Abstract
Autophagy is a conserved catabolic process critical for cell homeostasis with broad implications for aging and age-associated diseases. A defining feature of autophagy is the de novo formation of a specialized transient organelle, the double-membrane autophagosome. Autophagosomes originate from small vesicular precursors after rapid membrane expansion resulting in the engulfment of a broad spectrum of cytoplasmic cargoes within a few minutes for vacuolar or lysosomal degradation. Recent advances have provided exciting new insights into the molecular mechanisms underlying the assembly of autophagic membranes during autophagosome biogenesis. Specifically, the phospholipid biosynthesis activity of the endoplasmic reticulum and a dedicated membrane-tethering complex between nascent autophagosomes and the endoplasmic reticulum have emerged as key factors in autophagosome formation.
Keywords: Autophagy, autophagosome biogenesis, membrane contact sites, phospholipids
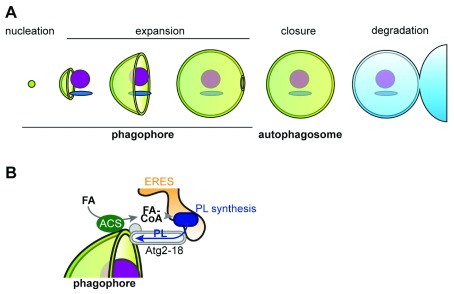
Macroautophagy, hereafter referred to as autophagy, is an intracellular mechanism critical for the maintenance of metabolic homeostasis and the removal of excess or dysfunctional cellular components in basal and stress conditions 1. Consistent with its central role, it has become clear that defects in autophagy are linked to aging and a broad spectrum of common age-associated diseases, including neurodegeneration, diabetes, and cancer 2– 4. Autophagy possesses an unparalleled scope of substrates ranging from protein aggregates to whole organelles and intracellular pathogens 5. The exceptional degradative capacity of autophagy is based on the formation of a specialized transient organelle, termed autophagosome, that surrounds and isolates substrates from the rest of the cytoplasm within a characteristic double-membrane structure. The biogenesis of an autophagosome begins with the nucleation of a small single-membrane vesicular structure, termed phagophore (or isolation membrane). The phagophore undergoes a stage of rapid membrane expansion and, within minutes, grows around the cargo in the shape of a large membranous cup. The cargo is topologically separated from the rest of the cytoplasm when the phagophore closes and divides its originally continuous single membrane into an inner and outer vesicle, the characteristic double-membrane structure of the autophagosome. The outer membrane of the autophagosome fuses with the lytic compartment (the vacuole in yeast and plants or the lysosome in mammals) to expose the inner vesicle and the engulfed cargo to degradation by resident hydrolases. The generated metabolites are recycled back to the cytoplasm. Thus, the life cycle of an autophagosome can be divided into five different stages: nucleation, expansion, closure of the phagophore, and maturation and fusion of the autophagosome with the lytic compartment ( Figure 1A) 6– 8. The origin of the autophagic membranes has been an outstanding question in the autophagy research field for many years.
Figure 1. Autophagosome biogenesis.
( A) The stages of the life cycle of an autophagosome. ( B) Model for the role of local fatty acid (FA) activation, de novo phospholipid (PL) synthesis, and Atg2-mediated PL transfer during phagophore expansion. ACS, acyl-coenzyme A synthetase; CoA, coenzyme A; ERES, endoplasmic reticulum exit site.
A core set of autophagy proteins is essential for the biogenesis of autophagosomes and cooperates with a variety of cellular factors. Upon induction of autophagy, core autophagy proteins assemble in a hierarchical manner to initiate autophagosome formation. The biochemical events underlying the initial assembly have been characterized in considerable detail 1, 9, 10. In short, the Atg1/ULK kinase complex (composed of Atg1, Atg13, Atg17, Atg29, and Atg31 in yeast and ULK1/2, ATG13, ATG101, and FIP200 in mammalian cells) organizes into phase-separating supramolecular structures, resulting in auto-transactivation of the serine/threonine kinase activity of Atg1/ULK 11, 12. The Atg1/ULK kinase targets a number of downstream factors, including the essential autophagy protein Atg9 13. Atg9 is a transmembrane protein that resides in small vesicles (Atg9 vesicles) that are derived from the Golgi apparatus and recycling endosomes 14– 16. Upon autophagy initiation, Atg9 vesicles are bound by the Atg1/ULK kinase complex and critically contribute to the nucleation of the phagophore 17– 20. In addition, the phosphatidylinositol 3 kinase complex I (PI3KI) (composed of Vps34, Vps15, Atg6, and Atg14 in yeast and VPS34, VPS15, Beclin1, and ATG14 in mammals) is recruited to the site of autophagosome formation and generates PI3P essential to autophagy 9, 21. PI3P is bound by the PROPPIN Atg18/WIPI proteins, which recruit Atg2/ATG2 to the phagophore membrane 22– 24. In addition, the action of the two Atg8 and Atg12 ubiquitin-like conjugation systems results in the covalent linkage of Atg8 proteins (Atg8 in yeast and LC3s and GABARAPs in mammals) to phosphatidylethanolamine within autophagic membranes with critical functions for phagophore elongation and substrate interactions 25.
