Summary
The evolution of animal behaviour is poorly understood1,2. Despite numerous correlations of behavioural and nervous system divergence, demonstration of the genetic basis of interspecific behavioural differences remains rare3–5. Here, we develop a novel neurogenetic model, Drosophila sechellia, a close cousin of D. melanogaster6,7 that displays profound behavioural changes linked to its extreme specialisation on noni fruit8–16. Using calcium imaging, we identify D. sechellia olfactory pathways detecting host volatiles. Mutational analysis indicates roles for different olfactory receptors in long- and short-range attraction to noni. Cross-species allele transfer demonstrates that tuning of one of these receptors is important for species-specific host-seeking. We identify the molecular determinants of this functional change, and characterise their evolutionary origin and behavioural significance. Through circuit tracing in the D. sechellia brain, we find that receptor adaptations are accompanied by increased sensory pooling onto interneurons and novel central projection patterns. This work reveals the accumulation of molecular, physiological and anatomical traits linked to behavioural divergence, and defines a powerful model for investigating nervous system evolution and speciation.
The genetic and neural basis by which animals adapt behaviourally to their ecological niche is largely unknown1,2. Insights have been gained from intraspecific variation in traditional model organisms, including anxiety behaviours in Mus musculus17 and exploration/exploitation decisions in Caenorhabditis elegans18. Interspecific differences are more dramatic: Peromyscus mice species display variations in burrowing and parental care3,5, while the predatory nematode Pristionchus pacificus exhibits distinct feeding behaviours to C. elegans19. Defining the molecular basis of interspecific differences is challenging as it requires that species are comparable molecularly and anatomically, and genetically manipulatable.
Drosophilid flies are attractive models to investigate behavioural evolution: D. melanogaster offers deep neurobiological knowledge in a numerically-simple brain, and closely-related drosophilid species show distinct behaviours linked to their diverse ecologies20. Several of these behavioural traits have been correlated to anatomical and/or physiological changes in sensory or central pathways4,11,13,15,21,22. One remarkable drosophilid is the Seychelles endemic, D. sechellia, which shares a recent common ancestor with the cosmopolitan, ecological generalists D. melanogaster and D. simulans6,7 (Fig. 1a). D. sechellia has evolved extreme specialism for Morinda citrifolia “noni” fruit (Fig. 1a), and displays unique olfactory11–13,15,16, gustatory14, and reproductive behaviours8–10. Mapping approaches have located causal loci for some D. sechellia-specific traits within typically large genomic regions8,10, while candidate approaches have correlated chemosensory phenotypes with changes in its peripheral sensory pathways11,14,15.
Fig. 1. Behavioural and physiological responses of D. sechellia to noni.
a, D. sechellia specialises on noni fruit (Morinda citrifolia) while D. simulans and D. melanogaster are food generalists (MYA = million years ago).
b, Behavioural responses to noni fruit or juice in a wind tunnel assay of D. sechellia, D. simulans and D. melanogaster wild-type strains (n = 20 experiments (10 females/experiment)). In this and other panels, boxplots show the median, first and third quartile of the data, overlaid with individual data points. Comparisons to Dsec.07 noni juice responses are shown (Kruskal-Wallis test, Dunn’s post-hoc correction): *** P < 0.001; n.s. P > 0.05.
c, Behavioural responses in a trap assay testing preferences between noni and grape, or between noni juice and grape juice (same strains as in b, n = 15-27 experiments, exact n in Source Data, (22-25 females/experiment)). Comparisons to Dsec.07 noni juice responses are shown (pairwise Wilcoxon rank-sum test, P values adjusted for multiple comparisons using the Benjamini and Hochberg (B&H) method): *** P < 0.001; n.s. P > 0.05.
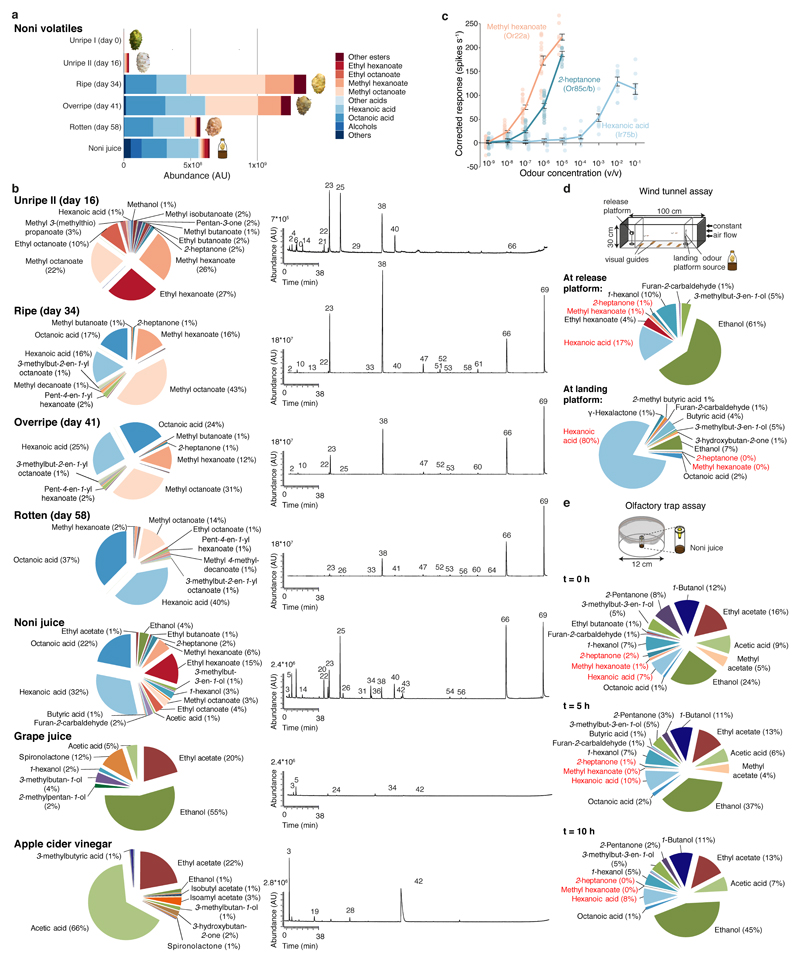
d, Odour bouquet of a ripe noni fruit determined by gas chromatography/mass spectrometry (see Methods, Extended Data Fig. 2, Supplementary Table 1).
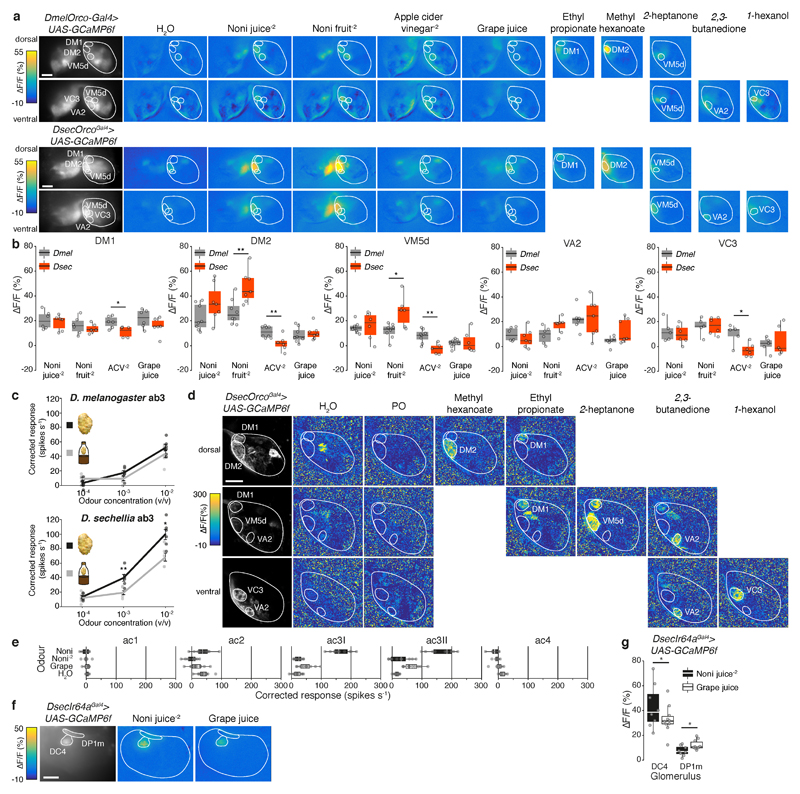
e, Representative odour-evoked calcium responses in the axon termini of Orco OSNs in the D. sechellia antennal lobe (genotype: UAS-GCaMP6f/UAS-GCaMP6f;;DsecOrcoGal4/+) acquired by two-photon imaging. Three focal planes are shown, revealing different glomeruli (outlined) along the dorsoventral axis. Left column: raw fluorescence images. Right columns: relative increase in GCaMP6f fluorescence (ΔF/F%) after stimulation with noni juice (10-2 in H2O) or grape juice. Scale bar, 25 μm.
f, Quantification of responses for the animals represented in e. Maximum response amplitudes for each experiment are plotted (n = 7-10 females). Wilcoxon signed-rank test: ** P < 0.01; * P < 0.05; n.s. P > 0.05.
Despite the potential D. sechellia presents for comparative neuroscience, investigations of D. sechellia’s behaviours have been limited by the lack of genetic tools. Here we develop D. sechellia into a genetic model system, moving from genotypic/phenotypic correlations to test the role of genetic changes in behavioural evolution.
Specific noni attraction of D. sechellia
Noni-derived volatiles are likely the initial cues that guide D. sechellia host-seeking16. We used two assays to compare attraction of wild-type strains of D. sechellia, D. simulans and D. melanogaster to noni at distinct spatial scales (Fig. 1b,c, Extended Data Fig. 1): In a long-range, wind tunnel assay23, D. sechellia displayed higher attraction to noni than its sister species (Fig. 1b); in a short-range trap assay15, only D. sechellia exhibits marked noni preference (Fig. 1c). The level of attraction/preference of this species to noni juice (a more reproducible odour stimulus) was comparable to that for ripe fruit (Fig. 1b,c), concordant with their qualitatively similar odour bouquets (Fig. 1d, Extended Data Fig. 2a-b, Methods). Assays with other natural odour sources, as previously described in field studies24, confirmed the unique attractiveness of noni for D. sechellia (Extended Data Fig. 1a-e,g).
Noni-sensing olfactory pathways
Drosophilids detect odours using olfactory sensory neurons (OSNs) in sensilla on their antennae and maxillary palps25. Most OSNs express a single Odorant Receptor (OR) or Ionotropic Receptor (IR) – which defines odour-tuning properties – along with an obligate co-receptor26–28. Neurons expressing the same tuning receptor converge onto a discrete glomerulus in the antennal lobe25. Electrophysiological analyses in D. sechellia have identified several OSN populations responding to individual noni odours11,13,15,29, but the global representation of the noni bouquet has not been examined.
We generated transgenic D. sechellia expressing GCaMP6f in the majority of OSNs under the control of Gal4 inserted at the Or co-receptor (Orco) locus (Extended Data Fig. 3). Using widefield imaging to compare this and an equivalent D. melanogaster line (Extended Data Fig. 4a-b), we did not detect novel noni-responsive olfactory channels in D. sechellia but rather quantitative differences in individual glomerular responses between species (Extended Data Fig. 4a). Two-photon calcium imaging highlighted two glomeruli (DM2 and VM5d) distinguished by their very high sensitivity to noni compared to grape juice in D. sechellia (Fig. 1e-f; Extended Data Fig. 4c-d). These glomeruli are innervated by OSNs housed in a common antennal basiconic sensillum class (ab3) that responds electrophysiologically to individual noni odours11,13.
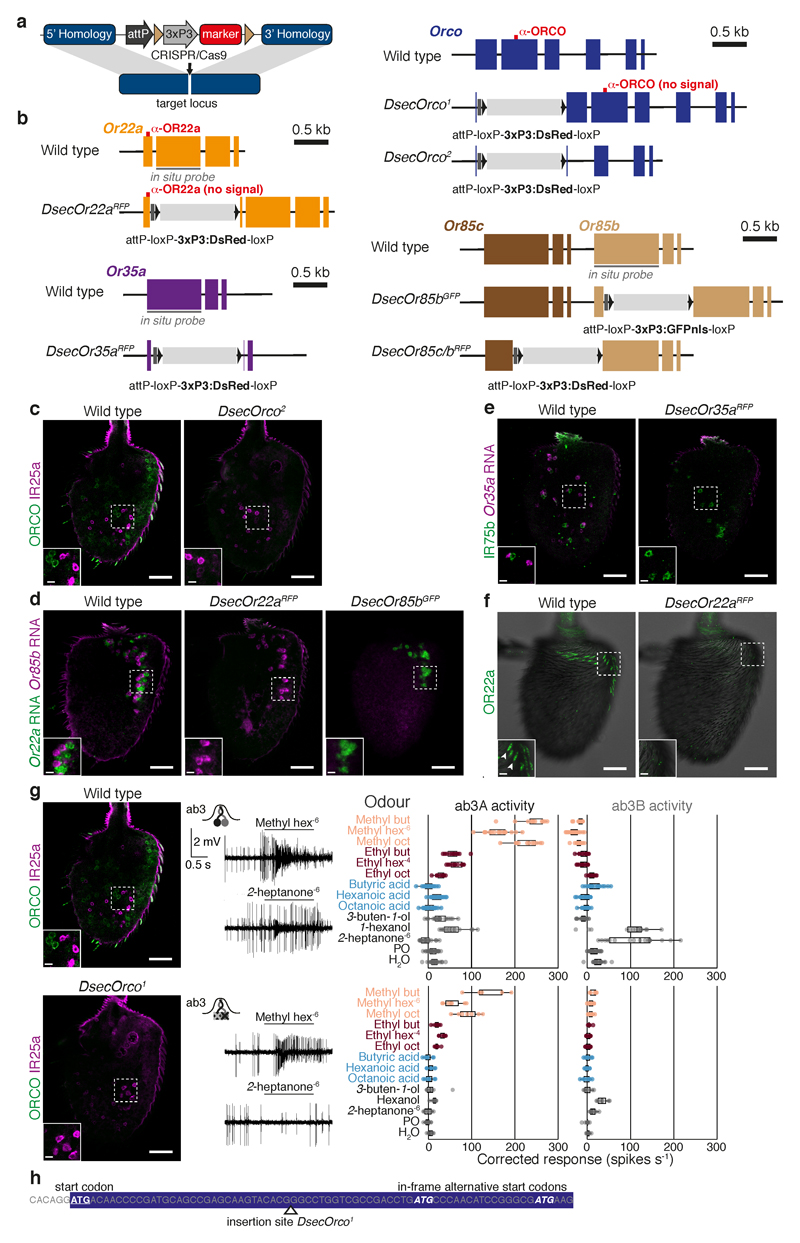
Genetic targeting of olfactory receptors
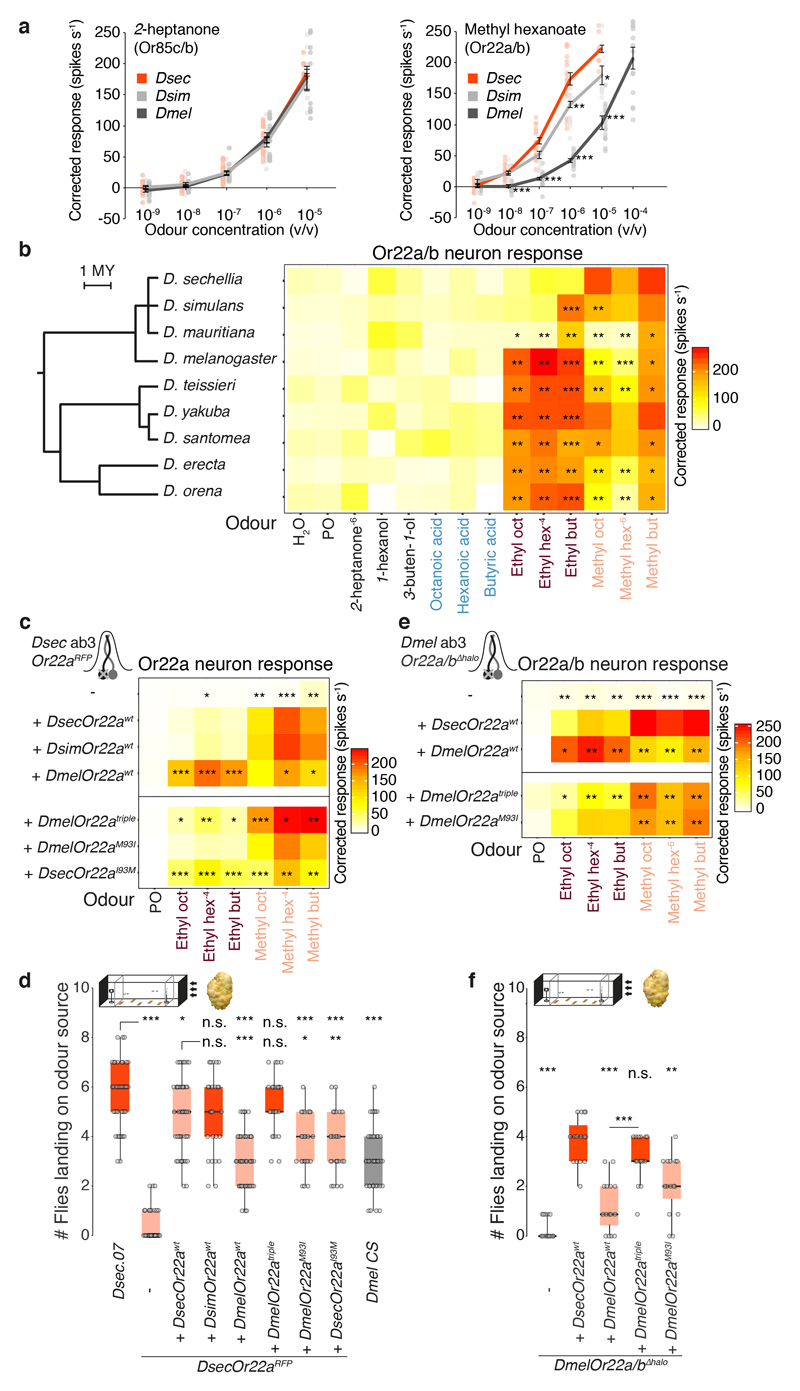
For noni-sensitive olfactory channels, we determined their electrophysiological responses to noni odours (Fig. 2a-b, Extended Data Fig. 4e, 5-6) and mutated candidate olfactory receptors (Extended Data Fig. 5-6). In wild-type ab3 sensilla, the larger-spiking ab3A neuron responded most strongly to methyl esters while the smaller-spiking ab3B neuron was highly stimulated by 2-heptanone and 1-hexanol (Fig. 2a). In DsecOrco mutants, all of these responses were lost (Fig. 2a), indicating their dependence upon OR signalling.
