Abstract
The amygdala plays an essential role in evaluating social information, threat detection, and learning fear associations. Yet, most of that knowledge comes from studies in adult humans and animals with a fully developed amygdala. Given the considerable protracted postnatal development of the amygdala, it is important to understand how early damage to this structure may impact the long-term development of behavior. The current study examined behavioral responses toward social, innate, or learned aversive stimuli among neonatal amygdala lesion (Neo-Aibo; males = 3, females = 3) or sham-operated control (Neo-C; males = 3, females = 4) rhesus macaques. Compared to controls, Neo-Aibo animals exhibited less emotional reactivity toward aversive objects, including faster retrieval of food reward, fewer fearful responses, and more manipulation of objects. This lower reactivity was only seen in response to social and innate aversive stimuli, whereas Neo-Aibo animals had similar responses to controls for learned aversive stimuli. The current study also detected sex differences in behavioral response to aversive stimuli, such that, as compared to males, females took longer to retrieve the food reward across all aversive stimuli types, but only expressed more hostility and more coo vocalizations during learned aversive trials. Interestingly, early amygdala damage impacted the expression of some, but not all, sex differences. For example, neonatal amygdala damage eliminated the sex difference in object manipulation. These findings add important information that broaden our understanding of the role of the amygdala in the expression of sexually dimorphic behaviors, as well as its role in learning fear associations and threat detection.
Keywords: Sex Differences, Amygdala, Emotional, Social, Innate
The amygdala is an almond shaped structure in the medial temporal lobe that is known to play a critical role in assessing social cues and detecting threat-related stimuli (Aggleton, 2000). Although the amygdala modulates both positive and negative cues, its role in the detection and processing of threat-related stimuli and the production of emotional responses to promote protection from those threats encountered in the environment has been extensively studied (Feinstein, Adolphs, Damasio, & Tranel, 2011). Damage to the amygdala in adult humans and animals results in impaired fear conditioning, decreased emotional reactivity, altered recognition of emotional facial expressions, as well as changes in social behavior (Adolphs, Tranel, Damasio, & Damasio, 1994; Adolphs, Tranel, Damasio, & Damasio, 1995; Brown & Schafer, 1888; Cheney & Seyfarth, 1990; De Martino, Camerer, & Adolphs, 2010; Kalin, Shelton, & Davidson, 2004; Kalin, Shelton, Davidson, & Kelley, 2001; Machado, Kazama, & Bachevalier, 2009). However, this knowledge was gained from lesion studies in adults that have a fully developed amygdala and have already acquired the skills needed for threat detection and promoting emotional responses toward threats. Thus, considering the extended postnatal development of the amygdala, which in macaques reaches volumetric stability at roughly 8 months of age (Payne, Machado, Bliwise, & Bachevalier, 2010), it is important to understand how early damage may impact the development of threat detection and emotional responses toward a variety of threats.
The development of threat detection and social competency in rhesus monkeys corresponds with postnatal growth of the amygdala that continues to develop from birth until two years of age (Chareyron, Lavenex, Amaral, & Lavenex, 2012; Payne et al., 2010). Previous studies have reported that early damage to the amygdala results in decreased emotional reactivity toward threatening object, including social (mammal like) and innate (snake) aversive objects (Bliss-Moreau, Bauman, & Amaral, 2011; Bliss-Moreau, Toscano, Bauman, Mason, & Amaral, 2010, 2011). Specifically, monkeys with neonatal amygdala lesions explored aversive objects more and exhibited fewer emotional expressions as compared to controls (Bliss-Moreau, Bauman, et al., 2011; Bliss-Moreau, Toscano, et al., 2011). Neonatal amygdala lesions also impacted the animal’s ability to modulate their emotional response based on the level of threat presented by the presence and direction of eye contact from an unfamiliar person (Raper, Wilson, Sanchez, Machado, & Bachevalier, 2013). These data might suggest that early amygdala damage impairs threat detection abilities and the learning of fear associations. Lesions in adult monkeys disrupt the ability to acquire new associations between a neutral cue and an aversive stimulus using a fear potentiated startle paradigm (Winslow, Noble, & Davis, 2008), whereas monkeys with neonatal amygdala lesions were able to learn such associations, but took significantly more trials to learn as compared to controls (Kazama, Heuer, Davis, & Bachevalier, 2012). Therefore, neonatal amygdala lesions in monkeys do not abolish the ability to learn fearful cues, but instead lengthens the learning curve.
The amygdala plays a role in sexually dimorphic behaviors, in part due to its high density of androgen receptors which have higher expression in males than females (Choate, Slayden, & Resko, 1998; McClure et al., 2004; Pomerantz & Sholl, 1987; Saint-Maurice, Welk, Silva, Siahpush, & Huberty, 2011; Zosuls et al., 2009). For example, female monkeys exhibited more fearful and hostile behavior than males (Mason, Green, & Posepanko, 1960) and this sex difference was detected in adulthood when gonadal hormones are high in circulation and pubertal changes in the brain have already occurred (Lidow, Goldman-Rakic, & Rakic, 1991; Mason et al., 1960). Sex differences prior to the emergence of those pubertal changes have been less frequently studied. Only a few studies have investigated sex differences in emotional reactivity during infancy. In infant monkeys, no sex differences were identified in freezing response during the human intruder paradigm (Kalin, Shelton, Rickman, & Davidson, 1998). Yet, vocalizations exhibited a sexually dimorphic expression, such that female infant monkeys emitted longer and more complex vocalizations than males (Tomaszycki, Davis, Gouzoules, & Wallen, 2001). Only one study so far demonstrated that neonatal amygdalectomy resulted in altered patterns of fearful defensive behaviors and vocalizations depending on the sex of the animal during infancy, with more defensive responses and vocalizations displayed by infant females than males (Raper, Wallen, et al., 2013). Thus, little is known about how early amygdala damage affects the expression of sex dimorphic behaviors in adulthood.
As part of a longitudinal study of cognitive and socioemotional development in rhesus monkeys with neonatal amygdala lesions, the current experiment focused on threat detection and behavioral responses toward social, innate, and learned aversive objects. Tests were conducted during adulthood to examine the long-term impact of early amygdala damage, as well as its potential to affect the expression of sexually dimorphic behaviors (Mason et al., 1960; Raper, Wallen, et al., 2013; Tomaszycki et al., 2001). Based on previous studies (Bliss-Moreau, Bauman, et al., 2011; Bliss-Moreau et al., 2010; Bliss-Moreau, Toscano, et al., 2011; Raper, Wilson, et al., 2013), we predicted that the neonatal amygdala lesioned monkeys will lack the ability to modulate their behavioral responses according to the level of potential threat from the aversive objects presented, resulting in fewer fearful responses as compared to controls. We predicted that sex differences will be found in fearful, hostile, and vocalizations expressions and that early amygdala damage will impact these sexually dimorphic behaviors (Raper, Stephens, Sanchez, Bachevalier, & Wallen, 2014; Raper, Wallen, et al., 2013).
Methods
Thirteen adult rhesus monkeys (Macaca mulatta) were used in this study. Animals received bilateral neonatal neurotoxic lesions of the amygdala (Neo-Aibo; males = 3, females = 3) or sham operations (Neo-C; males = 3, females = 4) between one and two weeks of age. As part of a longitudinal study to assess the impact of early amygdala damage on socioemotional and cognitive behaviors, animals were post-operatively tested on the following tasks not reported here: 1) Human Intruder Paradigm (at 2 months, 4.5 months, and 6-8 years of age(Raper, Wilson, et al., 2013)), 2) social behavior observations (at 3 months, 6 months, and 3 years of age (Goursaud & Bachevalier, 2007)), 3) goal-directed behaviors (at 3 months, 3 years and 4-5 years of age (Kazama & Bachevalier, 2012, 2013)), and 4) memory abilities (at 6, 8, 9, and 18 months, and 2, 4, 6-8 years of age). The current study used the Approach Avoidance Task to examine emotional responsivity to neural or aversive stimuli when the animals were reaching early adulthood between 4-6 years of age.
