Abstract
Background/Objectives
Non-alcoholic fatty liver disease (NAFLD) causes a wide spectrum of liver damage, from simple steatosis (SS) to cirrhosis. SS and non-alcoholic steatohepatitis (NASH) cannot be distinguished by clinical or laboratory features. Dysregulation of the gut microbiota is involved in NASH pathogenesis. The aim of this study was to assess the relationship between microbiota-derived metabolites and the degrees of NAFLD; also, to investigate whether these metabolites could be included in a panel of NASH biomarkers.
Subjects/Methods
We used liquid chromatography coupled to triple-quadrupole-mass spectrometry (LC-QqQ) analysis to quantify choline and its derivatives, betaine, endogenous ethanol, bile acids, short-chain fatty acids and soluble TLR4 in serum from women with normal weight (n = 29) and women with morbid obesity (MO) (n = 82) with or without NAFLD. We used real-time polymerase chain reaction (RT-PCR) analysis to evaluate the hepatic and intestinal expression level of all genes studied (TLR2, TLR4, TLR9, LXRα, SREBP1C, ACC1, FAS, PPARα, CPT1α, CROT, SREBP2, ABCA1, ABCG1 and FXR in the liver; TLR2, TLR4, TLR5, TLR9, GLP-1R, DPP-4, FXR and PPARɣ in the jejunum) in 82 women with MO with normal liver histology (NL, n = 29), SS (n = 32), and NASH (n = 21).
Results
Hepatic FAS, TLR2, and TLR4 expression were overexpressed in NAFLD patients. TLR2 was overexpressed in NASH patients. In women with MO with NAFLD, we found upregulation of intestinal TLR9 expression and downregulation of intestinal FXR expression in women with NASH. Circulating TMAO, glycocholic acid and deoxycholic acid levels were significantly increased in NAFLD patients. Endogenous circulating ethanol levels were increased in NASH patients in comparison to those in SS patients.
Conclusions
These findings suggest that the intestine participates in the progression of NAFLD. Moreover, levels of certain circulating microbiota-related metabolites are associated with NAFLD severity and could be used as a “liquid biopsy” in the noninvasive diagnosis of NASH.
Subject terms: Medical research, Diseases
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a health problem expanding in parallel with the global increase in obesity and metabolic syndrome, with an estimated global prevalence of 25%. A subgroup of individuals with NAFLD develops non-alcoholic steatohepatitis (NASH), which is characterized not only by hepatocellular lipid accumulation but also by varying severities of inflammation and fibrosis. NASH can lead to cirrhosis and even liver cancer [1]. The risk of liver-related mortality increases exponentially with an increase in fibrosis stage [2]. Currently, the diagnosis of NASH is biopsy-mediated, and it has become essential to improve the accuracy in its noninvasive diagnosis because biopsy is limited by cost, sampling error, and procedure-related morbidity and mortality [3].
NAFLD is a complicated metabolic disease with pathophysiological interactions between genetic and environmental factors [4]. In this regard, the most generally accepted hypothesis at present to explain the progression from simple steatosis (SS) to the concomitant presence of inflammation and ballooning, which defines NASH, is the “multiple hit” hypothesis. This hypothesis considers multiple insults acting together, including hormones/adipokines secreted from the adipose tissue, lipotoxicity, oxidative stress, mitochondrial dysfunction, genetic and epigenetic factors, and gut microbiota [5]. The dysregulation of gut microbiota has been found to be involved in a variety of metabolic diseases, such as diabetes, insulin resistance, obesity, and NAFLD [6].
The liver and the intestine are tightly linked through the portal circulation; consequently, gut microbial-derived products primarily arriving at the liver may have pathogenic implications [7]. In recent years, the role of the gut microbiota has been increasingly implicated in modulating risk factors for NAFLD, such as energy homeostasis dysregulation, insulin resistance, increase in intestinal permeability, endogenous production of ethanol, inflammation (innate immunity and inflammasomes), and choline and bile acid (BA) metabolism [8]. These factors likely act together to intervene in the pathogenesis of NAFLD [9].
First, gut microbiota play important roles in modulating host energy balance. In this sense, short-chain fatty acids (SCFAs) are generated by gut microbial fermentation of nondigestible carbohydrates and provide precursors for lipogenesis and gluconeogenesis, mechanisms involved in NAFLD [10]. Probiotics can inhibit small intestinal bacterial overgrowth (SIBO), leading to an improvement in insulin sensitivity in relation to incretin hormones [11] that have been shown diminished in patients with NAFLD [12]. Regarding intestinal permeability, patients with NAFLD or NASH are believed to have gut barrier dysfunction secondary to SIBO, with increased translocation of microbial products, allowing these products to reach the liver, where they may act as possible factors for NASH development [13]. In addition, both animal and human studies have suggested that gut microbiota is responsible for the increase in endogenous ethanol production in patients with NAFLD [14]. One bacteria-derived product, lipopolysaccharide (LPS), is able to activate Toll-like receptors (TLRs), and probably involved in the pathogenesis of NAFLD [15–17].
Moreover, the level of endogenous choline is influenced by gut microbiota and some studies have shown that choline deficiency induces fatty liver formation [18].
A common link among many NASH pathogenesis pathways is the disruption of BA homeostasis. Bile acids bind to farnesoid X receptor (FXR), which is critically involved in maintaining BA, glucose, and lipid homeostasis [19]. Also, intestinal dysbiosis is able to modify the profile of BAs in patients with NAFLD [20].
The co-metabolism of gut microbiota in a host means that a large number of microbial metabolites are excreted in blood, urine or feces. Some of these metabolites cannot be produced without bacterial fermentation, such as the choline metabolite trimethylamine (TMA), the secondary BAs, deoxycholic acid (DCA) and lithocholic acid (LCA), and also SCFAs. Therefore, metabolomics can be useful in identifying the systemic metabolic impact of the intestinal microbiota. The application of this technique to the study of the pathogenesis of NAFLD will increase the knowledge of the biochemical pathways involved in the progression of SS to NASH and will also help in the diagnosis of NASH. Therefore, different studies in animals have used metabolomics to understand mechanisms involved in NAFLD [21]. However, few studies in humans have been conducted in this regard [22].
