Abstract
To investigate the characteristics of the intestinal microbiota of mice treated with Folium senna decoction gavage combined with restraint and tail pinch stress. Ten healthy male Kunming mice were chosen and randomly divided into control group and model group, with five mice in each group. Mice in the control group were raised regularly, while mice in the model group were treated by feeding with Folium senna decoction, restraint in a constraint tube and tail pinch with a clip for 7 days. Intestinal contents from the jejunum to ileum were collected, and DNA was extracted from each mouse. The characteristics of the intestinal microbial species were analysed by PacBio Sequel-based 16S rRNA sequencing. Result showed that alpha diversity indices in the model group were higher than those in the control group, and the Simpson index differed significantly (P < 0.05). Based on the composition and abundances of species, there were differences between the control group and model group at the species level, but these differences were not significant (P > 0.05). In the control group, Candidatus arthromitus sp. SFB-mouse and Lactobacillus johnsonii were the dominant species. In the model group, Staphylococcus lentus, Lactobacillus johnsonii, Candidatus arthromitus sp. SFB-mouse and Lactobacillus murinus were included. Furthermore, LEfSe analysis showed that the relative abundances of Escherichia sp. BBDP27, Helicobacter ganmani, Bacteroides vulgatus and Lactobacillus intestinalis in the model group were higher than those in the control group (P < 0.05 or P < 0.01). In conclusion, Folium senna decoction gavage combined with restraint and tail pinch stress increased the intestinal microbiota diversity. Strains associated with intestinal diseases, including Bacteroides vulgatus, Helicobacter ganmani, Staphylococcus lentus and Lactobacillus murinus, were significantly enriched, while strains beneficial to health, such as Candidatus arthromitus sp. SFB-mouse and Lactobacillus johnsonii, were significantly depleted.
Keywords: Folium senna, Restraint, Intestinal microbiota, Third-generation sequencing
Introduction
The intestinal microbiota is called the “the second genome of human body” (Zhu et al. 2010), and the number of microbes in the human intestine is comparable to the total number of human cells. In recent years, the association between the intestinal microbiota and host has been gradually explored (Cani 2018). The intestinal microbiota is roughly divided into three categories: probiotics, opportunistic pathogens and pathogenic bacteria (He and Guo 2019). Probiotics, such as Bifidobacterium and Lactobacillus, are responsible for providing nutrition, promoting metabolism, and regulating immunity (Rezaee et al. 2019). Opportunistic pathogens, such as Enterobacteriaceae and Enterococcus, are usually symbiotic in the intestinal tract and are harmless under normal circumstances, but these bacteria can damage the intestinal tract and cause disease under conditions such as probiotic weakening and reduced immunity (Krammer et al. 2018). Pathogenic bacteria are mostly passerines, colonizing in large numbers and playing a role in the occurrence of diarrhoea in cases of dysbiosis (Zhang et al. 2019a).
Diarrhoea is one of the most common digestive system diseases. It is clinically characterized by increased stool frequency, sloppy stools and even watery stools (Zhang et al. 2019b). The balance of the intestinal microbiota is disrupted during diarrhoea caused by physical or psychological factors, such as oral administration or mental stress (Barko et al. 2018; Campos et al. 2016; Moloney et al. 2014). In addition, some phenomena accompany the occurrence of diarrhoea, such as loss of dominant probiotics, propagation of opportunistic pathogens and pathogenic invasion (Wu and Wang 2018). The original functions of the intestinal microbiota, such as nutrition, immunity and metabolism, are lost, resulting in product accumulation, excessive intestinal secretion and mucosal absorption disorder, causing a large amount of fluid to permeate into the intestinal lumen, resulting in increased intestinal peristalsis and aggravation of diarrhoea (Pickard et al. 2017; Piche 2014).
As a kind of traditional Chinese medicine for purging, Folium senna is mainly used to treat constipation, for intestinal preparation, and in other cases where cleansing is needed, as well as a common drug for diarrhoea modelling (Sun and Zhang 2017). Restraint and tail pinch stress are methods for establishing animal models with psychosocial symptoms such as anxiety and depression by restricting animal activities and pinching the tail (Chu 2017), causing forms of mental stress such as pain and restlessness (Zhao et al. 2017). Folium senna decoction gavage combined with restraint and tail pinch stress can effectively establish an animal model of diarrhoea accompanied by mental stress (Liu et al. 2020).
Third-generation sequencing technologies can accurately identify strains and investigate the diversity of the microbiota based on the 16S rRNA (Xu et al. 2019). The present research aimed to establish a diarrhoea model by Folium senna decoction gavage combined with restraint and tail pinch stress and use 16S rRNA high-throughput sequencing, quantitative insights into microbial ecology (QIIME) (https://qiime.org/) and R (https://www.r-project.org) software to elucidate the effects of Folium senna decoction gavage combined with restraint and tail pinch stress on the intestinal microbiota of mice. These findings could be of value, providing a scientific basis for related research in the future.
Materials and methods
Materials
Animals
Ten SPF male Kunming mice weighing 20 ± 2 g, with licence number SCXK (Xiang) 2016–0002, were purchased from Hunan Slaccas Jingda (SJA) Laboratory Animal Company (Hunan, China). All procedures involving animals were performed according to the protocols approved by the Institutional Animal Care and Use Committee of Hunan University of Chinese Medicine.
Feed
Qualified clean feed was purchased from Hunan SJA Laboratory Animal Company (Hunan, China).
