Abstract
Activation of acid sphingomyelinase (SMPD1) and the generation of ceramide is a critical regulator of apoptosis in response to cellular stress including radiation. Endothelial SMPD1 has been shown to regulate tumor responses to radiation therapy. We show here that the SMPD1 gene is regulated by a microRNA (miR), miR-15a, in endothelial cells (ECs). Standard low dose radiation (2 Gy) upregulates miR-15a and decreases SMPD1 levels. In contrast, high dose radiation (10 Gy and above) decreases miR-15a and increases SMPD1. Ectopic expression of miR-15a decreases both mRNA and protein levels of SMPD1. Mimicking the effects of high dose radiation with a miR-15a inhibitor decreases cell proliferation and increases active Caspase-3 & 7. Mechanistically, inhibition of miR-15a increases inflammatory cytokines, activates caspase-1 inflammasome and increases Gasdermin D, an effector of pyroptosis. Importantly, both systemic and vascular-targeted delivery of miR-15a inhibitor decreases angiogenesis and tumor growth in a CT26 murine colorectal carcinoma model. Taken together, our findings highlight a novel role for miR mediated regulation of SMPD1 during radiation responses and establish proof-of-concept that this pathway can be targeted with a miR inhibitor.
Subject terms: Tumour angiogenesis, Apoptosis
Introduction
Technological advances such as stereotactic body radiation therapy (SBRT) and stereotactic radiosurgery (SRS)1–5 have allowed significant improvements in therapeutic radiation dose escalation. These treatment modalities are able to ablate malignant tissue for excellent local control, however not all disease sites can be treated with these modalities due to toxicity concerns6–10. Dose escalation does not only augments DNA damage but also involves a vast number of tumor microenvironment (TME) regulators11. Within the TME, high dose radiation modulates the adjacent vasculature, stroma, and immune cells to contribute to the ionizing radiation (IR) response [4]. Radiation elicits endothelial cell dysfunction characterized by associated increased permeability, detachment from the underlying basement membrane, and apoptosis12,13. At ablative doses, greater than 8 Gy, there is rapid induction of sphingomyelinase-mediated production of ceramide, which triggers rapid onset of endothelial apoptosis14. Indeed, it is thought that endothelial apoptosis dictates the radiosensitivity of tumors. IR-mediated cell death combined with a pro-inflammatory state contributes to an immunostimulatory profile leading to further immunogenic cell death (ICD)15,16. In this context, we hypothesized that transcriptional programs in the endothelia triggered by different radiation doses dictated the overall tumor response to radiation.
MiRs play an important role in radiation responses of both malignant cells and the TME17,18. miRs are endogenous, short non-coding, single-stranded RNA spanning approximately 22 nucleotides. We and others have shown that radiation regulated miRs alter DNA damage repair pathways, pro-survival signaling pathways, cell-cycle checkpoint regulation, and apoptosis; functions which radiation therapy exploits for therapeutic gain19–23. Our previous work identified a group of miRs regulated in the tumor vasculature in response to radiation24. In particular, we have observed that some miRs in ECs are differentially regulated in response to different doses of radiation. We focused further attention on miRs predicted to target SMPD1. We found that miR-15a expressed the greatest magnitude difference between standard and ablative dose radiation with substantially lower miR-15a levels at higher doses. Our studies show that miR-15a targets SMPD1 in ECs and inhibition of miR-15a decreases EC and tumor cell proliferation, enhances cell death and diminishes tumor growth in a mouse CT26 colorectal carcinoma flank tumor model. Vascular-targeted nanoparticle delivery of miR-15a inhibitor is sufficient to both decrease tumor growth and angiogenesis. Consistent with the immunostimulatory role of miR-15a deficiency in autoimmune and infectious settings25,26, we found miR-15a inhibition increased cell death pathway proteins and caspase-1 activation. In summary, our findings establish a new miR based regulatory pathway that affects SMPD1 and therefore vascular cell death in response to radiation dose. Inhibition of this pathway may mimic features of high dose radiation and therefore offers avenues for the development of targeted therapeutics.
Materials and Methods
Methods have been adapted from our previous publications24,27–29.
miRNA profiling
RNA was extracted from HUVECs at 6 h post radiation with either 2 Gy or 20 Gy and miRs were profiled using TaqMan TLDA panels for human microRNAs. miRs proposed to target miR-15a as predicted by TargetScan were further analyzed. The average fold change after normalization to a housekeeping RNA, typically RNU48, is depicted.
Cell culture and reagents
HUVECs (Lonza) were cultured in EBM-2 media (Lonza) supplemented with 10% fetal calf serum (Hyclone). CT-26 cells (ATCC) were culture in RPMI media supplement with 10% fetal calf serum and antibiotics. HCT-116 cells (ATCC) were cultured in McCoy’s supplemented with 10% Fetal Calf Serum and antibiotics. Cells were tested and found negative for mycoplasma contamination before use in the assays described.
Transfections
Cells were reverse transfected with miR-15a-5p mimics, inhibitors and their respective controls using Lipofectamine RNAiMAX (Invitrogen) according to manufacturer’s instructions. miR mimics and inhibitors were purchased from Life Technologies or Exiqon.
Irradiation
Cells or mice were irradiated on a Shepherd Cesium-137 irradiator at a rate of approximately 1.34 cGy per minute. In tumor-targeted radiation experiments, mice were restrained in a lead shield (Brain Tree Scientific) to minimize exposure to the non-tumor areas.
