Each year in the United States, more than 30,000 patients undergo endovascular abdominal aortic aneurysm repair (EVAR).1 Guidelines from the Society for Vascular Surgery (SVS), and American College of Cardiology Foundation/American Heart Association as well as guidance from the Food and Drug Administration all recommend regular follow up imaging after endograft placement. 2, 3
However, adherence to annual surveillance after EVAR has been suboptimal. Prior reports suggest that nearly half of patients treated with EVAR do not receive the recommended imaging studies within five years after EVAR.4, 5 In this study, we evaluated patient level characteristics from the Vascular Quality Initiative (VQI) linked with longitudinal follow up from Medicare claims to better understand when and why surveillance failures occur after EVAR. We also examined geographic variation in surveillance failure by state.
Within the VQI, we identified patients who underwent EVAR from 2003 to 2015. We linked these patients to Medicare claims using individual identifiers. The initial EVAR was designated as the index operation and further procedures related to the EVAR were defined as reinterventions. We excluded patients who died during the index operation or were not enrolled in Medicare fee-for-service.
Surveillance failure was defined as any fifteen-month period in which a surveillance imaging study was not obtained (Figure 1, Panel A). We chose fifteen-months to include a three-month grace period in addition to the recommended yearly surveillance interval. Imaging studies were identified using Current Procedural Terminology codes (American Medical Association, Chicago, IL). We included abdominal imaging studies that could plausibly be used to provide surveillance for an indwelling endograft. Imaging modalities included computed tomography (CT), duplex ultrasound, and magnetic resonance imaging (MRI).
Figure.
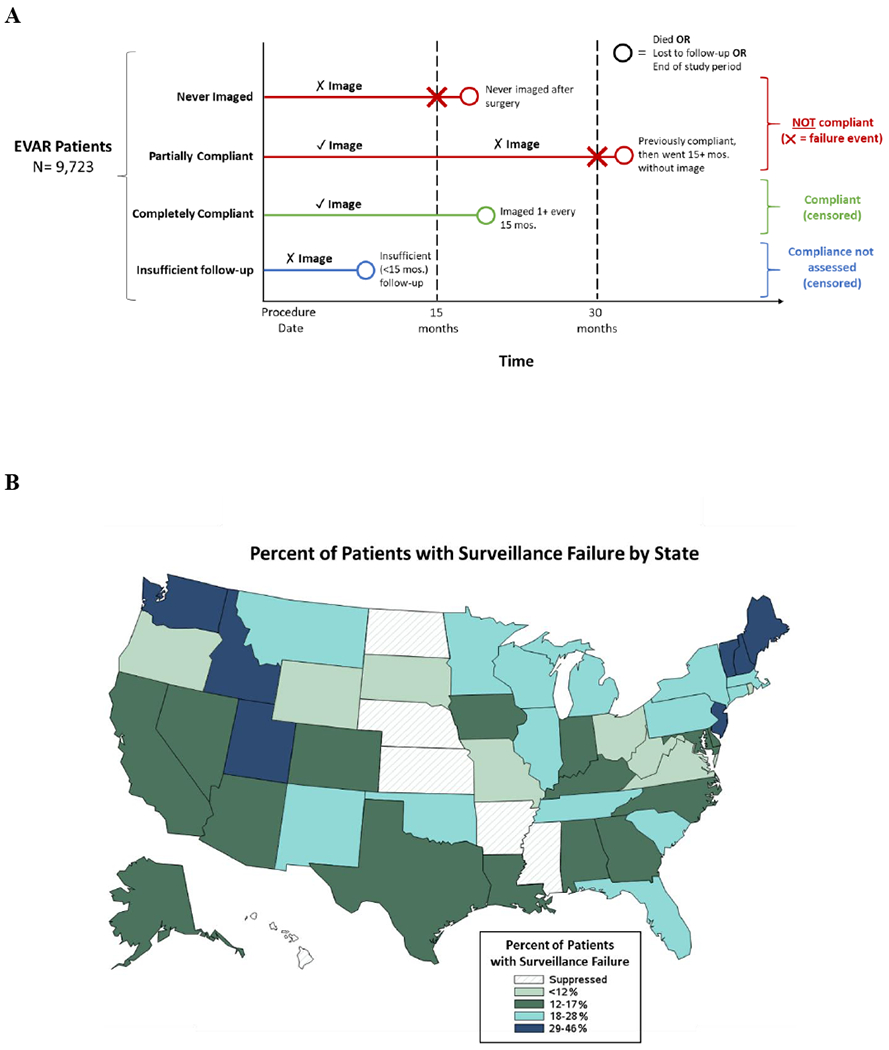
(A) Conceptual model of surveillance failure after EVR. ✓ marks indicate an image was obtained, while ✗ marks indicate no image was obtained. (B) Variation in rates of surveillance imaging failure by state. States with patient volume lower than Centers for Medicare and Medicaid Services reporting rules have been suppressed.
Kaplan-Meier survival analysis was used to assess freedom from surveillance failure. Patients were censored at the end of the study period or at death. Cox proportional hazards regression with backward, stepwise elimination was used to identify factors associated with surveillance failure. Informed consent was waived by the Committee for the Protection of Human Subjects at Dartmouth College.