The surgical procedures as well as the behavioral testing during infancy and adolescence were performed at the University of Texas Health Science Center (UTHSC, Houston, TX). Behavioral testing during adulthood was performed at the Yerkes National Primate Research Center (YNPRC, Atlanta, GA). At both institutions, animals were housed under a 12 h light/dark cycle and all procedures were approved by the respective Institutional Animal Care and Use Committees of the UTHSC and of Emory University. Procedures for rearing condition, neuroimaging, neurosurgery, and estimation of lesion extent have been previously described in detail elsewhere (Goursaud & Bachevalier, 2007; Kazama, Heuer, Davis, & Bachevalier, 2012; Raper, Wilson, et al., 2013) and will be briefly summarized below.
Rearing:
Animals were surrogate-peer reared and housed to promote species-specific socioemotional skills using procedures established by Sackett and colleagues (Sackett, Ruppenthal, & Davis, 2002). The major factors contributing to species-typical behavior in surrogate-peer rearing were extended care and interactions provided by human caregivers, socialization with age-matched peers, and extensive cognitive testing throughout development. Briefly, all animals were individually housed in cages under open radiant incubators and contact comfort was provided by a synthetic plush surrogate (approximately 30 cm in length) for infants. During this time, the cages were positioned to allow somatosensory contact with other infants. At 3 months of age, animals were transferred to a larger quad cage and individually housed, but visual and physical contact was possible between pairs of infants through the large central mesh separating two adjacent cages. At 7 months, animals were housed in quads, then moved to pair housing at 12 months of age.
Care of infants was given by a principal human caregiver, who interacted with the infants 6 hours per day, 5 days per week. A familiar human caregiver interacted with the animals on weekends for 2-4 hours per day. In addition to social contact with human caregivers, starting at one month of age until approximately 12 months of age, infants socialized with three other age- and sex-matched peers for 3-4 hours, 5 days per week in a large enclosure. Infants were hand-fed Similac formula until 3-4 weeks of age. Banana pellets (190 mg, P.J. Noyes, Cleveland, OH) were added to supplement the diet when animals were able to self-feed. Diet was changed to Purina monkey chow enriched with fresh fruit at 3 months of age.
Neuroimaging:
On the day of surgery, the infant was removed from its home cage and anesthetized (isoflurane, 1-2% to effect). Its head was shaved and secured in a nonferromagnetic stereotaxic apparatus. Three MRI sequences were obtained with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI) using a 3-inch surface coil. First, a high resolution T1-weighted spin-echo sequence (echo time [TE] = 11 ms, repetition time [TR] = 450 ms, contiguous 4 mm thick images, 12 cm field of view [FOV], 256 X 256 matrix) was taken in the sagittal plane and was used to align the two-subsequent series. A 3D T1-weighted fast spoiled gradient (FSPGR)-echo sequence (TE = 2.6 ms, TR = 10.2 ms, 258 flip angle, contiguous 1 mm thick images, 256 X 256 matrix) was used to determine the three-dimensional coordinates for each neurotoxin injection site in the amygdala for group Neo-Aibo. Animals in group Neo-Aibo also received a Fluid Attenuated Inversion Recovery (FLAIR) sequence (TE = 140 ms, TR = 10,000 ms, inversion time [TI] = 2200, contiguous 3 mm thick images, 12 cm FOV, 256 X 256 matrix). The FLAIR images reveal tissue T2 prolongation with cerebrospinal fluid suppression and were compared to the post-surgical FLAIR images to accurately indicate localized areas of edema and estimate the extent of lesion (Nemanic, Alvarado, Price, Jackson, & Bachevalier, 2002). Animals in group Neo-C received a Fast Spin-Echo — Inversion Recovery (FSE-IR) series (TE = 20 ms, TR = 4500/250 ms, ETL = 6, BW = 32 kHz, contiguous 1.5 mm thick images, 12 cm FOV, 256 X 256 matrix, 2 NEX). The latter series was used as part of a separate study to track the developmental trajectory of several brain structures (Payne, Cirilli, & Bachevalier, 2017; Payne et al., 2010).
Surgery:
After all structural neuroimaging was completed, the infant was kept anesthetized in the stereotaxic apparatus and immediately brought to the surgical suite. An intravenous drip of 5% dextrose and 0.45% sodium chloride was started to maintain normal hydration during surgery. Vital signs (heart rate, respiration, blood pressure, expired CO2) were monitored throughout the surgical procedures. Body temperature was maintained via a warm air blanket placed around the animal and attached to a Bair Hugger 1 Therapy warming unit. The scalp was disinfected with Nolvasan solution and a local anesthetic (Marcain 25%, 1.5 ml) was injected subcutaneously along the incision line to reduce pain during skin incision. Under aseptic conditions, the skin was opened, and connective tissue was gently displaced to expose the skull. For all animals, two small bilateral craniotomies were made above the amygdala. The dura was cut and retracted to expose the brain. For Neo-C animals, the surgical procedures stopped here, and no injections were made.
For Neo-Aibo animals, injections of ibotenic acid (Biosearch Technologies, Novato, CA; 10 mg/ml in phosphate buffer saline, pH 7.0-7.4) were made at 4-6 sites (2 mm apart) within the center of the amygdala using 10 ml Hamilton syringes held by a micromanipulator. The needles were lowered simultaneously in both hemispheres and ibotenic acid 0.2-0.6μl was manually injected at each site at a rate of 0.2 μl/30 s. After each injection, the needles were left in place for an additional 3-min period to allow complete diffusion of the neurotoxin at the tip of the needle and minimize its spread in the needle track during retraction of the needles. At completion of the surgical procedures, the dura was closed with silk sutures and the bone opening was covered with Surgical NU-KNIT (absorbable hemostat). The connective tissues and skin were closed in anatomical layers. The animal was removed from anesthesia and placed in a temperature-controlled incubator ventilated with oxygen until full recovery from anesthesia. All animals received acetaminophen (10 mg/kg), cephazolin (25 mg/kg), and dexamethasone sodium phosphate (0.4 mg/kg) to reduce pain, prevent infection and edema, respectively. A topical antibiotic ointment (bacitracin—neomycin—polymyxin) was applied to the wound daily.
Lesion Verification:
Estimation of the extent of intended and unintended damage for Neo-Aibo animals was accomplished using histological assessments. Following completion of the study, animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused transcardially with aldehyde fixatives. The brain was then removed from the skull, cryo-protected, and blocked in the coronal plane. Sections were cut in the coronal plane on a sliding microtome at a thickness of 50 μm, every 10th slice was mounted on premade gelatin-coated slides, and then air dried. Sections were defatted, stained with Nissl (1–3 min) to visualize cell bodies, rinsed with distilled water followed by ascending alcohol concentrations, and finally put into xylene and cover slipped with DPX mountant. A second series of sections at 500 μm intervals was processed with a Gallyas silver stain to visual fiber tracts.
Following staining and mounting, individual slides were matched to individual drawings of coronal sections from the standard rhesus monkey brain at 1 mm intervals. Matching was done separately for each hemisphere, on the basis of local landmarks, and with reference to an atlas of the rhesus brain when necessary (Salem & Logothestis, 2006). We then plotted areas of cell loss and gliosis on a normalized rhesus monkey brain (J. Bachevalier, unpublished atlas) using Adobe Photoshop software. Drawings were imported into an image analysis program (Image-J) to measure the surface area (in pixels2) of intended amygdala damage. Unintended damage for all surrounding structures (entorhinal and perirhinal cortices, and hippocampus) was also measured. The volume of amygdala damage was then divided by the normal volume of the amygdala (obtained from the template brain in a similar manner) and multiplied by 100 to estimate a percentage of the total volume damaged. The same procedure was applied to estimate potential damage to structures adjacent to the amygdala.
Approach Avoidance Task
Stimuli and testing procedures used in this study were the same as those described in Machado and colleagues (Machado et al., 2009), but will be briefly summarized below. Animals were wheeled to a Wisconsin General Testing Apparatus (WGTA) equipped with two opaque, vertically sliding panels: one between the animal and the test tray, and another between the experimenter and the test tray. This tray contained three food wells (one well at center, 16 cm from the animal, two lateral wells located 13 cm on either side, each 2 cm in diameter and 1 cm deep). Only the center well was used for this experiment. All animals had equal and extensive prior experience with the WGTA due to previous cognitive testing (Kazama & Bachevalier, 2013; Kazama et al., 2012).
