Abstract
Objective
The aim of the present study was to evaluate the efficacy of inhaled milrinone in controlling pulmonary arterial hypertension (PAH) in paediatric cardiac surgery and its effect on weaning from cardiopulmonary bypass (CPB).
Methods
A total of 40 patients with congenital heart diseases complicated by PAH submitted to cardiac surgery requiring CPB were included in the present study and were randomly classified into the control group (n=20) who received intravenous milrinone 0.5 μg kg−1 min−1 and the inhaled group (n=20) who received inhaled milrinone 50 μg kg−1 before initiation and just before weaning off CPB. Mean pulmonary artery pressure (mPAP), mean systemic arterial pressure (MAP), heart rate (HR), MAP/mPAP ratio, vasoactive drug requirements and time needed to wean the patients from CPB were collected.
Results
mPAP and HR were significantly lower, and MAP and MAP/mPAP ratio were significantly higher in the inhaled group than in the control group. Vasoactive drug requirements were significantly lesser, and the time needed to wean the patients was significantly shorter in the inhaled group than in the control group.
Conclusion
Milrinone inhalation facilitated the weaning from CPB as it significantly reduced mPAP and maintained MAP with subsequently less needs for vasoactive drugs.
Keywords: Cardiopulmonary bypass, congenial heart diseases, milrinone inhalation, pulmonary hypertension, vasoactive drugs
Introduction
Congenital heart diseases (CHDs) are the most common congenital anomalies worldwide (1). The global prevalence of CHDs is 8 out of 1000 live births that varies according to the geographic area (2). Pulmonary arterial hypertension (PAH) is a common finding in children with left to right shunts (3), resulting in an increase in perioperative complications, such as pulmonary hypertensive crisis and right ventricular dysfunction (4).
Pulmonary arterial hypertension in children is defined as mean pulmonary artery pressure (mPAP) >25 mmHg (5). It is a frequent condition that complicates cardiac surgery and affects its outcome. The closure of large left to right cardiac shunts and the use of cardiopulmonary bypass (CPB) in patents with pre-existing PAH may cause an acute increase in pulmonary artery pressure leading to right ventricular dysfunction that increases the risk of postoperative morbidity and mortality (6–9).
Milrinone is a specific phosphodiesterase-3 inhibitor that causes systemic and pulmonary vasodilatation and positive inotropic action caused by increase cyclic adenosine monophosphate in cardiac muscles without stimulation of cardiovascular β1-adrenergic receptors (10). The major side effect reported with intravenous milrinone is the high incidence of systemic arterial hypotension that is dose dependent with a subsequent increased need for vasoactive drugs (11).
Preoperative pulmonary hypertension is associated with difficult weaning from CPB (12). Inhalation of milrinone induces effective pulmonary vasodilatation with less adverse systemic effects and is proven to decrease the post-CPB associated right ventricular dysfunction (13). Inhaled milrinone has many advantages when used instead of nitric oxide. It does not require complex preparation, is already available in the operating room and needs only a simple nebuliser for administration. We hypothesised that inhaled milrinone before CPB could control PAH and may facilitate the weaning from CPB in children submitted to open heart surgery for total repair of CHDs complicated by PAH. Therefore, the primary goal of the present study was to evaluate the efficacy of inhaled milrinone in controlling PAH in children submitted for total correction of CHDs. The secondary goal was to evaluate its effect on weaning from CPB in children with regard to inotropic support and time needed to wean from CPB.
Methods
The study was approved by the local ethics committee of the Faculty of Medicine, Mansoura University (R 130/2015). Written consent was obtained from the patient’s parents or their guardians. This prospective randomised study was conducted between August 2015 and October 2018 in Mansoura University Children’s Hospital. A total of 40 patients of either sex aged between 6 and 24 months with left to right intracardiac shunts complicated by moderate to severe PAH submitted to corrective surgery requiring CPB were enrolled in the present study.
All patients included in the present study were evaluated by transthoracic echocardiography and cardiac catheterisation. Children with mPAP >25 mmHg were included in the current study. Patients with emergency surgery, previous cardiac surgery, heart failure, bronchial asthma, preoperative inotropic support, pre-existing thrombocytopenia and severe renal or hepatic diseases were excluded from the study.
