Abstract
Background
Results of medical research should be made publicly available in a timely manner to enable patients and health professionals to make informed decisions about health issues. We aimed to apply a multi-state model to analyze the overall time needed to publish study results, and to examine predictors of the timing of transitions within the research process from study initiation through completion/discontinuation to eventual publication.
Methods
Using a newly developed multi-state model approach, we analysed the effect of different study-related factors on each of the transitions from study approval to eventual publication, using a data set of clinical studies approved by a German research ethics committee between 2000 and 2002.
Results
Of 917 approved studies, 806 were included in our analyses. About half of the clinical studies which began were subsequently published as full articles, and the median time from study approval to publication was 10 years. Differences across model states were apparent; several factors were predictive of the transition from study approval to completion, while funding source and collaboration were predictive of the transition from completion to publication.
Conclusions
The proposed multi-state model approach permits a more comprehensive analysis of time to publication than a simple examination of the transition from approval to publication, and thus the findings represent an advance on previous studies of this aspect of the research process.
Introduction
Results of medical research should be made publicly available in a timely manner to enable patients and health professionals to make informed decisions about health issues [1]. However, there are several barriers inhibiting the translation of research knowledge into practice. Firstly, a large proportion of clinical studies are prematurely discontinued [2–9]. Secondly, not all research findings are ultimately published in peer-reviewed journals [10]. A systematic review of the extent of non-publication of studies approved by research ethics committees (REC) or included in trial registries showed that only about half of all studies were eventually published in a peer-reviewed journal [8, 11, 12].
A Health Technology Assessment report found that the main reason given by investigators for not publishing their studies was ‘lack of time or low priority’, followed by ‘results not important enough’ and ‘journal rejection’ [13, 14]. Lack of financial and personnel resources are also frequently-cited reasons for non-publication [15]. Specific study-related factors are also associated with whether or not a study is published, particularly the direction of the study results [11]. “Negative” study results, e.g. no significant difference between treatment arms, are less likely to be published than “positive” results, i.e. significant effects favouring the experimental treatment [16–20]. This is also associated with time to publication, with positive results published on average about 1–3 years more quickly than negative results [17–19, 21, 22]. Source of funding is also important; negative results are more likely to be published when studies are funded by non-profit institutions than when they are commercially funded [23]. In a cohort of biomedical research studies approved by a German REC we showed that multicentre and international collaboration, large sample size, a declared study funding source and the sponsor’s involvement in the study were also positively associated with subsequent publication [24, 25].
The ‘selective publication’ of studies results in a restricted and frequently over-optimistic body of evidence. Treatments are often erroneously accepted as effective, whereas null or adverse effects are underestimated. The problem is magnified when studies are combined in meta-analyses or systematic reviews, and can lead to inappropriate health care recommendations and ultimately non-optimal treatment that can prolong patients’ suffering, cause harm and in the worst case, cost lives [12, 26, 27].
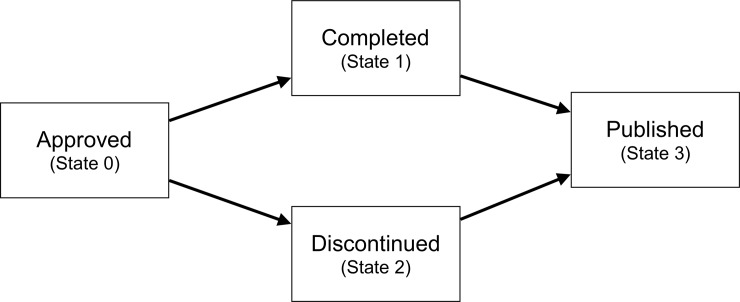
The process of conducting and publishing a study can be divided into multiple event types or “multi-states” occurring over a period of time. All studies start in an initial state, e.g. REC approved, may pass through one or more intermediate states, and may finally result in an absorbing state, e.g. published. The process is typically visualized by a directed graph, often called a multi-state model, where the states are drawn as nodes and the possible state transitions are indicated by arrows (Fig 1). For studies that have begun, two intermediate states are possible: a) the study is completed, i.e. the study has ended as planned and participants are no longer being followed up; or b) the study is discontinued (e.g. was stopped early due to adverse events). Either of these scenarios can then lead to eventual publication of the findings. This four-state model, originally developed in a Master’s thesis written by one of the co-authors (TH) [28], allows for the systematic examination of variations in the total time from ethical approval to publication, distinguishing between the time required to conduct the study (i.e., study initiation to either study completion or discontinuation), and the time required to analyse, write up and publish the study findings (i.e., study completion/discontinuation to publication).
Fig 1. Illustration of the four-state model.
The boxes indicate the states, the arrows the possible state transitions.
To demonstrate this novel analytic approach we conducted a secondary analysis of a data set collected for a previous investigation of study protocols submitted to a German REC [24]. In Germany (like in many countries), registration in a clinical trial registry is not mandatory, but all studies, regardless of their design, are required to be approved by an REC before they can legally begin. Using the aforementioned multi-state model, we analysed the effect of different factors on each of the illustrated transition intensities within the research process from study approval to eventual publication.
