Abstract
In eukaryotes, cellular genome is enclosed inside a membrane-bound organelle called the nucleus. The nucleus compartmentalizes genome replication, repair and expression, keeping these activities separated from protein synthesis and other metabolic processes. Each proliferative division, the duplicated chromosomes must be equipartitioned between the daughter cells and this requires precise coordination between assembly of the microtubule-based mitotic spindle and nuclear remodeling. Here we review a surprising variety of strategies used by modern eukaryotes to manage these processes and discuss possible mechanisms that might have led to the emergence of this diversity in evolution.
Introduction
The nucleus is delimited by the membranous nuclear envelope (NE) containing two apposed lipid bilayers. Of these, the outer nuclear membrane (ONM) is continuous with the endoplasmic reticulum (ER) and the inner nuclear membrane (INM) faces the intranuclear space and organizes an underlying protein meshwork of the nuclear lamina. The two membranes connect at the nuclear pores, communication channels between the nucleus and the cytoplasm decorated by the nuclear pores complexes (NPCs). The symmetrical NPC core is formed by nucleoporins that share common evolutionary roots with membrane-bending vesicle coat proteins. Peripheral Phe-Gly (FG)-repeat nucleoporins residing at the pore aperture ensure selectivity of nucleocytoplasmic transport along with the Ran GTPase system and other soluble factors. Remarkable conservation of most NPC components suggests that the last eukaryotic common ancestor (LECA) already had a functional nuclear envelope (for reviews see [1–3]).
A number of other proteins and protein complexes contribute to NE function. LINC (linkers of nucleoskeleton and cytoskeleton) complexes made of an INM-anchored SUN and the ONM-bound KASH proteins couple the chromatin to cytoplasmic cytoskeletal arrays and stabilize connections between the two membranes under mechanical load (for review see [4]). The SUN proteins also interact with other INM proteins and the nuclear lamina, which in turn organize chromatin at the nuclear periphery and supports NE structure and function (for review see [5]). Although functionality of the lamina is evolutionarily conserved, its molecular composition can vary substantially between species. Intermediate filaments lamins form the lamina meshwork in metazoans and proteins with similar structural motifs and functions have been recently discovered in other eukaryotic supergroups including protozoans, excavates and plants [6–8]. In organisms that lack lamin-like proteins altogether, lamina functions are likely performed by INM constituents, in particular the LEM-domain proteins. Yeast LEM proteins span the INM twice, with both the chromatin-interacting N-terminal LEM (or, rather its helix-extended loop-helix version) and the C-terminal MSC (MAN1–Src1p–C-terminal) domains facing the nucleoplasm. Proteins with similar architecture are found in all eukaryotic supergroups, indicating their ancient origin. The LEM proteins in yeast support NE integrity, organize chromatin at the nuclear periphery and regulate gene expression [9–14].
Eukaryotic chromosomes are segregated by a bipolar cytoskeletal array called the mitotic spindle. This structure containing microtubules, microtubule motors and other associated proteins, usually assembles at mitotic entry. Various spindle assembly mechanisms observed in different species can be categorized as either intranuclear or cytoplasmic. Intranuclear assembly - which often but not always accompanies a fully closed mitosis - implies that the spindle forms inside an intact nucleus. This requires that the mitotic microtubule organizing centers (MTOCs) are anchored at the inner side of the NE and that the tubulin dimers and microtubule-associated proteins are actively transported from the cytoplasm into the nucleus (for reviews see [15, 16]). Conversely, cytoplasmic spindle assembly relies on extranuclear microtubule nucleation and does not explicitly require delivery of the spindle constituents into the nucleus. This mechanism largely - but again not always - necessitates at least partial NE breakdown to enable access of spindle microtubules to the chromosomes.
The plethora of mitotic programs found in modern eukaryotes can be seen as a combination of these two spindle assembly strategies with distinct modes of NE remodeling (Fig. 1) [17, 18]. Here we examine natural variability in mitotic NE management focusing on the evolution of NE disassembly. Molecular mechanisms driving NE reformation after exit from mitosis have been recently discussed elsewhere [19, 20].
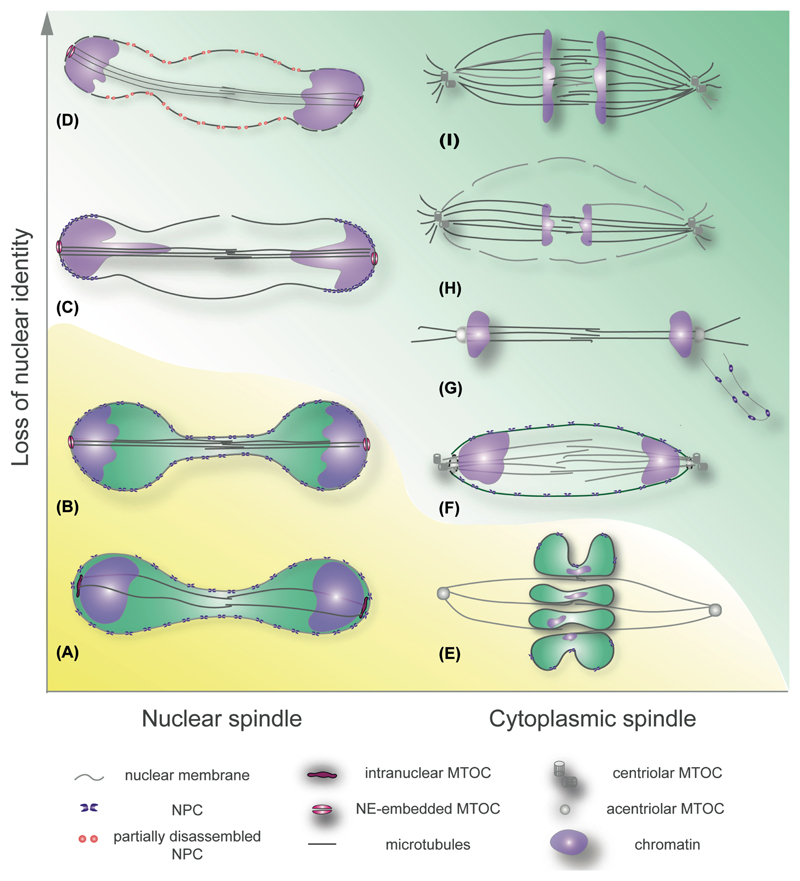
Figure 1.