Fig. 2. Olfactory receptors contribution to noni-sensing.
a, Electrophysiological responses of ab3 sensillum neurons to noni odours (n = 5-20, females, see Supplementary Table 7 for exact n and mean spike counts) in wild-type and receptor mutant D. sechellia (schematised in the cartoons), with representative traces for methyl hexanoate and 2-heptanone. Data points are the solvent-corrected activities of individual neurons (arrowheads in wild-type traces). Odours (oct, octanoate; hex, hexanoate; but, butanoate) are coloured according to chemical class: methyl esters (salmon), ethyl esters (dark red), acids (light blue), others (black), used at 10-2 v/v unless indicated otherwise. PO = paraffin oil.
b, Responses of ac3I neurons to noni juice, grape juice and noni odours (n = 5-11, females) in wild-type and receptor mutant D. sechellia, with representative traces for hexanoic acid and 1-hexanol. Data points are the summed, solvent-corrected activities of both neurons.
c, Behavioural responses to noni juice in the wind tunnel assay (n = 20 experiments). Comparisons to Dsec.07 responses are shown (Kruskal-Wallis test, Dunn’s post-hoc correction). c, d, e: red = no significant difference; salmon = significantly different response; *** P < 0.001; ** P < 0.01; * P < 0.05; n.s. P > 0.05.
d, Behavioural responses in the trap assay testing preference for noni juice or grape juice (n = 13-25 experiments). Comparisons to Dsec.07 responses are shown (d and e, pairwise Wilcoxon rank-sum test, P values adjusted for multiple comparisons using the B&H method).
e, Behavioural responses in the trap assay testing preference for noni juice or grape juice using wild-type D. sechellia, DsecOrco1/Ir8aGFP double mutants and antenna-less D. sechellia (n = 9-15 experiments (22-25 females or males, as indicated/experiment)). Average attraction indices for DsecOrco1/Ir8aGFP and antenna-less flies are not significantly different from zero (pairwise Wilcoxon rank-sum test).
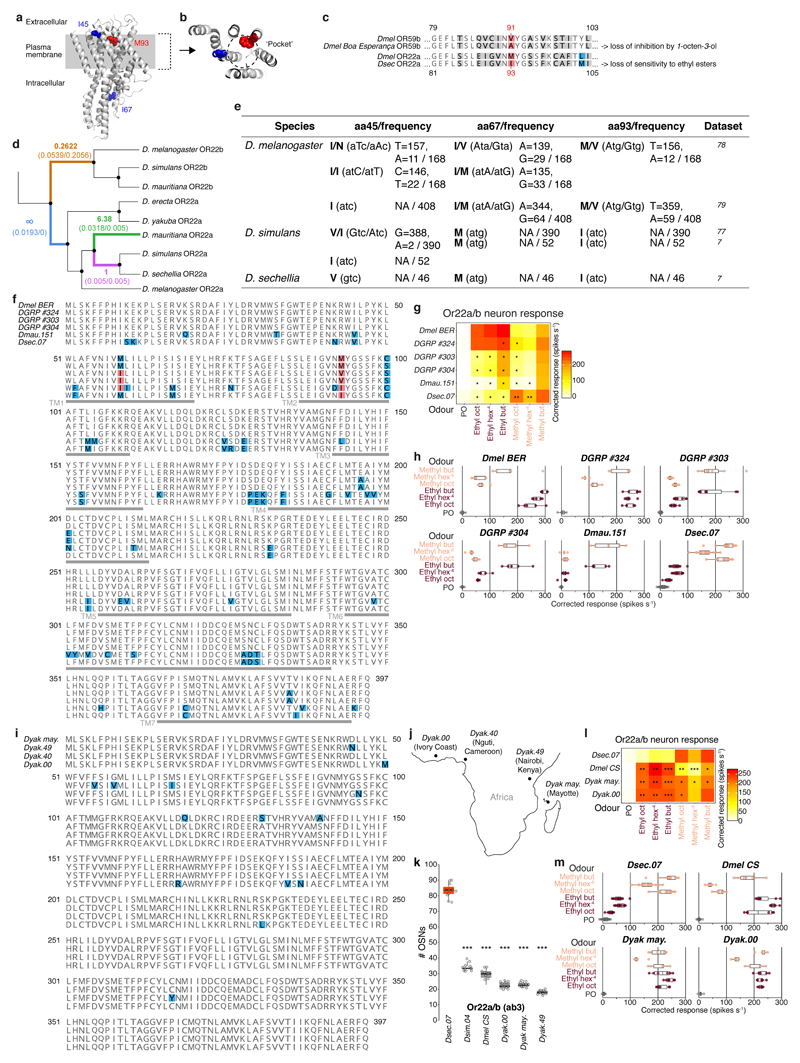
D. melanogaster ab3A expresses the Or22a and Or22b genes30 while D. sechellia possesses only Or22a11. Targeted mutation of this locus abolished odour-evoked responses of ab3A, but not ab3B (Fig. 2a). The receptor in D. melanogaster ab3B is thought to be OR85b25,31, but DsecOr85b mutant neurons retained some sensitivity to noni odours (Fig. 2a). Deletion of DsecOr85b and the neighbouring DsecOr85c, whose transcripts were detected in an antennal transcriptome32, led to complete loss of ab3B responses, arguing for partial receptor redundancy (Fig. 2a).
D. sechellia Ir75b neurons (in antennal coeloconic 3I (ac3I) sensilla) have evolved novel sensitivity to hexanoic acid15. Mutations in DsecIr75b or DsecIr8a (which encodes the acid-sensing IR co-receptor27) led to selective loss of hexanoic and butyric acid responses in ac3I (Fig. 2b, Extended Data Fig. 6). Mutation of DsecOr35a (expressed in the paired neuron) diminished responses to all odours except these acids, in-line with the broad tuning of this receptor in D. melanogaster33.
ORs required for long-range attraction
We used the receptor mutants to determine the behavioural role of individual olfactory pathways. In the long-range assay, DsecOrco exhibited no attraction to the odour source (Fig. 2c). Strikingly, both DsecOr22a and DsecOr85c/b mutants displayed similarly strong defects (Fig. 2c). By contrast, DsecOr35a mutants were not impaired (Fig. 2c). Loss of IR8a also led to a significant decrease in long-range attraction in D. sechellia (Fig. 2c). This does not appear to be primarily due to defects in the hexanoic acid-sensing pathway, as DsecIr75b mutants had either no or milder defects than DsecIr8a mutants (Fig. 2c). Mutations in DsecIr64a, which is broadly-tuned to acids in D. melanogaster34 and responded to noni in D. sechellia (Extended Data Fig. 4f-g), had no effect on this behaviour.
In the short-range assay, DsecOrco mutant flies displayed reduced, but not abolished, preference for noni (Fig. 2d). Individual Or pathway mutants had very slight (DsecOr22a) or no (DsecOr85c/b, DsecOr35a) defects in this behaviour (Fig. 2d, Extended Data Fig. 7). Mutants for DsecIr8a or DsecIr75b, but not DsecIr64a, displayed reduced preference, with notable frequent preference reversals in several trials (Fig. 2d). Orco/Ir8a double mutants, or antenna-less flies, displayed no noni preference (Fig. 2e, Extended Data Fig. 7c-d), indicating that this short-range behaviour depends upon multiple, partially redundant olfactory inputs. Consistent with these observations, individual noni odours promoted strong preference at short-range, while they triggered no or little flight attraction at long-range (Extended Data Fig. 7f-g)11,13,15. The relative contribution of individual channels to these behaviours may be related to their different detection thresholds (Extended Data Fig. 2c) and/or differential diffusion of cognate odours within each assay (Extended Data Fig. 2d-e).
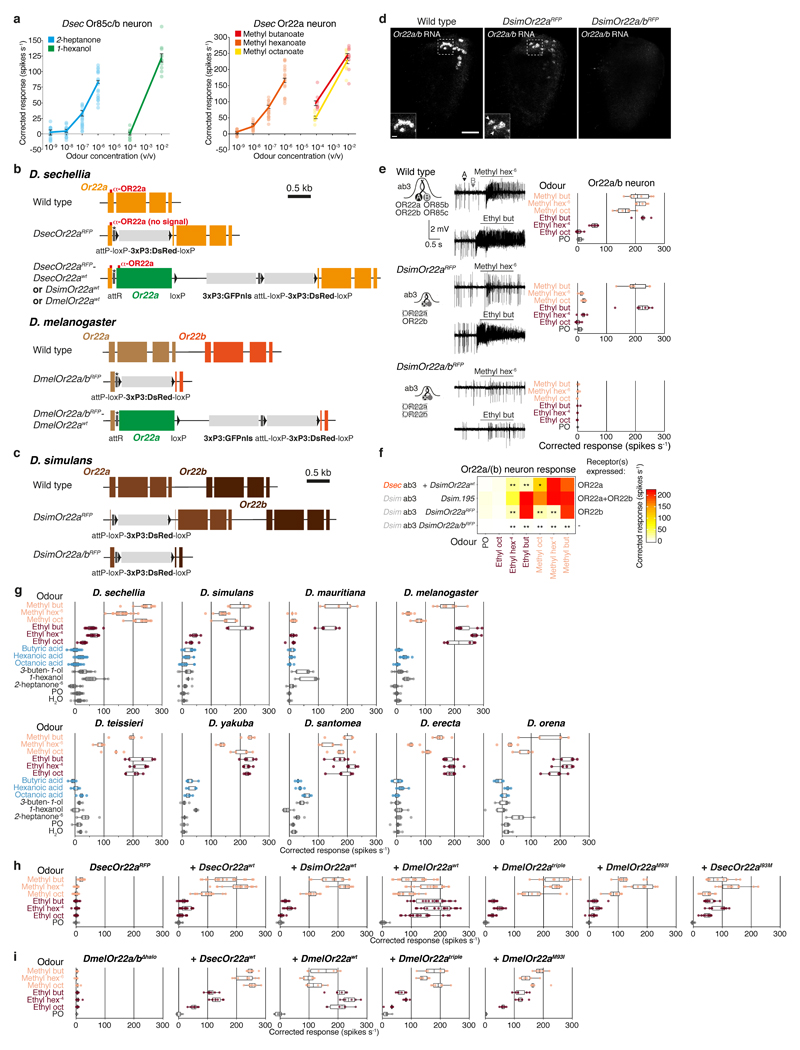
Tuning of OR22a impacts behaviour
Given the crucial role of OR22a and OR85c/b in long-range attraction, we explored the evolution of these pathways. Or85c/b neurons displayed an indistinguishable sensitivity across species to their best agonist, 2-heptanone. By contrast, both D. sechellia and D. simulans Or22a/(b) neurons exhibited increased sensitivity to methyl hexanoate compared to D. melanogaster (Fig. 3a, Extended Data Fig. 8a)11,29. Broader profiling of Or22a/(b) neurons in the D. melanogaster species subgroup (Fig. 3b, Extended Data Fig. 8g) revealed that D. sechellia was the only species with selective, high sensitivity to methyl esters; others, including D. simulans, also responded to ethyl esters (Fig. 3b). This suggests that changes in tuning sensitivity and/or breadth of OR22a – but not OR85c/b – contribute to the differential behaviour of D. sechellia.
