Abstract
Background:
The National Comprehensive Cancer Network and American Society of Clinical Oncology recommend consideration of the use of echocardiography 6-12 months after completion of anthracycline-based chemotherapy in at risk populations. Assessment of brain natriuretic peptide (BNP) has also been suggested by the American College of Cardiology/American Heart Association/Heart Failure Society of America for the identification of Stage A (at risk) heart failure (HF) patients. The real-world frequency of the use of these tests in patients after receipt of anthracycline therapy, however, has not been studied previously.
Methods and Results:
In this retrospective study, using administrative claims data from the OptumLabs Data Warehouse, we identified 31,447 breast cancer and lymphoma patients (age ≥ 18 years) who were treated with an anthracycline in the United States between January 1, 2008 and January 31, 2018. Continuous medical and pharmacy coverage was required for at least 6 months prior to the initial anthracycline dose and 12 months after the final dose. Only 36.1% of patients had any type of cardiac surveillance (echocardiography, BNP, or cardiac imaging) in the year following completion of anthracycline therapy (29.7% echocardiography). Surveillance rate increased from 37.5% in 2008 to 42.7% in 2018 (25.6% in 2008 to 40.5% echocardiography in 2018). Lymphoma patients had a lower likelihood of any surveillance compared to breast cancer patients (OR 0.79, 95% CI 0.74-0.85, p<0.001). Patients with pre-existing diagnoses of coronary artery disease and arrhythmia had the highest likelihood of cardiac surveillance (OR 1.54, 95% CI 1.39-1.69 and OR 1.42, 95% CI 1.31-1.53, p<0.001 for both), though no single comorbidity was associated with a >50% rate of surveillance.
Conclusions:
The majority of survivors of breast cancer and lymphoma who have received anthracycline-based chemotherapy do not undergo cardiac surveillance after treatment, including those with a history of cardiovascular co-morbidities such as heart failure.
Keywords: breast cancer, lymphoma, cardio-oncology, survivorship, health care use, outpatient, toxicity
Introduction
Anthracyclines are administered as part of curative-intent therapy for many patients with breast cancer and lymphoma. Importantly, this class of drugs carries a black box warning for cardiotoxicity. Algorithms for surveillance of cardiac function during anthracycline therapy were developed in the era of cardiac function assessment by multigated (MUGA) radionucleotide scans and were shown to improve heart failure (HF)-free survival (albeit with higher doses of anthracyclines than are usually given today).1 In October 2014, the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) released a consensus document for adult patients during and after cancer therapy,2 recommending echocardiogram with strain at completion of therapy and 6 months later. Other types of surveillance (including magnetic resonance imaging (MRI) and MUGA) are not preferred, but may be used in certain circumstances.
Since February 2015, the National Comprehensive Cancer Network (NCCN) Survivorship Guidelines have recommended echocardiography to identify asymptomatic cardiac dysfunction within 1 year of completion of anthracycline therapy for cancer patients at increased risk of cardiac dysfunction (including those with age >65, hypertension, diabetes, obesity, and history of other cardiovascular diseases).3 A similar recommendation is included in the 2017 American Society of Clinical Oncology (ASCO) Clinical Practice Guideline on Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers, published in December 2016.4 Neither ASCO nor NCCN suggests assessments after the one-year point for those who had normal results on the first test. While not included in the NCCN and ASCO guidelines, the 2017 update of the American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) heart failure (HF) guidelines, released in April 2017, included a class IIa recommendation for B-type natriuretic peptide (BNP) assessment for patients at risk of developing cardiac dysfunction (Stage A HF) in general.5 While this conceptually includes patients exposed to anthracyclines, this recommendation is based on the results of one single trial, the STOP-HF trial, which randomized patients >40 years of age and at least one of the following: hypertension, hyperlipidemia, diabetes, vascular disease, arrhythmia, or obesity to routine care without or with annual BNP testing and echocardiography and cardiology care in case of abnormal values.6 Patients in the BNP-direct care group had improved outcomes including lower rates of left ventricular dysfunction, HF and major adverse cardiac events. Of note, patients with anthracycline exposure were not included in this trial.
Until now, it has been unclear how providers in the U.S. apply the recommendations endorsed by cardiology and oncology societies pertaining to cardiac surveillance after receipt of anthracycline. The current study was designed to investigate the real-world use of cardiac function imaging and/or BNP testing after anthracycline therapy among survivors of breast cancer and lymphoma in the U.S.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We used de-identified administrative claims data from the OptumLabs® Data Warehouse (OLDW),7which includes medical and pharmacy claims, laboratory results and enrollment records for commercial and Medicare Advantage (MA) enrollees. The database contains longitudinal health information on enrollees and patients, representing a diverse mixture of ages, ethnicities and geographical regions across the United States. Adult patients (age ≥ 18 years) with breast cancer or lymphoma who were treated with an anthracycline between January 1, 2008 and January 31, 2018 were identified using Current Procedural Technology/Healthcare Common Procedure Coding System codes.5 Patients with both, breast cancer and lymphoma during the study timeframe, were excluded. Continuous medical and pharmacy coverage was required for at least 6-months prior to the initial anthracycline dose (baseline) and 12 months after the final dose. This study was exempt from Institutional Review Board approval (it used a pre-existing, de-identified data set).