Place
The experiment was carried out in a shielded environment at the Animal Experiment Center of the First Hospital of Hunan University of Chinese Medicine, ensuring ventilation, light avoidance, cleanliness and quietness, at a temperature of 23–25 °C and relative humidity of 47–53%, with licence number SYXK (Xiang) 2015–0003.
Medicine
Five hundred grams of Folium senna (Yunnan) was purchased from The First Hospital of Hunan University of Chinese Medicine and soaked for 10 min in boiling water after being cleaned and drained. The water was filtered with gauze after 10 min and concentrated by evaporation in a water bath at 75 °C to obtain a Folium senna decoction with a concentration of 1 g/mL. The decoction was cooled to 4 °C for the following experiment (Zou et al. 2009).
Methods
Animal grouping
After 2 days of adaptive feeding, all tested mice were randomly divided into 2 groups: 5 mice in the control group (gcn) and 5 mice in the model group (gmn).
Modelling
The mice in both groups were fasted for 12 h before gavage. At 9 am, the mice in the control group were administered 0.35 mL of distilled water, and the mice in the model group were treated with 0.35 mL of Folium senna decoction. At 3 p.m., the mice in the model group were restrained in a constraint tube, and the distal 1/3 of the tail was pinched with a clip, for 1 h each time and continuously for 7 days (Xiao et al. 2016). After being treated, the model mice became diarrhoeal.
Collection of intestinal contents
Once the model mice became diarrhoeal, they were sacrificed using cervical vertebra dislocation on a sterile operation platform. Intestinal contents from the jejunum to rectum were collected and immediately frozen at − 80 °C for DNA extraction.
DNA extraction
Total microbial genomic DNA samples were extracted using the D5625-01 OMEGA DNA Isolation Kit (Omega Bio-tek Inc., Norcross, GA, USA) according to the manufacturer’s instructions. The quantity and quality of the extracted DNA were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and 0.8% agarose gel electrophoresis, respectively. Then, the samples were stored at − 20 °C for subsequent use.
PCR amplification
To amplify the DNA, the universal primers for bacterial 16S rRNA were used: forward primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and reverse primer 1492R (5′-ACCTTGTTACGACTT-3′). The amplification system was prepared as follows: 5 μL of Q5 reaction buffer (5×), 5 μL of Q5 High-Fidelity GC Buffer (5×), 0.25 μL of Q5 High-Fidelity DNA Polymerase (5 U/μL), 2 μL (2.5 mM) of dNTPs, 1 μL (10 μmol/L) each of the forward and reverse primers, 2 μL of DNA template, and 8.75 μL of ddH2O. The amplification conditions were as follows: the thermal cycle consisted of initial denaturation at 98 °C for 2 min, followed by 25/10 cycles (for the first and second amplification steps, respectively) consisting of denaturation at 98 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 90 s, with a final extension at 72 °C for 5 min.
PacBio sequencing
A total of PCR amplicons were purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN) and quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, amplicons were pooled in equal amounts, and single-molecule real-time (SMRT) sequencing was performed using the PacBio Sequel platform at Shanghai Personal Biotechnology Co., Ltd.
Bioinformatic and statistical analysis
Operational taxonomic unit (OTU) (Blaxter et al. 2005) generation refers to the merging of sequences according to a certain similarity threshold. Sequences with similarity higher than the threshold are merged into one OTU. A rarefaction curve can be used to judge whether the sequencing depth of each sample is sufficient to reflect the microbial diversity in the community sample. The flatter the curve is, the more sufficient the sequencing result. Alpha diversity analysis, which included determination of the Chao1, ACE, Shannon and Simpson indices, was used to evaluate abundance and diversity. Principal components analysis (PCA) shows the natural distribution of samples in a way that reduces the dimensions and simplifies the data structure (Ramette 2007). LEfSe analysis is used to screen the key biomarkers based on the linear discriminant analysis (LDA) effect size (Segata et al. 2011). SPSS 21.0 software (IBM Corp., Armonk, NY, USA) was used to perform statistical analysis, and the measurement data were expressed as the means ± standard deviations. An independent t-test was used to compare the samples with normal distribution and homogeneity of variance, and P < 0.05 was regarded as a significant difference.
Results and analysis
OTU number of microbiota in the intestinal contents
All the raw data were submitted to NCBI (Accession number: SRP247284, https://www.ncbi.nlm.nih.gov/Traces/sra_sub/). QIIME software was used to merge and divide the effective sequences according to 97% similarity, and R software was used to draw the Venn diagram. Figure 1 shows that the number of intestinal microbial species in the model group was higher than that in the control group. There were 645 and 609 OTUs expressed in mice in the model and control groups, respectively. Among these OTUs, 298 were identical. These findings indicated that modelling by Folium senna decoction gavage combined with restraint and tail pinch stress had a certain effect on the number of OTUs in the intestinal contents, increasing the total number of intestinal microbes.
Fig. 1.