Although the role of gut microbiota in the development of NAFLD is well documented, the exact mechanisms by which gut microbiota contribute to NAFLD are not enough understood. Therefore, in the present project, we had a dual objective. First, we sought to improve our knowledge of the pathogenic mechanisms involved in NAFLD by studying the intestinal microbiota from a metabolomic point of view. In this sense, we studied the circulating levels of choline, betaine, endogenous ethanol, primary and secondary BAs, SCFA and soluble TLR4 in relation to the hepatic expression of FXR, hepatic lipid metabolism genes (LXRα, SREBP1C, ACC1, FAS, PPARα, CPT1α, CROT, SREBP2, ABCA1, and ABCG1) and TLRs (TLR2, TLR4, and TLR9), and in relation to the intestinal expression of FXR, TLRs, GLP-1, and DPP-4 receptors and PPARγ in a cohort of patients with MO and NAFLD. In addition, we sought to assess whether the circulating levels of microbiota-related metabolites are associated with the severity of the disease and can be used to indicate a diagnosis of NASH.
Materials and methods
Subjects
The study was approved by the institutional review board, and all participants gave written informed consent (23c/2015). The sample size has been calculated by establishing an α level of 0.0167 (0.05/3 groups to be compared), a β level of 0.80, an estimated maximum standard deviation of 0.9, and a difference between averages of 50%. The resulting sample size to detect possible differences between groups was n = 23 in each study group. The study population consisted of 111 Caucasian women: 29 normal-weight controls (BMI < 25 kg/m2) and 82 patients with MO (BMI > 40 kg/m2). Liver and jejunal biopsies from MO patients were obtained during planned laparoscopic bariatric surgery. All liver biopsies were indicated for clinical diagnosis. The exclusion criteria were as follows: (1) subjects who had alcohol consumption higher than 10 g/day; (2) patients who had acute or chronic hepatic diseases (with the exception of NAFLD), (3) patients with inflammatory, infectious or neoplastic diseases; (4) patients with history of pseudomembranous colitis; (5) women who were menopausal or undergoing contraceptive treatment; (6) diabetic women receiving pioglitazone, GLP-1 receptor agonists, DPP-4 inhibitors or insulin; (7) patients treated with antibiotics (including rifamixin) in the previous 4 weeks or receiving cholestyramine or ursodeoxycholic acid; (8) subjects taking probiotics; and (9) patients receiving fecal transplantation.
Liver pathology
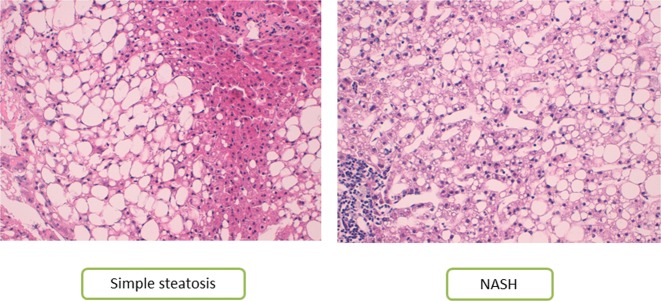
Liver samples were scored by experienced hepatopathologists using methods described elsewhere [23]. According to their liver pathology, women with MO were subclassified into three groups: normal liver (NL) histology (n = 29), SS (micro/macrovesicular steatosis without inflammation or fibrosis, n = 32), and NASH (Brunt Grades 1–3, n = 21). It is important to note that in our study, any patient had neither fibrosis nor cirrhosis. In order to give visual information, Fig. 1 shows the histologic features, grading, and staging of NAFLD with own images.
Fig. 1.
Histologic features, grading, and staging of NAFLD (own images). Histological evaluation of liver sections stained with Hematoxylin-eosin, 200×: a SS group: Normal architecture amb macrovesicular steatosis. b NASH group: Macrovesicular steatosis, ballooning degeneration, and lobular inflammation
Biochemical analyses
All of the subjects included underwent physical, anthropometric, and biochemical assessments. Blood samples were obtained from obese and control subjects. Biochemical parameters were analyzed using a conventional automated analyzer after 12 h of fasting. Insulin resistance (IR) was estimated using homeostasis model assessment of IR (HOMA2-IR).
Plasma measurements
Plasma samples, which were obtained from either the MO group or the control group, were stored at −80 °C. TLR4 levels were analyzed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Ref. SEA753Hu; USCN). Circulating levels of ethanol were assessed by colorimetric assay (Ref. MAK076; Sigma Aldrich). Absolute quantification of intestinal hormones (GLP1) in serum samples were analyzed by ELISA Milliplex (EZGLPHS-35K, MilliporeSigma, Burlington, Massachusetts). Absolute quantification of 15 BAs (CDCA, chenodeoxycholic acid; CA, cholic acid; GCDCA, glycochenodeoxycholic acid; GCA, glycocholic acid; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; DCA, deoxycholic acid; GDCA, glycodeoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid; TLCA, taurolithocholic acid; TDCA, taurodeoxycholic acid; and TUDCA, tauroursodeoxycholic acid) and relative quantification of 2 BAs (GLCA, glycolithocholic acid; GUDCA, glycoursodeoxycholic acid) were analyzed by liquid chromatography coupled to triple-quadrupole-mass spectrometry (LC-QqQ) at the Center for Omic Sciences (Rovira i Virgili University-Eurecat). Absolute quantification of choline, trimethylamine (TMA), trimethylamine N-oxide (TMAO), betaine, SCFAs (acetic, butyric and propionic acid), and BCFAs (isobutyric and isovaleric acid) in plasma samples were determined by LC-QqQ at the Center for Omic Sciences (see Supplementary information).
Gene expression in the liver and jejunum
Liver and jejunal samples collected after bariatric surgery were conserved in RNAlater (Qiagen, Hilden, Germany) at 4 °C and then processed and stored at −80 °C. Total RNA was extracted from both tissues by using the RNeasy mini kit (Qiagen, Barcelona, Spain). Reverse transcription to cDNA was performed with the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Madrid, Spain). Real-time quantitative PCR was performed with the TaqMan Assay predesigned by Applied Biosystems (Foster City, CA, USA) for the detection of TLR2, TLR4, TLR9, LXRα, SREBP1C, ACC1, FAS, PPARα, CPT1α, CROT, SREBP2, ABCA1, ABCG1, and FXR in the liver; TLR2, TLR4, TLR5, TLR9, GLP-1R, DPP-4, FXR, and PPARɣ in the jejunum. The expression of each gene was calculated relative to the expression of 18S RNA for liver genes and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for genes in the jejunum. All reactions were carried out in triplicate in 96-well plates using the 7900HT Fast Real-Time PCR system.