Diagrams summarizing representative modes of mitosis across eukaryotes. The mitotic apparatuses are shown in anaphase. Left column: (A) the excavate Trypanosoma brucei, (B) the fission yeast Schizosaccharomyces pombe, (C) the fission yeast Schizosaccharomyces japonicus, (D) the filamentous Ascomycete Aspergillus nidulans. Right column: (E) the dinoflagellate Crypthecodinium cohnii, (F) the green algae Chlamydomonas reinhardtii, (G) an yeast form of the Basidiomycete Ustilago maydis, (H) an embryo of the nematode Caenorhabditis elegans, (I) a cultured human cell. Yellow background indicates “closed” modes of mitosis and green background denotes mitotic NE breakdown. Pictorial legend is shown below.
Mitotic NE dynamics in cells with intranuclear spindle assembly
During fully closed mitosis, the NE remains functionally intact, which may limit the availability of cytoplasmic MTOCs for spindle nucleation. Some organisms, such as the excavate Trypanosoma brucei solve this problem by building specialized mitotic MTOCs at the inner side of the NE (Fig. 1A; see [21] for review). Yet, many other organisms have invented a way to use the same MTOCs to organize cytoplasmic microtubules in interphase and nuclear microtubules in mitosis. The budding yeast Saccharomyces cerevisiae permanently anchors its spindle pole bodies (SPBs) within the plane of the NE, so that microtubules can be nucleated at both nucleo- and cytoplasmic sides throughout the cell cycle. Each cell cycle, the SPB duplicates and the daughter SPB is inserted alongside the mother (for review see [22]). Another model Ascomycete, the fission yeast Schizosaccharomyces pombe (S. pombe), keeps the SPBs at the cytoplasmic side of the NE in interphase and relocates them into the NE for the duration of mitosis, necessitating spatially and temporally confined NE opening, or fenestration (Fig. 1B and [23]). Mechanisms underlying MTOC placement within the NE and their regulation by the cell cycle machinery have been extensively discussed [15, 24]. While specific requirements may differ between systems, SPB insertion and anchorage overall rely on functional interactions between the transmembrane SPB component Ndc1/Cut11 (incidentally, also required for NPC anchorage) and NE components such as LINCs, the membrane shapers reticulon and Yop1, along with TMEM33 and Brr6 proteins that may aid membrane bending required for insertion by promoting specific lipid composition of the NE [25–32].
Intranuclear assembly of the mitotic spindle does not necessarily mean that the nucleus will remain intact for the duration of mitosis. The fission yeast Schizosaccharomyces japonicus (S. japonicus), a relative of S. pombe [33], starts mitosis in a manner very similar to its sister species but breaks the NE at the nuclear equator in anaphase (Fig. 1C and [11, 34]). Curiously, the NE remains fully functional up until the moment it breaks, as assessed by fully assembled NPCs and active nucleocytoplasmic transport [35]. Although the mechanism driving the NE breakage remains unknown, it appears to be controlled by the cell cycle machinery and further fine-tuned by interplay between the LEM-domain proteins Lem2 and Man1 and the mitotic spindle [11, 12].
Another Ascomycete that assembles the mitotic spindle from NE-embedded SPBs, Aspergillus nidulans (A. nidulans) also breaks the nuclear membrane in late anaphase [36]. In addition, it loses nucleocytoplasmic compartmentalization in early mitosis, downstream of mitotic CDK and NIMA kinase signaling. In a manner reminiscent of early events in nuclear envelope breakdown (NEBD) in metazoans (see below), the peripheral nucleoporins disperse from the NPCs, disrupting nuclear transport selectivity and presumably allowing tubulin, microtubule-associated proteins and other mitotic regulators to access the nucleoplasm (Fig. 1D and [37–39]).
Thus, cells building intranuclear spindles tend to maintain an intact nuclear membrane at least until anaphase. The nucleocytoplasmic transport may be abolished and the membrane may rupture later in mitosis but spindle assembly and kinetochore attachment proceed within the confines of the NE.
Mitotic NE dynamics in cells with extranuclear spindle assembly
This form of spindle assembly is found throughout the eukaryotic domain. At its simplest, the spindle remains cytoplasmic and chromosomes are segregated within an intact NE. In the core dinoflagellate group an extranuclear spindle passes through NE invaginations and makes indirect contacts with the chromosomes attached to the inner side of the NE (Fig. 1E). It appears that the kinetochore-like structures may be integrated into the NE [40–42]. If and how accurate chromosome segregation is ensured in cells where microtubules do not establish direct contacts with chromosomes remain unknown.
