Abstract
Background:
A mandated reduction in the nicotine content of cigarettes could reduce smoking rate and prevalence. However, one concern is smokers may compensate by increasing the intensity with which they smoke each cigarette to obtain more nicotine. The present study assessed whether smokers engage in compensatory smoking by estimating the mouth-level nicotine intake of low nicotine cigarettes smoked during a clinical trial.
Methods:
Smokers were randomly assigned to receive cigarettes with one of five nicotine contents for six weeks. An additional group received a cigarette with the lowest nicotine content, but an increased tar yield. The obtained mouth level nicotine intake from discarded cigarette butts for a subset of participants (51–70/group) was estimated using solanesol as previously described. A compensation index was calculated for each group to estimate the proportion of nicotine per cigarette recovered through changes in smoking intensity.
Results:
There was no significant increase in smoking intensity for any of the reduced nicotine cigarettes as measured by the compensation index (an estimated 0.4 % of the nicotine lost was recovered in the lowest nicotine group, 95% CI=−0.1, 1.2). There was a significant decrease in smoking intensity for very low nicotine content cigarettes with increased tar yield.
Conclusions:
Reductions in nicotine content did not result in compensatory changes in how intensively participants smoked research cigarettes.
Impact:
Combined with data from clinical trials showing a reduction in cigarettes smoked per day, these data suggest that a reduction in nicotine content is unlikely to result in increased smoke exposure.
Introduction
The Food and Drug Administration is considering a mandated reduction in the nicotine content of cigarettes to a minimally-addictive or non-addictive level [1]. A recent simulation estimated that a nicotine reduction policy implemented by 2020 would save 1.7 million lives by the year 2050 [2]. Clinical trials surrounding nicotine reduction thus far have been encouraging—showing that a reduction in nicotine content in conventional cigarettes to 0.4 mg nicotine / g tobacco results in participants smoking fewer cigarettes per day, lower levels of nicotine dependence, more quit attempts, and greater smoking cessation [3–5].
However, one of the primary concerns regarding potential harms of a nicotine reduction policy has been the potential for compensation in order to mitigate the reduced nicotine content [6]. Compensation can take place via an increase in the number of cigarettes smoked per day or increase in intensity of smoking each cigarette (i.e., changes in smoking topography such as taking “larger” or more frequent puffs). Some of these concerns stem from an analogy to “light” cigarettes. Switching from regular to light cigarettes does result in compensatory smoking, driven primarily by an increase in intensity of smoking each cigarette [7]. Light cigarettes have the same nicotine content as full flavored cigarettes, but yield reduced nicotine via changes in the design of the cigarette (e.g., ventilated filters) under standardized smoking machine testing. However, smokers achieve much higher exposures than predicted by machine via compensatory smoking practices [8, 9].
In contrast, a nicotine reduction policy would reduce the nicotine content within the tobacco itself. In clinical trials, there is no evidence that compensation occurs through increases in the number of cigarettes smoked per day. When cigarette nicotine content is reduced to very low levels (<2.4 mg nicotine / g tobacco), participants smoke fewer, not more, cigarettes per day [3–5, 10]. Until now, the best evidence regarding the impact of nicotine reduction on changes in smoking intensity within each cigarette have come from lab-based puff topography assessments [11, 12] in which the total puff volume of each cigarette was measured by requiring participants to smoke research cigarettes through a machine that quantifies smoking behavior (e.g., number of puffs, volume of each puff, time between puffs). Lab-based puff topography assessments have shown that when nicotine content is reduced to low levels, there are increases in puff volume across the first few cigarettes [11, 12], but decreases in puff volume after extended use with the cigarettes [3]. In line with these data showing a lack of compensation when smokers switch to low nicotine cigarettes—clinical trials show that assignment to receive very low nicotine content (VLNC) cigarettes results in reduced biomarkers of exposure to smoke and tobacco toxicants [3–5].