Statistical analysis
The data were analyzed using the SPSS/PC+ for Windows statistical package (version 23.0; SPSS, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the distribution of variables. Continuous variables were reported as the mean ± SD; noncontinuous variables were reported as the median, and 25–75th percentile and categorical variables were shown as counts (percent). The different comparative analyses were performed using a nonparametric Mann-Whitney U test or Kruskal-Wallis test, according to the presence of two or more groups. The strength of the association between variables was calculated using Pearson’s method (parametric variables) and Spearman’s ρ correlation test (nonparametric variables). The area under the receiver operating characteristic curve (AUROC) was used as an accuracy index for evaluating the diagnostic performance of the selected variables. p-values < 0.05 were statistically significant.
Results
Baseline characteristics of subjects
The main characteristics of the study cohort, including anthropometric and biochemical parameters, are shown in Table 1. First, we classified the subjects, assigning them to two groups on the basis of their BMI: women with normal weight (NW) (BMI < 25 kg/m2; n = 29) and women with MO (BMI > 40 kg/m2; n = 82), which were comparable in terms of age. Biochemical analyses indicated that patients with MO had significantly higher levels of fasting glucose, insulin, glycosylated hemoglobin (HbA1c), HOMA2-IR and triglycerides (p < 0.05) than women with NW. The high-density lipoprotein cholesterol (HDL-C) level was significantly lower in the patients with MO than in the patients with NW (p < 0.001). Then, our cohort of women with MO was classified according to the hepatic histology, first as MO with NL histology (n = 29) and MO with NAFLD (n = 53); second, women with MO were classified into NL, MO with SS (n = 32), and MO with NASH (n = 21) (Table 1). In terms of age and anthropometric measurements (weight, BMI, and waist circumference [WC]), there were no significant differences between NL, SS, and NASH patients in the MO group. Biochemical analyses indicated that insulin and triglyceride levels were also significantly lower in women with NW than in NL and NAFLD women with MO. Although the levels of glucose, insulin, HOMA2-IR, and HbA1c were not significantly different between women with NW and women with MO with NL histology; the circulating levels were significantly lower in women with NW compared with women with MO with NAFLD. When we compared liver histologies in the MO group, we observed that glucose levels were significantly greater in SS subjects than in NL and NASH subjects. In the SS group, there were 10 patients with diabetes, and in the NASH group, there were 3. Diabetic patients were receiving treatment with diet and/or metformin. Moreover, triglycerides were significantly lower in women with MO with NL histology than in women with MO with NASH. For transaminases, Table 1 shows that levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), and alkaline phosphatase (ALP) were significantly higher in women with MO with SS and NASH than in women with NW and women with MO with NL histology.
Table 1.
Anthropometric and biochemical variables of the study cohort classified according to the BMI and histopathological characteristics
| Normal weight (n = 29) | Morbid obesity (n = 82) | NL (n = 29) | SS (n = 32) | NASH (n = 21) | |
|---|---|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Age (years) | 41.99 ± 9.20 | 46.31 ± 10.78 | 43.05 ± 10.35 | 47.49 ± 11.54 | 48.99 ± 9.45 |
| Weight (kg) | 57.01 ± 6.26a | 118.19 ± 16.10 | 119.10 ± 19.88 | 119.81 ± 13.94 | 114.45 ± 13.23 |
| BMI (kg/m2) | 21.56 ± 2.17a | 44.92 ± 5.03 | 44.38 ± 5.34 | 45.63 ± 5.42 | 44.57 ± 3.93 |
| Glucose (mg/dL) | 81.03 ± 6.79a | 109.57 ± 60.97 | 91.86 ± 42.51b | 135.15 ± 82.11c | 95.04 ± 18.79 |
| Insulin (mUI/L) | 6.15 ± 1.83a | 15.93 ± 14.30 | 11.98 ± 8.68 | 19.36 ± 18.03 | 16.61 ± 14.23 |
| HOMA2-IR | 0.78 ± 0.23a | 2.08 ± 1.87 | 1.54 ± 1.10 | 2.61 ± 2.42 | 2.10 ± 1.76 |
| HbA1c (%) | 5.34 ± 0.37a | 6.00 ± 1.17 | 5.63 ± 0.72 | 6.42 ± 1.50 | 5.92 ± 1.00 |
| Cholesterol (mg/dL) | 180.88 ± 33.74 | 175.39 ± 36.65 | 172.60 ± 35.49 | 173.55 ± 35.54 | 181.11 ± 40.64 |
| HDL-C (mg/dL) | 71.30 ± 13.47a | 41.85 ± 11.45 | 41.89 ± 10.84 | 43.96 ± 13.62 | 38.55 ± 7.84 |
| LDL-C (mg/dL) | 96.15 ± 28.20 | 103.79 ± 28.64 | 107.74 ± 27.33 | 100.90 ± 29.24 | 103.06 ± 30.59 |
| Triglycerides (mg/dL) | 64.88 ± 27.92a | 139.92 ± 70.17 | 114.36 ± 31.56 | 141.23 ± 59.13 | 167.73 ± 102.07d |
| AST (U/L) | 18.80 ± 5.15a | 28.50 ± 17.37 | 26.22 ± 14.72 | 26.73 ± 15.72 | 33.95 ± 21.88 |
| ALT (U/L) | 17.50 ± 7.45a | 30.81 ± 17.79 | 27.71 ± 15.34 | 32.19 ± 16.97 | 32.90 ± 21.89 |
| GGT (U/L) | 15.56 ± 8.12a | 29.18 ± 28.71 | 27.32 ± 30.84 | 31.74 ± 32.08 | 27.73 ± 19.07 |
| ALP (U/L) | 54.15 ± 13.24a | 67.45 ± 15.52 | 62.15 ± 14.90b | 75.00 ± 15.34c | 62.58 ± 12.36 |
Insulin resistance was estimated using homeostasis model assessment of IR (HOMA2-IR). Data are expressed as the mean ± SD