Anaesthetic technique and surgical team were the same for all patients. All patients received intramuscular ketamine 2 mg kg−1 and midazolam 0.1 mg kg−1 10 min before the induction of anaesthesia that was induced with fentanyl 5 μg kg−1, sevoflurane and rocuronium 0.9 mg kg−1 and maintained with sevoflurane 1%–3% in 50% oxygen in air, rocuronium 0.3 mg kg−1 h−1 and fentanyl 5 μg kg−1 h−1. Each patient was monitored using a non-invasive arterial blood pressure cuff that was recorded every 2 min until insertion of an arterial catheter, pulse oximetry, capnography, 5-lead electrocardiogram and a nasopharyngeal temperature probe.
Under complete aseptic precautions, the femoral artery was cannulated with a 20-gauge catheter, and a right internal jugular venous catheter was inserted. The patients were randomly classified into two groups by a computer-assisted programme.
Control group (n=20)
Patients in the control group received intravenous bolus of milrinone 50 μg kg−1, followed by continuous infusion of 0.5 μg kg−1 min−1, started before skin incision and continued postoperatively (14).
Inhaled group (n=20)
Patients received inhaled milrinone administered by a continuous output jet nebuliser placed on the inspiratory limb of the breathing circuit near the endotracheal tube using high oxygen flow (8 L min−1). Milrinone was administered by inhalation at a dose of 50–80 μg kg−1 (15) dissolved in 3 mL normal saline and applied to patients >10 min at two time events, the first was after induction of anaesthesia and the second was before weaning off CPB after declamping the aorta while the heart was beating and ejecting blood to the systemic and pulmonary circulation.
Ventilation was adjusted to maintain end-tidal carbon dioxide 30–35 mmHg, peak airway pressure <20 mmHg and positive end-expiratory pressure <5 mmHg. Surgery was achieved via median sternotomy. Heparin 3–4 mg kg−1 was administered via central venous catheter, followed by aortic and bicaval cannulation. The CPB circuit was primed with a combination of Ringer’s acetate, 20% albumin and whole blood to achieve a haematocrit 25%–30% and was initiated after obtaining an activated clotting time >480 s. The pump flow was non-pulsatile at a rate of 2.4 L m2−1 body surface area to maintain the arterial blood pressure at 40–70 mmHg. CPB was accompanied with normothermia (temperature ≥35°C) and ultrafiltration to maintain fluid balance. Myocardial preservation was done by the administration of a single dose cold crystalloid histidine-tryptophan-ketoglutarate, Bretschneider’s formula, cardioplegia (Custodiol 50 mL kg−1) in an antegrade manner after clamping the ascending aorta.
Weaning off CPB was started after repair of cardiac defect, cardiac de-airing, obtaining good myocardial contractility, suitable heart rate (HR), haematocrit >30%, nasopharyngeal temperature 37°C and normal arterial blood gases and electrolytes. Before weaning off CPB, dobutamine was infused at a dose of 5 μg kg−1 min−1 that was increased up to 20 μg kg−1 min−1 according to patient response. Norepinephrine infusion was added up to a dose of 0.2 μg kg−1 min−1 if the mean arterial blood pressure during weaning off CPB did not exceed 50 mmHg. After surgery, all patients were transferred to the intensive care unit (ICU) sedated and on controlled mechanical ventilation.
Recorded data
Data were recorded at the following time events: before initiating milrinone therapy as basal values (T0), 10 min after initiating milrinone and before starting CPB (T1), 30 min after weaning off CPB (T2) and 60 min thereafter (T3). The data recoded at these time events included HR, mean systemic arterial pressure (MAP), mPAP, central venous pressure (CVP), arterial oxygen tension (PaO2) and MAP/mPAP ratio. The following data were recorded: CPB time, aortic cross clamp time, spontaneous beating of the heart after removal of aortic cross clamp, need for direct current defibrillation and number of shocks, inotropic support and its dose and time needed to wean the patient from CPB. We could not measure the cardiac index as the proper sizes of Swan Ganz catheters for children were not available in Mansoura University Children’s Hospital, and mPAP was measured through intraoperatively placed lines.
