Abstract
Background
This is an update of the original Cochrane review published in Issue 4, 2000. Intestinal obstruction commonly occurs in progressive advanced gynaecological and gastrointestinal cancers. Management of these patients is difficult due to the patients' deteriorating mobility and function (performance status), the lack of further chemotherapeutic options, and the high mortality and morbidity associated with palliative surgery. There are marked variations in clinical practice concerning surgery in these patients between different countries, gynaecological oncology units and general hospitals, as well as referral patterns from oncologists under whom these patients are often admitted.
Objectives
To assess the efficacy of surgery for intestinal obstruction due to advanced gynaecological and gastrointestinal cancer.
Search methods
We searched the following databases for the original review in 2000 and again for this update in June 2015: CENTRAL (2015, Issue 6); MEDLINE (OVID June week 1 2015); and EMBASE (OVID week 24, 2015).
We also searched relevant journals, bibliographic databases, conference proceedings, reference lists, grey literature and the world wide web for the original review in 2000; we also used personal contact. This searching of other resources yielded very few additional studies. The Cochrane Pain, Palliative and Supportive Care Review Group no longer routinely handsearch journals. For these reasons, we did not repeat the searching of other resources for the June 2015 update.
Selection criteria
As the review concentrates on the 'best evidence' available for the role of surgery in malignant bowel obstruction in known advanced gynaecological and gastrointestinal cancer we kept the inclusion criteria broad (including both prospective and retrospective studies) so as to include all studies relevant to the question. We sought published trials reporting on the effects of surgery for resolving symptoms in malignant bowel obstruction for adult patients with known advanced gynaecological and gastrointestinal cancer.
Data collection and analysis
We used data extraction forms to collect data from the studies included in the review. Two review authors extracted the data independently to reduce error. Owing to concerns about the risk of bias we decided not to conduct a meta‐analysis of data and we have presented a narrative description of the study results. We planned to resolve disagreements by discussion with the third review author.
Main results
In total we have identified 43 studies examining 4265 participants. The original review included 938 patients from 25 studies. The updated search identified an additional 18 studies with a combined total of 3327 participants between 1997 and June 2015. The results of these studies did not change the conclusions of the original review.
No firm conclusions can be drawn from the many retrospective case series so the role of surgery in malignant bowel obstruction remains controversial. Clinical resolution varies from 26.7% to over 68%, though it is often unclear how this is defined. Despite being an inadequate proxy for symptom resolution or quality of life, the ability to feed orally was a popular outcome measure, with success rates ranging from 30% to 100%. Rates of re‐obstruction varied, ranging from 0% to 63%, though time to re‐obstruction was often not included. Postoperative morbidity and mortality also varied widely, although again the definition of both of these surgical outcomes differed between many of the papers. There were no data available for quality of life. The reporting of adverse effects was variable and this has been described where available. Where discussed, surgical procedures varied considerably and outcomes were not reported by specific intervention. Using the 'Risk of bias' assessment tool, most included studies were at high risk of bias for most domains.
Authors' conclusions
The role of surgery in malignant bowel obstruction needs careful evaluation, using validated outcome measures of symptom control and quality of life scores. Further information could include re‐obstruction rates together with the morbidity associated with the various surgical procedures.
Currently, bowel obstruction is managed empirically and there are marked variations in clinical practice by different units. In order to compare outcomes in malignant bowel obstruction, there needs to be a greater degree of standardisation of management.
Since the last version of this review none of the new included studies have provided additional information to change the conclusions.
Keywords: Female; Humans; Male; Gastrointestinal Neoplasms; Gastrointestinal Neoplasms/complications; Genital Neoplasms, Female; Genital Neoplasms, Female/complications; Intestinal Obstruction; Intestinal Obstruction/etiology; Intestinal Obstruction/mortality; Intestinal Obstruction/surgery; Prospective Studies; Recurrence; Retrospective Studies
Plain language summary
Surgery for resolving symptoms associated with malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers
Background
Advanced cancer causes a range of complex problems for patients. In gynaecological (for example ovarian and womb) and gastrointestinal (for example colon or bowel) cancers, the bowel can become blocked or obstructed by the original tumour, metastatic deposits or due to the side effects of previous treatments. The decision to operate on patients with bowel obstruction who are already very unwell because of their advanced cancer is difficult. Often, these people develop bowel obstruction as a sign that the cancer is progressing and they are in the process of dying. When the bowel obstructs in this situation, surgery might be useful for some patients, it might make no difference to how long the patient has to live, or it might make the situation worse due to the complications of surgery. When time is short, managing symptoms and maximising comfort for the patient is the priority. Different surgical teams adopt different approaches. We wanted to establish the evidence for the benefit and harm of surgery in these situations and therefore help patients and doctors make good decisions.
Key findings and quality of the evidence
We first looked at the evidence in 2000 and this is an update of the original review. In total we found 43 studies examining 4265 people. We looked at adults with advanced gynaecological or gastrointestinal cancer who developed bowel obstruction and had either surgical or non‐surgical treatment. The studies we found were of low quality and measured success and benefit in different ways. It was therefore not possible to compare these studies and conclude whether surgery was of benefit or harm in this situation. Research in this area is problematic and the type of study needed to answer this question would be very difficult to conduct.
Background
This is an update of the original Cochrane review published in Issue 4, 2000 (Feuer 2000). Gastrointestinal and ovarian cancers are common cancers. Bowel and rectal cancer is the third most common cancer for men and women (with an incidence in men of 58/100,000 and in women of 37.4/100,000, and an overall incidence of 47/100,000) and ovarian cancer is the fifth most common cancer in women (with an incidence of 17.1/100,000) within the United Kingdom (Cancer Research UK 2014). Bowel cancer alone accounts for 10% of all cancer deaths (Cancer Research UK 2014). In the UK, mortality from bowel cancer stands at 16.3/100,000 (men: 20.5/100,000 and women 13.0/100,000) and from ovarian cancer at 8.9/100,000 (Cancer Research UK 2014).
Worldwide, colorectal cancer is the third most common cancer in men (746,000 cases, 10.0% of the total) and the second most common in women (614,000 cases, 9.2% of the total) (GLOBOCAN 2012). There is wide geographical variation in incidence, with almost 55% of cases occurring in more developed regions were mortality is higher compared with more developed countries (GLOBOCAN 2012). Worldwide gynaecological cancer incidence and mortality data are not available.
Description of the condition
The incidence of malignant intestinal obstruction due to progressive disease (not at the primary diagnosis) in these patients is generally not known. Many retrospective and autopsy studies have estimated that this can occur in 5% to 51% of patients with ovarian malignancies (Dvoretsky 1988a; Dvoretsky 1988b; Ripamonti 1993; Rose 1978), and in 10% to 28% of patients with gastrointestinal cancer (Ripamonti 1993). These figures, however, are from highly selected groups of patients, and different diagnostic criteria for malignant intestinal obstruction are often used. The true incidence of obstruction may be even higher.
Two autopsy studies of patients with ovarian carcinoma, with 100 and 428 patients respectively, described involvement of the bowel with cancer. In one study 70% of patients had involvement of the small bowel and 78% involvement of the large bowel with an overall 51% incidence of intestinal obstruction (Dvoretsky 1988a; Dvoretsky 1988b). In the other study there was small bowel involvement in 42% of cases and large bowel involvement in 49% (Rose 1978).
Several pathophysiological mechanisms may be involved in gastrointestinal obstruction due to progressive disease and often more than one factor is responsible (Baines 1998).
Intraluminal obstruction. This is often caused by polypoid lesions, both primary and metastatic, which, if large enough, occlude the lumen or act as a point for intussusception. Tumours can also occlude the lumen in an annular fashion, particularly in the right and left colon.
Intramural obstruction. Lateral spread of the tumour within the muscular coats of the bowel wall causing an 'intestinal linitis plastic'.
Extramural obstruction. Mesenteric and omental masses and malignant adhesions are able to cause extrinsic compression of the bowel.
Motility disorders. Disordered or absent motility of a segment of bowel will lead to a similar clinical scenario, but with no occlusion of the bowel lumen. This is caused by tumour infiltration of the mesentery, bowel muscle or coeliac and enteric plexuses. Interleukin‐1 type factors may also be partly responsible (Watson 1997). Further important factors include the long‐term effects of opioids (causing hypersegmentation of the bowel), anticholinergics (interfering with parasympathetic nerve transmission) and chemotherapy agents (which can cause both peripheral and autonomic neuropathy). These agents may have a direct effect upon the bowel that may result in bowel perforation and dysfunction (Feuer 1999a).
Constipation/faecal impaction. Obstruction may be precipitated where an obstructing lesion, particularly in the large bowel, is able to reduce the lumen.
Side effects of radiotherapy on small bowel can cause obstruction due to a combination of stricture formation and an effect on peristalsis.
Management of bowel obstruction
Prior to a seminal paper by Baines et al in 1985 (Baines 1985), treatment for bowel obstruction due to progressive advanced malignancy was either palliative surgery or, if surgery was not possible, nasogastric tube suction together with administration of intravenous fluids. Both methods meant that the patient remained in hospital, often for prolonged periods of time, with their symptoms inadequately addressed. Baines 1985 demonstrated, however, that the symptoms of bowel obstruction in those patients where surgery was not possible could be managed by pharmacological means. In such patients they demonstrated an improvement in generalised abdominal pain, colic, nausea and vomiting by using regular analgesics, antispasmodics and antiemetics. Since this study, others have produced similar results in different settings, but often using different medication.
The presentation of malignant intestinal obstruction is often not the classical acute surgical abdomen where there is sudden onset of colicky abdominal pain, associated with vomiting and/or absolute constipation; more usually the onset is more insidious, over many weeks or months, with all the symptoms gradually worsening, becoming more continuous and severe (Ripamonti 1993). The natural history of obstruction can also be intermittent, with obstructive episodes resolving spontaneously, if temporarily (Baines 1998; Feuer 1999b).
In bowel obstruction, the bowel initially contracts, with increased peristaltic activity, which in turn causes release of prostaglandins, secretagogues and nociceptive mediators. Vasoactive intestinal polypeptide is also released, and may mediate some of the pathological alterations that accompany bowel obstruction (especially small bowel) such as hyperaemia, oedema and luminal accumulation of fluid. The abdominal distension often seen in distal small bowel obstruction is caused by accumulation of swallowed air and up to 8 litres of gastrointestinal secretions (saliva 1500 ml, gastric secretions 2500 ml, bile and pancreatic secretions 1000 ml and small bowel secretions 3000 ml). This sequestration of fluid is partially responsible for the picture of hypovolaemia, tachycardia, systemic hypotension and eventually multiorgan system failure often seen in bowel obstruction, which in turn leads to higher surgical morbidity and mortality. A vicious circle occurs, represented by this distension, secretion and contractile hyperactivity, which in turn can lead to epithelial damage. Due to this often gradual presentation, patients have often been prescribed a multitude of oral antiemetics, analgesics and laxatives, often with little or no effect (as nausea and then vomiting become more continuous). The patients are often anorexic and dehydrated, with uncontrolled nausea and vomiting (depending on the level of the obstruction), and may experience abdominal distension and pain.
On admission to hospital the initial management is to resuscitate the patient using intravenous fluids and electrolytes and keep them 'nil by mouth' to reduce vomiting. Analgesics and antiemetics are used to a varying extent in the hospital setting but are used more widely within the hospice and palliative care population. Nasogastric intubation, occasionally with continuous suction, is commonly performed within hospital, with the aim of decompressing the stomach and reducing the risk of vomiting (Butler 1991). This conservative management can continue for three to nine days, if not longer, and it is estimated that between 12% to 29% of symptoms may resolve spontaneously. Unfortunately, it is thought that symptoms recur in 32% to 45% of patients (Butler 1991; Osteen 1980). The patients in whom symptoms are not managed, or are inadequately addressed, may deteriorate, with uncontrolled vomiting (occasionally faeculant) and pain, and they will eventually die in this most distressing fashion.
Description of the intervention
The role of surgery
The role of surgery in malignant bowel obstruction due to advanced progressive gynaecological and gastrointestinal cancer remains controversial. The published literature is mainly retrospective and deals mostly with gynaecological (often ovarian) cancer. Individual narrative reviews, however, come to different conclusions regarding this evidence, suggesting for example that surgery has either a significant role (Beattie 1989), or no role in this group of patients (Farias‐Eisner 1994). Little has been written about the position in advanced gastrointestinal cancer.
Over time, changes in surgical, anaesthetic and oncological practices have taken place. These changes influence the decision to operate or not operate. In particular, the now routine use of chemotherapy with its resultant effect on the natural history of the cancer (Ozols 1997), and the change in philosophy in treating these patients, are important determinants in the different retrospective reviews that have been undertaken. The development of intra‐luminal stents offers a less invasive surgical option for a subgroup of patients. This further complicates decision‐making (Fiori 2012).
Many of the earlier reports stated that patient benefit occurred if they lived for 60 or more days following surgery (Castaldo 1981; Krebs 1983). Unfortunately, in a population of patients where the disease has been previously treated with chemotherapy, malignant bowel obstruction often represents progressive disease (Tunca 1981), usually with very limited chemotherapeutic options, and this points to a very poor prognosis ‐ usually less than 60 days. The decision to operate on these patients is often made on the assumption that obstruction always demands surgical intervention (Parker 1996), and that this is the best and only way to manage symptoms.
In the vast majority of retrospective reviews, the symptoms and the quality of life post‐surgery are rarely measured or reported (or, if included, unvalidated tools are often used), yet it is these factors that determine whether the palliative surgery has been successful. Equally, it is not stated how many patients were managed without surgery and, of the ones managed with surgery, the number who had an 'open and shut' laparotomy without any corrective surgery taking place. Another problem with the retrospective literature is the large number of variables that exist from each review that are all uncontrolled and biased in nature. In the absence of randomised controlled trials (RCTs), however, it is this literature that guides us as to whether or not we should offer surgery as a way of improving our patients' symptoms. Equally it would guide us as to what further research is needed. This is therefore the aim of this systematic review.
Why it is important to do this review
When this review was first performed, the National Confidential Enquiry into Perioperative Deaths in the United Kingdom, NCEPOD 1997, stated that surgeons were performing too many "inappropriate and aggressive" operations on patients who were frail or terminally ill. Although pressure to perform such procedures from the patient, relatives or medical colleagues was indisputable, the report was explicit that surgeons needed to be clear about the aims of each operation. The original report was unable to offer much guidance in this situation. In recent years, the desire for such procedures has grown while the volume and quality of data available to guide decision‐making has not.
The best management(s) of this group of patients remains controversial. There is uncertainty regarding both the benefits and possible harmful effects of surgery, and there are marked variations in clinical practice between different countries, gynaecological oncology units and general hospitals, as well as the referral patterns from oncologists under whom these patients are often admitted.
Currently, patients with bowel obstruction remain in hospital for long periods of time, due to uncertainty in both the diagnosis (due to the chronic recurrent non‐acute nature of symptoms) and management strategies, especially so if the patient remains symptomatic. The cost of numerous trials of unsuccessful medications and then often surgery with increased medical and nursing input is significant, as is the emotional distress to the carers and family. If appropriate symptom management is instigated rapidly, a patient's symptoms can be controlled satisfactorily, and discharge home (or to a hospice) with appropriate care is facilitated.
The original review, Feuer 2000, considered the role of surgery for the resolution of symptoms of malignant bowel obstruction in advanced gynaecological and gastrointestinal caners and found few data. Therefore an update is needed to evaluate the newest evidence available, to guide clinicians and patients and specify the areas for further research.
Since the original review, a Cochrane review comparing surgery to non‐surgical treatment for symptom relief in bowel obstruction in ovarian cancer has been published (Kucukmetin 2010). The review reported on one non‐randomised study comparing surgery to octreotide in women with ovarian cancer (Mangili 2005), highlighting once again the paucity of data in the palliative management of bowel obstruction in advanced cancer. Given the low quality of evidence and the limited outcome data, the review authors were unable to comment on the relative benefits or harms of these interventions.
Objectives
To assess the efficacy of surgery for intestinal obstruction due to advanced gynaecological and gastrointestinal cancer.
Methods
Criteria for considering studies for this review
Types of studies
The systematic review included studies that met the following criteria:
Randomised controlled trials (RCTs) ‐ randomisation was defined as studies that were described by the authors as 'randomised' anywhere in the manuscript.
Cohort studies ‐ where the comparability of cohorts has been established, or existing confounding factors adjusted for. (These may be prospective with historical controls or retrospective with concurrent controls).
Well‐designed case‐control retrospective studies ‐ where evidence is shown that selection by using confounding variables has been addressed or considered.
Longitudinal surveys or case histories.
Studies published in any language were included. All identified trials, published and unpublished, were eligible.
We drew conclusions from the non‐randomised, non‐controlled data if there was insufficient evidence from RCTs.
As this review concentrated on the 'best evidence', we graded the quality of all the research found using the Cochrane 'Risk of bias' tool (Higgins 2011).
Types of participants
Participants 18 years and over with a clinical diagnosis of intestinal obstruction due to known advanced gastrointestinal or gynaecological cancer where management of participants included the option of surgery.
Types of interventions
We considered studies that included any trial of surgery though ideally there would be a separation between corrective surgery and non‐corrective surgery. Although ideally randomised, placebo‐controlled trials are the most reliable evidence, we considered all available trials, even those without placebo, as 'best evidence available'.
Types of outcome measures
We included studies in comparison tables if there was an outcome in terms of the following.
Primary outcomes
Clinical resolution or improvement of the symptoms of bowel obstruction (nausea, vomiting, pain, constipation).
Secondary outcomes
Re‐obstruction rates in patients who undergo surgery.
Morbidity and mortality of patients who undergo surgery.
Change of quality of life scores in patients who undergo surgery.
Adverse effects.
The outcome measures, such as symptom control or re‐obstruction rates, had to be specific to those patients only operated on for advanced gastrointestinal or gynaecological cancer. If the results were described for a group of patients including other diagnoses as well as gastrointestinal or gynaecological cancer, and there was no separation according to cause, then we did not use these results. We also took further information about the stage and type of disease, number of patients studied, type of operation, study design, study duration and follow‐up, withdrawals and adverse effects (minor and major) from each report.