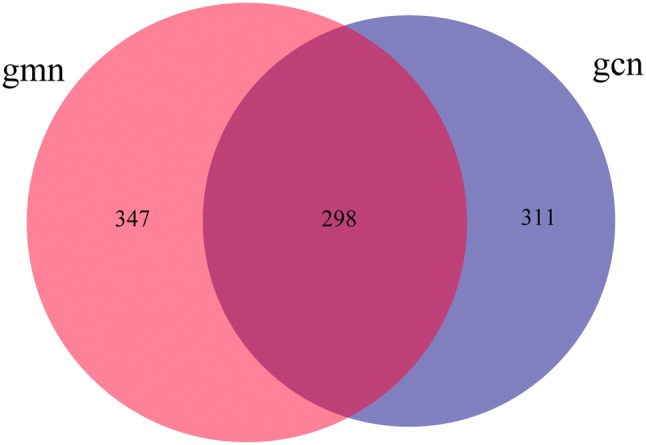
Effects of Folium senna decoction gavage combined with restraint and tail pinch stress on intestinal microbiota OUT number. Venn diagram of OTUs based on sequences with over 97% similarity under a similar level of clustering. The larger the number is, the more the number of intestinal microbes. The diagram shows that Folium senna decoction gavage combined with restraint and tail pinch stress increased the number of intestinal microbial OTUs. gmn model group, gcn control group
Diversity of the microbiota in the intestinal contents
Alpha diversity analysis
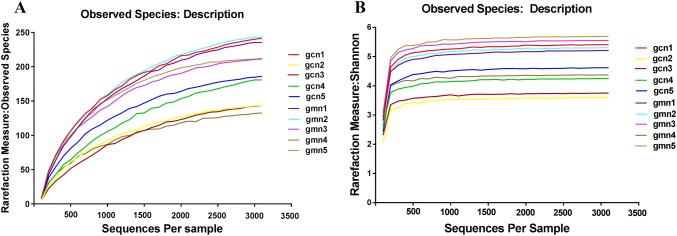
Alpha diversity was estimated by four indices and rarefaction curves, which could reflect the richness and uniformity of specific regions or ecosystems. Effective sequences were randomly sampled by QIIME software, and rarefaction curves were drawn by using the sequence number extracted at each depth and the corresponding OTU number. As shown in Fig. 2, most observed species and Shannon curves of the samples had a gentle trend, indicating that the diversity was close to saturation.
Fig. 2.
Rarefaction curve diagrams of OTUs. The abscissa represents the sequences randomly selected per sample, and the ordinate represents the number of OTUs found at the corresponding depth. The flatter the curve is, the more sufficient the sequencing result. gcn 1–5 control groups 1–5, gmn 1–5 model groups 1–5
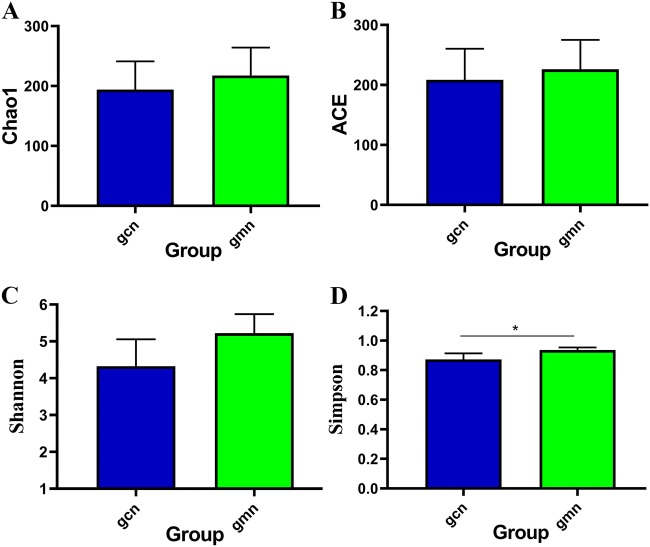
As indicated in Fig. 3, all indices in the model group were higher than those in the control group. There was no significant difference between the two groups in terms of the Chao1 index and ACE index, which reflect the richness of the community (P > 0.05). As indices that reflect the evenness of the community, the Shannon index had no significant difference (P > 0.05) but the Simpson index did (P < 0.05), indicating that modelling changed the diversity of the microbiota in intestinal contents and that the evenness was improved.
Fig. 3.
Effects of Folium senna decoction gavage combined with restraint and tail pinch stress on intestinal microbiota diversity. The alpha diversity analysis of Chao1, ACE, Shannon and Simpson indices. gcn control group, gmn model group. *P < 0.05
Beta diversity analysis
The distances in the coordinate system of PCA could indicate the actual differences among individuals to the greatest extent; the shorter the distance between two points is, the more similar the community structure and, therefore, the smaller the difference between samples. As shown in Fig. 4, the points in the control group were distributed in the first, second and fourth quadrants, and three of them were concentrated. The points in the model group were dispersed and distributed in the first, second and third quadrants. The discreteness of the sample distribution may be due to individual differences or responses to modelling in mice. In general, the distribution of the samples in both groups was relatively dispersed. These findings suggest that modelling had little impact on the community structure of the intestinal microbiota in mice.
Fig. 4.