Fig. 3. Tuning of OR22a is important for noni attraction.
a, Dose-dependent responses of Or85c/b (left) and Or22a/(b) neurons (right) in the indicated species to 2-heptanone and methyl hexanoate, respectively (mean ± SEM and individual data points, n = 7-20, females). Significant differences to D. sechellia responses are shown (a, b, c and e: pairwise Wilcoxon rank-sum test, P values adjusted for multiple comparisons using the B&H method): *** P < 0.001; ** P < 0.01; * P < 0.05.
b, Responses of Or22a/(b) neurons to noni odours across the D. melanogaster species subgroup; MY = million years (n = 5-20, females). D. sechellia responses as in Fig. 2a. Significant differences to D. sechellia ester responses are shown.
c, Responses of D. sechellia Or22a neurons expressing wild-type (top) or mutant (bottom) versions of Or22a at the Or22a locus (n = 10-11, females). Significant differences to DsecOR22awt responses are shown (c and e). Responses to methyl hexanoate at 10-4 here compared to 10-6 in b and e.
d, Behavioural responses to noni fruit in the wind tunnel assay (n = 25-45 experiments). Comparisons to Dsec.07 (top) and DsecOR22awt (below) responses are shown (d and f, Kruskal-Wallis test, Dunn’s post-hoc correction). Salmon = D. sechellia genotypes with significantly different response to Dsec.07; *** P < 0.001; ** P < 0.01; * P < 0.05.
e, Responses of D. melanogaster Or22a/b mutant neurons expressing wild-type (top) or mutant (bottom) versions of Or22a (n = 5-7, females). Boxplot representations of b, c and e are shown in Extended Data Fig. 8g, h and i, respectively.
f, Behavioural responses to noni fruit in the wind tunnel assay (n = 20 experiments). Comparisons to DsecOr22awt (top) and DmelOR22awt (below) responses are shown. Salmon = genotypes with significantly different responses.
We next reintroduced DsecOr22awt, DsimOr22awt or DmelOr22awt into the DsecOr22a endogenous locus (Extended Data Fig. 8b). DsecOr22awt and DmelOr22awt restored electrophysiological response profiles similar to native neuronal responses, indicating that the receptor is key for species-specific neuron tuning (Fig. 3c, upper part). DsimOr22awt conferred sensitivity to methyl but not ethyl esters (Fig. 3b-c, Extended Data Fig. 8h); genetic analysis in D. simulans indicated that detection of ethyl esters by the endogenous Or22a/b neurons depends upon the co-expressed OR22b (Extended Data Fig. 8c-f).
Concordant with their physiological properties, DsecOR22awt and DsimOR22awt but not DmelOR22awt, rescued long-range behavioural responses to almost wild-type levels (Fig. 3d). Reciprocally, expression of DsecOR22awt in Or22a/b mutant neurons in D. melanogaster (Fig. 3e, upper part) conferred higher noni sensitivity and long-range attraction than DmelOR22awt (Fig. 3f, Extended Data Fig. 8i, 9a).
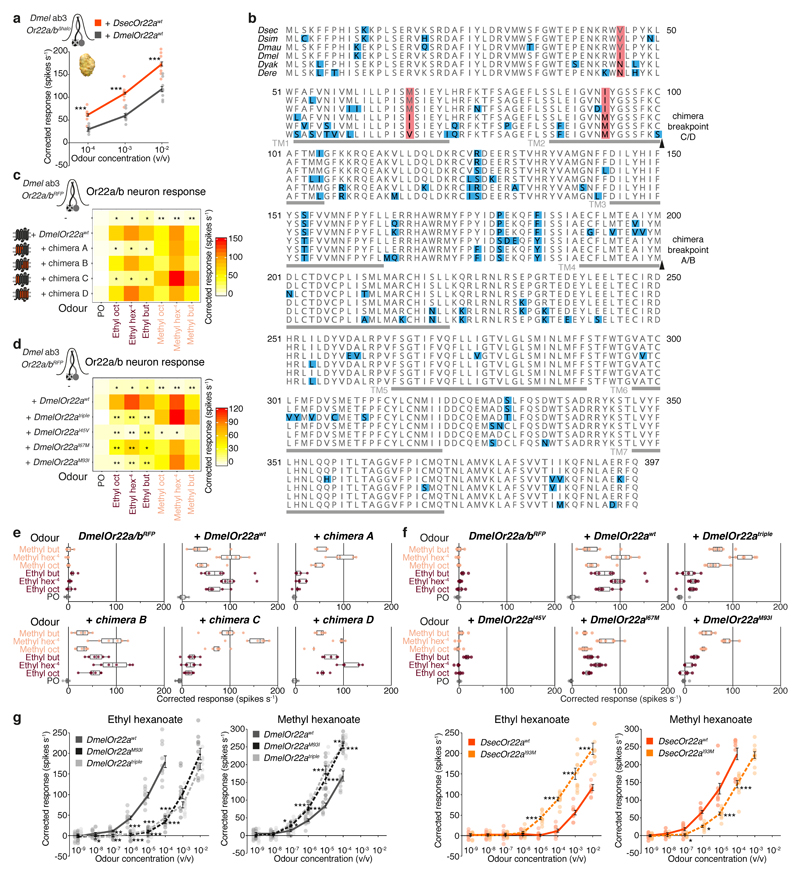
Molecular basis of OR22a tuning changes
We next sought the molecular basis of OR22a’s tuning differences. Expression of chimeric versions of DsecOR22awt and DmelOR22awt in D. melanogaster Or22a/b neurons (Extended Data Fig. 9c) indicated that high-sensitivity and selectivity for methyl esters were determined by the N-terminal 100 amino acids of DsecOR22a (chimera C) (Extended Data Fig. 9b-c,e). Within these, three positions (I45, I67 and M93) differ between DmelOR22a and its orthologues in species displaying narrowed tuning for methyl esters (Extended Data Fig. 9b). Exchange of these residues (DmelOR22aI45V,I67M,M93I (DmelOR22atriple)) narrowed responsiveness to methyl esters, similar to DsecOR22awt (Fig. 3c,e (lower parts), Extended Data Fig. 9d,f). Individual mutations revealed that DmelOR22aM93I most closely recapitulated the higher sensitivity of this receptor to methyl esters over ethyl esters (Fig. 3c,e, (lower parts), Extended Data Fig. 9d-g). Conversely, DsecOR22aI93M exhibited broadened sensitivity to both ester classes (Fig. 3c (lower part), Extended Data Fig. 9g).
In the long-range olfactory behaviour assay, expression of DmelOR22atriple in D. sechellia Or22a neurons restored D. sechellia-like attraction to noni, while both DmelOR22aM93I and DsecOR22aI93M displayed levels of attraction intermediate between those of the wild-type receptor rescues (Fig. 3d). Similarly, expression of DmelOR22atriple in D. melanogaster conferred noni attraction at equivalent levels to DsecOR22awt, while DmelOR22aM93I supported intermediate attraction levels (Fig. 3f). These results provide evidence that the molecular differences in OR22a orthologues contribute to species-specific olfactory behaviours.
Sensory representation of Or22a
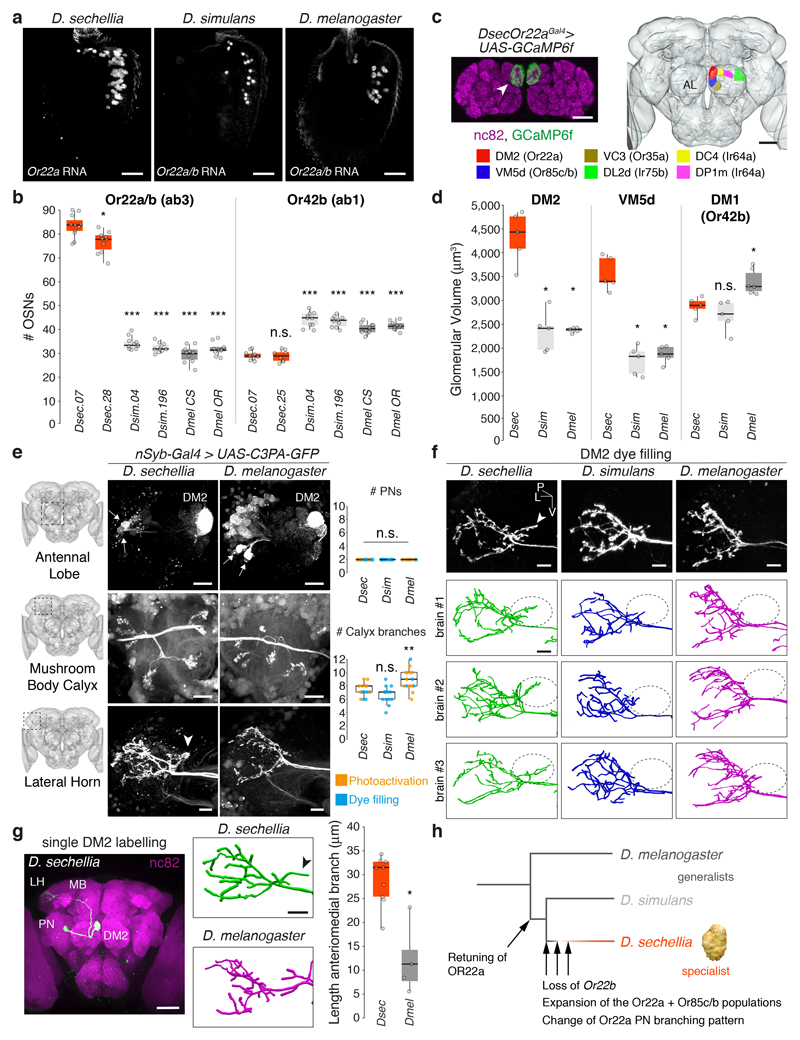
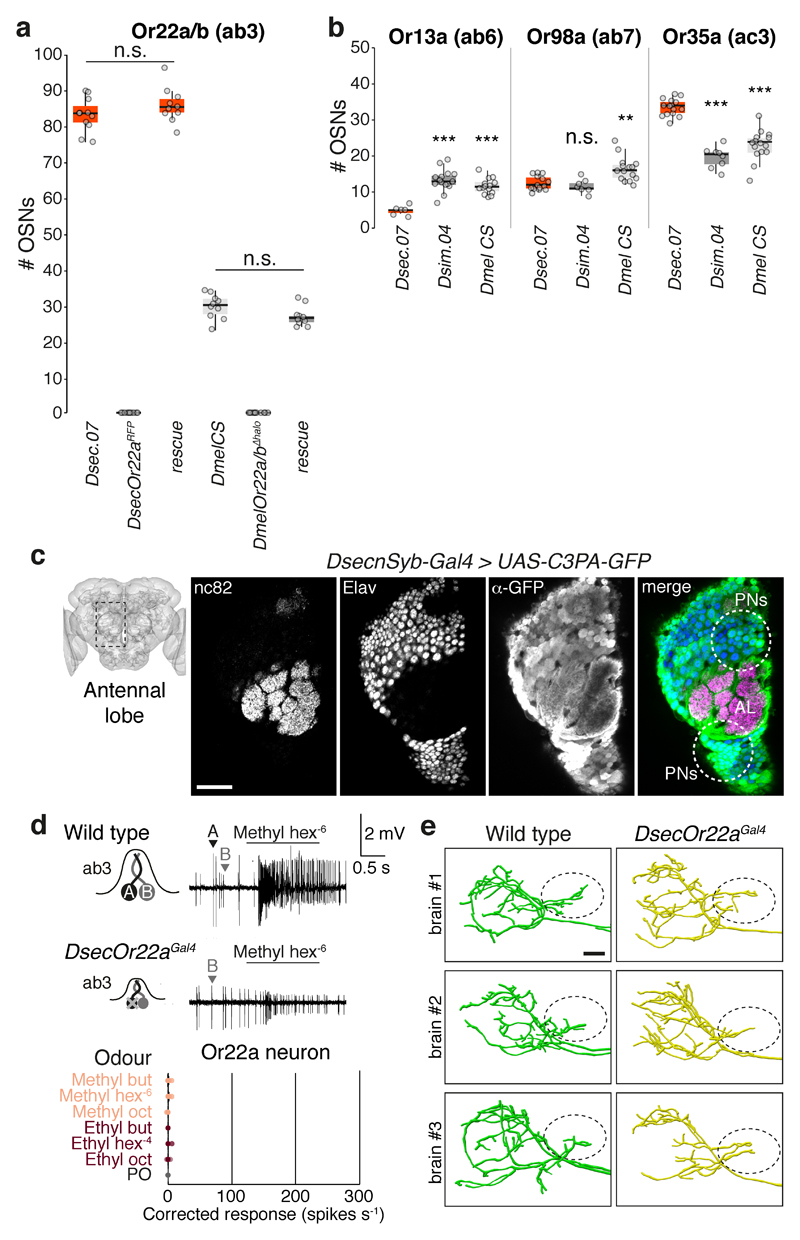
The functional similarity of OR22a orthologues in D. sechellia and D. simulans (Fig. 3c-d) indicates that additional changes occurred during speciation of D. sechellia. Concordant with ab3 sensilla counts11,29, D. sechellia exhibits a three-fold increase in the number of Or22a neurons (recapitulated in rescue experiments, Extended Data Fig. 10a) and the paired Or85c/b neurons, but not several other neuron classes (Fig. 4a-b, Extended Data Fig. 5d, 10b and 15)).
Fig. 4. Neuroanatomy of noni-sensing olfactory pathways.
a, Antennal Or22a/b RNA expression in different species. Scale bars, 25 μm.
b, Quantification of Or22a/(b) or Or42b OSNs (n = 8-11, females). Comparisons to Dsec.07 are shown (pairwise Wilcoxon rank-sum test, P values adjusted for multiple comparisons using the B&H method, b, d, e): *** P < 0.001; ** P < 0.01; * P < 0.05; n.s. P > 0.05.
c, Left: Or22aGal4-driven GCaMP6f expression in DM2 (arrowhead); neuropil visualised with nc82 (magenta). Scale bar, 25 μm. Right: Antennal lobe (AL) glomerular segmentation in D. sechellia (Extended Data Fig. 3). Scale bar, 50 μm.
d, Quantification of DM2, VM5d and DM1 volumes (n = 5 females).
e, DM2 projection neurons (PNs) labelled via photoactivation in D. sechellia (DsecnSyb-Gal4/UAS-C3PA-GFP) and D. melanogaster (UAS-SPA-GFP/UAS-C3PA-GFP;nSyb-Gal4/UAS-C3PA-GFP). Left: Image acquisition site. Top: AL with labelled PNs (arrows) and DM2 glomerulus; scale bar, 20 μm. Middle and bottom: PN innervation of mushroom body calyx and lateral horn (LH); scale bars, 10 μm. Arrowhead (e, f, g): extra anterio-medial branch in D. sechellia. Right: quantification of DM2 PNs (top) and calyx branches (bottom) (n = 14-17 females).
f, LH arbours of dye-filled DM2 PNs. Genotypes: D. sechellia, D. melanogaster as in e, D. simulans: DsimOr22aGFP. Below: representative LH DM2 arbour traces. Ovals: location of D. sechellia-specific branch. P, posterior; L, lateral; V, ventral. Scale bars, 10 μm.
g, Left: single dye-filled DM2 PN in D. sechellia; scale bar, 50 μm. Middle: representative LH arbour traces of DM2 PNs in D. sechellia and D. melanogaster; scale bar, 10 μm. Right: quantification of anteriomedial branch length (n = 4-9 females) (pairwise Wilcoxon rank-sum test): * P < 0.05.
h, Evolution of structural and physiological changes in the Or22a pathway.
To analyse OSN projections in D. sechellia, we inserted Gal4 at the corresponding receptor loci, and combined these with UAS-GCaMP6f as anatomical marker (Extended Data Fig. 3). Extending single-neuron dye-filling analyses11,13,15, OSN glomerular innervation patterns were indistinguishable between D. sechellia and D. melanogaster (Fig. 4c, Extended Data Fig. 3c-d). However, the glomerular targets of Or22a and Or85c/b neurons (DM2 and VM5d, respectively) were nearly doubled in volume in D. sechellia compared to both other species (Fig. 4d)11,13.
