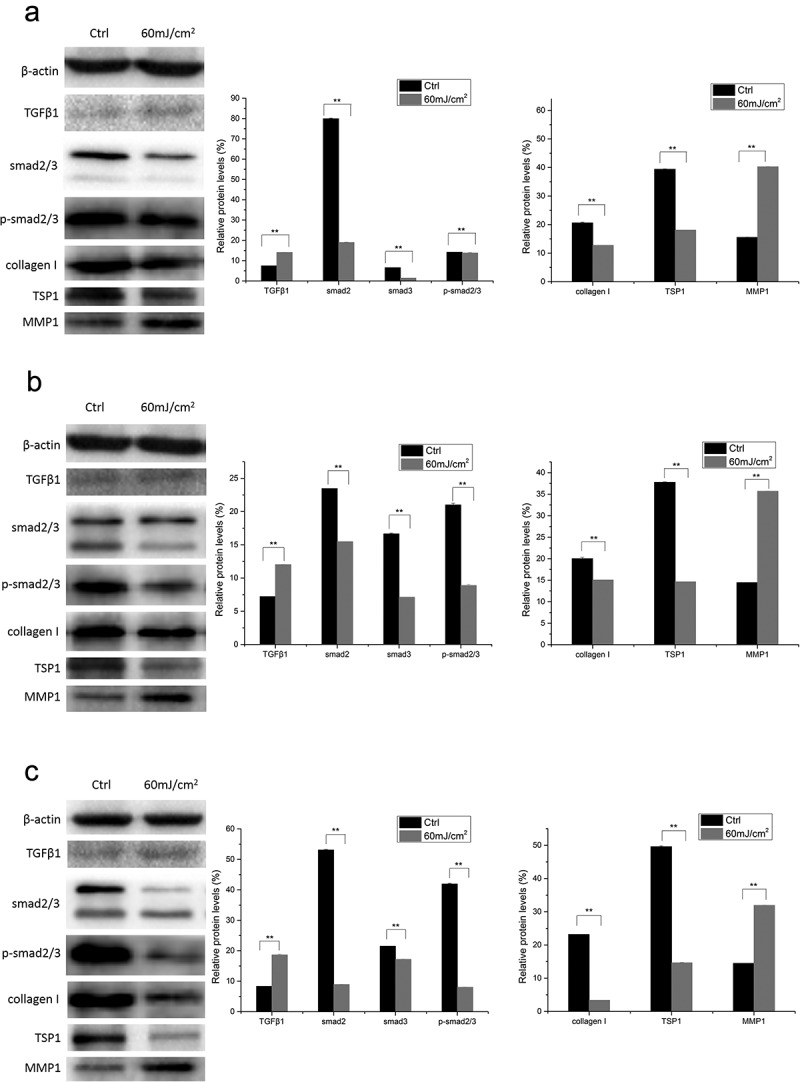
Protein expression of TSP1, TGF-β1, MMP1, smad2/3, p-smad2/3, and Collagen I at different time points after 60 mJ/cm2 UVB irradiation in dMSCs. (a) 6 h after 60 mJ/cm2 UVB irradiation; (b) 12 h after 60 mJ/cm2 UVB irradiation; (c) 24 h after 60 mJ/cm2 UVB irradiation. (All data are presented as the mean±SD across three-independent experiments. *p < 0.05, **p < 0.01).
4. Discussion
4.1. mSKPs alleviated UVB irradiation damage via TGF-β/smad pathway activation by TSP1
Latent TGF-β can be activated by molecules such as TSP1, integrins, MMPs, and plasmin. Activated TGF-β plays an essential role in wound healing through its pleiotropic effects on cell proliferation and differentiation, extracellular matrix production, and immune modulation [16]. Besides, TGF-β was revealed to facilitate the sphere formation and proliferation of the mSKPs in vitro without altering their phenotypical characteristics [17]. Thrombospondins (TSPs) represent extracellular matrix (ECM) proteins belonging to the TSP family that comprises five members, which play a major role in cardiovascular diseases. TSPs-mediated effects indeed depend on the availability of the binding partner and local micro-environment, which explains cell- and tissue-specific actions of TSPs. TSPs display different cellular distributions, different temporal expression profiles and have distinct functional responsibilities and modes of transcriptional regulation [18]. Smads are essential downstream signal proteins in TGF-β/Smad pathway. In mammals, smad2 and smad3 are TGF-β/activin-specific receptor-regulated smads (R-smads) [19]. In our study, increased p-smad2/3 protein expression confirmed the activation of TGF-β/Smad pathway. Our findings suggested that one of the wound-healing mechanisms in mSKPs might be TGF-β/Smad pathway activation by TSP1, if UVB irradiation damage, cell senescence, or cell death is considered as wound or injury.
4.2. TGF-β/smad pathway activation by TSP1 was an early-phase event after UVB irradiation
To our knowledge, this is the first study to report TGF-β/Smad pathway activation by TSP1 in a time sequence pattern. In mSKPs, cell viability decreased 24 h after the UVB irradiation, but recovered at the following time points. No similar tendency was noticed in dMSCs, which was consistent with the findings in morphology, β-galactosidase staining, and Calcein-AM/Propidium Iodide staining.
