Abstract
Merkel cell carcinoma (MCC) is a highly aggressive cutaneous malignancy, with a high metastasis rate and a significant proportion of cases affecting the eyelid or periocular region. Current treatments for periocular MCC include wide local excision (WLE) with or without adjuvant radiotherapy and can result in profound morbidity and visual deficit. Metastatic disease has been traditionally treated with chemotherapy, though durable responses are typically poor and toxicity is high. Avelumab (Bavencio®, Merck KgaA, Darmstadt, Germany and Pfizer Inc., New York, NY, USA), the first FDA-approved human anti-programmed death-ligand 1 (PD-L1) antibody for the treatment of metastatic MCC (mMCC), has demonstrated safety and efficacy as first-line treatment and in chemotherapy-refractory cases. This review summarizes pivotal clinical trial data for avelumab in the treatment of mMCC, including efficacy, safety and tolerability, and describes the efficacy of two other immune checkpoint inhibitors, pembrolizumab (Keytruda®, Merck & Co., Inc., Kenilworth, NJ, USA) and nivolumab (Opdivo®, Bristol‐Myers Squibb, New York, NY, USA and Ono Pharmaceuticals, Trenton, NJ, USA) for the treatment of advanced MCC. Our purpose is to provide the rationale to further investigate avelumab as a potential therapy for advanced or metastatic eyelid and periocular MCC.
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare, highly aggressive skin cancer of both epithelial and neuroendocrine origin.1 Most commonly affecting Caucasians after the sixth decade, it affects men twice as frequently as women.2 The estimated annual incidence of MCC in 2006 was 0.6 per 100,000 persons yet studies suggest a rising incidence in the US.2–5 Typically, MCC presents with local disease, but regional lymph node and distant metastasis may be present in up to 30% of new cases and overall mortality rates range from 25–32%.5
Risk factors for MCC include immunosuppression – particularly in individuals with chronic lymphocytic leukemia, human immunodeficiency virus infection, and in solid-organ transplant recipients – as well as exposure to ultraviolet light and the Merkel cell polyoma virus (MCPyV).6 MCC is considered an immunogenic cancer due to its increased prevalence and worse prognosis in immunosuppressed persons. 7 Additionally, evidence indicates that approximately 50% of MCC express PD-1 on tumor-infiltrating lymphocytes and express PD-L1 on tumor cells or infiltrating macrophages.8
Approximately 2.5–10% of MCC occur on the eyelid or periocular region,9,10 and represent 5–20% of all head and neck MCC.11–15 Wide local excision (WLE) with or without adjuvant radiotherapy has been a mainstay of treatment. Other therapeutic modalities include Mohs micrographic surgery, exenteration, or WLE with neoadjuvant chemotherapy.16,17 Delayed diagnosis can result in in significant morbidity – including blindness, loss of the eye, and cosmetic deformity – and mortality. Until recently, metastatic MCC (mMCC) has been managed with conventional chemotherapies. Durable responses to chemotherapy are uncommon, however, with a median progression-free survival of only 3 months.18,19
The advent of immunotherapy has heralded new and promising treatment paradigms for the management of advanced and mMCC. In 2017, avelumab (Bavencio®, Merck KgaA, Darmstadt, Germany and Pfizer Inc., New York, NY, USA), a human anti-programmed death-ligand 1 (PD-L1) antibody, was the first FDA-approved drug as an alternative to chemotherapy for the treatment of mMCC.20–22 Pembrolizumab (Keytruda®, Merck & Co., Inc., Kenilworth, NJ, USA), an IgG1 anti-programmed cell death protein 1 (PD-1) monoclonal antibody, was then approved for treatment of advanced MCC in 2018.23
Clinical trials with pembrolizumab and avelumab, both immune checkpoint inhibitors (ICIs), have demonstrated safety and efficacy for the treatment of advanced MCC and mMCC respectively. Avelumab, in particular, has demonstrated encouraging results for both primary treatment as well as in chemotherapy-refractory disease. Though no clinical trials have specifically assessed the use of ICIs for primary or metastatic periocular MCC, previous studies provide a strong rationale to further investigate avelumab as a therapeutic option for advanced eyelid and periocular MCC.
