ABSTRACT
N6-methyladenosine (m6A) is the most prevalent epigenetic modification of messenger RNA (mRNA) in higher eukaryotes; this modification is mainly catalyzed by a methyltransferase complex including methyltransferase-like 3 (METTL3) as a key factor. Although m6A modification has been proven to play an essential role in diverse biological processes, our knowledge of Mettl3 is still limited because Mettl3 mutations are lethal to embryos in both mammals and plants. In this study, we knocked down Mettl3 by microinjection of its specific short interfering RNAs (siRNAs) or morpholino into fully grown germinal vesicle (GV) oocytes. As a result, we demonstrated that knocking down Mettl3 in female germ cells severely inhibited oocyte maturation by decreasing mRNA translation efficiency and led to defects in the maternal-to-zygotic transition, probably due to its interference in disrupting mRNA degradation. The discovery from this study suggests that the reversible m6A modification has vital functions in mammalian oocyte maturation and pre-implantation embryonic development processes.
KEYWORDS: Mettl3, N6-methyladenosine, oocyte maturation, maternal-to-zygotic transition
Introduction
During oogenesis, maternal mRNA is actively transcribed and accumulated in growing oocytes, but this transcription stops before the oocytes grow to their full size. The accumulated maternal mRNA is used for protein synthesis in the oocytes during oocyte-to-embryo transition and even in the embryos to sustain development after fertilization [1]. Massive degradation of maternal mRNA begins when the oocytes resume meiosis. During meiotic maturation in the 12 h prior to ovulation, mouse oocytes become transcriptionally quiescent, and the majority of their polyadenylated mRNAs disappears [2]. This degradation continues after fertilization, and by the mid two-cell stage, most maternal mRNAs are degraded. Therefore, the transition of mRNA from stability to instability is a key step in oocyte cytoplasmic maturation and is critical for the oocyte-to-zygote transition, suggesting that post-transcriptional mRNA regulation plays an important role in oocyte maturation and maternal-to-zygotic transition.
In the past few years, More than 150 types of RNA modifications have been identified in eukaryotic RNA, among which m6A is the most prevalent RNA modification and is reversible in eukaryotic mRNA[3]. There are common reversible m6A modifications on mRNA within the RRACH consensus motif (R = A, G; H = A, C, U) that are vital for mammalian development [4,5]. In mammals, METTL3, METTL14, Wilms’ tumor 1-associating protein (WTAP) and KIAA1429 are indispensable for the methyltransferase complex [6–9], and AlkB homologue 5 (ALKBH5) and fat mass and obesity-associatedprotein (FTO) have been verified as the demethylase for adenosine methylation [10,11]. Meanwhile, m6A can be recognized by YTH-domain-containing family proteins, including YTHDF1, YTHDF2, YTHDF3, YTHDC1 and YTHDC2 [12–17]. These m6A methyltransferases, demethylases, and binding proteins are, respectively, “writers”, “erasers” and “readers” of m6A modification. Additionally, m6A modification processes many biological steps. m6A affects almost every stage of mRNA metabolism, from processing in the nucleus to translation and decay in the cytoplasm. Most METTL3-binding sites are situated in introns, and Mettl3 depletion in mouse embryonic stem cells generally favors exon skipping and intron retention, indicating that recruitment of METTL3 to pre-mRNA is a co-transcriptional event, with methylation potentially preceding and influencing splicing [18]. m6A was also suggested to promote mRNA export. A previous study showed that the depletion of METTL3 inhibited mRNA export, while depletion of ALKBH5 enhanced mRNA export to the cytoplasm [19]. Dynamic m6A modification is verified by YTHDF2 to affect the translation status and lifetime of mRNA [12]. YTHDC1 directly regulates mRNA splicing through bridging interactions of trans- and cis-regulatory elements [20]. YTHDF1 or YTHDF3-mediated translation promotion contributes to translation efficiency [13,15,16]. YTHDC1 preferentially recognizes m6A residues on X-inactive specific transcripts (XISTs) and is required for XIST-mediated transcriptional repression [21]. Moreover, current studies have revealed that FTO regulates mRNA splicing and is necessary for adipogenesis [22], and that specific inhibition of Mettl3 is sufficient to cause circadian period elongation and RNA processing delays [19]. Knocking down Mettl3 and Mett14 has been shown to induce increases in the expression of their respective target mRNAs in human and mouse cells [7,23].
In zebrafish, it was reported that approximately one-third of maternal mRNAs were highly methylated at m6A, and after reducing m6A levels, it was shown that m6A was indispensable for the maternal-to-zygotic transition [24]. To be specific, as the first identified component of the methyltransferase complex, METTL3 has effects on cell division, differentiation, and spermatogenesis in mammals. Mettl3−/- mice are embryonically lethal, and knocking out Mettl3 in mouse embryonic stem cells impedes differentiation [18,23,25]. A recent study found that germ cell conditional Mettl3 knockout mice had defects in spermatogenesis through controlling spermatogonial differentiation and meiosis [26]. And Mettl3 mutation disrupts gamete maturation and reduces fertility in zebrafish [27]. Herein, METTL3 is essential in mammals, implicating a basic character for m6A in mammalian embryonic development. However, due to the early lethality of Mettl3−/- mice, METTL3-mediated m6A modification in female mammalian reproduction is still unclear.
Here, we reduced the m6A modification level by knocking down METTL3 with a microinjection of its specific siRNAs or morpholino into GV oocytes, and we found that METTL3-mediated m6A was necessary for oocyte maturation and maternal-to-zygotic transition. Knocking down Mettl3 was accompanied by altered mRNA translation efficiency, mRNA degradation and zygotic gene activation (ZGA). This study reveals that METTL3-mediated m6A modification is essential for female mammalian reproduction.
Materials and methods
Mice and ethics statement
CD1 mice were housed under specific pathogen-free (SPF) conditions on a 12 h light-12 h dark cycle. All animal work was approved by the Animal Care and Use Committee of Nanjing Medical University.
