Abstract
This review summarizes the outcomes and known adverse effects of current immunosuppression strategies in use in pediatric intestinal transplantation. Intestinal transplantation has evolved from an experimental therapy to a highly successful treatment for children with intestinal failure who have complications with total parenteral nutrition. Because of continued success with intestinal transplantation over the past decade, the focus of clinicians and researchers is shifting from short-term patient survival to optimizing long-term outcomes.
Current 5-year patient and graft survival rates after intestinal transplantation are 58% and 40%, respectively, in the US; single centers have reported nearly 80% patient and 60% graft survival rates at 5 years. The immunosuppression strategy in intestinal transplantation includes a tacrolimus-based regimen, usually in conjunction with an antibody induction therapy such as rabbit-antithymocyte globulin, interleukin-2 receptor antagonists, or alemtuzumab. The use of these immunosuppressive regimens, along with improved medical and surgical care, has contributed significantly toward improved outcomes. Optimization of post-transplant immunosuppression strategies to reduce adverse effects while minimizing acute and chronic graft rejection is a strong clinical and research focus.
Keywords: Tacrolimus, Sirolimus, Alemtuzumab, Daclizumab, Graft Survival Rate
For over 2 decades, intestinal transplantation has presented a significant challenge for both clinicians and scientists worldwide because of the difficulty in controlling allograft rejection without imposing a significant risk of infection or drug-related morbidity. After initial case experience with isolated small bowel transplantation,[1–4] tacrolimus was introduced in 1990, allowing more pronounced clinical progress. The initial challenges[5] with intestinal transplantation included technical issues, sepsis, rejection, and development of necessary clinical expertise with immunosuppression for both maintenance therapies as well as for management of rejection episodes. Further technical improvements in both donor intestine recovery and recipient surgery,[6–8] better understanding of infectious complications,[9] and novel immunosuppression regimens[10–13] have significantly improved patient and graft survival. Other important achievements that have contributed to decreased mortality have been improved postoperative infectious prophylaxis and serial viral load monitoring with pre-emptive therapy for cytomegalovirus and Epstein-Barr virus infections, with subsequent reduction in the development of post-transplant lymphoproliferative disorders (PTLDs).[14]
Despite these advances, areas needing ongoing improvement include reduction of mortality and late graft loss. The Intestinal Transplant Registry (see section 1.1) reports sepsis (54%), rejection (7%), and PTLD (3%) as ongoing causes of death.[15] Although acute cellular rejection has been well managed with current immunosuppression regimens, graft loss due to chronic rejection is a newer concern as longer term survival is being achieved.[16]
The objective of this review is to summarize the outcomes and known adverse effects of current immunosuppression strategies in use in pediatric intestinal transplantation. The Intestinal Transplant Registry (ITR; see section 1.1), Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients (OPTN/SRTR; section 1.2), and single center reports (section 1.3) were used for compilation of data for this review.
1. Clinical Experience with Immunosuppressive Regimens
The first long-term survivor of pediatric intestinal transplantation was maintained under cyclosporine (ciclosporin) immunosuppression by Goulet et al[2] in 1989, and 22 years later the patient is still alive and well. Tacrolimus subsequently replaced cyclosporine and has become the primary maintenance immunosuppressant agent in pediatric intestinal transplantation.
Recently, induction immunosuppression with antibody preparations such as rabbit antithymocyte globulin [rATG] (Thymoglobulin®; Sangstat, Menlo Park, CA, USA), alemtuzumab (Campath®; Genzyme Corporation, Cambridge, MA, USA), basiliximab (Simulect®; Novartis Pharmaceuticals, East Hanover, NJ, USA), daclizumab (Zenapax®; Hoffmann La Roche, Nutley, NJ, USA), and muromonab CD3 (OKT3, Orthoclone OKT3®; Ortho Biotech, Raritan, NJ, USA) have been increasingly employed in intestinal transplantation in an effort to improve clinical outcomes, and patient and graft survival rates. Classification, pertinent mechanism of action, and adverse effects of common immunosuppressant drugs used in pediatric intestinal transplantation are reviewed in detail elsewhere[17–21] and summarized in table I.
Table I.
