Abstract
Sarcopenia, the age-related loss of skeletal muscle mass and function, increases the risk of developing chronic diseases in older individuals and is a strong predictor of disability and death. Because of the ongoing demographic transition, age-related muscle weakness is responsible for an alarming and increasing contribution to health care costs in Western countries. Exercise-based interventions are most successful in preventing the decline in skeletal muscle mass and in preserving or ameliorating functional capacities with increasing age. However, other treatment options are still scarce. In this review, we explore currently applied nutritional and pharmacological approaches to mitigate age-related muscle wasting, and discuss potential future therapeutic avenues.
Introduction
Sarcopenia is a geriatric syndrome characterized by the loss of skeletal muscle mass and function that develops gradually during aging [1]. Data from cross-sectional studies indicate that muscle mass and strength reach their peak values between the second and the fourth decade of life and start to decline continuously from between the third and fifth decade [1]. Besides neurodegenerative events and mental decline, sarcopenia is the main cause for loss-of-independence, the inability to perform daily tasks and social interactions, frailty and falls, admission to nursing homes, and thus reduced overall quality of life, morbidity and mortality. Muscle aging involves complex qualitative and quantitative changes in the neuromuscular system [2]. However, the etiology of sarcopenia is still poorly understood and it is unknown to what extent the development is an inevitable consequence of aging and/or the result of a combination of additional factors including a decrease in physical activity, nutrient deficiencies and genetic predisposition. Age-related co-morbidities such as cardiovascular diseases, obesity and type 2 diabetes, the decline of hormones [e.g. estrogens, androgens and growth hormone (GH)] as well as age-associated chronic, systemic low-grade inflammation likely also contribute to the development of sarcopenia and may account for inter-individual variations in the age of onset and slope of progression.
Due to the demographic transition, the world’s geriatric population is continuously expanding, resulting in an ever-increasing number of sarcopenic patients, tightly linked to enormous personal, social and financial burdens. To date, exercise and an active life-style are the most effective interventions for preventing the decline in skeletal muscle mass and preserving or even ameliorating functional capacities with increasing age. In fact, a recent systematic meta-analysis of muscle morphology and performance in master athletes suggests that exercise training preserves physical function, muscular strength and body fat levels with increasing age similar to that of young, healthy individuals [3]. However, to overcome issues with compliance, adherence, and, in the case of comorbidities, exercise intolerance, other approaches would be of high clinical relevance. Unfortunately, efficient pharmacological avenues are still lacking. In this review, we discuss applied and emerging nutritional and pharmacological strategies for the treatment and prevention of sarcopenia with regard to benefits, limitations and open questions.
Age-associated anabolic resistance
In general, skeletal muscle mass is determined by the balance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB) controlled by an overarching process called proteostasis. Thus, loss of muscle mass often is the result of a negative net protein balance arising from a reduction in MPS and/or increase in MPB. Dietary protein and exercise are two independent stimuli for MPS and act synergistically leading to a net increase of skeletal muscle mass when combined [4]. Aging is associated with an attenuated response of MPS to both, protein ingestion [5] and exercise [6], a phenomenon known as anabolic resistance. Thus, analogous to the impaired sensitivity to insulin in insulin-resistant patients (e.g. in type 2 diabetes), skeletal muscle becomes less sensitive to essential amino acids and/or training stimuli. A decline in muscular activity, chronic, systemic low-grade inflammation (including that of skeletal muscle) as well as impaired digestion and/or absorption of dietary protein are hypothesized to contribute to this reduced sensitivity. Therefore, exercise, anti-inflammatory interventions and specific dietary modifications may help to overcome age-related anabolic resistance.
Nutritional Strategies
For a variety of reasons, under- and malnutrition are often observed in elderly individuals, leading to inadequate caloric and/or deficient marco- and micronutrient intake [7, 8]. Accordingly, dietary interventions are of high interest to mitigate sarcopenia, even though only limited and inconsistent evidence exists, primarily based on cross-sectional studies [9]. Nevertheless, several micro- and macronutrients, including protein, essential amino acids, omega-3 (n-3) polyunsaturated fatty acids (PUFAs) as well as caloric restriction (CR) as dietary intervention have been suggested to exert beneficial effects on sarcopenia and therefore, are discussed in the following sections.
Protein and amino acids
Besides exercise, protein intake provides the main anabolic stimulus to skeletal muscle. Upon protein ingestion, amino acid levels rise in the blood (hyperaminoacidemia), stimulate MPS and suppress MPB. Basal (i.e. unstimulated) levels of MPS and MPB often do not change with increasing age [10, 11]. In contrast, there is emerging evidence that older individuals show a compromised stimulation of MPS (and maybe also a reduced inhibition of MPB) in response to protein ingestion [5], which may be further exacerbated by physical inactivity and short periods of bedrest [12]. However, this blunted response to dietary protein can partially be overcome with higher amounts of protein per single meal [5]. In particular, protein quality i.e. the content of the amino acid leucine appears to be crucial [13, 14]. Of all amino acids, leucine and its metabolite β-hydroxy-β-methylbutyrate (HMB) [15] have the most potent ability to activate the mammalian target of rapamycin complex 1 (mTORC1), which subsequently phosphorylates the downstream signaling targets ribosomal protein S6 kinase 1 (S6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) that facilitate translation initiation and stimulation of MPS (Figure 1) [16]. The effect of leucine can further be potentiated by co-ingestion of other branched-chain amino acids or the whole spectrum of essential amino acids [17]. Of note, Katsanos et al. [14] observed that the leucine content in a mixture of essential amino acids needed to be higher in older compared to younger men (1.7 g vs. 2.8 g) to stimulate MPS. Therefore, it has been proposed that older individuals may benefit not only from eating higher amounts of protein, but also from using high quality (i.e. leucine rich) sources and optimally, evenly distribute the intake throughout the day to robustly stimulate MPS in a sustained manner [18]. However, 6% of men and 12% of women aged 70 years or above do not meet the United States’ Estimated Average Requirement (EAR) and Recommended Dietary Allowance (RDA) for protein [18]. Moreover, since the current EAR and RDA for protein intake in elderly adults might be too low [18], the number of people getting insufficient amounts of protein may be underestimated. Nevertheless, the general recommendation of a higher protein intake for elderly adults as an antisarcopenic stimulus is still debated. Recent observational studies indicate that higher protein intake is associated with better preservation of muscle mass and function with aging [18] and results from short-term intervention studies suggest that improving dietary protein quality and quantity may partially reduce the negative effects of short-term physical inactivity on muscle mass [19, 20] and function [19–21]. On the other hand, clinical trials so far have failed to demonstrate the effectiveness of protein supplementation to promote muscle mass and function in elderly adults [22–24] and only a few long-term longitudinal trials assessing the potential protective effect of higher protein intake on muscle mass and function with aging exist [25]. Furthermore, results on the enhancing effect of protein supplementation on resistance training-mediated improvements in muscle mass and strength of older adults have been conflicting [26]. Thus, more long-term intervention trials will have to demonstrate the efficacy of protein intake at different levels and qualities to mediate benefits to the aging population.