Cell titer glo/caspase glo
Cells were transfected in a 6 well plate with miR-15a-5p mimic or inhibitor, and the corresponding controls from Exiqon (Qiagen) as previously described. Cells were transferred to a 96 well plate 16 hours post-transfection (1000 cells/well). In some studies, at 24 h post-transfection the cells were irradiated with 0, 2, or 5 Gy. Cell Titer-Glo and Caspase 3/7 Glo were analyzed at 48 h and 96 h, according to manufacturer’s instructions.
Western blot and densitometric analysis
After treatment, cells were washed in phosphate-buffered saline (PBS) and lysed in RIPA buffer (Sigma) supplemented with Complete Protease inhibitor cocktail (ROCHE) and Phosphatase inhibitors cocktail 2 and 3 (Sigma). Lysed cells were harvested by scraping, and proteins were analyzed by Western blot. Equivalent amounts of protein were loaded on a 4–12% gradient SDS-polyacrylamide gel (BioRAD) and transferred for 30 min in a TransBlot turbo (BioRAD) onto Nitrocellulose membranes. Membranes were blocked in 5% milk or 3% BSA and incubated with antibodies as indicated: Anti-SMPD1 (OTI3H7, NBP2-45889, Novus Biologicals, or Clone # 563418, R&D Systems 1:1000, overnight, 4 °C), Gasdermin D (96458, Cell Signaling, 1:1000, overnight, 4 °C), GAPDH (D16H11, Cell Signaling, 1:5,000, 1 h RT), β-actin (Sigma, A5316, 1: 5,000 1 h RT) was used as a housekeeping control for the total levels of protein loaded. Membranes were washed in TBST and incubated with secondary antibodies from Licor Biosciences. Licor antibodies used were goat anti mouse 925–68020 (1:15,000) and goat anti rabbit 925–32211 (1:15,000). Blots were scanned on the Licor Odyssey scanner or Azure scanner according to manufacturer’s instructions. Bands were quantified manually using ImageJ to calculate the Integrated Density values of the band of interest and normalizing it to the loading control in the same lane.
Immunohistochemistry
CT26 tumors were harvested in PBS, fixed with 4% PFA for 1 hr at room temperature, and soaked overnight in 30% Sucrose. On the following day, they were PBS washed, embedded in OCT, frozen, and stored at −80 °C until cryosectioning. 10 µm sections were blocked with 10% of normal goat serum in 1x PBS containing 0.5% saponin and 2% BSA for 2 hr at room temperature. Sections were then washed with PBS and incubated overnight with anti-CD31 from BD Biosciences (550274; lot 51627341, at 1:100 dilution) followed by secondary antibody Goat anti-Rat Alexa 546 from Invitrogen (A11081; lot 2045302; at 1:400) for 2 hr at room temperature. Sections were mounted in ProLong™ Gold Antifade Mountant with DAPI (P36931) and stored at 4 °C until imaging with Yokogawa CSU-W1 spinning disk confocal microscope with 20 × 0.45 Plan Fluor objective (Nikon). The images were analyzed with Fiji software. Specifically, 3–5 images from at least 3 mice per each treatment group were combined into a single virtual stack and inverted to generate black image on a white background. Thresholds were set to visualize most of the vessels in each image of the stack. The analyze particle function with a size limit of 1–25 micron2 was used to generate both area and density measurements. The area fractions from the CD31 channels were normalized to the area fractions from the DAPI channels.
Multiplex cytokine ELISA
Cytokines were profiled from supernatants from HUVECs at 24 or 72 h post transfection using a 60 cytokine, Human Cytokine/Chemokine Magnetic Bead Panel (Millipore/Sigma HCYTOMAG-60K) kit in a 96 well format per manufacturer’s recommendations.
3-D angiogenic sprouting assay
Early passage HUVECs were coated on cytodex-3 beads (GE Healthcare) at a density of 10 million cells/40 μl beads and incubated in suspension for 3–4 hours with gentle mixing every hour. They were plated on TC treated 6 well dishes overnight and resuspended in a 2 mg/ml fibrin gel with 200,000 human smooth muscle cells. The gel was allowed to polymerize and complete EGM-2 media was added. Sprouts were visualized from days 3–4 via confocal imaging after overnight incubation with FITC labeled Ulex europaeus lectin (Vector labs). Immunofluorescence imaging was performed on a Yokogawa CSU-W1 spinning disk confocal microscope with 20 0.45 Plan Fluor objective (Nikon).
In vivo assays
All animal work was approved by the OHSU Institutional Animal Use and Care Committee. All experiments were performed in accordance with the relevant guidelines and regulations. 8–10 week old Balb/C mice purchased from Jackson Labs were injected subcutaneously with 5 × 105 tumor cells in Matrigel (BD) per each flank. Tumor growth was measured with calipers, with volume computed as ½ * Length * Width2. Mice were randomized into groups once the average tumor volume reached 100 mm3, approximately 7–10 days after injection. miR inhibitors were delivered i.v. in either PBS or vascular targeted 7C1 nanoparticles every two days from randomization for a total of three doses.
Statistics
All statistical analysis was performed using Excel (Microsoft) or Prism (GraphPad). Two-tailed Student’s T-test or ANOVA with post-hoc corrections was used to calculate statistical significance. P values <0.05 were considered significant.