The broad scope of substrates of autophagy suggests that cells form autophagosomes at many different sites in their cytoplasm. In yeast and mammals, autophagy protein assembly and formation of autophagosomes occur in close association with the intricate and dynamic network of the endoplasmic reticulum (ER), a seemingly universally conserved feature of autophagy 26– 30. Although it has been unclear what determines the specific site of autophagosome formation at the ER, autophagosomes do form at specialized subregions of the ER often in proximity to ER contact sites with other organelles, including mitochondria or plasma membrane in yeast and mammalian cells 31– 33. Specifically, in yeast, nascent autophagosomes are quantitatively linked to ER exit sites (ERESs) dedicated to the formation of COPII transport vesicles 27, 28. The function of ERESs is required for the assembly of the autophagy protein machinery downstream of the Atg1 kinase complex and, as a consequence, for the nucleation of the phagophore 27, 34. Strikingly, autophagic structures are stably tethered to ERESs throughout autophagosome biogenesis with one or two ERESs localizing to the rim of the expanding phagophore 27, 28. In line with this spatial arrangement, recent work has demonstrated that COPII vesicles are incorporated into autophagosomal membranes in yeast 35. In mammals, autophagosome formation initiates in proximity to PI3P-enriched membrane compartments connected to the ER, termed omegasomes, which closely enwrap nascent autophagosomes 26. Similar to yeast, ERESs and the ER-Golgi intermediate compartment (ERGIC) can be found in proximity to forming autophagosomes in mammalian cells, and COPII vesicles play an important role in the early stages of autophagosome biogenesis 27, 36– 38.
Close physical association of membrane-bound organelles is generally established by protein tethers, which organize these sites into membrane contact sites 39. Strikingly, the Atg2–Atg18 complex (ATG2A/B and WIPI4) has emerged as a tether for ER–phagophore contacts 40– 43. Cryo-electron microscopy (Cryo-EM) analyses have uncovered an extended rod-shaped conformation for Atg2 bridging the distance between two apposing membranes at membrane contact sites 40, 43. Indeed, in vitro experiments revealed the ability of the Atg2–Atg18 complex to tether membrane vesicles 40, 42. A C-terminal amphipathic helix is required for Atg2 localization to the phagophore while the N-terminus plays an important role for ER binding critical to the expansion of the phagophore in yeast 42. At the same time, the Atg2–Atg18 complex physically interacts with Atg9, confining it to the rim of the expanding phagophore and colocalizing it with ERESs 27, 28, 41. This spatial arrangement has been highly suggestive of phospholipid transfer reactions occurring at these ER–phagophore contacts. However, the molecular nature of these potential phospholipid transfer reactions had remained unclear. Excitingly, three independent studies have demonstrated that Atg2 itself displays phospholipid transfer activity in vitro: Atg2 can extract phospholipids from one vesicular membrane, bind them, and transfer them to a separate vesicular membrane 44– 46. This behavior is reminiscent of other membrane-tethering complexes. Indeed, Atg2 shares sequence and structural homology with the conserved membrane-tethering and lipid transfer protein Vps13 47. Cryo-EM studies have revealed that Atg2 contains a cavity or groove along its extended conformation which can bind phospholipids indiscriminately in terms of headgroup or fatty acid chain composition 44, 45. The identification and first functional insights into the Atg2–Atg18 complex as a membrane tether and phospholipid transfer protein have provided a novel model of how at least parts of the membrane material required for the rapid expansion of the phagophore are transferred from the ER to the phagophore. In particular, the model of non-vesicular transport provides an attractive explanation for the strikingly low inner volume and protein concentration of the phagophore membrane.