Sixteen inanimate stimuli were chosen for this experiment from the Machado and colleagues (Machado et al., 2009) stimuli set. The emotional valence of these items varied such that eight objects were intended to be aversive or potentially dangerous, whereas the remaining eight were intended to be neutral items of similar size and shape. The aversive items were specifically selected to be either items that the animals innately feared (rubber snake, spider; (Chudasama, Izquierdo, & Murray, 2009; Izquierdo, Suda, & Murray, 2005; Mineka, 1987; Mineka, Keir, & Price, 1980)), items commonly used in handling or capture in nonhuman primate laboratories that, in our experience with this population, readily elicited fear (capture net and handling gloves) or items with a social component (girl doll, Mr. Potato Head, Elmo, and SpongeBob toys). Since direct eye contact is a highly threatening gesture, social stimuli such as these elicit behavioral expressions of fear, passive avoidance, and/or generalized tension in macaque monkeys (Bliss-Moreau et al., 2010; Bliss-Moreau, Toscano, et al., 2011; Chevalier-Skolnikoff, 1973; Machado et al., 2009; van Hooff, 1967). An example item from each aversive category can been seen in Figure 2a-c.
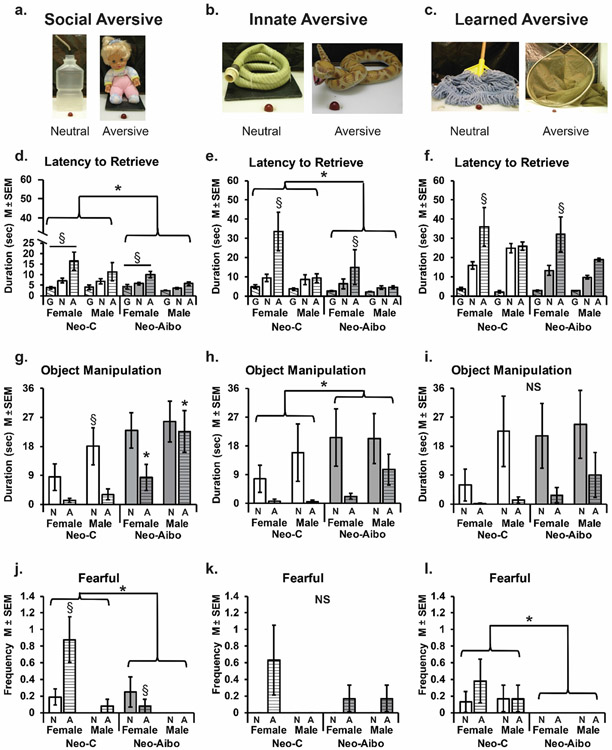
Figure 2.
Behavioral responses toward neutral and aversive stimuli. (a-c) illustrate an example of neutral (N, solid bars) and aversive (A, horizontal striped bars) stimuli across different category type. Mean ± SEM behavioral responses on Social (d,g,j), Innate (e,h,k), and learned (f,i,l) aversive categories. Latency to retrieve the grape (G, diagonal striped bars) reward (d-f), duration of object manipultion (g-i), and frequency of fearful behavior expression (j-l). Grey bars indicate neonatal amgydala lesions (Neo-Aibo) and white bars indicate sham-operated controls (Neo-C). § indicates a significant difference between sexes. * indicates a significant difference between groups (p < 0.05). NS indicates no significant differences.
Following previously published protocols (Machado et al., 2009), each of the eight neutral/aversive stimulus pairs were presented only once, without replication, to measure the animals emotional reactivity without the influence of experience or habituation. A seedless red grape was paired with each of the items to motivate approach. Prior to testing, experimenters determined that all animals would readily take grape food rewards within 1-min. A given pair of neutral and aversive objects was presented within a block of four 1-min trials: (a) Baseline Trial—nothing presented on the test tray, (b) Grape Only—grape presented in the center food well, (c) Neutral Item—a neutral item was positioned 2 cm behind the grape in the center food well, and (d) Aversive Item—an aversive item was positioned 2 cm behind the grape. Two four-trial blocks occurred each day, and each trial was separated by a 30-s intertrial interval (ITI). During each trial, animals could take or manipulate the grape and item freely, if present. During the ITI, the opaque panel between the animal and the test tray was lowered, the object and food reward were removed (if present), and the tray was reset for the next trial with the requisite stimuli. To control for circadian effects on animals’ motivation, all testing for this experiment occurred between 10:00 a.m. and noon (i.e., at least 18 hr. after their last feeding). Testing order was generated randomly, and that order was counterbalanced between groups.
Behavioral Measures
Animals’ responses toward the neutral/aversive stimuli were videotaped and later coded using a detailed ethogram (Table 2). Digital videos were coded using The Observer Video XT 10 software package (Noldus Inc., Netherlands) by one experimenter (JT) who was blind to the animals’ treatment. Prior to coding, the experimenter (JT) reached an average intra-rater reliability of Cohen’s Kappa = 0.96 and inter-rater reliability Cohen’s Kappa = 0.90 with another experimenter (JR).
Table 2.
Behavioral ethogram
| Category and Specific Behaviors |
Measurement | Brief Descriptions |
|---|---|---|
| Object Manipulation | Duration | Use of hands or mouth to nonaggressively grab, hold, and explore the stimulus object |
| Fearful | Cumulative Frequency | |
| Freeze1 | Frequency | Rigid, motionless posture (except slight head movement) for at least 3 seconds in duration |
| Crouch | Frequency | Whole body, or just front bent with head near floor |
| Withdrawal | Frequency | Quick, jerky motion away from the stimulus object (jump back) |
| Grimace | Frequency | Refracted lips, exposed clenched teeth |
| Hostile | Cumulative Frequency | |
| Threat bark | Frequency | Low pitch, high intensity, rasping, guttural |
| Threat | Frequency | Any of the following: open mouth (no teeth exposed), head-bobbing, or ear flapping |
| Cage aggression | Frequency | Vigorously slaps, shakes, or slams body against cage |
| Lunge | Frequency | A quick, jerky movement toward the stimulus |
| Coo vocalizations | Frequency | Clear soft pitch and intensity, sounds like “ooooh” |
| Stereotypies | Cumulative Duration | |
| Pacing | Duration | Repetitive motor pattern around the test cage |
| Motor stereotypy | Duration | Repetitive, abnormal voluntary or involuntary motor patterns (e.g. swinging, twirling, flipping) |
| Anxiety2 | Cumulative Frequency | |
| Scratch | Frequency | Rapid scratch of body with hands or feet |
| Body shake | Frequency | Shake of the whole body or just head and shoulders region |
| Tooth grind1 | Frequency | Repetitive, audible rubbing of upper and lower teeth |
| Yawn | Frequency | Open mouth widely, exposing teeth |
| Affiliative2 | Cumulative Frequency | |
| Grunt | Frequency | Deep, muffled, low intensity, almost gurgling sound |
| Lipsmack | Frequency | Rapid movement of pursed lips, accompanied by a smacking sound |
| Present | Frequency | Rigid posture (knees locked) with tail elevated and rump oriented toward the stimulus object |
List of all behaviors scored, how they are measured and a brief definition.
Behavior for which total duration was also measured.
Behavior that was primarily seen during Social Aversive Stimuli trials.
Data Analysis
Prior to analysis, Kolmogorov–Smirnov (K–S) tests were performed to verify whether the behavioral data was normally distributed. When behaviors were not normally distributed, they were transformed using a natural log plus constant to obtain normality. For the purposes of interpretation, raw data (means and variance indices) are presented in Figure 2; log transformed data are available upon request. On trials during which animals did not take the food or explore the objects, the latency score was replaced with the length of the trial (60s). The impact of early amygdala damage on the response toward neutral/aversive stimuli was examined separately for each aversive category (Social, Innate, Learned) using a General Linear Mixed-Models ANOVA with Group (Neo-C, Neo-Aibo) and Sex as between subjects’ factors, and Object Type (Neutral, Aversive, or Grape for Latency to Retrieve; Neutral or Aversive for all other behaviors) as the within subjects’ factor with repeating measures. A Huynh-Feldt correction was applied to the results because sphericity could not be assumed. All analyses were conducted with SPSS 24 for Windows, a p < 0.05 was considered significant, and effect sizes (partial eta squared) were calculated.