NL normal liver, SS simple steatosis, NASH non-alcoholic steatohepatitis, BMI body mass index, HOMA1 homeostatic model assessment method-insulin resistance, HbA1c glycosylated hemoglobin, HDL-C high-density lipoprotein cholesterol, LDL-C low density lipoprotein cholesterol, AST aspartate aminotransferase ALT alanine aminotransferase, GGT gamma-glutamyltransferase, ALP alkaline phosphatase
aSignificant differences between the normal weight group and morbidly obese group (p < 0.05)
bSignificant differences between NL and SS (p < 0.05)
cSignificant differences between SS and NASH (p < 0.05)
dSignificant differences between NL and NASH (p < 0.05)
Evaluation of the liver expression of the main genes related to hepatic lipid metabolism, farnesoid X receptor (FXR) and Toll-like receptors according to liver histology
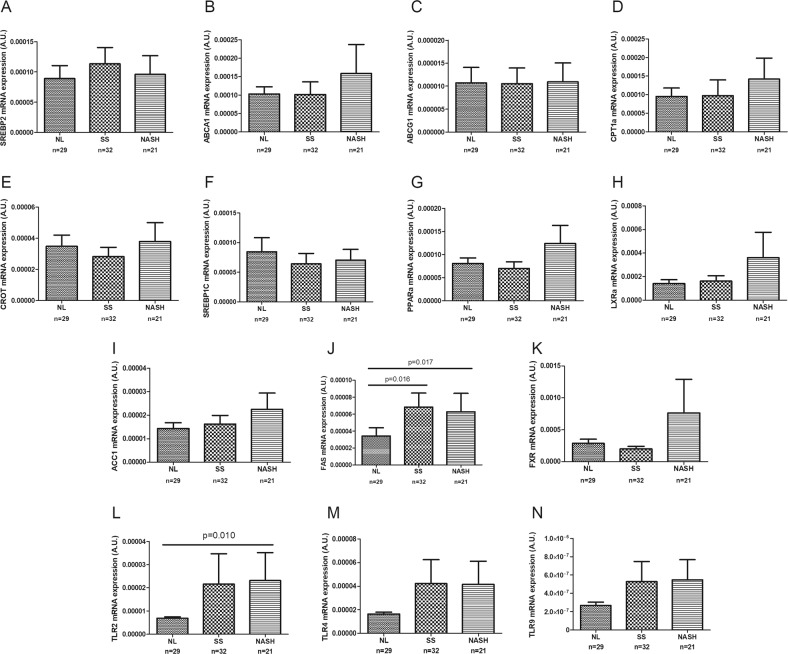
In our cohort of women with MO, we analyzed the expression of hepatic lipid metabolism genes (LXRα, SREBP1C, ACC1, FAS, PPARα, CPT1α, CROT, SREBP2, ABCA1, and ABCG1), FXR and TLRs (TLR2, TLR4, and TLR9). As stated previously, we classified the obese cohort first into NL and NAFLD. The results indicated that FAS, TLR2 and TLR4 were overexpressed in the livers of NAFLD patients. Then, we classified the patients into NL, SS, and NASH groups (Fig. 2). The results indicated that among the hepatic lipid metabolism genes analyzed, only FAS mRNA expression was significantly higher in women with MO with both SS and NASH compared with those with NL histology. The hepatic expression of FXR was upregulated in NASH patients; however, it did not show significant expression differences between patients with NL and SS. Finally, when we analyzed TLRs, TLR2 was overexpressed in the livers of women with NASH compared with those of women with MO with NL.
Fig. 2.
Hepatic expression of genes related to lipid metabolism, FXR and Toll-like receptors in women with morbid obesity (n = 82) classified according to liver histopathology: normal liver (NL), simple steatosis (SS), and non-alcoholic steatohepatitis (NASH). The Mann-Whitney U test or Kruskal-Wallis test was used to determine differences between groups. SREBP2, sterol regulatory element-binding protein 2; ABCA1, ATP-binding cassette A1; ABCG, ATP-binding cassette G; CPT1α, carnitine palmitoyl transferase 1 alpha; CROT, carnitine O-Octanoyltransferase; SREBP1C, sterol regulatory element-binding protein 1c; PPARα, peroxisome proliferator-activated receptor alpha; LXRα, liver X receptor alpha; ACC1, acetyl-CoA carboxylase 1; FAS, fatty acid synthase; FXR, farnesoid X receptor; TLR2, Toll-like receptor 2; TLR4, Toll-like receptor 4; and TLR9, Toll-like receptor 9. p < 0.05 was considered statistically significant
Evaluation of the jejunal expression of FXR, TLRs, glucagon-like-peptide-1 (GLP-1R) and dipeptidyl peptidase-4 (DPP-4) receptors and PPARγ according to liver histology
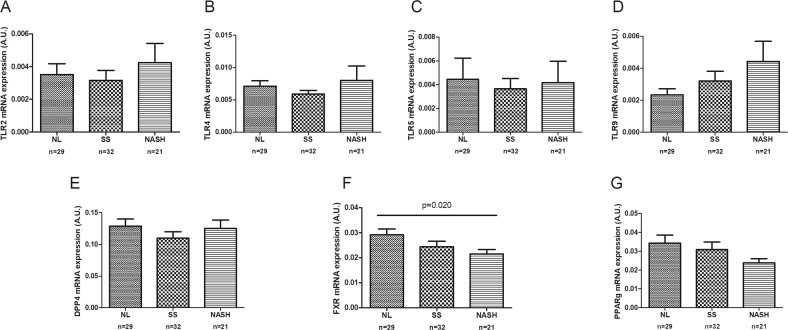
To add to the current knowledge about the role of intestinal FXR, TLRs, GLP-1, and DPP-4 receptors in the pathogenesis of NAFLD, we analyzed their jejunal expression according to liver histology (Fig. 3). First, it is important to note that we could not demonstrate expression of the GLP-1R in the jejunum. Then, we observed that in women with MO with NAFLD, the intestinal TLR9 expression was greater than in women with MO with NL histology (p = 0.05). When we classified the obese cohort into NL, SS, and NASH groups, only the FXR mRNA jejunal expression level was found to be significantly lower in women with MO with NASH when compared with women with MO with NL.
Fig. 3.
Intestinal mRNA expression of Toll-like receptors, DPP-4, FXR and PPARɣ in women with morbid obesity (n = 82) classified according to liver histopathology: normal liver (NL), simple steatosis (SS), and non-alcoholic steatohepatitis (NASH). Mann-Whitney’s U test or Kruskal-Wallis test was used to determine differences between groups. TLR2, Toll-like receptor 2; TLR4, Toll-like receptor 4; TLR5, Toll-like receptor 5; TLR9, Toll-like receptor 9; DDP-4, dipeptidyl peptidase-4; FXR, farnesoid X receptor; PPARγ, peroxisome proliferator-activated receptor gamma. p < 0.05 was considered statistically significant
Circulating levels of gut microbiota-derived metabolites in the population studied
First, we investigated circulating levels of gut microbiota-derived metabolites according to the presence of obesity. The results are summarized in Table 2. The circulating choline levels were significantly greater, and TMA levels were significantly lower in women with MO than in women with NW. Regarding SCFAs, isobutyrate levels were significantly lower and isovalerate levels significantly higher in women with MO than in women with NW. We also quantified the circulating levels of primary BAs, and we observed that circulating CDCA, CA, and GCDCA levels were significantly lower in women with MO compared with women with NW. Finally, circulating levels of secondary BAs were analyzed, and we found decreased levels of DCA, GDCA, TLCA, TDCA, and GLCA in women with MO in comparison with women with NW.