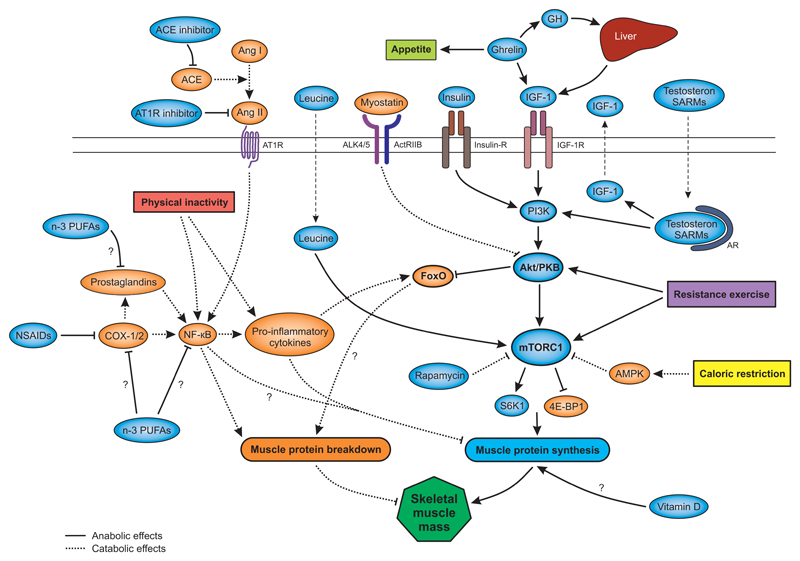
Figure 1.
Schematic overview on the nutritional and pharmacological targets and the related signaling pathways to control muscle protein biosynthesis (marked in light blue) and breakdown (marked in orange) in sarcopenia. Abbreviations: ACE, angiotensin-converting enzyme; ActRIIB, activin type IIB receptor; ALK4/5, activin receptor-like kinase 4 or 5; AMPK, AMP-dependent protein kinase, Ang I/II, angiotensin I or II; AT1R, angiotensin receptor type 1; AR, androgen receptor; COX-1/2, cyclooxygenase 1 or 2; FoxO, forhkead box O; GH, growth hormone; IGF-1, insulin-like growth factor 1; Insulin-R, insulin receptor; IGF-1R, IGF-1 receptor; mTORC1, mammalian target of rapamycin complex 1; NF-ҡB, nuclear factor ҡB; n-3 PUFAs, omega-3 poly unsaturated fatty acids; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; SARMs, selective androgen receptor modulators; S6K1, ribosomal protein S6 kinase 1; 4E-BP1, eukaryotic translation initiation factor 4E-binding protein 1.
Omega-3 polyunsaturated fatty acids
N-3 PUFAs modulate the biophysical properties of cell membranes and are involved in lipid signaling processes. The n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) exhibit anti-inflammatory properties [27] and are frequently consumed as fish oil supplements. Besides potential effects on chronic, systemic inflammation, n-3 PUFAs may also sensitize skeletal muscle to the anabolic stimuli of resistance exercise and dietary amino acids [28]. In an 8-week supplementation trial, n-3 PUFAs potentiated MPS and increased mTORC1-p70S6K1 phosphorylation in response to hyperaminoacidemic-hyperinsulinemic clamp in older adults [28]. Sedentary elderly men and women (60-85 years old) participating in a randomized controlled trial of 6-month fish oil-derived n-3 PUFA supplementation further functionally benefited from improved average isokinetic power, thigh muscle volume, handgrip strength, and one-repetition maximum [29]. However, despite these positive reports, the exact effects of n-3 PUFA supplementation on skeletal muscle of older individuals remain to be determined. In particular, the time course of EPA and DHA integration in muscle cell walls, as well as the exact dosing have to be evaluated [30]. For example, 3 months of daily supplementation with 1.3 g of n-3 PUFAs was not affecting muscle strength and physical function [31], whereas a similar 6-month treatment was sufficient [29]. Moreover, in a recent trial, a combination of 3 g daily fish oil with exercise training for 3 months enhanced exercise-induced gains in isometric torque and muscle quality (strength per unit muscle area) in female participants but, curiously, not in males [32].
In summary, there is increasing evidence that n-3 PUFAs can act as anabolic enhancers on skeletal muscle of elderly people. The effects of n-3 PUFAs have mainly been attributed to their anti-inflammatory properties, but the fact that n-3 PUFA supplementation regimens seem to have similar anabolic effects also in younger adults [33, 34] may indicate the presence of a more direct effect on skeletal muscle. Thus, further studies are required to elucidate the underlying mechanisms and to prove the efficacy of fish oil supplementation as sarcopenia treatment.