Oocyte and embryo collection and culture
GV oocytes were collected from the ovaries of 3 weeks old mice without hormonal stimulation. GV oocytes were either allowed to mature in vitro in M2 (Sigma, M7167) medium or incubated in the presence of 2.5 µM milrinone (Sigma, M4659) to inhibit spontaneous germinal vesicle breakdown. Metaphase II (MII) oocytes were collected at 14 h after transferring the oocytes to milrinone-free M2 medium. Next, zygote, two-cell, four-cell, morula, and blastocyst stage embryos were collected after 22–26 h, 48–50 h, 60–65 h, 70–75 h, and 96–100 h of culture in KSOM (Millipore, MR-020P-D) medium, respectively. All oocytes and embryos were maintained at 37°C in a humidified atmosphere of 5% CO2.
Sirna or morpholino microinjection
GV oocytes were microinjected with approximately 5 pl of either siRNA (Ribobio Co. China) (siRNA 1# 5ʹ- CAAGGAAGAGTGCATGAAA-3ʹ; siRNA 2# 5ʹ- GAAAGGTCTTGGAGAGGTA −3ʹ; siRNA 3# 5ʹ- CAGTGGATCTGTTGTGATA-3ʹ) or morpholino (Gene Tools, USA) (5ʹ-ACGTGTCCGACATCCTAGCTCCCAG-3ʹ) in M2 medium in the presence of 2.5 µM milrinone. Non-silencing siRNA (5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ) and morpholino (5ʹ-CCTCTTACCTCAGTTACAATTTATA-3ʹ) was used as the control. The ORF of eGFP was cloned into the pCS2+ vector. The constructs were linearized by SacII or KpnI and purified using a gel extract kit (Promega). The SP6 message machine (Ambion) was used for producing capped mRNAs, and the mRNAs were purified by the RNeasy cleanup kit (Qiagen). The eGFP was injected at 5 pl. GV oocytes were cultured for 24 h in 2.5 µM milrinone and collected to assess siRNA knockdown efficiency by quantitative real-time PCR (qRT-PCR). GV oocytes were collected for 3 h, 12 h and 24 h to ascertain morpholino knockdown efficiency and optimum effect time by western blot. For Mettl3, the forward primer was 5ʹ -TTTCATCTTGGCTCTATCCGGCTG-3ʹand the reverse primer was 5ʹ – AGTAGGCACGGGACTATCACTA-3ʹ. mRNA level was calculated using the Ct value of qRT-PCR: △Ct = Ct Value (gene)-Ct Value (18s), 2−△Ct was used to evaluate the relative mRNA level. mRNA level was shown relative to 18s and the value in NC oocytes was normalized to 1.
Parthenogenetic activation
Following morpholino microinjection, GV oocytes were cultured in M2 medium with 2.5 µM milrinone for 12 h before in vitro maturation to MII oocytes in M2 medium without milrinone. The MII oocytes were activated with 5 mM SrCl2 in modified CZB (Sigma, MR109) medium containing 2 mM Ethylenediaminetetraacetic acid (Sigma, E-6635) and 5 µg/ml of cytochalasin B (Sigma, C6762) for 4 h. They were then cultured in KSOM medium at 37°C in 5% CO2 in a humidified atmosphere.
RNA extraction and qRT-PCR
Total RNA was isolated from 50 oocytes or embryos using an RNeasy Plus Micro Kit (Qiagen, 73034) according to the manufacturer’s instructions, and total RNA was reverse-transcribed into cDNA using the reverse transcription enzyme (Takara, DRR036A) and diluted into 100 ng/µl for each example. qRT-PCR was performed using a SYBR Premix Ex Taq kit (Vazyme, Q141-02/03) and a 7300 Real Time PCR System (Applied Biosystems, Singapore). Relative gene expression was analyzed based on the 2−△△CT method with 18S as an internal control. 18S primers were designed as 5ʹ-GTAACCCGTTGAACCCCATT-3ʹ and 5ʹ- CCATCCAATCGGTAGTAGCG-3ʹ. At least three independent experiments were analyzed.
Immunofluorescence and confocal microscopy
Oocytes and embryos were fixed in 4% paraformaldehyde (PFA) (Sigma, P6148) in PBS overnight at 4°C and then incubated in permeabilization buffer for 30 min at 37°C. They were blocked with 5% bovine serum albumin (BSA) for 2 h at room temperature and then incubated with the primary antibody overnight at 4°C. The following antibodies were used at the following dilutions: rabbit anti-METTL3 (Invitrogen, PA5-41599, 1:1000), rabbit anti-m6A (Synaptic Systems, 202003, 1:1000), rabbit anti-RNA pol II CTD phospho Ser2 (Abcam, ab5095, 1:1000), and mouse anti-RNA pol II CTD phospho Ser5 (Abcam, ab5408, 1:1000). After washing with PBS three times, secondary antibodies (Invitrogen, a21202; a31572, 1:500) were added to the samples for 2 h at room temperature. This was followed by DAPI or Hoechst 33342 (Invitrogen, 1:3000) for DNA staining. Finally, oocytes and embryos were washed, coverslipped with diazabicyclooctane (DABCO), and examined using a ZEISSLS M710 confocal laser-scanning microscope (Carl ZEISS Micro Imaging GmbH, Jena, Germany). The fluorescence signal was detected using a ZEISSLS M710 confocal laser-scanning microscope. ZEN software was used to quantify the intensity of fluorescence. Each experimental group included 10–20 oocytes, and the experiments were done at least in triplicate.