The goal of the present study was to assess whether smokers assigned to receive cigarettes with a reduced nicotine content engage in compensatory smoking by changing their smoking intensity (i.e., changing how they smoke each cigarette to obtain more nicotine than expected). We used a novel approach that assessed changes in smoking intensity for cigarettes smoked outside of the lab during the clinical trial by analyzing the cigarette butts of these cigarettes. As part of a recent clinical trial, smokers were randomly assigned to receive research cigarettes with varying nicotine contents for six weeks. During Weeks 2 and 6, participants collected and returned a sample of their discarded cigarette butts. A subset of these butts was used to measure solanesol. Solanesol is a long-chain terpenoid that is naturally occurring in tobacco and is deposited in the filter when the cigarette is smoked, serving as a useful marker for smoke exposure. Solanesol levels in butts can be used to estimate the obtained mouth level of nicotine intake from each cigarette [13, 14]. Assessing nicotine exposure per cigarette using solanesol measured in discarded cigarette butts has an advantage over lab-based topography measurements because it is noninvasive—smokers can smoke as they normally would outside of the laboratory and mouth level nicotine intake can be estimated using the cigarette butts at a later time [13–15].
Materials and Methods
Participants
Full methods for the clinical trial, including the Consolidated Standards of Reporting Trials (CONSORT) flow-chart can be found in the primary paper [3]. Briefly, daily smokers were recruited from 10 U.S. sites (Brown University, Duke University, Johns Hopkins University, MD Anderson Cancer Center, Moffitt Cancer Center, University of California San Francisco, University of Minnesota Duluth, University of Minnesota Twin Cities, University of Pennsylvania, and University of Pittsburgh). Inclusion criteria included: at least 18 years old, at least five cigarettes per day, expired carbon monoxide > 8 parts per million or urine cotinine > 100 ng/ml. Exclusion criteria included intention to quit smoking in the next 30 days, use of other tobacco products, including e-cigarettes, on more than 9 days per month, binge drinking on more than 9 days per month, significant or unstable medical or psychiatric condition as determined by a licensed medical professional, positive illicit drug screen for drugs other than cannabis, pregnant or breastfeeding or exclusively smoking roll-your-own cigarettes.
Procedures
Participants paid for and smoked their usual brand during a two-week baseline period. Participants (n=839) were then randomly assigned to receive either their usual brand (not included here) or a research cigarette with one of five nicotine contents for six weeks (15.8 (normal nicotine content (NNC) control), 5.2, 2.4, 1.3, 0.4 mg nicotine /g tobacco, Spectrum, produced for National Institute on Drug Abuse by 22nd Century Group, Inc., St. Clarence, NY, USA) (n=119–123/group). To understand how tar yield might interact with nicotine content, two groups of participants were assigned to the lowest nicotine content; one received a cigarette with the same machine-measured tar level as the other groups (8–10 mg ISO), and one group received a cigarette with an increased tar yield (HT, 13 mg ISO). For research cigarette groups, nicotine content was double-blind. All cigarettes were provided for free. Participants were asked not to smoke cigarettes other than the research cigarettes but were encouraged to be honest if they used non-study cigarettes. Across all six weeks of study assessment, participants reported the number of study and non-study cigarettes smoked per day using an automated interactive voice-response system (InterVision Media). Participants returned to the lab each week during the experimental period to complete assessments and receive more cigarettes. A two-week supply of cigarettes was provided at each weekly visit to allow for the measurement of compensatory increases in smoking rate or to prevent participants from running out of cigarettes if they missed a visit.
At Baseline, Week 2, and Week 6, participants were asked to collect their discarded cigarette butts from the 24-hours prior to their first void urine on the day of their scheduled visit. For the purpose of this analysis, we focus on Week 6 as the timepoint most representative of stable smoking behavior (Baseline and Week 2 not shown). Participants were provided with individual metal tins for collecting up to 20 cigarette butts, which participants labeled with the time they smoked each cigarette and returned to the lab at their visit. If a participant forgot to collect a cigarette butt, they were instructed to skip a space in the collection box to indicate a missing sample. If participants smoked non-study cigarettes, they were instructed to collect those butts and mark them to indicate they were non-study. Cigarette tins and vials containing cigarette butts were sent to the Center for Disease Control and Prevention (CDC) for solanesol analysis.
Solanesol Analysis and Estimation of Mouth Level Nicotine Intake
Mainstream solanesol exposure was analyzed as previously described [13, 14, 16, 17] with slight modifications. A 1-cm portion of discarded cigarette filter, as measured from the mouth end, was cut and used for analysis. The cut portion of the butts were spiked with isotopically-labelled internal standard and solvent-extracted. The extracts were analyzed quantitatively for solanesol using liquid chromatography and quadrupole mass spectrometry. In order to correlate the solanesol from the smokers’ discarded filter butts to the corresponding mainstream smoke delivery, each of the six research cigarettes was smoked under varying conditions.