The flagellated green algae Chlamydomonas reinhardtii assembles a cytoplasmic spindle from the pair of polar organizing centers that form around cortex-associated basal bodies. The bulk of the NE remains unbroken but large polar fenestrae form to allow microtubule access to the chromosomes (Fig. 1F; for review see [43]). Similar strategy is utilized by the excavate Giardia intestinalis that divides its both diploid nuclei simultaneously by basal-body anchored spindles passing through large NE openings [44].
In fact, NE opening at the spindle poles allowing access of microtubules to mitotic chromosomes is widely spread in nature. Basidiomycetous budding yeasts Ustilago maydis and Cryptococcus neoformans undergo unusual mitoses as compared to their Ascomycete counterparts. In interphase U. maydis cells, SPBs lie on the outer side of the nucleus located in the mother cell. As cells enter mitosis, the NPCs disassemble and the nuclear membrane fenestrates in the vicinity of the SPBs. Astral microtubules emanating from the SPBs pull them together with chromosomes into the daughter cell, leaving the NE remnant behind (Fig. 1G). The spindle elongates in anaphase, pushing one set of chromosomes back into the mother cell [45, 46]. In C. neoformans a remnant of the mother NE is eventually utilized to enclose daughter genomes [47].
Early polar fenestration followed by further breakdown of the NE in anaphase occurs in some metazoan cells, e.g. in the embryos of the nematode Caenorhabditis elegans (Fig. 1H) and the fruit fly Drosophila melanogaster. Gradual NE breakdown in these cell types starts from complete NPC disassembly, with some INM and lamina constituents persisting at the nuclear periphery until late anaphase [48–50].
Finally, NE identity is completely lost in early mitosis in somatic metazoan and land plant cells. The process is best understood in cultured mammalian cells (Fig. 1I; see [51] for an excellent review). Phosphorylation of the peripheral nucleoporin Nup98 by mitotic CDK and other kinases triggers full NPC disassembly and a loss of nucleocytoplasmic compartmentalization [52].
CDK1 also phosphorylates lamins and lamin-associated proteins, promoting depolymerization of the lamin meshwork and chromatin detachment from the INM [53]. Lamina disassembly additionally destabilizes the nuclear membrane, making it more susceptible to microtubule motor-dependent tearing in the vicinity of spindle poles [54, 55]. Microtubules are not essential for NEBD but increase its efficiency, similarly to their function in fungi S. japonicus and U. maydis. LINC complexes [56] and ER proteins REEP3/4 [57] interact with microtubules to clear membranes away from chromatin.
Curiously, some degree of compartmentalization remains even in fully open mitosis. Membranes derived from the ER and the NE surround the mitotic spindle and may promote a specialized molecular crowding state in the vicinity of the mitotic apparatus [58]. This remaining compartmentalization may account for accumulation of soluble tubulin subunits and factors promoting microtubule assembly to assist timely spindle assembly and proper function [59].
Plasticity of NE remodeling in mitosis
A remarkable diversity of mitotic mechanisms that arose in evolution suggests that rewiring of NE behavior can be achieved with relative ease. In fact, as attested by organisms that switch between different types of NE remodeling and spindle assembly during their ontogenesis, mitotic plasticity can emerge form relatively minor tweaks in a conserved molecular machinery supporting nuclear integrity. A case in point is the myxomycete mold Physarum polycephalum that transits between a fully open mitosis with a cytoplasmic spindle in its uninucleate state and closed mitosis with an intranuclear spindle in syncytial plasmodium [60]. Thus, mitotic NE behavior can be viewed as a continuum of intermediate states, each reflecting a degree to which separate compartmental identity is maintained (Fig. 1).
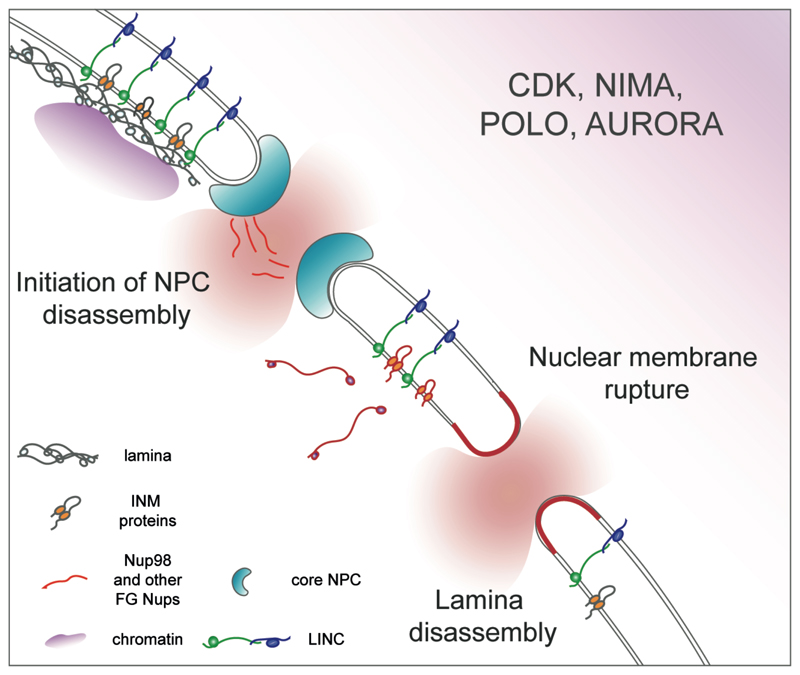
There are several recurring approaches to perturb nuclear identity that likely reflect NE “vulnerabilities” exploited by mitotic regulators (Fig. 2). One of them may center on mitotic phosphorylation of peripheral nucleoporins, in particular an evolutionarily conserved FG nucleoporin Nup98, which triggers its dissociation from the nuclear pore [52]. Since Nup98 enforces NPC transport selectivity [61, 62], its removal renders the NE permeable, allowing intermixing between the nuclear and cytosolic compartments and optionally, further phosphorylation-dependent NPC disassembly. Introducing discontinuities into the nuclear membrane will result in a similar outcome, even if the NPCs remain assembled. This strategy may require fusion between the outer and inner membranes generating NE breaches that can further expand through interaction with the cytoskeleton. Finally, disassembly of an INM-organized lamina causing dissociation of the chromatin from the nuclear periphery may reduce the ability of the NE to withstand the considerable pushing or pulling forces exerted by the cytoskeleton.
