ABSTRACT
Extensive research has shown that LINC00963 is aberrantly expressed in human cancers, and that dysregulation of LINC00963 is implicated in the initiation and progression of human cancers. The expression and functions of LINC00963 in breast cancer are still unclear. Our aims were to measure the expression of LINC00963 in breast cancer, determine its effects on malignant behaviors of tumor cells, and uncover the molecular events underlying the actions of LINC00963 in breast cancer. Herein, LINC00963 was found to be overexpressed in breast cancer samples, and its overexpression was correlated with lymph node metastasis, TNM stage and differentiation grade. Patients with breast cancer harboring higher LINC00963 expression showed shorter overall survival than did the patients with lower LINC00963 expression. Functional experiments revealed that depletion of LINC00963 inhibited breast cancer cell proliferation, migration, and invasion and facilitated apoptosis in vitro and impaired tumor growth in vivo. Mechanism investigation revealed that LINC00963 can interact with microRNA-625 (miR-625). LINC00963 worked as a competitive endogenous RNA for miR-625 to weaken the suppressive effect of miR-625 on high mobility group AT-hook 1 (HMGA1) in breast cancer cells. Furthermore, miR-625 inhibition and HMGA1 restoration both abrogated the effects of LINC00963 silencing on breast cancer cells. Our findings indicate that the LINC00963–miR-625–HMGA1 pathway plays an important role in the malignancy of breast cancer in vitro and in vivo. Hence, targeting this pathway may be a novel strategy against breast cancer.
KEYWORDS: High mobility group AT-hook 1, microRNA-625, LINC00963, breast cancer
Introduction
Breast cancer is the most frequently diagnosed human cancer and the second leading cause of cancer-associated deaths among women globally [1]. In China, breast cancer accounts for 12.2% of new cancer cases and 9.6% of all cancer-related deaths [2]. Currently, the first-line therapeutic strategies include surgical resection, adjuvant radiotherapy, hormone therapy, chemotherapy, and targeted biotherapy [3]. In spite of the noticeable progress in the diagnosis and treatment in the past decades, the clinical outcomes for patients with breast cancer are still devastating, especially for the patients presenting with recurrence and metastasis [4,5]. Thus, it is worthwhile to thoroughly characterize the mechanisms underlying the aggressive characteristics of breast cancer for developing novel targets for the diagnosis and treatment.
MicroRNAs (miRNAs or miRs) are a class of noncoding short RNA molecules that consist of ~22 nucleotides [6]. miRNAs have been proven to downregulate gene expression through partial or complete base pairing with the 3′ untranslated region (3'- UTRs) of their target mRNAs, thereby leading to translation inhibition and/or mRNA degradation [7]. Some studies on miRNA expression indicate that various miRNAs are dysregulated during breast carcinogenesis and progression of this cancer [8–10]. In breast cancer, miRNAs can be subdivided into two groups: oncogenic miRNAs and tumor-suppressive miRNAs. The functions of oncogenic miRNAs increase the malignancy of breast cancer, while tumor-suppressive miRNAs play the opposite roles [11–13].
Long noncoding RNAs (lncRNAs) are another group of transcripts; they are over 200 nucleotides long but lack the protein-coding ability [14]. LncRNAs modulate the expression of tumor suppressors or oncogenes through multiple mechanisms, e.g., by acting as an RNA decoy, via alternative splicing, and through epigenetic, transcriptional, and post-transcriptional modifications [15,16]. Accumulating evidence has revealed that lncRNAs play crucial roles in various fundamental biological processes and human diseases, including cancer [17–19]. The aberrant expression of lncRNAs has been reported to be widespread among various types of human cancer, suggesting that aberrantly expressed lncRNAs may be involved in carcinogenesis and cancer progression [20,21]. A number of lncRNAs are dysregulated in breast cancer and exert oncogenic or tumor-suppressive actions [22–24]. For instance, LINC01355 [25], PANDAR [26], and MIR503HG [27] are downregulated in breast cancer and inhibit cancer progression; on the contrary, SNHG1 [28], NEAT1 [29], and ADPGK-AS1 [30] are overexpressed in this cancer and increase the malignancy of breast cancer. Therefore, identification of cancer-related lncRNAs and miRNAs in breast cancer may allow for the identification of potential therapeutic targets to improve the prognosis for patients.
Extensive research suggests that LINC00963 is aberrantly expressed in human cancers, and that dysregulation of LINC00963 is implicated in the initiation and progression of human cancers [31–34]. Unfortunately, the expression and functions of LINC00963 in breast cancer remain largely unexplored. Hence, in this study, we attempted to characterize the expression profile of LINC00963 in breast cancer and determine the effects of LINC00963 on the malignant behaviors of breast cancer cells. In addition, a systematic approach was employed to illustrate the molecular events underlying the activities of LINC00963 in breast cancer.
Materials and methods
Clinical samples
In total, 53 patients with a diagnosis of breast cancer received in Liaocheng People’s Hospital were enrolled in this study, and none of these patients had been treated with preoperative radiotherapy, chemotherapy, or other relevant modalities. Breast cancer tissue samples and matched adjacent normal tissues were collected, immediately preserved in liquid nitrogen, and then stored at −80°C until analysis. Written informed consent was provided by all the patients. The work with human subjects was carried out with the approval of the Ethics Committee of Liaocheng People’s Hospital and in compliance with the principles of the Declaration of Helsinki.
Cell lines
Breast cancer cell lines (BT-474, MCF-7, MDA-MB-231, and SKBR3) and human normal breast epithelial cell line MCF-10A were bought from the Chinese Academy of Sciences (Shanghai, China) and cultured in accordance with the vendor’s instructions. In brief, cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% of fetal bovine serum (FBS) and 1% of a penicillin/streptomycin mixture (Gibco, Grand Island, NY, USA) at 37°C in a humidified chamber supplied with 5% of CO2.
Cell transfection
Small interfering RNA downregulating lncRNA LINC00963 (si-LINC00963) and negative control (NC) small interfering RNA (si-NC) were acquired from Shanghai GenePharma Co., Ltd. (Shanghai, China). The miR-625 mimics, NC miRNA mimics (miR-NC), miR-625 inhibitor, and NC inhibitor were purchased from Guangzhou Ribobio Co., Ltd. (Guangzhou, China). The full-length sequence of HMGA1 lacking the 3'-UTR was synthesized by Shanghai GenePharma Co., Ltd., and inserted into the pcDNA3.1 vector to produce the pcDNA3.1-HMGA1 plasmid (pc-HMGA1). The oligonucleotide and plasmid transfection was carried out using the Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse-transcription quantitative PCR (RT-qPCR)
The TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used for total-RNA isolation. The quality and concentration of total RNA were evaluated on a Nanodrop device (Invitrogen; Thermo Fisher Scientific, Inc.). To quantify LINC00963 expression, cDNA was prepared from total RNA by means of the PrimeScript RT-Reagent Kit (Takara Bio, Kusatsu, Japan), and then cDNA was subjected to qPCR with the SYBR Premix Ex Taq™ Kit (Takara Bio, Kusatsu, Japan). For miR-625 expression quantitation, total RNA was reverse-transcribed into cDNA using the miScript Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany). After that, qPCR was carried out with the miScript SYBR Green PCR Kit (Qiagen GmbH, Hilden, Germany). LINC00963 and miR-625 expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6, respectively. Relative gene expression was calculated by the 2−ΔΔCt method.