Statistical analysis
Sample size was done based on mPAP changes in the previous study by Wang et al. (14). In their study, mPAP decreased approximately 8 mmHg from the baseline after milrinone inhalation. The standard deviation used in the study by Wang et al. (14) was 8.9. Seventeen patients for each group would be required to obtain 80% power at a 5% significance level.
A dropout of 10% of patients was expected. Therefore, a total number of 40 patients (20 patients per group) would be needed.
Calculations were done using G power software for Windows, version 3.0.10 (Franz Faul, Christian-Albrechts-Universität Kiel, Kiel, Germany). IBM Statistical Package for the Social Sciences statistical software for Windows, version 25 (IBM SPSS Corp.; Armonk, NY, USA) was used for statistical analysis of the collected data. Shapiro-Wilk test was used to check the normality of the data distribution in continuous variables. Continuous variables were expressed as mean±SD, whereas categorical variables were expressed as number and percentage. One-way ANOVA and Kruskal-Wallis tests were used to compare normally and non-normally distributed continuous variables with no follow-up readings, respectively. Repeated measures ANOVA model with Bonferroni post hoc test and 95% confidence interval (CI) were used to compare the follow-up values of continuous data. Fisher’s exact test was used for intergroup comparison of nominal and ordinal data using the crosstabs function. Comparison of follow-up and basal values (intragroup) was conducted using Wilcoxon signed-rank test and McNemar test for ordinal and nominal data, respectively. All tests were conducted with 95% CI. Charts were generated using SPSS chart builder. A P (probability) value <0.05 was considered statistically significant.
Results
A total of 40 patients with CHDs were enrolled in the present study and classified into two groups, each of them included 20 patients, controlled group and inhaled milrinone group. There were no statistically significant differences between both studied groups with regard to demographic data (age, gender, weight and height) and the nature of cardiac anomalies (Table 1).
Table 1.
Patient demographic data and type of cardiac anomalies in the studied groups
| Variables | Control group n=20 | Inhaled group n=20 | p |
|---|---|---|---|
|
| |||
| Age (months) | 14.62±8.90 | 15.38±9.45 | 0.911 |
| Gender (M/F) | 12/8 | 11/9 | 0.71 |
| Weight (kg) | 9.11±4.92 | 10.61±5.62 | 0.832 |
| Height (cm) | 73.14±10.20 | 76.24 ±8.41 | 0.738 |
| Cardiac anomalies (n, %) | |||
| ASD | 2 (10%) | 1 (5%) | ns |
| VSD | 13 (65%) | 14 (70%) | ns |
| CAVC | 5 (25%) | 5 (25%) | ns |
Data are expressed as mean±SD, number (n) and percentage (%). M: male; F: female; ASD: atrial septal defect; VSD: ventricular septal defect; CAVC: common atrioventricular canal; ns: non-significant
The patient’s haemodynamics (HR, MAP, mPAP and CVP) are shown in Figures 1–4, respectively. HR and mPAP were significantly lower in the inhaled milrinone group than in the control group at the following time events: before starting CPB (T1), 30 min after weaning off CPB (T2) and 60 min thereafter (T3), as shown in Figures 1 and 3, respectively.
Figure 1.
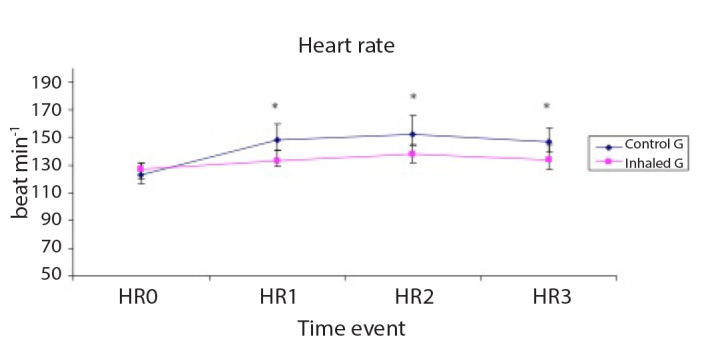
Heart rate (HR, beat min−1) of the studied groups
Data are expressed as mean±SD. *p<0.05 is significant compared with the control group.
Figure 2.