Differences in Or22a circuit wiring
To visualise higher-order elements of the Or22a pathway, we combined a pan-neuronal driver (Extended Data Fig. 10c) with a photoactivatable-GFP transgene to selectively photolabel DM2 projection neurons (PNs). Analysis with analogous genetic reagents in D. melanogaster – as well as targeted electroporation of a lipophilic dye35 into this glomerulus in D. simulans, D. sechellia and D. melanogaster – permitted cross-species comparisons. Two DM2 projection neurons (PNs) were consistently labelled in all three drosophilids (Fig. 4e).
PNs innervate the mushroom body (required for learning/memory) and the lateral horn (implicated in innate olfactory responses)36. Within the former, the number and arrangement of PN axonal branches were similar between species (Fig. 4e). In the lateral horn, global anatomy was conserved, with the main tract bifurcating into dorsal and ventral branches. However, dorsal to the bifurcation, D. sechellia DM2 PNs had a prominent branch innervating an area not targeted by the homologous D. melanogaster or D. simulans neurons (Fig. 4e-f). Using successive photo- and dye-labelling to visualise single DM2 PNs in D. sechellia and D. melanogaster, we confirmed quantitatively the presence of a D. sechellia-specific branch (Fig. 4g); this was also detected in DsecOr22a mutant animals (Extended Data Fig. 10d-e), indicating its independence of sensory input. These data raise the possibility that D. sechellia-specific central circuit changes form part of its olfactory specialisation towards noni.
Discussion
We have developed D. sechellia as a model to link genetic and neural circuit changes to behaviours relevant for its ecology. The characterisation of the Or22a pathway and comparison of this circuit’s functional and structural properties across closely-related species provides several insights into behavioural evolution (Fig. 4h).
The Or22a allele transfer experiments provide evidence that olfactory receptor tuning contributes to species-specific odour-evoked behaviour. Our definition of determinants of OR22a re-tuning also informs the molecular basis of odour/receptor interactions. When mapped onto a presumed homologous ORCO structure37, the key change (M93I) falls within a putative ligand-binding pocket, and may be a “hot-spot” for functional evolution (Extended Data Fig. 11a-c).
Although functional differences in this receptor are important, they cannot explain the behavioural differences of D. sechellia and D. simulans, as these receptors are interchangeable for supporting noni attraction. We note that the native Or22a/(b) neuron responses of these species are not identical (Fig. 3a-b); loss of Or22b in D. sechellia led to a narrowed (and possibly slightly increased) sensitivity to methyl esters, which could be behaviourally relevant. The D. sechellia-specific expansion of this neuron population is a likely key additional evolutionary innovation, although it is insufficient alone to restore D. sechellia-like host attraction when expressing DmelOR22a. The difference in D. sechellia PN axon projections suggests that central circuit connectivity changes form part of this species’ adaptation to noni. Future studies are necessary to understand the genetic bases and behavioural significance of these neuroanatomical differences.
The critical role of OR22a in host attraction in D. sechellia may account for the rapid molecular evolution of this locus (Extended Data Fig. 11d-h)38–40. D. erecta, a specialist on Pandanus fruit, also exhibits expansion of this OSN population21. However, a second noni-adapted drosophilid, D. yakuba mayottensis41 does not share the same receptor or OSN number changes we describe (Extended Data Fig. 11i-m) implying an independent evolutionary solution to locate a common host fruit.
Finally, other olfactory channels are important for noni attraction. These include Or85c/b neurons, which have conserved physiology while increasing in number in D. sechellia, and Ir75b neurons, which have both changed in function and number while apparently preserving partner PN projections15. Future application of the D. sechellia genetic toolkit should offer further fundamental insights into how genes and neurons control behaviour and enable evolution of novel traits.
Methods
Data reporting
Preliminary experiments were used to assess variance and determine adequate sample sizes in advance of acquisition of the reported data. Several experiments were carried out repeatedly because they served as controls for different genetic manipulations. In particular, we ran wild-type controls in parallel with mutant analyses in behavioural assays and therefore replicated them multiple times. In the wind tunnel assay the number of possible samples per day is rather low, leading to testing of flies of different genotypes on different days. In these cases experiments were started at the same time of the day under the stringently controlled conditions of temperature, humidity, light, and age of flies. For electrophysiological recordings, data were collected from multiple flies on multiple days in randomised order interleaving wild-type and mutant genotypes. Within datasets the same odour dilutions were used for acquisition of the dataset. In all cases the results were reliable and robust over the course of the many years it took to complete this study. For olfactory trap assays, the experiments were conducted with the experimenter blinded to the genotype. The experimenter was not blinded to the genotype of flies in the wind tunnel assay or physiological experiments. All replicates are biological replicates.
Volatile collection, gas chromatography and mass spectrometry
Volatiles were collected from 1 ml of fruit juice or 13 g of noni fruit at different ripening stages in capped 15 ml glass vials with poly-tetra-fluoroethylene-lined silicone septa (Sigma, 23242-U). After penetrating the septum of the cap with a Solid Phase Microextraction (SPME) fibre holder, the SPME fibre (grey hub plain, coated with 50/30 μm divinylbenzene/carboxen on polydimethylsiloxane on a StableFlex fibre (Sigma, 57328-U)) was exposed to the headspace of each vial for 30 min at room temperature. For collection of headspaces in the trap assay, a single noni juice trap was placed in the arena and odours were collected with SMPE for 5 min at 0 h, 5 h and 10 h after placement. For collection of headspaces in the wind tunnel assay, samples were captured with SPME for 10 min at the landing platform and the release platform directly after noni juice application. After each odour collection, the SPME fibre was retracted and immediately inserted into the inset of a Gas Chromatography-mass spectrometry (GC-MS) system (Agilent 7890B fitted with MS 5977A unit) for desorption at 260ºC in split mode (split ratio 100:1). The GC was operated with a HP-INNOWax column (Agilent 19091N-133UI). Samples were injected at an initial oven temperature of 50°C; this temperature was held for 1 min and gradually increased (3°C min-1) to 150°C before holding for 1 min. Subsequently, the temperature was increased (20°C min-1) to 260°C and held for 5 min. The MS-transfer-line was held at 260°C, the MS source at 230°C, and the MS quad at 150°C. MS spectra were taken in EI-mode (70 eV) in a 29-350 m/z range. Between different collections, the SPME fibre was conditioned at 270°C for 15 min. All chromatograms were processed using MSD ChemStation F.01.03.2357 software. Volatile compounds were identified using the NIST library and matched to standards of the Max-Planck-Institute for Chemical Ecology library. For quantification, peak areas were measured for 3 replicates for each sample. Note that SPME allows only for a qualitative analysis of odour compositions as well as for an estimate of changing ratios of odours across different samples. Vapour pressure values for hexanoic acid, methyl hexanoate and 2-heptanone were described previously (www.thegoodscentscompany.com)42,43.
Drosophila strains
Drosophila stocks were maintained on standard wheat flour/yeast/fruit juice medium under a 12 h light:12 h dark cycle at 25°C. For all D. sechellia strains, a few g of Formula 4-24® Instant Drosophila Medium, Blue (Carolina Biological Supply Company) soaked in noni juice (nu3 GmbH) were added on top of the standard food. Wild-type Drosophila strains are described in the corresponding figure legends or Supplementary Table 2. These strains do not show intraspecific sequence variation in OR22a or IR75b for known odour specificity-determining residues (Extended Data Fig. 12), suggesting that other polymorphisms (or non-genetic factors) underlie the observed minor intraspecific behavioural differences (Fig. 1b,c). The mutant and transgenic lines used and generated in this study are listed in Supplementary Table 2.
CRISPR/Cas9-mediated genome engineering
sgRNA expression vectors: for expression of single sgRNAs, oligonucleotide pairs (Supplementary Table 3) were annealed and cloned into BbsI-digested pCFD3-dU6-3gRNA (Addgene #49410), as described44. To express multiple sgRNAs from the same vector backbone, oligonucleotide pairs (Supplementary Table 4) were used for PCR and inserted into pCFD5 (Addgene #73914) via Gibson Assembly, as described45.
Donor vectors for homologous recombination: to generate an eGFP-expressing donor vector (pHD-Stinger-attP), the fluorophore was excised from pStinger46 with NcoI/HpaI and used to replace the DsRed sequence in NcoI/HpaI-digested pHD-DsRed-attP (Addgene plasmid #51019)47. Homology arms (1-1.6 kb) for individual target genes were amplified from D. sechellia (Drosophila Species Stock Center [DSSC] 14021-0248.07), D. simulans (DSSC 14021-0251.195) or D. melanogaster (Research Resource Identifier Database:Bloomington Drosophila Stock Center [RRID:BDSC]_58492) genomic DNA and inserted either into pHD-DsRed-attP or pHD-Stinger-attP via restriction cloning. Details and oligonucleotide sequences are available from the corresponding authors upon request.
Transgenic source of Cas9: pBac(nos-Cas9,3XP3-YFP) (gift of D. Stern) was integrated into D. sechellia (DSSC 14021-0248.07) via piggyBac transgenesis. The insertion was mapped to the fourth chromosome using TagMap48.
Transgene construction
Oligonucleotides for each cloning step are listed in Supplementary Table 5.
attB-nSyb-Gal4,miniW: 1.9 kb upstream sequence of the neuronal Synaptobrevin (nSyb) gene were amplified from D. sechellia genomic DNA (DSSC 14021-0248.07) and inserted into pGal4attB49 via restriction cloning using NotI and KpnI.
attB-Gal4,3XP3-Stinger: we first generated an eGFPnls-SV40 fragment via PCR (using pHD-Stinger-attP as template) and fused it to a minimal attB40 site50,51 before insertion into pCR-Blunt II-TOPO (Thermo Fisher). We added a 3XP3-Stinger fragment amplified from pHD-Stinger via restriction cloning using EcoRV and SalI. Subsequently, we placed a loxP site downstream of the initial SV40 sequence via oligonucleotide annealing and SpeI/KpnI restriction cloning, to produce pCR-TOPO-loxP-attB40-eGFPnlsSV40rev-3XP3:Stinger. We replaced the eGFPnls-SV40 sequence with an hsp70-Gal4-SV40 fragment via PCR amplification of the vector backbone and Gal4 from pGal4attB49 and subsequent Gibson Assembly resulting in attB-Gal4,3XP3-Stinger.
attB-Or22awt,3XP3-Stinger: in the pCR-TOPO-loxP-attB40eGFPnlsSV40rev-3XP3-Stinger plasmid described above, the eGFPnlsSV40 fragment was flanked by EcoRV and SalI sites, which were used to integrate the D. sechellia or D. melanogaster Or22a ORF+3’UTR after PCR amplification from cDNA, or the D. simulans Or22a ORF+3’UTR (synthesised by Eurofins Genomics), to produce attB-DsecOr22awt,3XP3-Stinger, attB-DmelOr22awt,3XP3-Stinger, and attB-DsimOr22awt,3XP3-Stinger, respectively.
Or22a chimeras: chimeric sequences of D. sechellia and D. melanogaster Or22a were generated by PCR amplification and fusion using the respective species’ Or22a gene templates. After subcloning into pCR-Blunt II-TOPO and sequence confirmation, the chimeras were integrated into pCR-TOPO loxP attB40eGFPnlsSV40rev-3XP3-Stinger via restriction cloning.
Or22a site-directed mutant constructs: point mutations were introduced via site directed mutagenesis following standard procedures.
attB-UAS-constructs: DsecOr22awt, DmelOr22awt, DmelOr22atriple and DmelOr22aM93I were amplified by PCR incorporating flanking EcoRI and SalI restriction sites using the constructs described above as template, and integrated into the EcoRI/XhoI-digested pUAST-attB52.
pDONR221-MCS: a pDONR221 entry vector carrying a multiple cloning site (MCS) was generated by amplification of the MCS of pCR-Blunt II-TOPO incorporating flanking attB1/2 sites and integration of the PCR fragment into pDONR221 via a BP reaction (Gateway, Thermo Fisher Scientific).
pDONR221-DsimOr22a: a 5.7 kb promoter region upstream of the Or22a start codon was amplified from D. simulans (DSSC 14021-0251.195) genomic DNA, subcloned into pCR-Blunt II-TOPO and transferred to pDONR221-MCS via BamHI/EcoRV restriction cloning.
pDEST-Hemmar-eGFPnls: the eGFPnls fragment of pStinger46 was amplified by PCR incorporating XhoI and SpeI restriction sites and integrated into the XhoI/XbaI-digested vector pDEST-HemmarG53 to replace eGFP.
pDsimOr22a-eGFPnls: pDONR221-DsimOr22a and pDEST-Hemmar-eGFPnls were combined using LR recombination (Gateway, Thermo Fisher Scientific).
Drosophila microinjections
Transgenesis of D. sechellia, D. simulans and D. melanogaster was performed in-house following standard protocols (http://gompel.org/methods), except for DsimOr22a-GFPnls (generated by Rainbow Transgenic Flies Inc.). For the D. sechellia egg-laying agar plates, we replaced grape juice with noni juice and added on the surface a few g of Formula 4-24® Instant Drosophila Medium, Blue (Carolina Biological Supply Company) soaked in noni juice (nu3 GmbH). Embryos were manually selected for the appropriate developmental stage prior to alignment and injection. For piggyBac transgenesis, we co-injected piggyBac vector (300 ng μl-1) and piggyBac helper plasmid54 (300 ng μl-1). For CRISPR/Cas9-mediated homologous recombination, we injected a mix of an sgRNA-encoding construct (150 ng μl-1), donor vector (400 ng μl-1) and pHsp70-Cas9 (400 ng μl-1) (Addgene #45945)55. The DsRed fluorescent marker was destroyed in DsecnSyb-Gal4 and DsecUAS-C3PA-GFP via injection of an sgRNA construct targeting DsRed (150 ng μl-1) and pHsp70-Cas9 (400 ng μl-1). Injections into Dsecnos-Cas9 were of a mix of an sgRNA construct (150 ng μl-1) and donor vector (500 ng μl-1). Site-directed integration into attP sites was achieved by co-injection of an attB-containing vector (400 ng μl-1) and either p3xP3-EGFP.vas-int.NLS (400 ng μl-1) (Addgene #60948)56 or pBS130 (encoding phiC31 integrase under control of a heat shock promoter (Addgene #26290)57). All concentrations are given as final values in the injection mix.