Figure 2.
A schematic diagram describing NE “vulnerabilities” (e.g. NPC selectivity, membrane integrity and lamina function, outlined in red) that can be attacked by mitotic regulators (e.g. CDK1, Aurora, Polo and NIMA kinases) to induce NE disassembly or remodeling. Pictorial legend is included.
These events are wired into the logic of the cell cycle, i. e. regulated by the mitotic driver CDK1 and other kinases active during mitosis (Fig. 2). Interestingly, CDK1, NIMA, Polo and Aurora family kinases implicated in NE remodeling in different experimentally tractable systems tend to be enriched at the spindle poles where nuclear membrane discontinuities are often initiated. Recent work suggests that the Polo kinase associated with mitotic chromatin may promote localized NE breakdown in C. elegans embryos [63], underscoring that the cell cycle machinery may regulate NE integrity in a spatially constrained manner. Evolution of networks determining NE fate during mitosis may proceed in cis-, by evolving proteins with somewhat different properties including distinct capacities for phosphoregulation. For instance, some of the mitotic phosphosites identified in mammalian Nup98 are conserved in A. nidulans but not in yeast species undergoing closed mitosis [52]. Alternatively, fairly identical biochemical machinery may be plugged into different regulatory circuits resulting in distinct functional outcomes, as in the lipin-centered pathway of NE expansion required for closed mitosis [64].
Factors affecting the choice between different mitotic mechanisms
What could be the reasons for mitotic diversity? First, it is worth considering that, in a given system, evolution of nuclear division may be constrained by factors unrelated to mitosis. Mitosis is often thought as “efficient” when chromosomes are segregated faithfully, rapidly and with minimum energy consumption. While this is generally true, ramping up the proliferation rate may lower the overall fitness of an organism due to a number of reasons, including, at its simplest, reallocation of finite resources between cell division and other critical processes. The mitotic machinery may also have functions beyond chromosome segregation. As an example, the mitotic spindle is important in determining the site and plane of cytokinesis in multicellular organisms [65] so NE breakdown could enable spindle-mediated signaling to cell division machinery located outside of the nucleus. Mitosis may further help to segregate structures and organelles other than chromosomes and specific spindle architectures and NE topologies may facilitate this functionality. For instance, emergence of the cytoplasmic spindle organized by centriole-structured MTOCs may have been an evolutionary cooption driven by pressure to segregate basal body-based cellular motility systems. Co-segregation of genomes and the basal bodies may have been economical but it also necessitated opening up the NE to allow cytoplasmic microtubules to access the kinetochores. This in turn generated a potential for evolving corresponding physiological and environmental regulation mechanisms.
There could be several general considerations driving selection of a particular mode of mitosis. Rapid and/or syncytial divisions tend to occur in a closed or semi-open manner, with the bulk of the NE maintaining its separate identity. For instance, the nuclear membrane remains largely intact in fast S/M cycles in green algae and cleaving embryos, in addition to many rapidly dividing unicellular organisms (Fig. 1). This strategy could have been selected to prevent entanglement of the neighboring spindles and chromosome mis-segregation in a multinucleate environment but also to accelerate resealing of the NE in the daughter nuclei. In addition to reducing the time between subsequent divisions, the latter may protect the genome from possible risks including reactive oxygen species and foreign genetic elements [66]. The sheer geometry of closed nuclear division could also contribute to daughter cell differentiation by promoting asymmetric segregation of episomes [67] and other nuclear components [68]. This could be particularly important for free-living microbes directly exposed to the environment.
But why not keep the nucleus always intact? In a syncytium, breaking down the nuclear selectivity barrier can enable fast mitotic signaling throughout the common cytoplasm and facilitate diffusion of spindle components inside each individual nucleus. Further considerations could include a high cost of nuclear membrane expansion required for fully closed division. Intact nuclei need to increase their surface area to accommodate division into two daughters – in fact, such mitotic NE expansion has been observed in a number of experimental systems including the fission yeast S. pombe [11, 69]. The NE does not expand in its sister species S. japonicus, necessitating membrane breakage to build two smaller daughter nuclei [11]. For these two organisms, divergent regulation of phosphatidic acid flux by the phosphatidic acid phosphatase lipin appears to underpin differences in mitotic NE expansion [64]. Energy cost may become prohibitive in cells with large genomes and correspondingly larger nuclei because of a sharp increase in absolute amount of membrane required to enclose DNA. Fully closed division may also rely on specific adaptations to prevent abnormal NE distortion by an elongating anaphase spindle, a function executed by the SPBs in fission yeast [70, 71].