HPG incorporation assay and EU incorporation assay
Oocytes were cultured in M2 medium with 2.5 µM milrinone and 50 µM HPG and then were fixed with 4% in PBS overnight at 4°C and then incubated in permeabilization buffer for 30 min at room temperature. Next, the samples were stained by the Click-iT™ HPG Alexa Fluor™ 488 Protein Synthesis Assay Kit (Invitrogen, C10428) according to the manufacturer’s instructions. Embryos were cultured in KSOM medium with 100µM 5-EU. Fixation, permeabilization, and staining were performed by the Click-iT RNA Alexa Fluor 594 Imaging Kit (Invitrogen, C10330) according to the manufacturer’s instructions. Followed by being washed with PBS and incubated with secondary antibodies. The oocytes and embryos were incubated with DAPI and observed by a ZEISSLS M710 confocal laser-scanning microscope (Carl ZEISS Micro Imaging GmbH, Jena, Germany). ZEN software was used to quantify the intensity of fluorescence. Each experimental group included 10–20 oocytes, and the experiments were done at least in triplicate.
Western blot
Fifty oocytes were extracted with 8 M urea lysis buffer (8 M urea, 75μM NaCl, 50μM Tris-Cl pH 8.2) containing 1 mM PMSF. Protein samples were separated in a 7% SDS-PAGE gel and transferred to PVDF membranes (Bio-Rad, 162–0177). Appropriate primary antibodies were incubated overnight at 4°C after blocking in 5% nonfat milk (BD, 232100). For this procedure, the following primary antibodies were diluted immediately before the incubation: mouse anti-MSY2 (Santa Cruz, sc-393840, 1:1000), rabbit anti-CLTC (Abcam, ab172958, 1:1000), rabbit anti-SPDL-1 (Bioss, bs-2321R, 1:500), rabbit anti-PCNT (Biorbyt, orb215500, 1:500), rabbit anti-METTL3 (Invitrogen, PA5-41599, 1:3000), and rabbit anti-ACTIN (Millipore, MAB1501, 1:3000). After washing three times in TBST, secondary antibodies (Invitrogen, 31800; 35560, 1:3000) were used for 2 h at room temperature. The signals were detected by SuperSignal™ West Femto Maximum Sensitivity Substrate (Invitrogen, 34095).
Chromosome spread
Oocytes were exposed to Tyrode’s buffer (Sigma, T1788) for 30 sec at 37°C to remove the zona pellucida. After recovery in M2 medium for 10 min at 37°C, oocytes were fixed in a drop of 0.15% Triton X-100 and 1% PFA on a glass slide. After air drying, oocytes were incubated with CREST (Davis, 15–234, 1:500) at 4°C overnight. They were then incubated with secondary antibody for 1 h for kinetochore labeling. The chromosomes were stained with DAPI, and samples were examined under a ZEISSLS M710 confocal laser-scanning microscope.
RNA-seq
The MII oocytes were collected into lysis buffer containing RNase inhibitor. Oocytes were amplified by the Smart-Seq2 method. An oligo-dT primer was used for reverse transcription of the 1st cDNA synthesis, followed by PCR amplification and then a magbead purification step to clean up the cDNA. The amplified cDNA was checked by a Qubit ® 3.0 fluorometer, and an Agilent 2100 Bioanalyzer was used to ensure that the expected product length was approximately 1–2 kbp. Then, the cDNA was sheared randomly by ultrasonic waves for the Illumina library preparation protocol, which involved DNA fragmentation, end repair, 3ʹ-end A-tailing, adapter ligation, PCR amplification and library validation. After library preparation, the PerkinElmer LabChip ® GX Touch and Step OnePlusTM Real-Time PCR System were used for library quality inspection. Suitable libraries were then loaded on the Illumina Hiseq platform for PE150 sequencing. Two independent experiments were done.
Gene ontology and pathway enrichment analysis
Gene ontology (GO) and pathway enrichment analysis were performed using the function enrich GO of the cluster Profiler package [28] in the R programming environment. The resulting GO terms were analyzed for semantic similarity (cutoff value of 0.7) with GOSemSim R package [29] to reduce redundancy. Selections of non-redundant enriched terms from the GO analysis were plotted. Visualization of results was performed using the ggplot2 R package [30].
Statistical analysis
Data were evaluated by an unpaired two-tailed t-test or chi-square test for comparisons between the experimental groups and control group. Statistical significance: *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Mettl3 expression and m6A levels are high in oocytes and pre-implantation embryos
To investigate the function of m6A modification in mammalian fetal development, we first identified the expression pattern of m6A in mouse oocytes and pre-implantation embryos by conducting immunofluorescence (IF) staining, and we found that m6A mainly localized at the cytoplasm from GV to blastocyst. We speculated that the accumulation of maternal RNAs in the cytoplasm is gradually degraded with the gradual weakening of the RNA modification signal from GV to 2 cell stage, after ZGA, the increase of cytoplasmic RNAs is accompanied by enhancement of m6A signaling from 2 cell to blastocyst stage. (Figure 1(a)). As a result, we subsequently used IF to detect the expression of METTL3 in mouse oocytes and pre-implantation embryos. METTL3 mainly localized at the nucleus of GV oocyte and METTL3 signal could be detected both in the nucleus and cytoplasm of all stages (Figure 1(b)). Meanwhile, qRT-PCR and western blot showed that Mettl3 mRNA and METTL3 protein were highly expressed in MII oocytes (Figure 1(c and d)). However, downregulation after fertilization suggested that Mettl3 was a maternal-effect gene.
Figure 1.

The expression of m6A and METTL3 in mouse oocytes and pre-implantation embryos.
(a) Immunofluorescence staining of m6A in mouse oocytes and pre-implantation embryos. DNA was stained with DAPI. Scale bar, 20 µm. IgG was used as a negative control. (b) Immunofluorescence of METTL3 in mouse oocytes and pre-implantation embryos stained with anti-Mettl3 antibody (red) and DAPI (blue) are shown as indicated. Scale bar, 20 µm. IgG was used as a negative control. (c) qRT-PCR results showing relative expression levels of Mettl3 in mouse oocytes and pre-implantation embryos. Error bars, mean ± SD, n = 3, technical replicates (Student’s t-test). (d) Western blot results show METTL3 protein expression in mouse oocytes and pre-implantation embryos.
