Abstract
Background
To date, no safe allergen-specific immunotherapy for patients with peanut allergy is available. Previous trials were associated with severe side effects.
Objective
We sought to determine the relative importance of conformational and linear IgE-binding epitopes of the major peanut allergen Ara h 2 and to produce a hypoallergenic variant with abolished anaphylactogenic activity.
Methods
Wild-type Ara h 2 and a mutant lacking the loops containing linear IgE epitopes were produced in insect cells. Conformational IgE epitopes were removed by unfolding these proteins through reduction and alkylation. IgE binding was tested by means of ELISA with sera from 48 Ara h 2–sensitized patients with peanut allergy. Basophil activation and T-cell proliferation were tested with blood samples from selected patients. Anaphylactogenic potency was tested by using intraperitoneal challenge of mice sensitized intragastrically to peanut extract.
Results
Patients’ IgE recognized conformational and linear epitopes in a patient-specific manner. The unfolded mutant lacking both types of epitopes displayed significantly lower IgE binding (median ELISA OD, 0.03; interquartile range, 0.01-0.06) than natural Ara h 2 (median ELISA OD, 0.99; interquartile range, 0.90-1.03; P < .01). Basophil activation by unfolded mutant Ara h 2 was low (median area under the curve, 72 vs 138 for native wild-type Ara h 2; P < .05), but its ability to induce T-cell proliferation was retained. Unfolded mutants without conformational epitopes did not induce anaphylaxis in peanut-sensitized mice.
Conclusions
By removing conformational and linear IgE epitopes, a hypoallergenic Ara h 2 mutant with abolished IgE binding and anaphylactogenic potency but retained T-cell activation was generated.
Keywords: Peanut allergy, Ara h 2, epitopes, hypoallergen, mouse model, immunotherapy
The prevalence of food allergies in developed countries is still on the increase. Peanut (Arachis hypogaea)–induced IgE-mediated allergy usually begins early in life and persists throughout life. During the past years, the frequency of peanut allergy has increased steadily.1 Because some patients experience serious adverse reactions after being exposed to even trace amounts of peanut, strict avoidance is the only preventive measure to avoid allergic reactions.2
Ara h 2 is the most important of the 16 peanut allergens accepted by the World Health Organization/International Union of Immunological Societies Allergen Nomenclature Sub-Committee (http://www.allergen.org/). More than 90% of peanut-sensitive patients recognize Ara h 2.3 Ara h 2 belongs to the 2S albumin seed storage protein family. It folds into a compact conformation consisting of 5 α-helices, which are stabilized by 4 disulfide bridges.4 Its core structure is highly resistant to proteolysis.5
There are conflicting data on the contribution of conformational and linear epitopes to binding of Ara h 2–specific IgE. Although most studies focused on the importance of linear IgE epitopes,6–9 several publications suggested that conformational epitopes of Ara h 2 were essential because disruption of its 3-dimensional structure resulted in significantly reduced IgE binding.10–12 Thus far, only one study compared the relative importance of conformational and linear epitopes and found varying contributions to IgE binding among individual patients.13
To date, no immunotherapy for peanut allergy exists.14–16 Sub-cutaneous immunotherapy with peanut extract (PE) failed because of systemic reactions.17 Oral immunotherapy was extensively studied but has recently been shown to lack efficacy.18 Sub-lingual and epicutaneous immunotherapies represent promising approaches, but further studies regarding long-term effectiveness and safety are required.19,20 Ara h 2, the most important peanut allergen, is an essential component of any vaccine for patients with peanut allergy. Therefore hypoallergenic Ara h 2 mutants with removed B-cell but retained T-cell epitopes are candidates for future immunotherapeutics.
Several hypoallergenic Ara h 2 variants have been developed. Van Hoffen and colleagues21,22 removed conformational epitopes by using reduction and alkylation to unfold the allergens, whereas King et al,7 Wood et al,16 and Bannon et al23 modified linear IgE-binding epitopes using site-directed mutagenesis. These mutants still bound Ara h 2–specific IgE, most likely because both conformational and linear epitopes contribute to IgE binding. None of the studies published to date aimed to remove both types of B-cell epitopes while retaining T cell–activating properties.
Hence we decided to analyze IgE binding and the ability to activate basophils and induce T-cell proliferation, as well as the in vivo anaphylactogenic potency of Ara h 2 mutants lacking linear and conformational epitopes. We expressed wild-type Ara h 2 (wtAra h 2), which we then reduced and alkylated (red/alk) to destroy its conformational epitopes. Furthermore, we produced a novel mutant Ara h 2 (mtAra h 2) in which we removed most of the previously identified linear IgE-binding epitopes6,7,13 in the unstructured N- and C-terminal regions and the central loop. The immunodominant T-cell epitopes were retained.24 Reduction and alkylation of mtAra h 2 led to complete loss of IgE binding, which qualifies this hypoallergen as a candidate vaccine component to be used in allergen-specific immunotherapy of patients with peanut allergy.