Genomes with large size and complexity may favor a fully open mode of mitosis for a number of reasons. First, it may be difficult to capture, align and segregate a large number of kinetochores in an enclosed compartment. Second, building a large spindle through active transport of tubulin monomers and accessory factors may be inadequately slow. Finally, differences in scaling between the nuclear diameter and spindle length as related to genome size may preclude assembly of an intranuclear spindle predicted to become too large to fit into the nucleus beginning from a certain genomic size [66].
How an ancestral eukaryote might have divided its nucleus remains an important question, albeit difficult to answer in the absence of fossil record. The abundance of semi-open mitoses in all modern eukaryotic supergroups, together with their relative simplicity as compared to fully closed or fully open divisions, suggests that an ancestral eukaryote might have divided its genome through partially breaking the NE. It may have used nuclear membrane elaborations to move chromosomes on extranuclear cytoskeletal arrays that could have been based on actin or microtubules. Specialized mitotic spindle, as we know it, is likely a later invention. The half-spindles of dinoflagellates interacting with chromosomes through the NE potentially represent a vestigial transition stage between ancient and modern chromosome segregation strategies. A shift towards spindle microtubules as a primary force to move chromosomes should have improved their segregation fidelity [18] and culminated in the emergence of a modern kinetochore and the spindle assembly checkpoint. Still, kinetochores remain associated with the MTOCs throughout the cell cycle in many modern unicellular eukaryotes and a number of NPC components moonlight on kinetochores during chromosome segregation in more complex organisms (see [42, 72] for reviews). The S. pombe nucleus can even divide without spindle microtubules, as long as kinetochores-SPB interactions are maintained [73].
An ancient membrane remodeler ESCRT-III has been recently implicated in NE reformation following mitosis in cultured mammalian cells [74, 75]. Given that it is involved in other NE-based processes such as NPC quality surveillance [76], it would be important to evaluate if it supports mitotic NE dynamics in other organisms. The fact that the machinery that executes final steps of cytokinesis in eukaryotes and archae is involved in membrane fusion events at the NE suggests intriguing insights into evolutionary origins of the NE and mitosis. A recent “inside-out” hypothesis on the origin of eukaryotes, postulates that the eukaryotic endomembrane system may have evolved from the plasma membrane protrusions of an archaeal host cell that eventually became the nucleus, co-evolving with an ensnared α-proteobacterium that became a proto-mitochondrion [77]. According to this hypothesis, nuclear division in an early proto-eukaryote would be similar to archaeal cytokinesis, indeed placing the ESCRT-III machinery at the core of nuclear membrane remodeling.
Outlook
To conclude, there is a lot of interesting biology to learn by studying how mitotic nuclear remodeling has evolved throughout the eukaryotic domain of life. First, it may inform our understanding of basic NE biology and indeed, nuclear origins. Second, it may tell us how variation arises at the cellular level, essentially linking cell biology to evolutionary analysis. Such studies can be particularly powerful when conducted mechanistically in related organisms that utilize distinct approaches to mitosis. As an example, comparative analyses using the fission yeasts S. pombe and S. japonicus have been instructive in illuminating functional requirements for closed mitosis and division site positioning [11, 64, 78, 79]. Importantly, retroengineering recombinant and synthetic mitotic systems with novel properties should improve our understanding of how specific mechanisms of nuclear division interact with other physiological pathways. Current advances in genome editing, genome analysis and other “omics” approaches will undoubtedly streamline such experiments, finally putting answers to long-standing evolutionary questions within our reach.
Acknowledgements
We are grateful to Oliferenko lab members for discussions and E. Makeyev for suggestions on the manuscript. Our work is supported by a Wellcome Trust Senior Investigator Award (103741/Z/14/Z) to S. O.
References
- 1.Mans BJ, Anantharaman V, Aravind L, Koonin EV. Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell cycle. 2004;3:1612–1637. doi: 10.4161/cc.3.12.1316. [DOI] [PubMed] [Google Scholar]
- 2.Devos DP, Graf R, Field MC. Evolution of the nucleus. Current opinion in cell biology. 2014;28:8–15. doi: 10.1016/j.ceb.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson KL, Dawson SC. Evolution: functional evolution of nuclear structure. The Journal of cell biology. 2011;195:171–181. doi: 10.1083/jcb.201103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapley EC, Starr DA. Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Current opinion in cell biology. 2013;25:57–62. doi: 10.1016/j.ceb.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amendola M, van Steensel B. Mechanisms and dynamics of nuclear lamina-genome interactions. Current opinion in cell biology. 2014;28:61–68. doi: 10.1016/j.ceb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.DuBois KN, Alsford S, Holden JM, Buisson J, Swiderski M, Bart JM, Ratushny AV, Wan Y, Bastin P, Barry JD, et al. NUP-1 Is a large coiled-coil nucleoskeletal protein in trypanosomes with lamin-like functions. PLoS biology. 2012;10:e1001287. doi: 10.1371/journal.pbio.1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruger A, Batsios P, Baumann O, Luckert E, Schwarz H, Stick R, Meyer I, Graf R. Characterization of NE81, the first lamin-like nucleoskeleton protein in a unicellular organism. Molecular biology of the cell. 2012;23:360–370. doi: 10.1091/mbc.E11-07-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciska M, Moreno Diaz de la Espina S. The intriguing plant nuclear lamina. Frontiers in plant science. 