Search methods for identification of studies
Electronic searches
In order to be as comprehensive as possible, we carried out the following search strategies to identify all relevant studies, irrespective of language. We did not systematically search for studies predating 1966.
We searched the following electronic databases using search strategies developed in close collaboration with a qualified librarian. Initial literature searches in MEDLINE using combinations of MeSH headings and free text words provided several thousand references. Difficulties arose in devising a search strategy that was both sensitive and specific enough to identify all important material, yet produce a manageable and relevant list, especially as all trials (not only randomised controlled trials) were required. Further examination of the indexing of known and important papers led to the eventual search strategy developed. We used no filter in any of the databases due to the requirement of the review that any report with surgery was possibly acceptable and the small number of final papers that we retrieved.
Databases searched in 2015 for this update
Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 6).
MEDLINE (OVID) searched from 1997 to June week 1 2015.
EMBASE (OVID) searched from 1997 to week 24, 2015.
The search strategies used for the 2015 searches can be found in Appendix 1.
Databases searched for the original review
MEDLINE ‐ on Silver Platter, searched from September 1966/1997. This was performed using two searches, which were then combined using the Boolean operator AND, i.e. one on intestinal obstruction and one on surgery. All searches combined the MeSH terms and an extensive free text search. These searches were further developed by identifying appropriate articles and noting the manner in which they had been indexed.
EMBASE ‐ on Silver Platter, searched from December 1980/1997. Two overlapping searches were combined, as for MEDLINE, and the searches were developed as for MEDLINE.
CANCERCD ‐ on Silver Platter, searched from December 1980/1997. Two overlapping searches were combined, as for MEDLINE. The searches were developed using only free text terms.
The Cochrane Controlled Trials Register (CENTRAL/CCTR 1998, Issue 1). This was searched using only one subject, i.e. obstruction, using both MeSH terms and text terms.
Science Citation Index on BIDS ISI from December 1991/1997. Two overlapping searches were combined as for MEDLINE, and developed using only free text terms.
CINAHL ‐ on Silver Platter. Searched from December 1982/1997. Two overlapping searches were combined as for MEDLINE. The searches were developed as for MEDLINE.
Grey literature ‐ electronic searches.
Dissertation Abstracts ‐ on Silver Platter, November 1961/1997. This was searched using only one subject, i.e. 'obstruction', using free text terms.
SIGLE ‐ via Blaze Line British Library Current Edition. This was searched as for Dissertation Abstracts.
Index to Scientific and Technical Proceedings ‐ on BIDS ISI, from February 1982 /1998. This was searched as for Dissertation Abstracts.
Boston Spa Conferences ‐ via Blaze Line British Library Current Edition. This was searched as for Dissertation Abstracts.
Inside Conferences ‐ via Blaze Line British Library Current Edition 1996‐1997. This was searched as for Dissertation Abstracts.
National Health Service National Research Registry ‐ via CD‐ROM August 1997. This was searched using MeSH terms and free text terms as for CENTRAL/CCTR.
Searching other resources
The searching of other resources was performed for the original review but yielded very few additional studies (see Appendix 2 for searching of other resources performed for the original review). The Cochrane Pain, Palliative and Supportive Care Review Group no longer routinely handsearch journals. For these reasons, we did not repeat the searching of other resources for the 2015 update.
Data collection and analysis
Selection of studies
We imported all records from each of the databases above to the bibliographic package Reference Manager and merged them into one core database where we inspected all titles, keywords and abstracts for relevance. Where it was not possible to classify an article and reject it with certainty on the basis of the information available from the databases, we obtained the full text of the article for further evaluation. We therefore created a final list of potentially relevant articles in Reference Manager.
Two review authors independently assessed this group of studies (original; KEB and DJF: update; ET and SC) against the above inclusion criteria to increase validity (Chalmers 1987). The reports were masked to journal publication, date, authorship and institution so as to produce more consistent results. Where differences existed we resolved them by consensus and, when necessary, in consultation with a third review author (DJF).
We documented the justification for excluding studies at this stage.
Data extraction and management
We used a data abstraction form that we specifically designed for this review. Two review authors independently abstracted the following data items, which were masked with respect to journal publication, date, authorship and institution (so as to produce more consistent scores) (Chalmers 1987; Jadad 1996). For the original review this was done by KEB and DJF. For the update this was done by ET and SC.
Trial quality characteristics.
Participants: number of participants at baseline.
Interventions: type of surgery.
Outcome data.
Potential confounding factors: any assessment of previous treatment.
Assessment of risk of bias in included studies
For the original review, the evaluation of the methodological quality of the included trials is described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (see Appendix 3 for details). We rated each randomised controlled trial according to the quality of allocation concealment categories. A table outlining these original review results can be found in Appendix 4.
2015 update
For the update, we use the 'Risk of bias' assessment tool to assess the methodological quality of all included papers. Two review authors (ET and SC) independently assessed the quality of included studies using the tool (Higgins 2011). Where consensus could not be reached, we referred to a third author (DJF).
We assessed the following domains as high, unclear or low risk and gave a brief explanation for each.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Selective outcome reporting (reporting bias).
Given the nature of the studies identified in the original review, we did not plan to exclude data from the update on the basis of high risk of bias.
Measures of treatment effect
The outcomes can be reported in a variety of ways, making comparisons challenging. We sought to describe the outcomes but we did not attempt to combine them. Data analysis was not possible.
Results
Description of studies
In the initial review, we identified for inclusion 25 studies with a total of 938 patients from a variety of sources: 22 from MEDLINE and three via handsearching and personal contact. They dated from 1970 to 1997. Further details of these eligible studies are in the 'Characteristics of included studies' table.
An additional 18 studies provided further data for inclusion (Abbas 2007; Blair 2001; Chi 2009; Fiori 2012; Furuya 2012; Goto 2012; Kolomainen 2012; Mangili 2005; Mooney 2013; Parveen 2009; Perri 2014; Pothuri 2003; Pothuri 2004; Sartori 2009; Sartori 2010; Winner 2013; Wong 2009; Zhang 2012). Details of those excluded can be found in the 'Characteristics of excluded studies' table. Two included papers were drawn from the same study but the participants were counted once (Sartori 2009; Sartori 2010). Two included studies were prospective (Chi 2009; Fiori 2012). One was a prospective outcomes analysis (Chi 2009), the other randomised participants to stent or surgery (Fiori 2012). Two studies were population‐based using an insurance database to investigate ovarian (Mooney 2013) and colon cancer (Winner 2013). These two studies contributed large numbers (1518 and 1004 respectively) and made up the vast majority of the review participants. The rest were all retrospective case series. In total the additional studies looked at 3327 participants. As before there was marked variability in the studies with no defined and consistent approach to outcome criteria. The information abstracted was similar to the original review. We included data from 43 studies (25 + 18) with a total of 4265 participants (938 + 3327).
Risk of bias in included studies
The risk of bias for each study is detailed in table form and included in the section 'Characteristics of included studies'. This assessment of risk of bias for each study is shown in Figure 1, while an overall summary is displayed graphically in Figure 2. The main reasons for being given a high risk of bias rating were a lack of randomisation and a lack of blinding. Additionally, as studies were small, surgical techniques varied within studies and the outcomes were often not reported according to the surgical technique used. Therefore we did not have enough information to assess selective reporting bias in detail and most are judged as at unclear or high risk of bias.
1.
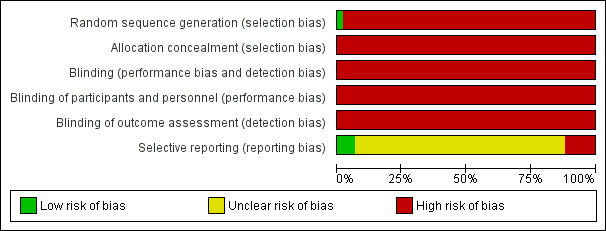
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
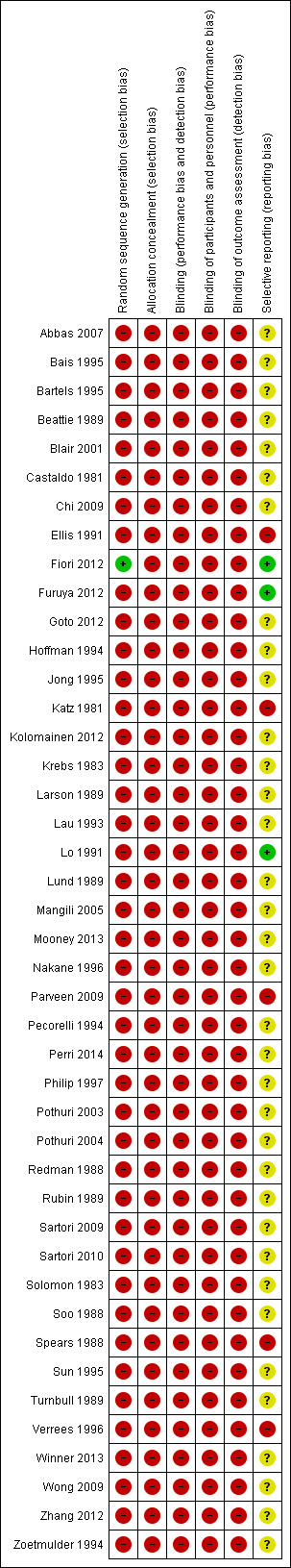
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Of those not receiving a rating of 'high' or 'unclear' risk, we assigned Fiori 2012 'low risk' for selection bias as patients were randomly assigned to stent or surgery and 'low risk' for selective reporting as a single surgical technique was used and reported on. Furuya 2012 and Lo 1991 reported outcomes by surgical technique used.
Effects of interventions
As all the studies identified were of low methodological quality, no statistical analysis was possible and we have presented a narrative description of the study results.
Clinical resolution or improvement of symptoms
Comparison of the resolution or improvement of symptoms proved challenging as the studies in both the original review and this update varied greatly in their outcome measures. No studies considered pain or quality of life as outcomes. A single paper, Larson 1989, considered symptom burden and examined nausea, vomiting, inability to eat and constipation in six patients who had died during the study. Symptomatic relief was specifically measured, if not defined, by several papers. The ability to tolerate oral intake was a frequent outcome measure. Often, survival/mortality data served as proxy markers for clinical resolution and symptoms.
Clinical resolution of obstruction was described in terms of undefined "clinical success" (Zhang 2012), or relieved/unrelieved obstruction or functioning bowel (Hoffman 1994; Lau 1993; Philip 1997; Redman 1988; Solomon 1983; Turnbull 1989). This varied from 100% clinical resolution for patients undergoing both self expanding metal stents (SEMS) and surgery (Zhang 2012), to a 63% return of normal bowel function (Lau 1993). Studies that stated symptom control or palliation in their outcomes frequently created a definition that failed to describe which symptoms (e.g. pain, nausea etc.) were managed (Chi 2009; Bais 1995; Beattie 1989; Jong 1995; Lo 1991; Lund 1989; Parveen 2009; Soo 1988). These tended to be composite measures that included various definitions of survival, normal bowel function, symptom relief, ability to tolerate oral intake, ability to return home, relief of obstruction and re‐obstruction. Parveen 2009 reported 26.7% success in symptomatic relief and return of bowel function with surgery, while Bais 1995 described 68% success in terms of restoration of intestinal function, discharge from hospital and survival for more than 60 days.
Despite being an inadequate proxy for symptom resolution or quality of life, the ability to feed orally was a popular outcome measure and featured in 18 studies. It was often described as "successful palliation" (Bartels 1995; Blair 2001; Ellis 1991; Fiori 2012; Furuya 2012; Goto 2012; Katz 1981; Kolomainen 2012; Lo 1991; Nakane 1996; Pecorelli 1994; Perri 2014; Pothuri 2003; Pothuri 2004; Rubin 1989; Sartori 2009;Turnbull 1989; Wong 2009). However, the definition of tolerating oral intake varied considerably and included improvement in oral intake (Nakane 1996), solid food at discharge (Blair 2001), average length of time for feeding to be achieved (Furuya 2012), and the ability to eat solid foods for at least 60 days (Goto 2012). Outcome success rates of oral feeding ranged from 30% (Pothuri 2004 ‐ ability to tolerate a regular or low residue diet 60 days postoperatively) to 100% (Fiori 2012 – oral feeding possible at 24 hours for SEMS and 96 hours for colostomy).
Surgical benefit in some of the papers was defined as survival, despite numerous papers having shown that this definition is too narrow. In three papers survival was the only outcome reported (Abbas 2007; Mangili 2005; Sartori 2010), and in seven studies it was the primary focus supplemented by days in hospital, unrelieved obstruction or re‐obstruction rates (Castaldo 1981; Krebs 1983; Redman 1988; Solomon 1983; Spears 1988; Sun 1995; Zoetmulder 1994). In contrast, several papers reported no survival/mortality data (Fiori 2012; Turnbull 1989; Zhang 2012).
The two prospective papers both compared operation with endoscopic procedures and reported on survival and undefined "symptom resolution and recurrence" or oral intake respectively (Chi 2009; Fiori 2012). Chi 2009 reported rates of symptom recurrence or death at 60 days as 4/14 for the operative group and 6/12 for the endoscopic group (percutaneous endoscopic gastrostomy or colonic stent). At 90 days the rate of symptom recurrence or death was 5/14 for the operative group and 7/12 for the endoscopic group. Fiori 2012 showed 100% oral intake tolerated by both the group undergoing self expanding metallic stenting at 24 hours and the group undergoing colostomy at 96 hours.
Two population‐based studies contributed almost 60% of the patient data (2522 patients) (Mooney 2013; Winner 2013). These used billing data for medical activities to examine hospitalisation for obstruction and length of stay, in addition to mortality data (see section below). Mooney 2013 identified 8607 women with ovarian cancer, of which 1518 (17.6%) were hospitalised for obstruction subsequent to their cancer diagnosis. While non‐surgical patients had a shorter hospital stay compared with surgically managed patients (non‐surgical median 17 days versus surgical median 24 days, P value < 0.001), this reflected survival and the ratio of days out to days in hospital were no different: surgical 6.2:1; non‐surgical 5.3:1 (P value = 0.28) (Mooney 2013). The ratio of days out to days in hospital for patients with colon adenocarcinoma and obstruction also showed no difference in the paper Winner 2013: 4.5:1 non‐surgical management and 4.7:1 surgical management (P value = 0.695). Surgical patients had significantly more days in the intensive care unit (ICU) and longer hospitalisation (Winner 2013).
Re‐obstruction
Seventeen retrospective reviews (Bais 1995; Beattie 1989; Hoffman 1994; Jong 1995; Kolomainen 2012; Krebs 1983; Lau 1993; Lo 1991; Lund 1989; Perri 2014; Pothuri 2003; Pothuri 2004; Redman 1988; Solomon 1983; Soo 1988; Spears 1988; Zhang 2012), two population‐based studies (Mooney 2013; Winner 2013), and one prospective review (Philip 1997), gave data on the re‐obstruction rates in those patients who had undergone surgery. The vast majority of papers did not include information on time to re‐obstruction, which is another important determinant of whether the surgery was useful. There were some individual data on whether further operations were carried out and their success (often poorly defined) for the patients that re‐obstructed.
Again there was a wide range of outcomes reported with re‐obstruction rates after surgery of zero to 63% (Fiori 2012; Pothuri 2003). Pothuri 2003 reported the highest re‐obstruction rate of 63% (36/57 corrective surgical procedures in women with advanced ovarian cancer) ‐ these occurred after a mean of 4.5 months (range 14 days to 16 months). The lowest re‐obstruction rate of zero was reported in 11 patients with colon or colorectal cancer undergoing colostomy (Fiori 2012). The second lowest re‐obstruction rate of 9% is reported in 89 patients with colon or colorectal cancer and surgical laparotomy – time to re‐obstruction was not given (Zhang 2012). These two papers compared self expanding metal stent insertion with surgery in patients with colon or colorectal cancer, showing a stent re‐obstruction rate of 9% (1/11) and 20.7% (20/97) respectively (Fiori 2012; Zhang 2012). In addition, Chi 2009 considered stenting in ovarian malignancy and reported that 36% of patients (5/12) either died or had recurrent symptoms within 90 days.
Of the population–based cohort studies, Mooney 2013 found that 33.5% of surgically managed and 35.9% of non‐surgically managed patients with ovarian cancer were readmitted to hospital with obstruction at least once after discharge and this rate did not differ statistically (P value = 0.403). Winner 2013 reported an overall re‐obstruction rate in those who survived of 24.5% in colon cancer.
Mortality and morbidity
The vast majority of studies gave data on survival/mortality and/or morbidity, with the exception of four papers (Fiori 2012; Turnbull 1989; Verrees 1996; Zhang 2012). Again the range of postoperative mortality data presented varied greatly in terms of the timing of assessment. For example, perioperative mortality was used by two studies and ranged from 4% to 16% (Nakane 1996; Pecorelli 1994), while Ellis 1991 reported death before discharge as 23%, and Zoetmulder 1994 presented 15‐day (10%) and 45‐day (33%) mortality.
Of those reporting postoperative 30‐day mortality (Bais 1995; Bartels 1995; Beattie 1989; Castaldo 1981; Goto 2012; Hoffman 1994; Kolomainen 2012; Krebs 1983; Larson 1989; Lau 1993; Lo 1991; Lund 1989; Mangili 2005; Mooney 2013; Parveen 2009; Perri 2014; Redman 1988; Rubin 1989; Sartori 2010; Solomon 1983; Soo 1988; Spears 1988; Sun 1995; Winner 2013), the range was 4% (Hoffman 1994) to 40% (Parveen 2009).
The two population‐based cohort studies showed that surgical management of obstruction was associated with lower 30‐day mortality than non‐surgical management (Mooney 2013; Winner 2013). In patients with ovarian cancer surgical 30‐day mortality was 13.1% compared with 24.2% (P value < 0.001) (Mooney 2013), and in patients with colon adenocarcinoma is was 18.5% compared with 30.8% who were non‐surgically managed (P value = 0.003) (Winner 2013).