For this study we constructed the correlation curves using smoking parameters reported in Table 1 to relate the filter solanesol level to the corresponding mainstream smoke deliveries of nicotine. Correlation equations (n=5), using linear regression were developed. R2 values ranged from 0.94 (NRC201) to 1.0 (NRC600) using a linear smoking machine to cover a wide range of nicotine deliveries and solanesol levels. Because of the very large number of cigarette butts collected, a subset of participants were selected at random for analysis (51–70 participants/group, 383 participants in total). In total, 16,000 butts were analyzed for solanesol and converted to nicotine “mouth level intake” values using the appropriate solanesol to nicotine correlation curves.
Table 1.
Smoking parameters used to create correlation curves relating filter solanesol level to corresponding mainstream smoke deliveries of nicotine.
| Regime Code | Regime Name | Description |
|---|---|---|
| ISO | Regular ISO |
|
| IN6 | IN 6 puffs |
|
| IN | Regular IN |
|
| B65 | Blocked vent, 65/20 |
|
| B70 | Blocked vent, 70/10 |
|
| IN | Regular IN |
|
| B65 | Blocked vent, 65/20 |
|
| B70 | Blocked vent, 70/10 |
|
Table Note: ISO= International Standards Organization, IN=Canadian Intensive
Data Analysis
Data analysis focused on testing whether participants changed their smoking behavior to obtain more nicotine from reduced nicotine cigarettes compared to what was expected from the extent of nicotine reduction in the cigarette (i.e., compensation). Our primary analysis focused on the average mouth level nicotine intake (as estimated from solanesol) for study cigarettes at Week 6. For each participant, we calculated the mean mouth level nicotine intake across all cigarette butts returned to the lab (1–20 butts). Because smokers might be more likely to compensate for reduced nicotine after a period of abstinence from smoking, a second analysis was conducted for the first study cigarette of the day separately from other cigarettes. However, the results of this first-cigarette analysis did not differ from the analysis of the average over all study cigarettes butts (data not shown). Average mouth level nicotine intake at Week 6 was analyzed on the natural log scale and analyzed using linear regression. Differences between treatment groups were summarized by the ratio of geometric means. We completed both an unadjusted analysis and an analysis that adjusted for age, race (white, black, other), gender, and salivary nicotine metabolite ratio. The pattern of results was similar across adjusted and unadjusted analyses, and only the unadjusted analysis has been reported here.
The compensation index (CI) was used to summarize compensation for each reduced nicotine cigarette. The CI has been used by prior researchers in a variety of contexts to test for the presence and degree of compensation when nicotine content or yield is reduced [18–22]. Here, the CI can be thought of as a measure of the proportion of nicotine that is recovered through changes in the smoking intensity of each cigarette when nicotine is reduced. The CI compares the actual reduction in mouth level nicotine intake for each reduced nicotine cigarette to the expected reduction in nicotine intake. A CI siginificantly greater than 0 would indicate that participants obtained more nicotine than would be expected given the change in nicotine content by increasing the intensity with which they smoked each cigarette (e.g., taking “larger” or more frequent puffs).
Calculation for CI was as follows:
Where eβVLNC is the regression coefficient for each treatment group from the regression model described above and NVLNC and NNNC are the nicotine yield for the VLNC and NNC cigarettes, respectively. To calculate the expected reduction in nicotine yield, we used the proportional change in nicotine as measured by the machine-based nicotine yield estimates for Spectrum Cigarettes using the International Standards Organization (ISO) methodology. We also considered relying on the Canadian Intensive (IN) methodology but ISO estimates were closer to the obtained mouth level nicotine intake, and thus we relied on ISO for the primary analysis. Calculations using IN are considered secondary and have been included in the supplementary materials. ISO and IN estimates for each cigarette have been previously published [23]. For this analysis, we averaged estimates for menthol and nonmenthol versions of the same nicotine content cigarette. However, we conducted a secondary analysis in which we conducted separate analyses for menthol and non-menthol cigarette types, and the results were the same as those presented here.
