Abstract
Patient: Male, 23-year-old
Final Diagnosis: Insulin overdose
Symptoms: Suicide attempt
Medication: —
Clinical Procedure: Conservative administration of potassium
Specialty: Endocrinology and Metabolic
Objective:
Management of emergency care
Background:
Insulin lowers not only blood glucose levels but also serum potassium levels by driving potassium into the cells. Hypokalemia can occur during aggressive treatment of hypoglycemia in patients with insulin overdose and is a well-documented clinical phenomenon; however, there are no studies describing delayed hyperkalemia occurring after initial treatment in patients with insulin overdose.
Case Report:
A 23-year-old male with a history of type 2 diabetes mellitus and self-medicating with insulin, attempted suicide by subcutaneously injecting 2100 units of insulin. He was admitted to our emergency department due to recurrent hypoglycemia. Continuous administration of 50% glucose and potassium via a central venous catheter was performed to maintain his glucose levels above 80 mg/dL and serum potassium level between 3.5 and 4.0 mEq/L. Because his serum potassium level exceeded 4.5 mEq/L at day 3 after admission, the dosage was adjusted accordingly. After his serum potassium level declined to 3.0 mEq/L, his potassium level abruptly increased to 6.0 mEq/L at day 5 after admission. The patient was placed on a potassium-restricted diet and administered furosemide. Potassium infusion was also discontinued. After serum potassium levels returned to the normal range without interventional therapies, the patient was discharged to home on day 14.
Conclusions:
In cases of high-dose insulin overdose, management of hyperkalemia following recovery from hypoglycemia is a critical aspect of patient management. Conservative administration of potassium to correct initial hypokalemia may be considered in patients with high-dose insulin overdose.
MeSH Keywords: Blood Glucose, Hyperkalemia, Hypokalemia, Insulin, Suicide
Background
Insulin self-injection is a common mode of treatment for patients with diabetes mellitus across the globe [1]. Several cases of intentional insulin overdose have been reported to date. Previous reports focus primarily on controlling blood glucose levels after insulin overdose [2–4]. Insulin administration lowers serum potassium concentrations by driving potassium into cells via enhancing the activity of the Na-K-ATPase pump [5]. Some studies have reported hypokalemia after aggressive treatment of hypoglycemia in patients with insulin overdose [1,6–8]. However, to our knowledge, there are no studies describing delayed hyperkalemia occurring after initial treatment. Here, we report a case of massive insulin overdose in a patient who developed hyperkalemia after initial treatment of hypoglycemia and hypokalemia.
Case Report
A 23-year-old Japanese male, with a history of type 2 diabetes mellitus and self-medicating with insulin, attempted suicide by subcutaneously injecting 2100 units of insulin: 900 units of insulin aspart (100 unit/mL) and 1200 units of insulin glargine (100 unit/mL). The patient became increasingly aware of his depreciating state of consciousness and managed to visit the local doctor’s office approximately 8 hours after the initial injection. His blood glucose level was 45 mg/dL, therefore he had 20 g of glucose administered intravenously. Improvements in his level of consciousness were noted and he was transferred to our emergency department 10 hours after the initial injection due to recurrent hypoglycemia. He had been diagnosed with dyslipidemia, fatty liver, and type 2 diabetes mellitus at the age of 19 years and had been placed on a regimen of 16 units of insulin at bedtime and 7 units of insulin aspart at meal time and oral vildagliptin (100 mg/day). The patient had never been diagnosed with any psychological disorders.
On admission, the patient had no specific complaints. He was 171 cm tall and weighed 65 kg. Glasgow Coma Scale was E4V5M6, blood pressure was 121/83 mm Hg, heart rate was 67 beats per minute, respiration rate 11 breaths per minute, oxygen saturations 100% on room air, and body temperature 36.7°C. Physical examination revealed bilateral soft swellings across the mid-section of the abdomen, which were suspected as the sites of massive insulin self-injection (Figure 1). The patient presented with no apparent neurological abnormalities.
Figure 1.
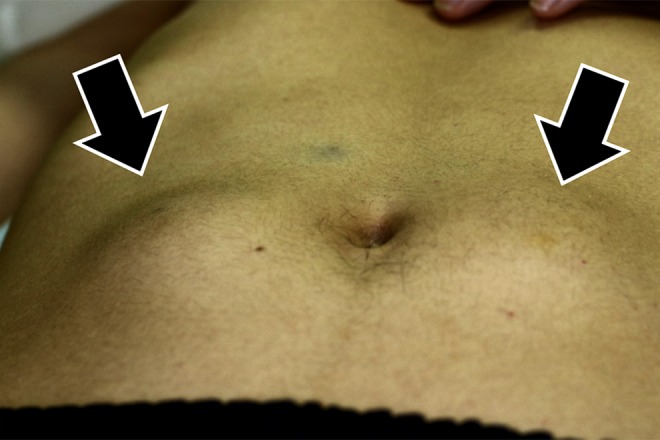
Physical findings of abdomen.