Comparison of survival information was complicated by how the data were presented, whether median or mean was used, and whether it included or excluded postoperative deaths. For patients undergoing surgical intervention, median survival ranged from two months (one to 31 months) (Spears 1988) to 8.4 months (Pothuri 2003). Three studies presented non‐surgically managed median survival data (Furuya 2012; Goto 2012; Sartori 2010), with a range of four weeks (0 to 102 weeks) (Sartori 2010) to 69 days (Goto 2012).
Postoperative morbidity rates were available for fewer papers (Bais 1995; Bartels 1995; Beattie 1989; Castaldo 1981; Ellis 1991; Fiori 2012; Goto 2012; Hoffman 1994; Kolomainen 2012; Lau 1993; Lo 1991; Lund 1989; Mangili 2005; Parveen 2009; Pothuri 2003; Redman 1988; Rubin 1989; Solomon 1983), with a range of 5% (Solomon 1983) to 86.6% (Parveen 2009). Frequently occurring causes of postoperative morbidity included infection, dehiscence, sepsis, enterocutaneous fistulae, deep vein thrombosis, pulmonary embolism and myocardial infarction.
Quality of life scores
There were no data on quality of life scores, though a few papers did mention that this was an aim of the study.
Adverse effects
Re‐obstruction rates, morbidity and mortality were included as secondary outcomes (see above). In our patient group, mortality may or may not be due to the intervention, especially in the absence of robust control groups. Where commented on in the papers, we have detailed surgical complications.
Discussion
Summary of main results
In summarising the results, the original review and the update are considered together.
Of primary importance to treatment in the palliative setting, there were no data on quality of life scores following surgical or non‐surgical management of intestinal obstruction in patients with advanced gastrointestinal or gynaecological cancer.
The heterogeneity of outcomes reported prevented comparison between surgical and non‐surgical management and severely limits the findings of this review.
Re‐obstruction rates post‐surgical laparotomy are an important determinant of the usefulness of surgery. When reported, this ranged from 10% to 63% and time to re‐obstruction data were limited.
In terms of adverse effects of treatment, the definition of surgical outcomes varied between papers. When reported, postoperative mortality (death within 30 days) ranged from 0% to 32% and postoperative morbidity data were limited and ranged from 22% to 87%.
Overall completeness and applicability of evidence
This review included 43 studies, the vast majority of which were retrospective, with patients allocated to surgical or non‐surgical management by clinician/patient choice. While the quality of the data was therefore low, the applicability of the evidence was complicated by other factors: the outcomes used; benign verses malignant causes of obstruction; variation of clinical profiles presenting; desire to establish good prognostic variables and the use of stents as an alternative to surgery.
As was alluded to in the results, one of the major difficulties in comparing the different series was the different denominators used. Even an outcome as simple as operability, defined as the ability to perform a definitive procedure, had a range from 76% to 100% (Larson 1989; Lund 1989). These figures are, however, inaccurate as several of the papers excluded patients from consideration of surgery when the life expectancy was limited or when there was evidence of diffuse intra‐abdominal disease. Equally not all of the reports separated benign or malignant causes of obstruction ‐ again a higher rate of success will inadvertently be found for the combined results.
The other major issue was of benign disease causing the obstruction ‐ benign causes of obstruction in advanced gynaecological or gastrointestinal cancer (caused by previous surgery, radiotherapy or intraperitoneal chemotherapy) are said to occur in up to 35% of cases (Soo 1988), and thus provide a rationale for surgical intervention: " ...these patients will have long‐term survival if managed appropriately" (Clarke‐Pearson 1994). This view has been challenged. Hogan and Boente state that benign intestinal obstruction is rare with advanced ovarian cancer in contrast to other malignancies (Hogan 1993). Woolfson et al showed that no patient with bowel obstruction developing more than five years after treatment of the primary tumour had carcinomatosis as a cause. Of the 75 patients whose obstruction occurred within five years, the longer the interval the greater the chance that it was benign (Woolfson 1997).
Many variables also affect operability in this group of patients. In advanced malignancy, patients may present with a variety of clinical pictures. In contrast to de novo presentation of acute obstruction, in advanced malignancy it is important that the treating team consider the wider clinical context and determine the patient's wishes. While the majority of the papers included in this review focused on treatment in the acute setting, Fiori et al looked at the management of patients with chronic subacute obstruction (Fiori 2012). The period of conservative management of 'drip and suck' is variable, as is the policy towards parenteral nutrition in this group of patients (Feuer 1999a). Other variables that may be related to an improved outcome include whether the patient is managed within a multidisciplinary setting (Higginson 1997; Junor 1994), and whether a gynaecological oncological surgeon carries out the primary surgery (in ovarian cancer) (Kehoe 1994).
Many groups have, by univariate and multivariate analysis, tried to analyse what are the 'good' prognostic variables in deciding whether surgery is appropriate. Suggestions have included low grade of tumour, long time interval since the original operation, age, ascites, albumin level and degree of secondary disease, as well as previous radiotherapy/chemotherapy and performance status (Clarke‐Pearson 1988; Fernandes 1988; Gallick 1986; Krebs 1983; Larson 1989; Rubin 1989; Zoetmulder 1994). Different groups, however, come to different conclusions, the evidence is all based on retrospective case series and mortality, not symptom control, is used as the primary endpoint. Without further validated outcome scores that reflect why the operation was carried out this information is clearly of limited value.
In updating the original review, it is clear that there is a new interest in the role of stenting in the management of obstruction. While some of these papers provided data for this review (Chi 2009; Fiori 2012; Zhang 2012), many more were not suitable for inclusion (Badgwell 2011; Huhtinen 2013; Lee 2011; Suarez 2010; Xinopoulos 2004). Patients with symptoms of malignant bowel obstruction in previously treated advanced gynaecological and gastrointestinal cancer are likely to have multiple levels of obstruction, making them less likely to be suitable for stenting.
Quality of the evidence
The vast majority of studies had unclear or high risk of bias. Selection bias within the non‐randomised studies was the main problem as treatment allocation depended on clinician/patient preference, with patients managed with surgery tending to be in better overall health. This, in addition to the difficulties with heterogeneity of outcomes discussed above, severely limits the conclusions that can be drawn.
Potential biases in the review process
A limitation of this review is the difficulty in identifying the population of palliative patients. Therefore we sought to include studies that involved patients with previously known advanced gastrointestinal or gynaecological cancer and not de novo presentation. However, it is still difficult to identify patient groups who are appropriate for inclusion as the focus of original studies is mainly on the management of obstruction rather than the decision‐making process in the palliative setting. It is therefore possible that data on palliative patients are hidden in studies not described as relevant to the palliative setting. Additionally, data on de novo diagnoses of advanced cancer or benign causes of gastrointestinal obstruction may have been included in some studies described in this review.
The Cochrane 'Risk of bias' assessment tool for non‐randomised studies of interventions, ACROBAT‐NRSI 2014, would have been a more suitable appraisal instrument but was not available at the time this review began. The use of the randomised controlled trial 'Risk of bias' tool presents a potential bias and carries two main problems. Firstly the RCT tool does not adequately assess how investigators in non‐randomised studies might have attempted to adjust analyses for known imbalances and secondly it does not encourage authors to consider how the direction of the effect is affected by the bias in question. As such, some confounders will operate in favour of the intervention and others against.
Clearly potential publication bias may effect the validity of this review, i.e. studies that did not find a statistically significant difference between treatments were not published, which can lead to an overestimation of intervention effects. We were unable to assess this as analysis was restricted to description only. However, we did seek to avoid duplicate publication bias and to identify all duplicate publications. We identified two publications as overlapping substantially and while we considered both studies, the participants were included only once. The impact of publication bias may also be due to the problems of designing and implementing a search strategy, so even published studies may have been missed due to the search terms having to exclude filters.
Agreements and disagreements with other studies or reviews
A systematic review of the management of malignant bowel obstruction in advanced ovarian cancer, Kucukmetin 2010, found one small non‐randomised study comparing medical and surgical treatment that met their inclusion criteria. The study showed a longer survival time in those treated with surgery. They adjusted for prognostic factors, but it was unclear how the better performance status of women chosen for surgery contributed to these results.
In contrast to this review, Kucukmetin 2010 considered intestinal obstruction secondary to ovarian cancer as pathophysiologically different from gastrointestinal malignancy and therefore this required separate evaluation. Additionally, they sought to minimise selection bias by only including studies that used statistical adjustment for baseline characteristics using multivariable analysis. Despite these attempts to maximise validity, the review encountered similar difficulties to ours in the limited empirical evidence available and the lack of quality of life data essential for palliative decisions.
Authors' conclusions
Implications for practice.
No clear implications for practice can be given. There are a range of options available to the clinician including: active medical management (e.g. use of steroids and prokinetic drugs to promote the resolution of obstruction), medical symptom management, oncological management, interventional radiological management (stenting) and a variety of surgical procedures. Patients may benefit from one or more of these approaches. We acknowledge that the decision‐making is difficult and advocate a multi‐professional approach, with individualised care and empowering patient choice.
Implications for research.
It is clear from the original systematic review that, without validated outcome measures for symptom control and quality of life in well‐designed prospective trials on all patients with malignant bowel obstruction, the decision to operate or not will still be left to individual preference and chance.
Between the original review, Feuer 2000, and this update there has been no progress. The need for well‐designed prospective trials remains. We recommend collaboration between palliative care physicians, oncologists and surgeons, to design and run relevant studies that inform the practice of all specialties. This would necessitate the use of existing validated quality of life and symptom burden outcome measures and may require the use of multiple centres to recruit adequate numbers.
What's new
| Date | Event | Description |
|---|---|---|
| 21 March 2016 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 22 June 2015 | New citation required but conclusions have not changed | The additional 18 studies added to this review update did not alter the original conclusions as the majority were retrospective case note reviews with poorly validated outcome criteria. |
| 22 June 2015 | New search has been performed | This updated review was originally published in 2000. It now includes 18 new studies (Abbas 2007; Blair 2001; Chi 2009; Fiori 2012; Furuya 2012; Goto 2012; Kolomainen 2012; Mangili 2005; Mooney 2013; Parveen 2009; Perri 2014; Pothuri 2003; Pothuri 2004; Sartori 2009; Sartori 2010; Winner 2013; Wong 2009: Zhang 2012). This review update also identified 18 further studies to exclude (Amikura 2010; Badgwell 2009; Badgwell 2011; Chakraborty 2011; Dalal 2011; Davis 2001; Fitzgerald 2012; Kucukmetin 2010; Lee 2011; Legendre 2001; McCahill 2003; Miller 2000; Miner 2004; Miner 2005; Podnos 2007; Suarez 2010; Ubogagu 2010; Xinopoulos 2004). We also updated the 'Risk of bias' assessment and added 'Risk of bias' summary tables. |
| 25 September 2008 | New search has been performed | Converted to new review format. |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in ten years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The Systematic Review Steering Group (A'Hern R, Blake P, Chinn R, Gore M, Hardy J, Laverty D, Louw G, Shaw C, Tate T) and the Systematic Review Training Unit, University of London.
Dr Karen E Broadley was an author on the original review but was not involved in the update.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. 2015 search strategies
CENTRAL (The Cochrane Library)
#1 MeSH descriptor: [Gastrointestinal Neoplasms] explode all trees
#2 MeSH descriptor: [Genital Neoplasms, Female] explode all trees
#3 MeSH descriptor: [Ovarian Neoplasms] explode all trees
#4 gynaecological or gynecological
#5 gastrointestin* or gastro‐intestin* or intestin* or bowel* or colon* or colorectal or rectal* or stomach* or gastric*
#6 ovarian or ovary or ovaries
#7 endometrial or endometrium
#8 uterine or uterus or vaginal or vulvar or vagina*
#9 cervix or cervical
#10 #4 or #5 or #6 or #7 or #8 or #9
#11 MeSH descriptor: [Neoplasms] explode all trees
#12 neoplasm* or cancer* or carcinoma* or tumor* or tumour*
#13 #11 or #12
#14 #10 and #13
#15 #1 or #2 or #3 or #14
#16 MeSH descriptor: [Intestinal Obstruction] explode all trees
#17 (bowel* or intestin* or gastrointestin* or gastro‐intestin* or colon* or colorect* or retrosigmoid*) and (obstruct* or blockage)
#18 #16 or #17
#19 MeSH descriptor: [Colorectal Surgery] this term only
#20 MeSH descriptor: [Surgical Procedures, Elective] this term only
#21 surgery or surgical* or resect*
#22 #19 or #20 or #21
#23 #18 and #22
#24 #15 and #23
MEDLINE (OVID)
1 exp Gastrointestinal Neoplasms/
2 exp Genital Neoplasms, Female/
3 exp Ovarian Neoplasms/
4 (gynaecological or gynecological).mp.
5 (gastrointestin$ or gastro‐intestin$ or intestin$ or bowel$ or colon$ or colorectal or rectal$ or stomach$ or gastric$).mp.
6 (ovarian or ovary or ovaries).mp.
7 (endometrial or endometrium).mp.
8 (uterine or uterus or vaginal or vulvar or vagina$).mp.
9 (cervix or cervical).mp.
10 or/4‐9
11 Neoplasms/
12 (neoplasm$ or cancer$ or carcinoma$ or tumor$ or tumour$).mp.
13 or/11‐12
14 10 and 13
15 1 or 2 or 3 or 14
16 exp Intestinal Obstruction/
17 ((bowel$ or intestin$ or gastrointestin$ or gastro‐intestin$ or colon$ or colorect$ or retrosigmoid$) and (obstruct$ or blockage)).mp.
18 or/16‐17
19 Colorectal Surgery/
20 Surgical Procedures, Elective/
21 (surgery or surgical$ or resect$).mp.
22 19 or 20 or 21
23 18 and 22
EMBASE (OVID)
1 Gastrointestinal tumor/
2 exp Female genital tract tumor/
3 Ovary cancer/
4 (gynaecological or gynecological).mp.
5 (gastrointestin$ or gastro‐intestin$ or intestin$ or bowel$ or colon$ or colorectal or rectal$ or stomach$ or gastric$).mp.
6 (ovarian or ovary or ovaries).mp.
7 (endometrial or endometrium).mp.
8 (uterine or uterus or vaginal or vulvar or vagina$).mp.
9 (cervix or cervical).mp.
10 or/4‐9
11 Neoplasms/
12 (neoplasm$ or cancer$ or carcinoma$ or tumor$ or tumour$).mp.
13 or/11‐12
14 10 and 13
15 1 or 2 or 3 or 14
16 exp Intestinal Obstruction/
17 ((bowel$ or intestin$ or gastrointestin$ or gastro‐intestin$ or colon$ or colorect$ or retrosigmoid$) and (obstruct$ or blockage)).mp.
18 or/16‐17
19 Colorectal Surgery/
20 exp intestine surgery/ or Colon Surgery/ or Rectum Surgery/ or Intestine Resection/ or Elective Surgery/
21 (surgery or surgical$ or resect$).mp.
22 19 or 20 or 21
23 18 and 22
24 15 and 23
24 15 and 23
Appendix 2. Original review 2000: searching other resources
The searching of other resources was performed for the original review but yielded very few additional studies. The Cochrane Pain, Palliative and Supportive Care Review Group no longer routinely handsearch journals. For these reasons, we did not repeat the searching of other resources for the 2015 update.
Handsearching
Using information from the above searches, the journals most likely to include trials were identified, and after discussion with the content experts, the following publications were searched.
a) Journals
Palliative Medicine ‐ from inception January 1987 to 1997.
Journal of Pain and Symptom Management ‐ from inception Winter 1986 to 1997.
Gynaecologic Oncology ‐ from January 1993 to 1997.
The Hospice Journal ‐ from inception Spring 1985 to 1997.
Journal of Palliative Care ‐ from inception September 1985 to 1997.
b) Bibliographic databases
Progress in Palliative Care ‐ from inception October 1993 to 1997.
Terminal Care Index ‐ via the British Library Health Care Information Service ‐ from inception (July 1988) to December 1990 and then continued as Palliative Care Index from January 1991 to 1997.
c) Conference proceedings
The following conference proceedings were searched for relevant abstracts:
European Congress on Palliative Care, Number IV and Number V.
The American Society of Clinical Oncology (ASCO) 1990 to 1997.
The European Cancer Conference (ECCO) 1990 to 1997.
d) Reference searching
Using chapters from standard textbooks on malignant bowel obstruction, reference lists were searched:
The Oxford Textbook of Palliative Medicine, edited by Doyle, Hanks and MacDonald (second edition).
Cancer. Principles and Practice of Oncology, edited by DeVita, Hellman and Rosenberg (fifth edition).
Symptom Management in Advanced Cancer, edited by Twycross (second edition).
Science Citation Index
Each included study was also found on the Science Citation Index and followed up for future studies.
RCT registries and ongoing trials
The following organisations were contacted for information about ongoing trials:
National Cancer Institute of America.
United Kingdom Co‐ordinating Committee on Cancer Research (UKCCCR).
The National Clinical Trials Registry of Australia: Cancer Trials.
French Cancer Clinical Trials Registry (FNCLCC).
Personal contact
Contact with every member of the Association for Palliative Medicine (651 members) was made by letter, asking for information, particularly unpublished work or research in progress.
Contact by letter with international associations of palliative medicine (26) (obtained via the Hospice Information Centre at St Christopher's Hospice). A covering letter was sent asking them to mail‐list their members using a standard letter which was also enclosed.
Contact with acknowledged experts (12), i.e. those authors who had previously published extensively in the palliative medicine literature or are active in palliative care research as defined by content experts.
Contact with the major gynaecological oncology units (18) as specified by the Royal College of Obstetricians and Gynaecologists.
Contact with the London Group of Medical Oncologists and Radiotherapists (30).
Contact with individual authors of the studies found.
Meeting with Dr Baines (author of seminal paper).