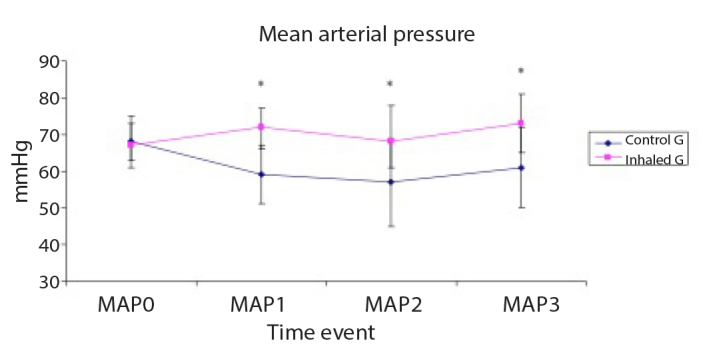
Invasive mean arterial pressure (MAP, mmHg) of the studied groups
Data are expressed as mean±SD. *p<0.05 is significant compared with the control group.
Figure 3.
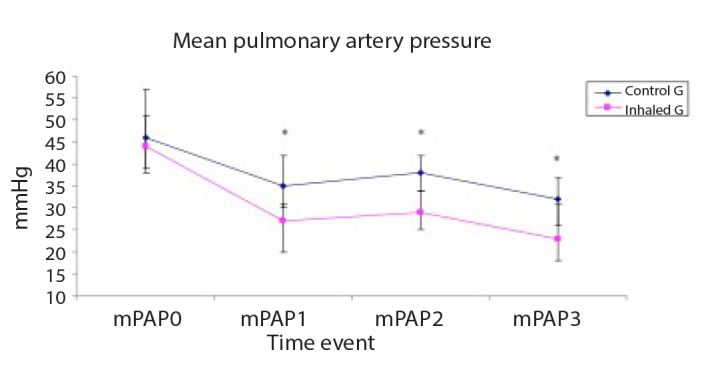
Mean pulmonary artery pressure (mPAP, mmHg) of the studied groups
Data are expressed as mean±SD. *p<0.05 is significant compared with the control group.
Figure 4.
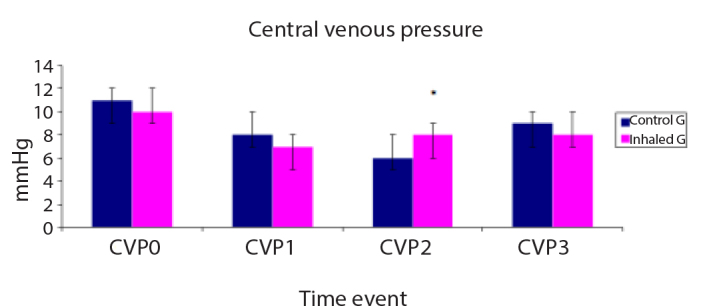
Central venous pressure (CVP, mmHg) of the studied groups
Data are expressed as mean±SD. *p<0.05 is significant compared with the control group.
MAP was significantly lower in the control group than in the inhaled group at T1, T2 and T3 time events (Figure 2), whereas CVP was significantly lower in the control group than in the inhaled group 30 min after weaning off CPB (T2) (Figure 4). The MAP/mPAP ratio was significantly lower in the control group than in the inhaled group at T1, T2 and T3 time events (Figure 5).
Figure 5.
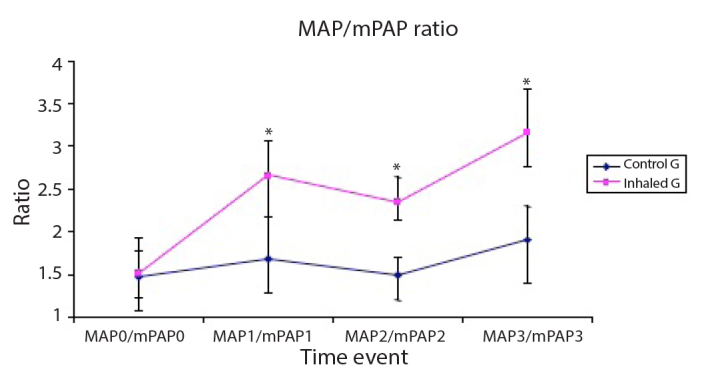
The ratio of mean arterial systemic pressure (MAP, mmHg) to mean pulmonary arterial pressure (mPAP, mmHg) of the studied groups
Data are expressed as mean±SD. *p<0.05 is significant compared with the control group.