Table 2.
Circulating levels of choline and its byproducts, betaine, ethanol, soluble TLR4, short-chain fatty acids, and primary and secondary bile acids in obese, and non-obese subjects
| Variable | Non-Obese (n = 29) | Obese (n = 82) | p-value |
|---|---|---|---|
| Choline (µM) | 9.82 (8.75–11.62) | 20.26 (13.71–24.19) | <0.001 |
| TMA (nM) | 58.02 (51.28–71.50) | 38.30 (25.59–61.05) | <0.001 |
| TMAO (µM) | 2.35 (1.82–5.46) | 2.45 (1.73–4.03) | 0.614 |
| Betaine (µM) | 33.64 (28.65–39.40) | 25.98 (21.78–34.92) | 0.003 |
| Ethanol (ng/µl) | 1.93 (1.01–12.03) | 3.04 (0.88–5.33) | 0.898 |
| TLR4 (ng/ml) | 2.810 (1.85–4.34) | 2.62 (1.58–3.26) | 0.152 |
| Short-chain fatty acids | |||
| Acetate (µM) | 29.82 (19.26–41.69) | 32.13 (16.30–46.76) | 0.911 |
| Propionate (µM) | 2.68 (2.28–2.98) | 2.70 (1.27–4.03) | 0.950 |
| Isobutyrate (µM) | 0.47 (0.39–0.51) | 0.33 (0.27–0.44) | <0.001 |
| Butyrate (µM) | 0.51 (0.32–0.80) | 0.63 (0.49–0.83) | 0.063 |
| Isovalerate (µM) | 0.25 (0.17–0.47) | 1.37 (0.08–0.20) | <0.001 |
| Primary bile acids | |||
| CDCA (nM) | 122.23 (59.51–340.75) | 32.00 (15.12–117–57) | <0.001 |
| CA (nM) | 84.35 (19.45–376.40) | 29.72 (14.53–83.27) | 0.022 |
| GCDCA (nM) | 376.37 (167.97–905.93) | 141.89 (79.03–289.98) | <0.001 |
| GCA (nM) | 102.09 (55.40–191.11) | 65.15 (37.26–114.16) | 0.054 |
| TCA (nM) | 15.84 (9.74–37.27) | 10.18 (6.47–28.78) | 0.234 |
| TCDCA (nM) | 89.26 (28.26–158.74) | 46.90 (26.86–91.04) | 0.091 |
| Secondary bile acids | |||
| DCA (nM) | 281.44 (100.87–727.28) | 101.82 (51.02–243.22) | 0.003 |
| GDCA (nM) | 131.45 (51.62–237.19) | 44.85 (25.16–91.44) | <0.001 |
| LCA (nM) | 12.75 (9.78–17.63) | 13.76 (8.45–17.58) | 0.705 |
| UDCA (nM) | 31.46 (18.09–62.53) | 29.21 (13.48–66.39) | 0.984 |
| TLCA (nM) | 2.38 (1.35–5.66) | 1.21 (0.81–1.97) | <0.001 |
| TDCA (nM) | 39.35 (14.08–79.20) | 10.03 (5.41–26.19) | <0.001 |
| TUDCA (nM) | 2.09 (1.57–4.03) | 3.09 (1.67–5.53) | 0.161 |
| GLCA (nM) | 82.17 (39.39–122.98) | 20.44 (12.13–36.25) | <0.001 |
| GUDCA (nM) | 334.46 (217.33–502.55) | 281.38 (124.17–659.76) | 0.428 |
Data are expressed as the median (25th–75th percentile). p < 0.05 was considered statistically significant
TMA trimethylamine, TMAO trimethylamine N-oxide, TLR4 toll-like receptor 4, CDCA chenodeoxycholic acid, CA cholic acid, GCDCA glycochenodeoxycholic acid, GCA glycocholic acid, TCA taurocholic acid, TCDCA taurochenodeoxycholic acid, DCA deoxycholic acid, GDCA glycodeoxycholic acid, LCA lithocholic acid, UDCA ursodeoxycholic acid, TLCA taurolithocholic acid, TDCA taurodeoxycholic acid, TUDCA tauroursodeoxycholic acid, GLCA glycolithocholic acid, GUDCA glycoursodeoxycholic acid
In order to assess the relationship between microbiota-derived metabolites and the degrees of NAFLD in MO patients, we analyzed circulating levels of these metabolites according to hepatic histology (Table 3). First, we observed that levels of TMAO and GCA and DCA were significantly higher in NAFLD than in NL patients. The levels of the same metabolites were also higher in SS than in NL. Interestingly, we found that circulating ethanol levels were increased in NASH patients in comparison with those in SS subjects.
Table 3.