Caloric restriction
CR increases lifespan in several species ranging from yeast to mammals and potentially even primates [35]. CR dampens mTORC1 activity [36] based on the reduced abundance of nutrients and insulin, as well as from the activation of the potent mTORC1 inhibitor AMP-dependent protein kinase (AMPK). Metformin, a putative AMPK activator, is currently being tested for sarcopenia [37]. In general though, CR as an anti-sarcopenic intervention seems counterintuitive, since activation of mTORC1 is linked to increased MPS and hypertrophy. However, mTORC1 activity might be pathologically elevated in old muscle, at least in mice [38] and one human cohort [11]. As chronic activation of mTORC1 signaling has been associated with muscle wasting [39], sarcopenic elderly could thus benefit from CR interventions aimed at inhibiting mTORC1 activity. Importantly however, inhibiting mTORC1 signaling with rapamycin also blocks essential amino acid induced stimulation of MPS [40], prevents or blunts the increase of MPS and/or muscle mass following resistance exercise in both rodents [41] and humans [42], but not basal, post-absorptive protein metabolism [43]. The use of dietary or pharmacological means to inhibit mTORC1 activity in old muscle thus remains to be explored. CR however elicits other, non-mTORC1-related potential benefits for sarcopenic patients, e.g. the ability to reduce chronic, systemic low-grade inflammation, as demonstrated in an aging rat model [44]. Furthermore, CR-mediated stimulation of mitochondrial function and oxidative metabolism could contribute to therapeutic effects [45], potentially mediated by sirtuins and the peroxisome proliferator activated receptor γ coactivator 1α (PGC-1α) [46]. Finally, the CR-associated reduction in obesity and other comorbidities such as cardiovascular pathologies and type 2 diabetes could further alleviate the health of elderly individuals.
Anti-inflammatory drugs
A persistent, systemic low-grade inflammation is highly associated with a number of chronic diseases [47, 48]. In skeletal muscle, inflammatory cytokines activate molecular pathways involved in muscle mass regulation, leading to an imbalance in anabolic and catabolic processes [49]. Aging has also been associated with increased levels of circulating pro-inflammatory markers [50, 51], which negatively correlated with muscle mass and strength in elderly adults [52]. In fact, this state of chronic, systemic low-grade inflammation has been termed “inflammaging” [53]. Non-steroidal anti-inflammatory drugs (NSAIDs) reduce systemic low-grade inflammation at least in part by inhibiting cyclooxygenases (COX-1 and COX-2), the key enzymes of leukotriene and prostaglandin synthesis, mediators of inflammation and pain. Long-term treatment of aged rats with the non-selective COX inhibitor ibuprofen improved inflammatory markers, preserved the anabolic response to food intake and increased muscle mass [54]. Notably however, concomitant administration of ibuprofen with exercise in young rodents impaired skeletal muscle regeneration [55], whereas the potent COX-1-selective NSAID indomethacin boosted inflammatory processes in skeletal muscle and brain [56]. Similarly, high doses of NSAIDs compromise muscle strength and hypertrophic adaptations to resistance exercise in young individuals [57]. In contrast, resistance training combined with daily ibuprofen consumption did either not affect [58, 59] or even increased muscle mass [60] and strength [61]in elderly populations. These findings indicate that the usage of NSAIDs in sarcopenia remains to be explored, but may potentially be relevant in later stages of sarcopenia or in conditions with inflammatory comorbidities [63].
Inhibitors of the renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone (RAA) regulatory circuit controls blood pressure and electrolyte balance. The enzyme renin converts angiotensinogen to angiotensin I, which in turn is cleaved by the angiotensin-converting enzyme (ACE) to the active hormone angiotensin II. The effects of RAA activity on skeletal muscle are significant, but yet still poorly understood. For example, reduced expression of ACE has been associated with a greater anabolic response to training [64]. Inversely, infusion of angiotensin II increases proteolysis and decreases local and systemic insulin-like growth factor 1 (IGF-1) levels [65]. Muscle-specific IGF-1 expression prevents angiotensin II-induced skeletal muscle wasting [66]. Besides their potent effects on cardiovascular and endothelial function, inhibitors of the RAA system could have anti-inflammatory effects by decreasing angiotensin II-induced NF-ҡB (nuclear factor ҡB) activation, thereby blocking the production of interleukin 6 (IL-6) and C-reactive protein (CRP) [63]. This effect might complement the improvements in cardiac output, hemodynamic parameters and endothelial function that positively affect muscle metabolism and function [67]. For example, in a cohort of 641 aging women with hypertension, the use of ACE inhibitors was associated with a lower decline in muscle strength and walking speed over a three-year period [68]. The ACE inhibitor perindopril improved six-minute walking distance in a double-blind randomized controlled trial with 130 geriatric patients [69]. In contrast, the ACE inhibitor fosinopril had no significant effect on the inflammatory profile (CRP, IL-6 and PAI-1 levels) [70] or physical performance [71] of adults with elevated cardiovascular risk. Discrepancies in the outcome might be explained by differences in inflammatory profiles of the participants at baseline [63]. In old mice, the angiotensin receptor type 1 (AT1R) inhibitor losartan prevented immobilization-induced muscle loss [72], improved measures of physical function and decreased IL-6 levels [73]. Whether losartan has similar effects in humans is currently investigated in a clinical trial with older adults [49].
Myostatin pathway and activin type II receptor function
Myostatin and its downstream signaling pathways are strong negative regulators of muscle mass. It is unclear whether myostatin and related factors, e.g. GDF11, contribute to the disease etiology of sarcopenia [74, 75]. Blocking myostatin signaling might nevertheless be a promising strategy to increase muscle mass in many wasting contexts, including sarcopenia [49]. In old mice, myostatin knockout results in muscle fiber hypertrophy, increased activation of satellite cells in vitro and improvements of muscle regeneration [76, 77]. Disappointingly though, neutralization of the myostatin protein with humanized myostatin antibodies (LY2495655 and REGN1033) only slightly increased appendicular lean body mass and gait speed in sarcopenic elderly, and did not ameliorate grip strength despite the achieved gains in muscle mass [78, 79]. Since the myostatin receptor complex (activin type IIB receptor and activin receptor-like kinase 4 or 5) is activated by multiple ligands, blockage of receptor function might be a more promising approach [80]. Different strategies to reduce activity of this receptor are currently being pursued and clinical trials with myostatin pathway inhibitors are in progress, including in sarcopenic patients [49].