Results
Lesion Extent
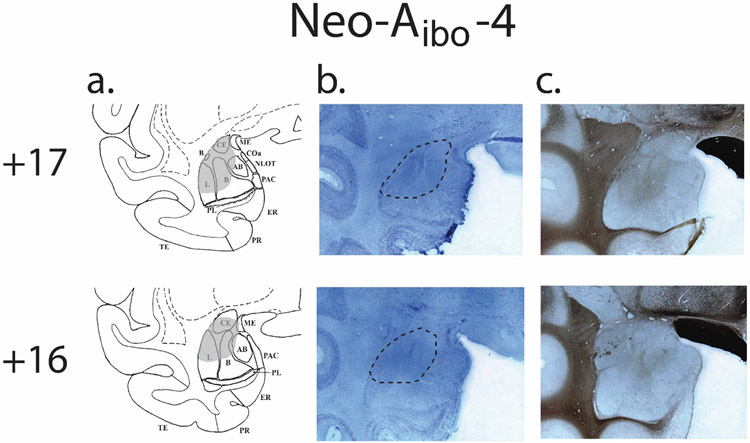
Histological examination of the lesion extent was reported in details in an earlier report (Payne & Bachevalier, 2019) and is briefly summarized here. Bilateral damage to the amygdala averaged 31.3% across both hemispheres (see Table 1) but varied from case to case. Five cases (Neo-Aibo-1, −2, −3, −4, and −6) received symmetrical damage to the amygdala in both hemispheres (from 35% to 65%), whereas the remaining case (Neo-Aibo-5), received less extensive damage to the left (17%) than to the right (30%) amygdala. In all cases, however, the damage included parts of the central, medial, accessory basal, and dorsal areas of the basal and lateral nuclei, but spared the ventral portion of the lateral and basal nuclei. Figure 1 illustrates the extent of amygdala damage in the left hemisphere of Neo-Abio-4 (but see also cases Neo-Aibo-2 and Neo-Aibo-5 illustrated on Figures 1 and 2 in (Payne & Bachevalier, 2019)). Extent of unintended damage to the perirhinal and entorhinal cortical areas, anterior portion of the hippocampus, and tails of the caudate and putamen were negligible for all cases (less than 3%, see Table 1).
Table 1.
Intended and unintended damage after neurotoxic lesions of the amygdala
| Intended damage | Unintended damage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala | Hippocampus | Perirhinal Cortex | ||||||||||
| Subjects | LT% | RT% | X% | W% | LT% | RT% | X% | W% | LT% | RT% | X% | W% |
| Neo-Aibo-1 | 61.2 | 35.0 | 48.1 | 21.4 | 4.5 | 1.7 | 3.1 | 0.1 | 5.2 | 6.0 | 5.6 | 0.3 |
| Neo-Aibo-2 | 45.2 | 65.0 | 55.1 | 29.4 | 0.0 | 2.4 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-3 | 40.3 | 40.0 | 40.1 | 16.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-4 | 42.9 | 51.4 | 47.2 | 22.1 | 0.0 | 2.1 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-5 | 17.6 | 30.0 | 23.8 | 5.3 | 0.0 | 3.2 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neo-Aibo-6 | 46.8 | 45.9 | 46.4 | 21.5 | 11.5 | 7.7 | 9.6 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mean | 42.3 | 44.6 | 43.4 | 19.3 | 2.7 | 2.8 | 2.8 | 0.2 | 0.9 | 1.0 | 0.9 | 0.1 |
Intended ibotenic acid damage to the amygdala (Neo-Aibo) and unintended damage to adjacent areas, as measured on postmortem, histological slices. LT%, percent damage in the left hemisphere; RT%, percent damage in the right hemisphere; X%, average damage to both hemispheres; W% weighted average damage to both hemispheres (W% = (LT% X RT%)/100).
Figure 1.
Amygdala lesion extent for a single case (Neo-Aibo-4): Atlas drawing outlining the extent to damage in grey (a), Corresponding photomicrogrpahs of 50μm coronal sections of the left amygdala stained for cell bodies with Nissl (b), and fiber tracts with silver impregnation (c). Dashed lines on the Nissl photomicrographs outline the borders of the cell loss (b).
Behavioral Responses Toward Aversive Stimuli
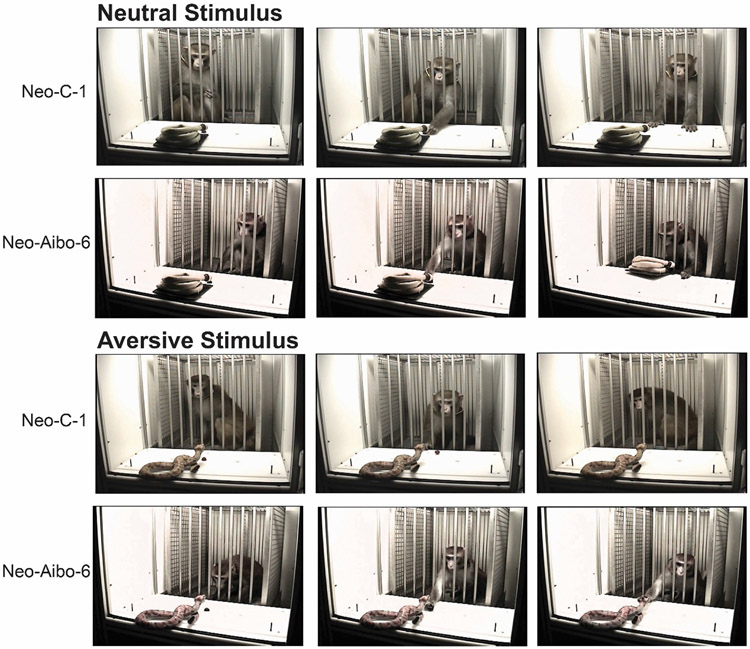
Figure 2 and Table 3 display the average (±SEM) for each category of behaviors obtained by males and females with sham operations or neonatal amygdala lesions. Figure 3 illustrates an example of one control and one neonatal amygdala lesion male responding to a neutral and aversive stimuli.
Table 3.