Methods
Study design and data
The data set we used to demonstrate our newly developed four-state model approach included all research protocols of human research studies submitted to and approved by the University of Freiburg REC between 2000 and 2002 [24]. The design of each study was determined according to predefined criteria (S1 Fig). Study characteristics were extracted by a research assistant (FV or AH, see acknowledgments). In cases of uncertainty, the issue was discussed with the lead author to reach a consensus. The lead author cross-checked the other database entries. Subsequent publications were identified via electronic literature searches (AH, PO) and a survey sent to the applicants (AH, AB, actively supported by the REC). The study was piloted by investigating protocols approved by the REC in 2000. The search for publications was conducted in 2006, the survey to applicants was sent in 2007 (followed by a reminder three months later) asking them to confirm the publications that had been identified and to add further publications. It was also asked whether the project (a) had been completed as planned, (b) had been discontinued, (c) had not been started at all or at the local study site, or (d) was still ongoing, and if so its current status (i.e., recruitment closed, data collection completed, or preliminary results published) [24]. The results of the pilot project were published in 2008 [25]. This was then complemented by protocols approved in 2001 and 2002. For those studies, the search for publications took place between August 2009 and January 2010, and surveys were sent in February 2010. For the pilot year an updated search was carried out between July 2011 and January 2012 (S2 Fig).
For the present study, in order to comply with the survey information received, we defined the study initiation date as the year of REC approval, the study completion date as the year in which data collection was completed, the study discontinuation date as the year in which the study was discontinued, and the study publication date as the year of electronic publication of the first manuscript presenting findings of the study.
Data from REC files, incl. application, protocol, correspondence with the REC, and from the survey were recorded for several covariates potentially associated with the occurrence of state transitions. These were: (a) whether the study was an RCT (y/n); (b) sample size; (c) funding source (commercial, non-commercial or not reported); (d) industry involvement in the conduct and/or analysis of the study (y/n); (e) a composite variable of number of study centres and international collaboration (categorized as ‘multi-centre, international collaboration’, ‘multi-centre, national collaboration’ and ‘single centre’); and (f) whether the study had a primary outcome (y/n). The only covariate for which there was a non-trivial amount of missing data was sample size; for these 37 (4.6%) studies the median number of participants (n = 120) was imputed.
For two covariates, funding source and industry involvement, information was also extracted from the final publication. There was occasional inconsistency between the REC protocol and the publication, likely because it was not an explicit requirement for the protocol to state the study funding source or (anticipated) involvement of the sponsor. In these cases we took the pragmatic approach of designating studies as (non-)commercially-funded if (non-)commercial funding was mentioned either in the protocol or the publication; the same applies for industry involvement.
Statistical analysis
We aim to analyze the time needed to publish study results, and to estimate predictors of the timing of transitions within the research process from study initiation through completion/discontinuation to eventual publication. To accomplish this, we employ time-to-event approaches, which make use of the information that (or whether) a certain event occurs and the time until which the transition to this event occurs. Thereby, the multi-state model [29] is central. Statistical inference is made on the basis of the transition intensities (hazards), the instantaneous risk of moving from one state to another. Even though they were developed for situations where transitions are observed in continuous time (potentially subject to right-censoring), multi-state model approaches can be used to deal with more challenging observation patterns, e.g., interval censoring [30], based on the assumption that patterns of missing time-to-event information are non-informative for the event history process. In our case, the observation pattern is retrospective event collection in a prospective cohort, which we have outlined along with the corresponding censoring mechanisms in the S1 Appendix and S1 Table.
We first investigated a simplified two-state model with only one possible transition, Approved → Published, corresponding to a standard survival analysis. We fitted the non-parametric Kaplan-Meier estimator to estimate the cumulative distribution function of total time to publication and the Cox model to estimate potential effects of the covariates on the hazard of publication using the R package survival. From the estimation procedure, we further obtained the median time to publication incl. corresponding confidence intervals based on the Brookmeyer-Crowley approach [31, 32].
Our main analysis was based on the four-state model, where we used numerical estimators based on the likelihood function to estimate covariate effects on each transition intensity from interval-censored data. We assumed piece-wise constant baseline intensities, allowing us to compute the full likelihood analytically (see section 2 in S1 Appendix). Maximization of the log-likelihood used the Method of Moving Asymptotes [33]. All covariates in the multivariable analyses were entered simultaneously, and no interactions were hypothesized.
The function to estimate the transition intensities as well as covariate effects was written in C by one of the authors (TH) and can be called from R [34] via a shared library object. Mathematical details as well as documentation of the function can be found in the appendix of [28].
Results
Baseline and outcome characteristics
Of 917 approved studies, 806 were eligible for inclusion in our analyses (Fig 2).
Fig 2. Flowchart of study protocols approved between 2000 and 2002 by the research ethics committee of the University of Freiburg/Germany.