Circulating levels of choline and its byproducts, betaine, ethanol, soluble TLR4, and primary and secondary bile acids in the obese group with liver histology
| Variable | NL (n = 29) | SS (n = 32) | NASH (n = 21) | p-value |
|---|---|---|---|---|
| Choline (µM) | 15.85 (12.61–23.86) | 20.85 (11.59–24.24) | 21.30 (16.13–24.38) | 0.418 |
| TMA (nM) | 31.68 (25.70–45.11) | 40.45 (26.07–67.19) | 46.84 (23.38–74.63) | 0.341 |
| TMAO (µM) | 1.95 (1.03–2.90) | 2.93 (1.84–5.95)b | 2.48 (1.79–4.65) | 0.031a |
| Betaine (µM) | 25.63 (21.57–32.73) | 26.05 (22.59–35.78) | 27.57 (20.81–36.39) | 0.866 |
| Ethanol (ng/µl) | 2.00 (0.53–6.13) | 1.80 (0.81–3.73) | 3.44 (2.31–9.80)c | 0.133 |
| TLR4 (ng/ml) | 2.62 (1.83–3.05) | 2.09 (1.18–3.26) | 2.69 (1.67–3.56) | 0.674 |
| Short-chain fatty acids | ||||
| Acetate (µM) | 33.53 (20.90–50.67) | 22.09 (13.00–34.87) | 35.42 (14.26–48.02) | 0.249 |
| Propionate (µM) | 2.65 (1.46–4.62) | 2.69 (1.24–3.75) | 3.08 (1.30–3.91) | 0.702 |
| Isobutyrate (µM) | 0.33 (0.26–0.42) | 0.32 (0.26–0.46) | 0.34 (0.28–0.43) | 0.983 |
| Butyrate (µM) | 0.58 (0.49–0.80) | 0.69 (0.49–0.87) | 0.63 (0.49–0.99) | 0.625 |
| Isovalerate (µM) | 0.14 (0.08–0.20) | 0.13 (0.08–0.21) | 0.15 (0.08–0.20) | 0.883 |
| Primary bile acids | ||||
| CDCA (nM) | 29.91 (14.61–88.59) | 48.20 (29.82–167.82) | 25.76 (1375–177.88) | 0.229 |
| CA (nM) | 21.60 (12.22–83.10) | 40.90 (14.89–114.82) | 30.49 (14.07–80.88) | 0.431 |
| GCDCA (nM) | 126.88 (79.10–283.23) | 154.86 (78.82–317.01) | 111.07 (58.80–221.22) | 0.559 |
| GCA (nM) | 50.33 (30.40–82.39) | 95.83 (42.61–156.34)b | 65.99 (47.62–99.10) | 0.036a |
| TCA (nM) | 10.09 (6.49–18.27) | 16.86 (7.11–32.17) | 9.61 (5.96–26.94) | 0.375 |
| TCDCA (nM) | 52.36 (27.40–86.68) | 47.13 (27.36–102.27) | 37.17 (18.32–90.24) | 0.784 |
| Secondary bile acids | ||||
| DCA (nM) | 66.18 (34.75–109.96) | 150.92 (76.61–302.18)b | 114.98 (45.39–252.32) | 0.014a |
| GDCA (nM) | 40.10 (23.27–54.63) | 58.21 (30.48–130.76) | 39.55 (21.15–89.90) | 0.158 |
| LCA (nM) | 13.81 (8.32–16.52) | 12.78 (7.86–16.17) | 16.43 (10.45–25.26) | 0.247 |
| UDCA (nM) | 23.12 (8.72–83.45) | 32.98 (25.08–63.92) | 23.67 (8.39–86.97) | 0.556 |
| TLCA (nM) | 1.16 (0.72–1.94) | 1.22 (0.80–2.39) | 1.21 (0.87–2.39) | 0.872 |
| TDCA (nM) | 7.49 (4.53–23.46) | 11.30 (6.54–34.10) | 11.17 (4.53–29.85) | 0.589 |
| TUDCA (nM) | 2.68 (1.79–7.63) | 3.67 (1.86–4.71) | 2.85 (1.42–5.72) | 0.775 |
| GLCA (nM) | 15.94 (11.02–35.64) | 22.52 (13.59–32.24) | 24.60 (13.78–47.71) | 0.557 |
| GUDCA (nM) | 242.11 (102.34–996.81) | 389.49 (160.74–651.31) | 201.64 (49.33–404.44) | 0.328 |
Data are expressed as the median (25th–75th percentile). p < 0.05 was considered statistically significant
TMA trimethylamine, TMAO trimethylamine N-oxide, TLR4 toll-like receptor 4, CDCA chenodeoxycholic acid, CA cholic acid, GCDCA glycochenodeoxycholic acid, GCA glycocholic acid, TCA taurocholic acid, TCDCA taurochenodeoxycholic acid, DCA deoxycholic acid, GDCA glycodeoxycholic acid, LCA lithocholic acid, UDCA ursodeoxycholic acid, TLCA taurolithocholic acid, TDCA taurodeoxycholic acid, TUDCA tauroursodeoxycholic acid, GLCA glycolithocholic acid, GUDCA glycoursodeoxycholic acid
aNL vs NAFLD: TMAO (p = 0.013), GCA (p = 0.022), DCA (p = 0.006)
bNL vs SS: TMAO (p = 0.009), GCA (p = 0.016), DCA (p = 0.004)
cSS vs NASH: ethanol (p = 0.045)
Evaluation of circulating microbiota-derived metabolites as biomarkers of non-alcoholic steatohepatitis
As a final step, we evaluated the diagnostic efficacy of a biomarker panel including circulating ethanol, betaine, GCA, and DCA levels as markers of NASH in a group of patients with liver histology indicative of NASH. A cutoff point and area under the curve were determined so that NASH could be diagnosed. To evaluate the extent to which these metabolites can predict histological features, a receiver operating characteristic (ROC) curve was obtained. The accuracy with which this panel discriminates NASH subjects from non-NASH subjects showed an AUROC of ~0.776 (0.632–0.921).
Discussion
The novelty of this work lies in the fact that we aimed to study, in a well-characterized cohort of women with MO with NAFLD, different mechanisms related to NAFLD-intestinal dysbiosis that could be involved in its pathogenesis. Moreover, we wondered whether any of the circulating microbiota-derived metabolites could be used in the construction of a novel scoring system that could be easily applied in the clinical diagnosis of NASH.
The main findings regarding hepatic expression indicate that the liver mRNA of FAS, TLR2, and TLR4 was overexpressed in NAFLD patients. Moreover, TLR2 was also overexpressed in NASH patients. Regarding FAS, our results are consistent with other publications showing dysregulation of lipogenesis [24, 25]. With respect to TLRs, recent data demonstrate that TLR signaling enhances hepatic injury in NASH and other chronic liver diseases [26]. The pathogenesis of NASH has been associated with TLRs, including TLR2, TLR4, TLR5, and TLR9, in animal studies [27–29], which recognize LPS, peptidoglycan, flagellin, and bacterial DNA, respectively. Kupffer cells respond to TLR ligands such as LPS, are activated, and produce inflammatory cytokines that induce lipid accumulation in hepatocytes, cell death and promote liver fibrosis by activating hepatic stellate cells [27]. In human studies, Kanuri et al. showed that hepatic TLR1–5 expression was significantly increased in the livers of NAFLD patients [30]. In another interesting study, Mridha et al. described that hepatic TLR9 and TLR4 mRNA levels were increased in human NASH but not in SS [28], proposing TLR as a possible therapeutic target for NASH. Currently, little data exist regarding TLR2 and NAFLD. However, some studies indicate that TLR2-mediated pathways crucially contribute to the progression of NAFLD/NASH [31]. The intestinal expression of TLRs has been well characterized in vitro and in vivo [32]. In human studies, increased intestinal expression of TLRs has been described in different bowel diseases [33, 34]. However, one of the novelties of our work is the study of intestinal TLR expression in women with MO with NAFLD. We found that intestinal TLR9 was overexpressed in this cohort, suggesting that the innate immune system may play an important role in the pathophysiology of NAFLD. In addition, in our study, jejunal FXR mRNA expression level was significantly lower in women with MO with NASH compared to in women with MO with NL. FXR is strongly expressed in the liver and intestine, where it is a regulator of BAs enterohepatic circulation. However, FXR seems to have a tissue-specific action: intestinal FXR antagonism inhibits SREBP1C with positive effects on lipid metabolism; however, hepatic FXR agonism increases insulin sensitivity and suppresses inflammation [35–37].