Table 1 shows the initial laboratory investigations. The patient’s potassium level was 3.8 mEq/L, blood sugar was 247 mg/dL, and hemoglobin (Hb)A1c (NGSP) was 10.2% (normal range, 4.6–6.2%). No renal, thyroidal or liver dysfunctions were noted. Chest radiograph and electrocardiogram showed no abnormalities.
Table 1.
Laboratory data on admission.
| Complete blood cell count | Biochemical examination | ||||
| White blood cells | 12,900 | /μL | Sodium | 136 | mmol/L |
| Red blood cells | 495 | ×104/μL | Potassium | 3.8 | mmol/L |
| Hemoglobin | 14.5 | g/dL | Chloride | 104 | mmol/L |
| Platelets | 24.5 | ×104/μL | Calcium | 9.2 | mg/dL |
| Total bilirubin | 1.0 | mg/dL | |||
| Arterial blood gas analysis (room air) | AST | 18 | U/L | ||
| pH | 7.410 | ALT | 14 | U/L | |
| PaCO2 | 39.6 | mmHg | Urea nitrogen | 14 | mg/dL |
| PaO2 | 104 | mmHg | Creatinine | 0.43 | mg/dL |
| Base excess | 0.0 | mmol/L | Creatine kinase | 80 | mg/dL |
| HCO3− | 24.5 | mmol/L | Glucose | 247 | mg/dL |
| Lactate | 1.43 | mmol/L | CRP | 0.15 | mg/dL |
| HbA1c (NGSP) | 10.2 | % | |||
| Hormone | Cholesterol | ||||
| TSH | 0.29 | µLU/mL | Total cholesterol | 162 | mg/dL |
| Free T3 | 3.13 | pg/mL | HDL | 59 | mg/dL |
| Free T4 | 0.97 | ng/dL | LDL | 98 | mg/dL |
Normal range: lactate, 0.50–2.00 mmol/L; TSH, 0.54–4.26; Free T3, 2.29–4.17; Free T4, 0.72–1.52; HbA1c, 4.9%–6.0%.TSH – thyroid stimulating hormone; AST – aspartate aminotransferase; ALT – alanine aminotransferase; CRP – C-reactive protein; HbA1c – hemoglobin A1c; NGSP – National Glycohemoglobin Standardization Program; HDL – high-density lipoprotein; LDL – low-density lipoprotein cholesterol.
We considered surgical removal of the subcutaneous pockets of insulin; however, the insulin deposits were confirmed to be in sedimentary pattern via ultrasonography and thus the surgical option was abandoned. Although the patient was capable of consuming an oral diet of 1800 kcal (carbohydrate 275 g, potassium 2540 mg) per day, continuous parenteral administration of glucose was required to maintain his blood glucose level above 80 mg/dL. Maximum dosage of glucose administration was 35 g/hour (0.54 g/kg/hour). The glucose requirement gradually decreased and ultimately the patient required over 90 hours of continuous administration at various concentrations (Figure 2). Additionally, intravenous potassium administration was used to maintain his serum potassium level between 3.5 and 4.0 mEq/L. A central venous catheter was performed for continuous infusion of highly concentrated glucose and potassium. Although we started with 1.67 mEq/hour of potassium administration, the patient’s serum potassium level did not increase and hovered between 3.3–3.5 mEq/L. Additionally, the patient required increased glucose supplementation in order to maintain plasma glucose levels. Therefore, we increased the potassium dosage on day 2 as a preventive measure against the hypokalemia expected to occur in the wake of the increased glucose and insulin administration. Since serum potassium levels exceeded 4.5 mEq/L at day 3 after admission, the dosage was reduced.
Figure 2.

Clinical course regarding glucose.
After serum potassium levels gradually declined to 3.0 mEq/L, they abruptly increased to 6.0 mEq/L at day 5 after admission. Although no symptoms and no significant changes in electrocardiograms were observed, the patient was placed on a potassium-restricted diet and potassium infusion was discontinued. Furosemide was consecutively administered intravenously for 3 days and orally for 4 days. After serum potassium levels stabilized without resorting to use of furosemide administration, the patient was discharged to home on day 14 (Figure 3). Ultimately, the total amount of potassium administered during this hospitalization reached 200 mEq.
Figure 3.

Clinical course regarding potassium.
Discussion
Insulin overdose is a life-threatening condition. An overdose of insulin due to intentional poisoning events such as suicides results in severe hypoglycemia [6]. Although suicide attempts using high doses of insulin have been reported, only a limited number of cases document the administration of >2000 units of insulin [6,9,10]. A previous study demonstrated the relationship between recovery time from hypoglycemia and the dose of insulin. Approximately 45 and 90 hours is required to recover from hypoglycemia in patients injected with 1000 and 2000 units of insulin, respectively [11]. In the present case, the high-dose of insulin required a high-dose and long-term administration of glucose and potassium in order to maintain nominal blood glucose and potassium levels. The recovery time from hypoglycemia in our case was approximately 60 hours, which is consistent with a previous report [11].