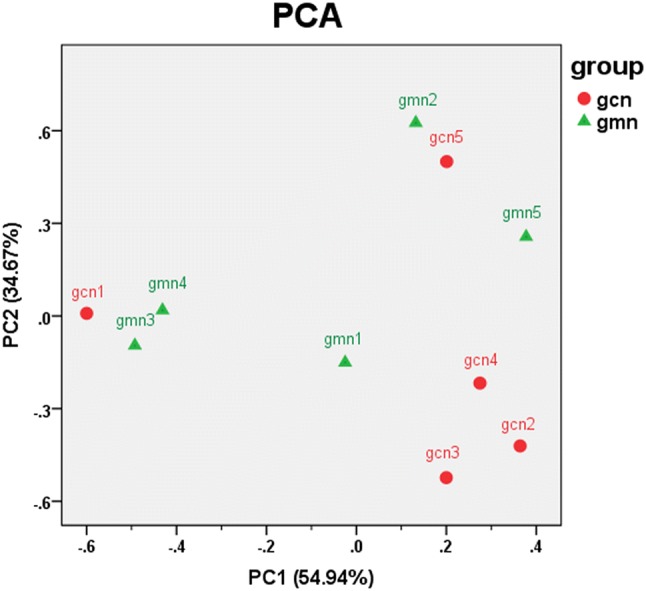
Effects of Folium senna decoction gavage combined with restraint and tail pinch stress on intestinal microbiota structure. Principal components analysis (PCA) shows the natural distribution of samples. The percentages in brackets on the axes represent the different proportions of raw data explained by the corresponding principal components. Each point represents a sample. The closer the points are, the more similar the structures of the communities. gcn control group, gmn model group
Abundance of the microbiota in the intestinal contents
Relative abundance of the microbiota at the species level
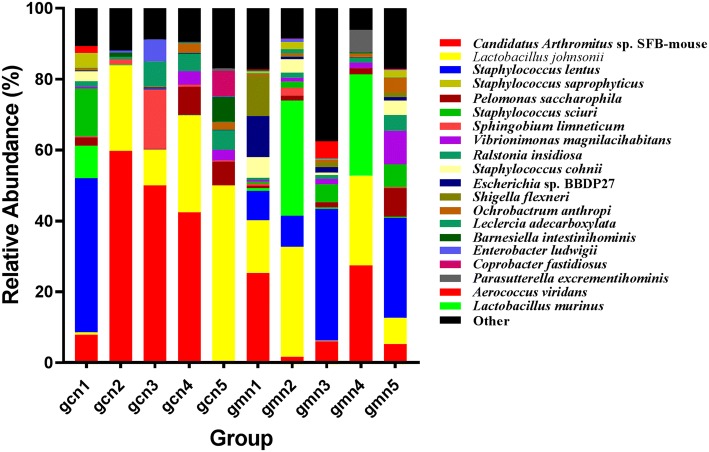
At the species level, there were significant dominant strains in the control group, namely, Candidatus arthromitus sp. SFB-mouse (32.0%) and Lactobacillus johnsonii (22.3%), accounting for 54.3% of the total strains. In the model group, the intestinal microbiota mainly contained Staphylococcus lentus (16.5%), Lactobacillus johnsonii (15.8%), Candidatus arthromitus sp. SFB-mouse (13.1%) and Lactobacillus murinus (12.5%). Although there were differences between the two groups at the same microbiota level, they showed no statistically significant difference (P > 0.05) (Table 1, Fig. 5).
Table 1.
Relative abundances of intestinal microbes at the species level
| Species | gcn | gmn |
|---|---|---|
| Candidatus arthromitus sp. SFB-mouse | 0.31982 ± 0.26478 | 0.13103 ± 0.12208 |
| Lactobacillus johnsonii | 0.22302 ± 0.18756 | 0.15772 ± 0.12598 |
| Staphylococcus lentus | 0.08814 ± 0.19399 | 0.16505 ± 0.15547 |
| Lactobacillus murinus | 0.01921 ± 0.04103 | 0.12526 ± 0.16546 |
| Pelomonas saccharophila | 0.03421 ± 0.03708 | 0.02703 ± 0.03046 |
| Staphylococcus sciuri | 0.02765 ± 0.06086 | 0.02778 ± 0.02816 |
| Sphingobium limneticum | 0.03854 ± 0.07174 | 0.00629 ± 0.00943 |
| Vibrionimonas magnilacihabitans | 0.01477 ± 0.01779 | 0.02818 ± 0.03751 |
| Ralstonia insidiosa | 0.02451 ± 0.02617 | 0.01779 ± 0.01523 |
| Staphylococcus cohnii | 0.00559 ± 0.01230 | 0.02864 ± 0.02458 |
| Escherichia sp. BBDP27 | 0.00160 ± 0.00101 | 0.02939 ± 0.04824 |
| Shigella flexneri | 0.00165 ± 0.00085 | 0.02801 ± 0.04722 |
| Ochrobactrum anthropi | 0.01020 ± 0.01147 | 0.01432 ± 0.01585 |
| Leclercia adecarboxylata | 0.01613 ± 0.03030 | 0.00341 ± 0.00465 |
| Staphylococcus saprophyticus | 0.00849 ± 0.01870 | 0.00970 ± 0.01007 |
| Barnesiella intestinihominis | 0.01659 ± 0.03075 | 0.00087 ± 0.00136 |
| Enterobacter ludwigii | 0.01402 ± 0.02688 | 0.00283 ± 0.00351 |
| Others | 0.00524 ± 0.00151 | 0.01057 ± 0.00584 |
| Coprobacter fastidiosus | 0.01437 ± 0.03173 | 0.00000 ± 0.00000 |
| Parasutterella excrementihominis | 0.00148 ± 0.00318 | 0.01282 ± 0.02816 |
| Aerococcus viridans | 0.00376 ± 0.00828 | 0.01040 ± 0.02092 |
Each value is the mean ± standard deviation, N = 5
gcn control group, gmn model group
Fig. 5.