Cell counting kit-8 (CCK-8) assay
After incubation for 24 h, transfected cells were collected to prepare a cell suspension. Then, 100 μl of the cell suspension (2 × 104 cells/ml) was seeded in each well of 96-well plates. The cells were kept at 37°C in a humidified chamber with 5% CO2, and the CCK-8 assay was performed 0, 24, 48, or 72 h later. At every time point, 10 μl of the CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added into each well prior to incubation at 37°C for 2 h, followed by measurement of absorbance at 450 nm wavelength on a microplate reader (Bio-Rad, Hercules, CA, USA).
Apoptosis detection by flow cytometry
After 48 h culture, transfected cells were centrifuged, washed twice with ice-cold PBS (Gibco, Grand Island, NY, USA), and subjected to the measurement of the cell apoptosis rate using the Annexin V–Fluorescein Isothiocyanate (FITC) Apoptosis Detection Kit (Biolegend, San Diego, CA, USA). Briefly, the cells were resuspended in 500 μl of 1× binding buffer and then stained with 5 μl of Annexin V-FITC and 5 μl of a propidium iodide solution that came with the kit. After 15 min incubation in the dark at room temperature, the proportion of apoptotic cells was determined on a FACScan flow cytometer (BD Biosciences).
Transwell migration and invasion assays
Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA) with membranes of 8 µm pore size were employed for the migration and invasion assays. For the invasion assay, the membranes were precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), whereas no Matrigel was applied in the migration assay. Transfected cells were harvested after 48 h incubation and were used to prepare cell suspension. The cells were resuspended in FBS-free DMEM, and the cell concentration was adjusted to 5 × 106 cells/ml. The upper compartments were covered with 200 μl of the cell suspension, while 500 μl of DMEM supplemented with 20% of FBS was added into the bottom compartments. At 24 h later, we gently removed nonmigratory and noninvasive cells by wiping the membranes with cotton swabs. Cells that passed through the pores to the underside of the membranes were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. The stained cells were subsequently counted in five randomly selected visual fields under an inverted microscope (Olympus Corp., Tokyo, Japan).
Tumor xenograft experiment
The procedures for the animal experiments were approved by the Animal Care and Use Committee of Liaocheng People’s Hospital and were compliant with the guidelines of the Animal Protection Law of the People’s Republic of China-2009 for experimental animals. MCF-7 cells transfected with either si-LINC00963 or si-NC were harvested at 24 h post-transfection, resuspended in 100 μl of PBS and injected subcutaneously into the flank of 4- to 6-week-old male BALB/c nude mice (Shanghai Experimental Animal Center of Chinese Academy of Sciences; Shanghai, China). The diameters of the tumor xenografts were measured using calipers once every 3 days. All the mice were euthanized after the last measurement. The tumor xenografts were excised, weighed, and subjected to RT-qPCR analysis and western blotting. Tumor volume was calculated via the following formula: tumor volume = 1/2 × tumor length × tumor width2.
Subcellular fractionation and RNA isolation
The cytoplasmic and nuclear fractions of breast cancer cells were isolated and purified by means of the PARIS Kit (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was extracted separately from cytoplasmic and nuclear fractions and then subjected to RT-qPCR.
RNA immunoprecipitation (RIP) assay
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore Inc., Billerica, MA, USA) was employed for the RIP assay. Cells were lysed in complete RIP lysis buffer, and the cell extract was collected and incubated with magnetic beads conjugated with an anti-Argonaute 2 (AGO2) or anti–immunoglobulin G (IgG) antibody (Millipore Inc., Billerica, MA, USA) at 4 °C for 6 h. Finally, RNA was isolated, and RT-qPCR analysis was performed to assess LINC00963 and miR-625 expression.
Bioinformatic analysis and luciferase reporter assay
The interaction between LINC00963 and miRNAs was predicted in starBase 3.0 (http://starbase.sysu.edu.cn/). The fragments of LINC00963 containing the predicted wild-type (WT) and mutant (MUT) miR-625–binding site were amplified by Shanghai GenePharma Co., Ltd., and separately cloned into the pmirGLO luciferase reporter vector (Promega Corporation, Madison, WI, USA), thus resulting in plasmids called LINC00963-WT and LINC00963-MUT, respectively. Cells were seeded in 24-well plates and allowed to attach for 24 h before transfection. Cotransfection of the constructed plasmids and either the miR-625 mimics or miR-NC was conducted with the Lipofectamine® 2000 reagent. At 48 h post-transfection, luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI, USA). Firefly luciferase activity was normalized to that of Renilla luciferase.
Western blot analysis
RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) was utilized for total-protein isolation. The concentration of total protein was measured by a bicinchoninic acid assay (Beyotime Institute of Biotechnology, Shanghai, China). Equal amounts of protein were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis in a 10% gel and were electroblotted to polyvinylidene difluoride membranes. Blocking was conducted in 5% fat-free milk for 2 h at room temperature. The membranes were probed with primary antibodies against HMGA1 (cat. No. ab129153; dilution 1:1,000; Abcam, Cambridge, UK) or GAPDH (cat. No. ab128915; dilution 1:1,000; Abcam) at 4°C overnight, and next incubated with a goat anti-rabbit immunoglobulin G horseradish peroxidase–conjugated secondary antibody (cat. No. ab205718; dilution 1:5,000; Abcam). Finally, the Pierce™ ECL Western Blotting Substrate Kit (Thermo Fisher Scientific, Inc.) was applied to develop the protein signals.
Statistical analysis
All the data were analyzed in the SPSS software (version 19.0; IBM Corp.). Student’s t test was performed to assess the differences between two groups, whereas one-way analysis of variance followed by Tukey’s post hoc test was carried out to evaluate the differences among multiple groups. The correlation between LINC00963 levels and clinical features of the patients with breast cancer was measured by the chi-squared test. The overall-survival curve was analyzed by the Kaplan–Meier method, and the log-rank test was conducted to compare the survival data. Spearman’s correlation analysis was performed to assess the expression correlation between LINC00963 and miR-625 among the breast cancer tissue samples. All results are presented as the mean ± SD. Data with P < 0.05 were considered statistically significant.