Covariates
We assessed demographic and clinical characteristics at baseline, including age (35-44 years, 45-54 years, 55-64 years and 65+ years), sex, race/ethnicity (Asian, Black, Hispanic, non-Hispanic white), region (Midwest, Northeast, South, West, Unknown) health plan type (Commercial, Medicare Advantage), year of last anthracycline dose, cancer type (breast cancer, lymphoma), and baseline cardiac comorbidities. Comorbidities (HF, hypertension, diabetes, cardiac arrhythmias, valvular disease, obesity, renal failure, pulmonary circulation disorder, peripheral vascular disease and CAD) previously defined Elixhauser algorithms were identified using both primary and secondary diagnoses in the 6 months prior to the initial anthracycline dose.8
Outcomes
The primary outcomes were the proportion of patients who had an echocardiogram, BNP, and/or cardiac imaging during the year after the final dose of anthracycline. Secondary outcomes were the proportion of patients who had these during each of the four subsequent years (for those who remained covered for each full year).
Statistical Analysis
The baseline patient characteristics were described by frequencies (percentages) and means (standard deviation) for each surveillance type (No surveillance, echo only, BNP only, CV imaging only and combination of surveillance types). Multivariable logistic regression was used to separately predict the provability of echocardiography, BNP testing, cardiac imaging and any cardiovascular surveillance in the first year after chemotherapy completion. Independent variables included baseline patient and clinical characteristics (age, sex, race/ethnicity, region, health plan, year of last anthracycline dose, cancer type and comorbidities. All comorbidities were chosen due to their relevance for prognosis, potential impact on surveillance rates, and as risk factors for cardiovascular diseases and in particular heart failure. Results were reported as odds ratios (OR) and 95% confidence intervals (CIs). All statistical analyses were performed using SAS® version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Patient population
The cohort included 31,447 patients with breast cancer or lymphoma who were treated with an anthracycline between January 1, 2008 and January 31, 2018. Demographic and clinical characteristics are presented in Table 1.
Table 1.
Characteristics of the study cohort
| Total (N=31447) |
|
|---|---|
| Age (years) | |
| Mean (SD) | 53.4 (12.7) |
| Median | 54 |
| Q1, Q3 | 45.0, 62.0 |
| Range | (18.0-87.0) |
| Age group | |
| 18-34 years | 2243 (7.1%) |
| 35-44 years | 5171 (16.4%) |
| 45-54 years | 9154 (29.1%) |
| 55-64 years | 9139 (29.1%) |
| 65+ years | 5740 (18.3%) |
| Gender | |
| Female | 25775 (82.0%) |
| Male | 5672 (18.0%) |
| Race | |
| Asian | 780 (2.5%) |
| Black | 3096 (9.8%) |
| Hispanic | 1945 (6.2%) |
| White | 17447 (55.5%) |
| Unknown | 8179 (26.0%) |
| Region | |
| Midwest | 9068 (28.8%) |
| Northeast | 4423 (14.1%) |
| South | 13747 (43.7%) |
| West | 4072 (12.9%) |
| Unknown | 137 (0.4%) |
| Health Plan | |
| Commercial | 26280 (83.6%) |
| Medicare Advantage | 5167 (16.4%) |
| Year of chemo completion | |
| 2008 | 2601 (8.3%) |
| 2009 | 3125 (9.9%) |
| 2010 | 3005 (9.6%) |
| 2011 | 3248 (10.3%) |
| 2012 | 3412 (10.9%) |
| 2013 | 3023 (9.6%) |
| 2014 | 3080 (9.8%) |
| 2015 | 3069 (9.8%) |
| 2016 | 3195 (10.2%) |
| 2017 | 3373 (10.7%) |
| 2018 | 316 (1.0%) |
| Cancer type | |
| Breast cancer only | 21728 (69.1%) |
| Lymphoma only | 9719 (30.9%) |
| Baseline comorbidities | |
| CHF | 1571 (5.0%) |
| HTN | 11904 (37.9%) |
| Diabetes | 4237 (13.5%) |
| Cardiac arrhythmias | 3215 (10.2%) |
| Valvular disease | 5918 (18.8%) |
| Obesity | 3297 (10.5%) |
| Renal failure | 904 (2.9%) |
| Pulmonary circulation disorder | 600 (1.9%) |
| Peripheral vascular disease | 1311 (4.2%) |
| CAD | 2035 (6.5%) |
| Trastuzumab use | 3202 (10.2%) |
| Surveillance | |
| None | 20094 (63.9%) |
| Echo only | 7398 (23.5%) |
| BNP only | 497 (1.6%) |
| CV imaging only | 1454 (4.6%) |
| Combination | 2004 (6.4%) |
Cardiac surveillance rates
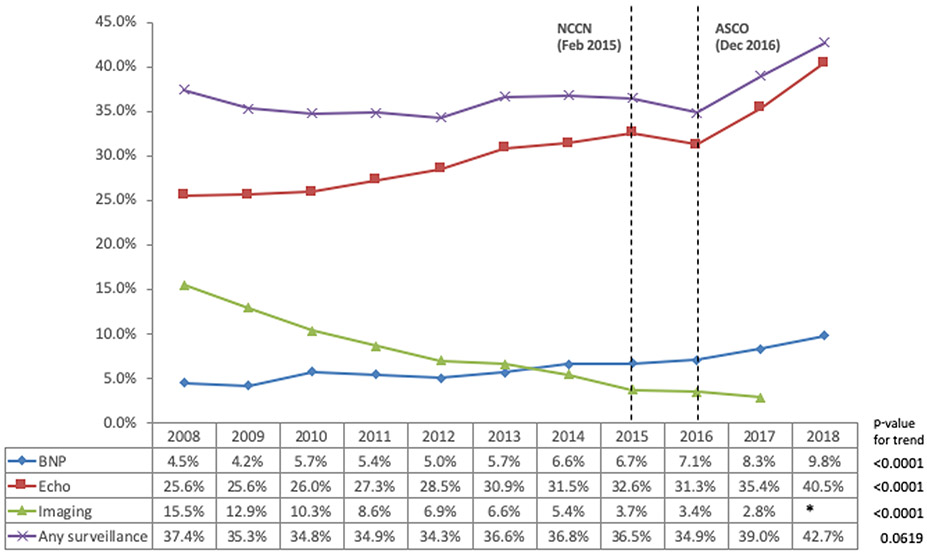
As shown in Figure 1, the frequency of cardiac surveillance by echocardiography, BNP, and/or other cardiac imaging was highest in the first year and declined over the following four years. Focusing on the year following anthracycline completion, nearly 29.7% had echocardiography, 7.4% had other types of cardiac imaging (MUGA or cardiac MRI) and 6% had BNP. As detailed in Figure 2, the rate of cardiac function testing by MUGA or cardiac MRI continuously declined over the study period whereas the rate of echocardiography and BNP increased. The rate of any type of cardiac surveillance remained rather stagnant from 2008 until 2016, only thereafter to increase.