Distribution of taxa at the species level. The abscissa is arranged according to the sample ID. The ordinate shows the relative abundance. The same colour represents the same classification unit, and longer bars indicate higher relative abundances of units
Absolute abundance of the microbiota at the species level
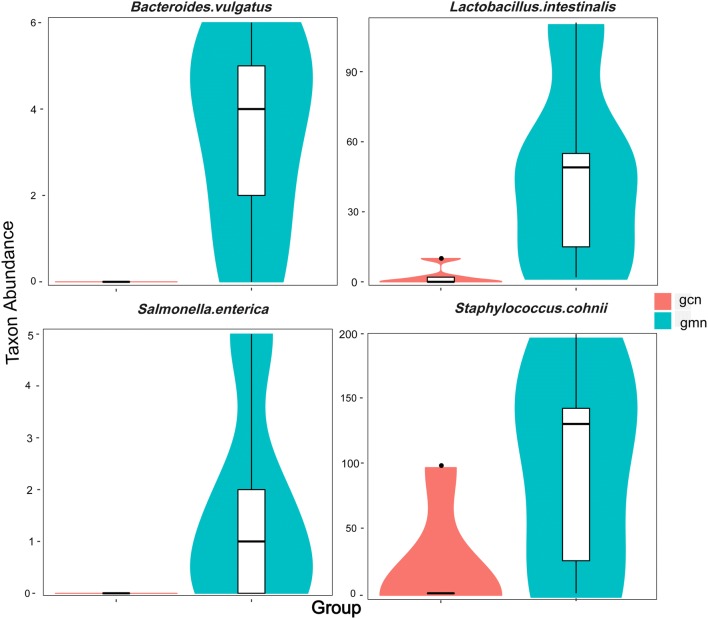
To confirm our finding, further analysis was performed by Mothur software, and the differences between sequences of samples at different levels were tested by pairwise comparison t-test using the Metastats statistical algorithm (White et al. 2009). As indicated in Fig. 6, the absolute abundances of Bacteroides vulgatus, Lactobacillus intestinalis, Salmonella enterica and Staphylococcus cohnii in the model group were significantly higher than those in the control group at the species level.
Fig. 6.
Abundance distribution between groups at the species level. The abscissa represents the groups, and the ordinate represents taxon abundance in each group. Based on the Metastats significant difference analysis results, the taxa that had significant differences were identified. gcn control group, gmn model group
Characteristic microbes at the species level
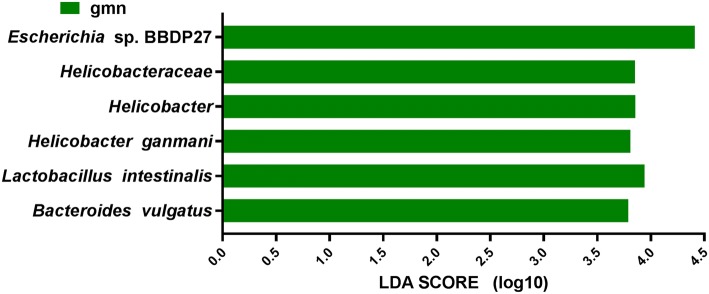
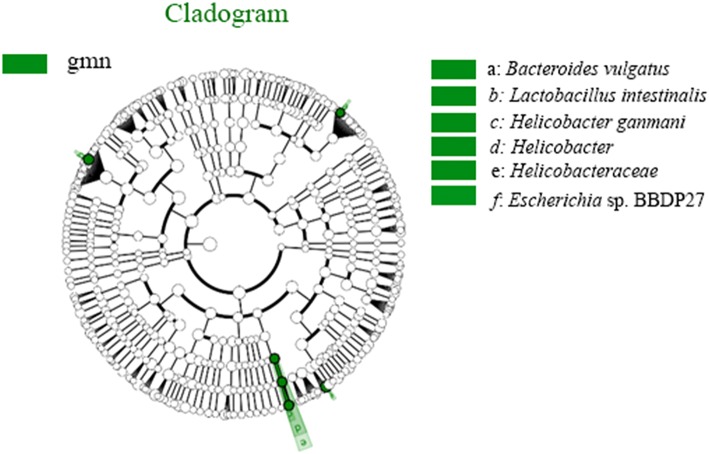
To further screen for key biomarkers, LEfSe analysis was performed. As seen in Fig. 7, at the species level, the relative abundances of Escherichia sp. BBDP27, Lactobacillus intestinalis, Helicobacter ganmani, and Bacteroides vulgatus in the model group were significantly higher than those in the control group, and these species could be used as characteristic microbes in the model group. The same results were obtained and are shown in Fig. 8.
Fig. 7.
Diagram of taxonomic units with significant differences between groups. The ordinate represents the taxa with significant differences, and the abscissa represents the LDA scores visualized as bars. The bars are ordered by score to describe the differences in each sample. The longer the bar is, the more significant the difference
Fig. 8.
Diagram of intergroup difference in taxonomic units based on the classification tree. The cladogram shows all the hierarchical relationships among the taxonomic units from phylum to species. The node sizes correspond to the average relative abundances of the taxa. The green colour indicates a significant difference in the taxa and high abundance in the sample. The letters identify taxa that had obvious differences
Discussion
Folium senna decoction gavage combined with restraint and tail pinch stress increased the intestinal microbiota diversity
The principle of Folium senna-induced diarrhoea is closely related to the intestinal microbiota. Folium senna modelling could significantly reduce the total number of intestinal microbes in mice, and the activities of cellulase, xylanase and lactase secreted by the microbiota in the intestine are significantly reduced (Hu et al. 2018; Xu et al. 2016). Cellulase, xylanase and lactase are important digestive enzymes in the intestine, and low activities of these enzymes can lead to weakened digestion, which is a critical cause of diarrhoea (He et al. 2018). In addition, mental stress caused by restraint and tail pinch are closely related to the intestinal microbiota. Restraint and tail pinch stress could induce depression-like and anxiety-like behaviour and simulate mental stress, which can change intestinal motility and mucosal permeability by influencing the immune, nervous and endocrine systems, resulting in changes in the locations or numbers of intestinal microbes (Wang and Wang 2017; Lotrich et al. 2011; Guo et al. 2017). It has been confirmed that emotional disorders are closely related to dysbiosis (Friedland 2015), and stressors can definitely induce abnormal behaviours and change the intestinal microbiota (Vuong et al. 2017). Folium senna decoction gavage combined with restraint and tail pinch stress leads to changes in the intestinal microbiota, and the release of a variety of active substances from the dysfunctional microbiota is different from the normal state, stimulating intestinal neurons and other factors related to gastrointestinal motility, inhibiting the expression of the intestinal mucosal barrier and the absorption ability of the intestine, inducing intestinal inflammatory responses, and further aggravating the diarrhoea (Kabouridis et al. 2015; Rooks and Garrett 2016; Long et al. 2018). In this study, the results show that the number of OTUs in the model group (645) was higher than that in the control group (609), and the Simpson index in the model group was significantly higher than that in the control group (P < 0.05), indicating that the evenness of the intestinal microbiota in the model group was higher than that in the control group and that the numbers of each species were similar. This indicated that Folium senna decoction gavage combined with restraint and tail pinch stress had a certain promotion effect on the diversity of the intestinal microbiota in mice.