Results
SMPD1 expression correlates with better overall survival in breast, ovaraian and lung cancers
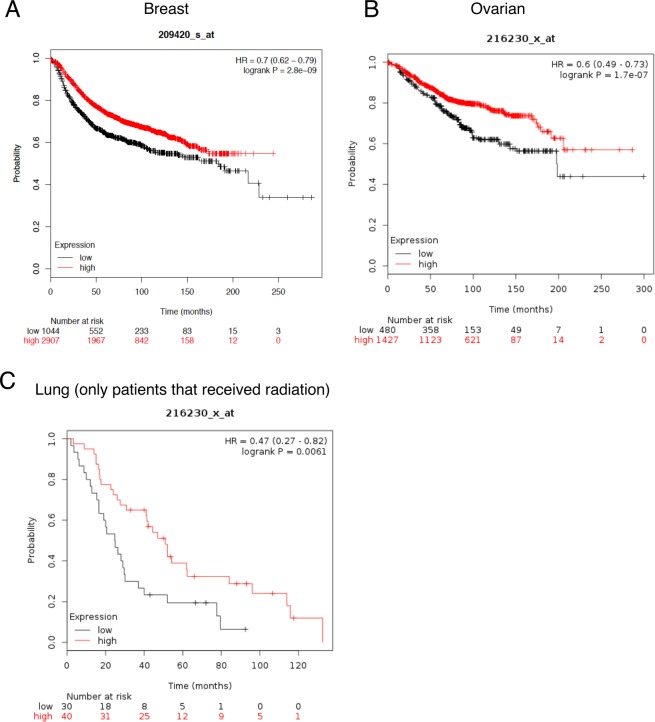
We first evaluated the expression of SMPD1 in human cancers and asked if the levels of SMPD1 correlated with overall survival (Fig. 1) using the online database KMplotter. We observed that in breast and ovarian cancers, SMPD1 high patients had significantly better overall survival. In lung cancer patients, data was available for patients that only received radiation therapy. In this subset, SMPD1 high patients had almost two-fold better overall survival than patients with low SMPD1 (Fig. 1C). Analysis of TCGA revealed that SMPD1 is seldom mutated or amplified suggesting transcriptional and/or post transcriptional mechanisms control the expression of SMPD1.
Figure 1.
SMPD1 expression correlates with better overall survival in human cancers. Kaplan-Meier plots (kmplotter) showing overall survival in (A) Breast, (B) Ovarian and (C) Lung cancer patients expressing high vs low SMPD1 levels. The expression levels were classified as high or low based on median expression of the gene. The lung cancer dataset was restricted to patients that received radiotherapy.
miRs regulating SMPD1 exhibit differential dose expression
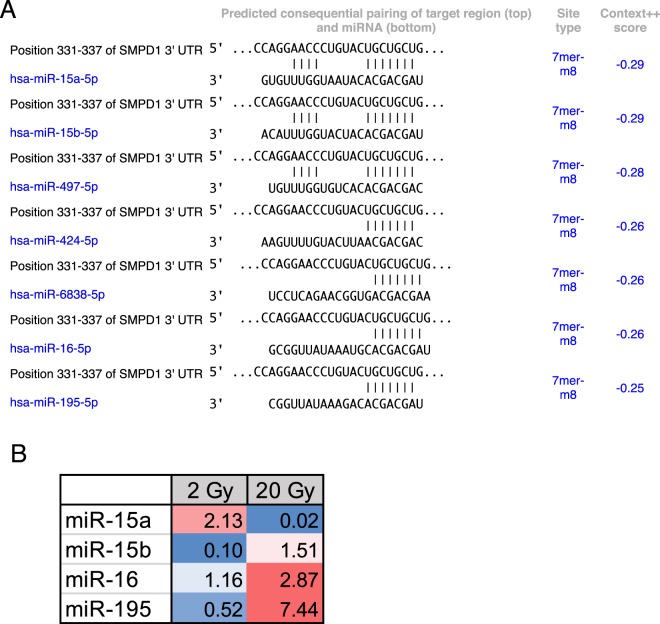
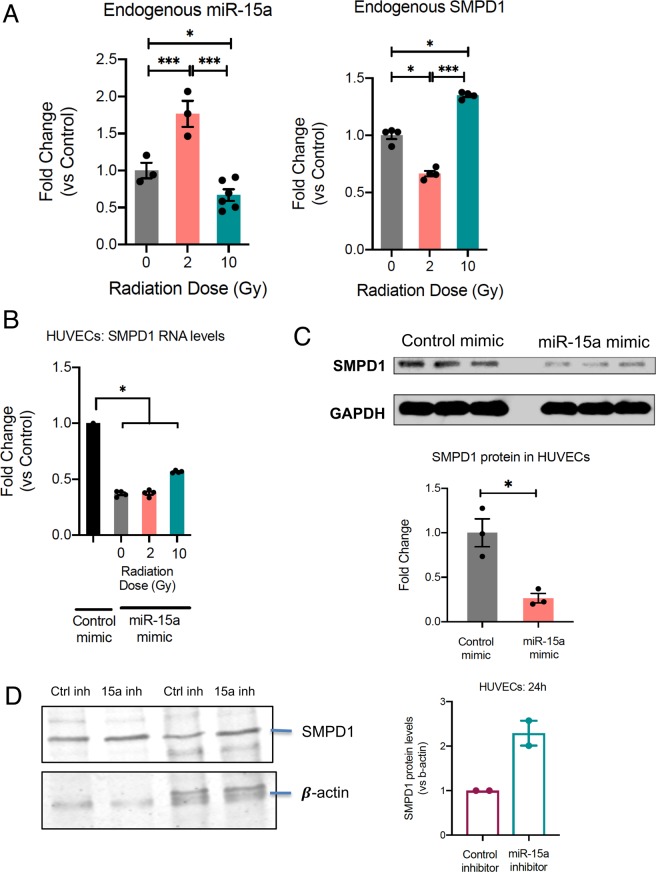
Given that miRs are a major mechanism for post-transcriptional control of gene expression, we sought to identify miRs that specifically targeted SMPD1. TargetScan analysis of the SMPD1 3′ untranslated region identified miR-15 family as putative regulators of SMPD1 (Fig. 2A). We chose to evaluate this using ECs as a model system since they express ~20 fold more SMPD1 than tumor cells. We asked if there was any miR-15a family member that was differentially regulated by radiation. HUVECs were treated with either a single 2 Gy or 20 Gy dose via Cs-137 and miRs were profiled at 6 h post treatment. miR-15a exhibited the greatest differential change at 6 hours post-IR between exposure of 2 Gy and 20 Gy radiation relative to non-irradiated samples (Fig. 2B). We first confirmed that endogenous miR-15a decreased at high dose radiation and the expression of SMPD1 was reciprocal to the amount of miR-15a (Fig. 3A) via qRT-PCR. Subsequently, we confirmed that exogenous transfection of miR-15a increased miR-15a levels in HUVECs (Supplementary Fig. 1), significantly reduced expression of SMPD1 mRNA (Fig. 3B) and protein levels (Fig. 3C, uncropped blot in Supplementary Fig. 2). Our data is consistent with other studies that have also shown that miR-15a targets SMPD1 directly30. These observations establish that miR-15a is differentially expressed at low vs high dose radiation and affects SMPD1 levels in ECs.