Methods
Patients
For this study, 48 Ara h 2–sensitized patients with peanut allergy (Austrian children and adults) were recruited. Each patient had a convincing history of peanut allergy, and 43 of 48 had positive skin prick test responses to PE. The presence of Ara h 2–specific IgE was determined by using ImmunoCAP (Thermo Fisher Diagnostics, Uppsala, Sweden). Clinical characteristics of the patients are shown in Table E1 in this article’s Online Repository at www.jacionline.org and summarized in Table I. The study was approved by the Ethics Committee of Lower Austria (GS4-EK-4/242-2013) and conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Table I. Summary of patients’ characteristics.
| No. of patients | 48 |
|---|---|
| Age (y) | |
| Median | 13 |
| Range | 1-47 |
| Male/female sex | 22/26 |
| Symptoms | |
| Oral allergy syndrome | 4 |
| Atopic dermatitis | 3 |
| Angioedema | 1 |
| Gastrointestinal | 1 |
| Anaphylaxis (grade 1) | 21 |
| Anaphylaxis (grade 2) | 14 |
| Anaphylaxis (grade 3) | 4 |
| Anaphylaxis (grade 4) | 1 |
| Skin prick test response to peanut (positive/negative/not done) | 43/0/5 |
| Total IgE (kU/L) | |
| Median | 388 |
| Range | 11-1990 |
| Peanut-specific IgE (kU/L) | |
| Median | 43 |
| Range | 4.1-99.3 |
| Ara h 2–specific IgE (kU/L) | |
| Median | 28 |
| Range | 1.1->100 |
Expression and purification of recombinant wtAra h 2 and mAra h 2
wtAra h 2 and mtAra h 2 were expressed as hexahistidine-tagged soluble proteins in Trichoplusia ni BTI-TN5B1-4 “HighFive” insect cells and purified by using an Ni-NTA resin (Qiagen, Hilden, Germany) under native conditions. Purity was analyzed by using SDS-PAGE and Coomassie Brilliant Blue staining. For experimental details, see the Methods section in this article’s Online Repository at www.jacionline.org.
Preparation of PE and natural Ara h 2
PE was prepared from ground roasted unsalted peanuts. Proteins were extracted overnight at 4°C with 20 mmol/L Tris-HCl (pH 8.5). Particulate matter was removed by means of centrifugation at 12,000 rcf for 30 minutes. Protein concentrations were determined by using the Pierce BCA protein assay (Thermo Fisher Scientific, Rockford, Ill). The Ara h 2 concentration was determined by using an Ara h 2 ELISA kit (Indoor Biotechnologies, Charlottesville, Va). Natural Ara h 2 (nAra h 2) was purified from roasted peanuts, as previously described.25
Reduction, alkylation, and physicochemical characterization of recombinant and natural proteins
nAra h 2, wtAra h 2, and mtAra h 2 underwent reduction and alkylation to destroy conformational IgE epitopes. All native and red/alk proteins were subjected to detailed characterization with respect to their identity, secondary structure, and aggregation. Experimental details are presented in the Methods section in this article’s Online Repository.
IgE ELISA and ELISA inhibition
IgE ELISAs were performed with sera from 48 patients with peanut allergy. Microtiter plates were coated overnight at 4°C with 2 μg/mL of the respective proteins in 50 mmol/L sodium carbonate buffer (pH 9.6). After blocking with TBS-Tween/3% BSA, sera were diluted to 1 kU/L Ara h 2–specific IgE according to ImmunoCAP data and incubated in duplicates overnight at 4°C. Specific IgE was detected by using an alkaline phosphatase-conjugated mouse anti-human IgE mAb (BD PharMingen, San Jose, Calif), followed by color development with p-nitrophenyl phosphate (Sigma FAST; Sigma-Aldrich Merck, Darmstadt, Germany). Plates were read at 405 nm. As negative controls, 3 individual sera from nonallergic and 2 from allergic, non–peanutsensitized donors were used. Values were considered positive if the OD exceeded the mean value of the negative controls by more than 3 SDs.
For inhibition ELISAs, nAra h 2 was immobilized. Inhibition was performed by preincubating sera for 2 hours with 50 μg/mL of the respective proteins before adding them to the plate. Inhibition values were calculated as follows:
Basophil activation test
Heparinized whole blood from 7 patients with peanut allergy and 2 atopic donors without peanut allergy was incubated for 15 minutes at 37°C with 10-fold serial dilutions of allergens (0.01-1000 ng/mL). Basophil activation was measured by using CD63 expression after gating for CCR3 and CD123 (BioLegend, San Diego, Calif) with flow cytometry.26 Data are represented as areas under the dose-response curves with logarithmic x-axes.27
T-cell proliferation in vitro
PBMCs were isolated from the blood of 9 patients with peanut allergy and cultivated with 2.5 μg/mL of the respective proteins. Proliferation was measured through incorporation of tritiated thymidine within 16 hours. Results are expressed as stimulation indices (SIs), which were calculated as the ratio of the amounts of radioactivity in antigen-stimulated PBMCs and unstimulated cells. Data are presented as the relative SI normalized to the SI of nAra h 2–stimulated cells.
For confirming antigen-specific proliferation of T cells, carboxyfluorescein N-succinimidyl ester staining was performed, and expression of cell-surface markers was measured by using flow cytometry. Experimental details are provided in the Methods section in this article’s Online Repository.
Mouse model of peanut-induced anaphylaxis
Female C3H/HeOuJ mice (Jackson Laboratory, Bar Harbor, Me) were housed in the animal facility of the Academic Medical Center (AMC), Amsterdam, The Netherlands, under specific pathogen-free conditions. All experiments were approved by the animal ethics committee of the AMC (approval no. 49AM-1) and performed in compliance with EU Directive 2010/63/EU for animal experiments.