Wind tunnel assay
Long-range attraction experiments were performed in a wind tunnel as described previously23 with a flight arena of 30 cm width, 30 cm height and 100 cm length. The airstream in the tunnel (0.3 m s-1) was produced by a fan (Fischbach GmbH, Neunkirchen, Germany), and filtered through an array of four activated charcoal cylinders (14.5 cm diameter × 32.5 cm length; Camfil, Trosa, Sweden). The wind tunnel was maintained within a climate chamber at 25°C and 50-55% relative humidity under white light. Flies were starved for approximately 20 h; to ensure the flight ability of assayed animals, flies were first released into a mesh cage (50 × 50 × 50 cm, maintained at the same conditions as the wind tunnel) and females escaping from the food vial were collected with an aspirator. For each assay, ten 4-6 day-old females were released from a plastic tube (with a mesh covering one end and the open end facing the landing platform) fixed horizontally in the centre of the first 5-10 cm of the downwind end of the tunnel. The landing platform was built by a filter paper (3 × 3 cm) charged with either 100 μl of juice (noni (Nu3 GmbH), grape (Beutelsbacher Fruchtsaftkelterei), pineapple (Andros), mango (Migros)), apple cider vinegar (Migros) or ~100 μl of homogenised ripe fruit (noni, fig) and fixed on a metal holder. Fruit homogenisation was performed by blending 10 mg of ripe fruit in 20 ml of distilled water, fruit particles were pelleted by centrifugation and the supernatant harvested for experiments. In two-choice assays two identical landing platforms were positioned with equal distance (7.5 cm) from the centre of the air stream alternating the position of noni fruit and apple cider vinegar between assays. The fly tube was placed within the centre of the airstream and 85 cm downwind of the odour source. An experimenter observed the landing platform(s) for the entire duration of the assay; flies arriving and staying on the landing platform(s) within the first 10 min after release were counted.
Olfactory trap assay
The two-choice olfactory trap assay was performed essentially as described15. For each experiment, traps contained either 300 μl of juice (noni (Nu3 GmbH), grape (Beutelsbacher Fruchtsaftkelterei), pineapple (Andros), mango (Migros)) or apple cider vinegar (Migros). When using fruits as stimuli (noni, grape, papaya, banana, fig), ripe fruits were peeled (banana, papaya) or used whole, homogenised with pestles, and each trap was filled with a spatula of the mix (to ~300 μl). Single odours (see below for CAS numbers) were used at a 10-2 dilution in grape juice. Triton X-100 (final concentration 0.2%) was added to all traps containing single odours and respective control traps to drown trapped flies. 25 fed, mated, ice-anesthetised female flies (3-5 day-old) were used for each experiment. D. sechellia flies were transferred to standard food without noni supplement 24 h prior to the start of the assay, unless stated otherwise. The distribution of flies was scored after 24 h at 25°C under red light at 60% relative humidity; experiments with >25% dead flies in the arena after 24 h were discarded. The attraction index was calculated as follows: (number of flies in treatment (e.g., noni juice) trap - number of flies in control (e.g., grape juice) trap)/number of trapped and untrapped flies alive. For the trap assay quantifications in Fig. 2e, all untrapped flies (including those that died during the assay) were counted due to the high mortality rate in these experiments (Extended Data Fig. 7c).
The multiple-choice olfactory trap assay was performed using eight traps containing mango, pineapple, noni and grape juices, apple cider vinegar and fig, banana and papaya fruits. Traps were placed equidistantly in a circle in random order for each experiment and conditions were as described for the two-choice assay. The percentage of flies per trap was calculated as follows: (number of flies in trap/number of trapped and untrapped flies alive)×100. Experiments with >25% dead flies in the arena after 24 h were discarded.
Two-photon calcium imaging
Flies were mounted and dissected as previously described58, and images were acquired using a commercial upright two-photon microscope (Zeiss LSM 710 NLO). In detail, an upright Zeiss AxioExaminer Z1 was fitted with a Ti:Sapphire Chameleon Ultra II infrared laser (Coherent) as excitation source. Images were acquired with a 20× water-immersion objective (W Plan-Apochromat 20×; NA 1.0, VIS-IR DIC), with a resolution of 128×128 pixels (1.1902 pixels μm-1) and a scan speed of 12.6 μs pixel-1. The excitation wavelength was set to 920 nm at a laser output of 64.1-70.2 mW measured at the exit of the objective. Emitted light was filtered with a 500-550 nm band-pass filter, and photons were collected by an external non-descanned detector. Each measurement consisted of 50 images acquired at 4.13 Hz, with stimulation starting ~5 s after the beginning of the acquisition and lasting for 1 s. Fly antennae were stimulated using a custom-made olfactometer as previously described59, with minor modifications. In brief, the fly’s antennae were permanently exposed to air flowing at a rate of 1.5 l min-1 and with 55% relative humidity obtained by combining a main stream of humidified room air (0.5 l min-1) and a secondary stream (1 l min-1) of normal room air. Both air streams were generated by vacuum pumps (KNF Neuberger AG) and the flow rate was controlled by two independent rotameters (Analyt). The secondary air stream was guided through either an empty 2 ml syringe or, to generate an odour pulse, a 2 ml syringe containing 20 μl of odour or solvent on a small cellulose pad (Kettenbach GmbH). Solvents were either double-distilled water (for noni juice and apple cider vinegar) or paraffin oil (for methyl hexanoate (CAS 106-70-7), 2-heptanone (CAS 110-43-0), 2,3-butanedione (CAS 431-03-8), ethyl propionate (CAS 105-37-3) and 1-hexanol (CAS 111-27-3)). To switch between control air and odour stimulus delivery, a three-way magnetic valve (The Lee Company, Westbrook, CT) was controlled using Matlab via a VC6 valve controller unit (Harvard Apparatus). Data were processed using Fiji60 and custom written scripts in Matlab and R as previously described59. Since bleaching was very strong at the beginning of each acquisition, the first 1.5 s were not considered for the analysis, and bleach correction was not applied. Colour-coded images and boxplots show the peak response calculated as the mean relative change in fluorescence (% ΔF/F) of three frames around the maximum during frames 19-30.
Widefield calcium imaging
Flies were mounted and dissected as previously described58. Images were acquired with a CCD camera (CoolSNAP-HQ2 Digital Camera System) mounted on a fluorescence microscope (upright fixed stage Carl Zeiss Axio Examiner D1) equipped with a 20× water-immersion objective (W Plan-Apochromat 20×; NA 1.0, VIS-IR DIC, Extended Data Fig. 4a) or 40× water-immersion objective (W Plan-Apochromat 40×; NA 1.0 VIS-IR DIC, Extended Data Fig. 4f). Excitation light of 470 nm was produced with an LED light (Cool LED pE-100, VisiChrome, intensity 1.8-3.9%). Light was guided through a filter block consisting of a 450-490 nm excitation filter, a dichroic mirror (T495LP), and a 500-550 nm emission filter (Chroma ET). Binned image size was 400×300 pixels (Extended Data Fig. 4a) or 266×200 pixels (Extended Data Fig. 4f) on the chip, corresponding to 465×349 μm (Extended Data Fig. 4a) or 149×112 μm (Extended Data Fig. 4f) in the preparation. Exposure time varied between 30-100 ms to adjust for different basal fluorescence values across preparations. Films (12.5 s duration) were recorded with an acquisition rate of 4 Hz. Metafluor software (Visitron) was used to control the camera, light, data acquisition and onset of odour stimulation. Odour stimulation and data analysis were otherwise performed as described for two-photon calcium imaging.
Electrophysiology
Single sensillum electrophysiological recordings were performed as described previously61. Noni and grape juice were purchased from Nu3 (nu3 GmbH) and Beutelsbacher (Beutelsbacher Fruchtsaftkelterei GmbH) and chemicals of the highest purity available from Sigma-Aldrich. Odorants were used at 10-2 (v/v) in all experiments unless noted otherwise in the figures or figure legends. Solvents were either double-distilled water (for noni juice, butyric acid (CAS 107-92-6), hexanoic acid (CAS 1821-02-9)) or paraffin oil (for octanoic acid (CAS 124-07-2), methyl butanoate (CAS 623-42-7), methyl hexanoate (CAS 106-70-7), methyl octanoate (CAS 111-11-5), ethyl butanoate (CAS 105-54-4), ethyl hexanoate (CAS 123-66-0), ethyl octanoate (CAS 106-32-1), 2-heptanone (CAS 110-43-0), 1-hexanol (CAS 111-27-3) and 3-buten-1-ol (CAS 627-27-0)). Odours for sensilla stimulation were used for a maximum of five consecutive trials. If noni juice or noni fruit extract (see Wind tunnel assay section for details) were used, odours were renewed after two stimulations or, for 10-4, 10-3 and 10-2 dilutions, after each stimulation. Corrected responses were calculated as the number of spikes in a 0.5 s window at stimulus delivery (200 ms after stimulus onset to take account of the delay due to the air path) subtracting the number of spontaneous spikes in a 0.5 s window 2 s before stimulation, multiplied by two to obtain spikes s-1. The solvent-corrected responses shown in the figures were calculated by subtracting from the response to each diluted odour the response obtained when stimulating with the corresponding solvent. Recordings were performed on a maximum of three sensilla per fly. Exact n and mean spike counts for all experiments are provided in Supplementary Data Table 7.
Immunohistochemistry
Fluorescent RNA in situ hybridisation using digoxigenin- or fluorescein-labelled RNA probes and immunofluorescence on whole-mount antennae were performed essentially as described59,62. D. sechellia probe templates were generated by amplification of regions of genomic DNA (DSSC 14021-0248.07) using primer pairs listed in Supplementary Data Table 6; these were cloned into pCR-Blunt II-TOPO and sequenced. D. sechellia OR22a antibodies were raised in rabbits against the peptide epitope PHISKKPLSERVKSRD (amino acids 7-22), affinity-purified (Proteintech Group Inc) and diluted 1:250. Other antibodies used were: guinea pig α-IR75b (RRID:AB_2631093)63 1:200, rabbit α-IR64a (RRID:AB_2566854)34 1:100, rabbit α-ORCO28 1:200, guinea pig α-IR8a (RRID:AB_2566833)27 1:500, rabbit α-IR25a (RRID:AB_2567027) 1:50064, rabbit α-GFP 1:500 (Invitrogen). Immunofluorescence on adult brains was performed as described65 (except for the D. sechellia reference brain samples; see below) using mouse monoclonal antibody nc82 1:10 (Developmental Studies Hybridoma Bank), rat monoclonal α-Elav 1:10 (Developmental Studies Hybridoma Bank) and rabbit α-GFP 1:500 (Invitrogen). Alexa488-, Cy3- and Cy5-conjugated goat α-guinea pig, goat α-mouse, goat α-rat and goat α-rabbit IgG secondary antibodies (Molecular Probes; Jackson Immunoresearch) were used at 1:500.
D. sechellia reference brain
D. sechellia (DSSC 14021-0248.07) brains (2-7 day-old animals) were stained with nc82 and imaged as described66. From 88 female brains imaged, 26 high-quality confocal stacks of the midbrain were selected for averaging on a selected “seed” brain, essentially as described67,68. Similarly, a male reference brain (not shown here) was constructed, using 20 high-quality confocal stacks (from 87 initially imaged). Reciprocal bridging registrations between D. melanogaster and D. sechellia references brains were also generated to permit comparison of homologous neurons within a common template, essentially as described68. The reference brains (DsecF and DsecM), bridging registrations and associated code are available for download via http://jefferislab.org/si/auer2019.
Image acquisition and processing
Confocal images of antennae and brains were acquired on an inverted confocal microscope (Zeiss LSM 710) equipped with an oil immersion 40× objective (Plan Neofluar 40× Oil immersion DIC objective; 1.3 NA), unless stated otherwise. Images were processed in Fiji60. D. sechellia brains were imaged and registered to a D. sechellia reference brain using the Fiji CMTK plugin (https://github.com/jefferis/fiji-cmtk-gui), as described69. For segmentation of individual glomeruli of the antennal lobe, glomerular identity was confirmed by location and labelling with Gal4 reporters (Extended Data Fig. 3) and segmentation performed using Amira 6.5 (Thermo Fisher Scientific). Glomerular volumes were calculated following segmentation with the Segmentation Editor plugin of Fiji using the 3D Manager Plugin. OSN numbers were counted using the Cell Counter Plugin in Fiji or Imaris (Bitplane). Projection neuron morphologies were reconstructed and measured in neuTube 1.0z70.
Projection neuron labelling
Photoactivation was performed as described35 on 3-5 day-old female flies. Brains were dissected in saline71 (low carbonate; 2 mM Mg2+ pH 7.2) and treated with collagenase (2 mg ml-1, 45 s). Dissected brains were initially imaged at 925 nm to identify the DM2 glomerulus based on anatomical position. Photoactivation was achieved through multiple cycles of exposure to 710 nm laser light with a 15 min rest period between each cycle to allow diffusion of the photoactivated fluorophore within the neuron. Photoactivation and imaging was performed on an Ultima two-photon laser scanning microscope (Bruker) equipped with galvanometers driving a Chameleon XR laser (Coherent). Emitted photons were collected with a GaAsP photodiode detector (Bruker) or a PMT detector through a 60× objective (Olympus 60× water immersion; 0.9 NA).
PN dye-fillings were performed as described35 with some modifications. Brains were dissected in saline, briefly treated with collagenase (2 mg ml-1, 45 s), washed and pinned with fine tungsten wires to a Sylgard sheet (World Precision Instruments) in a 35 mm Petri dish (Falcon) filled with saline. Pulled glass electrodes were backfilled with Texas Red Dextran (3000, lysine fixable, Thermo Scientific). The electrode was targeted to the DM2 glomerulus using as a guide either basal expression of the pan-neuronal, photoactivatable GFP (D. sechellia and D. melanogaster) or DM2-specific labelling (D. simulans, i.e., the Or22a-GFPnls strain, in which trace GFP levels were detected in OSN axon termini). The dye was electroporated by applying voltage pulses (30 V) until it became visible in distal neural processes of the PN, and left to diffuse for 60 min. Brains were subsequently imaged by two-photon microscopy, as described above.
To label single PNs, the DM2 glomerulus was first subjected to one cycle of exposure to 710 nm laser light to identify the cell bodies of DM2 PNs. Subsequently, the filled glass electrode was placed in the centre of the soma of one DM2 PN and the dye was electroporated by applying voltage pulses (30 V) until it became visible in distal neural processes of the neuron. The dye was left to diffuse for 60 min, before the brains were fixed with 2% paraformaldehyde for 45 min and subjected to immunofluorescence using nc82 (1:20) and α-mouse Alexa488 (1:500), as described above. Images were acquired on a Zeiss LSM 880 Airy scan confocal microscope using a 40× objective (Plan Neofluar 40× oil immersion DIC objective; 1.3 NA).
Molecular evolution and polymorphism analyses
The Or22a/b gene tree was inferred using annotations after manual verification by BLASTing OR22a/b protein sequences (using tblastn with default settings, BLAST+ v2.7.172) to the genomes of D. simulans (r2.01), D. mauritiana (r1), D. yakuba (r1.04), D. sechellia (r1.3) and D. erecta (r1.3). Genomic regions were annotated using Wise2 (v2.4.1)73, with D. melanogaster’s protein sequences as guides. All genes appeared intact and consistent with existing models, with one exception: D. mauritiana Or22b is truncated by ~240 bp due to a gap in the reference genome. The cDNAs were frame-aligned using TranslatorX74. The gene tree based on these alignments was inferred using MrBayes (v3.2.6)75 with the following settings: rate variation = invariable+gamma, number of substitutions = 6, substitution model = default, number of generations = 10, sample frequency = 10, burn-in = 250.
The Or22a/b topology outputted from MrBayes was used for PAML (v4.8)76 CodeML branch tests of protein evolution rate change. Seven models were tested, one with a freely varying ω value and six other models with 1, 4, 5, 7, 8 and 9 ω values (CodeML parameters were all set to zero except for the following: seqtype = 1, CodonFreq = 2, ndata = 1, model = 2, kappa = 2, omega = 0.4, fix_alpha = 1, ncatG = 5, getSE = 1, Small_Diff = 5e-7, cleandata = 1). Nested comparisons between these models using likelihood ratio tests identified a model with 5 ω values to provide the best fit (Supplementary Data Table 8, Extended Data Fig. 11d).