Figure 2.
Discovery of SMPD1 targeting miRs that are differentially regulated by radiation. (A) TargetScan prediction of miR candidates that harbor binding sites on the 3′ untranslated region of human SMPD1. (B) miR candidates targeting SMPD1 exhibit radiation dose-dependent differential expression at 6 h post-IR in HUVECs. Fold changes vs control untreated cells are indicated in colored cells relative to expression of the respective miRNA in non-irradiated samples. Red = increased expression. blue = decreased expression.
Figure 3.
miR 15a decreases SMPD1 expression in endothelial cells. (A) Reciprocal regulation of endogenous miR-15a (left) and SMPD1 (right) by high vs low dose radiation. HUVECs were irradiated as indicated and RNA was extracted at 18 h post RT. Bars show mean ± SEM of replicates. (B) HUVECs were transfected with either a control mimic or a miR-15a mimic. 24 h later HUVECs were irradiated with the indicated doses. 24 h after radiation, RNA was isolated and qRT-PCR was performed to measure the levels of SMPD1. (C) Cells were lysed at 48 h post transfection and SMPD1 protein levels were measured by western blotting. Lanes show biological replicates and bar graph shows mean band intensity ± SEM of replicates. (D) SMPD1 protein levels in HUVECs 24 h after transfection. Bars show normalized band intensity ± SEM. *P < 0.05, ***P < 0.001 per ANOVA with post hoc Tukey’s test for comparisons between more than 2 groups or two-tailed Student’s T-test for two groups.
miR-15a inhibition decreases HUVEC viability and increases caspase activity
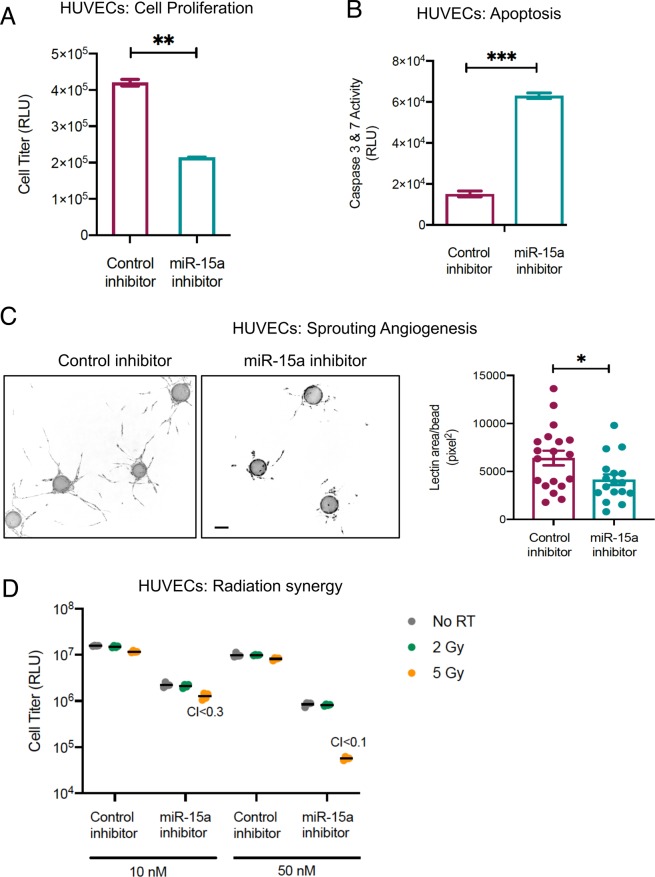
Since our data indicates that high dose radiation decreased miR-15a and increased SMPD1, we asked if inhibition of miR-15a affected cell viability. First, we established that miR-15a inhibition with a complementary, chemically stabilized oligonucleotide decreased miR-15a levels (Supplementary Fig. 1) and increased SMPD1 levels (Fig. 3D, uncropped blot Supplementary Fig. 3). HUVECs transfected this with miR-15a inhibitor demonstrated decreased cell proliferation and increased caspase activation (Fig. 4A,B). While noting SMPD1 is characterized by a 20 fold increased expression in ECs relative to other cell types31, we analyzed the effects of miR-15a inhibitor on malignant cell lines. Similar to HUVECs, miR-15a inhibitor dramatically decreased cell viability in HCT-116 cells and CT26 colorectal carcinoma cells (Supplementary Fig. 4).