PaO2 is shown in Figure 6 with no statistically significant difference between the two studied groups.
Figure 6.
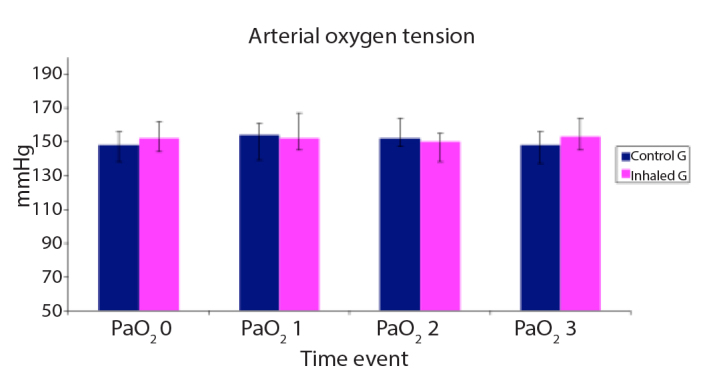
Arterial oxygen tension (PaO2, mmHg) of the studied groups
Data are expressed as mean±SD.
Table 2 shows intraoperative variables, CPB time, aortic cross clamp time and spontaneous beating of the heart after removal of aortic cross clamp. There were no statistically significant differences between the control and inhaled milrinone groups with regard to intraoperative variables.
Table 2.
Intraoperative variables
| Variables | Control group n=20 | Inhaled group n=20 | p |
|---|---|---|---|
|
| |||
| CPB time (min) | 81.3±14.2 | 66.4±22.6 | 0.62 |
| Aortic clamp time (min) | 67.5±14.8 | 71.7±13.6 | 0.76 |
| Spontaneous heart beat (n, %) | 18 (90) | 19 (95) | 0.93 |
| DC shock (n, %) | 2 (10) | 1 (5) | 0.86 |
| Dobutamine | |||
| (n, %) | 20 (100) | 20 (100) | 1 |
| Dose (μg kg−1 min−1) | 12.47±3.52 | 9.73±3.12 | 0.21 |
| Norepinephrine | |||
| (n, %) | 15 (75) | 4 (20)* | <0.01 |
| Dose (μg kg−1 min−1) | 0.14 (0.11–0.18) | 0.07 (0.04–0.09)* | 0.023 |
| Duration of weaning (min) | 5.72±3.21 | 3.22±2.41* | 0.021 |
Data are expressed as mean±SD, median and range, number (n) and percentage (%).
p<0.05 is significant when compared to the control group.
CPB: cardiopulmonary bypass; DC: direct current defibrillation
All patients in both groups received dobutamine without any statistically significant difference regarding its dose (Table 2). The number of patients requiring norepinephrine was significantly higher in the control group (n=15) than in the inhaled group (n=4) (Table 2). The dose of norepinephrine was significantly higher (p=0.023) in the control group than in the milrinone group (Table 2).
The time needed to wean the heart from CPB was significantly longer in the control group (5.72±3.21 min) than in the inhaled milrinone group (3.22±2.41 min) (Table 2). No patients developed pulmonary hypertensive crisis during surgery.
Discussion
The main findings of the current study showed that milrinone inhalation before initiation and during CPB just before weaning in children with CHDs suffering from PAH resulted in more stable haemodynamics with regard to HR and MAP and more effective reduction of mPAP with higher MAP/mPAP ratio than intravenous infusion of milrinone (control group). Patients in the control group required higher doses of vasoactive drugs and longer time to wean from CPB than patients in the inhaled milrinone group.
Wang et al. (14) compared the use of inhaled and intravenous milrinone in 48 patients undergoing mitral valve replacement complicated with pulmonary hypertension. They reported a comparable decrease in mPAP in both groups, whereas MAP was significantly higher in the inhaled group than in the intravenous group after initiation of milrinone therapy. The haemodynamic effects of inhaled milrinone lasted for approximately 60 min after its discontinuation as indicated by returning mPAP to the baseline.