Folium senna decoction gavage combined with restraint and tail pinch stress had little effect on the intestinal microbiota community structure
PCA of the beta diversity analysis was used to analyse the community structure of the intestinal microbiota and to identify the factors influencing the microbial composition in different groups. The results showed that the distributions of the samples in the two groups were all relatively discrete, and there was no significant difference between the two groups. This may be due to individual differences or the sensitivity of each experimental animal to the modelling, so the differences in samples offset each other. In the analysis of dominant species, differences in the composition of the intestinal microbiota were found at different taxonomic levels, but there was no significant difference between the control and model groups. These results were also confirmed in the LEfSe analysis and the diagram of intergroup differences in taxonomic units based on the classification tree. Only a few strains in the samples of the two groups showed significant differences. The effect of Folium senna decoction gavage combined with restraint and tail pinch stress on the intestinal microbiota community structure needs to be further studied and verified.
Folium senna decoction gavage combined with restraint and tail pinch stress was associated with a characteristic microbiota at the species level
Under the dual conditions of diarrhoea and emotional disturbance, intestinal microbiota disorder is inevitable. Patients diagnosed with diarrhoea-predominant irritable bowel syndrome (IBS-D) with symptoms of diarrhoea and emotional disturbances had an intestinal microbiota characterized by a reduction in probiotics, such as Bifidobacterium and Lactobacillus, and an increase in opportunistic pathogens, such as Enterococcus and Enterobacteriaceae (Li 2018). In this study, we established a diarrhoea model by Folium senna decoction gavage combined with restraint and tail pinch stress and used 16S rRNA high-throughput sequencing technology to analyse the changes in the intestinal microbiota, and significant differences in the proportions of four intestinal microbes were found at the species level. Among them, the abundances of Staphylococcus lentus and Lactobacillus murinus substantially increased from 8.9% to 16.5% and 1.9% to 12.5%, respectively, while those of Candidatus arthromitus sp. SFB-mouse and Lactobacillus johnsonii sharply decreased from 32.0% to 13.1% and 22.3% to 15.8%, respectively, after modelling. As a Staphylococcus sciuri spp., Staphylococcus lentus is considered an opportunistic pathogen associated with a disease called coagulase-negative Staphylococcus infection (Rivera et al. 2014), and enrichment of this pathogen could lead to inflammatory diarrhoea. Lactobacillus murinus is a probiotic that can induce the differentiation of Treg cells against dextran sulphate sodium (DSS) colitis (Tang et al. 2015). However, excessive growth and predominance of this species can lead to loss of intestinal vitamins and decreased utilization rate, weakening of metabolism, high susceptibility to infection by extrinsic factors, and diarrhoea (Hayashi et al. 2017). As an important intestinal commensal microbe in the immune system, Candidatus arthromitus sp. SFB-mouse can trigger an IgA response and activate CD4+ and CD8+ T cells (Bolotin et al. 2014). Lactobacillus johnsonii can produce several antimicrobial molecules, including bacteriocins (Bereswill et al. 2017), and reduced abundance of this species is bound to weaken immune function, leading to the development of inflammatory responses such as diarrhoea.
According to LEfSe analysis, there were significant differences in relative abundance at the species level, such as in the abundances of Escherichia sp. BBDP27, Lactobacillus intestinalis, Helicobacter ganmani, and Bacteroides vulgatus. As mentioned above, patients with depression exhibit elevated levels of Bacteroides. As a member of this genus, Bacteroides vulgates is a widely distributed opportunistic pathogen in the intestinal tract that may cause intestinal inflammation by activating NF-κB. In addition, this species is associated with Crohn’s disease (CD) and ulcerative colitis (UC), suggesting that its significant growth in the model group may have led to aggravation of diarrhoea (Roth et al. 2016; Cuív et al. 2017; Ramanan et al. 2016). Enzymes expressed by Lactobacillus intestinalis can convert daidzein to equol and have positive effects on human health, which may be due to the self-regulation of the intestinal microecology (Heng et al. 2019). Helicobacter ganmani is associated with chronic enteritis and hepatobiliary disease and can induce diarrhoea (Nilsson et al. 2008). Unfortunately, the effects of Escherichia sp. BBDP27 are rarely reported. It is not known whether Escherichia sp. BBDP27 is a probiotic, opportunistic pathogen or pathogenic bacterium, and the specific relationships between Escherichia sp. BBDP27 and diarrhoea and mental stress remain to be further explored. In addition, the relationships among Bacteroides vulgatus, Lactobacillus intestinalis, Escherichia sp. BBDP27 and Helicobacter ganmani and their suitability as biomarkers need to be further verified.