Figure 4.
Inhibition of miR-15a decreases endothelial cell proliferation and enhances cell death. (A) HUVECs were transfected with either a control negative inhibitor or a miR-15a inhibitor. 48 h later proliferation (A) or cell death (B) was measured using a luciferase-based Cell Titer glo assay (A) or Caspase 3 & 7 CasGlo assay (B). (C) Fibrin bead 3D angiogenesis assay. HUVECs were transfected as described in A and were coated onto cytodex beads and allowed to sprout in a fibrin gel in the presence of smooth muscle cells over 5 days. The images show representative beads with angiogenic sprouts stained with Ulex europaeus lectin for each condition. Bars depict mean +SEM of lectin area analyzed across at least 25 beads per group. Scale bar = 100 μm. D) HUVECs were transfected as described in A with the indicated concentrations of either control inhibitor or miR-15a inhibitor. 24 h later, cells were irradiated at the indicated doses. 48 h post irradiation, proliferation was measured using a Cell Titer glo assay. Synergy was calculated using the Chou-Talalay method with combination index < 1 considered synergistic. Bars indicate means ± SEM of 3 technical replicate wells. One of two independent experiments is shown.
We then asked if miR-15a inhibition affected angiogenesis in a physiologically relevant 3D sprouting angiogenesis assay. Consistent with the decreased proliferation and increased cell death, we observed fewer and less robust angiogenic sprouts in miR-15a inhibitor transfected HUVECs (Fig. 4C). Finally, we tested whether miR-15a inhibition synergized with radiation. We observed that in HUVECs, a combination of miR-15a inhibitor and 5 Gy dose of radiation was highly synergistic with a Chou-Talalay combination index less than 0.5 at both 10 and 50 nM doses. These observations indicate that miR-15a inhibition can decrease angiogenesis as well as act in concert with radiation.
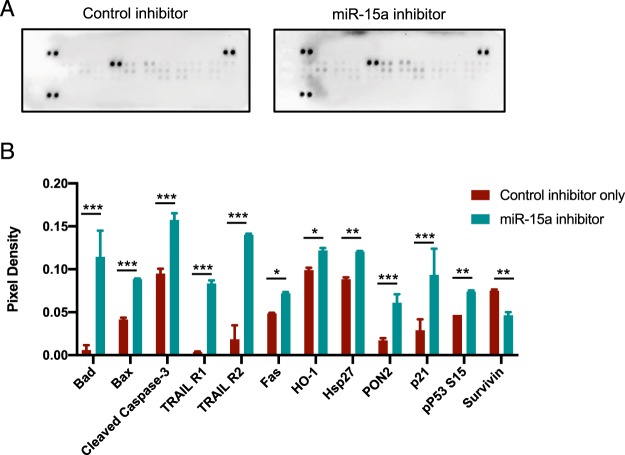
We observed that consistent with other reports, miR-15a inhibition affected pro-apoptotic pathways including induction of Bad, TRAIL receptors, p21 and decrease in pro-survival protein Survivin (Fig. 5). We also observed that miR-15a inhibition enhanced inflammasome activation. We found that miR-15a mimic decreased the expression of Gasdermin D a key regulator of pyroptosis32 while miR-15a inhibition increased Gasdermin D (Supplementary Fig. 5A). In addition, miR-15a inhibitor also increased the activity levels of executioner caspase Caspase-1(Supplementary Fig. 5B). Pyroptosis is a lytic, regulated cell death that requires the enzymatic activity of inflammatory caspases. Since pyroptosis releases intracellular danger associated molecular patterns (DAMPs) and cytokines such as IL-1, it is thought to be a more immunogenic form of cell death33. Recent evidence indicates there is significant cross-talk apoptosis and pyroptosis to regulate the inflammatory response34. Indeed, we found miR-15a inhibition increased a number of cytokines and chemokines including IL-1, PDGF while significantly downregulating IL-4, 5 and Fractalkine levels (Supplementary Fig. 6).
Figure 5.
miR-15a inhibition in endothelial cells drives cell death pathways. (A) Western blot on a membrane array for human apoptosis pathways (Proteome profiler, R&D Systems). HUVECs were transfected with either control inhibitor or miR-15a inhibitor as described in Fig. 4. 48 h later, cells were lysed and the lysates were incubated with a membrane containing duplicate spots with bound antibodies for different apoptotic proteins and developed with SA-HRP per manufacturer’s instructions. (B) Densitometry of duplicate spots from the membrane. Bars show mean + SD. Bars are shown only for proteins that were significantly different between the groups via two-tailed Student’s T-test followed by a Holms-Sidak post-hoc correction for multiple comparisons (Adjusted P-values <0.05). *** denotes adjusted P < 0.0001, ** denotes adjusted P < 0.005, * denotes adjusted P < 0.05.