Polymorphism data for Or22a/b was extracted from previously published data. The D. simulans and D. sechellia datasets were from7,77 and the D. melanogaster datasets from78,79. VCFtools (v0.1.17)80 was used to extract variable sites from the respective VCF files, as well as to output allele frequencies. Variant calling in7 was based on a subversion of the D. simulans r2 genome, which is slightly different than r2 on FlyBase. This subversion is available at https://github.com/kern-lab/FILET/blob/master/simSechResults/dsimV2-Mar2012_chrsonly.fa.bz2.
Statistics and reproducibility
Data were analysed and plotted using Excel and R (v3.2.3; R Foundation for Statistical Computing, Vienna, Austria, 2005; R-project-org).
Extended Data
Extended Data Fig. 1. Species-specific short- and long-range behavioural responses to diverse fruit stimuli.
a, Data reproduced from Fig. 1c. Behavioural responses in a trap assay testing preferences between noni and grape, or between noni juice and grape juice (n = 15-27 experiments; 22-25 females/experiment). Comparisons to Dsec.07 responses to noni juice are shown (a-d and h (right): pairwise Wilcoxon rank-sum test and P values adjusted for multiple comparisons using the B&H method): *** P < 0.001; n.s. P > 0.05.
b, Proportion of flies (mean ± SEM) in each stimulus trap for the assays shown in a. Comparisons to Dsec.07 responses are shown.
c, Behavioural responses in a trap assay testing preferences between noni juice and diverse fruit juices or fruits for D. sechellia (DSSC 14021-0248.07), D. simulans (DSSC 14021-0251.004) and D. melanogaster (Canton-S) (n = 10-11 experiments; 22-25 females/experiment). Comparisons to Dsec.07 responses are shown.
d, Proportion of flies (mean ± SEM) in each stimulus trap for the assays shown in c. Comparisons to Dsec.07 responses are shown.
e, Radar plot showing the mean percentage of flies per trap in a multiple choice trap assay with eight different stimuli for D. sechellia (DSSC 14021-0248.07), D. simulans (DSSC 14021-0251.004) and D. melanogaster (Canton-S) (n = 11 experiments; 22-25 females/experiment). ACV = Apple cider vinegar.
f, Left: Behavioural responses to noni juice in a wind tunnel assay of D. sechellia (DSSC 14021-0248.07) reared on standard food with (+) and without (-) noni supplement (Kruskal-Wallis test: n.s. P > 0.05). Right: Behavioural responses in a trap assay testing preferences between noni juice and grape juice for D. sechellia (DSSC 14021-0248.07) reared on standard food with (+) and without (-) noni supplement (pairwise Wilcoxon rank-sum test: n.s. P > 0.05).
g, Behavioural responses to apple cider vinegar, mango juice, pineapple or fig in a wind tunnel assay of D. sechellia (DSSC 14021-0248.07), D. simulans (DSSC 14021-0251.004) and D. melanogaster (Canton-S) (n = 10-12 experiments; 10 females/experiment). Comparisons to Dsec.07 responses are shown (g and h (left): Kruskal-Wallis test with Dunn’s post-hoc correction): * P < 0.05; n.s. P > 0.05.
h, Behavioural responses in a wind tunnel assay testing preference between noni fruit and apple cider vinegar of D. sechellia (DSSC 14021-0248.07), D. simulans (DSSC 14021-0251.004) and D. melanogaster (Canton-S) (n = 15 experiments; 10 females/experiment). Left: total number of flies landing on an odour source. Comparisons to Dsec.07 responses are shown. Right: attraction index calculated as: (flies landing on apple cider vinegar – flies landing on noni)/flies landing on either source. Comparisons to Dsec.07 responses are shown.
Extended Data Fig. 2. Chemicals emitted by natural odour sources and odour bouquet changes in behavioural assays.
a, Principal constituents of the odour bouquet of noni fruit at different ripening stages and commercial noni juice, as determined by gas chromatography/mass spectrometry. AU = arbitrary units.
b, Chemical composition of the odour bouquet of noni fruit at different stages of ripening, noni juice, grape juice and apple cider vinegar. Representative gas chromatograms are shown on the right. Numbers correspond to compounds as listed in Supplementary Table 1 (not all identified peaks are shown).
c, Dose-dependent electrophysiological responses of Or22a, Or85c/b and Ir75b neurons in wild-type D. sechellia (DSSC 14021-0248.07) to their best odour agonists (mean ± SEM and individual data points, n = 7-20, females). The contribution of Or35a neurons (whose spiking is difficult to separate from Ir75b neurons in ac3I) to hexanoic acid responses is likely to be minimal (Fig. 2b). D. sechellia Or22a and Or85c/b dose response data are replotted from Fig. 3a.
d, Chemical profile of odours collected by SPME at the release and landing platforms in the wind tunnel assay within the first 10 min after noni juice application.
e, Chemical profile of odours collected by SPME in the trap assay arena within 5 min after placement of a trap (i.e., t = 0 h), and after 5 h and 10 h, using noni juice as stimulus. “0%” indicates only trace proportions of the compound were detected.
Extended Data Fig. 3. Olfactory sensory neuron Gal4 driver lines in D. sechellia.
a, Schematic of the Gal4 reporter allele generation strategy, through CRISPR/Cas9-mediated integration of an attP site (marked by 3xP3:DsRed) into the desired Or or Ir gene (see Extended Data Fig. 5-6 for details on specific alleles), followed by introduction of a Gal4 open reading frame via phiC31-mediated transgenesis.
b, Co-expression of the indicated OrGal4-driven, IrGal4-driven or control background GCaMP6f signal (detected by α-GFP) with the corresponding receptor protein or RNA in whole-mount antennae. Arrowheads point to examples of co-labelled cells. Scale bars, 25 μm; inset scale bars, 5 μm. While Or22aGal4 and Ir64aGal4 completely recapitulate endogenous receptor expression, OrcoGal4 and Or85c/bGal4 lack expression in some receptor-expressing neurons. Or35aGal4 and Ir75bGal4 might be expressed in ectopic cells (see also c-d) or the protein/RNA signal for these receptor genes could be below the detection threshold.
c, Expression of the indicated OrGal4-driven, IrGal4-driven or control background GCaMP6f signal (detected by α-GFP) in glomeruli of whole-mount antennal lobes. Three focal planes of the neuropil (visualised with nc82 (magenta)) are shown. Images were registered to a D. sechellia reference brain (see Methods) for better comparison of antennal lobe structure. Scale bar, 25 μm.
d, Summary of the glomerular labelling by OrGal4 or IrGal4 drivers as characterised in c (dark blue indicates GCaMP6f signal was detected in at least 3/3 independent brains). Glomeruli are organised by the compartmentalisation of the corresponding OSN populations into different sensilla classes (based on data in D. melanogaster81, ab: antennal basiconic; at: antennal trichoid; ai; antennal intermediate; ac: antennal coeloconic, pb = palp basiconic, sac = sacculus, ? = OSN population unknown). OrcoGal4 is expressed in most but not all (e.g., Or67d/DA1) expected OSN populations; Or35aGal4 and Or85c/bGal4 display some ectopic expression as inferred by their labelling of more than one glomerulus.
Extended Data Fig. 4. Comparative olfactory representations of noni in D. melanogaster and D. sechellia.
a, Representative odour-evoked calcium responses in the axon termini of Orco OSNs in the antennal lobes of D. melanogaster (Orco-Gal4/Orco-Gal4;UAS-GCaMP6f/UAS-GCaMP6f) and D. sechellia (UAS-GCaMP6f/UAS-GCaMP6f;;DsecOrcoGal4/+;), acquired by widefield imaging. Left images: raw fluorescence signals. Right images: relative increase in GCaMP6f fluorescence (ΔF/F%) after stimulation with the indicated complex stimuli and single odours. Diagnostic odours: methyl hexanoate (10-6 (v/v)) for Or22a/(b)/DM2, ethyl propionate (10-4) for Or42b/DM1, 2-heptanone (10-5) for Or85c/b/VM5d, 2,3-butanedione (10-4) for Or92a/VA2 and 1-hexanol (10-4) for Or35a/VC3. Glomerular boundaries, and the entire antennal lobe, are outlined. Scale bars, 50 μm.
b, Quantification of odour-evoked calcium responses for the animals represented in a. Maximum calcium response amplitudes for each experiment are plotted (n = 5-8 females). Significantly different responses of species to the same stimulus are shown (Wilcoxon signed-rank test): ** P < 0.01; * P < 0.05.
c, Combined electrophysiological responses of neurons in the ab3 sensillum in D. melanogaster (top) and D. sechellia (bottom) upon stimulation with increasing concentrations of noni juice or noni fruit extract (mean ± SEM and individual data points, n = 6, females). Significant differences in responses are shown (pairwise Wilcoxon rank-sum test): ** P < 0.01; * P < 0.05. Note that responses of D. sechellia are stronger to noni fruit than noni juice, which may reflect the lower abundance of relevant ligands in the juice.
d, Representative odour-evoked calcium responses in the axon termini of Orco OSNs in the antennal lobe of D. sechellia (UAS-GCaMP6f/UAS-GCaMP6f;;DsecOrcoGal4/+) acquired by two-photon imaging. Three focal planes are shown, revealing different glomeruli along the dorsoventral axis. Left column: raw fluorescence images. Other columns: relative increase in GCaMP6f fluorescence (ΔF/F%) after stimulation with diagnostic odours. Scale bar, 25 μm.
e, Electrophysiological responses in the antennal coeloconic (ac) sensilla classes to the indicated stimuli (n = 6-11 sensilla, females) in D. sechellia (DSSC 14021-0248.07) representing the summed, solvent-corrected activities of the two or three neurons they house.
f, Representative odour-evoked calcium responses in the axon termini of Ir64a OSNs in the D. sechellia antennal lobe (genotype: UAS-GCaMP6f/UAS-GCaMP6f;;DsecIr64aGal4/+) acquired by widefield imaging. Left images: raw fluorescence signals. Right images: relative increase in GCaMP6f fluorescence (ΔF/F%) after stimulation with noni juice (10-2) or grape juice. Scale bar, 25 μm.
g, Quantification of odour-evoked calcium responses for the animals represented in f. Maximum calcium response amplitudes for each experiment are plotted (n = 7-10, females). Comparisons of responses to noni (10-2) and grape juice are shown (Wilcoxon signed-rank test): * P < 0.05.
Extended Data Fig. 5. Generation and validation of loss-of-function alleles of D. sechellia Or genes.
a, Schematic of the strategy for generating olfactory receptor mutant alleles, through integration of an eye-expressed fluorescent marker (3xP3:DsRed or 3xP3:GFPnls) into the desired locus via CRISPR/Cas9-cleavage induced homologous recombination. Brown triangles: loxP sites for removal of the fluorescent marker via Cre recombination.
b, Schematics depicting Or gene organisation, the structure of mutant alleles, and the location of the sequences encoding antibody epitopes. For DsecOrco1 the fluorescent marker was integrated into the first coding exon; for DsecOrco2 the marker replaces parts of exons 1 and 3 and the whole of exon 2. DsecOr22aRFP carries the fluorescent marker in the first coding exon close to the start codon. DsecOr35aRFP lacks most of exons 1 and 2. For DsecOr85bGFP the marker was integrated into exon 1; for DsecOr85c/bRFP the marker replaces most of the Or85c gene and part of exon 1 of Or85b.
c, Immunofluorescence for ORCO and IR25a (as internal staining control) on whole-mount antennae from wild-type and DsecOrco2 animals. c-g: Scale bars, 25 μm; inset scale bars, 5 μm.
d, RNA FISH for Or22a and Or85b on whole-mount antennae from wild-type, DsecOr22aRFP and DsecOr85bGFP mutant animals.
e, Immunofluorescence for IR75b and RNA FISH for Or35a on whole-mount antennae from wild-type and DsecOr35aRFP mutant animals. Arrowheads indicate Or35a-expressing cells. Note that Or35a neurons also pair with Ir75c neurons in ac3II sensilla15, which is reflected in Or35a-positive cells that are not paired with IR75b-expressing cells in wild-type antennae.
f, Immunofluorescence for OR22a on whole-mount antennae from wild-type and DsecOr22aRFP mutant animals. Arrowheads indicate sensilla housing Or22a neurons.
g, Left panel: immunofluorescence for ORCO and IR25a (as internal staining control) on whole-mount antennae from wild-type (same picture as shown in c) and DsecOrco1 animals. Central panel: electrophysiological responses in the two neurons of the ab3 sensillum (see Fig. 2a) to odours present in noni in D. sechellia (DSSC 14021-0248.07) and DsecOrco1 mutants (n = 5-20, females). Representative response traces to methyl hexanoate (10-6) and 2-heptanone (10-6) are shown to the left. Data points represent the solvent-corrected activities per neuron. D. sechellia wild-type responses are replotted from Fig. 2a. Surprisingly, even though ORCO expression is undetectable by immunofluorescence, weak electrophysiological responses in ab3 sensilla (and other ORCO-dependent sensilla (data not shown)) can be detected. These observations suggest that trace levels of functional ORCO are produced from this allele, potentially through use of in-frame start codons downstream of the marker insertion site (see h).
h, A schematic depicting the location of the Orco start codon, the fluorescent marker insertion site of the DsecOrco1 allele and downstream potential alternative in-frame start codons.
Extended Data Fig. 6. Generation and validation of loss-of-function alleles of D. sechellia Ir genes.
a, Schematics depicting Ir gene organisation, the structure of mutant alleles, and the sequences encoding antibody epitopes. For DsecIr8aRFP/GFP the fluorescent marker was integrated into the first coding exon. For DsecIr64aRFP the marker replaces parts of exon 2. DsecIr75bRFP lacks parts of exons 3 and 4, while for DsecIr75bGFP the marker was integrated into exon 3. For both alleles of Ir75b, the fluorophore was removed via Cre-mediated recombination to produce Ir75b1 and Ir75b2.
b, Immunofluorescence for IR64a and IR8a (as internal staining control) on whole-mount antennae from wild-type and DsecIr64aRFP mutant animals. Arrowheads indicate the Ir64a neuron dendrites innervating sensilla in the sacculus (sac). Scale bars, 25 μm; inset scale bars, 5 μm.
c, Left panel (top): immunofluorescence for IR8a and IR25a (as internal staining control) on whole-mount antennae from wild-type and DsecIr8aGFP animals. Left panel (bottom): immunofluorescence for IR75b and RNA FISH for Or35a on whole-mount antennae from DsecIr75b2 mutant animals. Scale bars, 25 μm; inset scale bars, 5 μm. Right panel: electrophysiological responses in the ac3I sensillum (neurons housed are indicated in the cartoon) to noni juice, grape juice and odours present in noni (n = 4-11, females) in D. sechellia (DSSC 14021-0248.07) and olfactory receptor mutants affecting the Ir75b neuron (DsecIr8aGFP, DsecIr75b2). Data points represent the summed, solvent-corrected activities of the sensillum. D. sechellia wild-type responses are replotted from Fig. 2b. Note that the Or35a neuron exhibits residual responses to hexanoic acid in the DsecIr8a and DsecIr75b olfactory receptor mutants (see also Fig. 2b).
d, Left panel: immunofluorescence for IR8a and IR25a (as internal staining control) on whole-mount antennae from wild-type (same picture as shown in c) and DsecIr8aRFP and DsecIr8aGFP (same picture as shown in c) animals. Scale bars, 25 μm; inset scale bars, 5 μm. Right panel: electrophysiological responses in the ac2 sensillum (neurons housed are indicated in the cartoon) to noni juice, grape juice and odours present in noni (n = 3-11, females) in D. sechellia (DSSC 14021-0248.07) and receptor mutants affecting the Ir75a neuron (DsecIr8aRFP, DsecIr8aGFP). Data points represent the summed, solvent-corrected neuronal activities of the sensillum. D. sechellia wild-type responses to noni and grape juice are as shown in Extended Data Fig. 4e.
e, Immunofluorescence for IR75b and RNA FISH for Or35a on whole-mount antennae from wild-type and DsecIr75b1 and DsecIr75b2 (same picture as shown in c) animals. Scale bars, 25 μm; inset scale bars, 5 μm.