Behavioral responses by category and stimulus type
| Neo-C | Neo-Aibo | |||||
|---|---|---|---|---|---|---|
| Behavior | Category | Type | Female | Male | Female | Male |
| Hostile | Social | Neutral | 0.13 ± 0.08 | 0.42 ± 0.23 | 0.75 ± 0.41 | 0.33 ± 0.22 |
| Aversive | 2.25 ± 1.06 | 2.75 ± 1.39 | 2.00 ± 1.40 | 4.75 ± 2.23 | ||
| Innate | Neutral | 0.50 ± 0.26 | 0.83 ± 0.47 | 0.33 ± 0.30 | 0.00 ± 0.00 | |
| Aversive | 0.88 ± 0.58 | 2.67 ± 1.60 | 1.67 ± 1.30 | 0.83 ± 0.40 | ||
| Learned | Neutral | 1.38 ± 0.98 | 3.67 ± 1.76 | 1.50 ± 0.67 | 1.83 ± 1.13 | |
| Aversive | 9.88 ± 7.27 | 4.33 ± 2.53 | 6.00 ± 2.79 | 4.00 ± 2.58 | ||
| Affiliative | Social | Neutral | 0.38 ± 0.27 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Aversive | 2.44 ± 1.30 | 0.08 ± 0.07 | 1.75 ± 1.70 | 1.00 ± 0.90 | ||
| Innate | Neutral | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Aversive | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Learned | Neutral | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.50 ± 0.49 | 0.00 ± 0.00 | |
| Aversive | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Anxiety | Social | Neutral | 0.00 ± 0.00 | 0.08 ± 0.07 | 0.17 ± 0.11 | 0.17 ± 0.11 |
| Aversive | 0.31 ± 0.25 | 0.67 ± 0.66 | 0.17 ± 0.11 | 0.17 ± 0.11 | ||
| Innate | Neutral | 0.00 ± 0.00 | 0.50 ± 0.34 | 0.33 ± 0.30 | 0.00 ± 0.00 | |
| Aversive | 0.25 ± 0.20 | 0.33 ± 0.30 | 0.33 ± 0.30 | 0.17 ± 0.16 | ||
| Learned | Neutral | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.33 ± 0.30 | |
| Aversive | 0.00 ± 0.00 | 0.33 ± 0.30 | 0.67 ± 0.49 | 0.50 ± 0.34 | ||
| Coo | Social | Neutral | 0.56 ± 0.22 | 0.08 ± 0.04 | 0.58 ± 0.29 | 0.00 ± 0.00 |
| Aversive | 0.44 ± 0.16 | 0.33 ± 0.26 | 1.00 ± 0.48 | 0.00 ± 0.00 | ||
| Innate | Neutral | 0.38 ± 0.18 | 0.00 ± 0.00 | 1.00 ± 0.45 | 0.00 ± 0.00 | |
| Aversive | 0.25 ± 0.16 | 0.33 ± 0.30 | 1.67 ± 0.67 | 0.00 ± 0.00 | ||
| Learned | Neutral | 0.25 ± 0.16 | 0.33 ± 0.30 | 0.50 ± 0.40 | 0.00 ± 0.00 | |
| Aversive | 0.38 ± 0.26 | 0.00 ± 0.00 | 1.17 ± 0.60 | 0.00 ± 0.00 | ||
| Stereotypy | Social | Neutral | 3.19 ± 2.03 | 12.39 ± 5.53 | 7.33 ± 4.31 | 2.47 ± 2.07 |
| Aversive | 1.21 ± 0.60 | 10.00 ± 4.90 | 8.94 ± 5.63 | 0.00 ± 0.00 | ||
| Innate | Neutral | 7.57 ± 4.09 | 4.37 ± 3.40 | 8.11 ± 8.01 | 9.49 ± 9.19 | |
| Aversive | 6.12 ± 4.29 | 2.70 ± 1.10 | 14.59 ± 9.90 | 0.00 ± 0.00 | ||
| Learned | Neutral | 1.12 ± 0.73 | 8.84 ± 8.04 | 4.58 ± 2.91 | 0.42 ± 0.40 | |
| Aversive | 6.10 ± 5.93 | 1.52 ± 1.12 | 7.89 ± 5.29 | 0.00 ± 0.00 | ||
Mean and SEM for behaviors across aversive stimuli category and stimulus type in females and males with neonatal amygdala lesions (Neo-Aibo) or sham-operated controls (Neo-C).
Figure 3.
Example responses toward neutral and aversive stimuli. Series of three video frames (from left to right) depicting when the stimuli is first visible, when the monkey retrieves the grape and their response to the stimulus after grape retrieval for one control male (Neo-C-1) and one neonatal amygdala lesioned male (Neo-Aibo-6). For the neutral stimulus (coiled water hose), although both monkeys are close the stimulus at the beginning and readily retrieve the grape, only the lesioned monkey manipulated the hose. For the aversive stimulus (rubber snake), the control monkey primarily remained in the back of the WGTA (first and third frames) and did a quick swiping motion to roll the grape close for retrieval. The lesioned monkey closely visually inspected and manipulated the snake (first and third frames), but unlike the control monkey, they calmly retrieved the grape similar to their neutral stimulus trial.
Latency to Retrieve:
Regardless of the aversive category, animals retrieved the grape faster when no stimulus was present and more slowly when the aversive object was present (Social: F(2,96) = 49.42, p < 0.001, ηp2 = 0.51; Innate: F(2,44) = 25.95, p < 0.001, ηp2 = 0.54; Learned: F(2,44) = 61.20, p < 0.001, ηp2 = 0.74; Figure 2d-f). However, the sex and lesion status of the animal did impact their willingness to retrieve the grape according to the aversive category. There was an overall effect of sex for social aversive stimuli (F (1,48) = 6.60, p = 0.013, ηp2 = 0.12) and a sex by object interaction for innate aversive stimuli (F (2,44) = 3.35, p = 0.044, ηp2 = 0.13), such that females took longer to retrieve the grape from aversive stimuli than males (Figure 2d). Neo-Aibo lesioned monkeys took less time to retrieve the grape during social (F (1,48) = 4.63, p = 0.037, ηp2 = 0.09) and innate (F (1,22) = 7.58, p = 0.012, ηp2 = 0.26) aversive stimuli trials as compared to control monkeys (Figure 2e). However, there were no effects of group, sex, or interactions for latency to retrieve the grape from learned aversive stimuli (Figure 2f).
Object manipulation:
Animals manipulated neutral objects longer than aversive stimuli for all categories (Social: F (1,48) = 22.05, p < 0.001, ηp2 = 0.32; Innate: F (1,22) = 19.49, p < 0.001, ηp2 = 0.47; Learned: F (2,44) = 38.06, p < 0.001, ηp2 = 0.63; Figure 2g-i). Interestingly, there was a Group X Sex X Object type interaction for the social aversive category (F (1,48) = 6.16, p = 0.017, ηp2 = 0.11), such that control males manipulated the neutral stimuli more than control females, whereas males and females with amygdala lesions equally manipulated the neutral social stimuli (Figure 2g). There were no other sex differences for innate or learned aversive stimulus categories. Yet, there were overall group effects for social (F (1,48) = 11.45, p = 0.001, ηp2 = 0.19) and innate aversive stimuli (F (1,22) = 5.62, p = 0.027, ηp2 = 0.20), such that Neo-Aibo monkeys manipulated objects longer than controls (Figure 2g, h). There were no group effects or interactions for learned aversive stimuli (Figure 2i).
Fearful behaviors:
The expression of fearful behavior differed by group and sex depending on the type of aversive stimuli. Females expressed more fear behaviors toward social aversive objects as compared to males (F (1,48) = 11.76, p = 0.001, ηp2 = 0.19; Figure 2j). Neonatal amygdala lesioned animals expressed fewer fearful behaviors compared to controls for social (F (1,48) = 4.36, p = 0.042, ηp2 = 0.08; Figure 2j) and learned aversive stimuli (F (1,48) = 5.07, p = 0.035, ηp2 = 0.19; Figure 2l). There were no sex or group differences for fearful responses toward innate aversive stimuli (Figure 2k).
Hostile behaviors:
Regardless of the aversive category, animals expressed more hostility when aversive stimuli were present as compared to neutral objects (Social: F (1,48) = 13.88, p = 0.001, ηp2 = 0.22; Innate: F (1,22) = 6.05, p = 0.022, ηp2 = 0.21; Learned: F (1,22) = 8.42, p = 0.008, ηp2 = 0.28; Table 3). There were no group differences in hostile expression across any of the aversive stimuli categories. Yet, there was a Sex X Object interaction for learned aversive stimuli (F (1,22) = 6.32, p = 0.02, ηp2 = 0.22), such that females expressed more hostility toward learned aversive stimuli as compared to males (Table 3).
Affiliative behaviors:
All animals tended to express more affiliative behaviors in the presence of an aversive social stimuli as compared to neutral stimuli; however, this effect did not reach statistical significance (Object: F(1,48) = 3.75, p = 0.059, ηp2 = 0.07; Table 3). No significant effects of Group, Sex, or interactions were detected in affiliative behaviors for social, innate or learned aversive stimuli (see Table 3).
Anxiety behaviors:
No significant effects of Group, Sex, Object, or interactions were detected in anxiety behaviors for social, innate, or learned aversive stimuli (see Table 3).
Coo vocalizations:
The emission of coo vocalizations differed based on sex, group, and aversive stimuli type. Female monkeys from both groups emitted more coo vocalizations compared to males for social aversive stimuli (F (1,48) = 7.30, p = 0.01, ηp2 = 0.13), yet for innate aversive stimuli Neo-Aibo lesioned females emitted the most coos (Group X Sex: F (1,22) = 4.21, p = 0.05, ηp2 = 0.16; Table 3). For learned aversive stimuli, there was a Sex X Object interaction (F (1,22) = 4.19, p = 0.05, ηp2 = 0.16), such that females of both groups emitted more coo vocalizations during the aversive stimuli as compared to males (Table 3).