Letter published in Palliative Medicine and Progress in Palliative Medicine, explaining study and requesting information.
Contact with editor of Palliative Medicine, to discuss if he was aware of any papers or reports not published.
Contact with Cochrane Review Groups: Colorectal Cancer Group and Gynaecological Cancer Group.
Contact and discussion with librarian at Halley Stewart Library at St Christopher's Hospice, and use of in‐house library database.
World wide web
The following websites were visited and searched using text words:
Doctors' Guide to the Internet.
Health on the Net Foundation.
Cancer News on the Net.
NCI Cancer Net Info.
Attempts were made to obtain full text translations of all relevant non‐English articles.
Appendix 3. Original review 2000: assessment of risk bias in included studies
For the original review, the evaluation of methodological quality of the trials included is described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Each trial was rated according to the quality of allocation concealment categories.
Category A: adequate concealment Category B: uncertain, indication of adequate Category C: inadequate concealment Category D: not used
The quality of the research was graded using the following criteria (Mann 1996):
Grade I (Strong evidence) Randomised controlled trial (RCT) or review of randomised controlled trials
IA: Calculation of sample size and accurate and standard definition of outcome variables
IB: Accurate and standard definition of outcome variables
IC: None of the above
Grade II (Fairly strong evidence) Prospective study with a comparison group (non‐randomised controlled trial or good observation study)
IIA Calculation of sample size and accurate, standard definition of outcome variables and adjustment for the effects of important confounding variables
IIB: One of the above
IIC: None of the above
Grade III (Weak evidence) Retrospective study
IIIA: Comparison group, calculation of sample size and accurate and standard definition of outcome variables
IIIB: Two of the above criteria
IIIC: None of the above
Grade IV (Weak evidence) Cross‐sectional study
These grades broadly correspond with the Clinical Outcomes of Group categories of evidence for use in clinical practice guidelines, where I=A, II or III=B, IV=C.
Differences in data extraction was resolved by referring back to the original article and utilising consensus between the two reviewers. Where necessary, information was sought from the authors of the primary study for clarification or missing information.
Appendix 4. Original review 2000: table of grade
For the original review, the evaluation of methodological quality of the trials included is described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) ‐ see Appendix 3 for details.
| Study |
Assessment of bias |
| Bais 1995 | IIIC |
| Bartels 1995 | IIIC |
| Beattie 1989 | IIIC |
| Castaldo 1981 | IIIC |
| Ellis 1991 | IIIC |
| Hoffman 1994 | IIIC |
| Jong 1995 | IIIC |
| Katz 1981 | IIIC |
| Krebs 1983 | IIIC |
| Larson 1989 | IIIC |
| Lau 1993 | IIIC |
| Lo 1991 | IIIC |
| Lund 1989 | IIIC |
| Nakane 1996 | IIIC |
| Pecorelli 1994 | IIIC |
| Philip 1997 | IIC |
| Redman 1988 | IIIC |
| Rubin 1989 | IIIC |
| Solomon 1983 | IIIC |
| Soo 1988 | IIIC |
| Spears 1988 | IIIC |
| Sun 1995 | IIIC |
| Turnbull 1989 | IIIC |
| Verrees 1996 | IIIC |
| Zoetmulder 1994 | IIIC |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbas 2007.
| Methods | Retrospective review | |
| Participants | 79 patients with any malignancy from 1992 to 2003; 31 colorectal and 19 gynaecological | |
| Interventions | All patients underwent surgery | |
| Outcomes | 11 colorectal and 0 gynaecological patients were inoperable. Median survival colorectal 7.4 months and gynaecological 3.7 months | |
| Notes | Complication data available but only for all‐cause malignancy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | None. "Patients undergoing laparotomy for resection of peritoneal metastases from recurrence of previous cancer between 1992‐2003 were reviewed retrospectively." |
| Allocation concealment (selection bias) | High risk | None. "Patients undergoing laparotomy for resection of peritoneal metastases from recurrence of previous cancer between 1992‐2003 were reviewed retrospectively." |
| Blinding (performance bias and detection bias) All outcomes | High risk | None. "Patients undergoing laparotomy for resection of peritoneal metastases from recurrence of previous cancer between 1992‐2003 were reviewed retrospectively." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None. "Patients undergoing laparotomy for resection of peritoneal metastases from recurrence of previous cancer between 1992‐2003 were reviewed retrospectively." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None. "Patients undergoing laparotomy for resection of peritoneal metastases from recurrence of previous cancer between 1992‐2003 were reviewed retrospectively." |
| Selective reporting (reporting bias) | Unclear risk | Mortality and morbidity data are for all diagnoses not by cancer type. Results not reported according to surgical intervention |
Bais 1995.
| Methods | Retrospective review | |
| Participants | 31 patients from 1974 to 1991 | |
| Interventions | 19 patients underwent surgery; definitive surgery in 17 patients | |
| Outcomes | Successful palliation: restoration of intestinal function, discharge from hospital, and survival > 60 days = 13/19 (68%) 2/19 (10%) patients required TPN Re‐obstruction: 4/19 (21%); 1 out of 4 re‐operated on and went home successfully Postoperative mortality: within 30 days 2/19 (11%); within 60 days 4/19 (22%) Postoperative morbidity: major 3/19 (16%) Mean survival: for 19 patients 109 days (15 to 775) | |
| Notes | Enteroenterostomy in 6/17, ileocolostomy in 8/17, resection in 3/17 Major morbidity defined as urosepsis, wound dehiscence and sepsis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported by surgery type |
Bartels 1995.
| Methods | Retrospective review | |
| Participants | 41 patients from 1978 to 1993 | |
| Interventions | Surgery ‐ see notes | |
| Outcomes | Surgical success: ability to take food within 30 days of operation; 56% of patients Survival: 3.5 months (median) for all patients Operative mortality: 17% of all patients Operative morbidity: 49% of all patients | |
| Notes | 83% of patients had a definitive surgical procedure (resection of bowel, stoma formation, lysis of adhesions at site of obstruction) No indication what morbidity included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported for morbidity but criteria for this unclear |
Beattie 1989.
| Methods | Retrospective review | |
| Participants | 105 patients from July 1981 to July 1986 | |
| Interventions | 43 patients had bowel obstruction, 11 patients had surgery (7 immediate, 4 failed conservative therapy) | |
| Outcomes | Complete responses: 7/11 (64%) No response: 4/11 (36%) Re‐obstruction: 1/11 (10%) Returned home: 7/11 (64%) Postoperative mortality: 1/11 (9%) Postoperative morbidity: 1 patient developed a faecal fistula Mean survival: surgery alone 211.4 days; conservative then surgery 99.6 days | |
| Notes | 3 patients who responded presented with obstruction at initial diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results only reported for patients undergoing surgery; no outcomes for conservative management Results not reported according to surgical intervention |
Blair 2001.
| Methods | Retrospective review | |
| Participants | 63 patients with any malignancy between 1995 and 2000; 31 colorectal, 1 small intestine and 5 GIST | |
| Interventions | Surgery in all patients | |
| Outcomes | 59% of colorectal patients tolerated solid food at the time of discharge Median survival for colorectal patients was 120 days (P value = 0.009) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | None. "Data on patients undergoing laparotomy for palliation of gastrointestinal MBO at City of Hope between 1995 and 2000 were retrospectively collected." |
| Allocation concealment (selection bias) | High risk | None. "Data on patients undergoing laparotomy for palliation of gastrointestinal MBO at City of Hope between 1995 and 2000 were retrospectively collected." |
| Blinding (performance bias and detection bias) All outcomes | High risk | None. "Data on patients undergoing laparotomy for palliation of gastrointestinal MBO at City of Hope between 1995 and 2000 were retrospectively collected." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None. "Data on patients undergoing laparotomy for palliation of gastrointestinal MBO at City of Hope between 1995 and 2000 were retrospectively collected." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None. "Data on patients undergoing laparotomy for palliation of gastrointestinal MBO at City of Hope between 1995 and 2000 were retrospectively collected." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes for all 63 patients reported Results not reported according to surgical intervention |
Castaldo 1981.
| Methods | Retrospective review | |
| Participants | 419 patients from 1968 to 1977 | |
| Interventions | 25 operations on 23 patients for bowel obstruction (2 patients underwent 2 operations) | |
| Outcomes | Successful palliation: defined survival > 8 weeks = 20/25 (80%) Hospital stay, post operation: mean 26 days (1 to 89 days) Postoperative mortality: 3/23 (13%) Postoperative morbidity: 10 patients suffered 22 complications | |
| Notes | No information on symptoms of successes Postoperative morbidity includes patients who died Complications: small bowel obstruction in 9, large bowel in 10 and combination in 6 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Successful palliation is defined but there is no information regarding symptoms in these patients Postoperative morbidity includes patients who died Results not reported according to surgical intervention |
Chi 2009.
| Methods | Prospective outcomes analysis | |
| Participants | 26 patients with ovarian cancer from July 2002 to July 2003 | |
| Interventions | Operation (14 patients), endoscopic intervention (12 patients) | |
| Outcomes | In the surgical group 4 patients (29%) either died or had recurrent symptoms within 60 days; in the endoscopic intervention group 5 (36%) either died or had recurrent symptoms within 90 days 9 patients (64%) of those undergoing surgery had symptom control for more than 90 days Median survival for those undergoing surgery was 191 days (33 to 902) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients underwent operative or endoscopic intervention as per local practice. There was no randomisation. Patients were identified once intervention had been decided and outcomes were looked at prospectively "...the operating room and endoscopy suite schedules were screened on a daily basis to identify all operative and endoscopic cases performed for symptomatic management of metastatic or advanced cancer." |
| Allocation concealment (selection bias) | High risk | Intervention was allocated as per local practice prior to inclusion in the study |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. "...the operating room and endoscopy suite schedules were screened on a daily basis to identify all operative and endoscopic cases performed for symptomatic management of metastatic or advanced cancer." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. "Evidence of symptom resolution and/or development of new symptoms was collected from the patient record." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported specifically for patients with bowel obstruction due to metastatic ovarian cancer Outcomes reported for all patients: "In this study, all patients were followed until death." Results not reported according to surgical intervention |
Ellis 1991.
| Methods | Retrospective review | |
| Participants | 134 patients (138 procedures) from 1979 to 1989 | |
| Interventions | 26 patients had a malignant cause of bowel obstruction confirmed | |
| Outcomes | Successful palliation: the ability to tolerate regular oral feeding at discharge; 20/26 (77%) Operative mortality: death occurring before discharge; 6/26 (23%) Morbidity: 14/26 (54%) | |
| Notes | Figures in the table do not add up (20/26 calculated from data in paper) All 26 patients had resection or anastomosis No indication what morbidity was |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | High risk | Figures in table do not add up |
Fiori 2012.
| Methods | Prospective randomised trial | |
| Participants | 22 patients January 2003 to May 2003. Stage IV unresectable colorectal cancer with symptoms of subacute obstruction > 3 months | |
| Interventions | Colostomy 11/22; transanal self expandable metallic stent (SEMS) 11/22 | |
| Outcomes | Oral feeding and bowel function: SEMS 100% 24 hours; colostomy 100% 96 hours SEMS group further symptoms 27% (3/11): average 100 days from procedure; 2 patients had faecal impaction and 1 patient had re‐obstruction with tumour in‐growth Colostomy group no further symptoms SEMS group complications Colostomy group complications: stoma prolapse: 9% (1/11), skin irritation 9% (1/11), anaemia requiring blood transfusion 18% (2/11) |
|
| Notes | Patients and families complained the stoma significantly interfered with lifestyle; none of the patients who had stent placement complained about the procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to 1 of the 2 forms of treatment according to random numbers table." Patients assigned to transanal self expanding metallic stent or diverting proximal colostomy |
| Allocation concealment (selection bias) | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients assigned to transanal self expanding metallic stent or diverting proximal colostomy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients assigned to transanal self expanding metallic stent or diverting proximal colostomy |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Selective reporting (reporting bias) | Low risk | All patient outcomes reported Only one surgical procedure used |
Furuya 2012.
| Methods | Retrospective review | |
| Participants | 24 patients with advanced gynaecological cancer, who developed bowel obstruction and later died with gynaecological cancer between 1996 and 2010 in a single centre | |
| Interventions | Surgical intervention 11 patients: 8 ileostomy; 3 percutaneous endoscopic gastrostomy Conservative management 13 patients |
|
| Outcomes | Average survival period: surgical 101 days; conservative 51 days Oral intake achieved: ileostomy 88%; PEG 100%; conservative 50% Oral intake length: ileostomy 50 days; PEG 48 days; conservative 10 days Rate of outpatient: ileostomy 50%; PEG 66%; conservative 21% Period at home: ileostomy 29 days; PEG 40 days; conservative 8 days |
|
| Notes | Poster abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective review of case notes. |
| Allocation concealment (selection bias) | High risk | Intervention clinical decision. Surgical, endoscopic or conservative |
| Blinding (performance bias and detection bias) All outcomes | High risk | Intervention clinical decision. Surgical, endoscopic or conservative |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention clinical decision. Surgical, endoscopic or conservative |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patient notes analysed retrospectively |
| Selective reporting (reporting bias) | Low risk | Outcomes reported for all 24 patients Results reported by surgical intervention |
Goto 2012.
| Methods | Retrospective review | |
| Participants | 53 patients with gynaecological malignancy and malignant bowel obstruction due to disease progression or recurrence between 2005 and 2010 Excluded: "cases with symptoms of bowel obstruction which were temporary and restorable with short medical treatment were excluded" |
|
| Interventions | 33 patients pharmacological treatment 20 patients laparotomy: colostomy 11; ileostomy 7; bypass 7 |
|
| Outcomes | Successful palliation defined as: ability to eat solid food for at least 60 days Surgical group: achieved 70% (14/20); symptoms unrelieved 10% (2/20) Postoperative complications 35% (7/20): infections and wound dehiscence 15% (3/20); abscess 20% (4/20); sepsis 5% (1/20); DVT 5% (1/20); short bowel syndrome 5% (1/20) Postoperative mortality within 30 days: 5% (1/20) Median survival following diagnosis of MBO operative: 146 days (61 to 294) Median survival following diagnosis of MBO non‐operative: 69 days |
|
| Notes | Operative group selected as fit for surgery and did better | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Of all the patients with gynaecological malignancy treated at our institute, medical records of patients who presented with MBO due to disease progression or recurrence between 2005 and 2010 were reviewed." |
| Allocation concealment (selection bias) | High risk | "Of all the patients with gynaecological malignancy treated at our institute, medical records of patients who presented with MBO due to disease progression or recurrence between 2005 and 2010 were reviewed." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Of all the patients with gynaecological malignancy treated at our institute, medical records of patients who presented with MBO due to disease progression or recurrence between 2005 and 2010 were reviewed." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Of all the patients with gynaecological malignancy treated at our institute, medical records of patients who presented with MBO due to disease progression or recurrence between 2005 and 2010 were reviewed." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Of all the patients with gynaecological malignancy treated at our institute, medical records of patients who presented with MBO due to disease progression or recurrence between 2005 and 2010 were reviewed." |
| Selective reporting (reporting bias) | Unclear risk | All patient outcomes reported for surgical group Conservative treatment group data limited Results not reported according to surgical intervention |
Hoffman 1994.
| Methods | Retrospective review | |
| Participants | 43 patients with recurrent/persistent cancer from July 1985 to June 1992 26 patients had intestinal obstruction due to disease or radiotherapy | |
| Interventions | Surgery on the 26 patients | |
| Outcomes | Return of bowel function: 22/26 (85%) Unrelieved obstruction: 2/26 (8%) discharged on liquid diet Re‐obstruction: 2/26 (8%) Postoperative mortality: 1/26 (4%) Postoperative morbidity: 1 patient developed rectovaginal/vesicovaginal fistulas | |
| Notes | All patients had received prior radiotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported according to surgical intervention |
Jong 1995.
| Methods | Retrospective review | |
| Participants | 53 patients from January 1982 to January 1992 | |
| Interventions | Surgery on 53 patients | |
| Outcomes | Successful palliation: defined as survival > 60 days, ability to return home and relief of obstruction > 60 days; 27/53 (51%) 28/53 (53%) were relieved of obstruction > 60 days 36/53 (67%) went home Unrelieved obstruction/re‐obstruction: 21/53 (40%) Median survival: 87.5 days (5 to 892) for 50 patients | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported according to surgical intervention |
Katz 1981.
| Methods | Retrospective review | |
| Participants | 2 patients with ovarian carcinoma | |
| Interventions | Surgery | |
| Outcomes | Descriptive results: both patients were able to tolerate a regular diet, with no postoperative vomiting | |
| Notes | Case reports of gastric outlet obstruction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Case report of 2 patients with ovarian carcinoma |
| Allocation concealment (selection bias) | High risk | Case report of 2 patients with ovarian carcinoma |
| Blinding (performance bias and detection bias) All outcomes | High risk | Case report of 2 patients with ovarian carcinoma |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Case report of 2 patients with ovarian carcinoma |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Case report of 2 patients with ovarian carcinoma |
| Selective reporting (reporting bias) | High risk | Descriptive results |
Kolomainen 2012.