Inhibition of miR-15a in the vasculature decreases tumor growth and angiogenesis
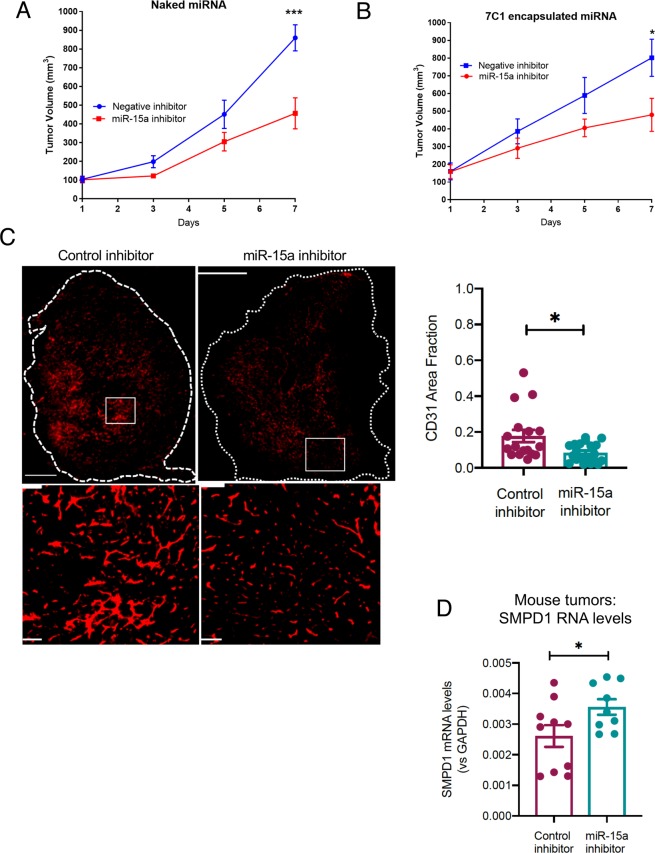
We next assessed whether miR-15a inhibitor had any effects on tumor growth in vivo and if these effects were dependent on its regulation of angiogenesis. In a murine CT26 colorectal carcinoma flank tumor model, systemic treatment with i.v. injected miR-15a inhibitor resulted in an approximately 50% decrease in tumor growth after 7 days (Fig. 6A).
Figure 6.
Systemic or vascular inhibition of miR-15a decreases tumor burden. (A) CT26 tumors were implanted subcutaneously in Balb/C mice (N = 5 mice per group, two tumors per mouse). Once tumors reached 100 mm3 volume, mice were randomly assigned to either a negative control inhibitor group or a miR-15a inhibitor (20 mg/kg, i.v. in PBS). Mice were treated every two days for a total of three treatments. ***P < 0.01; ANOVA. (B) In the same model, mice were randomly assigned to receive either a negative control inhibitor or a miR-15a inhibitor in vascular-targeted 7C1 nanoparticles (1 mg/kg, i.v.). *P < 0.05; ANOVA. (C) CD31 staining showing blood vessels in the tumors from B). Scale bar = 1000 μm. Dotted line shows whole tumor outline. Bottom panels show a magnified view of the boxed region. Scale bar = 100 μm. Bars show mean + SEM of 3–4 tumor sections from each mouse. Dots represent individual tumor sections. *P < 0.05, Mann-Whitney U-test. (D) SMPD1 mRNA levels at endpoint measured via qRT-PCR from tumors in B. *P < 0.05, Student’s T-test.
Since our in vitro experiments demonstrated miR-15a inhibition also affected CT26 proliferation, it is possible that this tumor delay was a result of direct tumor cell inhibition. To address this, we took advantage of a vascular-targeted nanoparticle that we have established as an efficient platform for delivering miRs to tumor vasculature and not tumor cells. We found that delivery of vascular-targeted miR-15a inhibitor in the same model was sufficient to decrease tumor burden (Fig. 6B). Importantly, the tumors treated with miR-15a inhibitor had a significant decrease in angiogenesis as measured by CD31 area (Fig. 6C). We noted that the tumors treated with miR-15a inhibition in this experiment also had higher levels of SMPD1 mRNA levels (Fig. 6D). Taken together, our observations indicate that miR-15a, a regulator of SMPD1, is inhibited by high dose radiation in ECs. A synthetic miR-15a inhibitor not only decreased EC proliferation in vitro but also decreased angiogenesis and tumor growth in vivo.
Discussion
The importance of the TME in radiation has been elucidated with the advent of new technologies and techniques allowing safer radiation dose escalation that engages the TME components11. Kolesnick et al. were among the first to demonstrate the importance of dose magnitude in eliciting rapid endothelial apoptosis via SMPD1 translocation to the plasma membrane. This translocation of SMPD1 produced ceramide thereby facilitating enhanced FAS-FASL and TNFRSF10-TNFLSF10 apoptotic signaling31. While earlier pre-clinical models focused attention on single high dose radiation, this is not directly clinically applicable to most disease sites given dose limitations to adjacent critical organs. With this constraint, total radiation dose is divided over several days to allow sublethal damage repair of normal tissue. Using a syngeneic CT26 colorectal cancer model, Zhu et al. compared fractionation between 6 Gy × 5 fractions and 12 Gy × 3 fractions. In the 6 Gy cohort, only a cumulative dose of 12 Gy or higher led to incremental increased SMPD1 activity, increased endothelial cell apoptosis, and decreased microvessel density. In contrast, multiple administrations of 12 Gy did not significantly change SMPD1 function or EC apoptosis rates35.
As radiation dose dictates SMPD1 activity, as well as the expression of distinct miRs, we asked whether miRs with predicted binding to the SMPD1 3′-UTR also exhibited dose dependent differential expression. Interestingly, among our miRNA microarray, there were three miRs targeting SMPD1, which increased with higher doses of radiation. However, just a single miR, miR-15a was increased nearly 2-fold at 2 Gy and decreased significantly with the ablative dose radiation of 20 Gy. Recent insight into vascular miR-15a, elucidates oxidative stress as an inhibitor of miR-15a expression and the subsequent rise in SMPD1 activity. In retinal ECs, Wang et al. confirmed that miR-15a binds directly to the 3′-UTR of SMPD1, and also that miR-15a inhibition significantly increases ceramide production. Indeed, miR-15a inhibition has been shown to increase expression of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α30 and increased leukostasis, elevated CD45, and NF-κB levels36 in different pathophysiological contexts.