Testosterone and selective androgen receptor modulators
Cross-sectional and longitudinal studies in aging men indicate that testosterone levels gradually decrease between the third and the ninth decade of life, with high inter-individual variability [81–84]. This aging-associated reduction in androgen production and/or bioavailability is thought to contribute to muscular atrophy and the development of sarcopenia. Testosterone treatment stimulates muscle protein synthesis and induces muscle fiber hypertrophy in a dose-dependent manner [85] and, therefore, testosterone replacement therapy has been used as treatment strategy for sarcopenia. For example, in hypogonadal pre-frail and frail elderly men, daily dermal testosterone gel application for six months increased serum testosterone levels and preserved muscle thickness [86]. Testosterone also improves muscle strength in women [87]. Even though the effects of testosterone on the balance between MPS and MPB are thought to be predominately mediated by its binding to the androgen receptor (AR) thereby inducing IGF-I transcription and/or interacting with phosphoinositide 3-kinase (PI3K) to activate the PI3K-Akt/protein kinase B (PKB)-mTORC1 pathway, the exact mechanism of testosterone action in skeletal muscle has not been fully elucidated so far [88]. Moreover, despite promising effects on muscle mass [89] and in some cases also force [90], improved muscle function was not achieved in all trials. Importantly, due to the high number of serious adverse effects (e.g. increased risk of prostate cancer and cardiovascular events, erythrocytosis, behavioral abnormalities, and virilization in women) [91–93], androgen replacement therapies are non-viable treatment options for general prescription if applied in a non-substituting manner. To circumvent the problem of adverse effects that are mostly linked to androgenic and/or non-skeletal muscle tissue-linked effects of androgens, selective androgen receptor modulators (SARMs) that retain anabolic, but lose androgenic properties might be a safer treatment option, even if skeletal muscle-specificity cannot be achieved. For example, the SARM enbosarm elicits a dose-dependent increase in total lean body mass as well as improvements in physical function in both older male and female participants, importantly without an increased risk of adverse effects [94]. Treatment with MK-0773, another SARM, increased lean body mass of sarcopenic elderly women but had no effect on muscle strength [95]. There was no evidence for androgenization induced by MK-0773, but several participants in the treatment group showed elevated transaminase levels. Different SARMS are currently being evaluated in clinical trials [49].
Growth hormone and insulin-like growth factor 1
GH is synthetized in the adenohypophysis and affects skeletal muscle indirectly by stimulating IGF-1 release by the liver, which in turn acts on skeletal muscle via the IGF-1-receptor (IGF-1R) and subsequent activation of the PI3K-Akt/PKB-mTORC1 pathway [96]. Accordingly, in mice with a skeletal muscle-specific ablation of the IGF-1R, GH administration does not increase muscle mass [97]. In humans, aging is associated with a decrease in GH secretion and lower levels of circulating IGF-1 [98]. In line, GH replacement therapy increases lean mass in older men with a relative GH-deficiency [99] and daily injection of recombinant GH prevented a decline in muscle cross-sectional area (CSA) during two weeks of single leg immobilization and led to a greater increase of muscle CSA during the following six weeks of rehabilitation training compared to a placebo treated group [100]. Similarly, various forms of human recombinant IGF-1 are being assessed for their therapeutic value in sarcopenia and other muscle wasting diseases [101, 102]. However, while GH and IGF-1 treatment may be beneficial in individuals with relative deficiencies, non-replacement therapy of both hormones might be hampered by severe adverse effects, including cardiovascular complications and glucose intolerance [103].
Vitamin D
The synthesis of the active form of the hormone vitamin D requires skin exposure to sunlight (i.e. UVB light) [104]. Accordingly, older adults are at higher risk of vitamin D deficiency due to limited sun exposure, decreased capacity for cutaneous vitamin D synthesis and reduced intake and/or absorbance of vitamin D from the diet [105]. Even though the main function of vitamin D entails the regulation of calcium homeostasis and bone metabolism, skeletal muscle tissue also expresses the vitamin D receptor (VDR) [106] and knockout of the VDR results in muscular abnormalities [107]. Moreover, an increasing body of literature suggests a relationship between serum 25-hydroxyvitamin D [25(HO)D] levels and skeletal muscle physiology in elderly adults. Vitamin D deficiency is associated with reduced muscle mass and function, whereas vitamin D treatment seems to improve these parameters [108, 109]. Moreover, VDR expression has been reported to decrease in muscle with age [110] and VDR gene polymorphisms may further modify the risk of sarcopenia, even though the exact contribution of different genotypes has yet to be identified [111].
Given the blunted responsiveness of elderly adults to dietary protein and resistance exercise that often correlates with low levels of vitamin D, it has been suggested that vitamin D supplementation may overcome anabolic resistance. For example, participants with higher 25(OH)D levels and higher dietary protein intake at baseline responded with greater gains in muscle mass to a vitamin D- and leucine-enriched whey protein diet for 13 weeks [112]. Furthermore, a one year vitamin D supplementation together with a HMB-, arginine- and lysine-containing drink resulted in increased fat-free mass in elderly adults without exercise training, but only in those individuals with a 25(HO)D status of ≥30 nmol/ml [113]. Similarly, vitamin D supplementation significantly increased muscle strength in older adults [114] and treating women with low vitamin D levels resulted in 10% increase in muscle fiber size [115]. In fact, resistance exercise and vitamin D supplementation provide reciprocally beneficial effects on strength gains [116] and bone mineral density compared to exercise or vitamin D supplementation alone [116]. Despite these observations, the underlying mechanisms of vitamin D action in skeletal muscle remain enigmatic. Moreover, while the effects of vitamin D supplementation may be more pronounced in individuals with lower levels [109, 117], the optimal dose, route of administration, dosing intervals and treatment duration require further investigations [118]. Finally, a recent analysis showed no benefit of vitamin D supplementation on clinically relevant outcome measures such as fractures, falls or bone mineral density [119].
Ghrelin
The gastrointestinal peptide hormone ghrelin has originally been discovered as an endogenous ligand for the growth hormone secretagogue receptor 1a that has the ability to stimulate GH release [120]. Subsequent studies revealed a broad spectrum of additional physiological functions of ghrelin, including the regulation of feeding behavior by stimulating appetite and food intake as well as the promotion of anti-inflammatory processes [121]. Ghrelin treatment has accordingly been suggested to counteract muscle wasting in sarcopenia in several different ways. First, given that elderly people often lose appetite, ghrelin may increase food intake, helping these individuals to meet adequate energy and nutrient goals to maintain their muscle mass. Accordingly, in studies on cancer patients suffering from cachexia, synthetic ghrelin receptor agonists increased food intake and muscle mass [49]. However, other functional parameters, such as grip strength, could not be ameliorated by ghrelin treatment. Second, ghrelin-stimulated GH release and the subsequent increase of hepatic IGF-1 production might help to enhance or restore IGF-1 signaling in sarcopenic elderly. Capromorelin, a ghrelin receptor agonist, elevated GH and IGF-1 levels and improved lean mass, tandem walk and stair climbing after one year of treatment in older adults with mild functional limitations [122]. In a one-year randomized double-blind placebo-controlled crossover trial with healthy elderly adults, the ghrelin receptor agonist ibutamoren (MK-677) restored GH and IGF-1 to levels observed in young adults [123]. However, reliable functional endpoints could not be assessed due to the short duration and low sample size of the study. In a later trial, MK-677 was given to people recovering from a hip fracture for a period of 24 weeks. Participants showed higher IGF-1 levels and mild improvements in stair climbing and gait speed but unfortunately, the trial had to be stopped early because of adverse effects [124]. Third, numerous animal studies suggest anti-inflammatory properties of ghrelin and ghrelin receptor agonists [121]. However, human trials demonstrating reduced inflammation with ghrelin treatment have yet to be conducted. Taken together, even though ghrelin may exert beneficial effects on sarcopenia by restoring food intake and increasing muscle mass, the applicability is limited as effects on functional endpoints are modest and clinical trials with long-term follow-ups to evaluate the risk of adverse effects are lacking.
Conclusions and future perspectives
To date, pharmacological therapies for sarcopenia remain elusive, either because clinical trials revealed little efficacy or unacceptable adverse effects. However, treatment options for muscle wasting disorders have expanded dramatically in recent years and a number of new compounds and approaches are currently being evaluated in patients [49]. Several of these are of potential interest for the prevention and treatment of sarcopenia, at least if certain limitations can be overcome. For example, besides the ongoing debate whether aging represents a disease or a natural, physiological process [125], the design of clinical trials for sarcopenia is not trivial: age of participants, length of the trial or the definition of soft and hard endpoints are only some of the open questions that have to be addressed. Nevertheless, besides the phase II and III compounds, other pharmacological and nutritional agents are currently in the experimental stage, and might enter clinical testing in the future, potentially expanding the repertoire of drugs for muscle wasting and sarcopenia [45, 49, 126]. Finally, multi-pronged approaches using combinations of pharmacological and nutritional interventions might result in a positive outcome for sarcopenia. Until then, exercise remains the primary and most efficient intervention to prevent and mitigate sarcopenia, in particular when combined with adequate dietary approaches and skin exposure to sunlight. Of note, while resistance exercise confers the main benefits on muscle mass and strength, endurance training can further help to increase muscular sensitivity to anabolic stimuli and by promoting tissue vascularization, cardiovascular and metabolic benefits. Accordingly, a combination of both types of exercise most likely contributes most to an optimized health span [48, 127, 128]. It thus should be the interest of all stake-holders, including patients, insurance companies and governments, to implement broad incentives for an active life-style to overcome the problems with adherence and compliance.
Acknowledgments
We thank Prof. Urs T. Rüegg for discussions on the manuscript. Work in our laboratory is funded by the Swiss National Science Foundation, the European Research Council (ERC) Consolidator grant 616830-MUSCLE_NET, Swiss Cancer Research grant KFS-3733-08-2015, the Swiss Society for Research on Muscle Diseases (SSEM), SystemsX.ch, the Novartis Stiftung für Medizinisch- Biologische Forschung and the University of Basel.
Footnotes
Conflict of interest: The authors have no conflict of interest related to this manuscript.
Reprint request
Unfortunately, due to copyright-relatedt issues, we are not able to post the post-print pdf version of our manuscript - in some cases, not even any version of our manuscript. Thus, if you would like to request a post-production, publisher pdf reprint, please click send an email with the request to christoph-dot-handschin_at_unibas-dot-ch (see http://www.biozentrum.unibas.ch/handschin).
Information about the Open Access policy of different publishers/journals can be found on the SHERPA/ROMEO webpage: http://www.sherpa.ac.uk/romeo/
Reprint Anfragen
Aufgrund fehlender Copyright-Rechte ist es leider nicht möglich, dieses Manuskript in der finalen Version, z.T. sogar in irgendeiner Form frei zugänglich zu machen. Anfragen für Reprints per Email an christoph-dot-handschin_at_unibas-dot-ch (s. http://www.biozentrum.unibas.ch/handschin).
Informationen zur Open Access Handhabung verschiedener Verlage/Journals sind auf der SHERPA/ROMEO Webpage verfügbar: http://www.sherpa.ac.uk/romeo/
References
- [1].Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, et al. Writing Group for the European Working Group on Sarcopenia in Older People (EWGSOP2), and the Extended Group for EWGSOP2, Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2018 [Google Scholar]
- [2].Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev. 2019;99(1):427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McKendry J, Breen L, Shad BJ, Greig CA. Muscle morphology and performance in master athletes: A systematic review and meta-analyses. Ageing Res Rev. 2018;45:62–82. doi: 10.1016/j.arr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- [4].Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- [5].Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70(1):57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- [6].Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(1):211–7. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Artaza-Artabe I, Saez-Lopez P, Sanchez-Hernandez N, Fernandez-Gutierrez N, Malafarina V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review, Maturitas. 2016;93:89–99. doi: 10.1016/j.maturitas.2016.04.009. [DOI] [PubMed] [Google Scholar]
- [8].Esquivel MK. Nutritional Assessment and Intervention to Prevent and Treat Malnutrition for Fall Risk Reduction in Elderly Populations. Am J Lifestyle Med. 2018;12(2):107–112. doi: 10.1177/1559827617742847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients. 2018;10(3) doi: 10.3390/nu10030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286(10):1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Markofski MM, Dickinson JM, Drummond MJ, Fry CS, Fujita S, Gundermann DM, Glynn EL, Jennings K, Paddon-Jones D, Reidy PT, Sheffield-Moore M, et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol. 2015;65:1–7. doi: 10.1016/j.exger.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol. 2015;593(18):4259–73. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130(10):2413–9. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- [14].Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- [15].Jakubowski JS, Wong EPT, Nunes EA, Noguchi KS, Vandeweerd JK, Murphy KT, Morton RW, McGlory C, Phillips SM. Equivalent Hypertrophy and Strength Gains in HMB- or Leucine supplemented Men. Med Sci Sports Exerc. 2018 doi: 10.1249/MSS.0000000000001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moberg M, Apro W, Ekblom B, van Hall G, Holmberg HC, Blomstrand E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am J Physiol Cell Physiol. 2016;310(11):C874–84. doi: 10.1152/ajpcell.00374.2015. [DOI] [PubMed] [Google Scholar]