| Methods | Retrospective review | |
| Participants | 90 women with relapsed epithelial ovarian cancer who underwent palliative surgery for bowel obstruction 1992 to 2008: both elective and emergency (surgery within 24 hours of decision to operate) | |
| Interventions | Gastrostomy 19/90 (alone 15/90, as part of procedure 4/90); bypass +/‐ stoma 5/90; stoma 56/90; anastomosis 17/90; bowel resection 22/90 No conservative treatment |
|
| Outcomes | "Successful palliation" as independent of IV fluids and adequate oral intake at 60 days postoperative: overall 66% (59/90); emergency 69% (34/49); elective 63% (25/40) Postoperative mortality: overall 18% (16/90); emergency 20% (10/49); elective 15% (6/40) Postoperative morbidity rate: 27% (24/90); return to operating room 2/90; high‐output stoma 9/90; retraction of stoma 3/90; parastomal abscess 1/90; superficial wound breakdown 2/90; wound infection 3/90; wound dehiscence 2/90; prolonged ileus 1/90; intra‐abdominal/sub‐sheath collections 1/90; medical complications 5/90 Median overall survival: 90.5 days (< 1 day to 6 years) Re‐obstruction rate: 10 patients further obstructed and no further surgery |
|
| Notes | Unclear outcome 1 patient | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No allocation. All patients included in study underwent surgery. "Indication of surgery was based on clinical findings of an acute abdomen, failure of bowel obstruction to resolved following a period of conservative management." |
| Allocation concealment (selection bias) | High risk | No blinding. All patients included in study underwent surgery. Outcomes identified from review of notes made by clinicians at time of treatment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. All patients included in study underwent surgery. Outcomes identified from review of notes made by clinicians at time of treatment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. All patients included in study underwent surgery. Outcomes identified from review of notes made by clinicians at time of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. All patients included in study underwent surgery. Outcomes identified from review of notes made by clinicians at time of treatment |
| Selective reporting (reporting bias) | Unclear risk | Unclear outcome 1 patient Results not reported according to surgical intervention Symptoms not included in "successful palliation" |
Krebs 1983.
| Methods | Retrospective review | |
| Participants | 118 procedures in 98 patients from 1960 to 1980 | |
| Interventions | 104 patients had corrective surgery; 14 patients had inoperable disease | |
| Outcomes | Successful palliation/benefit: defined by Castaldo: survival > 2 months = 65% Unrelieved obstruction: 2/104 (2%) Re‐obstruction: 20/98 (20%) Postoperative mortality: within 4 weeks: 12/104 (12%); within 8 weeks 15/92 Median survival: 12.5 weeks (1 to 78) | |
| Notes | Small bowel: bypass in 28 patients, bypass with enteroenterostomy in 11, resection in 25 Large bowel: bypass in 25 cases 15 patients had small and large bowel obstruction and had combined procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported for different tumour sites or surgical procedures |
Larson 1989.
| Methods | Retrospective review | |
| Participants | 33 patients from July 1980 to June 1987; 56 admissions for these 33 patients | |
| Interventions | 19 operations for obstruction took place | |
| Outcomes | Symptoms at death: in all 6/19 patients known to have died, all 6 had nausea, vomiting, inability to eat and constipation at death Postoperative mortality: (within 30 days) 15.8% Median survival: 102 days | |
| Notes | 12/19 had bypass, 2/19 colostomy, 5/19 had bypass + colostomy/ gastrostomy 17/33 patients spent 1/3 of their remaining life in hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported for different surgical procedures |
Lau 1993.
| Methods | Retrospective review | |
| Participants | 33 patients from 1980 to 1990 | |
| Interventions | 27 patients had corrective surgery 3 patients had open/shut laparotomy | |
| Outcomes | Return of bowel function: 19/30 (63%) Return home: 17/30 (56%) Unrelieved obstruction: 6/30 (20%) Re‐obstruction: 8/17 (47%) re‐laparotomy in 4: 1/4 died, 1/4 recovered, 2/4 repeated obstruction Postoperative mortality: 5/30 (17%) Postoperative morbidity: 8/30 (27%) | |
| Notes | 11 patients had bypass, 4 resection, 4 colostomy, 5 combination Mean interval to re‐obstruction was 120 days (30 to 186) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported by surgical procedure |
Lo 1991.
| Methods | Retrospective review | |
| Participants | 51 patients from January 1986 to December 1988; all had gastric outlet obstruction | |
| Interventions | Surgery ‐ see notes | |
| Outcomes | Palliation: defined as relief of symptoms, able to eat normal food with no recurrence of symptoms for > 6 weeks; 60% Time to resume diet: 3 to 18 days (all 40 patients who survived) Postoperative stay: mean 13.3 days (5 to 44) Re‐obstruction: 3/51; all 3 re‐operated, 2/3 died within 15 days, 1 died at 11 weeks Postoperative mortality: within 30 days, 11/51 (22%) Postoperative morbidity: 55% Median survival: (excluding postoperative death) 11 weeks (6 to 44) | |
| Notes | 51 patients underwent gastrojejunostomy ‐ 45 antecolic, 3 retrocolic, 3 roux‐en‐Y Postoperative morbidity: delayed gastric emptying, infection, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Low risk | Single surgical procedure although different techniques used |
Lund 1989.
| Methods | Retrospective review | |
| Participants | 45 patients from January 1981 to December 1986 | |
| Interventions | 25 patients had surgery ‐ 19 had corrective surgery; 16 patients conservative therapy; 4 patients not included in analysis | |
| Outcomes | Surgical benefit: defined as survival > 60 days without symptoms of incomplete or complete obstruction: 8/19 (42%) Re‐obstruction: 3/8 (38%) median time 106 days (100 to 200 days) Postoperative mortality: within 30 days: 32% Postoperative morbidity: 64% Median survival: 68 days (7 to 919) | |
| Notes | 23 had small intestinal obstruction, 13 had large intestinal obstruction, and 5 combined 13 patients had colostomy +/‐ resection, 4 had bypass +/‐ resection, 2 lysis of malignant adhesions 6 patients only had exploratory laparotomy Postoperative morbidity includes infection, herniation, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported by surgical procedure |
Mangili 2005.
| Methods | Retrospective case series | |
| Participants | 47 women with intestinal obstruction secondary to ovarian cancer | |
| Interventions | Non‐randomised 27 patients surgery: 4 inoperable, 2 gastrointestinal tubes placed, 8 colostomies, 9 intestinal bypass, 3 intestinal resections, 1 bypass and colostomy 20 patients medical management with octreotide: mean dosage of 0.48 mg/day; 1 required nasogastric tube |
|
| Outcomes | 30‐day mortality post‐surgery 22% Post‐surgery morbidity 22% (6 patients): 2 wound infection, 2 incisional dehiscence, 2 enterocutaneous fistula Mean survival (surgical and non‐surgical): 76 days Multivariant analysis showed women treated with surgery had significantly better survival than women treated with octreotide (P value < 0.001) after adjustment for performance status (and other prognotic factors) but no hazard ratio reported |
|
| Notes | Prognostic factors reported therefore possible to assess baseline imbalances; only performance status (PS 0, 1, 2) differed significantly (P value = 0.03) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported; probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported; probably not done |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes analysed with statistical adjustment for the differences in prognostic factors within the group and others not. When statistically significant, hazard ratio not presented |
Mooney 2013.
| Methods | Retrospective review | |
| Participants | 1518 1C‐IV ovarian carcinoma with bowel obstruction | |
| Interventions | Surgical management 373, non‐surgical management 1145 | |
| Outcomes | Median survival after first post‐diagnosis obstruction: surgical 162 days; non‐surgical 98 days, P value < 0.001 30‐day mortality; surgical 13.1%; non‐surgical 24.2%, P value < 0.001 Days in hospital, median (IQR): surgical 24 (16, 42), non‐surgical 17 (8, 32), P value < 0.001 Days out of hospital, median (IQR): surgical 137 (29, 536); non‐surgical 80 (17, 412), P value < 0.002 Ratio of days out to days in hospital: surgical 6.2:1; non‐surgical 5.3:1, P value = 0.28 Re‐admission for obstruction: surgical 33.5%; non‐surgical 35.9%, P value = 0.403 |
|
| Notes | Uses Surveillance, Epidemiology, and End Results ‐ Medicare database that tracks 26% of patients with cancer in the US population by searching for coded information on billing for procedures and services rendered Hospitalisation and re‐admission rates used as a proxy for quality of life markers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Not reported by surgical procedure ‐ unclear it this information was available via coding or not |
Nakane 1996.
| Methods | Retrospective review | |
| Participants | Retrospective review of 85 patients from January 1980 to December 1993 | |
| Interventions | 50 patients underwent laparotomy; 28 patients had peritoneal metastasis, 12 patients had local recurrence = 40 patients with recurrent disease | |
| Outcomes | Symptomatic relief: 24/31 (77%) after corrective surgery or catheter jejunostomy; 21/26 (80%) after corrective surgery Improvement of oral food intake: 17/26 (65%) Discharge home: 17/26 (65%) Perioperative death: 1/26 (4%) | |
| Notes | 10 had benign adhesions 9/40 patients had biopsy only Corrective surgery was bypass (14 patients), colostomy (7 patients) or resection (5 patients) No definition of symptomatic relief is present | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported by surgical procedure |
Parveen 2009.
| Methods | Retrospective case series | |
| Participants | 56 patients presenting to 3 surgical units with partial or complete obstruction on a background of ovarian cancer from March 1998 to April 2009 | |
| Interventions | Trial of conservative management to all patients first: IV fluids, anti‐spasmodics, nasogastric tube, nil by mouth 22 patients: conservative management successful 30 patients: conservative management unsuccessful and proceeded to laparotomy. Specifically: resection and anastomosis 9; bypass surgery 7; colostomy 3; Hartmann procedure 1; adhesiolysis 8; open/close 2 (advanced abdominal malignancy) 4 patients: conservative management unsuccessful but laparotomy not done due to uncontrolled ascites and cachexia |
|
| Outcomes | Conservative treatment: sufficient 39% (22/56) Surgical treatment "successful", i.e. symptomatic relief and return of bowel function: 26.7% (8/30) Postoperative mortality: 40% (12/30): mean survival after operation 4.2 months (range 2 to 6 months) Postoperative complications: 86.6% (26/30): wound infection 77% (22/30); UTI 23% (6/30); intra‐abdominal abscess 15% (4/30); burst abdomen 7.6% (2/30); enterocutaneous fistula 7.6% (2/30); pneumonia 7.6% (2/30); DVT 7.6% (2/30) |
|
| Notes | 8 of the 30 surgical patients had lysis of adhesions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Laparotomy was done if obstruction was not relieved by conservative treatment." |
| Allocation concealment (selection bias) | High risk | "Laparotomy was done if obstruction was not relieved by conservative treatment." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The record of all patients admitted in all three surgical units of Ayub Teaching Hospital from March 1998 to April 2008 with a diagnosis of intestinal obstruction were reviewed." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The record of all patients admitted in all three surgical units of Ayub Teaching Hospital from March 1998 to April 2008 with a diagnosis of intestinal obstruction were reviewed." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The record of all patients admitted in all three surgical units of Ayub Teaching Hospital from March 1998 to April 2008 with a diagnosis of intestinal obstruction were reviewed." |
| Selective reporting (reporting bias) | High risk | All patients accounted for Results not reported according to surgical intervention 8 patients underwent surgery for non‐malignant obstruction and were included in the outcomes |
Pecorelli 1994.
| Methods | Retrospective review | |
| Participants | 147 patients from 1973 to 1983; all patients had recurrent ovarian carcinoma | |
| Interventions | 117 patients had a definite procedure; 30 patients no surgical correction | |
| Outcomes | "Benefit of surgery" defined as able to leave hospital on a regular/low residue diet: 83/117 (71%) Perioperative mortality: 15/117 (13%) for corrective surgery; 23/147 (16%) for total Median survival: 3.5 months, mean 7.2 (? group) | |
| Notes | Intestinal resections in 13%, bypass in 17% and neostomy in 50% Benefit of surgery not synonymous with relief of symptoms as suggested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Median survival reported as 3.5 months but the group this is based on is not stated |
Perri 2014.
| Methods | Retrospective review | |
| Participants | 68 procedures on 62 patients with gynaecological malignancies between October 2004 and January 2013 at a single centre | |
| Interventions | 68 surgical procedures: colostomy 18, ileostomy 27, resection/bypass and anastomosis 13, colonic stent 1, gastrostomy 5, gastroenterostomy 4 | |
| Outcomes | Deaths prior to discharge: n = 18, within 3 to 81 days (median 25 days) Postoperative survival: median 106 days (range 3 to 1342) 30‐day mortality = 14.7% 60‐day mortality = 29.4% Postoperative oral intake = 65% Postoperative chemotherapy (additional) = 53% Re‐obstruction: 10/62 median 4.3 months (1 to 36 months) Severe complications: sepsis 5; leak from anastomosis 6; necrotising fascitis 2 |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if resolution of bowel obstruction is synonymous with relief of symptoms Not reported by procedure |
Philip 1997.
| Methods | Prospective review | |
| Participants | 33 patients (62 episodes of obstruction) over a 12‐month period | |
| Interventions | In 9 episodes surgery was used | |
| Outcomes | Resolution: 8/9 achieved resolution of obstruction Re‐obstruction: 4/8 in mean 5 months, median 2 months Survival: 35 weeks from time of first episode of bowel obstruction | |
| Notes | Not clear if resolution of bowel obstruction is synonymous with relief of symptoms Not clear how measured survival, mean or median |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and prospectively followed up |
| Allocation concealment (selection bias) | High risk | No comparison group |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and prospectively followed up |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and prospectively followed up |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and prospectively followed up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if resolution of bowel obstruction is synonymous with relief of symptoms Not clear how measured survival, mean or median |
Pothuri 2003.
| Methods | Retrospective review | |
| Participants | 68 operations on 64 patients; ovarian cancer | |
| Interventions | Surgery for all patients 1 exploratory laparotomy, 11 exploratory laparotomy and G‐tube/PEG insertion, 10 G tube insertions, 21 colostomies, 18 resections and 33 bypasses |
|
| Outcomes | 37 of 52 patients tolerated a regular or low residue diet 60 days postoperatively; 22% morbidity including 5 enteric fistulae, 5 abscesses, 2 pulmonary embolus, 2 bacterial peritonitis, 1 neutropenic fever 36/57 (63%) patients who had surgical correction re‐obstructed Mean time to re‐obstruction 4.5 months (14 days to 16 months) Median survival for all patients was 7.9 months For those in whom surgical correction was possible median survival was 8.4 months versus 3.5 months where surgical correction was not possible For those successfully palliated median survival was 11.6 months versus 3.9 months where it was not successful (P value < 0.01) For patients with small bowel obstruction median survival was 7.4 months, large bowel obstruction median survival was 7.7 months or both median survival was 9.7 months |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "We retrospectively reviewed all patients undergoing surgery for intestinal obstruction due to recurrent ovarian cancer from 1994‐1999." |
| Allocation concealment (selection bias) | High risk | "We retrospectively reviewed all patients undergoing surgery for intestinal obstruction due to recurrent ovarian cancer from 1994‐1999." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "We retrospectively reviewed all patients undergoing surgery for intestinal obstruction due to recurrent ovarian cancer from 1994‐1999." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "We retrospectively reviewed all patients undergoing surgery for intestinal obstruction due to recurrent ovarian cancer from 1994‐1999." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "We retrospectively reviewed all patients undergoing surgery for intestinal obstruction due to recurrent ovarian cancer from 1994‐1999." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported specifically for patients with bowel obstruction due to recurrent ovarian carcinoma Outcome reported for all 68 operations on 64 patients Results not reported according to surgical intervention |
Pothuri 2004.
| Methods | Retrospective review | |
| Participants | 10 patients with ovarian carcinoma | |
| Interventions | Surgery for all patients; 5 resections, 5 open close procedures | |
| Outcomes | 3 patents were able to tolerate a regular or low residue diet 60 days postoperatively 4 patients suffered complications (3 enterocutaneous fistulae and 1 wound infection) The 3 patients successfully palliated went on to have further chemotherapy 2 patients re‐obstructed at 3 and 6 months respectively Mean survival 4.5 months (3 to 17 months) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "We reviewed the records of all patients with ovarian cancer who underwent repeat surgery for recurrent, malignant bowel obstruction at our institution between 1994 and 2002." |
| Allocation concealment (selection bias) | High risk | "We reviewed the records of all patients with ovarian cancer who underwent repeat surgery for recurrent, malignant bowel obstruction at our institution between 1994 and 2002." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "We reviewed the records of all patients with ovarian cancer who underwent repeat surgery for recurrent, malignant bowel obstruction at our institution between 1994 and 2002." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "We reviewed the records of all patients with ovarian cancer who underwent repeat surgery for recurrent, malignant bowel obstruction at our institution between 1994 and 2002." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "We reviewed the records of all patients with ovarian cancer who underwent repeat surgery for recurrent, malignant bowel obstruction at our institution between 1994 and 2002." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported specifically for bowel obstruction due to ovarian carcinoma Outcomes reported for all 10 patients Results not reported according to surgical intervention |
Redman 1988.
| Methods | Retrospective review | |
| Participants | 38 patients from January 1976 to December 1986 | |
| Interventions | 26 patients went to operation and 20 had recurrent disease confirmed at operation | |
| Outcomes | Successful palliation: (as defined by Castaldo) = survival > 8 weeks: 16/26 (62%) Unrelieved obstruction: 2/26 (8%) Re‐obstruction: 4/26 (15%) Postoperative mortality: 4/26 (15%) 10‐week mortality: 10/26 (38%) Postoperative morbidity: 11/26 (42%) Median survival: 81 days | |
| Notes | 6 cases of obstruction in non‐recurrent disease were 4 non‐malignant adhesions (2 subsequently relapsed), 1 radiation stricture, 1 ileal carcinoid 11 patients underwent resection, 9 patients bypass No data on median time to re‐obstruction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | No information regarding symptom control of "successfully palliated patients" Included data on patients with non‐malignant obstruction |
Rubin 1989.
| Methods | Retrospective review | |
| Participants | 54 operations on 52 patients from 1983 to 1985 | |
| Interventions | Definitive surgical procedure in 43 operations (41 patients); exploratory operation in 11 cases (11 patients) | |
| Outcomes | On discharge taking regular/low residue diet: 34/43 (79%) On discharge taking oral diet: 3/43 On discharge receiving TPN: 2/43 Unrelieved obstruction: 4/43 (9%) Postoperative mortality: 4/43 (9%) Postoperative morbidity: 3 fistulas, 2 sepsis; 5/43 (12%) Mean survival: 6.8 months | |
| Notes | Small intestine in 18/43 and 24/54, large intestine in 16/43 and 18/54, mixed in 9/43 and 12/54 for definitive and total cases respectively 20/43 colostomies,13/43 resections, 14/43 bypasses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported by site or surgical procedure |
Sartori 2009.