Figure 1.
Rate of echocardiogram, other cardiac imaging, and B-type natriuretic peptide (BNP) assessment over time after completion of chemotherapy.
Figure 2.
Rate of echocardiogram, other cardiac imaging, and B-type natriuretic peptide (BNP) assessment in the first year after completion of chemotherapy by year of treatment completion (Cell size was n<11 for imaging in 2018 so value is suppressed to protect patient confidentiality).
Among subgroups (Table 2), the majority of patients were not tested regardless of age, but there was a continuous gradient from the youngest (least likely to be tested) to the oldest subgroup (most likely to be tested). CAD, lung disease, renal disease, HF, valve disease, and peripheral vascular disease were the comorbidities with the highest level of cardiac surveillance (45-50%). Cardiovascular testing was most common among patients who received additional trastuzumab therapy (76%), consisting mostly of echocardiography (56%). Excluding breast cancer patients who received trastuzumab (during which echocardiography is recommended every three months), the proportion who underwent echocardiography dropped to 24.8%. No ethnic disparities were seen (Supplemental Figure 1), but gender disparities were noted (Supplemental Figures 2 and 3). Specifically, cardiac surveillance rates were substantially higher in male lymphoma patients than female.
Table 2.
Cardiac surveillance and patient characteristics
| No CV Surveillance (N=20094) |
Echo only (N=7398) |
BNP only (N=497) |
Imaging Only (N=1454) |
Combination of CV Surveillance (N=2004) |
Total (N=31447) |
|
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Mean (SD) | 53.0 (12.7) | 54.0 (12.9) | 57.5 (12.2) | 51.4 (11.1) | 55.8 (13.1) | 53.4 (12.7) |
| Median | 53 | 54 | 57 | 51 | 56.5 | 54 |
| Q1, Q3 | 45.0, 61.0 | 45.0, 62.0 | 49.0, 66.0 | 44.0, 59.0 | 47.0, 64.0 | 45.0, 62.0 |
| Range | (18.0-87.0) | (18.0-87.0) | (22.0-85.0) | (19.0-82.0) | (18.0-87.0) | (18.0-87.0) |
| Age group | ||||||
| 18-34 | 1512 (67.4%) | 509 (22.7%) | 11 (0.5%) | 92 (4.1%) | 119 (5.3%) | 2243 (7.1%) |
| 35-44 | 3382 (65.4%) | 1170 (22.6%) | 57 (1.1%) | 293 (5.7%) | 269 (5.2%) | 5171 (16.4%) |
| 45-54 | 5981 (65.3%) | 2056 (22.5%) | 147 (1.6%) | 477 (5.2%) | 493 (5.4%) | 9154 (29.1%) |
| 55-64 | 5752 (62.9%) | 2162 (23.7%) | 148 (1.6%) | 443 (4.8%) | 634 (6.9%) | 9139 (29.1%) |
| 65+ | 3467 (60.4%) | 1501 (26.1%) | 134 (2.3%) | 149 (2.6%) | 489 (8.5%) | 5740 (18.3%) |
| Gender | ||||||
| Female | 16468 (63.9%) | 6090 (23.6%) | 402 (1.6%) | 1236 (4.8%) | 1579 (6.1%) | 25775 (82.0%) |
| Male | 3626 (63.9%) | 1308 (23.1%) | 95 (1.7%) | 218 (3.8%) | 425 (7.5%) | 5672 (18.0%) |
| Race | ||||||
| Asian | 514 (65.9%) | 194 (24.9%) | * | * | 34 (4.4%) | 780 (2.5%) |
| Black | 1950 (63.0%) | 752 (24.3%) | 62 (2.0%) | 141 (4.6%) | 191 (6.2%) | 3096 (9.8%) |
| Hispanic | 1296 (66.6%) | 437 (22.5%) | * | * | 118 (6.1%) | 1945 (6.2%) |
| White | 11098 (63.6%) | 4104 (23.5%) | 285 (1.6%) | 808 (4.6%) | 1152 (6.6%) | 17447 (55.5%) |
| Unknown | 5236 (64.0%) | 1911 (23.4%) | 112 (1.4%) | 411 (5.0%) | 509 (6.2%) | 8179 (26.0%) |
| Region | ||||||
| Midwest | 5715 (63.0%) | 1980 (21.8%) | 146 (1.6%) | 556 (6.1%) | 671 (7.4%) | 9068 (28.8%) |
| Northeast | 2670 (60.4%) | 1217 (27.5%) | 27 (0.6%) | 247 (5.6%) | 262 (5.9%) | 4423 (14.1%) |
| South | 8846 (64.3%) | 3270 (23.8%) | 261 (1.9%) | 496 (3.6%) | 874 (6.4%) | 13747 (43.7%) |
| West | 2767 (68.0%) | 905 (22.2%) | * | * | * | 4072 (12.9%) |
| Unknown | 96 (70.1%) | 26 (19.0%) | * | * | * | 137 (0.4%) |
| Health Plan | ||||||
| Commercial | 16979 (64.6%) | 6053 (23.0%) | 370 (1.4%) | 1326 (5.0%) | 1552 (5.9%) | 26280 (83.6%) |