2014;5:166. doi: 10.3389/fpls.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez Y, Saito A, Sazer S. Fission yeast Lem2 and Man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus. 2012;3:60–76. doi: 10.4161/nucl.18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrales RR, Forn M, Georgescu PR, Sarkadi Z, Braun S. Control of heterochromatin localization and silencing by the nuclear membrane protein Lem2. Genes & development. 2016;30:133–148. doi: 10.1101/gad.271288.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yam C, He Y, Zhang D, Chiam KH, Oliferenko S. Divergent strategies for controlling the nuclear membrane satisfy geometric constraints during nuclear division. Current biology : CB. 2011;21:1314–1319. doi: 10.1016/j.cub.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 12.Yam C, Gu Y, Oliferenko S. Partitioning and remodeling of the Schizosaccharomyces japonicus mitotic nucleus require chromosome tethers. Current biology : CB. 2013;23:2303–2310. doi: 10.1016/j.cub.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 13.Grund SE, Fischer T, Cabal GG, Antunez O, Perez-Ortin JE, Hurt E. The inner nuclear membrane protein Src1 associates with subtelomeric genes and alters their regulated gene expression. The Journal of cell biology. 2008;182:897–910. doi: 10.1083/jcb.200803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton LJ, Soshnev AA, Geyer PK. Networking in the nucleus: a spotlight on LEM-domain proteins. Current opinion in cell biology. 2015;34:1–8. doi: 10.1016/j.ceb.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Oliferenko S. Remodeling the nuclear membrane during closed mitosis. Current opinion in cell biology. 2013;25:142–148. doi: 10.1016/j.ceb.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Okada N, Sato M. Spatiotemporal Regulation of Nuclear Transport Machinery and Microtubule Organization. Cells. 2015;4:406–426. doi: 10.3390/cells4030406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath IB. Variant mitoses in lower eukaryotes: indicators of the evolution of mitosis. International review of cytology. 1980;64:1–80. doi: 10.1016/s0074-7696(08)60235-1. [DOI] [PubMed] [Google Scholar]
- 18.Kubai DF. The evolution of the mitotic spindle. International review of cytology. 1975;43:167–227. doi: 10.1016/s0074-7696(08)60069-8. [DOI] [PubMed] [Google Scholar]
- 19.Schellhaus AK, De Magistris P, Antonin W. Nuclear Reformation at the End of Mitosis. Journal of molecular biology. 2015 doi: 10.1016/j.jmb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Wandke C, Kutay U. Enclosing chromatin: reassembly of the nucleus after open mitosis. Cell. 2013;152:1222–1225. doi: 10.1016/j.cell.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Akiyoshi B, Gull K. Evolutionary cell biology of chromosome segregation: insights from trypanosomes. Open biology. 2013;3 doi: 10.1098/rsob.130023. 130023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaspersen SL, Ghosh S. Nuclear envelope insertion of spindle pole bodies and nuclear pore complexes. Nucleus. 2012;3:226–236. doi: 10.4161/nucl.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Molecular biology of the cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smoyer CJ, Jaspersen SL. Breaking down the wall: the nuclear envelope during mitosis. Current opinion in cell biology. 2014;26:1–9. doi: 10.1016/j.ceb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Winey M, Hoyt MA, Chan C, Goetsch L, Botstein D, Byers B. NDC1: a nuclear periphery component required for yeast spindle pole body duplication. The Journal of cell biology. 1993;122:743–751. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West RR, Vaisberg EV, Ding R, Nurse P, McIntosh JR. cut11(+): A gene required for cell cycle-dependent spindle pole body anchoring in the nuclear envelope and bipolar spindle formation in Schizosaccharomyces pombe. Molecular biology of the cell. 1998;9:2839–2855. doi: 10.1091/mbc.9.10.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friederichs JM, Ghosh S, Smoyer CJ, McCroskey S, Miller BD, Weaver KJ, Delventhal KM, Unruh J, Slaughter BD, Jaspersen SL. The SUN protein Mps3 is required for spindle pole body insertion into the nuclear membrane and nuclear envelope homeostasis. PLoS genetics. 2011;7:e1002365. doi: 10.1371/journal.pgen.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki Y, Lau CK, Maekawa H, Jaspersen SL, Giddings TH, Jr, Schiebel E, Winey M. The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Molecular biology of the cell. 2006;17:1959–1970. doi: 10.1091/mbc.E05-07-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casey AK, Dawson TR, Chen J, Friederichs JM, Jaspersen SL, Wente SR. Integrity and function of the Saccharomyces cerevisiae spindle pole body depends on connections between the membrane proteins Ndc1, Rtn1, and Yop1. Genetics. 2012;192:441–455. doi: 10.1534/genetics.112.141465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D, Oliferenko S. Tts1, the fission yeast homologue of the TMEM33 family, functions in NE remodeling during mitosis. Molecular biology of the cell. 2014;25:2970–2983. doi: 10.1091/mbc.E13-12-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamm T, Grallert A, Grossman EP, Alvarez-Tabares I, Stevens FE, Hagan IM. Brr6 drives the Schizosaccharomyces pombe spindle pole body nuclear envelope insertion/extrusion cycle. The Journal of cell biology. 2011;195:467–484. doi: 10.1083/jcb.201106076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walde S, King MC. The KASH protein Kms2 coordinates mitotic remodeling of the spindle pole body. Journal of cell science. 2014;127:3625–3640. doi: 10.1242/jcs.