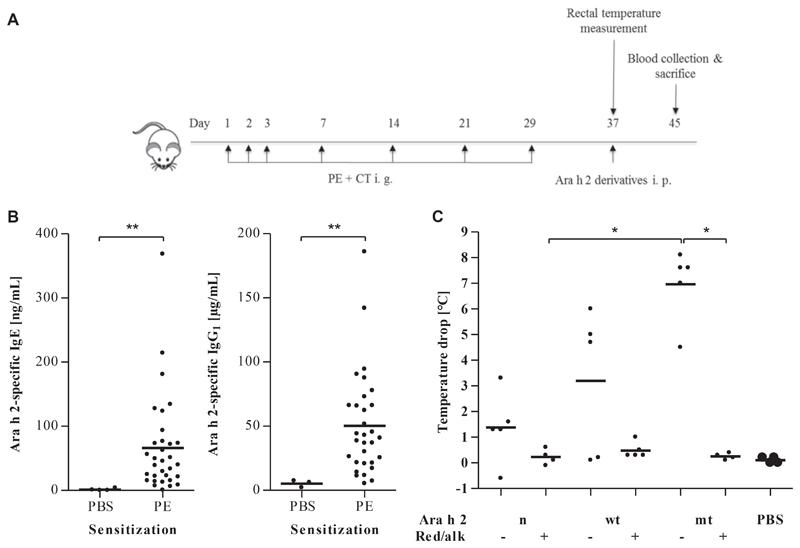
Mice were sensitized intragastrically with 1.8 mg of PE mixed with 15 μg of cholera toxin (List Biological Laboratories, Campbell, Calif) in 300 μL of PBS on days 0, 1, 2, 7, 14, 21, and 29 (7 groups, 4-6 per group). Control mice (n = 4) were treated with PBS alone. On day 37, mice were challenged intraperitoneally with 16.3 μg of one of the 6 Ara h 2 derivatives in 300 μL of PBS or PBS alone. Core body temperature was measured with a rectal probe (Physitemp Instruments, Clifton, NJ) before challenge and every 10 minutes after challenge for 1 hour. Blood samples were taken, and mice were killed on day 45 by means of exsanguination after achievement of anesthesia.
Mouse serum IgE and IgG1
Sera were analyzed for Ara h 2–specific IgE and IgG1 by using quantitative ELISA, as described in the Methods section in this article’s Online Repository.
Statistical analysis
Friedman tests with the Dunn posttest were performed to compare IgE binding, basophil activation, and T-cell activation of different proteins. Statistical analysis of the T-cell proliferation tests was performed by using absolute SI values. The Spearman rank correlation test was used to test correlation between the amount of Ara h 2–specific IgE and the recognition of conformational or linear epitopes. Anaphylactic response to different proteins was compared by using the Kruskal-Wallis test, followed by the Dunn posttest. Differences in Ara h 2–specific IgE levels between the control and PE-sensitized groups of mice were tested by using the Mann-Whitney U test. P values of less than .05 were considered significant. Analyses were performed with GraphPad Prism software (GraphPad Software, La Jolla, Calif).
Results
Design and characterization of Ara h 2 derivatives
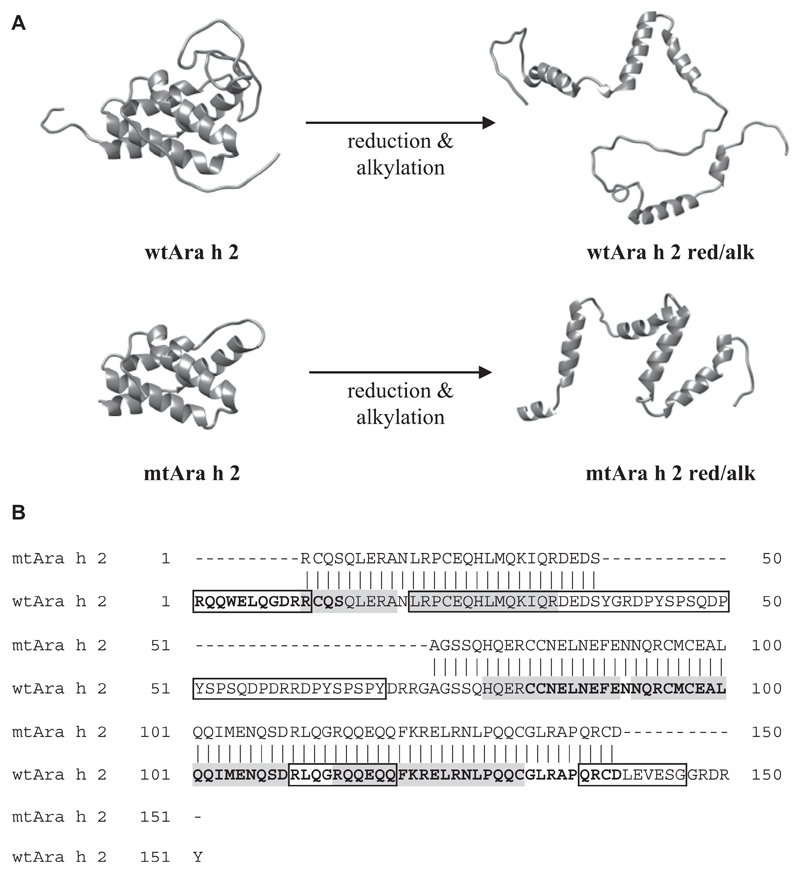
Based on the published literature,6,7,28 we designed mtAra h 2 with most of the linear IgE-binding epitopes removed (Fig 1, A and B). The α-helical core and published T-cell epitopes were left intact.7,24,29–32 To test the importance of conformational epitopes without destroying linear IgE epitopes and T-cell epitopes, nAra h 2, wtAra h 2, and mtAra h 2 were red/alk.
Fig 1.
Construction of Ara h 2 derivatives. A, Structural representations of native and red/alk proteins. B, Amino acid sequences of wtAra h 2 and mtAra h 2 showing the IgE-binding epitopes removed in mtAra h 2. Previously determined linear IgE epitopes are boxed, immunodominant T-cell epitopes are shown in boldface, and helices are shown in gray.
Reduction and alkylation of the proteins was confirmed by using SDS-PAGE under reducing and nonreducing conditions (see Fig E1, A, in this article’s Online Repository at www.jacionline.org). All nonreduced proteins had correct molecular masses (see Fig E1, B, and Table E2 in this article’s Online Repository at www.jacionline.org) and predicted α-helical structures (see Fig E1, C). The red/alk proteins showed CD-spectra characteristic of unstructured proteins, confirming full reduction and alkylation (see Fig E1, C). Dynamic light-scattering analysis demonstrated that nAra h 2 was monomeric (see Fig E1, D). Recombinant wtAra h 2 and mtAra h 2 contained small amounts of dimers (2% and 15%, respectively), whereas red/alk proteins aggregated to a large extent (see Fig E1, D, and Table E3 in this article’s Online Repository at www.jacionline.org).