In addition, a well-known feature associated with insulin overdose is hypokalemia [1,6–8]. The total content of potassium in the body is not reduced in patients with hypokalemia after overdose of insulin injection. The hypokalemia under these circumstances represents only a temporary shift from the intravascular fluid into cells. A possible explanation for the hyper-kalemia documented in this case could be the high dosage of glucose and potassium administration in the acute phases of treatment. After insulin overdose, the potassium initially shifts intracellularly, however, as stabilization of blood glucose and serum potassium levels occurs, this pooled potassium gradually shifts back into the intravascular fluid resulting in elevated serum potassium levels. A conservative administration of potassium may be considered in these cases.
Symptoms of hyperkalemia include arrhythmias, numbness of limbs, muscle weakness, and nausea. Among clinical manifestations, the most important is arrhythmia, which can lead to cardiac arrest due to ventricular fibrillation [12,13]. Emergency treatment for hyperkalemic patients involves administration of calcium gluconate which has antagonistic actions to potassium cardiomyotoxicity, and sodium bicarbonate, glucose and insulin, which temporarily transfers potassium into cells [12]. However, the effects of these treatments are temporary, as the total potassium content in the body does not change. In order to treat the root cause, it is essential to remove the excess potassium accumulated in the body with loop diuretics, enema/oral administration of cation exchange resin, and hemodialysis [13]. In the present case, serum potassium levels were corrected by infusion, diuretics, and potassium-restricted diet; however, it should be noted that the amount of time invested in potassium management outweighed that which was required for glucose control.
One important lesson learned from this case was the delayed hyperkalemia triggered by the large volumes of potassium administered. The present case serves as an important reminder to monitor these patients on a slightly extended basis even after blood glucose levels are stabilized because the patient could develop delayed hyperkalemia.
Conclusions
In cases of high-dose insulin overdose, management of hyperkalemia following recovery from hypoglycemia is a critical issue and a conservative administration of potassium to correct initial hypokalemia may be considered in these patients.
References:
- 1.Matsumura M, Nakashima A, Tofuku Y. Electrolyte disorders following massive insulin overdose in a patient with type 2 diabetes. Intern Med. 2000;39:55–57. doi: 10.2169/internalmedicine.39.55. [DOI] [PubMed] [Google Scholar]
- 2.Efrimescu C-I, Yagoub E, Doyle R. Intentional insulin overdose associated with minimal hypoglycemic symptoms in a non-diabetic patient. Maedica (Buchar) 2013;8:365–69. [PMC free article] [PubMed] [Google Scholar]
- 3.White M, Zacharin MR, Werther GA, Cameron FJ. Intravenous glucagon in a deliberate insulin overdose in an adolescent with type 1 diabetes mellitus. Pediatr Diabetes. 2016;17:66–69. doi: 10.1111/pedi.12210. [DOI] [PubMed] [Google Scholar]
- 4.El-Laboudi AH, Misra S, Martineau M, et al. Intentional large insulin overdose captured on a continuous glucose monitor: A novel case report. J Diabetes Sci Technol. 2015;9:929–31. doi: 10.1177/1932296815579691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boini KM, Graf D, Kuhl D, et al. SGK1 dependence of insulin induced hypokalemia. Pflugers Arch Eur J Physiol. 2009;457:955–61. doi: 10.1007/s00424-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 6.Johansen NJ, Christensen MB. A systematic review on insulin overdose cases: Clinical course, complications and treatment options. Basic Clin Pharmacol Toxicol. 2018;122:650–59. doi: 10.1111/bcpt.12957. [DOI] [PubMed] [Google Scholar]
- 7.Gradwohl-Matis I, Pann J, Schmittinger CA, et al. Prolonged bilateral reactive miosis as a symptom of severe insulin intoxication. Am J Case Rep. 2015;16:1–3. doi: 10.12659/AJCR.892324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibutani Y, Ogawa C. Suicidal insulin overdose in a Type 1 diabetic patient: Relation of serum insulin concentrations to the duration of hypoglycemia. J Diabetes Complications. 2000;14:60–62. doi: 10.1016/s1056-8727(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 9.Groth CM, Banzon ER. Octreotide for the treatment of hypoglycemia after insulin glargine overdose. J Emerg Med. 2013;45:194–98. doi: 10.1016/j.jemermed.2012.11.099. [DOI] [PubMed] [Google Scholar]
- 10.Warriner D, Debono M, Gandhi RA, et al. Acute hepatic injury following treatment of a long-acting insulin analogue overdose necessitating urgent insulin depot excision. Diabet Med. 2012;29:232–35. doi: 10.1111/j.1464-5491.2011.03385.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohyama T, Saisho Y, Muraki A, et al. Prediction of recovery time from hypoglycemia in patients with insulin overdose. Endocr J. 2011;58:607–11. doi: 10.1507/endocrj.k11e-018. [DOI] [PubMed] [Google Scholar]
- 12.Kovesdy CP. Management of hyperkalemia: An update for the internist. Am J Med. 2015;128:1281–87. doi: 10.1016/j.amjmed.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Palmer BF, Clegg DJ. Diagnosis and treatment of hyperkalemia. Cleve Clin J Med. 2017;84:934–42. doi: 10.3949/ccjm.84a.17056. [DOI] [PubMed] [Google Scholar]