A central question that arises from this model is whether Atg2–Atg18 bridges provide sufficient transfer capacity to explain the rapid and extensive growth of the phagophore membrane from a small vesicle to a double-membrane autophagosome. It seems clear from estimates that the transfer rate between vesicles mediated by Atg2–Atg18 in vitro is magnitudes to slow 45: an autophagosome can reach a diameter of 1 μm within a few minutes of biogenesis and thus contain around 25 million phospholipids requiring a total transfer rate of ≥50.000 phospholipid molecules * s −1, but the extrapolated maximal transfer rate in vitro is only 0.017 phospholipid molecules * s −1 per Atg2 molecule 45. Multiple Atg2 molecules contribute to the punctate structures seen at the ER–phagophore contact site by fluorescence light microscopy, but their number very likely cannot compensate for the low transfer rate in vitro. Thus, if Atg2-mediated transfer is the predominant way with which phospholipids are channeled from the ER into autophagic membrane assembly, mechanisms that dramatically accelerate phospholipid transfer via Atg2 in vivo must exist.
Very recent work has identified a pathway involving conserved acyl-coenzyme A synthetases (ACSs) and localized phospholipid synthesis in the ER that critically drives autophagic membrane assembly during phagophore expansion in yeast 48. ACSs are a conserved protein family of peripheral and transmembrane proteins that link coenzyme A to fatty acids to provide activated fatty acids for lipid synthesis, membrane editing, protein acylation, or vesicular fusion 49. In the context of autophagy, the conserved ACS Faa1 localizes to nucleated phagophores downstream of the assembled core autophagy proteins and locally activates fatty acids. These activated fatty acids are channeled into de novo phospholipid synthesis in the ER, which is essential for the efficient expansion of the phagophore and formation of functional autophagosomes 48. These data now show that newly synthesized phospholipids locally drive autophagic membrane formation. Thus, although a number of previously implicated organelles may contribute preformed membranes during phagophore expansion, they appear to be insufficient. These data indicate that localized de novo synthesis may constitute a driving force for the directed transfer of phospholipids across the Atg2–Atg18 complex from the ER into the membrane of the phagophore. Indeed, localized synthesis has been shown to accelerate the transfer of phospholipids across ER–mitochondria membrane contact sites 50. In line with these concepts, phospholipid-synthesizing enzymes appear to be enriched within the ER in proximity to forming autophagosomes and phosphatidylcholine synthesis promotes autophagy in mammalian cells 51, 52. In summary, although the mechanistic details have to be determined, the recent advances in our understanding of the mechanisms that drive the biogenesis of autophagosomes have shifted the focus to the principles of how cells modulate their lipid metabolism and transport across organelle contact sites in order to perform autophagy and maintain cellular health.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Nicholas Ktistakis, Signalling Programme, Babraham Institute, Cambridge, UK
Etienne Morel, Cell Biology Department, Institut Necker-Enfants Malades (INEM), Paris, France
Funding Statement
This work was supported by the Max Planck Society and the Deutsche Forschungsgesellschaft (DFG) (SFB 1218/TP A04).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Gross AS, Graef M: Mechanisms of Autophagy in Metabolic Stress Response. J Mol Biol. 2020;432(1):28–52. 10.1016/j.jmb.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 2. Hansen M, Rubinsztein DC, Walker DW: Autophagy as a promoter of longevity: Insights from model organisms. Nat Rev Mol Cell Biol. 2018;19(9):579–93. 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Dikic I, Elazar Z: Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–64. 10.1038/s41580-018-0003-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Leidal AM, Levine B, Debnath J: Autophagy and the cell biology of age-related disease. Nat Cell Biol. 2018;20(12):1338–48. 10.1038/s41556-018-0235-8 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Gatica D, Lahiri V, Klionsky DJ: Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20(3):233–42. 10.1038/s41556-018-0037-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Abada A, Elazar Z: Getting ready for building: Signaling and autophagosome biogenesis. EMBO Rep. 2014;15(8):839–52. 10.15252/embr.201439076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kraft C, Martens S: Mechanisms and regulation of autophagosome formation. Curr Opin Cell Biol. 2012;24(4):496–501. 10.1016/j.ceb.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 8. Wen X, Klionsky DJ: An overview of macroautophagy in yeast. J Mol Biol. 2016;428(9 Pt A):1681–99. 10.1016/j.jmb.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hurley JH, Young LN: Mechanisms of Autophagy Initiation. Annu Rev Biochem. 2017;86:225–44. 10.1146/annurev-biochem-061516-044820 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Noda NN, Inagaki F: Mechanisms of Autophagy. Annu Rev Biophys. 2015;44:101–22. 10.1146/annurev-biophys-060414-034248 [DOI] [PubMed] [Google Scholar]