Knocking down Mettl3 causes an oocyte maturation defect
To determine the functional role of METTL3-mediated m6A modification in murine oocyte maturation, GV oocytes were microinjected with siRNAs against Mettl3, and then incubated in milrinone-containing M2 medium to inhibit spontaneous germinal vesicle breakdown. The knockdown effect was confirmed by qRT-PCR and western blot (Fig. S1a, S1b). Thus, we chosen Mettl3 siRNA 1# and siRNA 3# in the following experiments. The IF experiment indicated that the m6A level was decreased after knocking down Mettl3 (Fig. S1c). After incubation in milrinone-containing M2 medium for 24 h, GV oocytes were washed and cultured in M2 medium for an additional 3 h to observe germinal vesicle breakdown (GVBD) oocytes without milrinone, and for additional 14 h to observe first polar body extrusion. Our results showed that Mettl3 knockdown had no effect on meiotic resumption, as evidenced by the similar GVBD rate, but the ratio of first polar body extrusion was significantly decreased in Mettl3 knockdown oocytes after 14 h of in vitro maturation compared with the control group (Figure 2(a and b)), suggesting an involvement of Mettl3 in oocyte meiotic maturation. We next assessed whether Mettl3 knockdown affected spindle organization at the MII oocyte stage using immunofluorescence with the a-tubulin antibody. Normal MII oocytes usually showed a typical barrel-shape spindle with the well-aligned chromosomes at the equator. While we found about 50% of Mettl3 knockdown MII oocytes had obvious spindle abnormalities including short, wide-polar and elongated spindles[31]. To our knowledge, spindle defects can lead to the errors in chromosome segregation during oocyte meiosis [31–33]. Chromosome spread experiment showed that most control oocytes had 20 univalent to maintain the euploidy. However, 52.38% of aneuploid oocytes appeared after siRNA 1# knockdown, and 52.17% of aneuploid oocytes in siRNA 3# knockdown oocytes (Figure 2(c and d)). Thus, these data suggest that METTL3-mediated m6A modulates mammalian oocyte meiotic maturation.
Figure 2.

METTL3 is maternally required for mouse oocyte maturation.
(a) Mettl3 depletion impaired oocyte maturation. Mettl3 siRNA 1# or siRNA 3# microinjected GV oocytes were cultured and quantified (siRNA1#, n = 80; siRNA3#, n = 90; three independent experiments). NC indicates negative control (n = 120, three independent experiments). Error bars, mean ± SD, ***P < 0.001 in an unpaired two-tailed t-test. (b) Representative images show oocyte maturation defects. The oocytes arrested at the GV or GVBD oocyte stage were indicated by arrows. Scale bar, 100 µm. (c) Representative images of spindle morphologies in control and Mettl3 siRNA microinjected oocytes. Oocyte swere stained with α-tubulin antibody to visualize spindle. Scale bar, 20 µm. 45 NC, 45 siRNA 1# and 36 siRNA 3# MII oocytes were analyzed. The percentages of normal spindles and aberrant spindles including short, wide polar and elongated spindles were recorded in NC, siRNA 1# and siRNA 3# oocytes. The experiment was performed three times. *P < 0.05 in a chi-square test. (d) Chromosome spread of control and Mettl3 siRNA microinjected oocytes. Chromosomes were stained with DAPI and kinetochores were labeled with CREST. Scale bar = 5 µm. 33 NC, 21 siRNA 1# and 23 siRNA 3# MII oocytes were analyzed. The percentages of euploid and aneuploidy MII oocytes were recorded in NC, siRNA 1# and siRNA 3# oocytes. The experiment was performed three times. *P < 0.05, **P < 0.01 in a chi-square test.
Knocking down Mettl3 decreases mRNA translation efficiency
Global transcriptional silencing in oocytes occurs in parallel with large-scale chromatin condensation and rearrangement around the nucleus to establish a chromatin configuration termed surrounded nucleolus (SN), versus non-surrounded nucleolus (NSN), NSN type oocytes present high transcriptional level and can synthesize various types of RNA, while SN type oocytes present global transcriptional inhibition [34]. We found that the ratio of GV oocytes after Mettl3 siRNA treatment in the NSN/SN chromatin configuration was not different compared to the control group by Hoechst staining (Fig. S2a, S2b), suggesting that METTL3 knockdown may not affect the process of transcriptional silencing during oocyte maturation. Moreover, according to a previous study, m6A modification has roles in mRNA translation efficiency. To ascertain whether mRNA translation efficiency of the GV oocyte stage was affected by the m6A level being lower, we selected genes which had near-complete depletion of m6A after Mettl3 ablation according to a previous study [18]. We chose a few representative genes including Cltc, Pcnt, Spdl-1 and Msy2, which were reported to be required for spindle formation and chromosome congression during mouse oocyte maturation respectively [35–38]. Then we assayed the translation efficiency of the representative selected genes in siRNA-microinjected GV oocytes by qRT-PCR and western blot, the procedure shown in Figure 3(a). The qRT-PCR results clearly indicated that for every instance, the relative mRNA level was increased after Mettl3 siRNA treatment (Figure 3(b)). Western blot analysis showed that the relative protein abundance of CLTC, PCNT, SPDL-1 and MSY2 in oocytes was lower after knocking down Mettl3 (Figure 3(c and d)). These results suggested that the translation efficiency in siRNA-microinjected GV oocytes was relatively decreased (Figure 3(e)). Additionally, L-homopropargylglycine (HPG), an amino acid analog of methionine that is incorporated into proteins during active protein synthesis, can be used to detect newly formed proteins in mouse oocytes[39]. So we did the HPG incorporation experiment and found that the HPG signal intensity decreased significantly after METTL3 knockdown compared with the control group (Figure 3(f)), suggesting that the overall translation efficiency of maternal mRNA in oocytes was reduced.
Figure 3.

Knocking down Mettl3 in mouse GV oocytes decreases mRNA translation efficiency.