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhind N, Chen Z, Yassour M, Thompson DA, Haas BJ, Habib N, Wapinski I, Roy S, Lin MF, Heiman DI, et al. Comparative functional genomics of the fission yeasts. Science. 2011;332:930–936. doi: 10.1126/science.1203357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoki K, Hayashi H, Furuya K, Sato M, Takagi T, Osumi M, Kimura A, Niki H. Breakage of the nuclear envelope by an extending mitotic nucleus occurs during anaphase in Schizosaccharomyces japonicus. Genes to cells : devoted to molecular & cellular mechanisms. 2011;16:911–926. doi: 10.1111/j.1365-2443.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 35.Gu Y, Yam C, Oliferenko S. Divergence of mitotic strategies in fission yeasts. Nucleus. 2012;3:220–225. doi: 10.4161/nucl.19514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govindaraghavan M, Lad AA, Osmani SA. The NIMA kinase is required to execute stage-specific mitotic functions after initiation of mitosis. Eukaryotic cell. 2014;13:99–109. doi: 10.1128/EC.00231-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Souza CP, Osmani AH, Hashmi SB, Osmani SA. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Current biology : CB. 2004;14:1973–1984. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 38.Davies JR, Osmani AH, De Souza CP, Bachewich C, Osmani SA. Potential link between the NIMA mitotic kinase and nuclear membrane fission during mitotic exit in Aspergillus nidulans. Eukaryotic cell. 2004;3:1433–1444. doi: 10.1128/EC.3.6.1433-1444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osmani AH, Davies J, Liu HL, Nile A, Osmani SA. Systematic deletion and mitotic localization of the nuclear pore complex proteins of Aspergillus nidulans. Molecular biology of the cell. 2006;17:4946–4961. doi: 10.1091/mbc.E06-07-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubai DF, Ris H. Division in the dinoflagellate Gyrodinium cohnii (Schiller). A new type of nuclear reproduction. The Journal of cell biology. 1969;40:508–528. doi: 10.1083/jcb.40.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhaud Y, Guillebault D, Lennon J, Defacque H, Soyer-Gobillard MO, Moreau H. Morphology and behaviour of dinoflagellate chromosomes during the cell cycle and mitosis. Journal of cell science. 2000;113(Pt 7):1231–1239. doi: 10.1242/jcs.113.7.1231. [DOI] [PubMed] [Google Scholar]
- 42.Drechsler H, McAinsh AD. Exotic mitotic mechanisms. Open biology. 2012;2 doi: 10.1098/rsob.120140. 120140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cross FR, Umen JG. The Chlamydomonas cell cycle. The Plant journal : for cell and molecular biology. 2015;82:370–392. doi: 10.1111/tpj.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagolla MS, Dawson SC, Mancuso JJ, Cande WZ. Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. Journal of cell science. 2006;119:4889–4900. doi: 10.1242/jcs.03276. [DOI] [PubMed] [Google Scholar]
- 45.Straube A, Weber I, Steinberg G. A novel mechanism of nuclear envelope break-down in a fungus: nuclear migration strips off the envelope. The EMBO journal. 2005;24:1674–1685. doi: 10.1038/sj.emboj.7600644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theisen U, Straube A, Steinberg G. Dynamic rearrangement of nucleoporins during fungal "open" mitosis. Molecular biology of the cell. 2008;19:1230–1240. doi: 10.1091/mbc.E07-02-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozubowski L, Yadav V, Chatterjee G, Sridhar S, Yamaguchi M, Kawamoto S, Bose I, Heitman J, Sanyal K. Ordered kinetochore assembly in the human-pathogenic basidiomycetous yeast Cryptococcus neoformans. mBio. 2013;4:e00614–00613. doi: 10.1128/mBio.00614-13. [*The authors describe interesting mitotic NE dynamics in C. neoformans and show that this important human pathogen uses a metazoan-like strategy to time kinetochore assembly.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Molecular biology of the cell. 2000;11:3089–3099. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hachet V, Busso C, Toya M, Sugimoto A, Askjaer P, Gonczy P. The nucleoporin Nup205/NPP-3 is lost near centrosomes at mitotic onset and can modulate the timing of this process in Caenorhabditis elegans embryos. Molecular biology of the cell. 2012;23:3111–3121. doi: 10.1091/mbc.E12-03-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsani KR, Karess RE, Dostatni N, Doye V. In vivo dynamics of Drosophila nuclear envelope components. Molecular biology of the cell. 2008;19:3652–3666. doi: 10.1091/mbc.E07-11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nature reviews Molecular cell biology. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 52.Laurell E, Beck K, Krupina K, Theerthagiri G, Bodenmiller B, Horvath P, Aebersold R, Antonin W, Kutay U. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell. 2011;144:539–550. doi: 10.1016/j.cell.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Torvaldson E, Kochin V, Eriksson JE. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus. 2015;6:166–171. doi: 10.1080/19491034.2015.1017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- 55.Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 56.Turgay Y, Champion L, Balazs C, Held M, Toso A, Gerlich DW, Meraldi P, Kutay U. SUN proteins facilitate the removal of membranes from chromatin during nuclear envelope breakdown. The Journal of cell biology. 2014;204:1099–1109. doi: 10.1083/jcb.201310116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlaitz AL, Thompson J, Wong CC, Yates JR, 3rd, Heald R. REEP3/4 ensure endoplasmic reticulum clearance from metaphase chromatin and proper nuclear envelope architecture. Developmental cell. 2013;26:315–323. doi: 10.1016/j.devcel.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schweizer N, Pawar N, Weiss M, Maiato H. An organelle-exclusion envelope assists mitosis and underlies distinct molecular crowding in the spindle region. The Journal of cell biology. 2015;210:695–704. doi: 10.1083/jcb.201506107. [**Using cell biology and modeling approaches the authors show that nucleocytoplasmic compartmentalization may persist even in open mitosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi H, Kimura K, Kimura A. Localized accumulation of tubulin during semi-open mitosis in the Caenorhabditis elegans embryo. Molecular biology of the cell. 2012;23:1688–1699. doi: 10.1091/mbc.E11-09-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burland TG, Solnica-Krezel L, Bailey J, Cunningham DB, Dove WF. Patterns of inheritance, development and the mitotic cycle in the protist Physarum polycephalum. Advances in microbial physiology. 1993;35:1–69. doi: 10.1016/s0065-2911(08)60096-x. [DOI] [PubMed] [Google Scholar]