CI provides an estimate of the proportion of nicotine that is recovered for each study cigarette, but it does not directly provide information about proportional change in smoking intensity. Larger changes in smoking intensity are required to recover lost nicotine for the lower nicotine content cigarettes. For example, a 15% increase in smoking intensity will recover less nicotine and result in a smaller compensation index for participants assigned to lower nicotine contents than participants assigned to higher nicotine contents. Because changes in smoking intensity are more important to the public health impact of nicotine reduction, we also calculated the change in smoking intensity that would be required to obtain each CI estimate. The change in intensity was calculated as follows:
Finally, we tested the impact of cigarette nicotine content on total cigarettes smoked per day during Week 6 of the trial among the participants included in the cigarette butt analysis. This is a replication of analyses that were completed for the primary paper using all participants. The goal was to confirm that the pattern of results for cigarettes per day in the primary paper was the same as the pattern of results for the subset of participants utilized for the cigarette butt analysis. As in the primary paper, the analysis utilized linear regression, with adjustment for the baseline number of cigarettes smoked per day. Results from a secondary analysis, adjusted for age, race (white, black, other), gender, and salivary nicotine metabolite ratio were consistent with those from the unadjusted analysis and are not included here. A Bonferroni adjustment was made to account for the comparison of the four groups to the control cigarettes (two-tailed test at an alpha of 0.0125).
Results
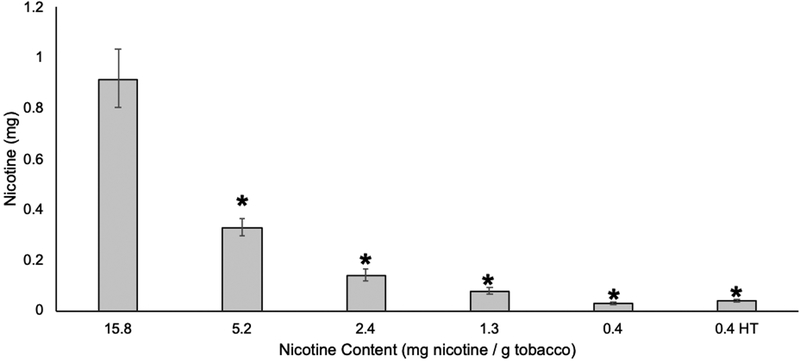
The overall impact of nicotine content on smoking behavior, nicotine biomarker exposure, dependence, withdrawal, and craving can be found in the primary paper [3]. Table 2 shows the demographic characteristics for the subset of participants in the trial who were included in the cigarette butt analysis. Figure 1 shows the average obtained mouth level intake for each Spectrum Cigarette. Mouth level nicotine intake was significantly lower for the 5.2, 2.4, 1.3, and 0.4 mg / g cigarettes, and 0.4 mg / g HT cigarettes than for the 15.8 mg / g control cigarette (Ratio of Geometric Means: 0.36, 0.15, 0.08, 0.03, 0.04, respectively, p < 0.001). See supplementary materials for statistical analyses comparing obtained mouth level intake to machine-measured nicotine yields using both ISO and IN methodologies.
Table 2.
Demographic characteristics for participants included in cigarette butt analysis
| Variable | Overall (N=378) | 15.8 mg (N=64) | 5.2 mg (N=66) | 2.4 mg (N=51) | 1.3 mg (N=64) | 0.4 mg (N=63) | 0.4 mg, HT (N=70) |
|---|---|---|---|---|---|---|---|
| 378 | 64 | 66 | 51 | 64 | 63 | 70 | |
| Age -yr (SD) | 42.5 (13.1) | 43 (13.2) | 44 (12.9) | 42.9 (11.7) | 41.9 (13.2) | 42.4 (14.6) | 40.8 (12.7) |
| Male sex – no. (%) | 219 (57.9%) | 41 (64.1%) | 34 (51.5%) | 33 (64.7%) | 36 (56.2%) | 36 (57.1%) | 39 (55.7%) |