Our cohort included 9,723 patients who underwent EVAR in 168 centers. Most patients were male (80%, n = 7,761) and Caucasian (92%, n = 8,950). A total of 38,524 surveillance imaging studies were identified during 23,177 person-years of follow up for an average rate of 1.7 imaging studies per person year. The most common method of postoperative imaging was CT (64%, n=24,680 studies), followed by duplex ultrasound (35%, n=13,456 studies), and MRI (1%, n=406 studies).
Most patients were initially compliant with surveillance after EVAR, as 93% (n = 9,061) of patients had a surveillance image within the first fifteen months after surgery. However, surveillance failure steadily increased over time. Kaplan-Meier survival analysis demonstrated that 50% of patients at risk experienced a surveillance imaging failure by 4.19 years, and three out of four patients (75%) at risk experienced a surveillance imaging failure by 6.35 years.
Cox proportional hazards regression identified several factors associated with surveillance failure. Age over 85 (hazard ratio [HR] 1.31 [95% confidence interval (CI) 1.14-1.50]), dual Medicare/Medicaid eligibility status (HR 1.40 [95%CI 1.24-1.58]), chronic kidney disease (HR 1.16 [95%CI 1.03-1.30]), and a diagnosis of dementia (HR 1.55 [95%CI 1.20-2.01]) were all associated with a higher risk of a surveillance failure. Alternatively, having a reintervention procedure (HR 0.80 [95%CI 0.72-0.90]), peripheral artery disease (HR 0.79 [95%CI 0.66-0.93]), or diagnosis of cancer (HR 0.85 [95%CI 0.76-0.95]) were associated with a lower likelihood of a surveillance failure.
In addition to patient-level factors associated with surveillance failure, we also examined state level variation in surveillance failure (Figure). States in the Northeast and Northwest exhibited the highest rates of surveillance failure, and states in the Midwest exhibited lower rates.
Follow up surveillance after EVAR continues to pose a challenge for patients, proceduralists, and regulators. Half of the patients in our study experienced a surveillance failure within four years of their EVAR operation. Schanzer et. al. found that older age and comorbidities including congestive heart failure, chronic kidney disease, and chronic obstructive pulmonary disease were associated with higher risk of failure, findings echoed in our report.5 Garg et. al. demonstrated that dual Medicare and Medicaid eligibility was associated with surveillance failure as well.4
Our study has several limitations. Our study was limited to Medicare patients and is an observational study which relies on clinical event detection identified from billing claims. Surveillance imaging studies were defined broadly, and we were unable to isolate vascular specific imaging from studies for non-vascular indications.
In summary, surveillance failures are common and persistent after EVAR. Before performing EVAR, proceduralists and patients should carefully consider risk factors for surveillance failure, and thorough pre-operative discussion is critical to ascertain patient preferences related to the need for surveillance. For those patients with a high risk of surveillance failure, open repair may be considered a favorable approach.
Acknowledgements:
This work was presented in the poster session at the Society for Vascular Surgery, Vascular Research Initiatives Conference 2019 and American Heart Association, Vascular Discovery 2019.
Funding Sources:
This work was supported by the American Heart Association Grant # 18SFRN33900147, 2018. Population Health Project, Philip Goodney.
This work was supported by the Food and Drug Administration (1U01FD005478-01)
Footnotes
Author disclosures:
Zachary Wanken – AHA SFRN Fellow funded under Dr. Goodney, no additional disclosures
Spencer Trooboff – no disclosures
Barbara Gladders – no disclosures
Jesse Columbo – no disclosures
Niveditta Ramkumar – no disclosures
Andrea Austin – no disclosures
David Stone – no disclosures
Matthew Mell – no disclosures
Art Sedrakyan – supported by Food and Drug Administration grant 1U01FD005478-0, no additional disclosures
Philip Goodney – supported by American Heart Association Grant # 18SFRN33900147, 2018. Population Health Project, no additional disclosures
Data Sharing: We would be happy to discuss data, methods, and study materials with any interested parties. Requests can be made to the corresponding author by email.
References
- 1.Dua A, Kuy S, Lee CJ, Upchurch GR Jr. and Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59:1512–1517. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW and Shen WK. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1425–1443. [DOI] [PubMed] [Google Scholar]
- 3.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, Timaran CH, Upchurch GR Jr. and Veith FJ. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: Executive summary. J Vasc Surg. 2009;50:880–896. [DOI] [PubMed] [Google Scholar]
- 4.Garg T, Baker LC and Mell MW. Adherence to postoperative surveillance guidelines after endovascular aortic aneurysm repair among Medicare beneficiaries. J Vasc Surg. 2015;61:23–27. [DOI] [PubMed] [Google Scholar]
- 5.Schanzer A, Messina LM, Ghosh K, Simons JP, Robinson WP, Aiello FA, Goldberg RJ and Rosen AB. Follow-Up Compliance After Endovascular Abdominal Aortic Aneurysm Repair in Medicare Beneficiaries. J Vasc Surg. 2015;61:16–22.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]