| Medicare Advantage | 3115 (60.3%) | 1345 (26.0%) | 127 (2.5%) | 128 (2.5%) | 452 (8.7%) | 5167 (16.4%) |
| Year of chemo completion | ||||||
| 2008 | 1627 (62.6%) | 494 (19.0%) | * | * | * | 2601 (8.3%) |
| 2009 | 2021 (64.7%) | 601 (19.2%) | 38 (1.2%) | 258 (8.3%) | 207 (6.6%) | 3125 (9.9%) |
| 2010 | 1959 (65.2%) | 594 (19.8%) | 51 (1.7%) | 204 (6.8%) | 197 (6.6%) | 3005 (9.6%) |
| 2011 | 2115 (65.1%) | 701 (21.6%) | 55 (1.7%) | 183 (5.6%) | 194 (6.0%) | 3248 (10.3%) |
| 2012 | 2241 (65.7%) | 786 (23.0%) | 42 (1.2%) | 147 (4.3%) | 196 (5.7%) | 3412 (10.9%) |
| 2013 | 1916 (63.4%) | 760 (25.1%) | 45 (1.5%) | 127 (4.2%) | 175 (5.8%) | 3023 (9.6%) |
| 2014 | 1947 (63.2%) | 788 (25.6%) | 53 (1.7%) | 102 (3.3%) | 190 (6.2%) | 3080 (9.8%) |
| 2015 | 1950 (63.5%) | 812 (26.5%) | 52 (1.7%) | 66 (2.2%) | 189 (6.2%) | 3069 (9.8%) |
| 2016 | 2080 (65.1%) | 800 (25.0%) | 55 (1.7%) | 54 (1.7%) | 206 (6.4%) | 3195 (10.2%) |
| 2017 | 2057 (61.0%) | 963 (28.6%) | 75 (2.2%) | 43 (1.3%) | 235 (7.0%) | 3373 (10.7%) |
| 2018 | 181 (57.3%) | 99 (31.3%) | * | * | * | 316 (1.0%) |
| Cancer type | ||||||
| breast cancer only | 13768 (63.4%) | 5216 (24.0%) | 331 (1.5%) | 1109 (5.1%) | 1304 (6.0%) | 21728 (69.1%) |
| lymphoma only | 6326 (65.1%) | 2182 (22.5%) | 166 (1.7%) | 345 (3.5%) | 700 (7.2%) | 9719 (30.9%) |
| Baseline comorbidities | ||||||
| CHF | 835 (53.2%) | 465 (29.6%) | 29 (1.8%) | 46 (2.9%) | 196 (12.5%) | 1571 (5.0%) |
| HTN | 7237 (60.8%) | 3017 (25.3%) | 259 (2.2%) | 444 (3.7%) | 947 (8.0%) | 11904 (37.9%) |
| Diabetes | 2517 (59.4%) | 1049 (24.8%) | 120 (2.8%) | 154 (3.6%) | 397 (9.4%) | 4237 (13.5%) |
| Cardiac arrhythmias | 1729 (53.8%) | 982 (30.5%) | 75 (2.3%) | 115 (3.6%) | 314 (9.8%) | 3215 (10.2%) |
| Valvular disease | 3432 (58.0%) | 1841 (31.1%) | 102 (1.7%) | 106 (1.8%) | 437 (7.4%) | 5918 (18.8%) |
| Obesity | 2000 (60.7%) | 845 (25.6%) | 75 (2.3%) | 117 (3.5%) | 260 (7.9%) | 3297 (10.5%) |
| Renal failure | 477 (52.8%) | 264 (29.2%) | 27 (3.0%) | 20 (2.2%) | 116 (12.8%) | 904 (2.9%) |
| Pulmonary circulation disorder | 310 (51.7%) | 179 (29.8%) | 18 (3.0%) | 15 (2.5%) | 78 (13.0%) | 600 (1.9%) |
| Peripheral vascular disease | 710 (54.2%) | 373 (28.5%) | 42 (3.2%) | 40 (3.1%) | 146 (11.1%) | 1311 (4.2%) |
| CAD | 1019 (50.1%) | 639 (31.4%) | 55 (2.7%) | 63 (3.1%) | 259 (12.7%) | 2035 (6.5%) |
| Trastuzumab use | * | 1794 (56.0%) | * | 624 (19.5%) | 574 (17.9%) | 3202 (10.2%) |
cell size suppressed to protect patient confidentiality
Predictors of cardiac surveillance
Multivariable modeling (Table 3) identified the following factors associated with any cardiac surveillance in the first year after anthracycline exposure: residence in the Northwest region of the U.S., and presence of any cardiovascular risk factor, cardiovascular disease, or non-cardiac co-morbidity. Significant negative predictors were: residence in the South region of the U.S., Hispanic ethnicity, year of completion of chemotherapy 2010-2012 and 2016, and diagnosis of lymphoma. Age ≥ 65 years was not a predictor for cardiac surveillance and echocardiography in particular despite being defined as a high-risk patient population in the NCCN and ASCO guidelines. However, age groups 45 and above were all significant predictors of BNP testing, as was more recent chemotherapy completion date, and all CV risk factors, diseases, and co-morbidities. For echocardiography, all chemotherapy completion years from 2012 to 2018 were identified as significant predictors as were all CV risk factors, diseases, and co-morbidities except for diabetes. Residence in the Northeast region was a positive predictor for echocardiography and a negative predictor for BNP testing.