Denault et al. (15) in 2014 studied the use of inhaled milrinone in patients with preoperative pulmonary hypertension undergoing complicated cardiac surgery. Their study included 21 patients who were randomised in a double-blind study to receive either inhaled milrinone or placebo. Milrinone was inhaled before surgical incision and CPB. They found that inhaled milrinone was not associated with systemic hypotension with reduced pulmonary artery pressure and pulmonary vascular resistance but not statistically significant. Denault et al. (16) in 2016 in a randomised clinical study included 140 patients and compared the use of inhaled milrinone versus placebo in high-risk cardiac surgical patients with pulmonary hypertension. They found that the use of inhaled milrinone was associated with an increase in cardiac output and a significant reduction of systolic pulmonary artery pressure (p=0.04) without significant effects on systemic arterial pressure or HR. The MAP/mPAP ratio was >20% in 10 patients.
Rong et al. (17) studied the benefits of inhaled and intravenous milrinone in adult cardiac surgery in a meta-analysis assessing the haemodynamic and clinical effects of both routes of administration. The meta-analyses involved 30 studies that included 1438 adult cardiac patients, 194 of them received inhaled milrinone and 521 received intravenous milrinone. The primary goals were mPAP and systemic vascular resistance, whereas the secondary goals were MAP, HR, cardiac output and index, CVP and pulmonary capillary wedge pressure. They found that intravenous milrinone was associated with significantly lower mPAP and systemic vascular resistance than placebo. On the other hand, there was no significant difference when inhaled milrinone was compared to placebo with regard to mPAP and systemic vascular resistance. Intravenous milrinone was associated with significantly lower MAP and shorter hospital length of stay than inhaled milrinone. No significant statistical difference was found between intravenous and inhaled milrinone with regard to mPAP (17).
Lamarche et al. (18) in a retrospective study included 70 cardiac surgical patients and compared the effects of milrinone inhalation before and after CPB. They found that a single dose of milrinone inhalation before going on CPB was associated with a significant reduction of the rate of re-initiation of CPB (3%) when compared with patients who received inhaled milrinone after CPB (23%).
As shown in the current study, the MAP/mPAP ratio was significantly lower in the control group than in the inhaled milrinone group. The MAP/mPAP ratio has been used as a predictor of the outcome of cardiac surgery. As demonstrated from previous studies, The MAP/mPAP ratio was an easy method that correlated with the severity of PAH during cardiac surgery (19, 20).
Preoperative MAP/mPAP ratio <4 refers to the presence of PAH with lower survival rates following cardiac surgery (19, 20). Robitaille et al. (19) in their retrospective and prospective studies in adult patients found that lower MAP/mPAP ratios have been associated with more haemodynamic complications after cardiac surgery that included cardiac arrest, the need for vasopressor support >24 h postoperatively or the use of intra-aortic balloon pump.
In the current study, the time needed to wean the patients from CPB was significantly shorter, and the dose of vasoactive agents was smaller in the inhaled group than in the control group as intravenous milrinone causes systemic vasodilatation and hypotension, whereas inhaled milrinone has minimal systemic effects as it acts only locally on pulmonary vascular bed (21).
Lamarche et al. (18) observed that a single bolus dose of inhaled milrinone administration before initiation of CPB was accompanied with a lower rate of CPB re-initiation than those who received inhaled milrinone after CPB.
Laflamme et al. (22) in their retrospective study observed that milrinone inhalation is associated with lower rate of difficult weaning from CPB and a significant reduction in the requirements of vasoactive agents in the first 24 h in the ICU after cardiac surgery.
Cardiac surgical patients may develop pulmonary reperfusion syndrome as a result of CPB that aggravates pulmonary hypertension (23), resulting in difficult weaning and right ventricular dysfunction with poor postoperative prognosis (22). Consequently, prevention and controlling pulmonary hypertension would be expected to minimise the incidence of pulmonary reperfusion syndrome.