| Race –no. (%) | |||||||
| White | 200 (52.9%) | 28 (43.8%) | 32 (48.5%) | 27 (52.9%) | 31 (48.4%) | 38 (60.3%) | 44 (62.9%) |
| Black | 133 (35.2%) | 28 (43.8%) | 25 (37.9%) | 18 (35.3%) | 24 (37.5%) | 22 (34.9%) | 16 (22.9%) |
| Hispanic ethnic group –no. (%) | 17 (4.5%) | 4 (6.2%) | 2 (3%) | 2 (3.9%) | 3 (4.7%) | 2 (3.2%) | 4 (5.7%) |
| Attended college –no. (%) | 212 (56.1%) | 30 (46.9%) | 39 (59.1%) | 32 (62.7%) | 30 (46.9%) | 36 (57.1%) | 45 (64.3%) |
| Use of menthol cigarettes-no. (%) | 210 (55.6%) | 40 (62.5%) | 35 (53%) | 32 (62.7%) | 39 (60.9%) | 28 (44.4%) | 36 (51.4%) |
| Cigarettes/day-no. (SD) | 16±8.1 | 16.2±9.2 | 17±8.2 | 15.3±8.9 | 16±8.2 | 14.8±7.1 | 16.3±7 |
| Total nicotine equivalents-geometric mean (range) | 43.4 (0.1,221.9) | 42.7 (0.1,197) | 47 (8.7,221.9) | 45.2 (0.7,176.2) | 48.5 (7,196.5) | 33.7 (3.8,144.9) | 44.9 (4.9,176.1) |
| Expired carbon monoxide-ppm (SD) | 15.2 (7.8) | 14.6 (8.3) | 15.2 (7.3) | 16.2 (8.7) | 16.6 (8.5) | 13.7 (7.1) | 14.8 (7) |
| Use of other tobacco product in path 30 days-no. (%) | 77 (20.4%) | 14 (21.9%) | 14 (21.2%) | 13 (25.5%) | 10 (15.6%) | 12 (19%) | 14 (20%) |
| Total score on Fagerstrom Test for Nicotine Dependence (SD) | 5.2±2.2 | 5.2±2.5 | 5.4±2.1 | 4.7±1.8 | 5.4±2.4 | 5.1±2.2 | 5.2±2.1 |
Table Note: yr=year, no.=number, ppm=parts per million,
Figure 1. Mouth level nicotine intake.
Mouth level nicotine intake and 95% confidence intervals calculated using solanesol deposited in cigarette butts collected during Week 6 of clinical trial (n=51–70 participants / group). * denotes significant reduction in mouth level nicotine intake compared to control group (15.8 mg nicotine / g tobacco). HT=high tar
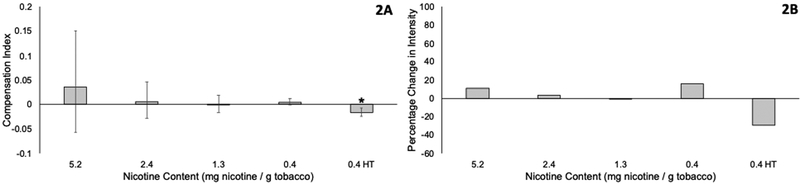
Figure 2A shows the CI and 95% confidence interval for each reduced nicotine cigarette. It is worth noting that confidence intervals will be wider for higher nicotine content cigarettes (e.g., the 5.2 mg cigarette) than for lower nicotine content cigarettes (e.g., the 0.4 mg cigarette) because changes in smoking intensity will have a greater impact on nicotine intake when the nicotine content of the cigarette is greater. Compensation was not significantly different from 0 for the 5.2, 2.4, 1.3, and 0.4 mg / g tobacco cigarettes, indicating that smokers did not change their smoking intensity for each cigarette to obtain more nicotine. Compensation was significantly below 0 for the 0.4 mg / g HT cigarette, indicating that participants may have decreased their smoking intensity of the high tar cigarette in such a way that they obtained less nicotine than would be predicted. Figure 2B shows the percentage change in smoking intensity that would be required to generate the estimated compensation index for each reduced nicotine cigarette. Changes in smoking intensity vary from a decrease of 1.1% in the 1.3 mg nicotine / g tobacco cigarette to increases in intensity of 15.6% in the 0.4 mg nicotine / g tobacco. All confidence intervals include 0, indicating that we cannot be confident that there was any change in smoking intensity.
Figure 2. Compensation Index (A) and Change in Smoking Intensity (B) for each reduced nicotine content cigarette.