In summary, diarrhoea and mental stress caused by Folium senna decoction gavage combined with restraint and tail pinch stress increased the diversity of the microbiota in the intestinal contents, and distinct characteristic species were detected. Changes in the intestinal microbiota caused by diarrhoea accompanied by mental stress could further aggravate diarrhoea.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81874460) and the Scientific Research Fund of Hunan Provincial Education Department (19A256).
Authors’ contributions
ZT designed the study; CZ and XP performed the experiments; ZY and LS analyzed the data; ZY wrote the paper; CL checked the paper. The decision to submit the manuscript for publication was made by all the authors. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Institutional animal care and use committee statement
The study was approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine.
Contributor Information
Chengxing Long, Email: longchengxing@163.com.
Zhoujin Tan, Email: tanzhjin@sohu.com.
References
- Barko PC, McMichael MA, Swanson KS, Williams DA. The gastrointestinal microbiome: a review. J Vet Intern Med. 2018;32(1):9–25. doi: 10.1111/jvim.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S, Ekmekciu I, Escher U, Fiebiger U, Stingl K, Heimesaat MM. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci Rep. 2017;7(1):2138. doi: 10.1038/s41598-017-02436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, Abebe E. Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc Lond B Biol Sci. 2005;360(1462):1935–1943. doi: 10.1098/rstb.2005.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin A, de Wouters T, Schnupf P, Bouchier C, Loux V, Rhimi M, Jamet A, Dervyn R, Boudebbouze S, Blottière HM, Sorokin A, Snel J, Cerf-Bensussan N, Gaboriau-Routhiau V, van de Guchte M, Maguin E. Genome sequence of “Candidatus arthromitus” sp. strain SFB-mouse-NL, a commensal bacterium with a key role in postnatal maturation of gut immune functions. Genome Announc. 2014 doi: 10.1128/genomeA.00705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Rocha NP, Nicoli JR, Vieira LQ, Teixeira MM, Teixeira AL. Absence of gut microbiota influences lipopolysaccharide-induced behavioral changes in mice. Behav Brain Res. 2016;312:186–194. doi: 10.1016/j.bbr.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XX (2017) The establishment of environmental stress animal models and their neurobiological mechanisms. Dissertation, Shanghai Jiao Tong University
- Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015;45(2):349–362. doi: 10.3233/JAD-142841. [DOI] [PubMed] [Google Scholar]
- Guo YA, Wang YM, He W, Sun CQ. Advances in intestinal flora and brain diseases. Contin Med Educ. 2017;31(7):89–92. doi: 10.3969/j.issn.1004-6763.2017.07.045. [DOI] [Google Scholar]
- Hayashi A, Mikami Y, Miyamoto K, Kamada N, Kanai T. Intestinal dysbiosis and biotin deprivation induce alopecia through overgrowth of Lactobacillus murinus in mice. Cell Rep. 2017;20(7):1513–1524. doi: 10.1016/j.celrep.2017.07.057. [DOI] [PubMed] [Google Scholar]
- He L, Liu YW, Guo YF, Shen KJ, Hui HY, Tan ZJ. Diversity of intestinal bacterial lactase gene in antibiotics-induced diarrhea mice treated with Chinese herbs compound Qi Wei Bai Zhu San. 3 Biotech. 2018;8(1):4. doi: 10.1007/s13205-017-1024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He QZ, Guo CX. Investigation and analysis on characteristics of dominant intestinal flora in patients with ulcerative colitis and crohn’s disease and its relationship with pathogenesis. Chin J Integr Trad West Med Dig. 2019;27(11):844–849. doi: 10.3969/j.issn.1671-038X.2019.11.10. [DOI] [Google Scholar]
- Heng Y, Kim MJ, Yang HJ, Kang S, Park S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch Microbiol. 2019;201(8):1009–1017. doi: 10.1007/s00203-019-01665-5. [DOI] [PubMed] [Google Scholar]
- Hu J, Peng MJ, Luo HH, Ou YN, Wu YG, Xiao NQ. Effects of senna on the intestinal microbiota and enzyme activity in mice with spleen-deficiency. Chin J Microecol. 2018;30(2):155–157. doi: 10.13381/j.cnki.cjm.201802007. [DOI] [Google Scholar]
- Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85(2):289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer EM, de Ruyck J, Roos G, Bouckaert J, Lensink MF. Targeting dynamical binding processes in the design of non-antibiotic anti-adhesives by molecular simulation—the example of FimH. Molecules. 2018;23(7):1641. doi: 10.3390/molecules23071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF. Analysis of the characteristics of intestinal flora in patients with different subtypes of irritable bowel syndrome. Heilongjiang Med J. 2018;31(4):148–150. doi: 10.14035/j.cnki.hljyy.2018.04.070. [DOI] [Google Scholar]
- Liu YW, Wu Y, Hui HY, Tan ZJ. Establishment of a mouse model of Ganqichengpi diarrhea and the efficacy of Tongxieyaofang prescription. Chin J Appl Environ Biol. 2020 doi: 10.19675/j.cnki.1006-687x.2019.09026. [DOI] [Google Scholar]