Regarding the circulating levels of gut microbiota-derived metabolites in obesity, we found that circulating choline levels were significantly greater and TMA levels were significantly lower in women with MO. In this sense, obese individuals under a hypocaloric diet showed decreases in circulating choline levels and greater improvements in adiposity and energy metabolism [38]. Regarding SCFA, isobutyrate levels were significantly lower, and isovalerate levels were significantly higher in women with MO than in women with NW. SCFAs, can act by sensing nutritional status, thereby maintaining body energy homeostasis. Numerous animal and some human studies suggest a beneficial role of these metabolites in the prevention and treatment of obesity and its comorbidities [39]. Finally, we described decreased levels of primary and secondary BA in our cohort of women with MO, according to Prinz et al. [40].
Then, in order to improve the accuracy of the noninvasive diagnosis of NASH, we analyzed circulating levels of these metabolites according to hepatic histology and observed that levels of TMAO, GCA, and DCA were significantly higher in NAFLD patients than in NL patients. Serum TMAO levels have been described to be significantly higher in patients with NAFLD than in healthy people and correlate with the severity of steatosis [41]. TMAO might contribute to the development of NAFLD by different mechanisms: modulating glucose metabolism, promoting inflammation in adipose tissue [42], and influencing lipid absorption and cholesterol homeostasis [43]. In regard to BAs, we found that levels of GCA, a primary BA, and DCA, a secondary one, were significantly higher in NAFLD patients than in NL patients at the expense of the SS group. Elevated total BA levels have been previously observed in the serum, plasma, urine and liver of patients with NAFLD [20, 44]. In addition, Lake et al. found increased protein expression levels of BA synthesis enzymes in human NASH livers [45]. In a population of patients with NASH, levels of unconjugated cholic acid and chenodeoxycholic acid were increased in relation to microbiota composition [46]. A metabolomic study in humans demonstrated differences in plasma concentrations of BAs between patients with SS and with NASH, suggesting that the fluctuation of these BAs could be used as a biomarker of disease [44]. However, in our study, we could not reproduce these results.
Of particular interest among our findings is that endogenous circulating ethanol levels were increased in NASH patients in comparison with SS patients; therefore, circulating ethanol levels could distinguish between SS and NASH. One of the most important studies in this sense is that of Zhu et al. who concluded that the increased abundance of alcohol-producing bacteria in NASH microbiomes, elevated blood-ethanol concentration in NASH patients, and the well-established role of alcohol metabolism in oxidative stress and liver inflammation suggest a role for alcohol-producing microbiota in the pathogenesis of NASH [47].
One of the most important objectives of the present study was to evaluate the diagnostic efficacy of a biomarker panel of NASH. Based on our results, we included circulating ethanol, betaine, GCA, and DCA levels as markers of NASH in a group of patients with liver histology indicative of NASH. The AUROC obtained was ~0.776. Although this predictive value is not sufficient for an ideal biomarker, it is similar to that of other studies [3, 48].
It is important to note here that although our cohort made it possible to establish clear relationships between women with morbid obesity with NAFLD and altered circulating microbiota-derived metabolites, without the interference of confounding factors such as gender or age, these results cannot be extrapolated to men or overweight subjects.
Conclusions
Taking into account all of our results, the intestine seems to be fundamental in the progression of NAFLD, in coordination with other organs that are already known to be involved, such as adipose tissue and muscle. Moreover, circulating levels of certain microbiota-related metabolites are associated with the severity of the disease and could be incorporated into biomarker panels to be used as a “liquid biopsy” in the noninvasive diagnosis of non-alcoholic steatohepatitis.