A) The proportion of nicotine that is recovered by changes in smoking intensity when smokers switch to reduced nicotine content cigarettes. Compensation Index was calculated using the expected reduction in nicotine estimated using ISO methodology. Error bars show 95% confidence intervals, and a confidence interval that does not include zero is indicated by *. B) The percentage change in smoking intensity that would be required to obtain the compensation index is shown in Figure 2B. HT=high tar
Table 3 shows the impact of cigarette nicotine content on total cigarettes smoked per day during Week 6 among the subset of participants included in the cigarette butt analysis. The pattern of results here is the same as in the primary paper—reductions in cigarette nicotine content to 2.4 mg nicotine / g tobacco or less reduce the number of cigarettes smoked per day, and the treatment effect sizes were similar to those reported in the primary paper (reductions between 4–7 cigaretes per day). As expected given the smaller sample size in this paper, only reductions to the lowest nicotine content (0.4 mg nicotine / g tobacco) met the Bonferroni criterion for significance (alpha=0.0125).
Table 3.
Difference in the mean of total cigarettes per day for the four VLNC conditions vs. NNC controls at the Week 6 visit among participants included in cigarette butt analysis.
| Unadjusted Linear Regression1 | Adjusted Linear Regression2 | ||||
|---|---|---|---|---|---|
| Treatment Group | N | Mean Difference (95% CI) | p-value | Mean Difference 5% CI) | p-value |
| 15.8 mg/g | 64 | ||||
| 5.2 mg/g | 66 | 1.04 (−3.22, 5.29) | 0.63 | 0.67 (−3.34, 4.69) | 0.74 |
| 2.4 mg/g | 51 | −5.32 (−9.87, −0.77) | 0.02 | −5.91 (−10.19, −1.62) | 0.007 |
| 1.3 mg/g | 64 | −4.56 (−8.84, −0.27) | 0.04 | −4.55 (−8.58, −0.51) | 0.03 |
| 0.4 mg/g | 62 | −6.59 (−10.91, −2.27) | 0.003 | −7.33 (−11.42, −3.23) | <0.001 |
| 0.4 mg/g HT | 70 | −4.61 (−8.81, −0.42) | 0.03 | −5.28 (−9.26, −1.3) | 0.0096 |
Adjusted for baseline cigarettes per day
Adjusted for age, race, gender and salivary nicotine metabolite ratio
Discussion
These data show that as nicotine content of cigarette tobacco is reduced, the mouth level intake of nicotine is dramatically reduced and the proportional reduction in nicotine delivered to the smoker is consistent with the machine estimated nicotine yields of the product. We used these mouth level nicotine intake estimates to calculate a per cigarette compensation index for each nicotine content—a measure that has been used widely to test compensation [18–22]. Average CI estimates were not significantly greater than zero for any reduced nicotine cigarettes. The CI estimate for the 0.4 mg/g high tar cigarette was significantly less than zero, indicating that participants likely smoked these cigarettes with less intensity than participants who smoked the NNC control cigarette. Together, these data suggest that a reduction in nicotine content, to levels tested here, does not result in compensatory smoking via increases in smoking intensity per cigarette.
It is important to think of the CI estimates in the context of how they relate to changes in smoking behavior and overall toxicant exposure. For the 0.4 mg/g cigarette for example, the CI was 0.004, meaning that on average participants recovered only 0.4% of the nicotine that is lost in the reduction from 15.8 mg/g to 0.4 mg/g through changes in smoking behavior. However, it is important to note that for very low nicotine contents, even a small level of compensation, if reliable, could be indicative of meaningful changes in smoking intensity and smoke exposure. For the 0.4 mg/g cigarette for example, recovering 0.4% of the nicotine lost would require a 15.6% increase in smoking intensity (Figure 2B). In this analysis, we only estimate changes in compensation through changes in the smoking intensity of each cigarette. The other way in which smokers can compensate is by smoking more cigarettes. Data from this clinical trial demonstrated a 30% reduction in the number of cigarettes smoked per day relative to normal nicotine control cigarettes (see supplementary materials), an effect size similar to other published studies [3–5]. Together, these data confirm that even if one were to argue that the non-significant and small compensation observed with 0.4 mg/g cigarettes was reliable and clinically significant, overall smokers are exposed to less, not more, smoke when they switch to very low nicotine content cigarettes.