Results
Overexpression of LINC00963 correlates with poor prognosis in breast cancer
To elucidate the expression profile of LINC00963 in breast cancer, we first measured the expression of this lncRNA in 53 pairs of breast cancer tissue samples and matched adjacent normal tissues. The results of RT-qPCR analysis suggested that the expression of LINC00963 was higher in breast tumors than in matched adjacent normal tissue samples (Figure 1(a), **P < 0.01). To confirm our finding, LINC00963 expression was also determined in breast cancer cell lines, including BT-474, MCF-7, MDA-MB-231, and SKBR3. In comparison with human normal breast epithelial cell line MCF-10A, LINC00963 was weakly expressed in all the four tested breast cancer cell lines, as evidenced by RT-qPCR analysis (Figure 1(b), *P < 0.05, **P < 0.01).
Figure 1.

LINC00963 expression is excessive in breast cancer and predicts poorer prognosis.
(a) RT-qPCR analysis was performed to determine LINC00963 expression in 53 pairs of breast cancer tissue samples and matched adjacent normal tissues. Paired student’s t-test is utilized for statistical analysis. **P < 0.01 vs. adjacent normal tissues. (b) Expression of LINC00963 in four breast cancer cell lines (BT-474, MCF-7, MDA-MB-231, and SKBR3) and in human normal breast epithelial cell line MCF-10A was tested via RT-qPCR. Unpaired student’s t-test is utilized for statistical analysis. *P < 0.05, **P < 0.01 vs. MCF-10A. (c) The Kaplan–Meier method and logrank test were utilized to analyze the correlation between LINC00963 expression and the overall survival among patients with breast cancer. The overall-survival curve was analyzed by the Kaplan–Meier method, and the log-rank test was conducted to compare the survival data. P = 0.040.
To assess the clinical value of LINC00963 in breast cancer, all the patients were assigned to either a high LINC00963 expression group (n = 27) or low LINC00963 expression group (n = 26) according to the median value of LINC00963 expression among the 53 breast cancer tissue samples. The correlation of LINC00963 expression and clinical parameters was analyzed by the chi-squared test. The analysis revealed that upregulation of LINC00963 correlated with lymph node metastasis (P = 0.002), TNM stage (P = 0.042) and differentiation grade (P = 0.024) among our 53 patients with breast cancer (Table 1). In addition, patients with breast cancer harboring high LINC00963 expression showed shorter overall survival than patients with low LINC00963 expression (Figure 1(c), p = 0.040). The above results suggested that LINC00963 was overexpressed in breast cancer and may be associated with cancer progression.
Table 1.
Correlation of clinicopathological parameters with LINC00963 expression in patients with breast cancer.
| LINC00963 expression |
|||
|---|---|---|---|
| Clinicopathological parameters | High | Low | P |
| Age (years) | 27 | 26 | 0.569 |
| < 50 | 11 (41%) | 8 (31%) | |
| ≥50 | 16 (59%) | 18 (69%) | |
| Tumor size (mm) | 0.583 | ||
| < 2 | 17 (63%) | 14 (54%) | |
| ≥ 2 | 10 (37%) | 12 (46%) | |
| ER status | 0.412 | ||
| Negative | 14 (52%) | 10 (39%) | |
| Positive | 13 (48%) | 16 (61%) | |
| PR status | 0.170 | ||
| Negative | 12 (44%) | 17 (65%) | |
| Positive | 15 (56%) | 9 (35%) | |
| Her-2 status | 0.785 | ||
| Negative | 16 (59%) | 14 (54%) | |
| Positive | 11 (41%) | 12 (46%) | |
| Lymph node metastasis | 0.002 | ||
| Negative | 14 (52%) | 21 (81%) | |
| Positive | 13 (48%) | 5 (19%) | |
| TNM stage | 0.042 | ||
| I–II | 18 (67%) | 24 (92%) | |
| III | 9 (33%) | 2 (8%) | |
| Differentiation grade | 0.024 | ||
| Well and moderately | 12 (44%) | 20 (77%) | |
| Poorly and undifferentiated | 15 (56%) | 6 (23%) | |
chi-squared test.
Disruption of the expression of LINC00963 decreases breast cancer cell growth and metastasis in vitro
Cell lines MCF-7 and MDA-MB-231 featured relatively higher LINC00963 expression among the four tested breast cancer cell lines; therefore, these two cell lines were chosen for the following experiments. MCF-7 and MDA-MB-231 cells were transfected with si-LINC00963 to silence LINC00963 expression. The decreased expression of LINC00963 in MCF-7 and MDA-MB-231 cells after si-LINC00963 transfection was confirmed via RT-qPCR analysis (Figure 2(a), ***P < 0.001). The influence of LINC00963 silencing on breast cancer cell proliferation was tested by the CCK-8 assay. Transfection with si-LINC00963 resulted in significant inhibition of MCF-7 and MDA-MB-231 cell proliferation (Figure 2b, **P < 0.01). Then, flow-cytometric analysis was conducted to examine the impact of the LINC00963 knockdown on the apoptosis of breast cancer cells. LINC00963 depletion dramatically increased the apoptosis of MCF-7 and MDA-MB-231 cells (Figure 2c, **P < 0.01). The migratory and invasive abilities of tumor cells are required for tumor metastasis; accordingly, the Transwell cell migration and invasion assays were conducted to test the migration and invasiveness of LINC00963-depleted breast cancer cells. The results showed that the numbers of migratory (Figure 2d, **P < 0.01) and invasive (Figure 2e, **P < 0.01) MCF-7 and MDA-MB-231 cells were remarkably reduced in the cell groups transfected with si-LINC00963. In a word, these results revealed that a reduction of LINC00963 expression may reduce the capacity of breast cancer cells for growth and metastasis in vitro.
Figure 2.

LINC00963 knockdown attenuates proliferation, migration, and invasiveness and increases apoptosis of breast cancer cells.
(a) Si-LINC00963 was designed to successfully knock down endogenous LINC00963 in MCF-7 and MDA-MB-231 cells. ***P < 0.001 vs. si-NC. (b) The CCK-8 assay shows the impact of the LINC00963 knockdown on the proliferation of MCF-7 and MDA-MB-231 cells. **P < 0.05 vs. si-NC. (c) Investigation of the apoptosis rate of LINC00963-depleted MCF-7 and MDA-MB-231 cells was performed by flow cytometry. **P < 0.05 vs. si-NC. (d, e) Influences of LINC00963 silencing on the migratory and invasive abilities of MCF-7 and MDA-MB-231 cells were evaluated by Transwell migration and invasion assays. **P < 0.01 vs. si-NC. Unpaired student’s t-test is utilized for statistical analysis.