In mSKPs, the qRT-PCR results implied that the elevated TSP1 and TGF-β1 gene expression was an early event after UVB irradiation. Similar tendency was noticed in MMP1 gene expression. The activation of TGF-β/Smad pathway by TSP1 might be considered as the acute response to UVB irradiation (especially one-single irradiation). The qRT-PCR results were consistent with ELISA results, also in concert with the statement “temporal and spatial expressions in the healing wound” by Agah et al. and Bornstein et al. [20,21]. The up-regulated gene expression of Collagen I at the early phase might be attributed to UVB irradiation damage alleviation through TGF-β/Smad pathway activation. The decreased Collagen I gene expression at the following time points might suggest that the pathway activation was not sufficient for counteracting the irradiation damage, or cell death, or it was exhausted during the process of pathway activation. Considering the gene expression would not be reflected as protein expression instantly, we selected the later time points (6 h, 12 h, and 24 h after UVB exposure) when detecting protein expression. Satisfactory correlation was present between qRT-PCR results and Western-blot results in mSKPs.
4.3. No dose-dependent correlation was present between UVB irradiation and TGF-β/smad pathway activation
The mSKPs ELISA results demonstrated the increase of TSP1 and TGF-β1 was not in direct proportion to the dose of UVB irradiation, which might be the cell senescence or cell death caused by larger dose UVB irradiation (for instance, 90 mJ/cm2). The elevated TGF-β1 in the supernatant at the early phase implied the activation of TGF-β/Smad pathway, while the decrease in TGF-β1 at the subsequent time points might be caused by cell senescence or cell death. Notably, the change of TGF-β1 was not coincident with that of TSP1, implying TSP1 might not be the only activator of TGF-β/Smad pathway, and some other factors might be involved as well.
4.4. TGF-β/smad pathway activation by TSP1 was not observed in dMSCs
Different from mSKPs, the divergent change in TSP1 and TGF-β1 was present in dMSCs. ELISA results demonstrated low amount of TSP1 but considerably high amount of TGF-β1 (even higher than that in mSKPs supernatant), suggesting the possibility that certain TGF-β-related pathway might be activated by some other molecules. The TGF-β1 generated from the activated TGF-β-related pathway was externally secreted into the supernatant, resulting in the low content of TGF-β1 in dMSCs (Western-blot).
The divergent change in TSP1 and TGF-β1 was also noticed in dMSCs qRT-PCR and Western-blot findings. Western-blot exhibited reduced protein expression of smad2/3 and p-smad2/3 at all time points. Taken together, in dMSCs, the increase in TGF-β1 might not result from TGF-β/Smad pathway activation, or there might be cross-talk between TGF-β/Smad pathway and some other pathways or cytokines, or TGF-β1 activated non-Smad pathways such as Erk, JNK, and p38 MAPK pathways [19].
Seo et al. found that in human skin dermis and dermal fibroblasts 70 mJ/cm2 and 90 mJ/cm2 UV (275 nm-380 nm) exposure induced TSP-1 mRNA and protein expression via the PI3K, Akt, and mTOR pathways, and TSP-1 upregulation prevented decrease of type I procollagen expression via TGF-β/pSmad3 signaling pathway. However, they mentioned UV exposure reduced TSP-1 expression in human skin epidermis and keratinocytes. TSP-1 expression could be differentially regulated by UV, depending on cell types [22]. Just as stated above, TSP-mediated effects could be cell- and tissue-specific [18]. Up-regulation of TSP1 expression in dMSCs was not observed in our study, which might be associated with cell types, wavelength, UV dose, or other factors.
4.5. Further consideration
Both qRT-PCR and Western-blot results indicated a decreased Collagen I expression at the final time points after UVB exposure, which might be explained as the increased TSP1 or (and) the activation of TGF-β/Smad pathway might not be sufficient for counteracting UVB irradiation damage. Whether the over-expression of TSP1 would have an impact on mSKPs’ anti-UVB irradiation damage capacity remains to be elucidated. In addition, mSKPs’ response to multiple UVB irradiation, UVA irradiation, and UVA+UVB irradiation needs further investigation.
In summary, the present study explored mSKPs’ possible application other than neurological function recovery. Our findings revealed that mSKPs significantly alleviated UVB irradiation damage through TGF-β/Smad pathway early activation by TSP1 (Figure 6). Given that mSKPs could differentiate into SFBs in vivo or with the presence of serum, mSKPs might serve as a potential cell-replacement therapeutic choice for fibroblasts supplement, UVB irradiation damage or even photo-aged skin treatment in clinic.
Figure 6.
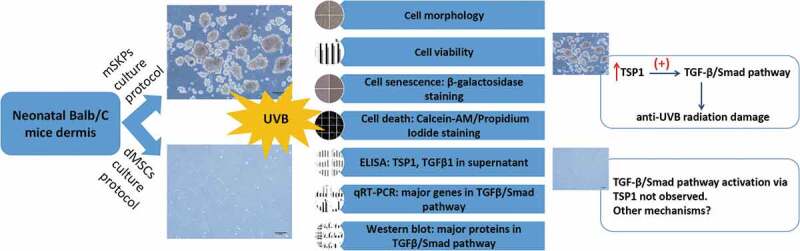
Schematic summary diagram. Summary of experiment design and key findings. Color should be used for Figure 6 in print.