In the oncogenic context, miR-15a inhibition has been shown to enhance the innate immune response in favor of anti-tumor immunity. Yang et al.37 found that miR-15a deficiency inhibited tumor growth and prolonged survival in an orthotopic glioma model. In these experiments, they demonstrated miR-15a deficiency led to an influx of CD8+ T cells, decreased expression of inhibitory receptors including PD-1, Tim-3, and LAG-3, and increased inflammatory cytokine production.
Given the heterogeneity of cancer and versatile nature of miRs, miR-15a’s role as either an oncogenic miR or a tumor suppressive miR does not lie firmly within one category. Several cancers including non-small cell lung cancer and breast cancer, express lower miR-15a levels. This decrease in the miR has been linked to increased tumor growth and radioresistance that is reversible through miR-15a overexpression38,39. In colorectal cancer, a recent analysis of 182 patients found that miR-15a overexpression is associated with a worse 5-year progression free survival and overall survival (68% vs 88%, p = 0.001; 60% vs 74%, p = 0.035, respectively)40. However, the dichotomic behavior is not unique to miR-15a, being a largely an oversimplified classification for this molecule able to regulate multiple targets in a context dependent fashion41.
We chose to use colorectal cancer as our model for in vivo studies given the above findings. However, the primary focus remains the influence of vascular miR-15a on the TME to effect anti-cancer activity. SMPD1 is also a known target of miR-15a, consistent with our findings, and they both exhibit dose dependent reciprocal expression. MiR-15a inhibition decreased cellular viability, increased endothelial caspase activity and enhanced both inflammasome activation and Gasdermin expression. These mechanisms suggest that miR-15a inhibition maybe potent due to its ability to drive pyroptosis in the vasculature, which would be beneficial in a therapeutic context. On the basis of these observations, we propose that inhibition of miR-15a offers a unique approach to suppress tumor growth.
Supplementary information
Acknowledgements
We thank Dr. Liana Tsikitis (OHSU) for useful discussions. We thank Drs Daniel G Anderson and Omar F. Khan (MIT) for 7C1 nanoparticles. We thank LaTroy Robinson for technical help. We acknowledge the OHSU Advanced Light Microscopy Core, Knight Cancer Institute Flow Cytometry Core and the Gene Profiling Shared Resource for technical help and useful discussions. This work was supported by US NIH grant R01HL137779 and R01HL143803 to S.A and a seed grant from ASTRO (Grant ID 534775) and RSNA to S.R.
Author contributions
S.R. and S.A. designed the study. S.R., C.E.D., N.C., C.H., E.F.B., A.A., S.K., R.R. performed the experiments, analyzed the data. S.R., S.A., C.R.T. analyzed and interpreted the data, drafted the manuscript. All authors reviewed the manuscript.
Competing interests
Dr. Anand receives grant funding from NIH, American Heart Association and institutional funds. Dr. Rana receives grant funding from American Society for Radiation Oncology (ASTRO) and Radiological Society of North America. Drs Anand, Thomas and Rana are named inventors on a provisional US patent application. Drs. Espinosa-Diez, Chatterjee, Fraile-Bethencourt, Agarwal, Khou and Ms Ruhl and Mr Hudson declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62621-8.
References
- 1.Lo, S. S. et al. The development of stereotactic body radiotherapy in the past decade: a global perspective. Future Oncol, 10.2217/fon.15.220 (2015). [DOI] [PubMed]
- 2.Rehman S, Roach MC, Bradley JD, Robinson CG. Lung Stereotactic Body Radiation Therapy. Mo Med. 2015;112:361–365. [PMC free article] [PubMed] [Google Scholar]
- 3.Song CW, Kim MS, Cho LC, Dusenbery K, Sperduto PW. Radiobiological basis of SBRT and SRS. Int J Clin Oncol. 2014;19:570–578. doi: 10.1007/s10147-014-0717-z. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847–2854. doi: 10.1200/JCO.2014.55.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu JB, Sandler HM. Stereotactic body radiation therapy: Let’s not give up on progress. Pract Radiat Oncol. 2015;5:193–196. doi: 10.1016/j.prro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Osmundson EC, et al. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys. 2015;91:986–994. doi: 10.1016/j.ijrobp.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Trakul N, Koong AC, Chang DT. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin Radiat Oncol. 2014;24:140–147. doi: 10.1016/j.semradonc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Dobbelstein M, Sorensen CS. Exploiting replicative stress to treat cancer. Nat Rev Drug Discov. 2015;14:405–423. doi: 10.1038/nrd4553. [DOI] [PubMed] [Google Scholar]
- 9.Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nature reviews. Clinical oncology. 2015;12:527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 11.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckmann M, Douwes K, Peter R, Degitz K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp Cell Res. 1998;238:148–154. doi: 10.1006/excr.1997.3826. [DOI] [PubMed] [Google Scholar]