LINC00963 works as a competitive endogenous RNA (cerna) for mir-625 in breast cancer cells
To explore the mechanisms by which LINC00963 exerted the oncogenic actions in breast cancer cells, we first investigated the subcellular localization of LINC00963 in MCF-7 and MDA-MB-231 cells. As depicted in Figure 3a, LINC00963 is mainly present in the cytoplasm of MCF-7 and MDA-MB-231 cells, suggesting that LINC00963 may function as a ceRNA for some miRNA in breast cancer cells. We, therefore, searched starBase 3.0 for the potential miRNAs that could be sponged by LINC00963. MiR-625 was found to have a high probability of binding to LINC00963 (Figure 3b). In addition, another study has revealed that miR-625 performs tumor-suppressive functions in breast cancer [35]. Hence, miR-625 was selected for further experiments. The luciferase reporter assay was conducted to dissect the direct interaction between LINC00963 and miR-625 in breast cancer cells. First, the transfection efficiency of the miR-625 mimics was validated by RT-qPCR; it was observed that the expression of miR-625 was markedly higher in miR-625 mimics–transfected MCF-7 and MDA-MB-231 cells (Figure 3c, ***P < 0.001). The luciferase activity of MCF-7 and MDA-MB-231 cells transfected with LINC00963-WT was decreased by miR-625 overexpression (*P < 0.05, **P < 0.01). By contrast, the miR-625 mimics transfection did not affect the luciferase activity in the cells cotransfected with LINC00963-MUT (Figure 3d). In addition, the RIP assay indicated that LINC00963 and miR-625 were preferentially enriched by the anti-AGO2 antibody after the immunoprecipitation in the lysates of MCF-7 and MDA-MB-231 cells, suggesting that miR-625 is a target of LINC00963 in breast cancer cells (Figure 3e, **P < 0.01, ***P < 0.001).
Figure 3.
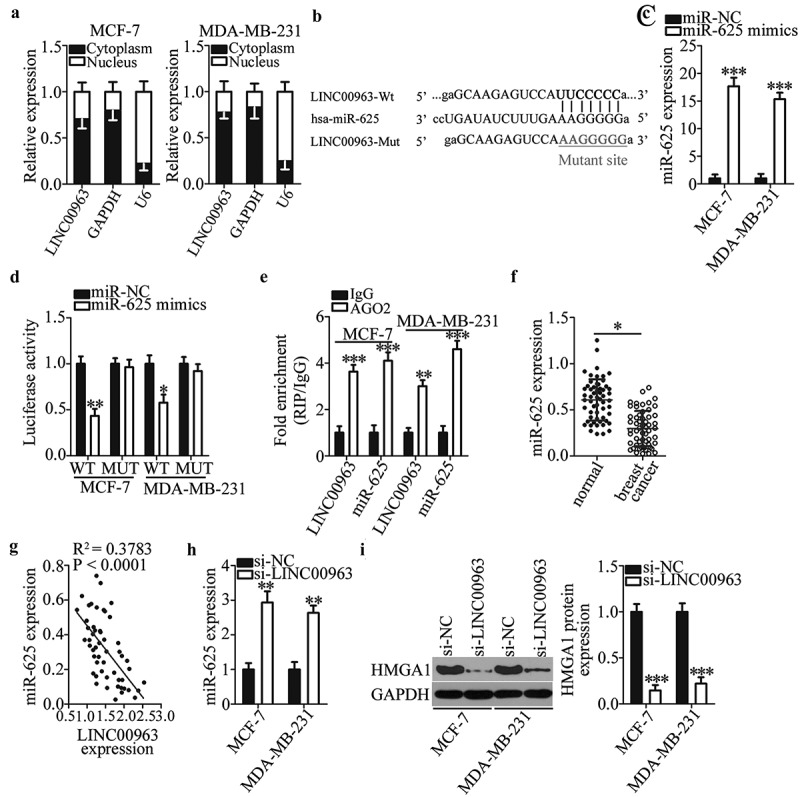
LINC00963 functions as a sponge for miR-625 in breast cancer cells.
(a) LINC00963 is mainly expressed in the cytoplasm of MCF-7 and MDA-MB-231 cells. (b) The wild-type miR-625–binding sequence in LINC00963 was predicted by starBase 3.0. The mutant miR-625–binding sequences are shown too. (c) The miR-625 mimics was introduced into MCF-7 and MDA-MB-231 cells to elevate the endogenous miR-625 expression. Unpaired student’s t-test is utilized for statistical analysis. ***P < 0.001 vs. miR-NC. (d) The luciferase reporter assay was conducted to confirm the interaction between miR-625 and LINC00963 in breast cancer cells. Luciferase activities were measured in MCF-7 and MDA-MB-231 cells after cotransfection with either the miR-625 mimics or miR-NC and either LINC00963-WT or LINC00963-MUT. Unpaired student’s t-test is utilized for statistical analysis. *P < 0.05, **P < 0.01 vs. miR-NC. (e) The RIP assay indicates that LINC00963 and miR-625 are preferentially enriched by the anti-AGO2 antibody after immunoprecipitation in the lysates of MCF-7 and MDA-MB-231 cells. Unpaired student’s t-test is utilized for statistical analysis. **P < 0.01, ***P < 0.001 vs. IgG. (f) MiR-625 expression in 53 pairs of breast cancer tissue samples and matched adjacent normal tissue samples, as determined by RT-qPCR. *P < 0.05 vs. adjacent normal tissues. Paired student’s t-test is utilized for statistical analysis. (g) Spearman’s correlation analysis was carried out to test the expression correlations between LINC00963 and miR-625 in breast cancer tissues. R2 = 0.3783, P < 0.0001. (h) The impact of the LINC00963 knockdown on miR-625 expression was examined in MCF-7 and MDA-MB-231 cells after either si-LINC00963 or si-NC transfection. Unpaired student’s t-test is utilized for statistical analysis. **P < 0.01 vs.si-NC. (i) Western blotting revealed that HMGA1 protein expression is suppressed by si-LINC00963 in MCF-7 and MDA-MB-231 cells. Unpaired student’s t-test is utilized for statistical analysis. ***P < 0.001 vs. si-NC.