- [18].Traylor DA, Gorissen SHM, Phillips SM. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv Nutr. 2018;9(3):171–182. doi: 10.1093/advances/nmy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89(9):4351–8. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- [20].Arentso--Lantz EJ, Galvan E, Ellison J, Wacher A, Paddon-Jones D. Improving Dietary Protein Quality Reduces the Negative Effects of Physical Inactivity on Body Composition and Muscle Function. J Gerontol A Biol Sci Med Sci. 2019 doi: 10.1093/gerona/glz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103(2):465–73. doi: 10.3945/ajcn.115.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu ZR, Tan ZJ, Zhang Q, Gui QF, Yang YM. Clinical effectiveness of protein and amino acid supplementation on building muscle mass in elderly people: a meta-analysis. PLoS One. 2014;9(9):e109141. doi: 10.1371/journal.pone.0109141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tieland M, Franssen R, Dullemeijer C, van Dronkelaar C, Kyung Kim H, Ispoglou T, Zhu K, Prince RL, van Loon LJC, de Groot L. The Impact of Dietary Protein or Amino Acid Supplementation on Muscle Mass and Strength in Elderly People: Individual Participant Data and Meta-Analysis of RCT's. J Nutr Health Aging. 2017;21(9):994–1001. doi: 10.1007/s12603-017-0896-1. [DOI] [PubMed] [Google Scholar]
- [24].Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, Huang G, Campbell WW, Li Z, Howland AS, Chen R, Knapp PE. Effect of Protein Intake on Lean Body Mass in Functionally Limited Older Men A Randomized Clinical Trial. JAMA Intern Med. 2018;178(4):530–541. doi: 10.1001/jamainternmed.2018.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McDonald CK, Ankarfeldt MZ, Capra S, Bauer J, Raymond K, Heitmann BL. Lean body mass change over 6 years is associated with dietary leucine intake in an older Danish population. Br J Nutr. 2016;115(9):1556–62. doi: 10.1017/S0007114516000611. [DOI] [PubMed] [Google Scholar]
- [26].Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW, Phillips SM. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- [28].Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402–12. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102(1):115–22. doi: 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Phillips SM. Nutritional supplements in support of resistance exercise to counter agerelated sarcopenia. Adv Nutr. 2015;6(4):452–60. doi: 10.3945/an.115.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krzyminska-Siemaszko R, Czepulis N, Lewandowicz M, Zasadzka E, Suwalska A, Witowski J, Wieczorowska-Tobis K. The Effect of a 12-Week Omega-3 Supplementation on Body Composition, Muscle Strength and Physical Performance in Elderly Individuals with Decreased Muscle Mass. Int J Environ Res Public Health. 2015;12(9):10558–74. doi: 10.3390/ijerph120910558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Da Boit M, Sibson R, Sivasubramaniam S, Meakin JR, Greig CA, Aspden RM, Thies F, Jeromson S, Hamilton DL, Speakman JR, Hambly C. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr. 2017;105(1):151–158. doi: 10.3945/ajcn.116.140780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond) 2011;121(6):267–78. doi: 10.1042/CS20100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McGlory C, Gorissen SHM, Kamal M, Bahniwal R, Hector AJ, Baker SK, Chabowski A, Phillips SM. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019 doi: 10.1096/fj.201801857RRR. fj201801857RRR. [DOI] [PubMed] [Google Scholar]
- [35].Lee SH, Min KJ. Caloric restriction and its mimetics. BMB Rep. 2013;46(4):181–7. doi: 10.5483/BMBRep.2013.46.4.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–62. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- [37].Long DE, Peck BD, Martz JL, Tuggle SC, Bush HM, McGwin G, Kern PA, Bamman MM, Peterson CA. Metformin to Augment Strength Training Effective Response in Seniors (MASTERS): study protocol for a randomized controlled trial. Trials. 2017;18(1):192. doi: 10.1186/s13063-017-1932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].White Z, White RB, McMahon C, Grounds MD, Shavlakadze T. High mTORC1 signaling is maintained, while protein degradation pathways are perturbed in old murine skeletal muscles in the fasted state. Int J Biochem Cell Biol. 2016;78:10–21. doi: 10.1016/j.biocel.2016.06.012. [DOI] [PubMed] [Google Scholar]
- [39].Castets P, Lin S, Rion N, Di Fulvio S, Romanino K, Guridi M, Frank S, Tintignac LA, Sinnreich M, Ruegg MA. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013;17(5):731–44. doi: 10.1016/j.cmet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- [40].Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141(5):856–62. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kubica N, Kimball SR, Jefferson LS, Farrell PA. Alterations in the expression of mRNAs and proteins that code for species relevant to eIF2B activity after an acute bout of resistance exercise. J Appl Physiol (1985) 2004;96(2):679–87. doi: 10.1152/japplphysiol.00962.2003. [DOI] [PubMed] [Google Scholar]
- [42].Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(Pt 7):1535–46. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dickinson JM, Drummond MJ, Fry CS, Gundermann DM, Walker DK, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin does not affect post-absorptive protein metabolism in human skeletal muscle. Metabolism. 2013;62(1):144–51. doi: 10.1016/j.metabol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19(6):668–70. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- [45].Handschin C. Caloric restriction and exercise "mimetics'': Ready for prime time? Pharmacological research. 2016;103:158–66. doi: 10.1016/j.phrs.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gill JF, Santos G, Schnyder S, Handschin C. PGC-1alpha affects aging-related changes in muscle and motor function by modulating specific exercise-mediated changes in old mice. Aging Cell. 2018;17(1) doi: 10.1111/acel.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15(1):12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- [48].Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Furrer R, Handschin C. Muscle Wasting Diseases: Novel Targets and Treatments. Annu Rev Pharmacol Toxicol. 2018 doi: 10.1146/annurev-pharmtox-010818-021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, Manzato E, Sergi G, Veronese N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- [51].Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39(5):687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- [52].Wahlin-Larsson B, Wilkinson DJ, Strandberg E, Hosford-Donovan A, Atherton PJ, Kadi F. Mechanistic Links Underlying the Impact of C-Reactive Protein on Muscle Mass in Elderly. Cell Physiol Biochem. 2017;44(1):267–278. doi: 10.1159/000484679. [DOI] [PubMed] [Google Scholar]
- [53].Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- [54].Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587(Pt 22):5483–92. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Machida M, Takemasa T. Ibuprofen administration during endurance training cancels running-distance-dependent adaptations of skeletal muscle in mice. J Physiol Pharmacol. 2010;61(5):559–63. [PubMed] [Google Scholar]