Funding Statement
This work was supported by Natural Science Foundation of China (No. 81673084) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
Highlights
Recovery of UVB irradiation damage was noticed in mSKPs
mSKPs alleviated irradiation damage via TGF-β/Smad pathway early activation by TSP1
mSKPs may serve as therapeutic potential for fibroblasts supplement, damage treatment
Recovery of UVB irradiation damage was not noticed in dMSCs
Author Contributions
YL designed the research, carried out the experiments, analyzed and discussed the data, drafted the manuscript. LX analyzed and discussed the data, reviewed the manuscript. JT analyzed and discussed the data. GZ carried out the experiments, analyzed and discussed the data. RD analyzed and discussed the data. LL designed the research, reviewed the manuscript.
All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Sparling JS, Bretzner F, Biernaskie J, et al. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J Neurosci. 2015;35(17):6714–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kumar R, Sinha S, Hagner A, et al. Adult skin-derived precursor Schwann cells exhibit superior myelination and regeneration supportive properties compared to chronically denervated nerve-derived Schwann cells. Exp Neurol. 2016;278:127–142. [DOI] [PubMed] [Google Scholar]
- [3].Krause MP, Dworski S, Feinberg K, et al. Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Reports. 2014;3:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhong J, Li L.. Skin-Derived Precursors against UVB-Induced Apoptosis via Bcl-2 and Nrf2 Upregulation. BioMed Res Int. 2016;11:6894743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xian D, Gao X, Xiong X, et al. Photoprotection against UV-induced damage by skin-derived precursors in hairless mice. J Photochem Photobiol B. 2017;175:73–82. [DOI] [PubMed] [Google Scholar]
- [6].Wang S, Zhong J, Li L.. Protective effect of skin-derived precursors on photoaging in nude mice. Australas J Dermatol. 2018. DOI: 10.1111/ajd.12867 [DOI] [PubMed] [Google Scholar]
- [7].Mao Y, Xiong L, Wang S, et al. Comparison of the transcriptomes of mouse skin derived precursors (SKPs) and SKP-derived fibroblasts (SFBs) by RNA-Seq. PLoS One. 2015;10(2):e0117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-β activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018;68–69:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69:249–256. [DOI] [PubMed] [Google Scholar]
- [10].He T, Quan T, Fisher GJ. Ultraviolet irradiation represses TGF-β type II receptor transcription through a 38-bp sequence in the proximal promoter in human skin fibroblasts. Exp Dermatol. 2014;23(Suppl 1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bige N, Shweke N, Benhassine S, et al. Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int. 2012;81:1226–1238. [DOI] [PubMed] [Google Scholar]
- [12].Li Y, Li X, Xiong L, et al. Comparison of phenotypes and transcriptomes of mouse skin-derived precursors and dermal mesenchymal stem cells. Differentiation. 2018;102:30–39. [DOI] [PubMed] [Google Scholar]
- [13].Nghiem DX, Kazimi N, Mitchell DL, et al. Mechanisms underlying the suppression of established immune responses by ultraviolet radiation. J Invest Dermatol. 2002;119(3):600–608. [DOI] [PubMed] [Google Scholar]
- [14].Maglio DHG, Paz ML, Ferrari A, et al. Alterations in skin immune response throughout chronic UVB irradiation-skin cancer development and prevention by naproxen. Photochem Photobiol. 2010;86:146–152. [DOI] [PubMed] [Google Scholar]
- [15].Biernaskie JA, McKenzie IA, Toma JG, et al. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1(6):2803–2812. [DOI] [PubMed] [Google Scholar]
- [16].Finnson KW, McLean S, Di Guglielmo GM, et al. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Adv Wound Care (New Rochelle). 2013;2(5):195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kawase Y, Yanagi Y, Takato T, et al. Characterization of multipotent adult stem cells from the skin: transforming growth factor-beta (TGF-beta) facilitates cell growth. Exp Cell Res. 2004;295(1):194–203. [DOI] [PubMed] [Google Scholar]
- [18].Chistiakov DA, Melnichenko AA, Myasoedova VA, et al. Thrombospondins: A Role in Cardiovascular Disease. Int J Mol Sci. 2017;18(7):E1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Miyazono K. Positive and negative regulation of TGF-beta signaling. J Cell Sci. 2000;113(Pt 7):1101–1109. [DOI] [PubMed] [Google Scholar]
- [20].Agah A, Kyriakides TR, Lawler J, et al. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. [DOI] [PubMed] [Google Scholar]
- [22].Seo JE, Kim S, Shin MH, et al. Ultraviolet irradiation induces thrombospondin-1 which attenuates type I procollagen downregulation in human dermal fibroblasts. J Dermatol Sci. 2010;59(1):16–24. [DOI] [PubMed] [Google Scholar]