Milrinone inhalation before initiation of CPB protects the pulmonary vascular endothelium from ischaemic reperfusion injury during weaning from CPB through a more homogenous distribution of pulmonary blood flow in mechanically ventilated lungs, minimising post-CPB atelectasis. The administration of inhaled milrinone before the initiation of CPB could prevent reperfusion syndrome (20).
Inhaled milrinone can be used to facilitate weaning from CPB in paediatric cardiac surgery by causing selective pulmonary vasodilatation, consequently reducing the degree of pulmonary hypertension. Inhalation of milrinone is simple, safe and easy to administer via the inspiratory limb of anaesthesia ventilator. Milrinone given by inhalation is delivered to both lungs directly with minimum systemic absorption, so it is not associated with systemic hypotension as in intravenous route, decreasing the need for vasoactive drugs.
Conclusion
As demonstrated from the results of the current study, we can conclude that milrinone inhalation before and during CPB facilitates the weaning from CPB as it significantly reduces mPAP and maintains MAP, resulting in less needs for vasoactive drugs and shorter time needed to wean the patients from CPB.
Main Points.
The use of inhaled milrinone is effective in reducing the elevated pulmonary artery pressure after weaning from cardiopulmonary bypass.
Milrinone inhalation is associated with hemodynamic stability as regard heart rate and blood pressure.
Inhaled milrinone facilitates the weaning from cardiopulmonary bypass in children with preexisting pulmonary hypertension.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Mansoura University (R 130/2015).
Informed Consent: Written informed consent was obtained from patients’ parents or guardians who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – I.A.; Design – I.A., Supervision – A.D.; Supervision – A.D.; Materials – A.D.; Analysis and/or Interpretation – A.D.; Writing Manuscript – I.A.; Critical Review – I.A
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Chelo D, Nguefack F, Menanga AP, Ngo Um S, Gody JC, Tatah SA, et al. Spectrum of heart diseases in children: an echocardiographic study of 1,666 subjects in a pediatric hospital, Yaounde, Cameroon. Cardiovasc Diagn Ther. 2016;6:10–9. doi: 10.3978/j.issn.2223-3652.2015.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diller GP, Kempny A, Inuzuka R, Radke R, Wort SJ, Baumgartner H, et al. Survival prospects of treatment naïve patients with Eisenmenger: a systematic review of the literature and report of own experience. Heart. 2014;100:1366–72. doi: 10.1136/heartjnl-2014-305690. [DOI] [PubMed] [Google Scholar]
- 4.Kameny RJ, Fineman J, Adatia I. Perioperative Management of Paediatric Pulmonary Hypertension. Advances in Pulmonary Hypertension. 2016;15:87–91. doi: 10.21693/1933-088X-15.2.87. [DOI] [Google Scholar]
- 5.Dimopoulos K, Wort SJ, Gatzoulis MA. Pulmonary hypertension related to congenital heart disease: a call for action. Eur Heart J. 2014;35:691–700. doi: 10.1093/eurheartj/eht437. [DOI] [PubMed] [Google Scholar]
- 6.Kaestner M, Schranz D, Warnecke G, Apitz C, Hansmann G, Miera O. Pulmonary hypertension in the intensive care unit. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102:57–66. doi: 10.1136/heartjnl-2015-307774. [DOI] [PubMed] [Google Scholar]
- 7.Serrano CV, Jr, Souza JA, Lopes NH, Fernandes JL, Nicolau JC, Blotta MH, et al. Reduced expression of systemic proinflammatory and myocardial biomarkers after off-pump versus on-pump coronary artery bypass surgery: a prospective randomized study. J Crit Care. 2010;25:305–12. doi: 10.1016/j.jcrc.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Le Hiress M, Tu L, Ricard N, Phan C, Thuillet R, Fadel E, et al. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension role of the macrophage migration inhibitory factor/ CD74 complex. Am J Respir Crit Care Med. 2015;192:983–97. doi: 10.1164/rccm.201402-0322OC. [DOI] [PubMed] [Google Scholar]