Stereotyped behaviors:
Only a Group X Sex interaction reached significance for the expression of stereotypies for social aversive stimuli (F (1,48) = 6.43, p = 0.015, ηp2 = 0.12), such that control males did more pacing stereotypy as compared to Neo-Aibo males and Neo-Aibo females paced more than control females (Table 3). No other group or sex differences were detected in stereotyped behaviors for innate or learned aversive stimuli.
Discussion
The current study focused on examining the long-term impact of early amygdala damage on threat detection and behavioral responses toward social, innate, and learned aversive stimuli. Animals with neonatal amygdala lesions exhibited less emotional reactivity toward aversive stimuli, including faster retrieval of food rewards, fewer fearful responses, and more manipulation of objects as compared to age-, sex-, and rearing-matched controls. Although overall, early lesions resulted in less reactivity, their responses differed by aversive stimulus type, such that their responses toward learned aversive stimuli was very similar to controls. The current study also detected sex differences in behavioral response to aversive stimuli. Interestingly, early amygdala damage impacted the expression of some, but not all, sex differences. These findings add important information that broaden our understanding of the role of the amygdala in the expression of sexually dimorphic behaviors. Lastly, qualitative comparisons between the effects of neonatal-onset versus adult-onset amygdala lesions will be provided in the discussion below to indicate whether the magnitude of the emotional changes noted after the early lesions were similar to that reported after adult-onset lesions.
Response to Social Aversive Stimuli:
The current study demonstrated that neonatal amygdala lesions in rhesus monkeys results in decreased emotional reactivity when confronted with social stimuli as shown by faster retrieval of a food reward, longer manipulation of the object, and fewer fearful behaviors. These emotional changes parallel the lack of ability of the same neonatal amygdala lesioned animals to modulate their behavior based on the salience of a threat during an acute social stress test (Raper, Wilson, et al., 2013). The results are also consistent with previous studies demonstrating that monkeys with neonatal amygdala lesions manipulate objects more than controls (Bliss-Moreau et al., 2010; Bliss-Moreau, Toscano, et al., 2011) and are disinhibited during social testing (Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004; Raper et al., 2014). Yet, the current study showed that Neo-Aibo animals did not lack the ability to exhibit fearful responses, they merely expressed fewer fearful behaviors than controls. Decreased expressiveness toward social videos was also previously reported after early amygdala damage (Bliss-Moreau, Bauman, et al., 2011). Similar findings are reported in humans with amygdala damage who show decreased eye gaze, impaired social judgements, and being uninhibited in social situations as compared to controls (Adolphs et al., 2005; Kennedy, Gläscher, Tyszka, & Adolphs, 2009; Spezio, Huang, Castelli, & Adolphs, 2007). Thus, the current study reaffirms the important role the amygdala plays in attention to social stimuli.
The present findings are also consistent with assessments of social interactions or acute social stress paradigms (e.g. human intruder) in adult monkeys with adult-onset amygdala lesions, in that adult-onset amygdala damage results in social disinhibition and decreased fear (Emery et al., 2001; Kalin et al., 2004; Machado & Bachevalier, 2006). Similar reduction of fear and increased exploration were reported after adult-onset amygdala lesions using inanimate social objects (Meunier, Bachevalier, Murray, Malkova, & Mishkin, 1999). Yet, the present findings contrast with those of Machado and colleagues (Machado et al., 2009). Although this later study used stimuli and procedures identical to those used in the present study, the authors reported no difference in food retrieval latency and defensive behaviors between adult-onset amygdala lesions and controls. The lack of effects with late-onset amygdala lesions on reactivity to inanimate social stimuli cannot be explained by lesion extent, since the extent of amygdala damage in the late-onset lesions was more extended than that of the early-onset lesions. It is possible that exposure to similar inanimate social stimuli prior to surgery influenced the lack of effect seen after surgery in adult-onset lesion animals. Overall, the current findings of reduced reactivity and social disinhibition are consistent with studies of human patients with amygdala damage, as well as some, but not all, neonatal- and adult-onset amygdala lesion studies in monkeys (Bauman et al., 2004; Birmingham, Cerf, & Adolphs, 2011; Bliss-Moreau et al., 2010; Raper et al., 2014; Raper, Wilson, et al., 2013).
Response to Innate Aversive Stimuli:
In the current study, neonatal amygdala lesions resulted in decreased defensive behaviors, such that lesioned animals retrieved the food faster and manipulated stimuli more than controls when confronted with innate aversive stimuli. These results are consistent with previous studies of neonatal amygdala lesions, demonstrating less responsiveness and more object manipulation of reptile-like objects (Bliss-Moreau, Bauman, et al., 2011; Bliss-Moreau et al., 2010; Bliss-Moreau, Toscano, et al., 2011). In addition, current findings are consistent with adult-onset amygdala lesions demonstrating decreased defensive responses toward innate aversive stimuli in monkeys (Chudasama et al., 2009; Feinstein et al., 2011; Izquierdo et al., 2005; Kalin et al., 2004; Kalin et al., 2001; Machado et al., 2009; Meunier et al., 1999) and humans (Feinstein et al., 2011). The present findings confirm that an intact amygdala is required for species-typical defensive responses toward real or fake snakes and spiders. Thus, neonatal- and adult-onset lesions have similar responses to innate aversive stimuli, suggesting that this highly evolutionarily conserved brain area (Abellán, Desfilis, & Medina, 2013; Amaral, Price, Pitkanen, & Carmichael, 1992) plays an essential role in threat detection of innately threatening stimuli with little difference due to the developmental timing of the damage.
Response to Learned Aversive Stimuli:
The current study examined animals’ reactivity to objects typically used in primate handling or capture (i.e. nonhuman primate leather handling gloves and capture net) for which animals may have learned a negative connotation. Interestingly, neonatal amygdala lesioned animals did not differ from controls on food retrieval, object manipulation, or hostility. In fact, neonatal amygdala lesioned animals only differed from controls by expressing less fearful behaviors when presented with a learned aversive stimulus. These findings are consistent with a previous report on these same Neo-Aibo animals indicated that neonatal amygdala lesions did not abolish the acquisition of a learned fear signal during a classical fear conditioning paradigm, i.e. the fear-potentiated startle (Kazama et al., 2012). Specifically, early amygdala damage simply retarded the acquisition of the association between a neutral stimulus and a fear signal. These results are also consistent with those of adult-onset amygdala lesions indicating no changes in emotional reactivity towards learned stimuli (Machado et al., 2009). Combined, these results demonstrate that the acquisition of a fear cue or learning of a negative associations with an object can happen in the absence of an intact amygdala, but that the fearful association may take longer to acquire. The current findings support the existence of amygdala-independent alternate pathways for learning negative associations. It has already been demonstrated that with extensive training on fear conditioned cues, brain activation in control human subjects shifts from the amygdala to other structures, including the medial prefrontal, anterior cingulate, and insular cortices (Buchel, Morris, Dolan, & Friston, 1998; Foilb, Flyer-Adams, Maier, & Christianson, 2016; Knight, Smith, Cheng, Stein, & Helmstetter, 2004; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Ponnusamy, Poulos, & Fanselow, 2007). Additionally, evidence from functional neuroimaging in nonhuman primates has indicated that the extended amygdala, specifically the bed nucleus of the stria terminalis (BNST) is involved in threat-associated freezing behaviors and anxiety (Fox, Shelton, Oakes, Davidson, & Kalin, 2008; Kalin, Shelton, Fox, Oakes, & Davidson, 2005). Given the very similar overlapping connectivity of the BNST and the amygdala, it is entirely possible that this area may serve a compensatory function in the case of early amygdala damage, although this possibility has yet to be confirmed (Goode & Maren, 2017; Haufler, Nagy, & Pare, 2013). This proposal could perhaps help understand the discrepancies between the latency to retrieve food in the presence of learned aversive stimuli than in the presence of innate stimuli. The longer retrieval latency in presence of the learned aversive stimuli may be related to the amount of exposure/experience that animals have had with the stimuli. Indeed, due to their housing, the animal have had no prior exposure to tarantulas or snakes, but it is likely that they have been exposed to the adverse effects of leather handling gloves or capture nets, either towards themselves or by observing the other animals responses to those items. Thus the proposed alternate neural pathway described above may have enabled them to learn the adverse effects of a capture net and drive the longer latencies to reach for the food in presence of these stimuli.