PHARMACOLOGY
Mechanism of Action
Avelumab’s antitumor activity may be achieved through a dual mechanism of action. First, it is a human IgG1 monoclonal antibody that binds to and inhibits PD-L1 on tumor cells which prevents PD-L1 from binding to thePD-1 receptor on T cells.24 PD-1 is a surface expressed T cell protein that acts as an immune checkpoint inhibitor upon interaction with PD-L1, a protein found on normal and tumor cells. Tumor cells and tumor infiltrating lymphocytes often show upregulation of PD-1 and PD-L1 thereby promoting tumor immune subversion. Blocking this interaction with PD-1 antibodies prevents tumor immuno-evasion and promotes T-cell mediated destruction of tumor cells.25 The drug’s mean target occupancy of PD-L1 on CD3+ T-cells has been demonstrated to be 93% two weeks after a dose of 10 mg/kg in individuals with solid tumors.26 Second, avelumab bears a crystallizable fragment (Fc) IgG1 region that targets the Fc-γ receptor on natural killer (Nk) cells and is believed to enable antibody-dependent cell-mediated cytotoxicity (ADCC) by these cells.27,28 No evidence has thus far demonstrated that ADCC contributes to the clinical activity of avelumab, nonetheless, this engagement of both the adaptive and innate immune system is believed to enable a robust antitumor response.
Pharmacokinetics
Avelumab is administered by intravenous (IV) infusion, with maximum concentrations achieved by one hour.26 A dose of 10 mg/kg IV once every two weeks has been established through developmental investigations based on pharmacokinetic, target occupancy, and immunological analysis.26 With repeated dosing at 2-week intervals, steady-state concentrations are reached after approximately 4–6 weeks.26,29,30 An acceptable toxicity profile of up to 20 mg/kg was established in clinical trials.26
The degradation of avelumab is believed to be carried out by the same proteolytic mechanisms by which native IgG is catabolized.30,31 Infusion of 10 mg/kg yields a terminal elimination half-life of 6.1 days, and systemic clearance of 0.59 liters/day.29,30 Clearance does not appear to significantly differ on the basis of age, gender, PD-L1 status, mild-moderate hepatic impairment, or renal impairment. 29,30
EFFICACY TRIALS
To date, there has been one pivotal clinical trial assessing avelumab for the treatment of mMCC (Table 1). No clinical trials to date have specifically assessed the use of avelumab for cases with primary or metastatic eyelid or periocular MCC. Adverse events data are addressed in Safety and Tolerability.
Table 1.
JAVELIN Merkel 200 trial data of avelumab for the treatment of mMCC. The trial comprises two cohorts: individuals with disease refractory to chemotherapy (Part A), and patients with no prior systemic therapy for mMCC (Part B).
| Trial | Design | Subjects | Dosage | Median Treatment Duration | Selected Outcomes |
|---|---|---|---|---|---|
| Phase II | |||||
| JAVELIN Merkel 200, Part A(Second-line or later therapy) | Multicenter, international,open-label,single-arm | 88 | 10 mg/kg | 3.9 m† | Confirmed ORR: 33.0%†
Complete response: 11.4%† 2-year PFS: 26%† 2-year OS: 36%† Median OS: 12.6 m† Clinical activity independent of PD-L1 or MCPyV status† |
| JAVELIN Merkel 200, Part B(First-line therapy) | Multicenter, international,open-label,single-arm, pre-planned interim analysis | 39 | 10 mg/kg | 12 w | Confirmed ORR: 62.1%‡
Complete response: 13.8%%‡ 3-month PFS: 67%a Median PFS: 9.1 ma |
Dosing of avelumab in both parts of this trial was by intravenous infusion every two weeks.
Number of subjects in the dose escalation cohort, 18 of whom were included in dose-limiting toxicity analysis set. 53 were then included in the dose-escalation safety analysis set, who were then combined with 33 patients from the dose-expansion part of the same trial to comprise 86 patients assess for pharmacokinetic parameters.
From expanded data from this cohort in patients with ≥ 2 years follow up
In subset of 29 patients with at least 3 months follow up
Of cohort of all 39 treated patients
mMCC, metastatic Merkel cell carcinoma; Mg, milligrams; kg, kilograms; w, weeks; m, months; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; PD-L1, programmed death-ligand 1; MCPyV, Merkel cell polyoma virus
JAVELIN Merkel 200 Trial32−36
JAVELIN Merkel 200 is a pivotal phase 2, prospective, multicenter, open-label, single-arm trial of avelumab in individuals with mMCC. The trial comprises two cohorts: individuals with disease refractory to chemotherapy (Part A), and patients with no prior systemic therapy for mMCC (Part B).
JAVELIN Merkel 200 Part A: Second-Line (or Later) Treatment
Eighty-eight immunocompetent adults (mean: 72.5 years) with refractory mMCC were followed for a median of 10.4 months.32 Primary cutaneous tumors were identified in 76% of participants, though the location of these primary tumors were not reported. Patients were treated with 10 mg/kg of IV avelumab every 2 weeks with a median of 7 doses, and a median treatment duration of 17 weeks.
Overall, the objective response rate (ORR) was 31.8%. Most responses (82%) were observed as early as the first post-treatment assessment (week 7). Almost a third (29%) of patients demonstrated a durable response of at least 6 months. Median progression-free survival (PFS) was 2.7 months, and median overall survival (OS) was 11.3 months. A subsequent analysis determined that early objective response to avelumab is of clinical importance, noting that subjects with objective response by weeks 7–13 had significantly longer overall survival (OS) compared to patients with early nonresponse.33 The proportion of responders with at least 1 year response duration was similar irrespective of MCPyV or PD-L1 status. However, higher ORRs were noted in subjects who had received fewer lines of prior chemotherapy, as well as those with lower disease burden and those with PD-L1 positive tumors. The investigators surmise that these subjects may be more likely to be immunocompetent and therefore demonstrate more robust response to immunotherapy.34
Expanded data from patients with ≥2 y of follow-up (median 29.2 months) demonstrated an ORR of 33%.35,36 Complete response was observed in 11.4% of patients. PFS rate at 1 year, 18 months, and 2 years were stable at 29%, 29%, and 26%, respectively. Median OS was 12.6 months, with a 2-year OS rate of 36%.
JAVELIN Merkel 200 Part B: First-Line Treatment37
Part B assessed the efficacy and safety of avelumab as a first-line treatment in 39 patients (median age, 75 years) with mMCC.37 The primary end point was durable response with at least 6 months duration and secondary endpoints included best overall response, duration of response, PFS, and safety.
Median treatment duration was 12 weeks (range, 2.0–49.9), with median follow-up of 5.1 months (range, 0.3–11.3). PFS at 3 months was 67%, with a median PFS of 9.1 months (range, 1.9-not estimable). Disease progression was observed in 17.9% of subjects, with two deaths noted (5.1%).
SAFETY AND TOLERABILITY
The avelumab clinical trials data have demonstrated an overall acceptable safety and tolerability profile. Unless otherwise specified, safety profiles discussed herein are derived from pooled data from a total of 1738 subjects from Part A of the JAVELIN Merkel 200 study and the entirety of the phase 1 JAVELIN Solid Tumor study,24 which evaluated the safety of avelumab in the treatment of metastatic or locally advanced solid tumors (n = 88 and n=1650, respectively).38 Overall incidence of treatment-related adverse events (TRAEs) of any grade was 67.0%. However, incidence of grade 3 or greater TRAEs was only 10.2%, the most common of which were fatigue (1%), elevated serum lipase (1%), infusion-related reactions (IRRs, 0.6%), and elevated serum gamma‐glutamyl transferase (0.6%). Permanent discontinuation of the drug resulting from TRAEs was uncommon, occurring in 6.2% of patients. This safety profile is similar to safety data reported for first-line pembrolizumab for advanced MCC.39. Additionally, this safety profile is similar to 127 subjects with mMCC (88 subjects in JAVELIN Merkel 200 Part A, 39 in Part B; Table 2) treated with avelumab.
Table 2.
Treatment-related adverse events (TRAEs) in patients with metastatic Merkel cell carcinoma treated with avelumab, as reported in Part A and Part B of the JAVELIN Merkel 200 Trial
| Part A | Part B | Pooled Total | |
|---|---|---|---|
| Number of subjects receiving at least one dose of avelumab | 88 | 39 | 127 |
| TRAEs*, N(%) | |||
| Any TRAE | 62 (70.0) | 28 (71.8) | 90 (70.9) |
| Fatigue | 21 (24.0) | 9 (23.1) | 30 (23.6) |
| Infusion-related reaction | 15 (17.0) | 9 (23.1) | 24 (18.9) |
| Rash†a | 11 (12.5) | 2 (5.1) | 13 (10.2) |
| Nausea | 8 (9.1) | 2 (5.1) | 10 (7.9) |
| Asthenia | 7 (8.0) | 3 (7.7) | 10 (7.9) |
| Diarrhea | 8 (9.1) | 2 (5.1) | 10 (7.9) |
| ALT increase | 3 (3.4) | 3 (7.7) | 6 (4.7) |
| Arthralgia | 4 (4.5) | 2 (5.1) | 6 (4.7) |
| Pruritusa | 4 (4.5) | 2 (5.1) | 6 (4.7) |
| Chills | 3 (3.4) | 2 (5.1) | 5 (3.9) |
| Decreased appetite | 5 (5.7) | 0 (0.0) | 5 (3.9) |
| Blood CPK increase | 2 (2.3) | 2 (5.1) | 4 (3.1) |
| AST increase | 3 (3.4) | 1 (2.6) | 4 (3.1) |
| Dry mouth | 2 (2.3) | 2 (5.1) | 4 (3.1) |
| Dyspnea | 2 (2.3) | 2 (5.1) | 4 (3.1) |
| Pyrexia | 2 (2.3) | 2 (5.1) | 4 (3.1) |
| Dizziness | 3 (3.4) | 0 (0.0) | 3 (2.4) |
| Dry skin | 2 (2.3) | 0 (0.0) | 2 (1.6) |
| Dysgeusia | 2 (2.3) | 0 (0.0) | 2 (1.6) |
| Headache | 2 (2.3) | 0 (0.0) | 2 (1.6) |
| Influenza-like illness | 2 (2.3) | 0 (0.0) | 2 (1.6) |
| Palpitations | 2 (2.3) | 0 (0.0) | 2 (1.6) |
| Lymphopenia | 2 (2.3) | 0 (0.0) | 2 (1.6) |
| Vomiting | 2 (2.3) | 0 (0.0) | 2 (1.6) |
| Decreased weight | 0 (0.0) | 2 (5.1) | 2 (1.6) |
| Eosinophilia | 0 (0.0) | 2 (5.1) | 2 (1.6) |
| Autoimmune nephritis | 0 (0.0) | 1 (2.6) | 1 (0.8) |
| Cholangitis | 0 (0.0) | 1 (2.6) | 1 (0.8) |
| Gait disturbance | 0 (0.0) | 1 (2.6) | 1 (0.8) |
| Paraneoplastic encephalomyelitis | 0 (0.0) | 1 (2.6) | 1 (0.8) |
| Paraneoplastic syndrome | 0 (0.0) | 1 (2.6) | 1 (0.8) |
| Polyneuropathy | 0 (0.0) | 1 (2.6) | 1 (0.8) |
| Troponin increase | 0 (0.0) | 1 (2.6) | 1 (0.8) |
| Blood cholesterol increased | 1 (1.1) | 0 (0.0) | 1 (0.8) |
| TRAEs leading to discontinuation of therapy | 2 (2.3) | 6 (15.4) | 8 (6.3) |
Includes adverse events of any grade
Includes both non-specified rash and maculopapular rash
One case of pruritus and two cases of maculopapular rash were additionally classified as immune-related adverse events in Part B.
ALT, alanine aminotransferase; CPK, creatine phosphokinase; AST aspartate aminotransferase.
Infusion-Related Reactions
IRR such as pyrexia, hypersensitivity, or dyspnea of any grade occurred in 25.3% of patients, most commonly at the time of first infusion. Two percent of patients required treatment discontinuation due to IRRs. As such, premedication with acetaminophen and diphenhydramine is recommended before administration of the first four doses.29,30,40 Grade 1 and 2 IRRs warrant slowing or interruption of the infusion, and treatment should be permanently discontinued in cases of grade 3 and 4 IRRs.
Immune-Related Adverse Events
ICIs have been associated with immune-related adverse events (IRAEs).41 Overall incidence of IRAEs with avelumab was 14.2%. Grade 3 or greater IRAEs were experienced by 2.2% of subjects, most commonly thyroid disorder (5.6%) and rash (5.2%).30 IRAE were considered serious in 2.5% of patients. Treatment with a systemic corticosteroid was required in 44.1% of IRAEs. Life-threatening immune events, though rare, have been described with avelumab, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, myocarditis, myositis, and Guillain-Barré Syndrome.29,30
Death Associated with Avelumab
TRAEs were considered to be the primary cause of death in 0.2% of patients treated with avelumab. These cases included (1) autoimmune hepatitis, ascites, and peritoneal metastases in an individual with gastric cancer, (2) liver metastases and acute liver failure in a patient with metastatic breast cancer, (3) respiratory distress in another individual with metastatic breast cancer, and (4) treatment-related pneumonitis in a subject with urothelial carcinoma. Of note, none of the treatment fatalities occurred in patients with mMCC.
Ocular and Periocular Adverse Events
Ocular side effects of ICIs are typically less common than systemic adverse events (AEs), and most frequently include dry eye (1–24%) and uveitis (1%).42 Warner et al. report new onset dry eye in four of eight patients treated with avelumab for visceral neoplasms.43 One of these four patients experienced severe dry eye while the other 3 were reported as mild dry eye. Uveitis has not been reported in association with avelumab therapy to date, though it is a known immune-related adverse event that can occur with other PD-1/PD-L1 checkpoint inhibitors.42,44 The only periocular AE reported in avelumab treated mMCC patients was an unspecified grade 3 eyelid function disorder classified as a treatment-emergent adverse event.32
PATIENT-CENTERED PERSPECTIVES
The psychological implications of the management of patients with a rare, aggressive, and potentially disfiguring or life-threatening disease such as MCC is clinically significant. Controlling for physical symptomatology, patients with advanced malignancies appear to experience less psychological morbidity when treated with immunotherapy as compared to chemotherapy.45 Similarly chemorefractive mMCC patients treated with avelumab reported a clinically-relevant improvement in health-related quality of life, and a perceived comparatively better experience with avelumab than chemotherapy.46,50 These relatively better psychological experiences may derive from lower toxicity, disease-related morbidity, and improved functional and survival outcomes with avelumab.
OTHER IMMUNE CHECKPOINT INHIBITORS FOR ADVANCED MCC
In addition to avelumab, two other ICIs – pembrolizumab and nivolumab (Opdivo®, Bristol‐Myers Squibb, New York, NY, USA and Ono Pharmaceuticals, Trenton, NJ, USA) – have been explored in clinical trials as treatment for advanced MCC.
Pembrolizumab, an anti-PD-1 antibody, was FDA approved for adult and pediatric patients with mMCC or recurrent locoregional disease. In a non-controlled phase 2 trial of 26 individuals (median age, 68 years) with advanced MCC, first-line pembrolizumab demonstrated an ORR of 56%.36 A higher response rate was noted in MCPyV-positive compared to MCPyV-negative tumors (62% versus 44%, respectively). TRAEs, most commonly fatigue and laboratory abnormalities, were observed in 77% of individuals. Grade 3 or 4 adverse events occurred in 15% of individuals, and these were managed by discontinuation of pembrolizumab and initiation of corticosteroid therapy when appropriate. Additionally, pembrolizumab has been reported in the treatment of a metastatic MCC to the orbit with a dramatic response to combined pembrolizumab and XRT.51
Nivolumab, an anti-PD-1 pathway inhibitor, has also shown durable responses in advanced MCC.52–54 Preliminary analyses from a non-comparative, open-label, phase 1/2 trial of 25 patients with advanced MCC (median follow up, 51 weeks) demonstrated an ORR of 64%. The neoadjuvant use of nivolumab has also been reported effective in advanced MCC. Topalian et al.,54 report substantial pathologic and radiologic tumor regression after a 4-week course prior to surgery.
In 2018, the National Comprehensive Cancer Network listed avelumab, pembrolizumab, and nivolumab as preferred first-line therapies for metastatic or unresectable MCC.55 To date, no direct comparisons of the efficacy and safety of avelumab, pembrolizumab, or nivolumab for the treatment of advanced MCC have been reported though efficacy data and safety profiles appear similar.
CONCLUSIONS
Avelumab is approved for the treatment of mMCC and represents a promising immunotherapeutic agent for the treatment of advanced MCC. Studies have shown significantly longer response duration, with some responses continuing at up to 2 years, when compared to standard chemotherapy. The clinical activity of avelumab does not appear to substantially differ based on tumor PD-L1 or MCPyV status, though additional studies are needed. Improved health-related quality of life and enhanced psychological outcomes compared to those experienced with chemotherapy may add to avelumab’s perceived benefits.
Pembrolizumab39,,56 and nivolumab52–54 have demonstrated efficacy in the treatment of advanced MCC though no reported cases specifically assess their use for advanced eyelid or periocular lesions. While no clinical trials to date have studied avelumab for advanced MCC or periocular MCC, the efficacy and safety of trials on avelumab for mMCC and pembrolizumab for advanced MCC provide rationale for the use of avelumab for the treatment of advanced or metastatic periocular MCC. Further investigation of the efficacy, safety and tolerability of avelumab for use in metastatic cases with eyelid or periocular involvement or for primary advanced eyelid and orbital lesions is warranted.
Figure 1. Merkel cell carcinoma of the upper eyelid.
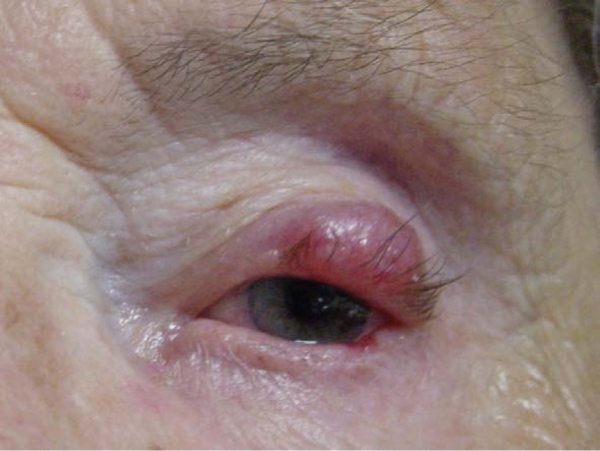
The mass often presents as a violaceous nodule that can masquerade as a chalazion, cyst, or basal cell carcinoma. The large lesion depicted here has induced a mechanical ptosis.
Reproduced, with permission, from DermNet New Zealand (www.dermnetnz.org), following licensing requirements from Creative Commons Attribution-NonCommercial-NoDerivs 3.0, New Zealand (https://creativecommons.org/licenses/by-nc-nd/3.0/nz/legalcode).
Acknowledgments
Sources of Support: This work was supported in part by: NIH Center Core Grant P30EY026877; Research to Prevent Blindness Unrestricted Grant, Inc, New York. The sponsor or funding organization had no role in the writing of this manuscript.
Abbreviations:
- ADCC
antibody-dependent cell-mediated cytotoxicity
- AE
adverse event
- CI
confidence interval
- Fc
crystallizable fragment
- ICI
immune-checkpoint inhibitor
- FDA
Food and Drug Administration
- IgG1
immunoglobulin G1
- IRAE
immune-related adverse event
- IRR
infusion-related reaction
- MCC
Merkel cell carcinoma
- mMCC
metastatic Merkel cell carcinoma
- MCPyV
Merkel cell polyoma virus
- Nk
Natural killer
- ORR
objective response rate
- OS
overall survival
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- TRAE
treatment-related adverse event
- WLE
wide local excision
REFERENCES
- 1).Xue Y, Thakuria M. Merkel cell carcinoma review. Hematol Oncol Clin North Am 2019;33:39–52. [DOI] [PubMed] [Google Scholar]
- 2).Tetzlaff MT, Nagarajan P. Update on Merkel cell carcinoma. Head Neck Pathol 2018;12:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Lyhne D, Lock-Andersen J, Dahlstrøm K, et al. Rising incidence of Merkel cell carcinoma. J Plast Surg Hand Surg 2011;45:274–280. [DOI] [PubMed] [Google Scholar]
- 4).Kieny A, Cribier B, Meyer N, et al. Epidemiology of Merkel cell carcinoma. A population-based study from 1985 to 2013, in northeastern of France. Int J Cancer 2018;144:741–745. [DOI] [PubMed] [Google Scholar]
- 5).Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. JAMA Dermatol 2014;150: 864–872. [DOI] [PubMed] [Google Scholar]
- 6).Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg 2013;39:232–238. [DOI] [PubMed] [Google Scholar]
- 7).Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol 2013;133:642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 2013;1:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Pan D, Narayan D, Ariyan S. Merkel cell carcinoma: five case reports using sentinel lymph node biopsy and a review of 110 new cases. Plast Reconstr Surg 2002;110:1259–1265. [DOI] [PubMed] [Google Scholar]
- 10).Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev 1999;8:153–158. [PubMed] [Google Scholar]
- 11).Kivelä T, Tarkkanen A. The Merkel cell and associated neoplasms in the eyelids and periocular region. Surv Ophthalmol 1990;35:171–87. [DOI] [PubMed] [Google Scholar]
- 12).Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 2010;63:751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Rice RD Jr, Chonkich GD, et al. Merkel cell tumor of the head and neck. Five new cases with literature review. Arch Otolaryngol Head Neck Surg 1993;119:782–6. [DOI] [PubMed] [Google Scholar]
- 14).Smith VA, Camp ER, Lentsch EJ. Merkel cell carcinoma: identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryngoscope 2012;122:1283–90. [DOI] [PubMed] [Google Scholar]
- 15).Merritt H, Sniegowski MC, Esmaeli B. Merkel cell carcinoma of the eyelid and periocular region. Cancers (Basel) 2014;6:1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Herbert HM, Sun MT, Selva D, et al. Merkel Cell Carcinoma of the eyelid: Management and prognosis. JAMA Ophthalmol 2014;132:197–204. [DOI] [PubMed] [Google Scholar]
- 17).Toto V, Colapietra A, Alessandri-Bonetti M, et al. Upper eyelid Merkel cell carcinoma treated with neoadjuvant chemotherapy and surgical excision. Arch Craniofac Surg 2019;20:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med 2016;5:2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Tai PT, Yu E, Winquist E, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol 2000;18:2493–9. [DOI] [PubMed] [Google Scholar]
- 20).Bavencio (avelumab) injection [package insert]. Darmstadt, Germany: Merck KGaA; 2017.