Supplementary information
Acknowledgements
This study was supported by the Fondo de Investigación Sanitaria and Fondo Europeo de Desarrollo Regional (FEDER, grant number PI16/00498, to Teresa Auguet), by funds from Agència de Gestió d’Ajuts Universitaris de Recerca (AGAUR 2017 SGR 357 to Cristóbal Richart) and the Grup de Recerca en Medicina Aplicada URV (2016PFR-URV-B2-72 to Cristóbal Richart), and by the Fundación Biociencia.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41366-019-0430-0) contains supplementary material, which is available to authorized users.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349–60. doi: 10.1002/hep.29721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–95. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–48. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10:S17–30. doi: 10.1093/advances/nmy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdou RM, Zhu L, Baker RD, Baker SS. Gut microbiota of nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61:1268–81. doi: 10.1007/s10620-016-4045-1. [DOI] [PubMed] [Google Scholar]
- 8.He X, Ji G, Jia W, Li H. Gut microbiota and nonalcoholic fatty liver disease: insights on mechanism and application of metabolomics. Int J Mol Sci. 2016;17:300. doi: 10.3390/ijms17030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aragonès G, González-García S, Aguilar C, Richart C, Auguet T. Gut microbiota-derived mediators as potential markers in nonalcoholic fatty liver disease. Biomed Res Int. 2019;2019:1–10. doi: 10.1155/2019/8507583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, et al. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur Gastroenterol J. 2018;6:1496–507. doi: 10.1177/2050640618804444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, et al. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110:650–7. doi: 10.1111/j.1365-2672.2010.04922.x. [DOI] [PubMed] [Google Scholar]
- 12.Junker AE. The role of incretin hormones and glucagon in patients with liver disease. Dan Med J. 2017;64. http://www.ncbi.nlm.nih.gov/pubmed/28552096. [PubMed]
- 13.Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556–60. doi: 10.1016/j.dld.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Kapil S, Duseja A, Sharma BK, Singla B, Chakraborti A, Das A, et al. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:213–21. doi: 10.1111/jgh.13058. [DOI] [PubMed] [Google Scholar]
- 15.Etienne-Mesmin L, Vijay-Kumar M, Gewirtz AT, Chassaing B. Hepatocyte Toll-like receptor 5 promotes bacterial clearance and protects mice against high-fat diet-induced liver disease. Cell Mol Gastroenterol Hepatol. 2016;2:584–604. doi: 10.1016/j.jcmgh.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Park S, Kim B, Kwon J. Toll-like receptor 7 affects the pathogenesis of non-alcoholic fatty liver disease. Sci Rep. 2016;6:27849. doi: 10.1038/srep27849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherriff JL, O’Sullivan TA, Properzi C, Oddo J-L, Adams LA. Choline, its potential role in nonalcoholic fatty liver disease, and the case for human and bacterial genes. Adv Nutr. 2016;7:5–13. doi: 10.3945/an.114.007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Thomsen M, Vitetta L. Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J Cell Biochem. 2019;120:2713–20. doi: 10.1002/jcb.27635. [DOI] [PubMed] [Google Scholar]
- 20.Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, et al. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:3318–28. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao J, Huo T-I, Cheng H-Y, Tsai J-C, Liao J-W, Lee M-S, et al. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE. 2014;9:e96969. doi: 10.1371/journal.pone.0096969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis CD, Gnani D, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451–64. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Auguet T, Berlanga A, Guiu-Jurado E, Martinez S, Porras JA, Aragonès G, et al. Altered fatty acid metabolism-related gene expression in liver from morbidly obese women with non-alcoholic fatty liver disease. Int J Mol Sci. 2014;15:22173–87. doi: 10.3390/ijms151222173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arendt BM, Comelli EM, Ma DWL, Lou W, Teterina A, Kim T, et al. Altered hepatic gene expression in nonalcoholic fatty liver disease is associated with lower hepatic n-3 and n-6 polyunsaturated fatty acids. Hepatology. 2015;61:1565–78. doi: 10.1002/hep.27695. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon N, Walsh L, Krüger B, Ward SC, Godbold JH, Radwan M, et al. A single nucleotide polymorphism of Toll-like receptor 4 identifies the risk of developing graft failure after liver transplantation. J Hepatol. 2010;53:67–72. doi: 10.1016/j.jhep.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1β in Mice. Gastroenterology. 2010;139:323–.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mridha AR, Haczeyni F, Yeh MM, Haigh WG, Ioannou GN, Barn V, et al. TLR9 is up-regulated in human and murine NASH: pivotal role in inflammatory recruitment and cell survival. Clin Sci. 2017;131:2145–59. doi: 10.1042/CS20160838. [DOI] [PubMed] [Google Scholar]
- 29.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanuri G, Ladurner R, Skibovskaya J, Spruss A, Königsrainer A, Bischoff SC, et al. Expression of toll-like receptors 1-5 but not TLR 6-10 is elevated in livers of patients with non-alcoholic fatty liver disease. Liver Int. 2015;35:562–8. doi: 10.1111/liv.12442. [DOI] [PubMed] [Google Scholar]
- 31.Chiu CC, Ching YH, Li YP, Liu JY, Huang YT, Huang YW, et al. Nonalcoholic fatty liver disease is exacerbated in high-fat diet-fed gnotobiotic mice by colonization with the gut microbiota from patients with nonalcoholic steatohepatitis. Nutrients. 2017;9. 10.3390/nu9111220. [DOI] [PMC free article] [PubMed]
- 32.Kamdar K, Nguyen V, DePaolo RW. Toll-like receptor signaling and regulation of intestinal immunity. Virulence. 2013;4:207–12. doi: 10.4161/viru.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dlugosz A, Zakikhany K, Acevedo N, D’Amato M, Lindberg G. Increased expression of toll-like receptors 4, 5, and 9 in small bowel mucosa from patients with irritable bowel syndrome. Biomed Res Int. 2017;2017:1–7. doi: 10.1155/2017/9624702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukla R, Ghoshal U, Ranjan P, Ghoshal UC. Expression of toll-like receptors, pro-, and antiinflammatory cytokines in relation to gut microbiota in irritable bowel syndrome: The evidence for its micro-organic basis. J Neurogastroenterol Motil. 2018;24:628–42. doi: 10.5056/jnm18130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguilar-Olivos NE, Carrillo-Córdova D, Oria-Hernández J, Sánchez-Valle V, Ponciano-Rodríguez G, Ramírez-Jaramillo M, et al. The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in non-alcoholic fatty liver disease. Ann Hepatol. 2015;14:487–93. [PubMed]
- 36.Wahlström A, Kovatcheva-Datchary P, Ståhlman M, Bäckhed F, Marschall H-U. Crosstalk between bile acids and gut microbiota and its impact on farnesoid X REceptor Signalling. Dig Dis. 2017;35:246–50. doi: 10.1159/000450982. [DOI] [PubMed] [Google Scholar]
- 37.Albert I, Tanaka N, Li F, Xie C, Krausz KW, Amin SG, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2014;125. 10.1172/jci76738. [DOI] [PMC free article] [PubMed]
- 38.Heianza Y, Sun D, Smith SR, Bray GA, Sacks FM, Qi L. Changes in gut microbiota-related metabolites and long-term successful weight loss in response to weight-loss diets: The POUNDS Lost trial. Diabetes Care. 2018;41:413–9. doi: 10.2337/dc17-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019. 10.1038/s41574-019-0156-z. [DOI] [PubMed]
- 40.Prinz P, Hofmann T, Ahnis A, Elbelt U, Goebel-Stengel M, Klapp BF, et al. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Front Neurosci. 2015;9:199. doi: 10.3389/fnins.2015.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Liu Y, Zhou R, Chen X, Wang C, Tan X, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–81. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–13. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lake AD, Novak P, Shipkova P, Aranibar N, Robertson D, Reily MD, et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol. 2013;268:132–40. doi: 10.1016/j.taap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS ONE. 2016;11:e0151829. doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–9. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 48.Jamali R, Arj A, Razavizade M, Aarabi MH. Prediction of nonalcoholic fatty liver disease via a novel panel of serum adipokines. Medicine (Baltimore) 2016;95:e2630. doi: 10.1097/MD.0000000000002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.