Table 3.
Adjusted Odds Ratios for surveillance services compared to no surveillance
| Year 1 BNP (ref= no BNP) |
Year 1 Echo (ref=no Echo) |
Year 1 Cardiac Imaging (ref=no cardiac imaging) |
Year 1 Any CV Surveillance (ref=no surveillance) |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age group (ref=18-34 years) | ||||||||
| 35-44 years | 1.11 (0.85, 1.45) | 0.4433 | 0.92 (0.82, 1.03) | 0.1576 | 1.06 (0.87, 1.28) | 0.5805 | 1.00 (0.90, 1.12) | 0.9608 |
| 45-54 years | 1.29 (1.01, 1.66) | 0.0410 | 0.88 (0.79, 0.99) | 0.0260 | 0.94 (0.78, 1.13) | 0.5057 | 0.96 (0.87, 1.07) | 0.4944 |
| 55-64 years | 1.52 (1.19, 1.94) | 0.0009 | 0.94 (0.84, 1.05) | 0.2520 | 0.99 (0.82, 1.19) | 0.9128 | 1.02 (0.92, 1.13) | 0.7685 |
| 65+ years | 1.35 (1.00, 1.82) | 0.0484 | 0.95 (0.82, 1.10) | 0.5166 | 0.95 (0.73, 1.25) | 0.7296 | 1.01 (0.88, 1.16) | 0.9044 |
| Gender (ref=Female) | ||||||||
| Male | 0.93 (0.80, 1.09) | 0.3612 | 1.04 (0.95, 1.14) | 0.3608 | 1.30 (1.10, 1.53) | 0.0020 | 1.06 (0.98, 1.15) | 0.1684 |
| Race (ref=White) | ||||||||
| Asian | 0.76 (0.52, 1.10) | 0.1454 | 0.97 (0.83, 1.14) | 0.7461 | 0.84 (0.62, 1.14) | 0.2752 | 0.94 (0.80, 1.09) | 0.3955 |
| Black | 0.97 (0.82, 1.14) | 0.6850 | 0.97 (0.89, 1.05) | 0.4435 | 1.07 (0.92, 1.25) | 0.3712 | 1.00 (0.92, 1.08) | 0.9342 |
| Hispanic | 1.00 (0.81, 1.22) | 0.9663 | 0.94 (0.84, 1.04) | 0.2310 | 0.88 (0.71, 1.07) | 0.1994 | 0.91 (0.82, 1.00) | 0.0575 |
| Unknown | 1.05 (0.93, 1.18) | 0.4533 | 1.01 (0.95, 1.07) | 0.8382 | 0.99 (0.89, 1.09) | 0.7747 | 1.01 (0.95, 1.06) | 0.8444 |
| Region (ref=Midwest) | ||||||||
| Northeast | 0.55 (0.46, 0.65) | <0.0001 | 1.19 (1.10, 1.29) | <0.0001 | 0.97 (0.85, 1.10) | 0.6449 | 1.10 (1.02, 1.18) | 0.0147 |
| South | 0.98 (0.88, 1.09) | 0.7132 | 1.03 (0.97, 1.10) | 0.3029 | 0.58 (0.53, 0.65) | <0.0001 | 0.92 (0.87, 0.97) | 0.0045 |
| West | 1.38 (0.73, 2.59) | 0.3227 | 0.86 (0.58, 1.27) | 0.4405 | 0.55 (0.24, 1.25) | 0.1540 | 0.80 (0.55, 1.16) | 0.2382 |
| Unknown | 0.72 (0.61, 0.86) | 0.0002 | 0.93 (0.85, 1.01) | 0.0854 | 0.55 (0.47, 0.64) | <0.0001 | 0.82 (0.76, 0.89) | <0.0001 |
| Health Plan (ref=Commercial) | ||||||||
| Medicare Advantage | 1.18 (0.97, 1.44) | 0.0981 | 0.94 (0.84, 1.06) | 0.3181 | 0.78 (0.62, 0.99) | 0.0379 | 0.95 (0.85, 1.06) | 0.3933 |
| Year of chemo completion (ref=2008) | ||||||||
| 2009 | 0.94 (0.72, 1.21) | 0.6097 | 1.00 (0.89, 1.13) | 0.9705 | 0.83 (0.71, 0.96) | 0.0129 | 0.91 (0.82, 1.02) | 0.1094 |
| 2010 | 1.25 (0.98, 1.60) | 0.0680 | 1.01 (0.90, 1.14) | 0.8580 | 0.65 (0.55, 0.76) | <0.0001 | 0.89 (0.80, 0.99) | 0.0345 |
| 2011 | 1.17 (0.92, 1.49) | 0.2083 | 1.07 (0.95, 1.20) | 0.2671 | 0.54 (0.46, 0.64) | <0.0001 | 0.89 (0.80, 0.99) | 0.0286 |
| 2012 | 1.06 (0.83, 1.36) | 0.6161 | 1.13 (1.00, 1.27) | 0.0415 | 0.42 (0.35, 0.50) | <0.0001 | 0.85 (0.77, 0.95) | 0.0040 |