- 11. Turco E, Fracchiolla D, Martens S: Recruitment and Activation of the ULK1/Atg1 Kinase Complex in Selective Autophagy. J Mol Biol. 2020;432(1):123–34. 10.1016/j.jmb.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Fujioka Y, Alam JM, Noshiro D, et al. : Phase separation organizes the site of autophagosome formation. Nature. 2020;578(7794):301–5. 10.1038/s41586-020-1977-6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Papinski D, Schuschnig M, Reiter W, et al. : Early Steps in Autophagy Depend on Direct Phosphorylation of Atg9 by the Atg1 Kinase. Mol Cell. 2014;53(3):471–83. 10.1016/j.molcel.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reggiori F, Tucker KA, Stromhaug PE, et al. : The Atg1-Atg13 Complex Regulates Atg9 and Atg23 Retrieval Transport from the Pre-Autophagosomal Structure. Dev Cell. 2004;6(1):79–90. 10.1016/s1534-5807(03)00402-7 [DOI] [PubMed] [Google Scholar]
- 15. Mari M, Griffith J, Rieter E, et al. : An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190(6):1005–22. 10.1083/jcb.200912089 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Young ARJ, Chan EY, Hu XW, et al. : Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119(Pt 18):3888–900. 10.1242/jcs.03172 [DOI] [PubMed] [Google Scholar]
- 17. Ragusa MJ, Stanley RE, Hurley JH: Architecture of the Atg17 Complex as a Scaffold for Autophagosome Biogenesis. Cell. 2012;151(7):1501–12. 10.1016/j.cell.2012.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Rao Y, Perna MG, Hofmann B, et al. : The Atg1–kinase complex tethers Atg9-vesicles to initiate autophagy. Nat Commun. 2016;7:10338. 10.1038/ncomms10338 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Yamamoto H, Kakuta S, Watanabe TM, et al. : Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol. 2012;198(2):219–33. 10.1083/jcb.201202061 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Suzuki SW, Yamamoto H, Oikawa Y, et al. : Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proc Natl Acad Sci U S A. 2015;112(11):3350–5. 10.1073/pnas.1421092112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kihara A, Noda T, Ishihara N, et al. : Two Distinct Vps34 Phosphatidylinositol 3–Kinase Complexes Function in Autophagy and Carboxypeptidase Y Sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152(3):519–30. 10.1083/jcb.152.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rieter E, Vinke F, Bakula D, et al. : Atg18 function in autophagy is regulated by specific sites within its β-propeller. J Cell Sci. 2013;126(Pt 2):593–604. 10.1242/jcs.115725 [DOI] [PubMed] [Google Scholar]
- 23. Krick R, Busse RA, Scacioc A, et al. : Structural and functional characterization of the two phosphoinositide binding sites of PROPPINs, a β-propeller protein family. Proc Natl Acad Sci U S A. 2012;109(30):E2042–E2049. 10.1073/pnas.1205128109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baskaran S, Ragusa MJ, Boura E, et al. : Two-site recognition of phosphatidylinositol 3-phosphate by PROPPINs in autophagy. Mol Cell. 2012;47(3):339–48. 10.1016/j.molcel.2012.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mizushima N: The ATG conjugation systems in autophagy. Curr Opin Cell Biol. 2019;63:1–10. 10.1016/j.ceb.2019.12.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Axe EL, Walker SA, Manifava M, et al. : Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182(4):685–701. 10.1083/jcb.200803137 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Graef M, Friedman JR, Graham C, et al. : ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell. 2013;24(18):2918–31. 10.1091/mbc.E13-07-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki K, Akioka M, Kondo-Kakuta C, et al. : Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J Cell Sci. 2013;126(Pt 11):2534–44. 10.1242/jcs.122960 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Hayashi-Nishino M, Fujita N, Noda T, et al. : A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11(12):1433–7. 10.1038/ncb1991 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Ylä-Anttila P, Vihinen H, Jokitalo E, et al. : 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5(8):1180–5. 