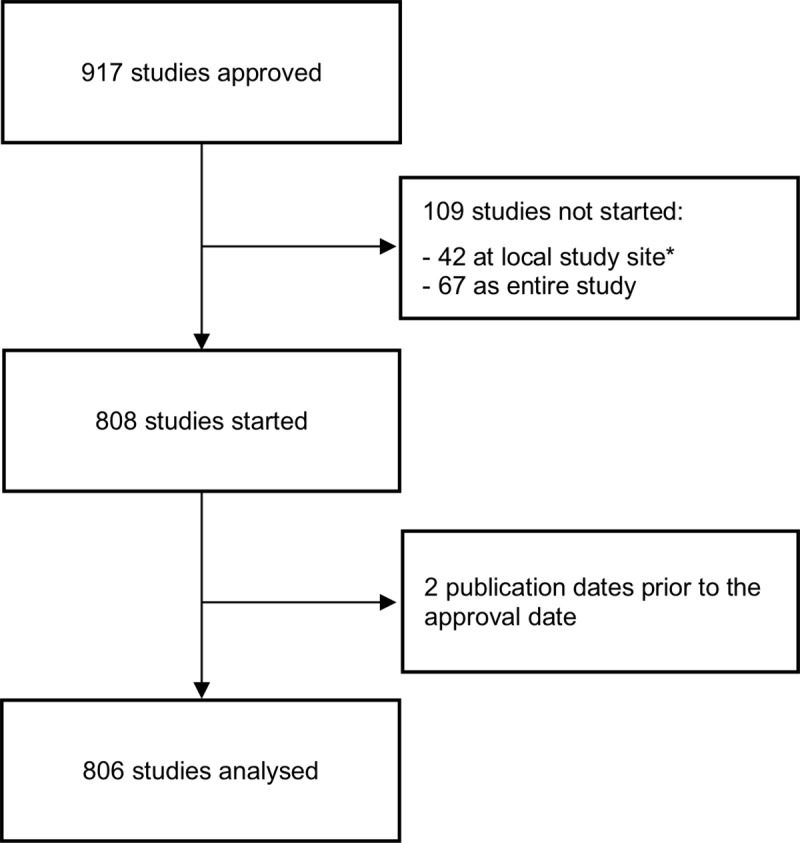
*We included one additional study compared to our previous analyses [24], because it later turned out that the survey results were incorrect (study started at local study site).
Study characteristics are displayed in Table 1.
Table 1. Baseline characteristics of included studies (n = 806).
| Characteristic | |
|---|---|
| Sample size | |
| Mean (sd) | 386 (909) |
| Median | 120 |
| Range | 3–3900 |
| Study design (n, %) | |
| RCT | 354 (43.9) |
| Other | 452 (56.1) |
| Collaboration | |
| Multi-centre, international | 189 (23.5) |
| Multi-centre, national | 275 (34.1) |
| Single-centre, national | 342 (42.4) |
| Primary outcome present (n, %) | |
| Yes | 482 (59.8) |
| No | 324 (40.2) |
| Funding (n, %) | |
| Commercial | 357 (44.3) |
| Non-commercial | 217 (26.9) |
| Not reported | 232 (28.8) |
| Industry Involvement (n, %) | |
| Yes | 348 (43.2) |
| No | 458 (56.8) |
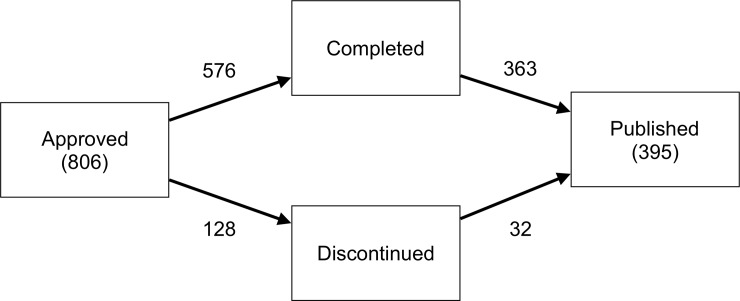
The overall survey response rate was 91%. By 2010 / 2012, 576 (71%) studies were completed and 128 (16%) had been discontinued. A further 41 were classified as ‘ongoing’ based on survey responses, while for 61 studies the status was unclear (i.e., no survey response, and no evidence of publication). Of the completed studies 363 (63%) had been published as a full article, as had 32 (25%) of the discontinued studies. Overall, 49% of studies had been published by 2010 / 2012. Fig 3 displays the number of studies observed to enter each state within the four-state model.
Fig 3. Four-state model.
The numbers next to the arrows indicate the total numbers of studies for each transition.
For the intermediate states of study completion and discontinuation, time-to-event information was only available in 23% and 13% of cases respectively, thus the majority of observations were interval-censored [35]. S1 Table summarizes the different observation cases within the four-state model with potential interval- or right-censoring and reports the corresponding counts and percentages in the data set.
Two-state model
The median time overall was 10 years (i.e., after 10 years half of all studies had been published) with an approximate 95% confidence interval of [8, ∞], and 4 years (95% CI [4, 5]) for studies that were actually published. The latter, however, is an underestimate of the true time to publication as it is biased by ‘conditioning on the future’ [36]; that is, only including studies for which the outcome was successful.