| 2013 | 1.22 (0.95, 1.55) | 0.1140 | 1.27 (1.13, 1.43) | 0.0001 | 0.40 (0.34, 0.48) | <0.0001 | 0.95 (0.85, 1.06) | 0.3525 |
| 2014 | 1.39 (1.09, 1.76) | 0.0068 | 1.27 (1.13, 1.43) | 0.0001 | 0.33 (0.27, 0.40) | <0.0001 | 0.94 (0.84, 1.05) | 0.2419 |
| 2015 | 1.37 (1.08, 1.74) | 0.0091 | 1.33 (1.18, 1.49) | <0.0001 | 0.22 (0.18, 0.28) | <0.0001 | 0.91 (0.82, 1.02) | 0.1123 |
| 2016 | 1.39 (1.10, 1.76) | 0.0056 | 1.23 (1.09, 1.38) | 0.0006 | 0.21 (0.17, 0.26) | <0.0001 | 0.84 (0.75, 0.94) | 0.0020 |
| 2017 | 1.59 (1.27, 2.00) | 0.0001 | 1.46 (1.30, 1.64) | <0.0001 | 0.17 (0.14, 0.22) | <0.0001 | 0.99 (0.89, 1.10) | 0.8735 |
| 2018 | 1.86 (1.22, 2.85) | 0.0040 | 1.79 (1.40, 2.29) | <0.0001 | 0.12 (0.05, 0.27) | <0.0001 | 1.14 (0.90, 1.45) | 0.2808 |
| Cancer type (ref=breast cancer) | ||||||||
| Lymphoma | 1.13 (0.98, 1.29) | 0.0866 | 0.82 (0.76, 0.89) | <0.0001 | 0.70 (0.60, 0.81) | <0.0001 | 0.79 (0.74, 0.85) | <0.0001 |
| Baseline comorbidities (ref=no) | ||||||||
| CHF | 1.54 (1.30, 1.84) | <0.0001 | 1.42 (1.28, 1.59) | <0.0001 | 1.10 (0.90, 1.35) | 0.3449 | 1.33 (1.20, 1.48) | <0.0001 |
| HTN | 1.20 (1.07, 1.34) | 0.0017 | 1.11 (1.05, 1.18) | 0.0004 | 0.94 (0.84, 1.04) | 0.2171 | 1.08 (1.02, 1.14) | 0.0062 |
| Diabetes | 1.37 (1.21, 1.55) | <0.0001 | 1.04 (0.96, 1.12) | 0.3506 | 1.02 (0.88, 1.17) | 0.8335 | 1.08 (1.00, 1.16) | 0.0470 |
| Cardiac arrhythmias | 1.37 (1.20, 1.57) | <0.0001 | 1.43 (1.32, 1.55) | <0.0001 | 1.08 (0.93, 1.26) | 0.3171 | 1.42 (1.31, 1.53) | <0.0001 |
| Valvular disease | 1.12 (1.00, 1.26) | 0.0472 | 1.49 (1.40, 1.58) | <0.0001 | 0.52 (0.45, 0.60) | <0.0001 | 1.28 (1.20, 1.36) | <0.0001 |
| Obesity | 1.27 (1.11, 1.46) | 0.0007 | 1.10 (1.02, 1.19) | 0.0199 | 0.95 (0.81, 1.11) | 0.5057 | 1.09 (1.01, 1.18) | 0.0222 |
| Renal failure | 1.38 (1.12, 1.70) | 0.0027 | 1.34 (1.16, 1.54) | 0.0001 | 0.87 (0.63, 1.20) | 0.3989 | 1.31 (1.14, 1.51) | 0.0001 |
| Pulmonary circulation disorder | 1.58 (1.23, 2.04) | 0.0004 | 1.36 (1.15, 1.61) | 0.0004 | 1.08 (0.76, 1.53) | 0.6626 | 1.37 (1.16, 1.62) | 0.0002 |
| Peripheral vascular disease | 1.34 (1.11, 1.61) | 0.0019 | 1.17 (1.03, 1.31) | 0.0130 | 1.31 (1.04, 1.65) | 0.0203 | 1.23 (1.09, 1.38) | 0.0007 |
| CAD | 1.58 (1.36, 1.85) | <0.0001 | 1.55 (1.40, 1.71) | <0.0001 | 1.09 (0.90, 1.32) | 0.3881 | 1.54 (1.39, 1.69) | <0.0001 |
Each multivariable model included age, gender, race, region, health plan, year of chemotherapy completion, cancer type and baseline comorbidities.
In terms of collinearity, age group/health plan and cancer type/gender were the two groups that showed strong correlations (r-value 0.91, p-value 0.0028, and r-value 0,94, p-value 0.0024, respectively, likely related to Medicare Advantage coverage of older enrollees and the inclusion of a female breast cancer patients.