Sex Differences:
In rhesus monkeys, the amygdala has been shown to be sexually dimorphic and to play a role in sexually dimorphic behaviors (Payne et al., 2010). The current study identified several sex differences in behavioral responses toward aversive stimuli. Specifically, female monkeys took longer to retrieve the food reward and thus expressed greater emotional reactivity to aversive stimuli as compared to males, and this was consistent across aversive stimulus type. Females also expressed more hostility and more coo vocalizations during learned aversive trials, yet more fearful behaviors during social aversive trials as compared to males. These findings are consistent with previous reports of sex differences in fearful, hostile, and vocalization behaviors in rhesus monkeys (Kalin et al., 1998; Mason et al., 1960; Raper, Wallen, et al., 2013; Tomaszycki et al., 2001). Coos are long duration high pitched calls that can transmit over greater distances making them ideal to communicate and reconnect with family and group members after separation from their social group (Hauser, 1991; Pfefferle, Kazem, Brockhausen, Ruiz-Lambides, & Widdig, 2014; Rowell & Hinde, 1962). Coos are emitted more frequently when retrieval or contact comfort from a conspecific is not immediately possible and may be motived by fear (Kalin, Shelton, & Snowdon, 1992). Therefore, increased cooing from females may indicate their increased fear toward the presence of aversive stimuli and increased attempts to reconnect with their cagemate. Interestingly, early amygdala damage did impact the normal expression of some of these sex differences. For example, control males manipulated neutral stimuli more than control females during social trials, whereas females with early amygdala damage exhibited increased manipulation of those objects equal to the lesioned males. Similar effects of neonatal lesions on sexually dimorphic behavior was reported in these same animals while looking at short videoclips of expressive conspecifics (Payne & Bachevalier, 2019). Specifically, normal control males looked equally toward the eyes and mouth of an unfamiliar conspecific, whereas control females exhibited a preference for looking at the eyes. This sex difference was reversed by early amygdala damage, such that Neo-Aibo females looked equally to the eyes and mouth of conspecifics, whereas Neo-Aibo males exhibited preferential looking toward the eyes. Interestingly, previous studies have also showed that male and female neonatal amygdala lesioned monkeys exhibited increased stereotypic behaviors compared to controls (Bauman, Toscano, Babineau, Mason, & Amaral, 2008; Bliss-Moreau, Moadab, Santistevan, & Amaral, 2017; Moadab, Bliss-Moreau, Bauman, & Amaral, 2017), although the current study showed that only the Neo-Aibo females exhibited increased pacing during social aversive trials. Finally, although females generally emitted more coos than males, Neo-Aibo females produced the highest frequency of coo vocalizations as compared to both male and female controls or Neo-Aibo males during innate aversive stimuli trials. In adult rhesus monkeys lesions of the amygdala central nucleus result in the emission of more coo vocalizations regardless of the salience of a threat presented (Kalin et al., 2004). Increased vocalization among neonatal amygdala lesioned female monkeys has also been reported in another study on an acute social stress test during infancy (Raper, Wallen, et al., 2013). Taken together, the current findings suggest that adult females with neonatal amygdala lesions may have been more distressed by the innate stimuli as compared to lesioned males, thus producing more coo vocalizations. Alternatively, amygdala damage early in life may create disinhibition of vocalizations, thereby increasing the normal sex difference in coos during the innate stimuli condition. Overall, findings from the current study further support the role of the amygdala in the expression of sexually dimorphic behaviors.
Limitations:
One important caveat in developmental research is that brain maturation and behavior development are the result of complex interactions between genetic and environmental factors, in that changes in these factors may impact normal development. In monkeys, rearing condition has been shown to alter brain maturation and socioemotional behavior (Rommeck, Capitanio, Strand, & McCowan, 2011; Sánchez, Hearn, Do, Rilling, & Herndon, 1998). Thus, a short-coming of this study is that rearing conditions could have affected the expression of behaviors, although this seems unlikely for several reasons. First, we have previously shown that our surrogate-peer rearing protocol is similar to ‘continuous rotation peer rearing’, which is known to produce behavioral and temperament measures similar to those of mother-reared monkeys (Rommeck et al., 2011). Second, we recently reported similar reduction of defensive behaviors during the human intruder task in neonatal amygdala lesioned juvenile monkeys raised with their mother in a large semi free-ranging social group (Raper, Wallen, et al., 2013). Third, our peer-nursery reared monkeys exhibit species-typical caregiver attachment as well as age dependent changes in emotional behavior on the human intruder task and cognitive skills similar to those reported in mother-reared monkeys (Goursaud & Bachevalier, 2007; Kalin, Shelton, & Takahashi, 1991; Raper, Wallen, et al., 2013; Raper, Wilson, et al., 2013; Zeamer, Heuer, & Bachevalier, 2010).
The relatively small sample size could be another limitation of our study. However, with the use of only seven control monkeys (Neo-C; males = 3, females = 4) and six monkeys with neonatal amygdala lesions (Neo-Aibo; males = 3, females = 3), we were able to detect sex differences that accounted for a significant portion of variance (ranging between 8 to 16 percent) according to our reported partial eta squared effect sizes.
Conclusions:
All in all, the present findings inform our understanding on the role of the amygdala in the development of responsiveness to social and innate threats, as well as learning fear associations. Results differed based on the type of aversive stimulus, such that early amygdala damage decreased fear responses for social and innate aversive stimuli but not for learned aversive stimuli. The current study suggests that the amygdala plays an important role in the expression of some, but not all, sexually dimorphic behaviors. Considering that altered structure and function of the amygdala is common among many neurodevelopmental disorders, the current study helps to inform how perturbing amygdala development results in specific alterations in emotional behaviors (Häfner, 2003; Rinehart et al., 2011; Schumann et al., 2011; Zahn-Waxler et al.,2008).