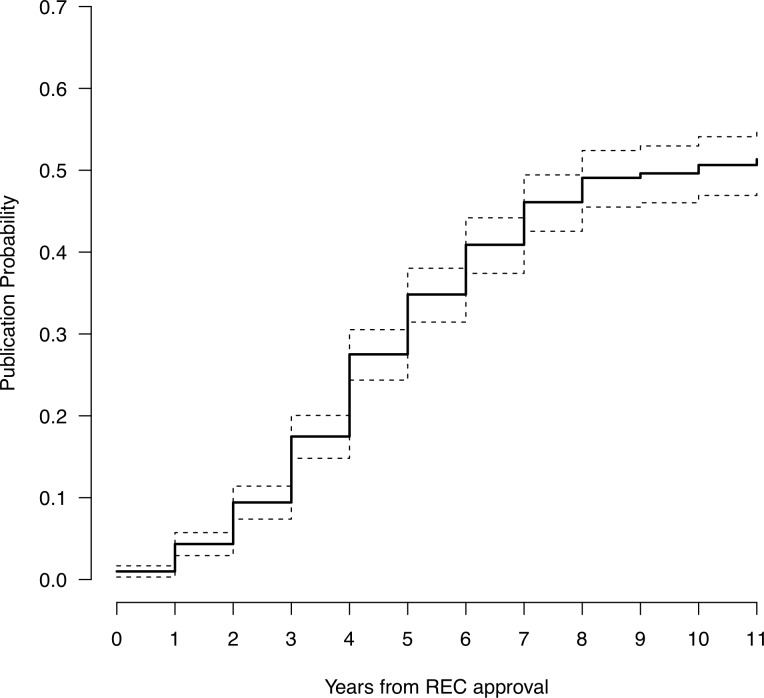
The publication rate, i.e., the probability that a previously unpublished study is published, is highest four to seven years after REC approval. Studies that have not been published after nine years are very unlikely to be subsequently published (publication probability < 10%) (Fig 4).
Fig 4. Complement of Kaplan-Meier estimate with its 95% confidence interval of the cumulative distribution function of the total time to publication.
Time point 0 is the REC’s positive vote, i.e. study approval.
Table 2, displaying the findings of the two-state model predicting the transition from approval to publication, shows that non-commercially funded studies were published faster than both commercially-funded studies and those in which the funding source was not reported. Studies with industry involvement in the conduct or analysis were published faster than those without industry involvement, as were studies with a primary outcome. Finally, single-centre studies tend to be published faster than multi-centre studies with an international partner. We note here that the variance inflation factors from the covariance matrix of the parameter estimates in the two-state Cox model were overall below three, i.e., there are probably no strong issues due to multicollinearity.
Table 2. Estimated covariate effects with their 95% confidence intervals (two-state model).
| Hazard ratio | 95% CI | |
|---|---|---|
| Approved → Published | ||
| log Sample Size | 1.038 | [0.959, 1.123] |
| RCT vs. other | 0.884 | [0.693, 1.128] |
| Funding: Commercial vs. non-commercial | 0.337 | [0.246, 0.461] |
| Funding: Not reported vs. non-commercial | 0.298 | [0.221, 0.404] |
| Industry involvement: Yes vs. no | 1.829 | [1.302, 2.567] |
| Primary outcome: Yes vs. no | 1.365 | [1.041, 1.790] |
| Collaboration: Multi-centre, national vs. single | 0.900 | [0.648, 1.249] |
| Collaboration: Multi-centre, international vs. single | 0.619 | [0.454, 0.844] |
Four-state model
We now consider each transition in the four-state model in detail. As shown in Table 3, several factors predicted study completion. Funding source was strongly predictive of subsequent study completion; non-commercially-funded studies were completed faster than commercially-funded studies and those in which the funding was unstated. Studies in which the sponsor was involved in the conduct or analysis were completed considerably faster than others. Also, studies with a predefined primary outcome tended to be completed faster than others. The main factors predicting discontinuation included study design and sample size: the hazard for an RCT to be discontinued after approval was higher than that for a non-RCT type study, while studies with a smaller sample size were also more likely to be discontinued. Funding source also tended to be predictive (borderline significant), with commercial funding or unstated funding increasing the hazard for study discontinuation as compared to non-commercial funding. Once completed, funding source was strongly predictive of subsequent publication; again, non-commercially-funded studies were published faster than commercially-funded studies and those in which the funding was unstated. Single-centre studies were published faster after completion than multi-centre/international studies. Prediction of publication following discontinuation was limited by the low number of studies in this category (n = 32), which led to wide confidence intervals around some of the estimates. However, among discontinued studies those with a larger sample size tended to be published considerably faster than others, whereas the hazard for an RCT to be published after discontinuation was considerably lower than that for a non-RCT type study.