Extended Data Fig. 7. Genetic and chemical contributions promoting attraction of D. sechellia to noni.
a, Data reproduced from Fig. 2d. Behavioural responses in a trap assay testing preference of the indicated genotypes for noni juice or grape juice (n = 13-25 experiments; 22-25 females/experiment). Comparisons to Dsec.07 responses are shown (a-d and f: pairwise Wilcoxon rank-sum test and P values adjusted for multiple comparisons using the B&H method): red bars, no significant difference; salmon bars, significantly different response; *** P < 0.001; ** P < 0.01; * P < 0.05; n.s. P > 0.05.
b, Proportion of flies (mean ± SEM) in each stimulus trap for the assays shown in a. Comparisons to Dsec.07 responses are shown.
c, Olfactory responses in a trap assay testing preferences between noni juice and grape juice of D. sechellia (DSSC 14021-0248.07), DsecOrco1/Ir8aGFP double mutants and D. sechellia (DSSC 14021-0248.07) whose third antennal segments were removed (antenna-less) (n = 9-15 experiments; 22-25 females or males, as indicated/experiment). These data represent the same experiments shown in Fig. 2e, but attraction indices were calculated here taking only alive flies into account. The percentages of flies alive at the end of the assay are indicated below, revealing the high mortality rate of antenna-less flies and DsecOrco1/Ir8aGFP double mutants (DsecOrco2/Ir8aGFP mutants appeared to be non-viable). Normally, trap assay experiments with >25% animal mortality were discarded; see Methods. Comparisons to Dsec.07 responses are shown.
d, Proportion of flies (mean ± SEM) in each stimulus trap for the assays shown in c. Comparisons to Dsec.07 responses are shown.
e, Behavioural responses in a trap assay testing preferences between noni fruit and grape juice of Dsec.07 and DsecOr22aRFP flies. Comparisons to Dsec.07 responses are shown (pairwise Wilcoxon rank-sum test): n.s. P > 0.05.
f, Behavioural responses in a trap assay testing preferences of D. sechellia (DSSC 14021-0248.07) between grape juice and 10-2 dilutions of the indicated odours in grape juice of D. sechellia (DSSC 14021-0248.07), DsecOr22aRFP, D. simulans (DSSC 14021-0251.004) and D. melanogaster (Canton-S). Comparisons to Dsec.07 responses to methyl hexanoate are shown.
g, Behavioural responses in a wind tunnel assay testing attraction of D. sechellia (DSSC 14021-0248.07) to three single noni odours (10-2 in water), a mix of all three in the approximate proportions of ripe noni fruit (1:0.04:1, methyl hexanoate:2-heptanone:hexanoic acid) and noni juice (n = 10 experiments; 10 females/experiment). Comparisons to Dsec.07 responses to noni juice are shown (Kruskal-Wallis test with Dunn’s post-hoc correction): *** P < 0.001.
Extended Data Fig. 8. Odour-tuning properties of drosophilid Or85c/b and Or22a/(b) neurons and genomic modifications of the Or22a/(b) loci.
a, Left: dose-dependent electrophysiological responses of Or85c/b neurons (ab3B) in wild-type D. sechellia (DSSC 14021-0248.07) to 2-heptanone and 1-hexanol (mean ± SEM and individual data points; n = 10-20, females). Right: dose-dependent electrophysiological responses of Or22a neurons (ab3A) in wild-type D. sechellia (DSSC 14021-0248.07) to methyl butanoate, methyl hexanoate and methyl octanoate (mean ± SEM and individual data points; n = 10-20, females). The dose response curves for 2-heptanone and methyl hexanoate are replotted from Fig. 3a.
b, Schematics depicting the arrangement of wild-type, mutant and rescue allele versions of DsecOr22a (top) and DmelOr22a/Or22b (bottom). Asterisk = stop codon preventing read through from the endogenous Or22a open reading frame.
c, Schematics depicting the arrangement of wild-type and mutant alleles of DsimOr22a/Or22b.
d, RNA FISH for Or22a on whole-mount antennae from wild-type D. simulans (DSSC 14021-0251.195), DsimOr22aRFP and DsimOr22a/bRFP mutant animals. As Or22a shares 85% sequence similarity with Or22b, the Or22a probe hybridises with transcripts from both genes. Arrowheads indicate Or22b-expressing cells in DsimOr22aRFP. Scale bar, 25 μm; inset scale bar, 5 μm.
e, Electrophysiological responses of Or22a/b neurons to different esters in wild-type D. simulans (DSSC 14021-0251.195) and receptor mutants (DsimOr22aRFP, DsimOr22a/bRFP) (n = 6-10, females). Representative response traces to methyl hexanoate (10-6) and ethyl butanoate (10-2) are shown to the left.
f, Heat map representation of the data shown in e, together with the data of the DsimOr22awt response profile when expressed in DsecOr22aRFP (replotted from Fig. 3c). The receptors expressed in the analysed neurons are listed to the right. Significant differences to D. simulans wild-type responses are shown (pairwise Wilcoxon rank-sum test and P values adjusted for multiple comparisons using the B&H method): *** P < 0.001; ** P < 0.01; * P < 0.05; n.s. P > 0.05. The equivalent responses to ethyl butanoate of D. simulans wild-type and Or22a mutant neurons (but complete loss in Or22a/b mutant neurons) suggests that this odour is detected principally by OR22b.
g, Boxplots with individual data points of the electrophysiological data presented in Fig. 3b.
h, Boxplots with individual data points of the electrophysiological data presented in Fig. 3c.
i, Boxplots with individual data points of the electrophysiological data presented in Fig. 3e.
Extended Data Fig. 9. Mapping of odour-specificity determinants of OR22a.
a, Electrophysiological responses of D. melanogaster Or22a/b mutant neurons expressing DsecOr22awt or DmelOr22awt upon stimulation with increasing concentrations of noni fruit extract (mean ± SEM and individual data points; n = 9, females). Significantly different values are indicated (pairwise Wilcoxon rank-sum test): *** P < 0.001.
b, Protein sequence alignment of OR22a orthologues of six species within the D. melanogaster species subgroup. Red shading: amino acid differences between D. melanogaster and D. sechellia, D. simulans and D. mauritiana that were analysed by mutagenesis in this study; blue shading: all other sequence differences. Arrowheads: chimera breakpoints (see c). Predicted transmembrane (TM) domains are indicated with grey lines (location as in11).
c, Electrophysiological responses to a panel of noni odours conferred by chimeric OR22a proteins encoded by transgenes integrated at the Or22a/b locus of D. melanogaster (n = 5-6, females). Schematics on the left indicate the relative proportions of D. sechellia (red) and D. melanogaster (dark grey) sequences in each chimera (precise chimera breakpoints are shown in b). Significant differences to DmelOr22awt responses are shown (c, d and g: pairwise Wilcoxon rank-sum test and P values adjusted for multiple comparisons using the B&H method): *** P < 0.001; ** P < 0.01; * P < 0.05; n.s. P > 0.05.
d, Electrophysiological responses of D. melanogaster Or22a/b mutant neurons expressing different OR22a variants (n = 5-7, females). The location of each mutated residue is indicated in b. Data for Or22a/b mutant and DmelOr22awt responses are replotted from c. Significant differences to DmelOr22awt responses are shown.
e, Boxplots with individual data points showing the same data as in c.
f, Boxplots with individual data points showing the same data as in d.
g, Dose-dependent electrophysiological responses of D. sechellia Or22a neurons expressing the indicated transgenes to ethyl or methyl hexanoate (mean ± SEM and individual data points; n = 10, females). Significant comparisons to either the DmelOr22awt (left graphs) or the DsecOr22awt (right graphs) transgene responses are shown.
Extended Data Fig. 10. Peripheral and central olfactory circuit changes in D. sechellia.
a, Quantification of the number of OSNs expressing Or22a/(b) in antennae of wild-type D. sechellia (DSSC 14021-0248.07) and D. melanogaster (Canton-S) (data as shown in Fig. 4b), Or22a/(b) mutants in both species, and rescue lines expressing DsecOr22awt (n = 9-11, females). Comparisons of rescue and wild-type genotypes for each species are shown (pairwise Wilcoxon rank-sum test). n.s. P > 0.05. No significant differences in Or22a cell number were observed for different rescue transgenes (data not shown).
b, Quantification of the number of OSNs expressing Or13a (ab6), Or98a (ab7) or Or35a (ac3I/II) in D. sechellia (DSSC 14021-0248.07), D. simulans (DSSC 14021-0251.004), and D. melanogaster (Canton-S) (n = 10-15, females). Comparisons to Dsec.07 cell number counts are shown (pairwise Wilcoxon rank-sum test and P values adjusted for multiple comparisons using the B&H method): *** P < 0.001; ** P < 0.01; n.s. P > 0.05.
c, Immunofluorescence with nc82 (neuropil), α-Elav (neurons) and α-GFP in a DsecnSyb-Gal4/UAS-C3PA-GFP transgenic line, which expresses photoactivatable GFP pan-neuronally. The schematic on the left indicates the region of image acquisition. An anterior section through the antennal lobe (AL) is shown to reveal the position of the labelled projection neuron (PN) soma (circled in the right panel). Scale bar, 25 μm.
d, Electrophysiological responses of the Or22a neuron to odours present in noni in homozygous DsecOr22aGal4 (mutant) transgenic animals (n = 6, females). Data points represent the solvent-corrected activities. Representative response traces to methyl hexanoate (10-6) in wild-type and transgenic animals are shown on top.
e, Tracing of axonal branches in the lateral horn of dye-filled DM2 PNs in D. sechellia wild-type and homozygous DsecOr22aGal4 mutant flies. Three representative samples are shown. The circles depict the position of the D. sechellia-specific axonal branch. Scale bar, 10 μm. Samples could not be discriminated by genotype when presented to six independent researchers blindly.
Extended Data Fig. 11. Phylogenetic and functional analysis of odour-specificity determinants in OR22a.
a, Side view of the ORCO monomer structure (determined by cryo-electronic microscopy37); the approximate location of the plasma membrane is indicated. The location of the residues corresponding to the odour-specificity determinants of OR22a analysed in this study (based on alignments generated in37) are highlighted as spheres.
b, Top view of a cross-section through the putative ligand-binding pocket of the ORCO structure shown in a.
c, Partial protein sequence alignment of OR22a and OR59b. The equivalent residue to D. melanogaster OR22a M93 in OR59b is V91, which exhibits intraspecific sequence variation impacting odour sensitivity82.
d, Results of branch-based models of molecular evolution that tested for changes in the rates of protein evolution among OR22a and OR22b orthologues (see Methods and Supplementary Table 8): the rate of protein changes within the OR22a/OR22b phylogenetic tree highlights dN/dS ratios (ω) that differ from the “background rate” (ω = 0.1772). Most branches exhibited low ω, arguing for strong purifying selection to maintain protein function over much of the tree. The two ω values that are >1 indicate an excess of protein changes, consistent with positive selection. The branch leading to D. simulans and D. sechellia OR22a displays nearly equal rates of silent and replacement substitutions, consistent with relaxed constraint during this period.
e, Allele frequencies within population datasets for D. melanogaster78,79, D. simulans7,77 and D. sechellia7 at the three sites of OR22a that were functionally characterised in this study. The table displays amino acid (aa) positions 45, 67 and 93 of OR22a and the frequencies at which variants within the corresponding codons are segregating (number of alleles with respective variant/number of alleles analysed). “NA” (not applicable) indicates that positions within the codon are invariant. Datasets analysed are referenced on the right. Selected Or22a variants from the Drosophila melanogaster Genetic Reference Panel (DGRP)79 were confirmed by sequencing (f) and Or22a neuron physiology analysed (g,h).
f, Protein sequence alignment of OR22a orthologues of D. melanogaster83, three lines of the DGRP, D. mauritiana (DSSC 14021-0241.151) and D. sechellia (DSSC 14021-0248.07). Red shading: shared amino acid differences of DGRP #303, DGRP #304 and D. mauritiana (compared to other sequences) at position 59 and the key odour specificity-determinant at residue 93; blue shading: all other sequence differences. No line within the DGRP with a polymorphism only at position 93 was identified.
g, Electrophysiological responses of the Or22a/b neuron to odours present in noni (n = 5-20, females) in the strains shown in f. The similarity between the response profiles of DGRP #303, DGRP #304 and D. mauritiana suggests that their only shared polymorphism (at position 59) modifies OR22a response properties in these strains. Comparisons to Dmel BER responses are shown (pairwise Wilcoxon rank-sum test and P values adjusted for multiple comparisons using the B&H method): *** P < 0.001; ** P < 0.01; n.s. P > 0.05. D. mauritiana and D. sechellia data are replotted from Fig. 3b.
h, Boxplots with individual data points showing the same data as in g. D. mauritiana and D. sechellia data are replotted from Extended Data Fig. 8g.
i, Protein sequence alignment of OR22a orthologues of the noni-specialised D. yakuba mayottensis41 (Dyak may.) and three other D. yakuba strains (DSSC 14021-0261.00, 14021-0261.40, 14021-0261.49). Blue shading: differences between these sequences.
j, Collection sites of D. yakuba strains shown in i.
k, Quantification of the number of OSNs expressing Or22a/(b) in D. sechellia (DSSC 14021-0248.07), D. simulans (DSSC 14021-0251.004), D. melanogaster (Canton-S) (data as shown in Fig. 4b), D. yakuba (DSSC 14021-0261.00, 14021-0261.49) and D. yakuba mayottensis (n = 10-12, females). Comparisons to Dsec.07 cell number counts are shown (k and l: pairwise Wilcoxon rank-sum test and P values adjusted for multiple comparisons using the B&H method): *** P < 0.001; ** P < 0.01; * P < 0.05; n.s. P > 0.05.
l, Electrophysiological responses to odours present in noni of the Or22a/(b) neurons in D. sechellia (DSSC 14021-0248.07), D. melanogaster (Canton-S), D. yakuba mayottensis (n = 5-20, females) and D. yakuba (DSSC 14021-0261.00). Comparisons to Dsec.07 responses are shown. D. sechellia, D. melanogaster and D. yakuba.00 data are replotted from Fig. 3b.
m, Boxplots with individual data points showing the same data as in l. D. sechellia, D. melanogaster and D. yakuba.00 data are replotted from Extended Data Fig. 8g.