10.4161/auto.5.8.10274 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Böckler S, Westermann B: Mitochondrial ER contacts are crucial for mitophagy in yeast. Dev Cell. 2014;28(4):450–8. 10.1016/j.devcel.2014.01.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Hamasaki M, Furuta N, Matsuda A, et al. : Autophagosomes form at ER–mitochondria contact sites. Nature. 2013;495(7441):389–93. 10.1038/nature11910 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Nascimbeni AC, Giordano F, Dupont N, et al. : ER-plasma membrane contact sites contribute to autophagosome biogenesis by regulation of local PI3P synthesis. EMBO J. 2017;36(14):2018–33. 10.15252/embj.201797006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Tan D, Cai Y, Wang J, et al. : The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc Natl Acad Sci U S A. 2013;110(48):19432–7. 10.1073/pnas.1316356110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shima T, Kirisako H, Nakatogawa H: COPII vesicles contribute to autophagosomal membranes. J Cell Biol. 2019;218(5):1503–10. 10.1083/jcb.201809032 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Ge L, Melville D, Zhang M, et al. : The ER–Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. eLife. 2013;2:e00947. 10.7554/eLife.00947 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Ge L, Zhang M, Kenny SJ, et al. : Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO Rep. 2017;18(9):1586–603. 10.15252/embr.201744559 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Ge L, Zhang M, Schekman R: Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. eLife. 2014;3:e04135. 10.7554/eLife.04135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prinz WA, Toulmay A, Balla T: The functional universe of membrane contact sites. Nat Rev Mol Cell Biol. 2020;21(1):7–24. 10.1038/s41580-019-0180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Chowdhury S, Otomo C, Leitner A, et al. : Insights into autophagosome biogenesis from structural and biochemical analyses of the ATG2A-WIPI4 complex. Proc Natl Acad Sci U S A. 2018;115(42):E9792–E9801. 10.1073/pnas.1811874115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gómez-Sánchez R, Rose J, Guimarães R, et al. : Atg9 establishes Atg2-dependent contact sites between the endoplasmic reticulum and phagophores. J Cell Biol. 2018;217(8):2743–63. 10.1083/jcb.201710116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Kotani T, Kirisako H, Koizumi M, et al. : The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc Natl Acad Sci U S A. 2018;115(41):10363–8. 10.1073/pnas.1806727115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Zheng JX, Li Y, Ding YH, et al. : Architecture of the ATG2B-WDR45 complex and an aromatic Y/HF motif crucial for complex formation. Autophagy. 2017;13(11):1870–83. 10.1080/15548627.2017.1359381 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Valverde DP, Yu S, Boggavarapu V, et al. : ATG2 transports lipids to promote autophagosome biogenesis. J Cell Biol. 2019;218(6):1787–98. 10.1083/jcb.201811139 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Maeda S, Otomo C, Otomo T: The autophagic membrane tether ATG2A transfers lipids between membranes. eLife. 2019;8:E3179. 10.7554/eLife.45777 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Osawa T, Kotani T, Kawaoka T, et al. : Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat Struct Mol Biol. 2019;26(4):281–8. 10.1038/s41594-019-0203-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Kumar N, Leonzino M, Hancock-Cerutti W, et al. : VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol. 2018;217(10):3625–39. 10.1083/jcb.201807019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Schütter M, Giavalisco P, Brodesser S, et al. : Local Fatty Acid Channeling into Phospholipid Synthesis Drives Phagophore Expansion during Autophagy. Cell. 2020;180(1):135–149.e14. 10.1016/j.cell.2019.12.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Grevengoed TJ, Klett EL, Coleman RA: Acyl-CoA metabolism and partitioning. Annu Rev Nutr. 2014;34:1–30. 10.1146/annurev-nutr-071813-105541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kannan M, Lahiri S, Liu LK, et al. : Phosphatidylserine synthesis at membrane contact sites promotes its transport out of the ER. J Lipid Res. 2017;58(3):553–62. 10.1194/jlr.M072959 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Nishimura T, Tamura N, Kono N, et al. : Autophagosome formation is initiated at phosphatidylinositol synthase-enriched ER subdomains. EMBO J. 2017;36(12):1719–35. 10.15252/embj.201695189 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Andrejeva G, Gowan S, Lin G, et al. : De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy. Autophagy. 2019;1–17. 10.1080/15548627.2019.1659608 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation