Abstract
We report a case of fatal immune checkpoint inhibitor (ICI) associated myocarditis in a 77-year-old male with metastatic Non-Small Cell Lung Cancer (NSCLC) who presented for mediport placement at our outpatient surgical center. He denied any cardiac complaints and had a previously normal electrocardiogram (EKG) off treatment. Intraoperatively and postoperatively, he displayed cardiac rhythm abnormalities. The patient was then transferred to a tertiary facility where he expired within 48 hours. As cancer immunotherapy becomes increasingly prominent, ICI-associated myocarditis should be considered a potentially critical contributor to perioperative cardiac morbidity and mortality.
Introduction
Immune checkpoint inhibitors (ICI), such as ipilimumab, nivolumab, and pembrolizumab, have shown great promise in the oncological care of melanoma, bladder, and lung cancer.1,2 Despite their success, there are a growing number of cases reporting ICI-associated myocarditis due to inflammatory reactions caused by suppression of immune system regulators. These cases range from low grade and manageable immune-related adverse events (irAE) to rapid onset of profound hemodynamic compromise progressing to death.3 Data regarding ICI-associated myocarditis are limited. As a result, a standardized definition to enable consistent and reliable diagnosis is lacking.4
We describe a single center’s experience with an ambulatory patient in the perioperative setting who was ultimately diagnosed with ICI-associated myocarditis. This manuscript adheres to the applicable Case Report Guidelines (CARE) guidelines of the Enhancing the Quality and Transparency of Health Research (EQUATOR) network. Written HIPAA permission was obtained from the deceased patient’s relative for submission of the clinical case report for potential publication.
Case Description
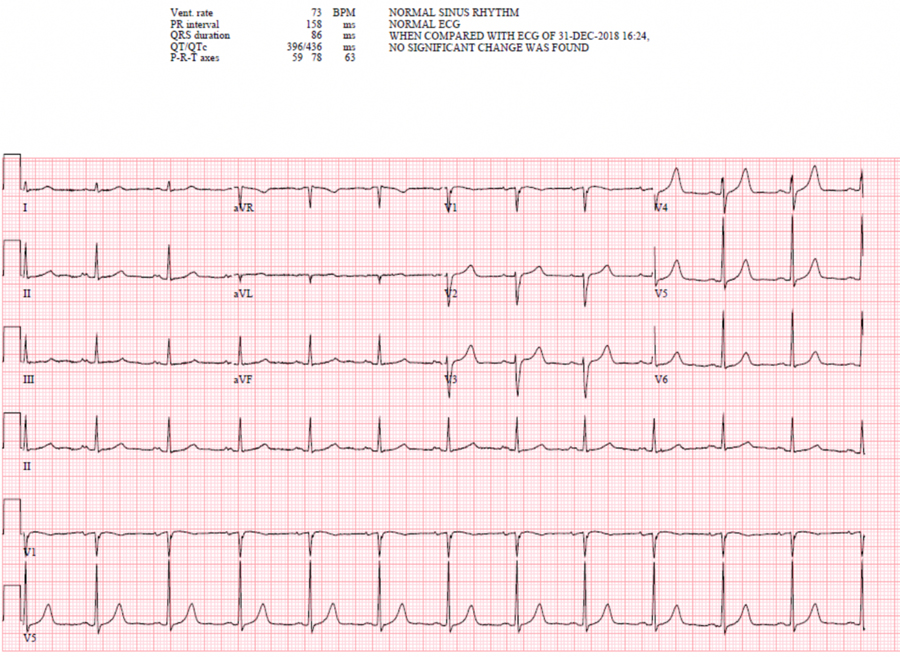
A 77-year-old male American Society of Anesthesiologists (ASA) class 3 with history of metastatic Epidermal Growth Factor Receptor positive (EGFR+) Non-Small Cell Lung Cancer (NSCLC), hypertension (HTN), hyperlipidemia, benign prostatic hyperplasia (BPH), and deep vein thrombosis (DVT), currently undergoing chemotherapy treatment, presented for mediport placement under monitored anesthesia care (MAC) at our freestanding outpatient cancer surgical center. Chemotherapy management for lung cancer included two cycles of carboplatin, pemetrexed, and pembrolizumab, an ICI which was begun two months prior to surgical presentation. During preoperative evaluation, the patient denied any cardiac history or complaints, and reported that he could climb one flight of stairs without stopping. He had an unremarkable electrocardiogram (EKG) before initiation of ICI and chemotherapy treatment three months prior (Figure 1).
Figure 1.
Preoperative electrocardiogram (EKG) from 3 months prior displaying normal sinus rhythm
Before starting sedation, tachycardia with a heart rate in the 110’s, frequent premature atrial contractions (PACs) and premature ventricular contractions (PVCs) were noted on the anesthesia operating room monitor. As a result, the anesthesia team contacted cardiology to evaluate the intraoperative 5-lead EKG and confirmed the presence of PACs and PVCs. MAC anesthesia with an intravenous (IV) propofol bolus of 30 mg followed by an IV infusion of 140 mcg/kg/min was begun. After 20 minutes, the propofol infusion was discontinued for a total of 230 mcg. Cardiology was consulted again to check the ST wave morphology and confirmed that there were no changes. The patient was awake at this time and denied any chest pain or shortness of breath. MAC anesthesia was resumed but with 2mg of IV versed and 50 mcg of IV fentanyl and the procedure was started. During the case, a total of 30 mg of esmolol and 240 mcg of phenylephrine were administered for management of blood pressure and tachycardia.
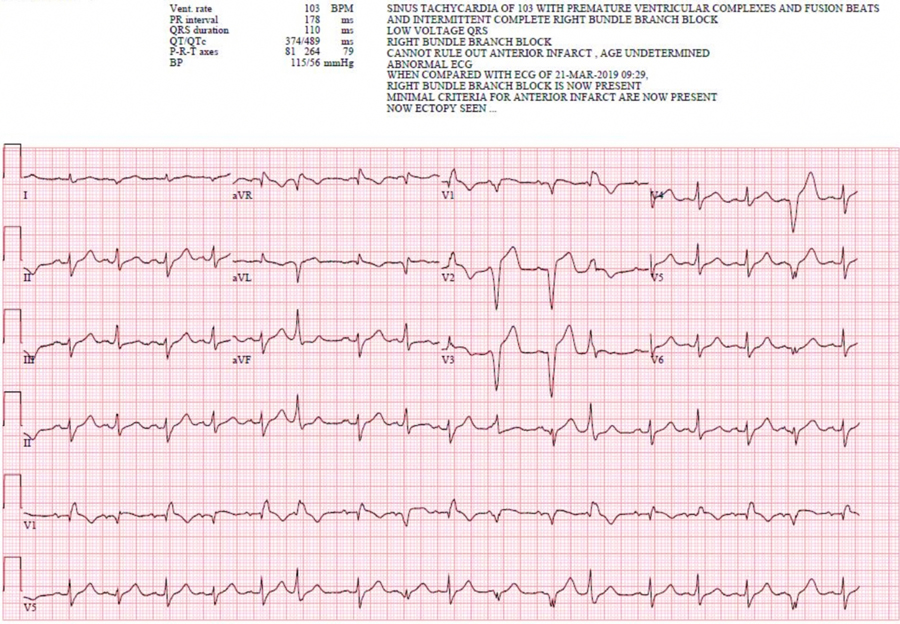
In the post-anesthesia care unit (PACU), the patient was hemodynamically stable and denied any cardiac complaints. A 12-Lead EKG was performed which displayed fusion beats and an intermittent right bundle branch block (Figure 2). The patient was then transferred to a tertiary facility at the recommendation of the cardiology service.
Figure 2.
Post-anesthesia care unit (PACU) EKG displaying fusion beats and intermittent right bundle branch block
Upon arrival in the Emergency Department (ED), the patient continued to remain asymptomatic. A 12-Lead EKG displayed sinus rhythm with mild ST-elevation in the anteroseptal leads. The patient’s initial troponin was reported at 37. The differential diagnosis at this time included acute coronary syndrome, pulmonary embolism and myocarditis. The patient subsequently received a computed tomography (CT) angiogram which did not show evidence of a pulmonary embolism. During his ED course, the patient had sinus tachycardia with downward trending blood pressures and a repeat troponin of 45. Repeat EKG displayed intraventricular conduction delay with more pronounced anteroseptal ST-elevation. Bedside echocardiogram showed left ventricle (LV) wall motion abnormality with LV systolic function between 45–50% and underfilling of the LV. The patient urgently underwent a cardiac catherization which displayed only mild systolic hypertension with no significant coronary artery disease.
The patient was admitted to the cardiac care unit where his hospital course was complicated by frequent runs of ventricular tachycardia (VT) and persistently elevated troponins ranging from 41 to 45. A diagnosis of ICI-associated myocarditis was made based on the lack of pulmonary embolus on CT, unremarkable cardiac catheterization, abnormal EKGs, elevated troponin levels and a clinical history of ICI treatment. Medical management with methylprednisolone and amiodarone was initiated, however, the patient continued to clinically deteriorate. Milrinone was begun in response to a repeat echocardiogram that displayed global akinesis with gross reduction of ejection fraction. Despite all treatments, the patient quickly developed biventricular heart failure and unstable VT with a blood pressure of 50/40 mmHg. Synchronized cardioversion was performed with sedation resulting in a return of sinus rhythm. Following a discussion with the family, a do-not-resuscitate (DNR) order was placed. Quickly thereafter, the patient developed pulseless VT and was pronounced dead less than 48 hours following admission.
Discussion
The use of ICIs has revolutionized cancer care and their oncological use continues to grow in scope and prevalence. In this case, we discuss a patient with NSCLC treated with pembrolizumab, an ICI that blocks the programed cell death protein 1 (PD-1) receptor expressed on cancer cells. By downregulating the function of anti-tumor T-cells, the PD-1 receptor enables cancer proliferation. As a result, PD-1 receptor antagonization with ICI therapy restores the anti-tumor immune response.1,5 However, this selected targeting of PD-1 receptor expression does not solely affect the cancerous cells. Consequently, it is not uncommon for patients undergoing immunotherapy to experience an irAE, yet such events as they pertain to cardiac myocytes are associated with the greatest mortality.6 The inhibition of PD-1 is believed to contribute to ICI-associated myocarditis as numerous studies have expanded upon the cardio-protective role of PD-1 in preventing auto-immune mediated damage targeting cardiac myocytes.7,8 Nevertheless, the pathophysiological mechanisms of cardiotoxicity related to immunotherapy remains unclear and further research is needed.
Reports estimate an ICI-associated myocarditis incidence rate of 0.1–1%, yet true incidence is likely underreported as a result of nonspecific diagnostic testing, variable clinical presentation and lack of awareness.4,9 Such challenges contribute to the difficulty of screening and identifying patients at risk for developing this irAE. Despite these challenges, it has been reported that patients with ICI-associated myocarditis have fatality rates between 25–50%.9 For this reason, early recognition and diagnosis is crucial to reverse the potentially fatal events caused by cardiotoxicity.
Most cases report the presentation of adverse cardiac related events roughly 3–6 months following treatment, yet delayed immune effects have been reported as late as 2 years after treatment.10,11 Patients that present with ICI-associated myocarditis may have cardiac complaints, however, they may be vague and nonspecific as evident in this case report.6,12 While presenting symptoms related to ICI-associated myocarditis are variable, a recent study has reported that nearly 25% of patients may have symptoms of myositis which includes muscle weakness, elevated creatine kinase levels, and ptosis.6,9 A diagnosis of ICI-associated myositis therefore warrants a cardiac evaluation as patients are not regularly screened for myocarditis while on ICI treatment.6 If cardiotoxicity is suspected, EKG, troponin measurements, and echocardiography are suggested to obtain important diagnostic information. A study of 35 patients with ICI-associated myocarditis reported that 89% presented with abnormal EKG findings, which included tachycardia, ST segment abnormalities, and QT prolongation amongst others. Ninety four percent had an elevated troponin, 51% had a preserved left ventricular ejection fraction (LVEF) on echocardiography, while 35% had severe LV dysfunction.12
Once ICI-associated myocarditis has been identified, studies recommend prompt discontinuation of ICI therapies, initiation of corticosteroids and immunosuppression to manage the toxicity.9,13 However, as a result of the low incidence rates of ICI-associated myocarditis, there remains a lack of pertinent literature available to guide appropriate therapy. It is suggested for all patients with ICI-associated myocarditis that ICI therapy be discontinued and steroid therapy initiated with the cardiac stability and response to treatment dictating further management.9 A systematic review of immunosuppressive therapies such as infliximab and intravenous immunoglobin utilized in 12 patients with ICI-associated myocarditis resulted in recovery for 9 patients, yet further research into such therapy is warranted.14
This case highlights how nonspecific EKG abnormalities in an asymptomatic patient presenting for mediport placement prompted further evaluation. Despite the patient’s clinical deterioration following mediport placement, we do not believe that the anesthetic exacerbated the myocarditis. Cardiologists at our cancer institution concluded that this patient would have likely decompensated due to the ICI-associated myocarditis regardless of this scheduled procedure and anesthetic. Nevertheless, it is suggested that any type of cardiac abnormality noted perioperatively in a patient recently started on an ICI be evaluated further due to the high case fatality rate related to myocarditis.
As leaders in perioperative care, anesthesiologists play a critical role in recognizing cardiac abnormalities prior to any surgical procedure. The need for greater awareness of ICI-associated myocarditis from anesthesia teams during preoperative evaluation will become more critical as cases of immunotherapy for cancer treatment in conjunction with surgical management become increasingly prevalent. This report contributes to the dissemination of information related to ICI-associated myocarditis beyond the specialties of cardiology and oncology and can assist in the development of evidence-based protocols to achieve prompt diagnosis and treatment of this lethal irAE.
Acknowledgements
The authors thank Rebecca Twersky MD MPH and Joanna Serafin PhD from the Department of Anesthesiology and Critical Care at Memorial Sloan Kettering Cancer Center New York, New York for their insights and edits pertaining to this manuscript.
Funding: The authors’ work was supported and funded in part by the National Institutes of Health/NCI Cancer Center Support Grant P30-CA008748.
Glossary of Terms
- ASA
American Society of Anesthesiologists
- BPH
Benign Prostatic Hyperplasia
- CT
Computed Tomography
- DNR
Do-Not-Resuscitate
- DVT
Deep Vein Thrombosis
- ED
Emergency Department
- EGFR +
Epidermal Growth Factor Receptor positive
- EKG
Electrocardiogram
- HTN
Hypertension
- ICI
Immune Checkpoint Inhibitor
- irAE
Immune-Related Adverse Event
- IV
Intravenous
- LV
Left Ventricle
- LVEF
Left Ventricular Ejection Fraction
- MAC
Monitored Anesthesia Care
- NSCLC
Non-Small Cell Lung Cancer
- PAC
Premature Atrial Contraction
- PACU
Post-Anesthesia Care Unit
- PD-1
Programed Cell Death Protein 1
- PVC
Premature Ventricular Contraction
- VT
Ventricular Tachycardia
Footnotes
Conflicts of interest: None
References
- 1.Cho JH. Immunotherapy for non-small-cell lung cancer: Current status and future obstacles. Immune Netw 2017. 17(6):378–391. doi: 10.4110/in.2017.17.6.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015. 161(2):205–14. doi: 10.1016/j.cell.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajiri K, Aonuma K, Sekine I. Immune checkpoint inhibitor-related myocarditis. Jpn J Clin Oncol 2018. 48(1):7–12. doi: 10.1093/jjco/hyx154 [DOI] [PubMed] [Google Scholar]
- 4.Neilan TG, Rothenberg ML, Amiri-Kordestani L, et al. Myocarditis Associated with Immune Checkpoint Inhibitors: An Expert Consensus on Data Gaps and a Call to Action. Oncologist. 2018. 23(8):874–878. doi: 10.1634/theoncologist.2018-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: The beginning of the end of cancer? BMC Med 2016. 14:73. doi: 10.1186/s12916-016-0623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anquetil C, Salem JE, Lebrun-Vignes B, et al. Immune checkpoint inhibitor–associated myositis: Expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation. 2018. 138:743–745. doi: 10.1161/CIRCULATIONAHA.118.035898 [DOI] [PubMed] [Google Scholar]
- 7.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001. 291(5502):319–22. doi: 10.1126/science.291.5502.319 [DOI] [PubMed] [Google Scholar]
- 8.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000. 192(7):1027–34. doi: 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Jones-O’Connor M, Awadalla M, et al. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr Treat Options Cardiovasc Med 2019. 21(7):32. doi: 10.1007/s11936-019-0731-6 [DOI] [PubMed] [Google Scholar]
- 10.Weber JS, Dummer R, De Pril V, Lebbé C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013. 119(9): 1675–82. doi: 10.1002/cncr.27969 [DOI] [PubMed] [Google Scholar]
- 11.Nishino M, Sholl LM, Hodi FS. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 2015. 373(3):288–90. doi: 10.1056/NEJMc1505197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood SS, Fradley MG, Cohen JV., et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018. 71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015. 3:11. doi: 10.1186/s40425-015-0057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mir H, Alhussein M, Alrashidi S, et al. Cardiac Complications Associated With Checkpoint Inhibition: A Systematic Review of the Literature in an Important Emerging Area. Can J Cardiol 2018. 34(8):1059–1068. doi: 10.1016/j.cjca.2018.03.012 [DOI] [PubMed] [Google Scholar]