Table 3. Estimated covariate effects with their 95% confidence intervals (four-state model).
| Hazard ratio | 95% CI | |
|---|---|---|
| Approved → Completed (n = 576) | ||
| Log sample size | 0.972 | [0.951, 0.993] |
| RCT vs. other | 1.077 | [0.855, 1.358] |
| Funding: Commercial vs. non-commercial | 0.523 | [0.359, 0.762] |
| Funding: Unstated vs. non-commercial | 0.648 | [0.485, 0.866] |
| Industry: Involved vs. not involved | 2.294 | [1.595, 3.298] |
| Primary outcome: Yes vs. no | 1.422 | [1.102, 1.836] |
| Collaboration: National multi-centre vs. national single-centre | 0.848 | [0.614, 1.171] |
| Collaboration: International multi-centre vs. national single-centre | 0.694 | [0.521, 0.924] |
| Approved → Discontinued (n = 128) | ||
| Log sample size | 0.857 | [0.819, 0.897] |
| RCT vs. other | 2.375 | [1.529, 3.689] |
| Funding: Commercial vs. non-commercial | 1.795 | [1.001, 3.220] |
| Funding: Unstated vs. non-commercial | 1.565 | [1.065, 2.301] |
| Industry: Involved vs. not involved | 1.093 | [0.541, 2.208] |
| Primary outcome: Yes vs. no | 1.532 | [0.924, 2.542] |
| Collaboration: National multi-centre vs. national single-centre | 0.751 | [0.429, 1.316] |
| Collaboration: International multi-centre vs. national single-centre | 0.815 | [0.482, 1.379] |
| Completed → Published (n = 363) | ||
| Log sample size | 1.086 | [0.999, 1.179] |
| RCT vs. other | 0.832 | [0.620, 1.117] |
| Funding: Commercial vs. non-commercial | 0.429 | [0.286, 0.644] |
| Funding: Unstated vs. non-commercial | 0.374 | [0.254, 0.551] |
| Industry: Involved vs. not involved | 1.124 | [0.716, 1.766] |
| Primary outcome: Yes vs. no | 0.998 | [0.711, 1.400] |
| Collaboration: National multi-centre vs. national single-centre | 0.825 | [0.527, 1.290] |
| Collaboration: International multi-centre vs. national single-centre | 0.597 | [0.392, 0.908] |
| Discontinued → Published (n = 32) | ||
| Log sample size | 1.481 | [1.468, 1.495] |
| RCT vs. other | 0.275 | [0.105, 0.724] |
| Funding: Commercial vs. non-commercial | 0.504 | [0.163, 1.559] |
| Funding: Unstated vs. non-commercial | -- | -- |
| Industry: Involved vs. not involved | 0.437 | [0.142, 1.338] |
| Primary outcome: Yes vs. no | 1.988 | [0.411, 9.625] |
| Collaboration: National multi-centre vs. national single-centre | 2.397 | [0.501, 11.481] |
| Collaboration: International multi-centre vs. national single-centre | 1.790 | [0.507, 6.317] |
Sensitivity analyses
Given the high proportion of missing information on the timing of model transitions, analyses were repeated using logistic regression. The outcomes were successful completion of each state transition, without taking into account time to event. Overall the results of the logistic regression analysis predicting transition from approval to publication show no discrepancies to the results of the two-state model (S2 Table).
The results (S3 Table) were similar to those obtained in the multi-state model analysis, but with larger standard errors. Funding source emerged as strongly predictive of the transition from approved to completed, with both commercially-funded studies and those in which the funding was unstated less likely to be completed than non-commercial studies. Moreover, two additional covariates emerged as predictive of the transition from completed to published: a pre-specified primary outcome, and industry involvement. With these changes, predictors of the transition from approved to completed were largely identical to those of the transition from completed to published.
Discussion
In this study, we applied a newly-developed four-state model to examine the research process from ethical approval to publication among clinical studies approved by a German REC between 2000 and 2002. We determined the overall time needed to publish the study results, distinguishing between the time required to conduct the study (i.e., study initiation to either study completion or discontinuation), and the time required to analyse, write up and publish the study findings (i.e., study completion/discontinuation to publication). We also examined predictors of the timing of transitions within the research process as described by the four-state model, i.e. from study initiation through the completion/discontinuation of data collection to eventual publication. About half of initiated clinical studies were subsequently published as full articles by 2010 / 2012, with the median time from study approval to publication 10 years. Discontinued studies were less often (25%) published than completed studies (63%). There is little consensus in the literature regarding time to publication, with wide variation depending on the cohorts investigated [3, 15, 37–39]. Previous evaluations, however, excluded studies with unknown completion date, which likely led to underestimation of time to publication [37].
The primary findings of this study were that a number of study-related factors which predicted transitions in the conduct of research studies from initiation to eventual publication could be identified, and that these factors differed across the transitions specified in the four-state model. The latter finding demonstrates the value of differentiating the time required to collect data from the time required to analyse and write up the findings for publication. The use of a four-state model permits a more comprehensive analysis of the research process than is possible if one simply examines the period from approval to publication in a single analysis. It thus represents an advance on previous studies of time to publication [11].