After that, the expression of miR-625 was measured in the 53 pairs of breast cancer tissue samples and matched adjacent normal tissue samples via RT-qPCR analysis. MiR-625 turned out to be significantly downregulated in breast cancer tissue samples relative to the adjacent normal tissues (figure 3f, p < 0.05). Furthermore, an inverse correlation between LINC00963 and miR-625 levels was identified in breast cancer tissue samples (Figure 3g, R2 = 0.3783, P < 0.0001), as revealed by Spearman’s correlation analysis. Moreover, the regulatory influence of LINC00963 silencing on endogenous miR-625 expression was examined via RT-qPCR. Downregulation of LINC00963 caused a significant increase of miR-625 expression in both MCF-7 and MDA-MB-231 cells (Figure 3h, **P < 0.01). HMGA1 has been previously validated as a direct target gene of miR-625 in breast cancer cells [35]. Hence, we next tested whether LINC00963 participates in the regulation of HMGA1 expression in breast cancer cells. The LINC00963 knockdown notably decreased the HMGA1 protein expression in MCF-7 and MDA-MB-231 cells (Figure 3i, ***P < 0.001). Taken together, these findings suggested that LINC00963 functioned as a ceRNA for miR-625 in breast cancer cells and thereby upregulated HMGA1.
Influence of LINC00963 knockdown on the characteristics of breast cancer cells is dependent on the mir-625–HMGA1 axis
We demonstrated above that a reduction in LINC00963 expression attenuated breast cancer progression, and that LINC00963 positively regulates HMGA1 expression by sponging miR-625 in breast cancer. Accordingly, we next performed rescue experiments to test whether the miR-625–HMGA1 axis is required for the oncogenic activities of LINC00963 in breast cancer cells. To this end, the LINC00963-depleted MCF-7 and MDA-MB-231 cells were next treated with the miR-625 inhibitor. RT-qPCR analysis confirmed that transfection with the miR-625 inhibitor led to a significant knockdown of miR-625 expression in MCF-7 and MDA-MB-231 cells (Figure 4a, **P < 0.01, ***P < 0.001). The LINC00963 downregulation–mediated increase in miR-625 expression (Figure 4b, **P < 0.01, ##P < 0.01) was reversed in MCF-7 and MDA-MB-231 cells that were cotransfected with the miR-625 inhibitor. Simultaneously, the results of functional experiments showed that silencing of LINC00963 expression inhibited MCF-7 and MDA-MB-231 cell proliferation (Figure 4c, **P < 0.01, ##P < 0.01), promoted their apoptosis (Figure 4d, **P < 0.01, ##P < 0.01), and decreased their migration (Figure 4e, **P < 0.01, ##P < 0.01) and invasiveness (figure 4f, **P < 0.01, ##P < 0.01), in agreement with what we observed above. By contrast, the influence of LINC00963 inhibition on breast cancer cells was abrogated by treatment with the miR-625 inhibitor.
Figure 4.
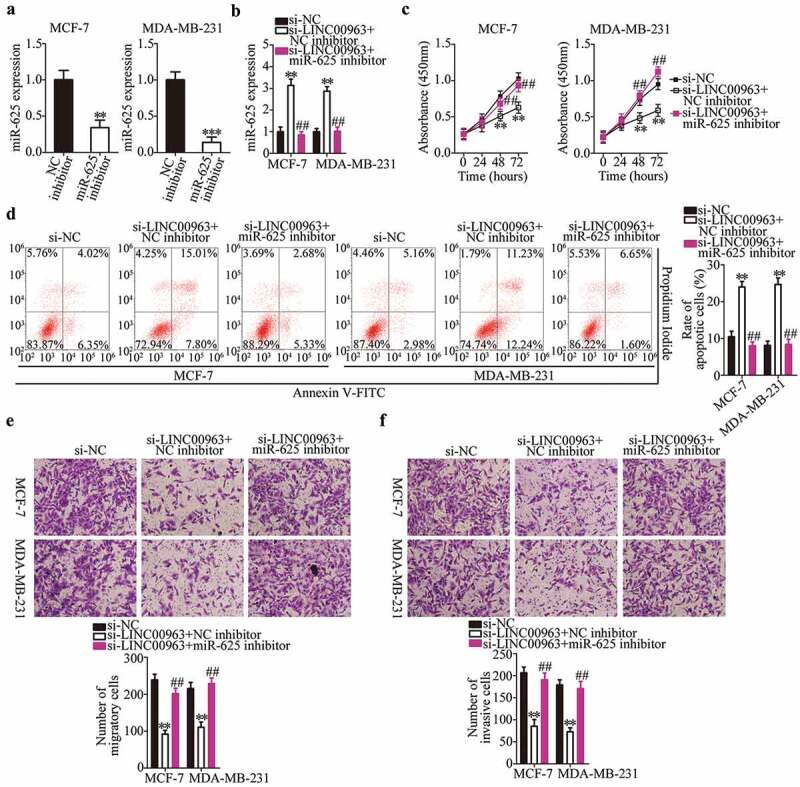
A reduction in LINC00963 expression suppresses the proliferation, migration, and invasiveness and accelerates the apoptosis of breast cancer cells via direct modulation of miR-625.
(a) MCF-7 and MDA-MB-231 cells transfected with either the miR-625 inhibitor or NC inhibitor were collected and subjected to RT-qPCR analysis for the determination of miR-625 expression. Unpaired student’s t-test is utilized for statistical analysis. **P < 0.01, ***P < 0.001 vs. NC inhibitor. (b) Si-LINC00963 and either the miR-625 inhibitor or NC inhibitor were cotransfected into MCF-7 and MDA-MB-231 cells. After 48 h culture, RT-qPCR analysis was conducted to assess miR-625 expression. *P < 0.01 vs. si-NC. ##P < 0.01vs. si-LINC00963+ NC inhibitor. (c, d) The CCK-8 assay and flow-cytometric analysis were performed to prove that the effects of si-LINC00963 on the proliferation and apoptosis of MCF-7 and MDA-MB-231 cells are abrogated by the miR-625 inhibitor. **P < 0.01 vs. si-NC. ##P < 0.01 vs. si-LINC00963+ NC inhibitor. (e, f) Transwell migration and invasion assays were conducted to analyze the migration and invasion status of MCF-7 and MDA-MB-231 cells cotransfected with si-LINC00963 and either the miR-625 inhibitor or NC inhibitor. **P < 0.01 vs. si-NC. ##P < 0.05 vs. si-LINC00963+ NC inhibitor. One-way analysis of variance followed by Tukey’s post hoc test was utilized for statistical analysis.
To confirm that the downregulation of LINC00963 has an inhibitory action on the malignancy of breast cancer cells via the miR-625–HMGA1 axis, either the HMGA1-overexpressing plasmid (pc-HMGA1) or the empty vector was cotransfected with si-LINC00963 into MCF-7 and MDA-MB-231 cells. The suppression of HMGA1 protein expression induced by the LINC00963 knockdown was reversed in MCF-7 and MDA-MB-231 cells after cotransfection with pc-HMGA1 (Figure 5a, ***P < 0.001, ###P < 0.001). Unsurprisingly, subsequent functional experiments indicated that the effects of LINC00963 silencing on the proliferation (Figure 5b, **P < 0.01, ##P < 0.01), apoptosis (Figure 5c, **P < 0.01, ##P < 0.01), migration (Figure 5d, **P < 0.01, ##P < 0.01), and invasiveness (Figure 5e, **P < 0.01, ##P < 0.01) of MCF-7 and MDA-MB-231 cells were partly neutralized by the reintroduction of HMGA1 expression. Overall, these data suggested that the LINC00963–miR-625–HMGA1 pathway regulates the malignant progression of breast cancer cells in vitro.