| Methods | Retrospective review | |
| Participants | 75 patients between 1984 and 2005 | |
| Interventions | 25 patients underwent surgery; 50 patients treated conservatively | |
| Outcomes | Median survival of all patients was 19.33 weeks (0 to 102 weeks) 72% of those who underwent surgery returned to oral feeding compared with 60% treated conservatively |
|
| Notes | The authors also try to define a scoring system to determine the decision for surgery Same patient group as Sartori 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Unclear. "We retrospectively reviewed 270 patients with epithelial ovarian cancer treated at the division of Gynaecologic Oncology, Department of obstetrics and Gynaecology, School of Medicine, University of Brescia between 1984 and 2005. Of these patients, 75 patients (28%) developed bowel obstruction related to progressive disease after initial treatment." |
| Allocation concealment (selection bias) | High risk | "Information for both relevant pathological and clinical features and follow‐up data were obtained from medical records of all patients." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Information for both relevant pathological and clinical features and follow‐up data were obtained from medical records of all patients." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Information for both relevant pathological and clinical features and follow‐up data were obtained from medical records of all patients." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Information for both relevant pathological and clinical features and follow‐up data were obtained from medical records of all patients." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported specifically for patients with bowel obstruction due to advanced ovarian cancer Outcomes reported for all patients Results not reported according to surgical intervention |
Sartori 2010.
| Methods | Retrospective review | |
| Participants | 75 patients between 1984 and 2005 | |
| Interventions | 25 patients underwent surgery; 50 patients treated conservatively | |
| Outcomes | Postoperative deaths 8% (2 patients) All patients ‐ median survival 8 weeks, mean survival 19.6 weeks Surgical group ‐ median survival 28 weeks, mean survival 34 weeks (0 to 94 weeks), 8‐week survival 80% (20 patients) Conservative group ‐ median survival 4 weeks, mean survival 12 weeks (0 to 102 weeks), 8‐week survival 34% (17 patients) |
|
| Notes | 16% were found to be inoperable intraoperatively The authors also try to define a scoring system to determine the decision for surgery Same patient group as Sartori 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "From 1984 to 2005, 270 patients with epithelial ovarian cancer were diagnosed and treated at the Department of Obstetrics and Gynaecology, University of Brescia. Seventy‐five (28%) cases developed bowel obstruction related to progression/ recurrence of disease." |
| Allocation concealment (selection bias) | High risk | "All clinical records of ovarian cancer‐related bowel obstruction were retrospectively reviewed and the necessary clinical data obtained." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "All clinical records of ovarian cancer‐related bowel obstruction were retrospectively reviewed and the necessary clinical data obtained." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "All clinical records of ovarian cancer‐related bowel obstruction were retrospectively reviewed and the necessary clinical data obtained." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "All clinical records of ovarian cancer‐related bowel obstruction were retrospectively reviewed and the necessary clinical data obtained." |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported specifically for patients with bowel obstruction due to advanced ovarian cancer Outcomes reported for all 77 patients Results not reported according to surgical intervention |
Solomon 1983.
| Methods | Retrospective review | |
| Participants | 163 patients from September 1978 to July 1982 | |
| Interventions | 27 episodes of bowel obstruction; 3 episodes conservatively treated 23 operations on 21 patients occurred (18 had recurrent disease, 3 radiotherapy causes, 2 endometriosis) | |
| Outcomes | Unrelieved obstruction: 3/21 (14%); re‐laparotomy in 3:2/3 successful, 1/3 unsuccessful Re‐obstruction: 2/21 (10%) Postoperative mortality: 1/21 (5%) Postoperative morbidity: 1/21 (5%) | |
| Notes | Postoperative morbidity was a short bowel syndrome due to an ileostomy No information on symptoms or what was a successful operation Difficulty with data analysis from paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | No information regarding symptom control of "successfully palliated patients" |
Soo 1988.
| Methods | Retrospective review | |
| Participants | Retrospective study of 2000 patients from 1974 to 1984 | |
| Interventions | 92 patients had intestinal obstruction; 64 patients went to operation, 42/64 due to recurrence | |
| Outcomes | Surgical palliation: defined as effective palliation of the symptoms with no subsequent episodes; 45/64 (70%) Partial palliation 19% (no definition) No palliation 11% Re‐obstruction: 12/64 (19%); 2 re‐operated on Median survival: only those with malignant causes 10 weeks, mean 27 weeks Postoperative mortality: 7/64 (11%), includes 4 patients where no surgical operation possible | |
| Notes | 59 ovarian, 18 cervical, 12 uterine, 3 fallopian tube out of 92 37 ovarian, 17 cervical, 8 uterine, 2 fallopian tube out of 64 Resection +/‐ colostomy in 16, bypass in 23, colostomy in 17, lysis of adhesions in 4 No surgical operation possible in 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported by surgical procedure |
Spears 1988.
| Methods | Retrospective review | |
| Participants | 143 patients from 1975 to 1985 | |
| Interventions | 96 patients were analysed 62 patients were operated on (early and late) 32 patients had cancer as cause of obstruction 30 patients had benign cause | |
| Outcomes | Re‐obstruction: 8/62 (13%); 6 had further laparotomy Postoperative mortality: within 30 days 17/62 (27%) Mortality in cancer patients: 31/32 patients died, median survival 2 months (1 to 31 months) | |
| Notes | 11 operated on early postoperatively, 51 late postoperatively Mortality due to infection in 8/17 cases, MI 2/17, obstruction unrelieved in 3/17 and 3/17 progressive disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | High risk | 30 patients had a non‐malignant cause for obstruction and included in the results Results not reported according to surgical technique |
Sun 1995.
| Methods | Retrospective review | |
| Participants | 57 patients from March 1987 to February 1993 | |
| Interventions | 23 patients treated by surgery; 6/23 (26%) had inoperable disease at laparotomy; 20/23 cases obstruction due to disease; 3/23 cases obstruction due to adhesions | |
| Outcomes | Benefit of surgery: if patient survived > 2 months without recurrence of intestinal obstruction ‐ 14/23 (61%) Postoperative mortality: 2/23 (9%) Median survival: 96 days | |
| Notes | 3 patients had resection of involved bowel and anastomoses, 6 had intestinal bypass, 8 had lysis of adhesions, 6 had laparotomy alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported according to surgical procedure Does not comment on symptoms, only survival without re‐obstruction |
Turnbull 1989.
| Methods | Retrospective review | |
| Participants | 89 patients from 1977 to 1986 | |
| Interventions | Data on 59 patients with colonic carcinoma and 19 patients with gastric carcinoma | |
| Outcomes | Improved: defined as obstruction relieved by surgery and able to resume a normal diet; 48/59 (81%) colonic, 10/19 (53%) gastric Duration of functioning bowel: defined as the ability to evacuate and eat without vomiting: median 84 days colon, 33 days gastric | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported according to surgical intervention |
Verrees 1996.
| Methods | Retrospective review | |
| Participants | 20 patients (21 operations) from December 1989 to January 1995 | |
| Interventions | 2 patients had small bowel obstruction | |
| Outcomes | Resolution: both patients had reversal of obstruction Improvement of oral food intake: both patients had return of normal alimentation | |
| Notes | Operative technique of suturing transferred tissue over sites of potential recurrent disease was felt to prevent "further intestinal obstruction" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | High risk | Descriptive reporting of 2 patients |
Winner 2013.
| Methods | Retrospective review | |
| Participants | 1004 patients between January 1991 and December 2005 with stage IV colon adenocarcinoma | |
| Interventions | 281 patients (28.0%) underwent surgery | |
| Outcomes | Hospitalisation length of stay: non‐surgical 7 days; surgical 11 days, P value < 0.001 30‐day mortality: non‐surgical 30.8%; surgery 18.5%, P value = 0.003 Absolute difference in medical survival between treatment surgery verses non‐surgery 56 days |
|
| Notes | Uses Surveillance, Epidemiology, and End Results ‐ Medicare database, which tracks 26% of patients with cancer in US population by searching for coded information on billing for procedures and services rendered Reduction in use of surgery over time ‐ 32.4% 1991 and 26.2% in 2005 Stenting not included as not coded for |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Coding/billing information reflected that patients were allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Not reported by surgical procedure ‐ unclear if this information was available via coding or not |
Wong 2009.
| Methods | Retrospective review | |
| Participants | 2 patients with bowel obstruction from gynaecological malignancy underwent 3 operations from September 2004 to August 2006 Part of larger group of 27 patients with stomach, colon (7), ovary, lung, gallbladder, small bowel, breast, bladder malignancy and thigh sarcoma |
|
| Interventions | All patients had undergone surgery | |
| Outcomes | No postoperative deaths (within 30 days) in 2 patients with gynaecological malignancy All discharged patients able to tolerate oral medications and feeding |
|
| Notes | All other data not identifiable by malignancy type No data identifiable for those with colon cancer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Clinical records for patients admitted over a two year period to our department for surgical palliation of bowel obstruction in advanced abdominal malignancy were reviewed retrospectively." |
| Allocation concealment (selection bias) | High risk | "These patient details were reviewed from morbidity and mortality presentations in the department weekly audit meetings." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "These patient details were reviewed from morbidity and mortality presentations in the department weekly audit meetings." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Clinical records for patients admitted over a two year period to our department for surgical palliation of bowel obstruction in advanced abdominal malignancy were reviewed retrospectively." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Clinical records for patients admitted over a two year period to our department for surgical palliation of bowel obstruction in advanced abdominal malignancy were reviewed retrospectively." |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes identifiable by malignancy type |
Zhang 2012.
| Methods | Retrospective review | |
| Participants | 186 patients with colon or colorectal cancer | |
| Interventions | Self expanding metal stent (n = 97) or surgery (n = 89) | |
| Outcomes | Technical success: SEMS 94.8%; surgery 100% Clinical success: SEMS 100%; surgery 100% (not defined) Minor postoperative complications: SEMS 5/97; surgery 16/89, P value < 0.05 Major postoperative complications: SEMS 8/97; surgery 7/87, no statistical difference Length of hospital admission: 8.2 ± 4.5; surgery 12.5 ± 9.7, P value < 0.05 (no units) Postoperative chemotherapy: SEMS 57.1%; surgery 84.2% Time to chemotherapy days (mean, SD): SEMS 15.7 ± 9.8; surgery 22.7 ± 13.2, no statistical difference Late obstruction: SEMS 20.7%; surgery 9%, P value < 0.05 |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and followed up |
| Selective reporting (reporting bias) | Unclear risk | Clinical success reported as 100% but not defined 2 difference values given for time to chemotherapy ‐ unclear |
Zoetmulder 1994.
| Methods | Retrospective review | |
| Participants | 60 patients from 1984 to 1961 | |
| Interventions | 58 patients were analysed 30 patients operated on | |
| Outcomes | Success: defined as patient alive without recurrent bowel obstruction: 18/30 (64%) Full oral intake was restored; 2 patients were on TPN Postoperative mortality: within 15 days 3/30 (10%), within 45 days 10/30 (33%) (most had persistent bowel obstruction) | |
| Notes | Lysis of adhesions in 5, small bowel resection/bypass in 16, ileostomy in 10, colostomy in 9 8/18 patients who had successful procedures presented with bowel obstruction as first sign of ovarian cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Allocation concealment (selection bias) | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients allocated to surgery by clinician/patient choice and retrospectively reviewed |
| Selective reporting (reporting bias) | Unclear risk | Results not reported according to surgical intervention 8 patients were de novo presentations of ovarian cancer Results not reported separately |
DVT: deep vein thrombosis
GIST: gastrointestinal stromal tumour
IQR: interquartile range
IV: intravenous
MBO: malignant bowel obstruction
MI: myocardial infarction
PEG: percutaneous endoscopic gastrostomy
SD: standard deviation
SEMS: self expandable metallic stent
TPN: total parenteral nutrition
UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aabo 1984 | Results not specific to gastrointestinal or gynaecological cancers |
| Amikura 2010 | Results not specific to gastrointestinal or gynaecological tumours |
| Annest 1979 | Results not specific to gastrointestinal or gynaecological cancers |
| Aranha 1981 | No primary outcomes included. Results not specific to gastrointestinal or gynaecological cancers |
| Badgwell 2009 | Results not specific to gastrointestinal or gynaecological cancers |
| Badgwell 2011 | Results not specific to gastrointestinal or gynaecological cancers. Outcome reported by site of obstruction |
| Barnhill 1991 | Results not specific to gastrointestinal or gynaecological cancers |
| Bizer 1981 | Results not specific to gastrointestinal or gynaecological cancers |
| Brown 1977 | Results not specific to gastrointestinal or gynaecological cancers |
| Butler 1991 | Results not specific to gastrointestinal or gynaecological cancers |
| Chakraborty 2011 | Results not specific to gastrointestinal cancer; gynaecological cancer excluded |
| Chan 1992 | Results not specific to gastrointestinal or gynaecological cancers |
| Clarke‐Pearson 1987 | No primary outcomes included |
| Clarke‐Pearson 1988 | No primary outcomes included |
| Dalal 2011 | Included de novo cases; unable to extract outcome data for known advanced disease |
| Davis 2001 | Review ‐ no original data |
| Ellis 1971 | No primary outcomes included |
| Emmert 1996 | Results not specific to gastrointestinal or gynaecological cancers. No primary outcomes included |
| Englert 2012 | Results not specific to gastrointestinal or gynaecological cancers |
| Fernandes 1988 | No primary outcomes included |
| Fitzgerald 2012 | Results not specific to gastrointestinal or gynaecological cancers |
| Fotopoulou 2013 | Subset of patients who develop short bowel syndrome and long‐life total parental nutrition after surgery for bowel obstruction. Participants too different for inclusion. Also both de novo and known advanced cancer |
| Gallick 1986 | Results not specific to gastrointestinal or gynaecological cancers |
| Glass 1973 | No primary outcomes included. Results not specific to gastrointestinal or gynaecological cancers |
| Henry 2012 | Results not specific to gastrointestinal or gynaecological cancers |
| Hirakawa 2014 | Stent only. Results not separated by palliative or bridge‐to‐surgery |
| Huhtinen 2013 | Stents only; no surgery |
| Hwang 2013 | No surgical arm ‐ medical treatment only |
| Kaltiala 1972 | Results not specific to gastrointestinal or gynaecological cancers |
| Ketcham 1970 | Results not specific to gastrointestinal or gynaecological cancers |
| Krebs 1987 | No primary outcomes included |
| Krebs 1989 | Review ‐ no original data |
| Kucukmetin 2010 | Review ‐ no original data |
| Landercasper 1993 | Results not specific to gastrointestinal or gynaecological cancers |
| Lee 2011 | De novo treatment |
| Legendre 2001 | Results not specific to gastrointestinal or gynaecological cancers |
| Maeda 2014 | Primary management |
| Mahteme 1996 | No primary outcomes included |
| Makela 1986 | Results not specific to gastrointestinal or gynaecological cancers |
| Makela 1990 | Not clear what number of patients were obstructed |
| Makela 1991 | Results not specific to gastrointestinal or gynaecological cancers |
| McCahill 2003 | Results not specific to gastrointestinal or gynaecological cancers |
| Miller 2000 | Results not specific to gastrointestinal or gynaecological cancers |
| Miner 2004 | Results not specific to gastrointestinal or gynaecological cancers |
| Miner 2005 | Review ‐ no original data. Results not specific to gastrointestinal or gynaecological cancers |
| Miner 2011 | Results reported for all tumour groups and multiple surgical diagnoses |
| Osteen 1980 | Results not specific to gastrointestinal or gynaecological cancers |
| Paganelli 1990 | No primary outcomes included |
| Pathak 1980 | No primary outcomes included. Results not specific to gastrointestinal or gynaecological cancers |
| Piver 1982 | No primary outcomes included |
| Podnos 2007 | Results not specific to gastrointestinal or gynaecological cancers |
| Scarabelli 1985 | No primary outcomes included |
| Simion 2014 | Both primary and secondary disease; all causes of malignancy (not stated); unclear de novo presentation or known advanced malignancy |
| Suarez 2010 | De novo treatment |
| Sugarbaker 1989 | No primary outcomes included. Results not specific to gastrointestinal or gynaecological cancers |
| Tang 1995 | Results not specific to gastrointestinal or gynaecological cancers |
| Tunca 1981 | No primary outcomes included |
| Ubogagu 2010 | Results not specific to gynaecological cancers; gastrointestinal cancers not included |
| Van Ooijen 1993a | Results not specific to gastrointestinal or gynaecological cancers |
| Van Ooijen 1993b | Results not specific to gastrointestinal or gynaecological cancers |
| Walsh 1984 | No primary outcomes included |
| Webb 1987 | No primary outcomes included |
| Weiss 1984 | Results not specific to gastrointestinal or gynaecological cancers |
| Wheeless 1973 | No primary outcomes included |
| Woolfson 1997 | Results not specific to gastrointestinal or gynaecological cancers |
| Xinopoulos 2004 | Not specific to advanced malignancy |
| Yanar 2014 | Stents only. De novo verses advanced not known at the time of stenting |
| Yeung 1993 | No primary outcomes included |
| Yoon 2013 | Stent occlusion specifically ‐ then re‐stenting or surgery |
Differences between protocol and review
For the 2015 update, we performed the 'Risk of bias' assessment on all included papers, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For the 2015 update, we did not perform 'searching of other resources'.
Contributions of authors
Original idea KB; developed further by DF, KB, Professor John Shepherd and Professor Desmond Barton; primary researcher DF; review authors DF and KB; written by DF with additional help from KB, Professor John Shepherd and Professor Desmond Barton.
Updated by DF, ET and SC.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Executive National Cancer Research and Development Programme NCP2/1211, UK.
Declarations of interest
Dr Sarah E Cousins has no relevant conflict of interest to declare.
Dr Emma Tempest has no relevant conflict of interest to declare.