- 21).Pfizer. European Commission approves Bavencio (avelumab) for metastatic Merkel cell carcinoma [Pfizer web site]. September 21, 2017. Available at: http://www.pfizer.com/news/press-release/press-release-detail/european_commission_approves_bavencio_avelumab_for_metastatic_merkel_cell_carcinoma
- 22).Pfizer. Bavencio (avelumab) approved for Merkel cell carcinoma in Japan [Pfizer web site]. September 21, 2017. Available at: http://www.pfizer.com/news/press-release/press-release-detail/bavencio_avelumab_approved_for_merkel_cell_carcinoma_in_japan
- 23).US Food and Drug Administration. FDA approves pembrolizumab for Merkel cell carcinoma [US FDA web site]. December 19, 2018. Available at: http://www.fda.gov/drugs/fda-approves-pembrolizumab-merkel-cell-carcinoma
- 24).Chin K, Chand VK, Nuyten DSA. Avelumab: clinical trial innovation and collaboration to advance anti-PD-L1 immunotherapy. Ann Oncol 2017;28:1658–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Heery CR, O’Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN solid tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18:587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Boyerinas B, Jochems C, Fantini M. et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015;3:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Fujii R, Friedman ER, Richards J, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget 2016;7:33498–33511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).European Medicines Agency. Bavencio (avelumab): summary of product characteristics [European Medicine Agencies web site]. 2018. Available at: https://www.ema.europa.eu/en/documents/product-information/bavencio-epar-product-information_en.pdf
- 30).US Food and Drug Administration. Bavencio® (avelumab): Highlights of prescribing information [US FDA web site]. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761049s003lbl.pdf
- 31).European Medicines Agency. European public assessment report: Bavencio (avelumab) [European Medicine Agencies web site]. 2017. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004338/WC500236649.pdf
- 32).Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).D’Angelo SP, Hunger M, Brohl AS, et al. Early objective response to avelumab treatment is associated with improved overall survival in patients with metastatic Merkel cell carcinoma. Cancer Immunol Immunother 2019;68:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).D’Angelo SP, Russell JS, Bhatia S, et al. 18-month efficacy and safety update from JAVELIN Merkel 200 part A: a phase II study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy [abstract no. 192]. J Clin Oncol 2018;36(Suppl 5S). [Google Scholar]
- 36).Nghiem P, Bhatia S, Scott Brohl A, et al. Two-year efficacy and safety update from JAVELIN Merkel 200 part A: a registrational study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy [abstract no. 9507]. J Clin Oncol 2018;36(Suppl):15. [Google Scholar]
- 37).D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol 2018;4:e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 2018;124:2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 2016;374:2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Shirley M Avelumab: A review in metastatic Merkel cell carcinoma. Target Oncol 2018;13:409–416. [DOI] [PubMed] [Google Scholar]
- 41).Wang P-F, Chen Y, Song S-Y, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol 2017;8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Dalvin LA, Shields CL, Orloff M, et al. Checkpoint inhibitor immune therapy: Systemic indications and ophthalmic side effects. Retina 2018;38:1063–1078. [DOI] [PubMed] [Google Scholar]
- 43).Warner BM, Baer AN, Lipson EJ, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 2019. pii:theoncologist.2018–0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).EMD Serono Inc. Bavencio (Avelumab) injection, for intravenous use [prescribing information]. 2017. Rockland, MD: EMD Serono, Inc and Pfizer, Inc. [Google Scholar]
- 45).McFarland DC. New lung cancer treatments (immunotherapy and targeted therapies) and their associations with depression and other psychological side effects as compared to chemotherapy. Gen Hosp Psychiatry 2019. pii:S0163–8343(18)30503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Bharmal M, Guillemin I, Marrel A, et al. How to address the challenges of evaluating treatment benefits-risks in rare diseases? A convergent mixed methods approach applied within a Merkel cell carcinoma phase 2 clinical trial. Orphanet J Rare Dis 2018;13:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Bharmal M, Marrel A, Hennessy M, et al. Comparative effectiveness of avelumab versus chemotherapy in Merkel cell carcinoma: innovative use of patient insights. J Comp Eff Res 2018;7:881–890. [DOI] [PubMed] [Google Scholar]
- 51).Cugley DR, Roberts-Thomson SJ, McNab AA. Biopsy-proven metastatic merkel cell carcinoma to the orbit: Case report and review of literature. Ophthalmic Plast Reconstr Surg 2018;34:e86–e88. [DOI] [PubMed] [Google Scholar]
- 52).Walocko FM, Scheier BY, Harms PW, et al. Metastatic Merkel cell carcinoma response to nivolumab. J Immunother Cancer 2016;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Topalian SL, Bhatia S, Hollebecque A, et al. Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC) [Abstract CT074]. Am Assoc Cancer Res 2017;77(suppl):13. [Google Scholar]
- 54).Topalian SL, Bhatia S, Kudchadkar RR, et al. Nivolumab (Nivo) as neoadjuvant therapy in patients with resectable Merkel cell carcinoma (MCC) in CheckMate 358 [Abstract 9505]. J Clin Oncol 2018;36(suppl):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Bichakjian CK, Olencki T, Aasi SZ, et al. Merkel cell carcinoma, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:742–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Nghiem P, Bhatia S, Lipson EJ, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol 2019;37:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]