Modified allergens showed low IgE-binding capacities
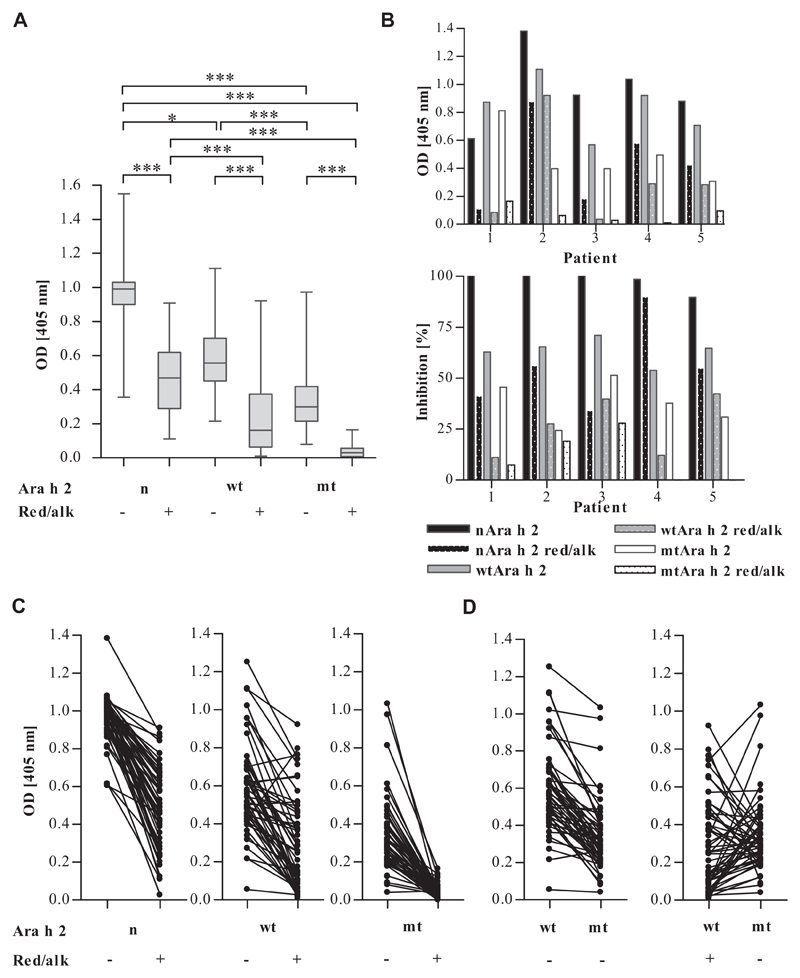
IgE binding to the proteins was tested by using 48 sera from patients with peanut allergy (Table 1 and see Tables E1 and E4 in this article’s Online Repository at www.jacionline.org). Absence of linear IgE epitopes resulted in significantly lower IgE binding to mtAra h 2 than to wtAra h 2 (median OD405nm = 5 0.30 and 0.56, P < .001; Fig 2, A). Removal of conformational epitopes by means of reduction and alkylation significantly diminished binding of IgE of almost all sera (median OD405nm = 0.99 and 0.47 for native and red/alk nAra h 2, 0.56 and 0.16 for native and red/alk wtAra h 2, and 0.30 and 0.03 for native and red/alk mtAra h 2; P < .001; Fig 2, A and C). Red/alk mtAra h 2 with removed conformational and linear epitopes showed the lowest IgE-binding capacity for all sera (P < .001; Fig 2, A) and bound IgE from only 17 sera (35%; see Table E4). Fig 2, B and D, shows heterogeneous patterns of epitope recognition among patients, with 67% and 27% preferentially recognizing conformational and linear epitopes, respectively, whereas 6% recognized conformational and linear epitopes to the same extent. None of the proteins were recognized by IgE from the atopic and nonallergic donors’ sera.
Fig 2.
IgE binding to Ara h 2 derivatives tested by means of ELISA with 48 Ara h 2–sensitized patients with peanut allergy. A, Distributions of ELISA OD values in all patients. *P < .05 and ***P < .001. B, IgE ELISA and inhibition ELISA results of 5 representative patients. For the inhibition ELISA, nAra h 2 was immobilized, and residual IgE binding after preincubating sera with Ara h 2 derivatives was measured. C, Comparison of IgE binding to native and red/alk proteins (lacking conformational epitopes) in individual patients. D, Individual epitope recognition patterns demonstrated by comparing IgE binding to wtAra h 2, mtAra h 2 (lacking linear epitopes), and red/alk wtAra h 2 (lacking conformational epitopes).
Reduced IgE-binding capacities of the modified Ara h 2 derivatives were confirmed by assaying their abilities to inhibit IgE binding to immobilized nAra h 2 (Fig 2, B). Although native mtAra h 2 and red/alk wtAra h 2 showed patient-specific inhibitions of between 11% and 51%, red/alk mtAra h 2 did not inhibit IgE binding to nAra h 2 in 2 sera and showed low inhibitions of 7% to 28% with the other 3 tested sera.
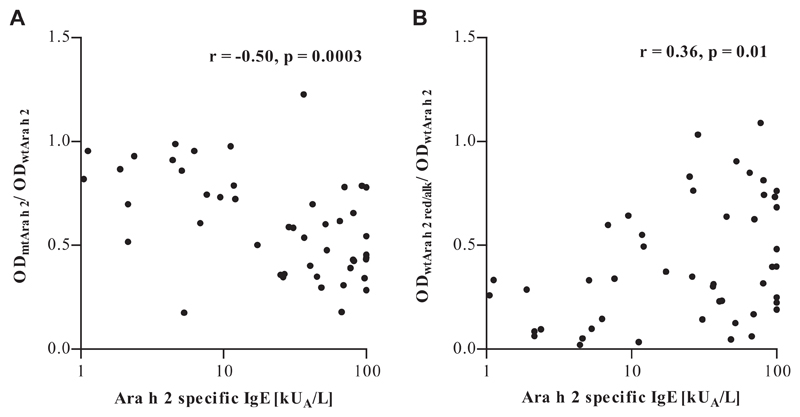
We calculated the ratio of IgE binding to native mtAra h 2 and native wtAra h 2 to assess the contribution of conformational epitopes to Ara h 2–specific IgE binding (Fig 3, A). Likewise, the ratio of IgE binding to red/alk and native wtAra h 2 indicated the contribution of linear epitopes (Fig 3, B). Greater amounts of Ara h 2–specific IgE, as measured by using ImmunoCAP, correlated with increased recognition of linear and decreased recognition of conformational epitopes. Patterns of epitope binding did not correlate with age or symptom severity (data not shown).
Fig 3.
Correlation of Ara h 2–specific IgE levels with measures of epitope recognition patterns derived from IgE ELISA data. A, The mtAra h 2 to wtAra h 2 ratio represents IgE binding to conformational epitopes. B, The red/alk to native wtAra h 2 ratio represents IgE binding to linear epitopes.
Red/alk mtAra h 2 showed reduced basophil activation
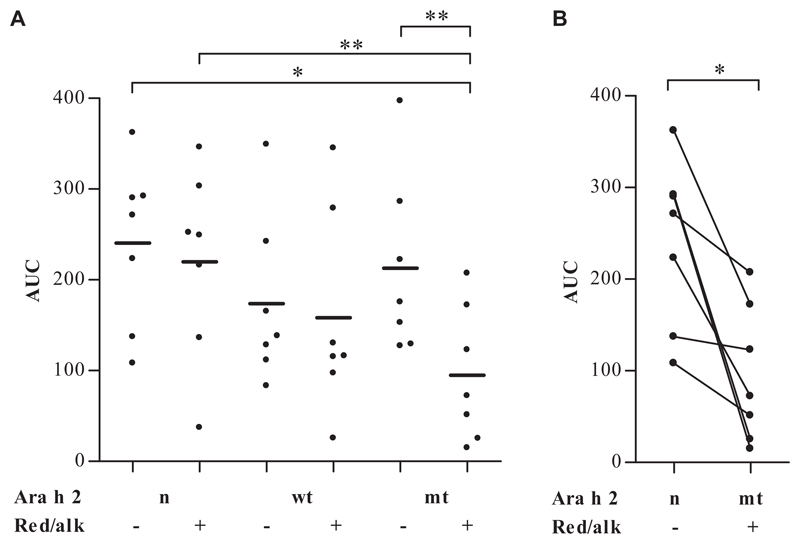
Basophils of 7 patients with peanut allergy were stimulated with Ara h 2 derivatives at concentrations of between 0.01 and 1000 ng/mL. Areas under the curve (AUCs) in Fig 4, A, and Table E5 in this article’s Online Repository at www.jacionline.org demonstrated that all proteins except red/alk mtAra h 2 induced basophil activation to a similar extent (median AUC value range, 116-271). In contrast, red/alk mtAra h 2 (median AUC value, 72) showed a significantly decreased basophil-activating capacity compared with native and red/alk nAra h 2 (P < .05; Fig 4, B), as well as with native mtAra h 2 (P < .01). For 2 of 7 patients, even 1000 ng/mL red/alk mtAra h 2 did not induce basophil activation (patients 3 and 4; see Fig E2 in this article’s Online Repository at www.jacionline.org), whereas for 3 of 7 patients (patients 2, 8, and 11; see Fig E2), the potency of red/alk mtAra h 2 was reduced by at least 100-fold compared with nAra h 2. The abilities of the different proteins to induce basophil activation were heterogeneous in a patient-specific manner (see Fig E2). Basophil activation test results were negative for both atopic control subjects with all 6 proteins (see Fig E3, A, in this article’s Online Repository at www.jacionline.org). Cells of all patients demonstrated basophil activation on stimulation with N-formyl-methionyl-leucyl-phenylalanine and anti-IgE positive controls (see Fig E3, B).
Fig 4.
Basophil activation tests (n = 7) with native and red/alk proteins. Areas under the concentration-dependent activation curves are shown. *P < .05 and **P < .01.
Modified allergens retained their ability to stimulate T-cell proliferation in PBMCs
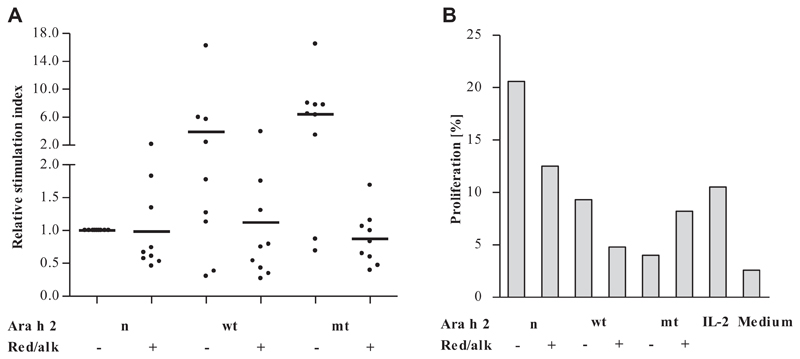
PBMCs from 9 Ara h 2–sensitized patients with peanut allergy were tested in proliferation assays. Fig 5, A, shows that all tested proteins, including red/alk mtAra h 2, were able to induce T-cell proliferation to a similar extent.