Dr David J Feuer has no relevant conflict of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Abbas 2007 {published data only}
- Abbas SM, Merrie AEH. Resection of peritoneal metastases causing malignant small bowel obstruction. World Journal of Surgical Oncology 2007;5:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bais 1995 {published data only}
- Bais JJ, Schilthuis MS, Slors JM, Lammes FB. Intestinal obstruction in patients with advanced ovarian cancer. International Journal of Gynecological Cancer 1995;5:346‐50. [DOI] [PubMed] [Google Scholar]
Bartels 1995 {published data only}
- Bartels CJ, Reed E, Sindelar WF. Intestinal obstruction due to advanced ovarian cancer: results of surgical intervention. Surgical Forum 1995;46:564‐6. [Google Scholar]
Beattie 1989 {published data only}
- Beattie GJ, Leonard R, Smyth JF. Bowel obstruction in ovarian carcinoma: a retrospective study and review of the literature. Palliative Medicine 1989;3:275‐80. [Google Scholar]
Blair 2001 {published data only}
- Blair SL, Chu DZJ, Schwarz RE. Outcome of palliative operations for malignant bowel obstruction patients with peritoneal carcinomatosis from nongynaecological cancer. Annals of Surgical Oncology 2001;8(8):632‐7. [DOI] [PubMed] [Google Scholar]
Castaldo 1981 {published data only}
- Castaldo TW, Petrilli ES, Ballon SC, Lagasse LD. Intestinal operations in patients with ovarian carcinoma. American Journal of Obstetrics and Gynecology 1981;139:80‐4. [DOI] [PubMed] [Google Scholar]
Chi 2009 {published data only}
- Chi DS, Phaëton R, Miner TJ, Kardos SV, Diaz JP, Leitao MM Jr, et al. A prospective outcomes analysis of palliative procedures performed for malignant intestinal obstruction due to recurrent ovarian cancer. The Oncologist 2009;14:835‐9. [DOI] [PubMed] [Google Scholar]
Ellis 1991 {published data only}
- Ellis CN, Boggs HW Jr, Slagle GW, Cole PA. Small bowel obstruction after colon resection for benign and malignant diseases. Diseases of the Colon and Rectum 1991;34:367‐71. [DOI] [PubMed] [Google Scholar]
Fiori 2012 {published data only}
- Fiori E, Lamazza A, Schillaci A, Femia S, DeMasi E, DeCesare A, et al. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy verses endoscopic stenting: final results of a prospective randomised trial. American Journal of Surgery 2012;204:321‐6. [DOI] [PubMed] [Google Scholar]
Furuya 2012 {published data only}
- Furuya K, Shiki Y. Palliative treatment of malignant bowel obstruction in patients with gynecological cancer: comparison between surgical intervention and conservative management. O264. International Journal of Gynecology and Obstetrics 2012;119S3:S353. [Google Scholar]
Goto 2012 {published data only}
- Goto T, Takano M, Aoyama T, Miyamoto M, Watanabe A, Kato M, et al. Outcomes of palliative bowel surgery for malignant bowel obstruction in patients with gynecological malignancy. Oncology Letters 2012;4:883‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 1994 {published data only}
- Hoffman MS, Gleeson NC, Finan MA, Roberts WS, Fiorica JV, Cavanagh D. Palliative intestinal surgery for patients with carcinoma of the cervix. Journal of Gynecological Surgery 1994;10:63‐9. [Google Scholar]
Jong 1995 {published data only}
- Jong P, Sturgeon J, Jamieson CG. Benefit of palliative surgery for bowel obstruction in advanced ovarian cancer. Canadian Journal of Surgery 1995;38:454‐7. [PubMed] [Google Scholar]
Katz 1981 {published data only}
- Katz LB, Frankel A, Cohen C, Slater G. Ovarian carcinoma complicated by gastric outlet obstruction. Journal of Surgical Oncology 1981;18:261‐4. [DOI] [PubMed] [Google Scholar]
Kolomainen 2012 {published data only}
- Kolomainen DF, Dapinte A, Barton DPJ, Pennert K, Ind TEJ, Bridges JE, et al. Outcomes of surgical management of bowel obstruction in relapsed epithelial ovarian cancer (EOC). Gynecologic Oncology 2012;125:31‐6. [DOI] [PubMed] [Google Scholar]
Krebs 1983 {published data only}
- Krebs HB, Goplerud DR. Surgical management of bowel obstruction in advanced ovarian carcinoma. Obstetrics and Gynecology 1983;61:327‐30. [PubMed] [Google Scholar]
Larson 1989 {published data only}
- Larson JE, Podczaski ES, Manetta A, Whitney CW, Mortel R. Bowel obstruction in patients with ovarian carcinoma: analysis of prognostic factors. Gynecologic Oncology 1989;35:61‐5. [DOI] [PubMed] [Google Scholar]
Lau 1993 {published data only}
- Lau PW, Lorentz TG. Results of surgery for malignant bowel obstruction in advanced, unresectable, recurrent colorectal cancer. Diseases of the Colon and Rectum 1993;36:61‐4. [DOI] [PubMed] [Google Scholar]
Lo 1991 {published data only}
- Lo NN, Kee SG, Nambiar R. Palliative gastrojejunostomy for advanced carcinoma of the stomach. Annals of the Academy of Medicine, Singapore 1991;20:356‐8. [PubMed] [Google Scholar]
Lund 1989 {published data only}
- Lund B, Hansen M, Lundvall F, Nielsen NC, Sorensen BL, Hansen HH. Intestinal obstruction in patients with advanced carcinoma of the ovaries treated with combination chemotherapy. Surgery, Gynecology and Obstetrics 1989;169:213‐8. [PubMed] [Google Scholar]
Mangili 2005 {published data only}
- Mangili G, Aletti G, Frigerio L, Franchi M, Panacci N, Viganò R, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. International Journal of Gynecological Cancer 2005;15(5):830‐5. [DOI] [PubMed] [Google Scholar]
Mooney 2013 {published data only}
- Mooney SJ, Winner M, Hershman DL, Wright JD, Feingold DL, Allendorf JD, et al. Bowel obstruction in elderly ovarian cancer patients: a population‐based study. Gynecologic Oncology 2013;129:107‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakane 1996 {published data only}
- Nakane Y, Okumura S, Akehira K, Okamura S, Boku T, Okusa T, et al. Management of intestinal obstruction after gastrectomy for carcinoma. British Journal of Surgery 1996;83:113. [DOI] [PubMed] [Google Scholar]
Parveen 2009 {published data only}
- Parveen Z, Qureshi AN, Akbar M, Zafar A, Subhani A. Palliative surgery for intestinal obstruction due to recurrent ovarian cancer. Journal of Ayub Medical College, Abbottabad 2009;21(1):135‐6. [PubMed] [Google Scholar]
Pecorelli 1994 {published data only}
- Pecorelli S, Sartori E, Santin A. Follow‐up after primary therapy: management of the symptomatic patient ‐ surgery. Gynecologic Oncology 1994;55:S138‐42. [DOI] [PubMed] [Google Scholar]
Perri 2014 {published data only}
- Perri T, Korach J, Ben‐Baruch G, Jakobson‐Setton A, Ben‐David Hogen L, Kalfon S, et al. Bowel obstruction in recurrent gynecological malignancies: defining who will benefit from surgical intervention. Journal of Cancer Surgery 2014;40:889‐904. [DOI] [PubMed] [Google Scholar]
Philip 1997 {published data only}
- Philip J, Do T, Hacker N, Grant P, Lickiss N. Outcomes in patients with GIT obstruction ‐ 62 episodes in 33 patients in a 12 month period. International Journal of Gynecological Cancer 1997;7(Suppl 2):86 (Abstr). [Google Scholar]
Pothuri 2003 {published data only}
- Pothuri B, Vaidya A, Aghajanian C, Venkatraman E, Barakat RR, Chi DS. Palliative surgery for bowel obstruction in recurrent ovarian cancer: an updated series. Gynaecologic Oncology 2003;89:306‐13. [DOI] [PubMed] [Google Scholar]
Pothuri 2004 {published data only}
- Pothuri B, Meyer L, Gerardi M, Barakat RR, Chi DS. Reoperation for palliation of recurrent malignant bowel obstruction in ovarian carcinoma. Gynaecologic Oncology 2004;95(1):193‐5. [DOI] [PubMed] [Google Scholar]
Redman 1988 {published data only}
- Redman CW, Shafi MI, Ambrose S, Lawton FG, Blackledge GRP, Luesley DM, et al. Survival following intestinal obstruction in ovarian cancer. European Journal of Surgical Oncology 1988;14:383‐6. [PubMed] [Google Scholar]
Rubin 1989 {published data only}
- Rubin SC, Hoskins WJ, Benjamin I, Lewis JL Jr. Palliative surgery for intestinal obstruction in advanced ovarian cancer. Gynecologic Oncology 1989;34:16‐9. [DOI] [PubMed] [Google Scholar]
Sartori 2009 {published data only}
- Sartori E, Chiudinelli F, Pasinetti B, Maggino T. Bowel obstruction and survival in patients with advanced ovarian cancer. International Journal of Gynaecological Cancer 2009;19(1):54‐7. [DOI] [PubMed] [Google Scholar]
Sartori 2010 {published data only}
- Sartori E, Chiudinelli F, Pasinetti B, Sostegni B, Maggino T. Possible role of palliative surgery for bowel obstruction in advanced ovarian cancer patients. European Journal of Gynaecological Oncology 2010;31(1):31‐6. [PubMed] [Google Scholar]
Solomon 1983 {published data only}
- Solomon HJ, Atkinson KH, Coppleson JV, Elliot PM, Houghton CR, Tattersall MH, et al. Bowel complications in the management of ovarian cancer. Australian and New Zealand Journal of Obstetrics and Gynaecology 1983;23:65‐8. [DOI] [PubMed] [Google Scholar]
Soo 1988 {published data only}
- Soo KC, Davidson T, Parker M, Paterson I, Paterson A. Intestinal obstruction in patients with gynaecological malignancies. Annals of the Academy of Medicine, Singapore 1988;17:72‐5. [PubMed] [Google Scholar]
Spears 1988 {published data only}
- Spears H, Petrelli NJ, Herrera L, Mittelman A. Treatment of bowel obstruction after operation for colorectal carcinoma. American Journal of Surgery 1988;155:383‐6. [DOI] [PubMed] [Google Scholar]
Sun 1995 {published data only}
- Sun X, Li X, Li H. Management of intestinal obstruction in advanced ovarian cancer an analysis of 57 cases. Chung Hua Chung Liu Tsa Chih 1995;17:39‐42. [PubMed] [Google Scholar]
Turnbull 1989 {published data only}
- Turnbull AD, Guerra J, Starnes HF. Results of surgery for obstructing carcinomatosis of gastrointestinal, pancreatic, or biliary origin. Journal of Clinical Oncology 1989;7:381‐6. [DOI] [PubMed] [Google Scholar]
Verrees 1996 {published data only}
- Verrees JF, Fernandez‐Trigo V, Sugarbaker PH. Rectal cancer recurrence after prior resection and radiotherapy: palliation following additional surgery. International Journal of Colorectal Disease 1996;11:211‐6. [DOI] [PubMed] [Google Scholar]
Winner 2013 {published data only}
- Winner M, Mooney SJ, Hershman DL, Feingold DL, Allendorf JD, Wright JD, et al. Management and outcomes of bowel obstruction in patients with stage IV colon cancer: a population‐based cohort study. Diseases of the Colon and Rectum 2013;56(7):834‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2009 {published data only}
- Wong TH, Tan YM. Surgery for the palliation of intestinal obstruction in advanced abdominal malignancy. Singapore Medical Journal 2009;50(12):1139‐44. [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang YX. Effect of self‐expanding metal stents verses surgery for malignant colorectal obstruction in patients with advanced colorectal cancers. Chinese Journal of Tissue Engineering Research 2012;16(34):6453‐6. [Google Scholar]
Zoetmulder 1994 {published data only}
- Zoetmulder FA, Helmerhorst TJ, Coevorden F, Wolfs PE, Leyer JP, Hart AA. Management of bowel obstruction in patients with advanced ovarian cancer. European Journal of Cancer 1994;30A:1625‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aabo 1984 {published data only}
- Aabo K, Pederia H, Bach F, Knudsen J. Surgical management of intestinal obstruction in the late course of malignant disease. Acta Chirurgica Scandinavica 1984;150:173‐6. [PubMed] [Google Scholar]
Amikura 2010 {published data only}
- Amikura K, Sakamoto H, Yatsuoka T, Kawashima Y, Nishimura Y, Tanaka Y. Surgical management for malignant bowel obstruction with recurrent gastrointestinal carcinoma. Journal of Surgical Oncology 2010;101(3):228‐32. [DOI] [PubMed] [Google Scholar]
Annest 1979 {published data only}
- Annest L, Jolly PC. The results of surgical treatment of bowel obstruction caused by peritoneal carcinomatosis. The American Surgeon 1979;November:718‐21. [PubMed] [Google Scholar]
Aranha 1981 {published data only}
- Aranha GV, Folk FA, Greenlee HB. Surgical palliation of small bowel obstruction due to metastatic carcinoma. American Journal of Surgery 1981;47:99‐102. [PubMed] [Google Scholar]
Badgwell 2009 {published data only}
- Badgwell BD, Smith K, Liu P, Bruera E, Curley SA, Cormier JN. Indicators of surgery and survival in oncology inpatients requiring surgical evaluation for palliation. Supportive Care in Cancer 2009;17(6):727‐34. [DOI] [PubMed] [Google Scholar]
Badgwell 2011 {published data only}
- Badgwell BD, Contreras C, Askew R, Krouse R, Feig B, Cormier JN. Radiological and clinical factors associated with improved outcomes in advanced cancer patients with bowel obstruction. Journal of Palliative Medicine 2011;14(9):990‐6. [DOI] [PubMed] [Google Scholar]
Barnhill 1991 {published data only}
- Barnhill D, Doering D, Remmenga S, Bosscher J, Nash J, Park R. Intestinal surgery performed on gynaecologic cancer patients. Gynecologic Oncology 1991;40:38‐41. [DOI] [PubMed] [Google Scholar]
Bizer 1981 {published data only}
- Bizer LS, Lieblimg RW, Delany HM, Gliedman ML. Small bowel obstruction. The role of nonoperative treatment in simple intestinal obstruction and predictive criteria for strangulation obstruction. Surgery 181;89(4):407‐13. [PubMed] [Google Scholar]
Brown 1977 {published data only}
- Brown PW, Terrz JJ, Lawrence W, Blievernicht SW. Survival after palliative surgery for advanced intraabdominal cancer. American Journal of Surgery 1977;134:575‐8. [DOI] [PubMed] [Google Scholar]
Butler 1991 {published data only}
- Butler JA, Cameron BL, Morrow M, Kahng K, Tom J. Small bowel obstruction in patients with a prior history of cancer. American Journal of Surgery 1991;162:624‐8. [DOI] [PubMed] [Google Scholar]
Chakraborty 2011 {published data only}
- Chakraborty A, Selby D, Gardiner K, Myers J, Moravan V, Wright F. Malignant bowel obstruction: natural history of a heterogeneous patient population followed prospectively over two years. Journal of Pain and Symptom Management 2011;41(2):412‐20. [DOI] [PubMed] [Google Scholar]
Chan 1992 {published data only}
- Chan A, Woodruff RK. Intestinal obstruction in patients with widespread intraabdominal malignancy. Journal of Pain and Symptom Management 1992;7(6):339‐42. [DOI] [PubMed] [Google Scholar]
Clarke‐Pearson 1987 {published data only}
- Clarke‐Pearson DL, Chin NO, DeLong ER, Rice R, Creasman WT. Surgical management of intestinal obstruction in ovarian cancer. 1. Clinical features, postoperative complications and survival. Gynecologic Oncology 1987;26:11‐8. [DOI] [PubMed] [Google Scholar]
Clarke‐Pearson 1988 {published data only}
- Clarke PD, DeLong ER, Chin N, Rice R, Creasman WT. Intestinal obstruction in patients with ovarian cancer. Variables associated with surgical complications and survival. Archives of Surgery 1988;123:42‐5. [DOI] [PubMed] [Google Scholar]
Dalal 2011 {published data only}
- Dalal KM, Gollub MJ, Miner TJ, Wong WD, Gerdes H, Schattner MA, et al. Management of patients with malignant bowel obstruction and stage IV colorectal cancer. Journal of Palliative Medicine 2011;14(7):822‐8. [DOI] [PubMed] [Google Scholar]
Davis 2001 {published data only}
- Davis MP, Nouneh C. Modern management of cancer‐related intestinal obstruction. Current Pain and Headache Reports 2001;5(3):257‐64. [DOI] [PubMed] [Google Scholar]
Ellis 1971 {published data only}
- Ellis H. Curative and palliative surgery in advanced carcinoma of the large bowel. BMJ 1971;July:291‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmert 1996 {published data only}
- Emmert C, Schenker U, Kohler U. Intestinal obstruction in patients with advanced gynecological cancer. A study of 62 cases. Archives of Gynecology and Obstetrics 1996;258:213‐8. [DOI] [PubMed] [Google Scholar]
Englert 2012 {published data only}
- Englert ZP, White MA, Fitzgerald TL, Vadlamudi A, Zervoudakis G, Zervos EE. Surgical management of malignant bowel obstruction: at what price palliation?. The American Surgeon 2012;78:647‐52. [PubMed] [Google Scholar]
Fernandes 1988 {published data only}
- Fernandes JR, Seymour RJ, Suissa S. Bowel obstruction in patients with ovarian cancer: a search for prognostic factors. American Journal of Obstetrics and Gynecology 1988;158:244‐9. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2012 {published data only}
- Fitzgerald JEF, Bonello V, Bhangu A, Tekkis PP, Antoniou A. P149. Survival in patients undergoing palliative defunctioning stoma surgery for gastrointestinal obstruction secondary to known advanced malignancy. Colorectal Disease 2012; Vol. 14, issue Suppl 2:46.