Figure 5.

Recovery of HMGA1 expression rescinds the effects of the LINC00963 knockdown on MCF-7 and MDA-MB-231 cells.
Either the HMGA1-overexpressing plasmid (pc-HMGA1) or the empty pcDNA3.1 vector in combination with si-LINC00963 was introduced into MCF-7 and MDA-MB-231 cells. The transfected cells were studied in the following experiments. (a) The protein expression of HMGA1 in MCF-7 and MDA-MB-231 cells treated as described above was examined by western blotting. ***P < 0.001 vs. si-NC. ###P < 0.001 vs. si-LINC00963+ pcDNA3.1. (b–e) The CCK-8 assay, flow cytometry, and Transwell migration and invasion assays were carried out to determine the proliferation, apoptosis, migration, and invasiveness of MCF-7 and MDA-MB-231 cells that were cotransfected with si-LINC00963 and either pc-HMGA1 or pcDNA3.1. **P < 0.01 vs. si-NC. ##P < 0.01 vs. si-LINC00963+ pcDNA3.1. One-way analysis of variance followed by Tukey’s post hoc test was utilized for statistical analysis.
Inhibition of LINC00963 restricts breast cancer growth in vivo
To further illustrate the effects of LINC00963 silencing on breast carcinogenesis and on the growth of breast cancer cells in vivo, the tumor xenograft experiment was conducted via injection of either si-LINC00963–transfected or si-NC–transfected MCF-7 cells into the flank of nude mice. Four weeks after the inoculation, the tumor growth curves revealed that the downregulation of LINC00963 significantly slowed the tumor growth of breast cancer cells in vivo (Figure 6a,b, *P < 0.05, **P < 0.01). The weight of tumor xenografts was lower in the si-LINC00963 group than in the si-NC group (Figure 6c, **P < 0.01). In addition, the expression of LINC00963 was still low (Figure 6d, *P < 0.05) while miR-625 expression was high (Figure 6e, **P < 0.01) in tumor xenografts derived from si-LINC00963–transfected MCF-7 cells. The protein level of HMGA1 was lower in the si-LINC00963 group than in the si-NC group (figure 6f, **P < 0.01), as determined by western blotting. These results implied that the LINC00963 knockdown hindered the tumor growth of breast cancer cells through regulation of the miR-625–HMGA1 axis.
Figure 6.
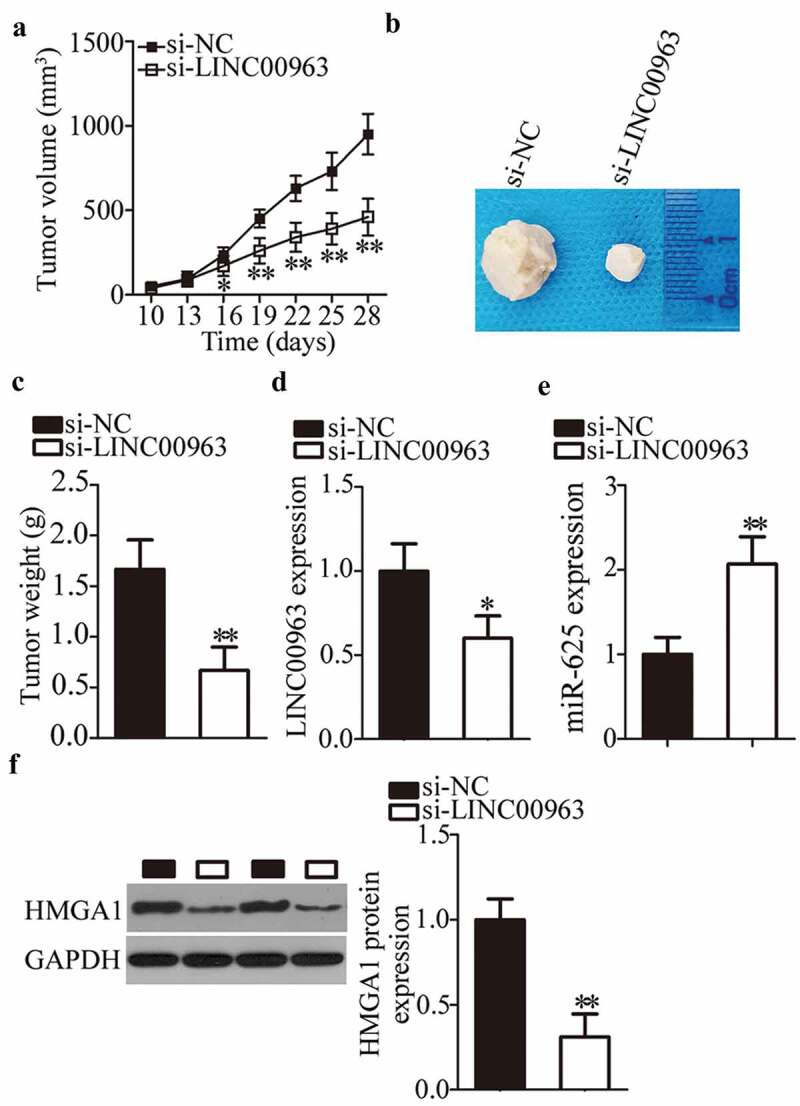
LINC00963 silencing restrains breast tumor growth in vivo.
(a) MCF-7 cells transfected with either si-LINC00963 or si-NC were injected into nude mice. Tumor growth curves revealed that the tumor xenografts grew at a markedly slower rate in the si-LINC00963 group than in the si-NC group. *P < 0.05. **P < 0.01 vs. si-NC. (b) At the end of this experiment, the mice were anesthetized, and tumor xenografts were excised. The representative images of tumor xenografts are presented. (c) The weight of tumor xenografts obtained from groups si-LINC00963 and si-NC was measured. **P < 0.01 vs. si-NC. (d, e) The levels of LINC00963 and miR-625 in the tumor xenografts were assessed by RT-qPCR. *P < 0.05, **P < 0.01 vs. si-NC. (f) Western blotting was carried out to measure HMGA1 protein expression in the tumor xenografts. **P < 0.01 vs. si-NC. Unpaired student’s t-test is used for all above statistical analysis.