Fig 5.
T cell–stimulating abilities of Ara h 2 derivatives. A, In vitro proliferation assays with PBMCs from Ara h 2–sensitized patients with peanut allergy (n = 9). Data are presented as relative SIs normalized to the SIs of cells treated with nAra h 2. B, Flow cytometry–based proliferation assay with PBMCs of a representative patient. Data represent percentages of proliferating (carboxyfluorescein N-succinimidyl ester–low) cells among CD4+ T cells (CD3+CD8− cells).
To confirm that the measured proliferation originated from CD4+ T cells, we performed a flow cytometry–based assay with PBMCs from a representative patient, measuring specifically the proliferation of CD3+CD4+ cells (Fig 5, B). All proteins were able to stimulate CD4+ T cells. nAra h 2 induced proliferation of 21% of CD4+ T cells, whereas stimulation with the modified allergens resulted in rates of proliferation between 4% and 13%.
Conformational epitopes mediated anaphylaxis in mice
PE-sensitized mice were intraperitoneally challenged with the modified proteins or PBS as a control to test the anaphylactogenic potencies of the Ara h 2 variants (Fig 6, A). Significantly increased levels of Ara h 2–specific IgE and IgG1 in PE-sensitized compared with PBS-treated mice confirmed sensitization to Ara h 2 (P < .01; Fig 6, B). Challenge with nAra h 2, wtAra h 2, and mtAra h 2 (decrease in body temperature of 1.5°C–7.0°C after challenge) but not with red/alk protein induced anaphylaxis (Fig 6, C).
Fig 6.
Anaphylactic response in C3H/HeOuJ mice with peanut allergy after challenge with native and red/alk proteins. A, Mice were sensitized intragastrically (i. g.) with PE and challenged intraperitoneally (i. p.) on day 37 with native and red/alk proteins. B, Ara h 2–specific IgE and IgG1 levels of peanut-sensitized mice and control mice sensitized by PBS. **P < .01. C, Maximum core body temperature decrease after intraperitoneal challenge measured over the period of 1 hour every 10 minutes. *P < .05.
Discussion
To date, no safe and efficacious allergen-specific immunotherapy for patients with peanut allergy is available.14,15 Injection-based immunotherapy was evaluated in human subjects but discontinued because of severe side effects.17 Hence other routes of application (oral, sublingual, and epicutaneous) were developed, but these approaches require further studies to prove their safety and long-term efficacy.18–20 Several designs of hypoallergenic Ara h 2 derivatives were tested in vitro and in animals to produce safe vaccine components.7,12,33 These studies aimed to elucidate the contribution of either linear or conformational epitopes of Ara h 2 to IgE binding and effector cell activation. We aimed to abolish IgE binding to linear and conformational epitopes using sequence modifications and chemical treatments, respectively. We found that only the combination of both methods resulted in a hypoallergenic Ara h 2 with no IgE binding (Fig 2) and highly reduced basophil activating capacity (Fig 4) but retained T cell–activating ability (Fig 5). Loss of allergenicity was confirmed in a mouse model of peanut-induced anaphylaxis (Fig 6).
To test Ara h 2–specific epitope recognition patterns, we removed the linear epitopes located in the unstructured loop between helices 2 and 3 and at the N- and C-termini and destroyed conformational epitopes by reducing the disulfide bonds, which stabilize the native structure of the allergen (Fig 1). We studied IgE binding to Ara h 2 and Ara h 2 derivatives in a large group of 48 patients, which included 24 children (age range, 1-12 years) and 24 adolescents and adults (age range, 13-47 years). Our results confirmed that both types of epitopes were important for recognition of Ara h 2 by patients’ IgE (Fig 2).
In agreement with Starkl et al12 and Apostolovic et al,11 we showed that destruction of conformational epitopes by means of reduction and alkylation decreased IgE binding in most patients (Fig 2, C), but unfolded Ara h 2 still bound IgE specific to linear epitopes. Bernard et al13 and King et al7 used removal of the central unstructured loop or site-directed mutagenesis of key residues to destroy linear IgE epitopes. In line with these studies, we observed that our Ara h 2 variant with removed linear IgE epitopes still displayed about 30% IgE-binding capacity compared with nAra h 2 (Fig 2) and also showed anaphylactogenic potency (Fig 6).
In our study we found that the IgE epitope repertoire of patients with peanut allergy was heterogeneous. Two thirds (32/48) of the patients primarily recognized conformational epitopes. The remaining third (16/48) recognized linear epitopes to a similar or greater extent than conformational ones. Heterogeneous epitope recognition was also observed by Starkl et al12 and Bernard et al.13 In our cohort patients with greater amounts of Ara h 2–specific IgE tended to have IgE recognition profiles biased toward linear epitopes (Fig 3). The importance of linear epitopes for Ara h 2 recognition could be the reason why peanut allergy is not outgrown like allergy to egg or milk. Patients with egg or milk allergy who recognize conformational epitopes usually lose their allergy by adulthood.34
Red/alk mtAra h 2 with removed conformational and linear epitopes was recognized by IgE from only 17 (35%) of 48 patients with peanut allergy with very low OD405nm levels close to the values obtained with nonallergic donors’ sera (see Table E4). Basophil activation tests (Fig 4) revealed a significantly decreased basophil-activating capacity for red/alk mtAra h 2 (see Table E5). The area under the dose-response curve has been used successfully as a measure of basophil activation in other studies of peanut allergy27 and shown to be a precise tool to quantify basophil reactivity.35 Determining the effect of the destruction of conformational epitopes has previously yielded conflicting results. Red/alk PE showed a significantly reduced potency in basophil activation with most patients,22 whereas red/alk nAra h 2 displayed no reduction of basophil activation for most patients.12 Moreover, King et al7 showed that an Ara h 2 derivative with mutated key residues of linear epitopes still possessed basophil-activating capacity. The design of our red/alk mtAra h 2 combined both strategies and was administered at concentrations of up to 1000 ng/mL without inducing degranulation of basophils from 2 of 7 patients and showed a potency reduced by at least 100-fold in 3 of 7 patients (see Fig E2). Another important characteristic of a hypoallergen, the ability to induce T-cell proliferation, was present in all of our nonreduced and red/alk allergen derivatives (Fig 5). Despite some patient-specific variability, the stimulating capacities of the Ara h 2 derivatives were similar to those of nAra h 2. In summary, red/alk mtAra h 2 proved to be hypoallergenic for the majority of Ara h 2–sensitized patients with peanut allergy.
In our mouse model of peanut allergy, mice were intragastrically sensitized with PE. On challenge with native wtAra h 2 or mAra h 2, mice underwent anaphylaxis, whereas challenge with the reduced proteins did not induce any change in body temperature (Fig 6, C). The more pronounced reaction to mtAra h 2 compared with wtAra h 2 can be explained by the increased aggregation of the mutant protein (see Fig E1, D) and therefore more efficient cross-linking of mast cell–bound IgE.36 The administered amount of 16.3 μg corresponds to 56 mg in a human weighing 65 kg. This is slightly lower than the maximum maintenance doses used in peanut oral immunotherapy (4000 mg of peanut protein,18 corresponding to 400 mg of Ara h 237) and much greater than allergen doses used in subcutaneous immunotherapy, which are in the microgram range. Our in vivo data confirmed the in vitro results and suggest that red/alk mtAra h 2 will be safe when used as a hypoallergen in immunotherapy. The next step will be to analyze the ability of this protein to desensitize mice with peanut allergy. Nevertheless, the successful application of a vaccine in a mouse model will not always translate to a similar efficacy and safety in human subjects, as exemplified by a rectally administered vaccine containing a mixture of Escherichia coli–encapsulated modified peanut allergens.16,38
In conclusion, we showed that our large group of patients recognized linear and conformational epitopes of Ara h 2 in a patient-specific manner. The most important outcome of this study is the successful generation of a novel hypoallergenic Ara h 2, which is a promising template for the further development of a vaccine for specific immunotherapy because of its abolished anaphylactogenic potency.
Key messages.
Ara h 2–specific IGE from patients with peanut allergy recognizes both conformational and linear epitopes.
The contribution of linear and conformational epitopes to IgE binding of Ara h 2 is patient specific.
A hypoallergenic mutant with abolished anaphylactogenic potency was developed.
Acknowledgments
Supported by the Austrian Science Fund (FWF; Doctoral Program W1248-B30 [MCCA] and grant P 30936-B30), the Medical University of Vienna, and the European Union (Horizon 2020 COST Action FA1402 [ImpARAS]).
Abbreviations used
- AUC
Area under the curve
- mtAra h 2
Mutant Ara h 2
- nAra h 2
Natural Ara h 2
- PE
Peanut extract
- red/alk
Reduced and alkylated
- SI
Stimulation index
- wtAra h 2
Wild-type Ara h 2
Footnotes
Disclosure of potential conflict of interest: R. van Ree has consultant agreements with HAL Allergy BV and Citeq BV and receives speaker’s fees from HAL Allergy BV and Thermo Fisher Scientific. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35:45–59. doi: 10.1016/j.iac.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker S. New medications in the treatment of peanut allergy. Hosp Pharm. 2018;53:369–70. doi: 10.1177/0018578718797266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuang Y, Dreskin SC. Redefining the major peanut allergens. Immunol Res. 2013;55:125–34. doi: 10.1007/s12026-012-8355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palladino C, Breiteneder H. Peanut allergens. Mol Immunol. 2018;100:58–70. doi: 10.1016/j.molimm.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann K, Schweimer K, Reese G, Randow S, Suhr M, Becker WM, et al. Structure and stability of 2S albumin-type peanut allergens: implications for the severity of peanut allergic reactions. Biochem J. 2006;395:463–72. doi: 10.1042/BJ20051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997;342:244–53. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- 7.King N, Helm R, Stanley JS, Vieths S, Luttkopf D, Hatahet L, et al. Allergenic characteristics of a modified peanut allergen. Mol Nutr Food Res. 2005;49:963–71. doi: 10.1002/mnfr.200500073. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Bruni FM, Fu Z, Maloney J, Bardina L, Boner AL, et al. A bioinformatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129:1321–8.e5. doi: 10.1016/j.jaci.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otsu K, Guo R, Dreskin SC. Epitope analysis of Ara h 2 and Ara h 6: characteristic patterns of IgE-binding fingerprints among individuals with similar clinical histories. Clin Exp Allergy. 2015;45:471–84. doi: 10.1111/cea.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht M, Kuhne Y, Ballmer-Weber BK, Becker WM, Holzhauser T, Lauer I, et al. Relevance of IgE binding to short peptides for the allergenic activity of food allergens. J Allergy Clin Immunol. 2009;124:328–36.e1-6. doi: 10.1016/j.jaci.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Apostolovic D, Luykx D, Warmenhoven H, Verbart D, Stanic-Vucinic D, de Jong GA, et al. Reduction and alkylation of peanut allergen isoforms Ara h 2 and Ara h 6; characterization of intermediate- and end products. Biochim Biophys Acta. 2013;1834:2832–42. doi: 10.1016/j.bbapap.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Starkl P, Felix F, Krishnamurthy D, Stremnitzer C, Roth-Walter F, Prickett SR, et al. An unfolded variant of the major peanut allergen Ara h 2 with decreased anaphylactic potential. Clin Exp Allergy. 2012;42:1801–12. doi: 10.1111/cea.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard H, Guillon B, Drumare MF, Paty E, Dreskin SC, Wal JM, et al. Allergenicity of peanut component Ara h 2: contribution of conformational versus linear hydroxyproline-containing epitopes. J Allergy Clin Immunol. 2015;135:1267–74.e1-8. doi: 10.1016/j.jaci.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–23. doi: 10.1016/j.jaci.2013.12.1040. [DOI] [PubMed] [Google Scholar]