The hazard from completion to publication was mainly predicted by funding source, with the findings consistent with previous research finding industry-funded studies to have a lower publication rate than studies funded by medical centres [38]. This may suggest that commercial funders have lower incentive to publish findings in academic journals. Interestingly, Goldacre et al recently found that among clinical trials registered in the European Union clinical trials registry, those with a commercial sponsor were more likely to include result data in the registry [40]. For commercial funders, journal publication may not always be the preferred means of communicating study findings to the wider community. It may also point to characteristics of the findings, e.g. whether or not the main hypotheses were supported, influencing the decision whether or not to publish to a greater extent for commercially-funded studies, as has been found previously [11]. The influence of funding source is also reflected in the transition from approved to discontinued, where commercial studies were more likely to be discontinued. Again, this is suggestive of emerging study findings influencing the decision whether or not to continue a study to a greater extent if the study is commercially funded.
Consistent with Ioannidis [17], we did not find sample size affected overall time to publication in the two-state model analysis, and in the four-state model analysis sample size was related only to the time from approval to discontinuation, with smaller studies more likely to be discontinued than larger studies.
The major limitation of our study was that in over three-quarters of studies the year of completion or discontinuation was unavailable. As it was not a focus of investigation at that time, survey respondents were not asked to specify the year in which the study was completed or discontinued. Rather, this information was sourced from annual reports submitted to the REC, where it was inconsistently reported. The underlying data set was, therefore, insufficient to perform an adequate time-to-event analysis (with 2 or more states). However, the method we used to maximise the log-likelihood in the time-to-event analyses, the Method of Moving Asymptotes [33], has been extensively studied with simulated data assuming different underlying baseline intensities and a similarly large amount of missing information on the timing of intermediate states still resulting in almost unbiased covariate effect estimates [28]. It is also important to note that we were mainly missing only time-to-event data for these state transitions, rather than whether or not the study was completed or discontinued. It is reassuring that the sensitivity analyses we performed, which did not use time-to-event information, mostly resulted in similar findings to the primary analyses with regard to the predictors of state transitions. Moreover, while model estimates in the time-to-event analyses had wider confidence intervals than we would have had with full data, even with limited data the estimates were more precise than in the logistic regression analyses, which did not take into account time-to-event data. Despite the presence of missing time-to-event data, Cox regression is preferable to logistic regression because of the long follow-up period we considered and the large heterogeneity in follow-up times between our two cohorts. With extended follow-up, the odds ratio is a poor estimate of the hazard ratio, tending to overestimate the risk factor effect particularly when there are many observed events [41].
A further limitation was that the exact timing of events was often not determinable. One problem is that it is not always easy to define the completion date of a study. For example, the study completion date may be the date on which the last patient completed the final follow-up, the date on which the database was closed, or the date of the study end report, e. g. final report to the funder. Therefore, we decided not to consider specific dates, but rather to restrict our analysis to the year in which events occurred. We note here that instead of fitting a continuous-time model, a model for time-discrete or grouped time-to-event data would also have been an adequate choice towards fitting this kind of data observed in time periods [42]. Still, our approach can be seen as a suitable approximation. Since REC approval always occurs prior to data collection, this would have had the effect of increasing the estimate of the time required from study initiation to completion/discontinuation. In most cases, we expect the difference would be minimal or non-existent, however it is conceivable that studies of complex behavioural interventions may require a considerable period of intervention development work before participants can be recruited. Additionally, while the 2000 cohort of studies was followed over 11 years, the 2001/2002 cohort was only followed until 2010, see S2 Fig. This reduced the number of studies from these cohorts which we were able to identify, which may have led to an overestimate of the median time to publication. However, this would not lead to a systematic bias if the assumption of independency between the publication process and the censoring mechanism is not violated. There were also a number of studies for which the planned sample size was unknown. Imputing the median sample size likely led to underestimating the variance of this predictor, thus reducing our capacity to find an effect. Finally, only a few trials were published following discontinuation, and confidence intervals for the effect estimates for this transition were partly broad (indeed, no estimate could be computed for the comparison between non-commercially funded studies and funding unstated). These findings should therefore be interpreted with caution.
We believe that poor availability of such data is typical, at least from 2000–2002 in Germany. Things have since changed for the better, particularly due to the founding of the German Registry of Clinical Trials (DRKS) https://www.drks.de/drks_web/ in 2008. Study investigators who apply for an ethics approval are now requested to register there, such that a study is identifiable by a unique study ID. This is intended to ensure it is easier to track studies over time, allowing more reliable data on time to publication to be collected.
Finally, we are obliged to call attention to the importance of careful data collection. Many of the limitations of this study we have discussed are a direct consequence of our failure (in retrospect) to ask the right questions in the survey to ensure we could establish the existence and timing of state transitions. Readers who are considering undertaking a multi-state model analysis should ensure that a data analysis plan is drafted before data collection begins, to help prevent important factors in the analysis being overlooked.