Discussion
In recent years, much attention was given to the expression and functions of lncRNAs in tumorigenesis and during tumor progression [36–38]. The dysregulation of lncRNAs has been confirmed in breast cancer and may be closely related to the formation and progression of breast cancer [39–41]. Therefore, exploring the important roles of lncRNAs in breast carcinogenesis and during breast cancer progression may offer novel insights into breast cancer pathogenesis and may facilitate the identification of potential targets for anticancer therapies. In this study, we first measured LINC00963 expression in breast cancer, and then the clinical value of LINC00963 was examined in patients with breast cancer. In addition, the regulatory effects of LINC00963 on the aggressive characteristics of breast cancer cells were investigated in detail. The mechanisms underlying the activity of LINC00963 in breast cancer cells were explored at the molecular level.
LINC00963 is upregulated in osteosarcoma, and this upregulation significantly correlates with poor clinical outcomes among patients with osteosarcoma [31]. The expression of LINC00963 is also high in melanoma. Patients with melanoma featuring high LINC00963 expression exhibit shorter overall survival than do the patients with low LINC00963 expression [32]. LINC00963 is reported to be overexpressed in hepatocellular carcinoma [33] and prostate cancer [34]. LINC00963 overexpression correlates with tumor size and TNM stage in patients with hepatocellular carcinoma [33]. However, whether LINC00963 is aberrantly expressed in breast cancer has been unknown until our study. Herein, our results showed that LINC00963 expression is higher in breast tumors and breast cancer cell lines in comparison with the adjacent normal tissues and human normal breast epithelial cell line MCF-10A, respectively. Besides, LINC00963 overexpression was found to correlate with the lymph node metastasis, TNM stage and differentiation grade among our 53 patients with breast cancer. Patients with breast cancer featuring high LINC00963 expression showed shorter overall survival than did the patients with low LINC00963 expression. These findings suggest that LINC00963 may serve as a biomarker for the diagnosis and prognosis of breast cancer.
LINC00963 plays oncogenic roles in carcinogenesis and cancer progression. For instance, upregulation of LINC00963 accelerates osteosarcoma cell growth and metastasis in vitro by deactivating the miR-204-3p–FN1 pathway [31]. Inhibition of LINC00963 restricts melanoma cell proliferation, migration, and invasion because of weaker sponging of miR-608 and therefore greater inhibition of NACC1 expression [32]. In hepatocellular carcinoma, LINC00963 overexpression promotes cell proliferation and shortens the cell cycle G0–G1 transition by promoting PI3K–AKT pathway activation [33]. In prostate cancer, silencing of LINC00963 expression restrains cell proliferation, motility, and invasion and promotes apoptosis in vitro [34]. Nevertheless, the functions of LINC00963, if any, in the malignancy of breast cancer have not been studied until our work. In this study, functional investigation revealed that the LINC00963 knockdown represses cell proliferation, migration, and invasion and induces apoptosis in vitro as well as attenuates tumor growth in vivo.
Next, a systemic approach was employed to elucidate the mechanisms behind the oncogenic activities of LINC00963 in breast cancer. First, our investigation of the subcellular localization of LINC00963 in breast cancer revealed that LINC00963 is mainly present in the cytoplasm of breast cancer cells, suggesting that LINC00963 may work as a ceRNA for certain miRNA in breast cancer. Second, bioinformatics prediction identified a high-probability binding site for miR-625 in LINC00963. Third, luciferase reporter and RIP assays indicated that miR-625 is a target of LINC00963 in breast cancer cells. Fourth, miR-625 was found to be downregulated in breast cancer, with an inverse correlation with LINC00963 levels. Fifth, LINC00963 inhibition increased miR-625 expression and downregulated the HMGA1 protein in breast cancer cells. Finally, the miR-625 knockdown and HMGA1 restoration both attenuated the effects of LINC00963 downregulation on breast cancer cells. Collectively, these data mean that LINC00963 functions as a ceRNA for miR-625 and thereby promotes HMGA1 expression in breast cancer.
MiR-625 levels are reported to be low in breast cancer tissues and cell lines [35]. This downregulation is associated with estrogen receptor, human epidermal growth factor receptor 2, and clinical stage among patients with breast cancer [35]. In addition, Kaplan–Meier and multivariate analyzes identified miR-625 as an independent factor predicting the poor clinical outcomes in patients with breast cancer [35]. MiR-625 plays tumor-suppressive roles in the progression of breast cancer by directly targeting HMGA1 mRNA and downregulating its expression [35]. HMGA1, a member of the HMGA protein family, regulates chromatin structure by directly binding to the A/T-rich DNA sequences located in the promoter and enhancer regions of multiple human genes [42]. HMGA1 exerts a crucial effect on the malignancy of breast cancer and is implicated in the regulation of various malignant characteristics [43–45]. Our current study revealed that the depletion of LINC00963 restrains the aggressiveness of breast cancer cells in vitro and in vivo; these influences turned out to be mediated by decreased sponging of miR-625 and consequent HMGA1 upregulation. Hence, targeting of the LINC00963–miR-625–HMGA1 pathway might be a promising method for the treatment of breast cancer.