Discussion
The current analysis from a large U.S. insurance claims database shows that the majority of survivors of breast cancer and lymphoma who have received anthracycline-based chemotherapy do not undergo annual cardiac surveillance (two thirds in the first year after completion of anthracycline and 90% after three years). Importantly, even among patients considered to be at high risk based on cardiovascular co-morbidities, such as those with a history of heart failure, echocardiographic surveillance after anthracycline therapy is seen in only half in the first year. The rarity of cardiac surveillance in this setting may be related in part to the inadequacy of currently available randomized clinical trial data supporting the current guidelines.
Soon after anthracyclines were introduced into clinical practice, cardiotoxicity was recognized as a dose-limiting side effect. It became evident that a decline in cardiac function preceded the development of heart failure, and could be seen at lower doses and with less predictability than early symptomatic heart failure. This led to the development of algorithms to monitor patients during anthracycline exposure, producing significantly higher HF-free survival.1 Based on serial echocardiographic data, the likelihood of ejection fraction decline is thought to be most pronounced in the first year after completion of anthracycline.9 Hence, current cardiology consensus and oncology guidelines have recommended a follow-up echocardiogram 6-12 months after completion of chemotherapy.
In the current analysis, only one third of patients who finished anthracycline-based chemotherapy for breast cancer or lymphoma 2008-2018 went on to have an echocardiogram in the year following. In the last two years (2017 and 2018), this proportion did rise somewhat, possibly related to the publication dates of the consensus and guidelines, i.e. October 2014 for the ASE/EACVI consensus recommendations, February 2015 for the NCCN guidelines, and December 2016 for the ASCO guidelines. For oncology and hematology providers, who are likely responsible for most survivorship care during the year following chemotherapy completion, the NCCN and ASCO guidelines may be the most impactful. Of note, the odds of echocardiographic testing have persistently been higher since 2012, preceding any publication of guidelines or consensus documents, possibly related to the growth of cardio-oncology as a field and increasing cross-talk between providers of survivorship care.
Lack of awareness has been previously shown to be the most common barrier to the uptake of other practice guidelines, with lack of familiarity and lack of agreement next in importance.10 Provider factors including age may be relevant, with those in the second half of their career relying more on personal experience.11 For serial follow-up echocardiograms for patients on trastuzumab, younger care providers have been found to be more likely to order regular three-monthly cardiac function tests as recommended.12 It is of note that trastuzumab therapy did increase the likelihood for cardiac surveillance in the first year, though still remaining at <80% overall and <60% for echocardiography. Accordingly, there is some awareness among providers that the combination of trastuzumab and anthracyclines is most problematic. It is likely due to the trastuzumab dynamics that patients with breast cancer were more likely to receive echocardiography than those with lymphoma (despite usually receiving lower anthracyclines doses). Indeed, when trastuzumab recipients were removed from the breast cancer cohort, the rate of echocardiography was similar to the overall cohort.
One might argue that many of the patients assessed in this study may not have qualified for consideration of echocardiography based on NCCN and ASCO guidelines because they were young and healthy and thus not perceived to be at high risk. It is true that only 18% of patients were in the age group considered as being at higher risk, e.g., age 65 or over. However, burden of (qualifying) pre-existing cardiovascular diseases and risk factor, including hypertension, CAD, valvular heart disease, arrhythmias, and heart failure was high, and these patients more frequently did undergo surveillance. Interestingly, patients with a history of heart failure, who might be considered to be at the highest risk of developing cardiac complications from an anthracycline, were not the population with the highest rate of cardiac surveillance. In fact, patients with co-morbidities not listed in the guidelines and characteristic of high risk, e.g. pulmonary disease and renal disease, had a higher likelihood of being monitored by cardiac imaging or BNP. This suggests that guideline recommendations are not the main drivers of cardiac surveillance test orders in the studied patient cohort.
While an upward trend was noted after the publication of the ASCO guidelines, this was not seen when stratified for ethnicities. Furthermore, though cardiac surveillance increased in male lymphoma patients, especially after the ASCO guideline publication date, a similar dynamic was not seen in female lymphoma patients. In fact, the most recent trend in these women showed a notable decline.This disparity might be related to the greater cardiovascular risk factors seen in men, and men’s earlier onset of cardiovascular disease, but it is unclear why cardiac surveillance rates have declined over time in women.Female lymphoma patients receive the same chemotherapy and dose regimens as men, standardized to body size, so the existing guidelines do not recommend any gender-related differences in cardio-oncology surveillance strategies.
At present, neither the NCCN nor the ASCO guideline provides any guidance on long-term surveillance for adult cancer survivors beyond the 6-12 months follow-up period. Accordingly, the sharp decline in surveillance rates from 36% in the first year to 19% in the second and 13-15% thereafter is not surprising. Because we do not have access to the results of the testing due to our administrative claims-based methodology, we do not know what proportion of the post-1-year testing was done to follow-up on a prior abnormal result, and additional clinical studies will be needed to assess if a normal echocardiogram result at 6-12 months is truly adequate to omit the need for any further cardiac surveillance. Studies in breast cancer survivors who have received anthracycline indicate that the average time to a diagnosis of HF is 5 years,13 but the point in time when cardiac function started to decline in these patients is unknown.14 Furthermore, the value of BNP testing is particularly under-studied in this population, and our data suggest that providers are not routinely assessing BNP post-chemotherapy at present.