- 15.Tscheppe A, Breiteneder H. Recombinant allergens in structural biology, diagnosis, and immunotherapy. Int Arch Allergy Immunol. 2017;172:187–202. doi: 10.1159/000464104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood RA, Sicherer SH, Burks AW, Grishin A, Henning AK, Lindblad R, et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013;68:803–8. doi: 10.1111/all.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–51. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 18.Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393:2222–32. doi: 10.1016/S0140-6736(19)30420-9. [DOI] [PubMed] [Google Scholar]
- 19.Fleischer DM, Greenhawt M, Sussman G, Begin P, Nowak-Wegrzyn A, Petroni D, et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA. 2019;321:946–55. doi: 10.1001/jama.2019.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73:799–815. doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]
- 21.van Hoffen E, van der Kleij HP, den Hartog Jager CF, van Doorn WA, Knol EF, Opstelten DJ, et al. Chemical modification of peanut conglutin reduces IgE reactivity but not T cell reactivity in peanut-allergic patients. Clin Exp Allergy. 2014;44:1558–66. doi: 10.1111/cea.12319. [DOI] [PubMed] [Google Scholar]
- 22.van der Kleij HPM, Warmenhoven HJM, van Ree R, Versteeg SA, Pieters RHH, Dreskin SC, et al. Chemically modified peanut extract shows increased safety while maintaining immunogenicity. Allergy. 2019;74:985–95. doi: 10.1111/all.13687. [DOI] [PubMed] [Google Scholar]
- 23.Bannon GA, Cockrell G, Connaughton C, West CM, Helm R, Stanley JS, et al. Engineering, characterization and in vitro efficacy of the major peanut allergens for use in immunotherapy. Int Arch Allergy Immunol. 2001;124:70–2. doi: 10.1159/000053672. [DOI] [PubMed] [Google Scholar]
- 24.Prickett SR, Voskamp AL, Dacumos-Hill A, Symons K, Rolland JM, O’Hehir RE. Ara h 2 peptides containing dominant CD4+ T-cell epitopes: candidates for a peanut allergy therapeutic. J Allergy Clin Immunol. 2011;127:608–15.e1-5. doi: 10.1016/j.jaci.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Palladino C, Narzt MS, Bublin M, Schreiner M, Humeniuk P, Gschwandtner M, et al. Peanut lipids display potential adjuvanticity by triggering a pro-inflammatory response in human keratinocytes. Allergy. 2018;73:1746–9. doi: 10.1111/all.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–38. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 27.Santos AF, Du Toit G, Douiri A, Radulovic S, Stephens A, Turcanu V, et al. Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J Allergy Clin Immunol. 2015;135:179–86. doi: 10.1016/j.jaci.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller GA, Gosavi RA, Pomes A, Wunschmann S, Moon AF, London RE, et al. Ara h 2: crystal structure and IgE binding distinguish two subpopulations of peanut allergic patients by epitope diversity. Allergy. 2011;66:878–85. doi: 10.1111/j.1398-9995.2010.02532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascal M, Konstantinou GN, Masilamani M, Lieberman J, Sampson HA. In silico prediction of Ara h 2 T cell epitopes in peanut-allergic children. Clin Exp Allergy. 2013;43:116–27. doi: 10.1111/cea.12014. [DOI] [PubMed] [Google Scholar]
- 30.Glaspole IN, de Leon MP, Rolland JM, O’Hehir RE. Characterization of the T-cell epitopes of a major peanut allergen, Ara h 2. Allergy. 2005;60:35–40. doi: 10.1111/j.1398-9995.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 31.Birrueta G, Tripple V, Pham J, Manohar M, James EA, Kwok WW, et al. Peanut-specific T cell responses in patients with different clinical reactivity. PLoS One. 2018;13:e0204620. doi: 10.1371/journal.pone.0204620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renand A, Farrington M, Whalen E, Wambre E, Bajzik V, Chinthrajah S, et al. Heterogeneity of Ara h Component-Specific CD4 T Cell Responses in Peanut-Allergic Subjects. Front Immunol. 2018;9:1408. doi: 10.3389/fimmu.2018.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003;170:3289–95. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- 34.Jarvinen KM, Beyer K, Vila L, Chatchatee P, Busse PJ, Sampson HA. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J Allergy Clin Immunol. 2002;110:293–7. doi: 10.1067/mai.2002.126080. [DOI] [PubMed] [Google Scholar]
- 35.Patil SU, Shreffler WG. Immunology in the Clinic Review Series; focus on allergies: basophils as biomarkers for assessing immune modulation. Clin Exp Immunol. 2012;167:59–66. doi: 10.1111/j.1365-2249.2011.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahajan A, Youssef LA, Cleyrat C, Grattan R, Lucero SR, Mattison CP, et al. Allergen valency, dose, and fcepsilonri occupancy set thresholds for secretory responses to pen a 1 and motivate design of hypoallergens. J Immunol. 2017;198:1034–46. doi: 10.4049/jimmunol.1601334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koppelman SJ, Vlooswijk RA, Knippels LM, Hessing M, Knol EF, van Reijsen FC, et al. Quantification of major peanut allergens Ara h 1 and Ara h 2 in the peanut varieties Runner, Spanish, Virginia, and Valencia, bred in different parts of the world. Allergy. 2001;56:132–7. doi: 10.1034/j.1398-9995.2001.056002132.x. [DOI] [PubMed] [Google Scholar]
- 38.Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112:159–67. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]