Conclusion
In summary, LINC00963 serves as a ceRNA for miR-625 thereby weakening the suppressive effect of miR-625 on HMGA1 expression in breast cancer. Thus, LINC00963 plays oncogenic roles in the progression of breast cancer. Our observations may form the basis for further research into the application of LINC00963 as a prognostic and diagnostic biomarker as well as a possible therapeutic target in breast cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [3].Liu H, Ye H.. Screening of the prognostic targets for breast cancer based co-expression modules analysis. Mol Med Rep. 2017;16:4038–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cancer Genome Atlas N . Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012;37:460–465. [DOI] [PubMed] [Google Scholar]
- [7].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- [8].Bahmanpour Z, Sheervalilou R, Choupani J, et al. A new insight on serum microRNA expression as novel biomarkers in breast cancer patients. J Cell Physiol. 2019;234:19199–19211. [DOI] [PubMed] [Google Scholar]
- [9].Qi Y, Wang X, Kong X, et al. Expression signatures and roles of microRNAs in inflammatory breast cancer. Cancer Cell Int. 2019;19:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vahidian F, Mohammadi H, Ali-Hasanzadeh M, et al. MicroRNAs and breast cancer stem cells: potential role in breast cancer therapy. J Cell Physiol. 2019;234:3294–3306. [DOI] [PubMed] [Google Scholar]
- [11].Zhou Y, Wang B, Wang Y, et al. miR-140-3p inhibits breast cancer proliferation and migration by directly regulating the expression of tripartite motif 28. Oncol Lett. 2019;17:3835–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang L, Chen T, Yan L, et al. MiR-155-3p acts as a tumor suppressor and reverses paclitaxel resistance via negative regulation of MYD88 in human breast cancer. Gene. 2019;700:85–95. [DOI] [PubMed] [Google Scholar]
- [13].Xie Q, Wang S, Zhao Y, et al. MicroRNA-216a suppresses the proliferation and migration of human breast cancer cells via the Wnt/beta-catenin signaling pathway. Oncol Rep. 2019;41:2647–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murugan AK, Munirajan AK, Alzahrani AS. Long noncoding RNAs: emerging players in thyroid cancer pathogenesis. Endocr Relat Cancer. 2018;25:R59–R82. [DOI] [PubMed] [Google Scholar]
- [16].Chen S, Wu DD, Sang XB, et al. The lncRNA HULC functions as an oncogene by targeting ATG7 and ITGB1 in epithelial ovarian carcinoma. Cell Death Dis. 2017;8:e3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tang Y, Zhou T, Yu X, et al. The role of long non-coding RNAs in rheumatic diseases. Nat Rev Rheumatol. 2017;13:657–669. [DOI] [PubMed] [Google Scholar]
- [18].Botchkareva NV. The molecular revolution in cutaneous biology: noncoding RNAs: new molecular players in dermatology and cutaneous biology. J Invest Dermatol. 2017;137:e105–e11. [DOI] [PubMed] [Google Scholar]
- [19].Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li X, Wang S, Li Z, et al. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int J Biol Macromol. 2017;105:346–353. [DOI] [PubMed] [Google Scholar]
- [21].Heilmann K, Toth R, Bossmann C, et al. Genome-wide screen for differentially methylated long noncoding RNAs identifies Esrp2 and lncRNA Esrp2-as regulated by enhancer DNA methylation with prognostic relevance for human breast cancer. Oncogene. 2017;36:6446–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Y, Shao A, Wang L, et al. The role of lncRNAs in the distant metastasis of breast cancer. Front Oncol. 2019;9:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Youness RA, Gad MZ. Long non-coding RNAs: functional regulatory players in breast cancer. Noncoding RNA Res. 2019;4:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bin X, Hongjian Y, Xiping Z, et al. Research progresses in roles of LncRNA and its relationships with breast cancer. Cancer Cell Int. 2018;18:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ai B, Kong X, Wang X, et al. LINC01355 suppresses breast cancer growth through FOXO3-mediated transcriptional repression of CCND1. Cell Death Dis. 2019;10:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li Y, Su X, Pan H. Inhibition of lncRNA PANDAR reduces cell proliferation, cell invasion and suppresses EMT pathway in breast cancer. Cancer Biomarkers. 2019;25:185–192. [DOI] [PubMed] [Google Scholar]
- [27].Fu J, Dong G, Shi H, et al. LncRNA MIR503HG inhibits cell migration and invasion via miR-103/OLFM4 axis in triple negative breast cancer. J Cell Mol Med. 2019;23:4738–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zheng S, Li M, Miao K, et al. SNHG1 contributes to proliferation and invasion by regulating miR-382 in breast cancer. Cancer Manag Res. 2019;11:5589–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li X, Deng S, Pang X, et al. LncRNA NEAT1 silenced miR-133b promotes migration and invasion of breast cancer cells. Int J Mol Sci. 2019;20(15). pii: E3616. doi: 10.3390/ijms20153616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang J, Wu W, Wu M, et al. Long noncoding RNA ADPGK-AS1 promotes cell proliferation, migration, and EMT process through regulating miR-3196/OTX1 axis in breast cancer. In Vitro Cell Dev Biol Anim. 2019;55:522–532. [DOI] [PubMed] [Google Scholar]
- [31].Zhou Y, Yin L, Li H, et al. The LncRNA LINC00963 facilitates osteosarcoma proliferation and invasion by suppressing miR-204-3p/FN1 axis. Cancer Biol Ther. 2019;20:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jiao H, Jiang S, Wang H, et al. Upregulation of LINC00963 facilitates melanoma progression through miR-608/NACC1 pathway and predicts poor prognosis. Biochem Biophys Res Commun. 2018;504:34–39. [DOI] [PubMed] [Google Scholar]
- [33].Wu JH, Tian XY, An QM, et al. LINC00963 promotes hepatocellular carcinoma progression by activating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22:1645–1652. [DOI] [PubMed] [Google Scholar]
- [34].Wang L, Han S, Jin G, et al. Linc00963: a novel, long non-coding RNA involved in the transition of prostate cancer from androgen-dependence to androgen-independence. Int J Oncol. 2014;44:2041–2049. [DOI] [PubMed] [Google Scholar]
- [35].Zhou WB, Zhong CN, Luo XP, et al. miR-625 suppresses cell proliferation and migration by targeting HMGA1 in breast cancer. Biochem Biophys Res Commun. 2016;470:838–844. [DOI] [PubMed] [Google Scholar]
- [36].Zhou Y, Xu S, Xia H, et al. Long noncoding RNA FEZF1-AS1 in human cancers. Clin Chim Acta. 2019;497:20–26. [DOI] [PubMed] [Google Scholar]
- [37].Shi C, Sun L, Song Y. FEZF1-AS1: a novel vital oncogenic lncRNA in multiple human malignancies. Biosci Rep. 2019;39(6). pii: BSR20191202. doi: 10.1042/BSR20191202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Flippot R, Beinse G, Boileve A, et al. Long non-coding RNAs in genitourinary malignancies: a whole new world. Nat Rev Urol. 2019;16:484–504. [DOI] [PubMed] [Google Scholar]
- [39].Wang G, Liu C, Deng S, et al. Long noncoding RNAs in regulation of human breast cancer. Brief Funct Genomics. 2016;15:222–226. [DOI] [PubMed] [Google Scholar]
- [40].Soudyab M, Iranpour M, Ghafouri-Fard S. The role of long non-coding RNAs in breast cancer. Arch Iran Med. 2016;19:508–517. [PubMed] [Google Scholar]
- [41].Yang Y, Qian J, Xiang Y, et al. The prognostic value of long noncoding RNA HOTTIP on clinical outcomes in breast cancer. Oncotarget. 2017;8:6833–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Benecke AG, Eilebrecht S. RNA-mediated regulation of HMGA1 function. Biomolecules. 2015;5:943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Penzo C, Arnoldo L, Pegoraro S, et al. HMGA1 modulates gene transcription sustaining a tumor signalling pathway acting on the epigenetic status of triple-negative breast cancer cells. Cancers (Basel). 2019;11:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zanin R, Pegoraro S, Ros G, et al. HMGA1 promotes breast cancer angiogenesis supporting the stability, nuclear localization and transcriptional activity of FOXM1. J Exp Clin Cancer Res. 2019;38:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang S, Lei R, Wu J, et al. Role of high mobility group A1 and body mass index in the prognosis of patients with breast cancer. Oncol Lett. 2017;14:5719–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]