- 9.Aljure OD, Fabbro M., 2nd Cardiopulmonary Bypass and Inflammation: The Hidden Enemy. J Cardiothorac Vasc. 2019;33:346–7. doi: 10.1053/j.jvca.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen DV, Torp-Pedersen C, Skals RK, Gerds TA, Karaliunaite Z, Jakobsen CJ. Intraoperative milrinone versus dobutamine in cardiac surgery patients: A retrospective cohort study on mortality. Crit Care. 2018;22:51. doi: 10.1186/s13054-018-1969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mladěnka P, Applová L, Patočka J, Costa VM, Remiao F, Pourová J, et al. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev. 2018;38:1332–403. doi: 10.1002/med.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg. 2009;108:422–33. doi: 10.1213/ane.0b013e31818d8b92. [DOI] [PubMed] [Google Scholar]
- 13.Kundra TS, Prabhakar V, Kaur P, Manjunatha N, Gandham R. The Effect of Inhaled Milrinone Versus Inhaled Levosimendan in Pulmonary Hypertension Patients Undergoing Mitral Valve Surgery - A Pilot Randomized Double-Blind Study. J Cardiothorac Vasc Anesth. 2018;32:2123–9. doi: 10.1053/j.jvca.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Gong M, Zhou B, Dai A. Comparison of inhaled and intravenous milrinone in patients with pulmonary hypertension undergoing mitral valve surgery. Adv Ther. 2009;26:462–8. doi: 10.1007/s12325-009-0019-4. [DOI] [PubMed] [Google Scholar]
- 15.Denault AY, Haddad F, Lamarche Y, Nguyen AQ, Varin F, Levesque S, et al. Pilot randomized controlled trial of inhaled milrinone in high-risk cardiac surgical patients. Surgery Curr Res. 2014;4:192. [Google Scholar]
- 16.Denault AY, Bussières JS, Arellano R, Finegan B, Gavra P, Haddad F, et al. A multicentre randomized-controlled trial of inhaled milrinone in high-risk cardiac surgical patients. Can J Anaesth. 2016;63:1140–53. doi: 10.1007/s12630-016-0709-8. [DOI] [PubMed] [Google Scholar]
- 17.Rong LQ, Rahouma M, Abouarab A, Di Franco A, Calautti NM, Fitzgerald MM, et al. Intravenous and inhaled milrinone in adult cardiac surgery patients: A pairwise and network meta-analysis. J Cardiothorac Vasc Anesth. 2019;33:663–73. doi: 10.1053/j.jvca.2018.08.208. [DOI] [PubMed] [Google Scholar]
- 18.Lamarche Y, Perrault LP, Maltais S, Tétreault K, Lambert J, Denault AY. Preliminary experience with inhaled milrinone in cardiac surgery. Eur J Cardiothorac Surg. 2007;31:1081–7. doi: 10.1016/j.ejcts.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Robitaille A, Denault AY, Couture P, Belisle S, Fortier A, Guertin MC, et al. Importance of relative pulmonary hypertension in cardiac surgery: the mean systemic to pulmonary artery pressure ratio. J Cardiothor Vasc Anesth. 2006;20:331–9. doi: 10.1053/j.jvca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Kaw R, Pasupuleti V, Deshpande A, Hamieh T, Walker E, Minai OA. Pulmonary hypertension: An important predictor of outcomes in patients undergoing non-cardiac surgery. Respir Med. 2011;105:619–24. doi: 10.1016/j.rmed.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Lamarche Y, Malo O, Thorin E, Denault A, Carrier M, Roy J, et al. Inhaled but not intravenous milrinone prevents pulmonary endothelial dysfunction after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;130:83–92. doi: 10.1016/j.jtcvs.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Laflamme M, Perrault LP, Carrier M, Elmi-Sarabi M, Fortier A, Denault AY. Preliminary experience with combined inhaled milrinone and prostacyclin in cardiac surgical patients with pulmonary hypertension. J Cardiothorac Vasc Anesth. 2015;29:38–45. doi: 10.1053/j.jvca.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 23.St-Pierre P, Deschamps A, Cartier R, Basmadjian A, Denault AY. Inhaled milrinone and epoprostenol in a patient with severe pulmonary hypertension, right ventricular failure and reduced baseline brain saturation value from a left atrial myxoma. J Cardiothorac Vasc Anesth. 2014;28:723–9. doi: 10.1053/j.jvca.2012.10.017. [DOI] [PubMed] [Google Scholar]