This analysis has several limitations. First, we only present data from the study cigarettes participants smoked, and most participants in the lowest nicotine content group smoked at least some non-study cigarettes [3, 24]. Although we collected non-study cigarette butts, we were unable to estimate mouth level nicotine intake from these cigarettes because the correlation between solanesol and nicotine yield is different for each brand. Thus, although these data show a reduction in the nicotine yields from each reduced nicotine cigarette, the levels of smoke exposure from non-study cigarettes is not available. It is possible that participants, when smoking non-study cigarettes, may smoke those cigarettes more intensely in order to obtain nicotine when they are unable to do so from very low nicotine content cigarettes. However, even in clinical trials where participants use non-study cigarettes, biomarkers of toxicant exposure are decreased rather than increased, suggesting that any compensation does not result in increased exposure [5]. Second, the data presented here were collected during the 24 hours prior to the Week 6 visit. Thus, they represent the final day of a six week trial from the subset of participants who completed the trial. Compensation might be more likely early in the trial, and it is possible that compensatory changes in smoking early in the trial could be associated with use of non-study cigarettes later in the trial. Third, in order to calculate a CI, a measure of expected reduction in intake is required, and the accuracy of the CI could be influenced by which measure is chosen. In this case, we relied on the machine-based ISO methodology for nicotine intake. As shown in the supplementary materials, neither the ISO or IN methodologies closely predicted the obtained mouth level nicotine intake. However, in this case, we have calculated CI using both methodologies, and the conclusions are similar. We chose not to rely on estimates of nicotine reduction based on nicotine content given that these estimates would assume that the transfer rate of nicotine from content to yield is the same across the research cigarettes.
These data are encouraging from a policy perspective. Compensatory smoking could lead to higher exposure from toxicants present in mainstream smoke. A lack of significant per cigarette compensation is reassuring for nicotine reduction because it indicates that smokers are unlikely to be exposed to greater smoke levels as a result of changes in how they smoke each cigarette. This study is in line with data from multiple other sources showing that reductions in nicotine content to 2.4 mg nicotine / g tobacco or less do not result in compensatory smoking. Data from clinical trials show that smokers who are assigned to these cigarettes smoke fewer, not more, cigarettes per day [3–5, 25]. Data from clinical trials also show reductions in expired carbon monoxide and other smoke exposure biomarkers when participants are assigned to these cigarettes [4, 5]. Finally, lab-based puff topography assessments do not show compensation when nicotine is reduced to low levels beyond the first few cigarettes [3].
The data included here are the first to show a lack of compensation associated with smoking VLNC cigarettes outside of the laboratory at the per cigarette level. This analysis is advantageous because participants are able to smoke as they normally would in the natural environment, and we are able to estimate nicotine exposure using their discarded cigarette butts. These data, along with the larger body of work surrounding nicotine reduction, show that a mandated reduction in nicotine content to 2.4 mg nicotine / g tobacco or less does not result in per cigarette compensatory changes in smoking behavior.
Supplementary Material
Acknowledgments:
The authors would like to thank all the co-investigators, students, fellows, staff, and participants involved in the Center for the Evaluation of Nicotine in Cigarettes.
Financial Support: The research reported in this manuscript was supported by the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products (U54DA031659). Research was also supported by the National Cancer Institute (P30CA77598) utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002494). Salary support during the preparation of this manuscript was provided by the National Institute on Drug Abuse (U54DA031659, U54DA036114, K01DA047433). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Food and Drug Administration, or the Centers for Disease Control and Prevention. The discarded filter analysis work was supported by funding from the Centers for Disease Control and Prevention, an operating division of the U.S. Department of Health and Human Services.
Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the U.S. Department of Health and Human Services.
Footnotes
Conflicts of Interest:
Dr. Benowitz is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has been a paid expert witness in litigation against tobacco companies. All other authors have no conflicts to report.
Contributor Information
Tracy T. Smith, Department of Psychiatry and Behavioral Sciences, and Hollings Cancer Center, Medical University of South Carolina, 103D Bioengineering Building, 68 President Street, Charleston, South Carolina, 29425
Joseph S. Koopmeiners, Division of Biostatistics, School of Public Health, and Masonic Cancer Center, University of Minnesota, Minneapolis, Minnesota
Dorothy K. Hatsukami, Department of Psychiatry and Masonic Cancer Center, University of Minnesota, Minneapolis, Minnesota
Katelyn M. Tessier, Masonic Cancer Center, University of Minnesota, Minneapolis, Minnesota
Neal L. Benowitz, Departments of Medicine and Bioengineers & Therapeutic Sciences, University of California, San Francisco, San Francisco, California
Sharon E. Murphy, Department of Biochemistry Molecular Biology and BioPhysics and Masonic Cancer Center, University of Minnesota, Minneapolis, Minnesota
Andrew A. Strasser, Department of Psychiatry, Perelman School of Medicine, and Abraham Cancer Center, University of Pennsylvania, Philadelphia, Pennsylvania
Jennifer W. Tidey, Department of Behavioral and Social Sciences, Brown University, Providence, Rhode Island
Eric C. Donny, Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, North Carolina
References
- 1.Food and Drug Administration (FDA), FDA accounces comprehensive regulatory place to shift trajectory of tobacco-related disease, death. 2017.