Fotopoulou 2013 {published data only}
- Fotopoulou C, Braicu EI, Kwee SL, Kuhbery M, Richter R, Pietzner K, et al. Salvage surgery due to bowel obstruction in advanced or relapsed ovarian cancer resulting in short bowel syndrome and long‐life total parental nutrition. International Journal of Gynecological Cancer 2013;23(8):1495‐500. [DOI] [PubMed] [Google Scholar]
Gallick 1986 {published data only}
- Gallick HL, Weaver DW, Sachs RJ, Bouwman DL. Intestinal obstruction in cancer patients. An assessment of risk factors and outcome. American Journal of Surgery 1986;52:434‐7. [PubMed] [Google Scholar]
Glass 1973 {published data only}
- Glass RL, LeDuc RJ. Small intestinal obstruction from peritoneal carcinomatosis. American Journal of Surgery 1973;125:316‐7. [DOI] [PubMed] [Google Scholar]
Henry 2012 {published data only}
- Henry JC, Pouly S, Sullivan R, Sharif S, Klemanski D, Abdel‐Misih S, et al. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery 2012;152(4):747‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirakawa 2014 {published data only}
- Hirakawa T, Katou Y. Endoscopic stenting for patients with malignant colorectal obstruction in our hospital. Gan to Kangaku Ryoho: Cancer & Chemotherapy 2014;41(12):1485‐7. [PubMed] [Google Scholar]
Huhtinen 2013 {published data only}
- Huhtinen H, Varpa P, Karvonen J, Rantala A, Gronroos JM. Late complications related to palliative stenting in patients with obstructing colorectal cancer. Minimally Invasive Therapy & Allied Technologies 2013;22(6):352‐8. [DOI] [PubMed] [Google Scholar]
Hwang 2013 {published data only}
- Hwang M, Pirrello R, Pu M, Messer K, Roeland E. Octreotide prescribing patterns in the palliation of symptomatic inoperable malignant bowel obstruction patients at a single US academic hospital. Supportive Care in Cancer 2013;21(10):2817‐24. [DOI] [PubMed] [Google Scholar]
Kaltiala 1972 {published data only}
- Kaltiala EH, Lenkkeri H, Larmi TKI. Mechanical intestinal obstruction. An analysis of 577 cases. Annales Chirurgiae et Gynaecologiae Fenniae 1972;60:87‐93. [PubMed] [Google Scholar]
Ketcham 1970 {published data only}
- Ketcham AS, Hoye RC, Pilch YH, Morton DL. Delayed intestinal obstruction following treatment for cancer. Cancer 1970;25:406‐10. [DOI] [PubMed] [Google Scholar]
Krebs 1987 {published data only}
- Krebs HB, Goplerud DR. Mechanical intestinal obstruction in patients with gynecologic disease: a review of 368 patients. American Journal of Obstetrics and Gynecology 1987;157(3):577‐83. [DOI] [PubMed] [Google Scholar]
Krebs 1989 {published data only}
- Krebs HB, Helmkamp BF. Management of intestinal obstruction in ovarian cancer. Oncology 1989;3(5):25‐31. [PubMed] [Google Scholar]
Kucukmetin 2010 {published data only}
- Kucukmetin A, Naik R, Galaal K, Bryant A, Dickinson HO. Palliative surgery versus medical management for bowel obstruction in ovarian cancer. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007792.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landercasper 1993 {published data only}
- Landercasper J, Cogbill TH, Merry WH, Stolee RT, Strutt PJ. Long‐term outcome after hospitalization for small‐bowel obstruction. Archives of Surgery 1993;128:765‐70. [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee HJ, Hong SP, Cheon JH, Kim TI, Kim WH. Long term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus palliative surgery. Gastrointestinal Endoscopy 2011;73(3):535‐42. [DOI] [PubMed] [Google Scholar]
Legendre 2001 {published data only}
- Legendre H, Vanhuyse F, Caroli‐Bosc FX, Pector JC. Survival and quality of life after palliative surgery for neoplastic gastrointestinal obstruction. European Journal of Surgical Oncology 2001;27(4):364‐7. [DOI] [PubMed] [Google Scholar]
Maeda 2014 {published data only}
- Maeda H, Okamoto K, Uemura S, Okabayashi T, Osaki S, Akimori T, et al. Staged surgery after colonic decompression may be safer for the treatment of obstructive left‐sided colorectal cancer in a non‐specialized hospital. Hepato‐gastroenterology 2014;61(135):1938‐41. [PubMed] [Google Scholar]
Mahteme 1996 {published data only}
- Mahteme H, Pahlman L, Glimelius B, Graf W. Prognosis after surgery in patients with incurable rectal cancer: a population‐based study. British Journal of Surgery 1996;83:1116‐20. [DOI] [PubMed] [Google Scholar]
Makela 1986 {published data only}
- Makela J. Surgery for recurrent intra‐abdominal cancer. Annales Chirurgiae et Gynaecologia 1986;75:196‐200. [PubMed] [Google Scholar]
Makela 1990 {published data only}
- Makela J, Haukipuro K, Laitinen S, Kairaluoma MI. Palliative operations for colorectal cancer. Diseases of the Colon and Rectum 1990;33(10):846‐50. [DOI] [PubMed] [Google Scholar]
Makela 1991 {published data only}
- Makela J, Kiviniemi H, Laitinen S, Kairaluoma MI. Surgical management of intestinal obstruction after treatment for cancer. European Journal of Surgery 1991;157:73‐6. [PubMed] [Google Scholar]
McCahill 2003 {published data only}
- McCahill LE, Smith DD, Borneman T, Juarez G, Cullinane C, Chu DZ, et al. A prospective evaluation of palliative outcomes for surgery of advanced malignancies. Annals of Surgical Oncology 2003;10(6):654‐63. [DOI] [PubMed] [Google Scholar]
Miller 2000 {published data only}
- Miller G, Boman J, Shrier I, Gordon PH. Small‐bowel obstruction secondary to malignant disease: an 11‐year audit. Canadian Journal of Surgery 2000;43(5):353‐8. [PMC free article] [PubMed] [Google Scholar]
Miner 2004 {published data only}
- Miner TJ, Brennan MF, Jaques DP. A prospective symptom related outcome analysis of 1022 palliative procedures for advanced cancer. Annals of Surgery 2004;240(4):719‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miner 2005 {published data only}
- Miner TJ. Palliative surgery for advanced cancer. American Journal of Clinical Oncology 2005;28(4):411‐4. [DOI] [PubMed] [Google Scholar]
Miner 2011 {published data only}
- Miner TJ, Cohen J, Charpentier K, McPhilips, Marvel L, Cioffi WG. The palliative triangle: improved patient selection and outcomes associated with palliative operations. Archives of Surgery 2011;146(5):517‐22. [DOI] [PubMed] [Google Scholar]
Osteen 1980 {published data only}
- Osteen RT, Guyton S, Steele G, Wilson RE. Malignant intestinal obstruction. Surgery 1980;87:611‐5. [PubMed] [Google Scholar]
Paganelli 1990 {published data only}
- Paganelli AMM, Leone V, Malagutti V, Vescovo M, Sallusto A. Intestinal surgery in patients with ovarian cancer. European Journal of Gynaecological Oncology 1990;XI(2):157‐60. [PubMed] [Google Scholar]
Pathak 1980 {published data only}
- Pathak V, Swaminathan AP, Ghuman SS, Raina S, Rush BF. Intestinal obstruction in carcinomatosis. The American Surgeon 1980;December:691‐3. [PubMed] [Google Scholar]
Piver 1982 {published data only}
- Piver MS, Barlow JJ, Lele SB, Frank A. Survival after ovarian cancer induced intestinal obstruction. Gynecologic Oncology 1982;13:44‐9. [DOI] [PubMed] [Google Scholar]
Podnos 2007 {published data only}
- Podnos YD, Juarez G, Pameijer C, Choi K, Ferrell BR, Wagman LD. Impact of surgical palliation on quality of life in patients with advanced malignancy: results of the decisions and outcomes in palliative surgery trial. Annals of Surgical Oncology 2007;14(2):922‐8. [DOI] [PubMed] [Google Scholar]
Scarabelli 1985 {published data only}
- Scarabelli C, Campagnutta E, Settin GC. Surgical treatment of intestinal occlusions in advanced ovarian cancer. European Journal of Gynaecological Oncology 1985;VI(1):66‐70. [PubMed] [Google Scholar]
Simion 2014 {published data only}
- Simion L, Straja ND, Alecu M, Poroch V, Mosoiu D, Pantis C, et al. Intestinal obstruction management in patients with advanced abdominal neoplasia. Chirurgia 2014;109:527‐33. [PubMed] [Google Scholar]
Suarez 2010 {published data only}
- Suarez J, Jimenez J, Vera R, Tarifa A, Balen E, Arrazubi V, et al. Stent or surgery for incurable obstructive colorectal cancer: an individualised decision. International Journal of Colorectal Disease 2010;25(1):91‐6. [DOI] [PubMed] [Google Scholar]
Sugarbaker 1989 {published data only}
- Sugarbaker PH. Surgical treatment of peritoneal carcinomatosis. Canadian Journal of Surgery 1989;32(3):164‐70. [PubMed] [Google Scholar]
Tang 1995 {published data only}
- Tang E, Davis J, Silberman H. Bowel obstruction in cancer patients. Archives of Surgery 1995;130:832‐6. [DOI] [PubMed] [Google Scholar]
Tunca 1981 {published data only}
- Tunca JC, Buchler DA, Mack EA, Ruzicka FF, Crowley JJ, Carr WF. The management of ovarian‐cancer‐caused bowel obstruction. Gynecologic Oncology 1981;12:186‐92. [DOI] [PubMed] [Google Scholar]
Ubogagu 2010 {published data only}
- Ubogagu EA, Urch C. P236. Malignancy related bowel obstruction: challenging the convention. Palliative Medicine 2010; Vol. EAPC:S125‐6.
Van Ooijen 1993a {published data only}
- Ooijen B, Burg MEL, Planting AST, Wiggers T. Management of intestinal obstruction in patients with advanced intraabdominal cancer. EJC. 1993; Vol. 29A (Suppl 6‐7):S205.
Van Ooijen 1993b {published data only}
- Ooijen B, Burg MEL, Planting AS, Siersema PD, Wiggers T. Surgical treatment or gastric drainage only for intestinal obstruction in patients with carcinoma of the ovary or peritoneal carcinomatosis of other origin. Surgery Gynaecology and Obstetrics 1993;176:469‐74. [PubMed] [Google Scholar]
Walsh 1984 {published data only}
- Walsh HPJ, Schofield PF. Is laparotomy for small bowel obstruction justified in patients with previously treated malignancy. British Journal of Surgery 1984;71(12):933‐5. [DOI] [PubMed] [Google Scholar]
Webb 1987 {published data only}
- Webb MJ, Weaver EW. Intestinal surgery in gynaecological oncology. Australian and New Zealand Journal of Obstetrics and Gynaecology 1987;27:299‐303. [DOI] [PubMed] [Google Scholar]
Weiss 1984 {published data only}
- Weiss SM, Skibber JM, Rosato FE. Bowel obstruction in cancer patients: performance status as a predictor of survival. Journal of Surgical Oncology 1984;25:15‐7. [DOI] [PubMed] [Google Scholar]
Wheeless 1973 {published data only}
- Wheeless CR. Small bowel bypass for complications related to pelvic malignancy. Obstetrics and Gynecology 1973;42(5):661‐6. [PubMed] [Google Scholar]
Woolfson 1997 {published data only}
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Archives of Surgery 1997;132:1093‐7. [DOI] [PubMed] [Google Scholar]
Xinopoulos 2004 {published data only}
- Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Paraskevas I, Vassilopoulos P, et al. Palliation of inoperable malignant colonic obstruction. Annals of Gastroenterology 2004;17(3):294‐9. [DOI] [PubMed] [Google Scholar]
Yanar 2014 {published data only}
- Yanar H, Ozcinar B, Yanar F, Sivrikoz E, Dagoglu N, Agcaoglu O, et al. The role of colorectal stent placement in the management of acute malignant obstruction. Turkish Journal of Trauma & Emergency Surgery 2014;20(1):23‐7. [DOI] [PubMed] [Google Scholar]
Yeung 1993 {published data only}
- Yeung RS, Moffat FL, Falk RE. Pelvic exenteration for recurrent and extensive primary colorectal adenocarcinoma. Cancer 1993;72(6):1853‐8. [DOI] [PubMed] [Google Scholar]
Yoon 2013 {published data only}
- Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Outcomes of secondary self‐expandable metal stents versus surgery after delayed initial palliative stent failure in malignant colorectal obstruction. Digestion 2013;88(1):46‐55. [DOI] [PubMed] [Google Scholar]
Additional references
ACROBAT‐NRSI 2014
- Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ACROBAT‐NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions (ACROBAT‐NRSI). Available from http://www.riskofbias.info 24 September 2014 (accessed 23 August 2015); Vol. Version 1.0.0.
Baines 1985
- Baines M, Oliver DJ, Carter RL. Medical management of intestinal obstruction in patients with advanced malignant disease. A clinical and pathological study. Lancet 1985;2:990‐3. [DOI] [PubMed] [Google Scholar]
Baines 1998
- Baines M. The pathophysiology and management of malignant intestinal obstruction. In: Doyle D, Hanks GWC, MacDonald N editor(s). Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press, 1998:526‐34. [Google Scholar]
Cancer Research UK 2014
- Cancer Research UK. UK Cancer Incidence 2011 and Mortality 2012 Summary ‐ Rates. http://publications.cancerresearchuk.org/downloads/Product/CS_REPORT_TOP10INCMORT.pdf September 2014.
Chalmers 1987
- Chalmers TC, Levin H, Sacks HS, Reitman D, Berrier J, Nagalingam R. Meta‐analysis of clinical trials as a scientific discipline. I: Control of bias and comparison with large co‐operative trials. Statistics in Medicine 1987;6:315‐25. [DOI] [PubMed] [Google Scholar]
Clarke‐Pearson 1994
- Clarke‐Pearson DL, Kohler MF, Hurteau JA, Elbendary A. Surgery for advanced ovarian cancer. Clinical Obstetrics and Gynecology 1994;37(2):439‐60. [DOI] [PubMed] [Google Scholar]
Dvoretsky 1988a
- Dvoretsky PM, Richards KA, Angel A, Rabinowitz L, Beecham JB, Bonfiglio TA. Survival time, causes of death, and tumor. Treatment‐related morbidity in 100 women with ovarian cancer. Human Pathology 1988;19:1273‐9. [DOI] [PubMed] [Google Scholar]
Dvoretsky 1988b
- Dvoretsky PM, Richards KA, Angel A, Rabinowitz L, Stoler MH, Beecham JB, et al. Distribution of disease at autopsy in 100 women with ovarian cancer. Human Pathology 1988;19:57‐63. [DOI] [PubMed] [Google Scholar]
Farias‐Eisner 1994
- Farias‐Eisner R, Kim YB, Berek JS. Surgical management of ovarian cancer. Seminars in Surgical Oncology 1994;10:268‐75. [DOI] [PubMed] [Google Scholar]
Feuer 1999a
- Feuer DJ. Management of intestinal obstruction. CME Bulletin: Palliative Medicine 1999;1:35‐40. [Google Scholar]
Feuer 1999b
- Feuer DJ, Broadley KE. Systematic review and meta‐analysis of corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancers. Annals of Oncology 1999;10(9):1035‐41. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2012
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. International Agency for Research on Cancer. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Available from: http://globocan.iarc.fr 2013 (accessed 30 January 2015).
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higginson 1997
- Higginson I, Hearn J. Multi‐disciplinary team. Guidance on Commissioning Cancer Services. Improving Outcomes in Colorectal Cancer. The Research Evidence. London: NHS Executive, 1997:42‐61. [Google Scholar]
Hogan 1993
- Hogan WM, Boente MP. The role of surgery in the management of recurrent gynaecologic cancer. Seminars in Oncology 1993;20(5):462‐72. [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Junor 1994
- Junor E, Hole DJ, Gillis CR. Management of ovarian cancer: referral to a multidisciplinary team matters. British Journal of Cancer 1994;70:363‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehoe 1994
- Kehoe S, Powell J, Wilson S, Woodman C. The influence of the surgeons specialisation on patient survival in ovarian cancer. British Journal of Cancer 1994;70:1014‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 1996
- Mann T. Clinical Guidelines: Using Clinical Guidelines to Improve Patient Care Within the NHS. Leeds: NHS Executive, 1996. [Google Scholar]
NCEPOD 1997
- NCEPOD. Report of the National Confidential Enquiry into Perioperative Deaths. Havard Associates Ltd, 1996/1997. [Google Scholar]
Ozols 1997
- Ozols RF, Vermorken JB. Chemotherapy of advanced ovarian cancer: current status and future directions. Seminars in Oncology 1997;24:S2‐1‐S2‐9. [PubMed] [Google Scholar]
Parker 1996
- Parker MC, Baines MJ. Intestinal obstruction in patients with advanced malignant disease. British Journal of Surgery 1996;83:1‐2. [DOI] [PubMed] [Google Scholar]
Ripamonti 1993
- Ripamonti C, Conno F, Ventafridda V, Rossi B, Baines MJ. Management of bowel obstruction in advanced and terminal cancer patients. Annals of Oncology 1993;4:15‐21. [DOI] [PubMed] [Google Scholar]
Rose 1978
- Rose PG, Piver S, Tsukada Y, Lau T. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer 1978;64:1508‐13. [DOI] [PubMed] [Google Scholar]
Watson 1997
- Watson JP, Mannix KA, Matthewson K. Percutaneous endoscopic gastroenterostomy and jejunal extension for gastric stasis in pancreatic carcinoma. Palliative Medicine 1997;11:407‐10. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Feuer 1999c
- Feuer DJ, Broadley KE, Sheperd JH, Barton DPJ. Systematic review of surgery in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Gynaecologic Oncology 1999;75:313‐22. [DOI] [PubMed] [Google Scholar]
Feuer 2000
- Feuer DDJ, Broadley KE. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002764] [DOI] [PubMed] [Google Scholar]