- Long CX, Liu YW, He L, Yu R, Li DD, Tan ZJ, Hui HY. Bacterial lactase genes diversity in intestinal mucosa of dysbacterial diarrhea mice treated with Qiweibaizhu powder. 3 Biotech. 2018;8:423. doi: 10.1007/s13205-018-1460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, El-Gabalawy H, Guenther LC, Ware CF. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48–54. doi: 10.3899/jrheum.110903. [DOI] [PubMed] [Google Scholar]
- Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome. 2014;25(1–2):49–74. doi: 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Sturegård E, Barup B, Willén R, Al-Soud WA, Hultberg A, Wadström T. Helicobacter ganmani infection associated with a spontaneous outbreak of inflammatory bowel-like disease in an IL-10-deficient mouse colony. Scand J Lab Anim Sci. 2008;35(1):13–24. doi: 10.23675/sjlas.v35i1.134. [DOI] [Google Scholar]
- Ó Cuív P, de Wouters T, Giri R, Mondot S, Smith WJ, BlottiŔre HM, Begun J, Morrison M. The gut bacterium and pathobiont Bacteroides vulgatus activates NF-κB in a human gut epithelial cell line in a strain and growth phase dependent manner. Anaerobe. 2017;47:209–217. doi: 10.1016/j.anaerobe.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Piche T. Tight junctions and IBS - the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol Motil. 2014;3:296–302. doi: 10.1111/nmo.12315. [DOI] [PubMed] [Google Scholar]
- Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, Honda K, Gause WC, Blaser MJ, Bonneau RA, Lim YA, Loke P, Cadwell K. Helminth infection promotes colonization resistance via type 2 immunity. Science. 2016;352(6285):608–612. doi: 10.1126/science.aaf3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol. 2007;62(2):142–160. doi: 10.1111/j.1574-6941.2007.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee P, Kermanshahi RK, Falsafi T. Antibacterial activity of lactobacilli probiotics on clinical strains of Helicobacter pylori. Iran J Basic Med Sci. 2019;22(10):1118–1124. doi: 10.22038/ijbms.2019.33321.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M, Dominguez MD, Mendiola NR, Roso GR, Quereda C. Staphylococcus lentus peritonitis: a case report. Perit Dial Int. 2014;34(4):469–470. doi: 10.3747/pdi.2012.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C, Petricevic M, John A, Goddard-Borger ED, Davies GJ, Williams SJ. Structural and mechanistic insights into a Bacteroides vulgatus retaining N-acetyl-β-galactosaminidase that uses neighbouring group participation. Chem Commun. 2016;52(74):11096–11099. doi: 10.1039/c6cc04649e. [DOI] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SF, Zhang YY. Progress in research on chemical component and pharmacological activities of Cassia Angustifolia Vahl. Shandong Chem Ind. 2017;46(13):44–45. doi: 10.3969/j.issn.1008-021X.2017.13.017. [DOI] [Google Scholar]
- Tang C, Kamiya T, Liu Y, Kadoki M, Kakuta S, Oshima K, Hattori M, Takeshita K, Kanai T, Saijo S, Ohno N, Iwakura Y. Inhibition of Dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015;18(2):183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Annu Rev Neurosci. 2017;40:21–49. doi: 10.1146/annurev-neuro-072116-031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang ZH. Alteration of rat intestinal pathology and flora based on the chronic stress model. Curr Biotech. 2017;7(1):52–57. doi: 10.3969/j.issn.2095-2341.2017.01.09. [DOI] [Google Scholar]
- White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5(4):e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wang H. Chronic diarrhea and intestinal flora imbalance. Chin J Gen Pract. 2018;17(10):778–780. doi: 10.3760/cma.j.issn.1671-7368.2018.10.006. [DOI] [Google Scholar]
- Xiao XY, Deng YL, Liu YJ, Li DD, Tan ZJ. Effects of Folium Sennae on blood routine in rats with diarrhea of splenic deficiency type. J Hubei Univ Chin Med. 2016;18(6):49–51. doi: 10.3969/j.issn.1008-987x.2016.06.15. [DOI] [Google Scholar]
- Xu Q, Liu YJ, Deng YL, Xiao XY, Tan ZJ, Shu L. Influence of senna on intestinal lactase activity in mice with diarrhea. Human J Tradit Chin Med. 2016;32(8):189–191. doi: 10.16808/j.cnki.issn1003-7705.2016.08.089. [DOI] [Google Scholar]
- Xu YK, Ma Y, Hu XX, Wang J. Analysis of prospective microbiology research using third-generation sequencing technology. Biodiv Sci. 2019;27(5):534–542. doi: 10.17520/biods.2018201. [DOI] [Google Scholar]
- Zhang H, Wang YE, Chen HY. Role and mechanism of gut microbiota involved in host immune response. Acta Microbiol Sin. 2019 doi: 10.13343/j.cnki.wsxb.20190318. [DOI] [Google Scholar]
- Zhang LJ, Jiang Y, Liu BH, Zhang ZG. Advances in the etiology of chronic diarrhea in adults. Chin Gen Prac. 2019;22(22):2760–2765. doi: 10.12114/j.issn.1007-9572.2019.00.378. [DOI] [Google Scholar]
- Zhao Y, Luo DN, Chen Y, Huang C, Zhou SY. Dose-effect and time-effect relationship of chronic restraint stress combined with senna extract gavage in inducing diarrhea-predonimant irritable bowel syndrome in rats. World Chin J Dig. 2017;25(15):1360–1367. doi: 10.11569/wcjd.v25.i15.1360. [DOI] [Google Scholar]
- Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1(8):718–725. doi: 10.1007/s13238-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zheng X, Dai S, Ye Q, Wu D. Establishment of spleen deficiency model in mice. Beijing J Tradit Chin Med. 2009;28(01):60–62. doi: 10.16025/j.1674-1307.2009.01.029. [DOI] [Google Scholar]