Limitations of the current study include those inherent to claims-based data analyses. This being said, for health care utilization analyses an approach as taken herein is useful based on the premise that bills will be generated for any tests ordered. For this reason, the current work should reflect accurately the testing pattern in the patient cohort analyzed. This patient cohort may not be fully representative of the entire population, but insurance status was integrated in the review to the degree possible. Details on the cancer stage and biology cannot be ascertained, such that some of these patients may have had stage 4 disease or may have later developed recurrent/progressive disease, either of which could have been treated with ongoing therapies beyond the anthracycline-containing regimen. Likewise, due to the claims-based methodology, the reasons for testing or lack thereof could not be defined in the current work.
In conclusion, cardiac function testing including echocardiography and/or BNP testing is used within a year of completion anthracycline-based chemotherapy in less than half of all survivors of breast cancer or lymphoma. Barriers to guideline implementation for cancer survivors at risk of cardiomyopathy and HF should be further studied. One aspect to consider is that neither guideline provides a class I recommendation for cardiac surveillance. NCCN and ASCO guidelines recommending consideration of echocardiography 6-12 months after anthracycline in patients at increased risk of cardiotoxicity are consistent with a class IIb recommendation in cardiology guidelines, and the ACCF/AHA/HFSA guidelines provide a class IIa recommendation for BNP in Stage A HF patients, but these are not specific for cancer patients, and in fact, cancer patients were not included in the trial supporting the ACCF/AHA/HFSA guideline recommendation. Thus, clinical trials are needed to further elucidate optimal cardiac surveillance in cancer survivors, leading to stronger guideline recommendations.
Supplementary Material
WHAT IS KNOWN
Early intervention (e.g., beta-blockers, ACE inhibitors) in patients with asymptomatic cardiac dysfunction protect against progression to symptomatic heart failure.
Echocardiography and brain natriuretic peptide testing may identify patients at risk of cardiomyopathy and heart failure after receipt of anthracycline.
WHAT THE STUDY ADDS
Cardiac function testing including echocardiography and/or brain natriuretic peptide testing is used in less than half of all patients who receive an anthracycline for breast cancer or lymphoma within a year of completion chemotherapy.
Barriers to the implementation of oncology and cardiology guideline recommendations for cancer patients at risk of cardiomyopathy and HF need to be identified.
Clinical trials are needed that would allow raising the strength of recommendation for cardiac surveillance in at risk cancer patients in professional guidelines.
Acknowledgments
Sources of Funding: KR and JH were supported by the National Cancer Institute of the NIH (CA233610-01) and previously by KL2 TR002379 from the National Center for Advancing Translational Sciences (NCATS) to KR and a K08 HL116952 from the National Heart Lung and Blood Institute of the NIH. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NIH. LS was supported by a 2016 NCCN Foundation Young Investigator Award (PI: Ruddy). JM was supported by R56 HL141466 and R01 HL 141466.
Disclosures:
Dr Ruddy is supported by the National Institutes of Health (RO1 CA233601and) is co-inventor on technology licensed by Mayo Clinic to AliveCor (MountainView, CA). Dr. Moslehi has served on an advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca, GlaxoSmithKline, Intrexon, and Regeneron and is supported by National Institutes of Health grants R56 HL141466 and R01 HL141466. Dr. Herrmann is supported by the National Institutes of Health (RO1 CA233601), has served on an advisory boards for Bristol-Myers Squibb, Takeda, and Amgen and has received research support from Amgen. The other authors report no conflicts.
References
- 1.Schwartz RG, McKenzie WB, Alexander J, Sager P, D'Souza A, Manatunga A, Schwartz PE, Berger HJ, Setaro J, Surkin L, Wackers FJT, Zaret BL. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109–18. [DOI] [PubMed] [Google Scholar]
- 2.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR and Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denlinger CS, Sanft T, Baker KS, Broderick G, Demark-Wahnefried W, Friedman DL, Goldman M, Hudson M, Khakpour N, King A, Koura D, Lally RM, Langbaum TS, McDonough AL, Melisko M, Montoya JG, Mooney K, Moslehi JJ, O'Connor T, Overholser L, Paskett ED, Peppercorn J, Pirl W, Rodriguez MA, Ruddy KJ, Silverman P, Smith S, Syrjala KL, Tevaarwerk A, Urba SG, Wakabayashi MT, Zee P, McMillian NR and Freedman-Cass DA. Survivorship, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1216–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K and Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin, Drazner, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 6.Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B and McDonald K. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. Jama. 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 7.Wallace PJ, Shah ND, Dennen T, Bleicher PA and Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood). 2014;33:1187–94. [DOI] [PubMed] [Google Scholar]
- 8.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE and Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 9.Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM, Margulies KB and Ky B. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations With Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up. Circulation. 2017;135:1397–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA and Rubin HR. Why don't physicians follow clinical practice guidelines? A framework for improvement. Jama. 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 11.Barth JH, Misra S, Aakre KM, Langlois MR, Watine J, Twomey PJ and Oosterhuis WP. Why are clinical practice guidelines not followed? Clin Chem Lab Med. 2016;54:1133–9. [DOI] [PubMed] [Google Scholar]
- 12.Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, Smith BD, Hortobagyi GN and Giordano SH. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin A, Thompson CL and Silverman P. Predictors of late-onset heart failure in breast cancer patients treated with doxorubicin. J Cancer Surviv. 2015;9:252–9. [DOI] [PubMed] [Google Scholar]
- 14.Feola M, Garrone O, Occelli M, Francini A, Biggi A, Visconti G, Albrile F, Bobbio M and Merlano M. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol. 2011;148:194–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.