- 61.Iwamoto M, Mori C, Kojidani T, Bunai F, Hori T, Fukagawa T, Hiraoka Y, Haraguchi T. Two distinct repeat sequences of Nup98 nucleoporins characterize dual nuclei in the binucleated ciliate tetrahymena. Current biology : CB. 2009;19:843–847. doi: 10.1016/j.cub.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt HB, Gorlich D. Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity. eLife. 2015;4 doi: 10.7554/eLife.04251. [*An interesting study suggesting that intrinsic phase separation properties of Nup98 alone may account for selective NPC permeability.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman MM, Munzig M, Kaneshiro K, Lee B, Strome S, Muller-Reichert T, Cohen-Fix O. Caenorhabditis elegans polo-like kinase PLK-1 is required for merging parental genomes into a single nucleus. Molecular biology of the cell. 2015;26:4718–4735. doi: 10.1091/mbc.E15-04-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makarova M, Gu Y, Chen JS, Beckley JR, Gould KL, Oliferenko S. Temporal Regulation of Lipin Activity Diverged to Account for Differences in Mitotic Programs. Current biology. 2016;26:237–243. doi: 10.1016/j.cub.2015.11.061. [**The authors use a comparative biology approach to provide a mechanistic basis for understanding NE expansion required in closed mitosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliferenko S, Chew TG, Balasubramanian MK. Positioning cytokinesis. Genes & development. 2009;23:660–674. doi: 10.1101/gad.1772009. [DOI] [PubMed] [Google Scholar]
- 66.Sazer S, Lynch M, Needleman D. Deciphering the evolutionary history of open and closed mitosis. Current biology : CB. 2014;24:R1099–1103. doi: 10.1016/j.cub.2014.10.011. [*This paper posits an interesting hypothesis on the origins of open mitosis, including possible role for transposons in promoting NE permeabilization.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gehlen LR, Nagai S, Shimada K, Meister P, Taddei A, Gasser SM. Nuclear geometry and rapid mitosis ensure asymmetric episome segregation in yeast. Current biology : CB. 2011;21:25–33. doi: 10.1016/j.cub.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Boettcher B, Marquez-Lago TT, Bayer M, Weiss EL, Barral Y. Nuclear envelope morphology constrains diffusion and promotes asymmetric protein segregation in closed mitosis. The Journal of cell biology. 2012;197:921–937. doi: 10.1083/jcb.201112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witkin KL, Chong Y, Shao S, Webster MT, Lahiri S, Walters AD, Lee B, Koh JL, Prinz WA, Andrews BJ, et al. The budding yeast nuclear envelope adjacent to the nucleolus serves as a membrane sink during mitotic delay. Current biology : CB. 2012;22:1128–1133. doi: 10.1016/j.cub.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng L, Schwartz C, Magidson V, Khodjakov A, Oliferenko S. The spindle pole bodies facilitate nuclear envelope division during closed mitosis in fission yeast. PLoS biology. 2007;5:e170. doi: 10.1371/journal.pbio.0050170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim HWG, Huber G, Torii Y, Hirata A, Miller J, Sazer S. Vesicle-like biomechanics governs important aspects of nuclear geometry in fission yeast. PloS one. 2007;2:e948. doi: 10.1371/journal.pone.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forbes DJ, Travesa A, Nord MS, Bernis C. Nuclear transport factors: global regulation of mitosis. Current opinion in cell biology. 2015;35:78–90. doi: 10.1016/j.ceb.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castagnetti S, Oliferenko S, Nurse P. Fission yeast cells undergo nuclear division in the absence of spindle microtubules. PLoS biology. 2010;8:e1000512. doi: 10.1371/journal.pbio.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. ESCRT-III controls nuclear envelope reformation. Nature. 2015;522:236–239. doi: 10.1038/nature14503. [*This is one of the two studies showing that ESCRT-III membrane remodeling complex performs annular fusion step to seal the NE after mitosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 2015;522:231–235. doi: 10.1038/nature14408. [*The authors show that mitotic spindle disassembly and ESCRT-III-driven NE resealing are coordinated in mammalian cells.] [DOI] [PubMed] [Google Scholar]
- 76.Webster BM, Colombi P, Jager J, Lusk CP. Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell. 2014;159:388–401. doi: 10.1016/j.cell.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baum DA, Baum B. An inside-out origin for the eukaryotic cell. BMC biology. 2014;12:76. doi: 10.1186/s12915-014-0076-2. [**The authors propose a conceptually novel “inside-out” hypothesis on the origin of eukaryotes, arguing that the NE and the rest of the endomembrane system may have arose from the the plasma membrane protrusions of an archaeal cell.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu Y, Yam C, Oliferenko S. Rewiring of cellular division site selection in evolution of fission yeasts. Current biology : CB. 2015;25:1187–1194. doi: 10.1016/j.cub.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu Y, Oliferenko S. Comparative biology of cell division in the fission yeast clade. Current opinion in microbiology. 2015;28:18–25. doi: 10.1016/j.mib.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]