- 2.Apelberg BJ, et al. , Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med, 2018. 378(18): p. 1725–1733. [DOI] [PubMed] [Google Scholar]
- 3.Donny EC, et al. , Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med, 2015. 373(14): p. 1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatsukami DK, et al. , Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction, 2010. 105(2): p. 343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatsukami DK, et al. , Effect of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Biomarkers of Smoke Exposure: A Randomized Clinical Trial. JAMA, 2018. 320(9): p. 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health and Human Services, Food and Drug Administration (FDA), Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Advanced Notice of Proposed Rulemaking, Federal Register, 2018. 83(52): p. 11818–11843. [Google Scholar]
- 7.Benowitz NL, et al. , Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev, 2005. 14(6): p. 1376–83. [DOI] [PubMed] [Google Scholar]
- 8.Kozlowski LT, Pillitteri JL, and Sweeney CT, Misuse of “light” cigarettes by means of vent blocking. J Subst Abuse, 1994. 6(3): p. 333–6. [DOI] [PubMed] [Google Scholar]
- 9.Kozlowski LT and O’Connor RJ, Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control, 2002. 11 Suppl 1: p. I40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donny EC, Houtsmuller E, and Stitzer ML, Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction, 2007. 102(2): p. 324–34. [DOI] [PubMed] [Google Scholar]
- 11.Macqueen DA, et al. , Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology (Berl), 2012. 223(1): p. 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strasser AA, et al. , New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend, 2007. 86(2–3): p. 294–300. [DOI] [PubMed] [Google Scholar]
- 13.Polzin GM, et al. , Estimating smokers’ mouth-level exposure to select mainstream smoke constituents from discarded cigarette filter butts. Nicotine Tob Res, 2009. 11(7): p. 868–74. [DOI] [PubMed] [Google Scholar]
- 14.Watson C, et al. , Development of a method to assess cigarette smoke intake. Environ Sci Technol, 2004. 38(1): p. 248–53. [DOI] [PubMed] [Google Scholar]
- 15.Watson CV, et al. , Mouth Level Nicotine in a Clinical Setting versus Non-clinical Setting. Tobacco Regulatory Science, 2019. 5(3): p. 229–241. [Google Scholar]
- 16.Ashley DL, et al. , Effect of differing levels of tobacco-specific nitrosamines in cigarette smoke on the levels of biomarkers in smokers. Cancer Epidemiol Biomarkers Prev, 2010. 19(6): p. 1389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bravo Cardenas R, et al. , Determination of Solanesol Levels in Cigarette Filters by Liquid Chromatography-Mass Spectrometry. Unpublished material. [DOI] [PMC free article] [PubMed]
- 18.Scherer G, Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl), 1999. 145(1): p. 1–20. [DOI] [PubMed] [Google Scholar]
- 19.Scherer G and Lee PN, Smoking behaviour and compensation: a review of the literature with meta-analysis. Regul Toxicol Pharmacol, 2014. 70(3): p. 615–28. [DOI] [PubMed] [Google Scholar]
- 20.Benowitz NL, Jacob P 3rd, and Herrera B, Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther, 2006. 80(6): p. 703–14. [DOI] [PubMed] [Google Scholar]
- 21.Grebenstein PE, et al. , Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alcohol Depend, 2015. 151: p. 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AC, et al. , Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol, 2008. 16(1): p. 86–97. [DOI] [PubMed] [Google Scholar]
- 23.Ding YS, et al. , Chemical Characterization of Mainstream Smoke from SPECTRUM Variable Nicotine Research Cigarettes. Tob Regul Sci, 2017. 3(1): p. 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardone N, et al. , Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction, 2016. 111(12): p. 2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatsukami DK, et al. , Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev, 2013. 22(6): p. 1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.