(a) Illustration of siRNA microinjection and oocyte culture in (b, c). NC, Mettl3 siRNA 1# and siRNA 3# fragments were microinjected into mouse full-grown GV oocytes, and then incubated overnight in M2 medium supplemented with 2.5 µM milrinone to maintain meiotic arrest. Then the oocytes were harvested to examine. (b) qRT-PCR analysis of Cltc, Pcnt, Spdl-1 and Msy2 mRNA in Mettl3 siRNA-microinjected oocytes, with normalization to 18S as an internal control. Error bars, mean ± SD. *P < 0.05, **P < 0.01, *** P < 0.001 in an unpaired two-tailed t-test. (c) Representative western blot images show protein expression after Mettl3 siRNA microinjection. Western blot probes with CLTC, PCNT, SPDL-1, MSY2 and ACTIN. Quantitative analysis of CLTC, PCNT, SPDL-1 and MSY2 protein expression in Mettl3 siRNA-microinjected GV oocytes. Error bars, mean ± SD. *P < 0.05, ***P < 0.001 in an unpaired two-tailed t-test. (d) Translation efficiency of Cltc, Pcnt, Spdl-1 and Msy2 in Mettl3 siRNA-microinjected GV oocytes. Calculated as, for example, translation efficiency = CLTC protein level/Cltc mRNA expression level. The CLTC protein level was determined by western blot and normalized to actin. Error bars, mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 in an unpaired two-tailed t-test. (f) Representative staining images of experimental and control GV oocytes. Scale bar, 20 µm. the HPG signal intensity decreased significantly after METTL3 knockdown in GV embryos. Three independent experimental replicates were performed. Error bars, mean ± SD. ***P < 0.001 in an unpaired two-tailed t-test.
Next, eGFP mRNA was constructed by in vitro transcription and fused with siRNAs at the same volume ratio, then the mixture was microinjected into GV oocytes [40], the procedure shown in Fig. S3a. And we detected eGFP levels in GV-arrested oocytes in milrinone-containing M2 medium after 24 h by qRT-PCR. We found that eGFP relative mRNA levels were higher in siRNA-microinjected GV oocytes (Fig. S3b). In addition, eGFP protein signals were lower compared with those of the control group (Fig. S3c, S3d), as well as the reduced level of translation efficiency (Fig. S3e). Thus, we speculated that translation efficiency was relatively reduced after knocking down Mettl3. These data demonstrate that METTL3-mediated m6A is required for mRNA translation efficiency during mammalian oocyte maturation.
Knocking down Mettl3 impedes the maternal-to-zygotic transition and zygotic genome activation
Mammalian oocyte maturation triggers the degradation of thousands of different mRNAs, which leads to a transition from mRNA stability to instability [41,42]. For detection of whether mRNA degradation was affected, we injected eGFP mRNA into MII oocytes which were grown from GV oocytes after siRNA microinjection treatment [43,44], the procedure shown in Fig. S4a. The eGFP mRNA relative levels were higher after culture for 6 h by qRT-PCR (Fig. S4b). Thus, we can speculate that mRNA decay is affected during the MII oocyte stage.
As mammalian embryonic development proceeds, two processes are triggered that together form the maternal-to-zygotic transition (MZT). First, abundant maternal mRNA is degraded. Second, zygotic gene activation of transcription begins [45]. The transition from mRNA stability to instability is a key step in the oocyte-to-zygote transition in mammals [46]. To ascertain whether METTL3-mediated m6A reduction of mRNA degradation is essential for the oocyte-to-zygote transition, we used METTL3 morpholino which can directly knock down the protein expression as a substitute for Mettl3 siRNA to reduce the culture time of GV oocytes in vitro [43]. The knockdown effect and optimum effect time were confirmed by western blot (Fig. S5a). By microinjecting GV oocytes with the METTL3 morpholino, and after maturation, the MII oocytes were activated by SrCl2 and diploidized by treatment with cytochalasin B [47], the procedure was shown in Fig. S5b. We found that nearly half of the two-cell embryos microinjected with METTL3 morpholino could not normally develop into the four-cell stage, while almost all two-cell embryos could develop into the four-cell stage in the controls (Figure 4(a and b)). Global transcription in the two-cell embryos was then assessed by EU incorporation [43,46], and the results demonstrated that knocking down Mettl3 reduced relative global transcription by 45% (Figure 4(c)). A similar decrease was observed when the two-cell embryos were stained with antibodies, Phosphor-pol II Ser2 and Phosphor-pol II Ser5, which were markers for RNA polymerase engaged in transcription (Figure 4(d)).
Figure 4.

METTL3 is maternally required for preimplantation embryonic development.
(a) Mettl3 depletion impaired early embryonic development. METTL3 morpholino-microinjected GV oocytes were cultured, developed and parthenogenetic activated. (METTL3 morpholino, n = 70, three independent experiments). NC indicates negative control. (n = 85, three independent experiments). Error bars, mean ± SD. *P < 0.05 in unpaired two-tailed t-test. (b) Representative images were to show preimplantation embryonic development defects. The embryos arrested at two-cell stage were indicated by arrows. Scale bar, 100 µm. (c) Global transcription in two-cell embryos following Mettl3 knockdown using a morpholino. Representative staining images of experimental and control two-cell embryos. Scale bar, 20 µm. The relative amount of EU was reduced following inhibition of Mettl3 in activated two-cell embryos. Three independent experimental replicates were performed. Error bars, mean ± SD. ***P < 0.001 in an unpaired two-tailed t-test. (d) Representative immunofluorescence of phosphor-polII ser2 and phospho-polII ser5 signals after knocking down Mettl3 by morpholino microinjection. Scale bar, 20 µm. Relative amount of phosphor-polII ser2 and phosphor-polII ser5 were reduced following inhibition of Mettl3 in activated two-cell embryos. Three independent experimental replicates were performed. Error bars, mean ± SD. ***P < 0.001 in an unpaired two-tailed t-test.
For further detection of the effect of METTL3 knockdown on mRNA processing in mouse oocytes, we performed RNA-seq analysis on the MII oocyte stage from METTL3 morpholino-microinjected oocytes and the controls by the procedure shown in Figure 5(a), using a 2.0-fold change in mRNA reads per kb million. Three hundred and twenty-seven genes were upregulated in morpholino-microinjected oocytes compared with the control group (P < 0.05) (Table S1). Gene ontology (GO) analysis of these upregulated revealed several distinct gene clusters (Figure 5(b)). Forty seven of these genes are enriched under the GO term of cell cycle, and thirty two of these genes are involved in negative regulation of transcription from RNA polymerase II promoter. GO analysis suggested the probable reason of affected oocyte-to-zygote transition. We assayed the relative abundance of seven typical selected transcripts in morpholino-microinjected oocytes by qRT-PCR. The relative mRNA level of typical genes was highly, consistent with the results of RNA-seq analysis (Figure 5(c)). Furthermore, the verified genes, Rnf126, Aldh2, Cdc42ep3 and Ube2m are overlapped with genes with near-complete depletion of m6A after Mettl3 ablation [18] and the downregulated transcripts (Abce1, Nlrp4e, Ywhah) were reported to participate in the process of oocyte maturation or maternal to zygote transition previously[48–50]. Then, besides the seven genes, we randomly selected several upregulated genes to detect the relative degradation level from GV oocyte to the MII oocyte stage by qRT-PCR, and we found that they also were degraded during meiotic maturation (Figure 5(d)). Collectively, these results demonstrate that METTL3-mediated m6A affects maternal-to-zygotic transition and zygotic genome activation probably by impeding mRNA degradation.
Figure 5.
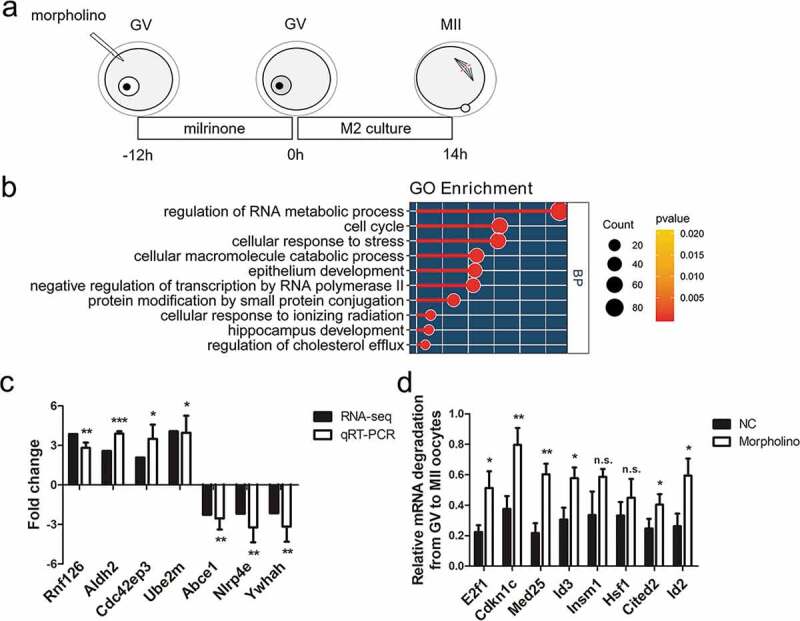
RNA-seq analysis on the MII oocyte from METTL3 morpholino-microinjected GV oocytes.
(a) Illustration of morpholino microinjection and oocyte culture. Morpholino was microinjected into mouse full-grown GV oocytes. After being incubated 12h in M2 medium supplemented with 2.5 µM milrinone, the GV oocytes were collected, the remaining oocytes were washed 5 times to remove the milrinone and then incubated for 14h in M2 medium. The oocytes developed to MII phase were collected. (b) GO analysis for the upregulated genes in METTL3 morpholino-microinjected oocytes compared with the control group; the top ten most significant processes identified are shown. (c) qRT-PCR analysis of genes selected from RNA-seq expression profiles. Rnf126, Aldh2, Cdc42e3p and Ube2m are in the list of upregulated genes. Abce1, Nlrp4e and Ywhah are in the list of downregulated genes. Error bars, mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 in an unpaired two-tailed t-test. (d) The relative mRNA degradation of eight randomly selected upregulated genes in METTL3 morpholino-microinjected were determined from the GV oocyte to the MII oocyte stage by qRT-PCR. The value is calculated as MII oocyte expression/GV oocyte expression. Error bars, mean ± SD. *P < 0.05, **P < 0.01 in an unpaired two-tailed t-test. n.s.: non-significant.
Discussion
In the present study, we showed that METTL3 was expressed in murine oocytes and pre-implantation embryos. To explore the roles of METTL3 in the mouse oocyte maturation process, microinjection of Mettl3-targeting siRNAs or morpholino were used in GV oocytes. Here, we found that knocking down Mettl3 perturbed meiotic progression and disrupted spindle formation and chromosome movement, consequently inducing the high frequency of aneuploidy in oocytes. Oocyte maturation involves the meiotic maturation of germinal vesicles to a metaphase II nucleus and the acquisition of developmental competence [51]. The global transcriptional repression related to chromatin configuration transformation is a crucial developmental transition and essential post-transcriptional event during oocyte maturation [52]. In line with this concept, we observed the chromatin configuration in GV oocytes following Mettl3 knockdown. The results showed no difference in chromatin configuration, indicating no influence of Mettl3 knockdown on oocyte nuclear maturation, which is consistent with the result of the GVBD rate. The SN type oocytes are transcriptionally quiescent and maternal mRNAs are remarkably stable. Knocking down Mettl3 did not alter the chromatin configuration of GV oocytes, suggesting that METTL3 knockdown may not affect the process of transcriptional silencing during oocyte maturation. But we observed an obvious decrease in the proportion of oocytes reaching MII, and then we investigated the cause. We found that some genes regulated by Mettl3 to modulate m6A levels that have been shown to play a role in oocyte cytoplasmic maturation by data mining and previous studies [18,35–38]. We next detected the translation efficiency of a few representative selected genes and found that knocking down Mettl3 led to lower translation efficiency. Besides these endogenous genes, we also evaluated the translation efficiency by injecting exogenous eGFP mRNA. Furthermore, the results of HPG incorporation and eGFP mRNA injection both proved the decrease of translational efficiency in GV oocytes after Mettl3 knockdown. Collectively, in keeping with the understanding of m6A modification, these findings strongly suggest that knocking down Mettl3 reduces the level of m6A modification to lower mRNA translation efficiency, which lead to oocyte maturation defects[53].