- 13.Langley RE, Bump EA, Quartuccio SG, Medeiros D, Braunhut SJ. Radiation-induced apoptosis in microvascular endothelial cells. Br J Cancer. 1997;75:666–672. doi: 10.1038/bjc.1997.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 16.Krysko DV, et al. Immunogenic cell death and DAMPs in cancer therapy. Nature reviews. Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 17.Mao A, Liu Y, Zhang H, Di C, Sun C. microRNA expression and biogenesis in cellular response to ionizing radiation. DNA and cell biology. 2014;33:667–679. doi: 10.1089/dna.2014.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer A, et al. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat Res. 2011;176:575–586. doi: 10.1667/RR2638.1. [DOI] [PubMed] [Google Scholar]
- 19.Czochor JR, Glazer PM. microRNAs in cancer cell response to ionizing radiation. Antioxid Redox Signal. 2014;21:293–312. doi: 10.1089/ars.2013.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandellini P, Rancati T, Valdagni R, Zaffaroni N. miRNAs in tumor radiation response: bystanders or participants? Trends Mol Med. 2014;20:529–539. doi: 10.1016/j.molmed.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Metheetrairut C, Slack FJ. MicroRNAs in the ionizing radiation response and in radiotherapy. Curr Opin Genet Dev. 2013;23:12–19. doi: 10.1016/j.gde.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright CM, Dan T, Dicker AP, Simone NL. microRNAs: The Short Link between Cancer and RT-Induced DNA Damage Response. Front Oncol. 2014;4:133. doi: 10.3389/fonc.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley KA, et al. Understanding and Resetting Radiation Sensitivity in Rectal Cancer. Annals of Surgery. 2017;266:610–616. doi: 10.1097/sla.0000000000002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson R, et al. MicroRNA regulation of endothelial TREX1 reprograms the tumour microenvironment. Nature Communications. 2016;7:13597. doi: 10.1038/ncomms13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X-F, et al. MiR-15a contributes abnormal immune response in myasthenia gravis by targeting CXCL10. Clinical Immunology. 2016;164:106–113. doi: 10.1016/j.clim.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Moon H-G, Yang J, Zheng Y, Jin Y. miR-15a/16 Regulates Macrophage Phagocytosis after Bacterial Infection. The Journal of Immunology. 2014;193:4558–4567. doi: 10.4049/jimmunol.1401372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand S, et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espinosa-Diez C, et al. MicroRNA regulation of the MRN complex impacts DNA damage, cellular senescence, and angiogenic signaling. Cell Death Dis. 2018;9:632. doi: 10.1038/s41419-018-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruhl R, et al. microRNA-451a regulates colorectal cancer proliferation in response to radiation. BMC Cancer. 2018;18:517. doi: 10.1186/s12885-018-4370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, et al. Dual Anti-Inflammatory and Anti-Angiogenic Action of miR-15a in Diabetic Retinopathy. EBioMedicine. 2016;11:138–150. doi: 10.1016/j.ebiom.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Meerleer G, et al. Radiotherapy for renal-cell carcinoma. The Lancet Oncology. 2014;15:e170–e177. doi: 10.1016/s1470-2045(13)70569-2. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs, S. B. & Miao, E. A. Gasdermins: Effectors of Pyroptosis. Trends in Cell Biology27, 673–684, 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed]
- 33.Kolb, J. P., Oguin, T. H., III, Oberst, A. & Martinez, J. Programmed Cell Death and Inflammation: Winter Is Coming. Trends in Immunology38, 705–718, 10.1016/j.it.2017.06.009. [DOI] [PMC free article] [PubMed]
- 34.Taabazuing, C. Y., Okondo, M. C. & Bachovchin, D. A. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell chemical biology24, 507–514.e504, 10.1016/j.chembiol.2017.03.009 Epub 2017 Apr 6. (2017). [DOI] [PMC free article] [PubMed]
- 35.Zhu H, et al. The Effects of ASMase Mediated Endothelial Cell Apoptosis in Multiple Hypofractionated Irradiations in CT26 Tumor Bearing Mice. Asian Pac J Cancer Prev. 2015;16:4543–4548. doi: 10.7314/APJCP.2015.16.11.4543. [DOI] [PubMed] [Google Scholar]
- 36.Ye, E.-A. et al. miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. Journal of Neuroinflammation13, 10.1186/s12974-016-0771-8 (2016). [DOI] [PMC free article] [PubMed]
- 37.Yang J, et al. MiR-15a/16 deficiency enhances anti-tumor immunity of glioma-infiltrating CD8+ T cells through targeting mTOR. International Journal of Cancer. 2017;141:2082–2092. doi: 10.1002/ijc.30912. [DOI] [PubMed] [Google Scholar]
- 38.Lan, F. et al. miR-15a/16 Enhances Radiation Sensitivity of Non-Small Cell Lung Cancer Cells by Targeting the TLR1/NF-κB Signaling Pathway. International Journal of Radiation Oncology*Biology*Physics91, 10.1016/j.ijrobp.2014.09.021 (2015). [DOI] [PubMed]
- 39.Mei, Z. et al. The miR-15 Family Enhances the Radiosensitivity of Breast Cancer Cells by Targeting G2 Checkpoints. Radiation Research183, 10.1667/rr13784.1 (2015). [DOI] [PubMed]
- 40.Kontos CK, Tsiakanikas P, Avgeris M, Papadopoulos IN, Scorilas A. miR-15a-5p, A Novel Prognostic Biomarker, Predicting Recurrent Colorectal Adenocarcinoma. Mol Diagn Ther. 2017;21:453–464. doi: 10.1007/s40291-017-0270-3. [DOI] [PubMed] [Google Scholar]
- 41.Svoronos AA, Engelman DM, Slack FJ. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Research. 2016;76:3666–3670. doi: 10.1158/0008-5472.can-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.