Acknowledgments
Funding Support: National Institute for Mental Health (MH58846 and MH050268)
National Institute for Child Health and Development (NICHD35471)
Center for Behavioral Neuroscience (NSF IBN 9876754)
Integrated Training in Psychobiology and Psychopathology Fellowship (NIMH T32 MH732525)
Yerkes National Primate Research Center, NIH Office of the Director (P51-OD011132)
Footnotes
Disclosures: None of the authors have any conflicts to disclose
References
- Abellán A, Desfilis E, & Medina L (2013). The Olfactory Amygdala in Amniotes: An Evo-Devo Approach. Anat Rec, 296, 1317–1332. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, & Damasio AR (2005). A mechanism for impaired fear recognition after amygdala damage. Nature., 433(7021), 68–72. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, & Damasio A (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372(6507), 669–672. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, & Damasio AR (1995). Fear and the human amygdala. . J Neurosci, 15(9), 5879–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP (2000). The Amygdala. A Functional Analysis (2nd ed.). New York, NY.: Oxford University Press. [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, & Carmichael ST ( 1992). Anatomical organization of the primate amygdaloid complex In Aggleton JP (Ed.), The amygdala (pp. 1–67). New York: Wiley. [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, & Amaral DG (2004). The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J Neurosci, 24(3), 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, & Amaral DG (2008). Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behav Neurosci, 122(5), 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham E, Cerf M, & Adolphs R (2011). Comparing social attention in autism and amygdala lesions: effects of stimulus and task condition. Soc Neurosci, 6(5-6), 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, & Amaral DG (2011). Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci, 125(6), 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Santistevan A, & Amaral DG (2017). The effects of neonatal amygdala or hippocampus lesions on adult social behavior. Behav Brain Res, 322(Pt A), 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, & Amaral DG (2010). Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Dev Psychobiol, 52(5), 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, & Amaral DG (2011). Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience, 178, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, & Schafer EA (1888). An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. . Philos Trans R Soc Lond, 179B, 303–327. [Google Scholar]
- Buchel C, Morris J, Dolan RJ, & Friston KJ (1998). Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron, 20, 947–957. [DOI] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Amaral DG, & Lavenex P (2012). Postnatal development of the amygdala: A stereological study in macaque monkeys. J Comp Neurol, 520(9), 1965–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, & Seyfarth RM (1990). How monkeys see the world. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Chevalier-Skolnikoff S (1973). Facial expression of emotion in nonhuman primates In Ekman P (Ed.), Darwin and facial expression: A century of research In review (pp. 11–89). New York: Academic Press. [Google Scholar]
- Choate JV, Slayden OD, & Resko JA (1998). Immunocytochemical localization of androgen receptors in brains of developing and adult male rhesus monkeys. Endocrine, 8(1), 51–60. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, & Murray EA (2009). Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci, 30(12), 2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Camerer CF, & Adolphs R (2010). Amygdala damage eliminates monetary loss aversion. Proc Natl Acad Sci U S A, 107(8), 3788–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, & Amaral DG (2001). The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav Neurosci, 115(3), 515–544. [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, & Tranel D (2011). The Human Amygdala and the Induction and Experience of Fear. Current Biology, 21, 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Flyer-Adams JG, Maier SF, & Christianson JP (2016). Posterior insular cortex is necessary for conditioned inhibition of fear. Neurobiol Learn Mem 134, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, & Kalin NH (2008). Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE 3, 3, e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, & Maren S (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn Mem, 24(9), 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud A-PS, & Bachevalier J (2007). Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala, and orbital frontal cortex. Behav Brain Res, 176, 75–93. [DOI] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, & Pare D (2013). Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem, 20(11), 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MD (1991). Sources of acoustic variation in rhesus macaque (Macaca mulatta) vocalizations. Ethology, 89(29–46). [Google Scholar]
- Izquierdo A, Suda RK, & Murray EA (2005). Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci, 25(37), 8534–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Davidson RJ (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in primate. J Neurosci, 24, 5506–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, & Kelley AE (2001). The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. . J Neurosci, 21, 2067–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, & Davidson RJ (2005). Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry, 58, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, & Davidson RJ (1998). Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav Neurosci, 112, 251–254. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Snowdon CT (1992). Affiliative vocalizaions in infant rhesus macaques (Macaca mulatta). Journal of Comparative Psychology, 106(3), 254–261. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Takahashi LK (1991). Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child Development, 62(1175–1183). [PubMed] [Google Scholar]
- Kazama AM, & Bachevalier J (2012). Preserved stimulus-reward and reversal learning after selective neonatal orbital frontal areas 11/13 or amygdala lesions in monkeys. Dev Cogn Neurosci, 2(3), 363–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, & Bachevalier J (2013). Effects of Selective Neonatal Amygdala Damage on Concurrent Discrimination Learning and Reinforcer Devaluation in Monkeys. J Psychol Psychother, Suppl 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Heuer E, Davis M, & Bachevalier J (2012). Effects of neonatal amygdala lesions on fear learning, conditioned inhibition, and extinction in adult macaques. Behav Neurosci, 126(3), 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Gläscher J, Tyszka JM, & Adolphs R (2009). Personal space regulation by the human amygdala. Nat Neurosci, 12(10), 1226–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, & Helmstetter FJ (2004). Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci, 4, 317–325. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, & Phelps EA (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron, 20, 937–945. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, & Rakic P (1991). Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci U S A, 88, 10218–10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, & Bachevalier J (2006). The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav Neurosci, 120(4), 761–786. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, & Bachevalier J (2009). Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion, 9(2), 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Green PC, & Posepanko CJ (1960). Sex differences in affective–social responses of rhesus monkeys. Behaviour, 16, 74–83. [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, … Pine DS (2004). A developmental examaination of gender differences in the brain engagement during evaluation of threat. Biol Psychiatry, 55, 1047–1055. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Malkova L, & Mishkin M (1999). Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci, 11, 4403–4418. [DOI] [PubMed] [Google Scholar]
- Mineka S (1987). A primate model of phobic fears In Eysenck HJ & Martin I (Eds.), Theoretical foundations of behavior therapy (pp. 81–111). New York: Plenum. [Google Scholar]
- Mineka S, Keir R, & Price V (1980). Fear of snakes in wild- and laboratoryreared rhesus monkeys (Macaca mulatta). Anim Learn Behav, 8, 653–663. [Google Scholar]
- Moadab G, Bliss-Moreau E, Bauman MD, & Amaral DG (2017). Early amygdala or hippocampus damage influences adolescent female social behavior during group formation. Behav Neurosci, 131(1), 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, & Bachevalier J (2002). Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication J Neurosci Methods, 121, 199–209. [DOI] [PubMed] [Google Scholar]
- Payne C, & Bachevalier J (2019). Early amygdala damage alters the way rhesus macaques process species-specific audio-visual vocalizations. Behav Neurosci., 133(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Cirilli L, & Bachevalier J (2017). An MRI study of the corpus callosum in monkeys: Developmental trajectories and effects of neonatal hippocampal and amygdala lesions. Dev Psychobiol, 59(4), 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, & Bachevalier J (2010). Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus, 20(8), 922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle D, Kazem AJN, Brockhausen RR, Ruiz-Lambides AV, & Widdig A (2014). Monkeys spontaneously discriminate their unfamiliar paternal kin under natural conditions using facial cues. Current Biology, 24, 1806–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz SM, & Sholl SA (1987). Analysis of sex and regional differences in androgen receptors in fetal rhesus monkey brain. Dev Brain Res, 36, 151–154. [DOI] [PubMed] [Google Scholar]
- Ponnusamy R, Poulos AM, & Fanselow MS (2007). Amygdaladependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience, 147, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Stephens SBZ, Sanchez M, Bachevalier J, & Wallen K (2014). Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Dev Psychobiol, 56, 1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wallen K, Sanchez MM, Stephens SB, Henry A, Villareal T, & Bachevalier J (2013). Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Horm Behav, 63, 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wilson ME, Sanchez M, Machado C, & Bachevalier J (2013). Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. . Psychoneuroendocrinololgy, 38, 1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommeck I, Capitanio JP, Strand SC, & McCowan B (2011). Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta). Am J Primatol, 73, 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TE, & Hinde RA (1962). Vocal communication by the rhesus monkey (Macaca mulatta). Proc. Zool. Soc. Lond, 138, 279–294. [Google Scholar]
- Sackett GP, Ruppenthal GC, & Davis AE (2002). Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtail monkeys (Macaca nemestrina). Am J Primatol, 56, 165–183. [DOI] [PubMed] [Google Scholar]
- Saint-Maurice PF, Welk GJ, Silva P, Siahpush M, & Huberty J (2011). Assessing children's physical activity behaviors at recess: a multi-method approach. Pediatr Exerc Sci, 23, 585–599. [DOI] [PubMed] [Google Scholar]
- Salem KS, & Logothestis NK (2006). A combined MRI and histology atlas of the rhesus monkey brain in sterotaxiccordinates. Boston: Academic Press, Elsevier Ltd. [Google Scholar]
- Sánchez MM, Hearn EF, Do D, Rilling JK, & Herndon JG (1998). Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res, 812, 38–49. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Huang PY, Castelli F, & Adolphs R (2007). Amygdala damage impairs eye contact during conversations with real people. J Neurosci, 27(15), 3994–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszycki ML, Davis JE, Gouzoules H, & Wallen K (2001). Sex differences in infant rhesus macaque separation-rejection vocalizations and effects prenatal androgens. Horm Behav, 39, 267–276. [DOI] [PubMed] [Google Scholar]
- van Hooff J (1967). The facial displays of the catarrhine monkeys and apes In Morris D (Ed.), Primate ethology (pp. 7–69). Chicago: Aldine Publishing Company. [Google Scholar]
- Zeamer A, Heuer E, & Bachevalier J (2010). Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci, 30, 9157–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zosuls KM, Ruble DN, Tamis-Lemonda CS, Shrout PE, Bornstein MH, & Greulich FK (2009). The acquisition of gender labels in infancy: implications for gendertyped play. Dev Psychol, 45, 688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]