Extended Data Fig. 12. Protein sequence alignments of OR22a and IR75b.
a, OR22a orthologues of strains used for behavioural assays as well as genome-sequenced strains (“gen.”) of each species (version: D. sechellia (r1.3), D. simulans (r2.01), D. melanogaster (r6.28)). Blue/red shading: differences between species/strains. Green boxes: residues tested in this study for their role in defining ester tuning specificity.
b, IR75b orthologues of same strains as in a. Blue/red shading: differences between species/strains. Black boxes: residues predicted to be located within the ligand-binding domain that contribute to odour tuning specificity63. Note that the premature stop codon of the Canton-S strain (position 169, marked by an asterisk) does not impair receptor function, as shown in other strains63.
Supplementary Material
Acknowledgements
We thank Y. Bellaïche, B. Deplancke, K. S. Douglas, S. Lavista-Llanos, K. O’Connor-Giles, J. Posakony, V. Ruta, D. Stern, G. Suh, A. Yassin, the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Developmental Studies Hybridoma Bank (NICHD of the NIH, University of Iowa) for reagents, B. Prud’homme for instruction on Drosophila microinjections, B. Sutcliffe and S. Cachero for advice on the generation of reference brains, I. Alali and K. Weniger for technical assistance, J. Simpson for details of the nSyb promoter construct and P. C. Chai for assistance with glomerular identification. We thank I. Rentero Rebollo, J. Sánchez-Alcañiz, L. Prieto-Godino and members of the Benton laboratory for discussions and comments on the manuscript. T.O.A. is supported by a Human Frontier Science Program Long-Term Fellowship (LT000461/2015-L). M.A.K., B.S.H., and M.K. are supported by the Max Planck Society. K.E. and S.J.C.C. are supported by a National Institute of Health Award (1 R01 NS 167970) and a Eunice Kennedy Shriver National Institute of Child Health & Human Development Award of the National Institutes of Health (5 T32 HD 007491). J.R.A. is supported by a Swiss National Science Professorship Grant (PP00P3 176956). G.S.X.E.J. is supported by an ERC Consolidator Grant (649111) and the MRC (MC_U105188491). Research in R.B.’s laboratory is supported by ERC Consolidator and Advanced Grants (615094 and 833548, respectively), the Swiss National Science Foundation and the Fondation Herbette.
Footnotes
Data, code and biological material availability statement
All relevant data supporting the findings of this study are available from the corresponding authors upon request or, for behavioural experiments, included here as source data (Fig. 1b,d; Fig. 2c,d,e; Fig. 3d,e; ED Fig. 1c,e,f,g,h; ED Fig. 7e,f,g). Supplementary Table 7 lists the exact n and mean values for all electrophysiological data. Code used for analyses and all unique biological materials generated in this study (e.g., mutant and transgenic Drosophila strains) are available from the corresponding authors upon request.
Author Contributions
T.O.A. and R.B. conceived the project. All authors contributed to experimental design, analysis and interpretation of results. T.O.A. generated all molecular reagents and new drosophilid mutants and transgenic lines; other experimental contributions were as follows: T.O.A. (Fig. 1c; Fig. 2a,b,d,e; Fig. 3a-e; Fig. 4a-d,g; ED Fig. 1a-f; ED Fig. 2c; ED Fig. 3; ED Fig. 4c,e; ED Fig. 5; ED Fig. 6; ED Fig. 7a-f; ED Fig. 8; ED Fig. 9; ED Fig. 10a-d; ED Fig. 11f-m; ED Fig. 12), M.A.K. with input from B.S.H. and M.K. (Fig. 1b,d; Fig. 2c; Fig. 3d,f; ED Fig. 1f-h; ED Fig. 2a,b,d,e; ED Fig. 7g, Sup. Table 1), G.Z. (Fig. 1c; Fig. 2d; Fig. 4b; ED Fig. 1a,b; ED Fig. 3b; ED Fig. 5c,e,g; ED Fig. 6; ED Fig. 7a,b,f; ED Fig. 10b; ED Fig. 11k), A.F.S. (Fig. 1e,f; ED Fig. 4d,f,g), K.E. with input from S.J.C.C. (Fig. 4e-g; ED Fig. 10e), R.A.O. (ED Fig. 4a,b), J.R.A (ED Fig. 11d,e), G.S.X.E.J. (Fig. 4c), R.B. (Fig. 4c). T.O.A. and R.B. wrote the paper with input from all other authors.
Competing interests
The authors declare no competing interests.
References
- 1.Arguello JR, Benton R. Open questions: Tackling Darwin's "instincts": the genetic basis of behavioural evolution. BMC Biol. 2017;15:26. doi: 10.1186/s12915-017-0369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendesky A, Bargmann CI. Genetic contributions to behavioural diversity at the gene-environment interface. Nat Rev Genet. 2011;12:809–820. doi: 10.1038/nrg3065. [DOI] [PubMed] [Google Scholar]
- 3.Bendesky A, et al. The genetic basis of parental care evolution in monogamous mice. Nature. 2017;544:434–439. doi: 10.1038/nature22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding Y, Berrocal A, Morita T, Longden KD, Stern DL. Natural courtship song variation caused by an intronic retroelement in an ion channel gene. Nature. 2016;536:329–332. doi: 10.1038/nature19093. [DOI] [PubMed] [Google Scholar]
- 5.Weber JN, Peterson BK, Hoekstra HE. Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature. 2013;493:402–405. doi: 10.1038/nature11816. [DOI] [PubMed] [Google Scholar]
- 6.Garrigan D, et al. Genome sequencing reveals complex speciation in the Drosophila simulans clade. Genome Res. 2012;22:1499–1511. doi: 10.1101/gr.130922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrider DR, Ayroles J, Matute DR, Kern AD. Supervised machine learning reveals introgressed loci in the genomes of Drosophila simulans and D. sechellia. Plos Genetics. 2018;14 doi: 10.1371/journal.pgen.1007341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amlou M, Moreteau B, David JR. Genetic analysis of Drosophila sechellia specialization: Oviposition behaviour toward the major aliphatic acids of its host plant. Behav Genet. 1998;28:455–464. doi: 10.1023/a:1021689312582. [DOI] [PubMed] [Google Scholar]
- 9.Cobb M, Burnet B, Blizard R, Jallon JM. Courtship in Drosophila sechellia - its structure, functional aspects, and relationship to those of other members of the Drosophila melanogaster species subgroup. J Insect Behav. 1989;2:63–89. [Google Scholar]
- 10.Coyne JA. Genetics of sexual isolation in females of the Drosophila simulans species complex. Genet Res. 1992;60:25–31. doi: 10.1017/s0016672300030639. [DOI] [PubMed] [Google Scholar]
- 11.Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr Biol. 2006;16:101–109. doi: 10.1016/j.cub.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 12.Higa I, Fuyama Y. Genetics of Food Preference in Drosophila sechellia. 1. Responses to Food Attractants. Genetica. 1993;88:129–136. doi: 10.1007/BF02424469. [DOI] [PubMed] [Google Scholar]
- 13.Ibba I, Angioy AM, Hansson BS, Dekker T. Macroglomeruli for fruit odours change blend preference in Drosophila. Die Naturwissenschaften. 2010;97:1059–1066. doi: 10.1007/s00114-010-0727-2. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo T, Sugaya S, Yasukawa J, Aigaki T, Fuyama Y. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol. 2007;5:e118. doi: 10.1371/journal.pbio.0050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto-Godino LL, et al. Evolution of acid-sensing olfactory circuits in Drosophilids. Neuron. 2017;93:661–676. doi: 10.1016/j.neuron.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 16.R'Kha S, Capy P, David JR. Host-plant specialization in the Drosophila melanogaster species complex: a physiological, behavioural, and genetical analysis. Proc Natl Acad Sci U S A. 1991;88:1835–1839. doi: 10.1073/pnas.88.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yalcin B, et al. Genetic dissection of a behavioural quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat Genet. 2004;36:1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]
- 18.Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI. Catecholamine receptor polymorphisms affect decision-making in C. elegans. Nature. 2011;472:313–318. doi: 10.1038/nature09821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bumbarger DJ, Riebesell M, Rodelsperger C, Sommer RJ. System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes. Cell. 2013;152:109–119. doi: 10.1016/j.cell.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Markow TA, O'Grady P. Reproductive ecology of Drosophila. Functional Ecology. 2008;22:747–759. [Google Scholar]
- 21.Linz J, et al. Host plant-driven sensory specialization in Drosophila erecta. Proc Biol Sci. 2013;280 doi: 10.1098/rspb.2013.0626. 20130626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeholzer LF, Seppo M, Stern DL, Ruta V. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature. 2018;559:564–569. doi: 10.1038/s41586-018-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dweck HK, et al. Olfactory channels associated with the Drosophila maxillary palp mediate short- and long-range attraction. Elife. 2016;5 doi: 10.7554/eLife.14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matute DR, Ayroles JF. Hybridization occurs between Drosophila simulans and D. sechellia in the Seychelles archipelago. J Evol Biol. 2014;27:1057–1068. doi: 10.1111/jeb.12391. [DOI] [PubMed] [Google Scholar]
- 25.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 26.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Abuin L, et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stensmyr MC, Dekker T, Hansson BS. Evolution of the olfactory code in the Drosophila melanogaster subgroup. Proc Biol Sci. 2003;270:2333–2340. doi: 10.1098/rspb.2003.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odour coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 31.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 32.Shiao MS, et al. Expression divergence of chemosensory genes between Drosophila sechellia and its sibling species and its implications for host shift. Genome Biol Evol. 2015;7:2843–2858. doi: 10.1093/gbe/evv183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao CA, Ignell R, Carlson JR. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci. 2005;25:8359–8367. doi: 10.1523/JNEUROSCI.2432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ai M, et al. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruta V, et al. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- 36.Grabe V, Sachse S. Fundamental principles of the olfactory code. Bio Systems. 2018;164:94–101. doi: 10.1016/j.biosystems.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Butterwick JA, et al. Cryo-EM structure of the insect olfactory receptor ORCO. Nature. 2018;560:447–452. doi: 10.1038/s41586-018-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguade M. Nucleotide and copy-number polymorphism at the odorant receptor genes Or22a and Or22b in Drosophila melanogaster. Mol Biol Evol. 2009;26:61–70. doi: 10.1093/molbev/msn227. [DOI] [PubMed] [Google Scholar]
- 39.Goldman-Huertas B, et al. Evolution of herbivory in Drosophilidae linked to loss of behaviours, antennal responses, odorant receptors, and ancestral diet. Proc Natl Acad Sci U S A. 2015;112:3026–3031. doi: 10.1073/pnas.1424656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nozawa M, Nei M. Evolutionary dynamics of olfactory receptor genes in Drosophila species. Proc Natl Acad Sci U S A. 2007;104:7122–7127. doi: 10.1073/pnas.0702133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yassin A, et al. Recurrent specialization on a toxic fruit in an island Drosophila population. Proc Natl Acad Sci U S A. 2016;113:4771–4776. doi: 10.1073/pnas.1522559113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambrose D, Ellender JH, Lee EB, Sprake CHS, Townsend R. Thermodynamic properties of organic oxygen compounds XXXVIII. Vapour pressures of some aliphatic ketones. The Journal of Chemical Thermodynamics. 1975;7:453–472. [Google Scholar]
- 43.Daubert TE, Danner RP. Physical and thermodynamic properties of pure chemicals data compilation. Data compilation. 1997 [Google Scholar]
- 44.Port F, Chen HM, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Port F, Bullock SL. Augmenting CRISPR applications in Drosophila with tRNA-flanked sgRNAs. Nat Methods. 2016;13:852–854. doi: 10.1038/nmeth.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726–732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- 47.Gratz SJ, et al. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics. 2014;196:961–971. doi: 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern DL. Tagmentation-Based Mapping (TagMap) of Mobile DNA Genomic Insertion Sites. bioRxiv. 2016 doi: 10.1101/037762. [DOI] [Google Scholar]
- 49.Croset V, et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6:e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bateman JR, Wu CT. A simple polymerase chain reaction-based method for the construction of recombinase-mediated cassette exchange donor vectors. Genetics. 2008;180:1763–1766. doi: 10.1534/genetics.108.094508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci U S A. 2000;97:5995–6000. doi: 10.1073/pnas.090527097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc Natl Acad Sci U S A. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnoult L, et al. Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science. 2013;339:1423–1426. doi: 10.1126/science.1233749. [DOI] [PubMed] [Google Scholar]
- 55.Gratz SJ, et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194:1029–1035. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Koolhaas WH, Schnorrer F. A versatile two-step CRISPR- and RMCE-based strategy for efficient genome engineering in Drosophila. G3 (Bethesda) 2014;4:2409–2418. doi: 10.1534/g3.114.013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gohl DM, et al. A versatile in vivo system for directed dissection of gene expression patterns. Nat Methods. 2011;8:231–237. doi: 10.1038/nmeth.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knecht ZA, et al. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife. 2016;5 doi: 10.7554/eLife.17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silbering AF, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benton R, Dahanukar A. Electrophysiological recording from Drosophila olfactory sensilla. Cold Spring Harb Protoc. 2011;2011:824–838. doi: 10.1101/pdb.prot5630. [DOI] [PubMed] [Google Scholar]
- 62.Saina M, Benton R. Visualizing olfactory receptor expression and localization in Drosophila. Methods Mol Biol. 2013;1003:211–228. doi: 10.1007/978-1-62703-377-0_16. [DOI] [PubMed] [Google Scholar]
- 63.Prieto-Godino LL, et al. Olfactory receptor pseudo-pseudogenes. Nature. 2016;539:93–97. doi: 10.1038/nature19824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez-Alcaniz JA, Zappia G, Marion-Poll F, Benton R. A mechanosensory receptor required for food texture detection in Drosophila. Nat Commun. 2017;8 doi: 10.1038/ncomms14192. 14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ostrovsky A, Cachero S, Jefferis G. Clonal analysis of olfaction in Drosophila: immunochemistry and imaging of fly brains. Cold Spring Harb Protoc. 2013;2013:342–346. doi: 10.1101/pdb.prot071720. [DOI] [PubMed] [Google Scholar]
- 67.Jefferis GS, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manton JD, et al. Combining genome-scale Drosophila 3D neuroanatomical data by bridging template brains. bioRxiv. 2014 doi: 10.1101/006353. [DOI] [Google Scholar]
- 69.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng L, Zhao T, Kim J. neuTube 1.0: A New Design for Efficient Neuron Reconstruction Software Based on the SWC Format. eNeuro. 2015;2 doi: 10.1523/ENEURO.0049-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caron SJ, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497:113–117. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abascal F, Zardoya R, Telford MJ. TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010;38:W7–13. doi: 10.1093/nar/gkq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ronquist F, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 77.Signor SA, New FN, Nuzhdin S. A large panel of Drosophila simulans reveals an abundance of common variants. Genome Biol Evol. 2018;10:189–206. doi: 10.1093/gbe/evx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grenier JK, et al. Global diversity lines - a five-continent reference panel of sequenced Drosophila melanogaster strains. G3 (Bethesda) 2015;5:593–603. doi: 10.1534/g3.114.015883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang W, et al. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 2014;24:1193–1208. doi: 10.1101/gr.171546.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grabe V, et al. Elucidating the neuronal architecture of olfactory glomeruli in the Drosophila antennal lobe. Cell Rep. 2016;16:3401–3413. doi: 10.1016/j.celrep.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 82.Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature. 2011;478:511–514. doi: 10.1038/nature10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.