- [56].Enos RT, Davis JM, McClellan JL, Murphy EA. Indomethacin in combination with exercise leads to muscle and brain inflammation in mice. J Interferon Cytokine Res. 2013;33(8):446–51. doi: 10.1089/jir.2012.0157. [DOI] [PubMed] [Google Scholar]
- [57].Lilja M, Mandic M, Apro W, Melin M, Olsson K, Rosenborg S, Gustafsson T, Lundberg TR. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol (Oxf) 2018;222(2) doi: 10.1111/apha.12948. [DOI] [PubMed] [Google Scholar]
- [58].Dideriksen K, Boesen AP, Kristiansen JF, Magnusson SP, Schjerling P, Holm L, Kjaer M. Skeletal muscle adaptation to immobilization and subsequent retraining in elderly men: No effect of anti-inflammatory medication. Exp Gerontol. 2016;82:8–18. doi: 10.1016/j.exger.2016.05.009. [DOI] [PubMed] [Google Scholar]
- [59].Dideriksen K, Reitelseder S, Malmgaard-Clausen NM, Bechshoeft R, Petersen RK, Mikkelsen UR, Holm L. No effect of anti-inflammatory medication on postprandial and postexercise muscle protein synthesis in elderly men with slightly elevated systemic inflammation. Exp Gerontol. 2016;83:120–9. doi: 10.1016/j.exger.2016.07.016. [DOI] [PubMed] [Google Scholar]
- [60].Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R655–62. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Trappe TA, Standley RA, Jemiolo B, Carroll CC, Trappe SW. Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304(3):R198–205. doi: 10.1152/ajpregu.00245.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Buford TW, Manini TM, Hsu FC, Cesari M, Anton SD, Nayfield S, Stafford RS, Church TS, Pahor M, Carter CS. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60(7):1244–52. doi: 10.1111/j.1532-5415.2012.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Alturki M, Beyer I, Mets T, Bautmans I. Impact of drugs with anti-inflammatory effects on skeletal muscle and inflammation: A systematic literature review. Exp Gerontol. 2018;114:33–49. doi: 10.1016/j.exger.2018.10.011. [DOI] [PubMed] [Google Scholar]
- [64].Puthucheary Z, Skipworth JR, Rawal J, Loosemore M, Van Someren K, Montgomery HE. The ACE gene and human performance: 12 years on. Sports Med. 2011;41(6):433–48. doi: 10.2165/11588720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [65].Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, Delafontaine P. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001;142(4):1489–96. doi: 10.1210/endo.142.4.8082. [DOI] [PubMed] [Google Scholar]
- [66].Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115(2):451–8. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sartiani L, Spinelli V, Laurino A, Blescia S, Raimondi L, Cerbai E, Mugelli A. Pharmacological perspectives in sarcopenia: a potential role for renin-angiotensin system blockers? Clin Cases Miner Bone Metab. 2015;12(2):135–8. doi: 10.11138/ccmbm/2015.12.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, Carter C, Di Bari M, Guralnik JM, Pahor M. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359(9310):926–30. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- [69].Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177(8):867–74. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cesari M, Kritchevsky SB, Atkinson HH, Penninx BW, Di Bari M, Tracy RP, Pahor M. Angiotensin-converting enzyme inhibition and novel cardiovascular risk biomarkers: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. Am Heart J. 2009;157(2):334 e1–8. doi: 10.1016/j.ahj.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc. 2010;11(1):26–32. doi: 10.1016/j.jamda.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3(82):82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lin CH, Yang H, Xue QL, Chuang YF, Roy CN, Abadir P, Walston JD. Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp Gerontol. 2014;58:174–8. doi: 10.1016/j.exger.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015;22(1):164–74. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, Moore MM, Bruce CJ, Greason KL, Suri RM, Khosla S, Miller JD, et al. Quantification of GDF11 and Myostatin in Human Aging and Cardiovascular Disease. Cell Metab. 2016;23(6):1207–1215. doi: 10.1016/j.cmet.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Siriett V, Platt L, Salerno MS, Ling N, Kambadur R, Sharma M. Prolonged absence of myostatin reduces sarcopenia. J Cell Physiol. 2006;209(3):866–73. doi: 10.1002/jcp.20778. [DOI] [PubMed] [Google Scholar]
- [77].Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8(5):573–83. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, Hochberg MC, Ferrari SL, Blain H, Binder EF, Rolland Y, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3(12):948–57. doi: 10.1016/S2213-8587(15)00298-3. [DOI] [PubMed] [Google Scholar]
- [79].Ebner N, von Haehling S. Unlocking the wasting enigma: Highlights from the 8th Cachexia Conference. J Cachexia Sarcopenia Muscle. 2016;7(1):90–4. doi: 10.1002/jcsm.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, Brachat S, Rivet H, Koelbing C, Morvan F, Hatakeyama S, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34(4):606–18. doi: 10.1128/MCB.01307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- [82].Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, A. Baltimore Longitudinal Study of Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- [83].Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, Lapauw B, Fiers T, Matsumoto AM, Bhasin S. Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161–1173. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- [85].Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283(1):E154–64. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- [86].Atkinson RA, Srinivas-Shankar U, Roberts SA, Connolly MJ, Adams JE, Oldham JA, Wu FC, Seynnes OR, Stewart CE, Maganaris CN, Narici MV. Effects of testosterone on skeletal muscle architecture in intermediate-frail and frail elderly men. J Gerontol A Biol Sci Med Sci. 2010;65(11):1215–9. doi: 10.1093/gerona/glq118. [DOI] [PubMed] [Google Scholar]
- [87].Morley JE, Perry HM., 3rd Androgens and women at the menopause and beyond. J Gerontol A Biol Sci Med Sci. 2003;58(5):M409–16. doi: 10.1093/gerona/58.5.m409. [DOI] [PubMed] [Google Scholar]
- [88].Rossetti ML, Steiner JL, Gordon BS. Androgen-mediated regulation of skeletal muscle protein balance. Mol Cell Endocrinol. 2017;447:35–44. doi: 10.1016/j.mce.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Neto WK, Gama EF, Rocha LY, Ramos CC, Taets W, Scapini KB, Ferreira JB, Rodrigues B, Caperuto E. Effects of testosterone on lean mass gain in elderly men: systematic review with meta-analysis of controlled and randomized studies. Age (Dordr) 2015;37(1):9742. doi: 10.1007/s11357-014-9742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, Wu FC. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- [91].Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, Salcido-Montenegro A, Benkhadra R, Prokop LJ, Bhasin S, Brito JP. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: A systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2018 doi: 10.1210/jc.2018-00404. [DOI] [PubMed] [Google Scholar]