Maternal mRNAs are stored in the oocytes before fertilization, after zygotic transcription is initiated, and in the transition from maternal to zygotic control of embryogenesis, there is a massive degradation of maternal mRNAs and full activation of zygotic transcription. In mammals, maternal mRNA begins to degrade during meiotic maturation, after fertilization, but it still remains and is translated to support development until the two-cell stage [54,55]. Since METTL3 plays a role in m6A methylation, we used exogenous eGFP mRNA to detect whether knocking down Mettl3 has an effect on mRNA degradation in the present study. The results clearly showed that eGFP mRNA degradation was affected after knocking down Mettl3. Besides, we found that knocking down Mettl3 increased the rate of two-cell arrest and reduced global transcriptional activity at the two-cell stage, when there was robust embryonic genome activation. Supporting this results, RNA-seq analysis on the MII oocyte stage from METTL3 morpholino microinjected GV oocytes revealed that three hundred and twenty-seven genes were upregulated in the Mettl3 knockdown group compared with the control group. Among the upregulated genes, we selected several genes which transcripts were determined via microarray to be degraded during the transcriptionally silent germinal vesicle stage to the metaphase II stage transition[41]. In addition, m6A levels of these four genes in Mettl3-KO ESCs were decreased sharply[18]. What is more, Abce1, Nlrp4e and Ywhah are in the list of the downregulated genes, which were reported to be necessary for oocyte maturation or maternal to zygote transition respectively [48–50]. The relative mRNA level of these genes between Mettl3 knockdown and control groups was verified by qRT-PCR, maybe helpful to understand the phenotypes and provide emerging evidence suggested that METTL3-mediated m6A has effects on maternal mRNA degradation. We also randomly selected several upregulated genes, E2f1, Cdkn1c, Med25, Id3, Insm1, Hsf1, Cited2 and Id2, to detect the relative degradation level from the GV oocyte to the MII oocyte stage, and we found that they were degraded during meiotic maturation as well. And these randomly selected eight genes are under the GO term of cell cycle and negative regulation of transcription from RNA polymerase II promoter, which further suggested the probable reason of affected oocyte-to-zygote transition. Together, it can be inferred that in addition to these genes, there are more genes regulated by METTL3 involved in the degradation of oocyte maturation, but the previous microarray approach was limited [41]. Therefore, we proposed that METTL3-mediated m6A was required to bridge the interregnum between the maternal and embryonic control of development. Taken together, it is tempting to conclude that the significant role of m6A during oocyte maturation leads to further effects on the maternal-to-zygotic transition and zygotic genome activation.
There is a growing consensus that RNA methylation during development might act as a developmental switch, a powerful means to switch from one programme to another at pivotal developmental transitions [56]. Our findings demonstrate such a role for one m6A writer, METTL3, in oocyte maturation and maternal-to-zygotic transition. In particular, our demonstration that METTL3-mediated m6A effects mRNA efficiency and mRNA degradation during oocyte maturation is a convincing finding that opens exciting avenues of investigation into the role of m6A in regulating oocyte and embryo development. However, the limited amount of RNA that can be isolated from microinjected oocytes excludes the possibility of performing m6A-seq to quantitatively determine if the consensus-containing changed genes in Mettl3 microinjected oocytes are methylated. In conclusion, m6A modification is crucial for the process of mouse oocyte maturation and maternal-to-zygotic transition. This study reveals that METTL3-mediated m6A is required for this process, providing new evidence regarding the control of oocyte maturation and pre-implantation embryonic development processes.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2018YFC1004002 and 2017YFC1001301), National Nature Science Foundation of China (31871505) and Postgraduate Education Reform Project of Jiangsu Province (KYCX181450).