Conclusions
The use of a four-state model permitted a comprehensive examination of the research process, allowing for the identification of predictive relationships between states that were not apparent from a simple two-state analysis. While the presence of missing time-to-event information was an important limitation, it did not appear to have an undue influence on the findings. Since this study was initiated, the proportion of studies which are registered in a clinical trial registry prior to data collection has greatly increased [43], and therefore future investigations of time to publication should have access to more complete information regarding the timing of state transitions. We recommend using modern statistical methods of event history analysis (multi-state models) to permit a detailed study of the publication process in medicine, epidemiology, or other areas of research.
Supporting information
(TIFF)
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The development and application of the multi-state model for the publication process was conducted as part of Tobias Haag’s Master Thesis. Data extraction of REC protocols and applications was carried out by Florian Volz (FV) and Alexander Hellmer (AH). Patrick Oeller performed the update of the literature search.
Data Availability
The file is available from Freidok Plus repository (accession number 14703).
Funding Statement
The authors received no specific funding for this work. The data of this project was collected within the research project funded by the German Research Foundation (DFG) [grant number EL 544/1-1]. The article processing charge was funded by the German Research Foundation (DFG) and the Albert-Ludwigs-University Freiburg in the funding programme Open Access Publishing.
References
- 1.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. Bmj. 1996;312(7023):71–2. 10.1136/bmj.312.7023.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Bogert CA, Souverein PC, Brekelmans CTM, Janssen SWJ, Koeter GH, Leufkens HGM, et al. Recruitment failure and futility were the most common reasons for discontinuation of clinical drug trials. Results of a nationwide inception cohort study in the Netherlands. J Clin Epidemiol. 2017;88:140–7. 10.1016/j.jclinepi.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 3.Chapman PB, Liu NJ, Zhou Q, Iasonos A, Hanley S, Bosl GJ, et al. Time to publication of oncology trials and why some trials are never published. PLoS One. 2017;12(9):e0184025 10.1371/journal.pone.0184025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldi I, Lanera C, Berchialla P, Gregori D. Early termination of cardiovascular trials as a consequence of poor accrual: analysis of ClinicalTrials.gov 2006–2015. BMJ open. 2017;7(6):e013482 10.1136/bmjopen-2016-013482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amstutz A, Schandelmaier S, Frei R, Surina J, Agarwal A, Olu KK, et al. Discontinuation and non-publication of randomised clinical trials supported by the main public funding body in Switzerland: a retrospective cohort study. BMJ open. 2017;7(7):e016216 10.1136/bmjopen-2017-016216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumle A, Schandelmaier S, Oeller P, Kasenda B, Briel M, von Elm E, et al. Premature Discontinuation of Prospective Clinical Studies Approved by a Research Ethics Committee—A Comparison of Randomised and Non-Randomised Studies. PLoS One. 2016;11(10):e0165605 10.1371/journal.pone.0165605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasenda B, von Elm E, You J, Blumle A, Tomonaga Y, Saccilotto R, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311(10):1045–51. 10.1001/jama.2014.1361 [DOI] [PubMed] [Google Scholar]
- 8.Chapman SJ, Shelton B, Mahmood H, Fitzgerald JE, Harrison EM, Bhangu A. Discontinuation and non-publication of surgical randomised controlled trials: observational study. BMJ. 2014;349:g6870 10.1136/bmj.g6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardez-Pereira S, Lopes RD, Carrion MJM, Santucci EV, Soares RM, de Oliveira Abreu M, et al. Prevalence, characteristics, and predictors of early termination of cardiovascular clinical trials due to low recruitment: Insights from the ClinicalTrials.gov registry. American Heart Journal. 2014;168(2):213–9.e1. 10.1016/j.ahj.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal R. The "file drawer problem" and tolerance for null results. Psychological Bulletin. 1979;86(3):638–41. [Google Scholar]
- 11.Schmucker C, Schell LK, Portalupi S, Oeller P, Cabrera L, Bassler D, et al. Extent of Non-Publication in Cohorts of Studies Approved by Research Ethics Committees or Included in Trial Registries. PLoS ONE. 2014;9(12):e114023 10.1371/journal.pone.0114023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AW, Song F, Vickers A, Jefferson T, Dickersin K, Gotzsche PC, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257–66. 10.1016/S0140-6736(13)62296-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14(8):iii, ix-xi, 1–193. [DOI] [PubMed] [Google Scholar]
- 14.Scherer RW, Ugarte-Gil C, Schmucker C, Meerpohl JJ. Authors report lack of time as main reason for unpublished research presented at biomedical conferences: a systematic review. J Clin Epidemiol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berendt L, Petersen LG, Bach KF, Poulsen HE, Dalhoff K. Barriers towards the publication of academic drug trials. Follow-up of trials approved by the Danish Medicines Agency. PLoS One. 2017;12(5):e0172581 10.1371/journal.pone.0172581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–72. 10.1016/0140-6736(91)90201-y [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279(4):281–6. 10.1001/jama.279.4.281 [DOI] [PubMed] [Google Scholar]
- 18.Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315(7109):640–5. 10.1136/bmj.315.7109.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sune P, Sune JM, Montoro JB. Positive outcomes influence the rate and time to publication, but not the impact factor of publications of clinical trial results. PLoS One. 2013;8(1):e54583 10.1371/journal.pone.0054583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dwan K, Gamble C, Williamson PR, Kirkham JJ, Reporting Bias G. Systematic review of the empirical evidence of study publication bias and outcome reporting bias—an updated review. PLoS One. 2013;8(7):e66844 10.1371/journal.pone.0066844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decullier E, Lheritier V, Chapuis F. Fate of biomedical research protocols and publication bias in France: retrospective cohort study. BMJ. 2005;331(7507):19 10.1136/bmj.38488.385995.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopewell S, Clarke M, Stewart L, Tierney J. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007(2):MR000011 10.1002/14651858.MR000011.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzoli L, Flacco ME, D'Addario M, Capasso L, De Vito C, Marzuillo C, et al. Non-publication and delayed publication of randomized trials on vaccines: survey. BMJ. 2014;348:g3058 10.1136/bmj.g3058 [DOI] [PubMed] [Google Scholar]
- 24.Blümle A, Meerpohl JJ, Schumacher M, von Elm E. Fate of Clinical Research Studies after Ethical Approval–Follow-Up of Study Protocols until Publication. PLoS ONE. 2014;9(2):e87184 10.1371/journal.pone.0087184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blümle A, Antes G, Schumacher M, Just H, von Elm E. Clinical research projects at a German medical faculty: follow-up from ethical approval to publication and citation by others. J Med Ethics. 2008;34(9):e20 10.1136/jme.2008.024521 [DOI] [PubMed] [Google Scholar]
- 26.Meerpohl JJ, Schell LK, Bassler D, Gallus S, Kleijnen J, Kulig M, et al. Evidence-informed recommendations to reduce dissemination bias in clinical research: conclusions from the OPEN (Overcome failure to Publish nEgative fiNdings) project based on an international consensus meeting. BMJ open. 2015;5(5):e006666 10.1136/bmjopen-2014-006666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510–20. 10.1258/jrsm.2011.110180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haag T. Multi-state models for analyzing the publication process of clinical studies FreiDok plus (https://freidok.uni-freiburg.de/data/14703): Freiburg; 2015. [Google Scholar]
- 29.Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11(2):91–115. 10.1191/0962280202SM276ra [DOI] [PubMed] [Google Scholar]
- 30.Commenges D. Inference for multi-state models from interval-censored data. Stat Methods Med Res. 2002;11(2):167–82. 10.1191/0962280202sm279ra [DOI] [PubMed] [Google Scholar]
- 31.Barker C. The mean, median, and confidence intervals of the Kaplan-Meier survival estimate—computations and applications. The American Statistician. 2009;63(1):78–80. [Google Scholar]
- 32.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982:29–41. [Google Scholar]
- 33.Svanberg K. The method of moving asymptotes—a new method for structural optimization. Int J Numer Methods Eng 1987;24:359–373. [Google Scholar]
- 34.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. 2018. [Google Scholar]
- 35.Van Den Hout A. Multi-state survival models for interval-censored data: Chapman and Hall/CRC; 2016. [Google Scholar]
- 36.Andersen PK, Keiding N. Interpretability and importance of functionals in competing risks and multistate models. Stat Med. 2012;31(11–12):1074–88. 10.1002/sim.4385 [DOI] [PubMed] [Google Scholar]
- 37.Korevaar DA, van Es N, Zwinderman AH, Cohen JF, Bossuyt PM. Time to publication among completed diagnostic accuracy studies: associated with reported accuracy estimates. BMC Med Res Methodol. 2016;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepshelovich D, Goldvaser H, Wang L, Abdul Razak AR. Comparison of published and unpublished phase I clinical cancer trials: an analysis of the CliniclTrials.gov database. Invest New Drugs. 2017. [DOI] [PubMed] [Google Scholar]
- 39.Strand LB, Clarke P, Graves N, Barnett AG. Time to publication for publicly funded clinical trials in Australia: an observational study. BMJ open. 2017;7(3):e012212 10.1136/bmjopen-2016-012212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldacre B, DeVito NJ, Heneghan C, Irving F, Bacon S, Fleminger J, et al. Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource. Bmj. 2018;362:k3218 10.1136/bmj.k3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green MS, Symons MJ. A comparison of the logistic risk function and the proportional hazards model in prospective epidemiologic studies. J Chronic Dis. 1983;36(10):715–23. 10.1016/0021-9681(83)90165-0 [DOI] [PubMed] [Google Scholar]
- 42.Tutz G and Schmid M (2016) Modelling Discrete Time-to-event Data. New York: Springer. [Google Scholar]
- 43.Trinquart L, Dunn AG, Bourgeois FT. Registration of published randomized trials: a systematic review and meta-analysis. BMC Med. 2018;16(1):173 10.1186/s12916-018-1168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The file is available from Freidok Plus repository (accession number 14703).