- [92].Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, et al. Effects of Testosterone Treatment in Older Men. N Engl J Med. 2016;374(7):611–24. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bhasin S, Ellenberg SS, Storer TW, Basaria S, Pahor M, Stephens-Shields AJ, Cauley JA, Ensrud KE, Farrar JT, Cella D, Matsumoto AM, et al. Effect of testosterone replacement on measures of mobility in older men with mobility limitation and low testosterone concentrations: secondary analyses of the Testosterone Trials. Lancet Diabetes Endocrinol. 2018;6(11):879–890. doi: 10.1016/S2213-8587(18)30171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, Steiner MS. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2(3):153–161. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Papanicolaou DA, Ather SN, Zhu H, Zhou Y, Lutkiewicz J, Scott BB, Chandler J. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17(6):533–43. doi: 10.1007/s12603-013-0335-x. [DOI] [PubMed] [Google Scholar]
- [96].Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49(1):59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology. 2005;146(4):1772–9. doi: 10.1210/en.2004-0906. [DOI] [PubMed] [Google Scholar]
- [98].Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9(6):366–376. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- [100].Boesen AP, Dideriksen K, Couppe C, Magnusson SP, Schjerling P, Boesen M, Aagaard P, Kjaer M, Langberg H. Effect of growth hormone on aging connective tissue in muscle and tendon: gene expression, morphology, and function following immobilization and rehabilitation. J Appl Physiol (1985) 2014;116(2):192–203. doi: 10.1152/japplphysiol.01077.2013. [DOI] [PubMed] [Google Scholar]
- [101].Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol. 1997;272(1 Pt 1):E94–9. doi: 10.1152/ajpendo.1997.272.1.E94. [DOI] [PubMed] [Google Scholar]
- [102].Philippou A, Barton ER. Optimizing IGF-I for skeletal muscle therapeutics. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2014;24(5):157–63. doi: 10.1016/j.ghir.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146(2):104–15. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- [104].Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- [105].Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115–24. doi: 10.2340/00015555-0980. [DOI] [PubMed] [Google Scholar]
- [106].Kupr B, Schnyder S, Handschin C. Role of Nuclear Receptors in Exercise-Induced Muscle Adaptations. Cold Spring Harb Perspect Med. 2017;7(6) doi: 10.1101/cshperspect.a029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wagatsuma A, Sakuma K. Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. Biomed Res Int. 2014;2014 doi: 10.1155/2014/121254. 121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22(3):859–71. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- [110].Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19(2):265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- [111].Xia Z, Man Q, Li L, Song P, Jia S, Song S, Meng L, Zhang J. Vitamin D receptor gene polymorphisms modify the association of serum 25-hydroxyvitamin D levels with handgrip strength in the elderly in Northern China. Nutrition. 2018;57:202–207. doi: 10.1016/j.nut.2018.05.025. [DOI] [PubMed] [Google Scholar]
- [112].Verlaan S, Maier AB, Bauer JM, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo MET, Mets T, Seal C, Wijers SLJ, et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults - The PROVIDE study. Clin Nutr. 2018;37(2):551–557. doi: 10.1016/j.clnu.2017.01.005. [DOI] [PubMed] [Google Scholar]
- [113].Fuller JC, Jr, Baier S, Flakoll P, Nissen SL, Abumrad NN, Rathmacher JA. Vitamin D status affects strength gains in older adults supplemented with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and lysine: a cohort study. JPEN J Parenter Enteral Nutr. 2011;35(6):757–62. doi: 10.1177/0148607111413903. [DOI] [PubMed] [Google Scholar]
- [114].Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59(12):2291–300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- [115].Ceglia L, Niramitmahapanya S, da Silva Morais M, Rivas DA, Harris SS, Bischoff-Ferrari H, Fielding RA, Dawson-Hughes B. A randomized study on the effect of vitamin D(3) supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98(12):E1927–35. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Antoniak AE, Greig CA. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e014619. doi: 10.1136/bmjopen-2016-014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyere O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- [118].Bruyere O, Cavalier E, Souberbielle JC, Bischoff-Ferrari HA, Beaudart C, Buckinx F, Reginster JY, Rizzoli R. Effects of vitamin D in the elderly population: current status and perspectives. Arch Public Health. 2014;72(1):32. doi: 10.1186/2049-3258-72-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847–858. doi: 10.1016/S2213-8587(18)30265-1. [DOI] [PubMed] [Google Scholar]
- [120].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- [121].Collden G, Tschop MH, Muller TD. Therapeutic Potential of Targeting the Ghrelin Pathway. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].White HK, Petrie CD, Landschulz W, MacLean D, Taylor A, Lyles K, Wei JY, Hoffman AR, Salvatori R, Ettinger MP, Morey MC, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94(4):1198–206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- [123].Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE, Jr, Clasey JL, Heymsfield SB, Bach MA, Vance ML, Thorner MO. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601–11. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Adunsky A, Chandler J, Heyden N, Lutkiewicz J, Scott BB, Berd Y, Liu N, Papanicolaou DA. MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase IIb study. Arch Gerontol Geriatr. 2011;53(2):183–9. doi: 10.1016/j.archger.2010.10.004. [DOI] [PubMed] [Google Scholar]
- [125].Blagosklonny M. Disease or not, aging is easily treatable. Aging (Albany NY) 2018;10(11):3067–78. doi: 10.18632/aging.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Weihrauch M, Handschin C. Pharmacological targeting of exercise adaptations in skeletal muscle: Benefits and pitfalls. Biochem Pharmacol. 2018;147:211–220. doi: 10.1016/j.bcp.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25 Suppl 3:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- [128].Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol Rev. 2017;97(4):1351–1402. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]