Author contributions
R Huo and W Shu conceived the project. X Sui, S Zhou and M Li designed experiments. X Sui, Y Cao, Y Hu and M Li performed experiments. C Ren analysed data. X Sui, Q Cao Y Hu and R Huo wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Alizadeh Z, Kageyama S, Aoki F.. Degradation of maternal mRNA in mouse embryos: selective degradation of specific mRNAs after fertilization. Mol Reprod Dev. 2005;72:281–290. [DOI] [PubMed] [Google Scholar]
- [2].Li L, Zheng P, Dean J.. Maternal control of early mouse development. Development. 2010;137:859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. [DOI] [PubMed] [Google Scholar]
- [5].Wei CM, Gershowitz A, Moss B. 5ʹ-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976;15(2):397–401. [DOI] [PubMed] [Google Scholar]
- [6].Bokar JA, Shambaugh ME, Polayes D, et al. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. Rna. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- [7].Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schwartz S, Mumbach MR, Jovanovic M, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5ʹ sites. Cell Rep. 2014;8:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu N, Dai Q, Zheng G, et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li A, Chen YS, Ping XL, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsu PJ, Zhu Y, Ma H, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Geula S, Moshitch-Moshkovitz S, Dominissini D, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. [DOI] [PubMed] [Google Scholar]
- [19].Fustin JM, Doi M, Yamaguchi Y, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. [DOI] [PubMed] [Google Scholar]
- [20].Xiao W, Adhikari S, Dahal U, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. [DOI] [PubMed] [Google Scholar]
- [21].Patil DP, Chen CK, Pickering BF, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao X, Yang Y, Sun BF, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Y, Li Y, Toth JI, et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhao BS, Wang X, Beadell AV, et al. m(6)A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature. 2017;542:475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen T, Hao YJ, Zhang Y, et al. m(6)A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16:289–301. [DOI] [PubMed] [Google Scholar]
- [26].Xu K, Yang Y, Feng GH, et al. Mettl3-mediated m(6)A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xia H, Zhong C, Wu X, et al. Mettl3 mutation disrupts gamete maturation and reduces fertility in Zebrafish. Genetics. 2018;208:729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yu G, Wang LG, Han Y, et al. Clusterprofiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yu G, Li F, Qin Y, et al. GOSemSim: an R package for measuring semantic similarity among GO terms and gene products. Bioinformatics. 2010;26:976–978. [DOI] [PubMed] [Google Scholar]
- [30].Ito K, Murphy D. Application of ggplot2 to pharmacometric graphics. CPT Pharmacometrics Syst Pharmacol. 2013;2:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lu Y, Li S, Cui Z, Dai X, Zhang M, Miao Y, Zhou C, Ou X, Xiong B. The cohesion establishment factor esco1 acetylates alpha-tubulin to ensure proper spindle assembly in oocyte meiosis. Nucleic Acids Res. 2018;46:2335-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jones KT, Lane SI . Molecular causes of aneuploidy in mammalian eggs. Development. 2013;140:3719–3730. [DOI] [PubMed] [Google Scholar]
- [33].Howe K, FitzHarris G. Recent insights into spindle function in mammalian oocytes and early embryos. Biol Reprod. 2013;89:71. [DOI] [PubMed] [Google Scholar]
- [34].De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;292:1–12. [DOI] [PubMed] [Google Scholar]
- [35].Ma W, Viveiros MM. Depletion of pericentrin in mouse oocytes disrupts microtubule organizing center function and meiotic spindle organization. Mol Reprod Dev. 2014;81:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Medvedev S, Pan H, Schultz RM. Absence of MSY2 in mouse oocytes perturbs oocyte growth and maturation, RNA stability, and the transcriptome. Biol Reprod. 2011;85:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao J, Wang L, Zhou HX, et al. Clathrin heavy chain 1 is required for spindle assembly and chromosome congression in mouse oocytes. Microsc Microanal. 2013;19:1364–1373. [DOI] [PubMed] [Google Scholar]
- [38].Zhang QH, Wei L, Tong JS, et al. Localization and function of mSpindly during mouse oocyte meiotic maturation. Cell Cycle. 2010;9:2230–2236. [DOI] [PubMed] [Google Scholar]
- [39].Sha QQ, Yu JL, Guo JX, et al. CNOT6L couples the selective degradation of maternal transcripts to meiotic cell cycle progression in mouse oocyte. Embo J. 2018;37:e99333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sha QQ, Dai XX, Dang Y, et al. A MAPK cascade couples maternal mRNA translation and degradation to meiotic cell cycle progression in mouse oocytes. Development. 2017;144:452–463. [DOI] [PubMed] [Google Scholar]
- [41].Su YQ, Sugiura K, Woo Y, et al. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol. 2007;302:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen J, Melton C, Suh N, et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ma J, Flemr M, Strnad H, et al. Maternally recruited DCP1A and DCP2 contribute to messenger RNA degradation during oocyte maturation and genome activation in mouse. Biol Reprod. 2013;88:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Balboula AZ, Blengini CS, Gentilello AS, et al. Maternal RNA regulates Aurora C kinase during mouse oocyte maturation in a translation-independent fashion. Biol Reprod. 2017;96:1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development. 2009;136:3033–3042. [DOI] [PubMed] [Google Scholar]
- [46].Hamatani T, Carter MG, Sharov AA, et al. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6:117–131. [DOI] [PubMed] [Google Scholar]
- [47].Kubiak J, Paldi A, Weber M, et al. Genetically identical parthenogenetic mouse embryos produced by inhibition of the first meiotic cleavage with cytochalasin D. Development. 1991;111:763–769. [DOI] [PubMed] [Google Scholar]
- [48].Jiao XF, Huang CJ, Wu D, Zhang JY, Long YT, Chen F, Li X, Huo LJ. Abce1 orchestrates M-phase entry and cytoskeleton architecture in mouse oocyte. Oncotarget. 2017;8:39012––39020.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chang BH, Liu X, Liu J, Quan FS, Guo ZK, Zhang Y. Developmental expression and possible functional roles of mouse Nlrp4e in preimplantation embryos. In Vitro Cell Dev Biol Anim. 2013;49:548––553.. [DOI] [PubMed] [Google Scholar]
- [50].De S, Kline D. Evidence for the requirement of 14-3-3eta (YWHAH) in meiotic spindle assembly during mouse oocyte maturation. BMC Develop Biol. 2013;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fert Develop. 1996;8:485––489.. [DOI] [PubMed] [Google Scholar]
- [52].Watson AJ. Oocyte cytoplasmic maturation: a key mediator of oocyte and embryo developmental competence. Journal Of Ani Sci. 2007;85:E1––3.. [DOI] [PubMed] [Google Scholar]
- [53].Frye M, Harada BT, Behm MHe C. RNA modifications modulate gene expression during development. Science. 2018;361:1346––1349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schultz RM. Regulation of zygotic gene activation in the mouse. Bioessays : News and Reviews in Molecular, Cellular and Develop Biol. 1993;15:531––538.. [DOI] [PubMed] [Google Scholar]
- [55].Telford NA, Watson Aj Fau - Schultz GA, SchultzGA. Transition from maternal to embryonic control in early mammalian development: a comparison of several species. Mol Reprod Develop. 1990;26(1):90–100. [DOI] [PubMed] [Google Scholar]
- [56].Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Gen Develop. 2015;29:1343––1355.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