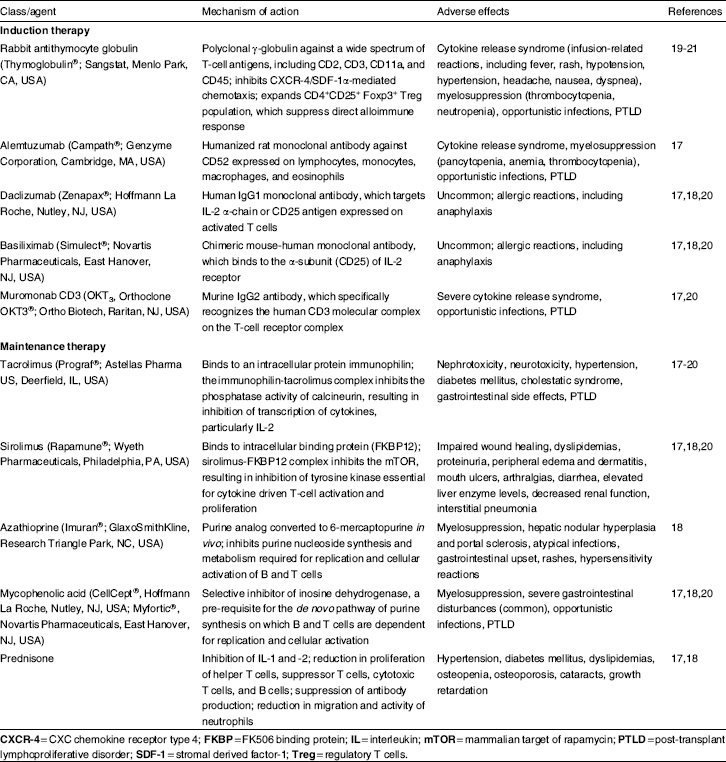
Classification, mechanism of action, and adverse effects of common immunosuppressive drugs used in pediatric intestinal transplantation
1.1 Intestinal Transplant Registry
Overall, the ITR[15] has summarized 25 years of experience with intestinal transplantation from April 1985 through 31 May 2009. The database includes information on 2188 transplants performed on 2038 patients at 73 centers worldwide. These transplants comprised small bowel (n = 926), liver-small bowel (n = 725), and multivisceral (grafts inclusive of stomach; n = 537). More than half (1238/2038) of the recipients are currently alive.
1236 pediatric intestine transplants were performed in 1151 children aged <18 years. 542 of these recipients are currently alive, of whom 464 (86%) are currently off total parenteral nutrition. Over time, pediatric intestine transplants have grown to a current rate of more than 120 transplants per year. Indications for pediatric intestinal transplantation include gastroschisis (24%), necrotizing enterocolitis (16%), volvulus (15%), intestinal atresia (9%), motility disorders (14%), mucosal defects (10%), re-transplantation (5%), neoplasm (1%), and others (2%). The use of pre-transplant immunosuppression with interleukin (IL)-2 receptor antibodies or antilymphocyte products has gradually increased over the past 2 decades. IL-2 receptor antibodies remain the most common pre-transplant immunosuppressant recorded in the registry.
Intestinal transplants continue to have challenging post-operative courses, with a mean hospital stay of 1–2 months. Because the level of immunosuppression required is relatively high compared with other transplants, the reported incidence of PTLD remains approximately 11–14%. Sepsis continues to be the leading cause of death in pediatric intestine transplantation.
1.2 US Data
According to the OPTN/SRTR,[22] 1848 intestinal transplants were performed in the US between 1 January 1988 and 30 September 2009. 1075 (58%) of these transplants were carried out in children <18 years of age, of which 841 (78%) were carried out in children <6 years of age. Liver-intestine-pancreas transplants constituted the majority of intestine-comprising transplants in the US because most transplanted patients with intestinal failure presented with accompanying irreversible parenteral nutrition-associated liver disease.
Recent developments in immunosuppressive regimens in the US from 1999 to 2008 have been tabulated in the 2009 OPTN/Scientific Registry of Transplant Recipients (SRTR) annual report[22] and were summarized by Mazariegos et al.[23] Over the past decade, newer trends in immunosuppression have been seen and immunosuppressive regimens continue to evolve. Along with single-center reports of successful experience with rATG[19] and alemtuzumab,[24,25] the 2009 OPTN/SRTR annual report also documents increasing use of induction therapy with antibody preparations.[22] For example, the most common induction regimen[22] in 2008 was rATG, which was used in 34% of intestine recipients, followed by alemtuzumab (18%), muromonab CD3 (15%), and daclizumab (8%). The use of induction immunosuppression peaked in 2003 and stabilized to its current use in 60% of transplant recipients in 2008.[23] The immunosuppression regimen used for maintenance therapy prior to hospital discharge[22] in 2008 (figure 1) included tacrolimus plus corticosteroids in 55% of intestinal transplant recipients, tacrolimus plus mycophenolate and corticosteroids in 13%, tacrolimus plus mycophenolate in 11%, and tacrolimus alone in 11%; the maintenance regimen at 1-year post-transplant[22] for transplants carried out in 2007 (figure 2) was tacrolimus and corticosteroids in 47% of recipients and tacrolimus alone in 33%.
Fig. 1.
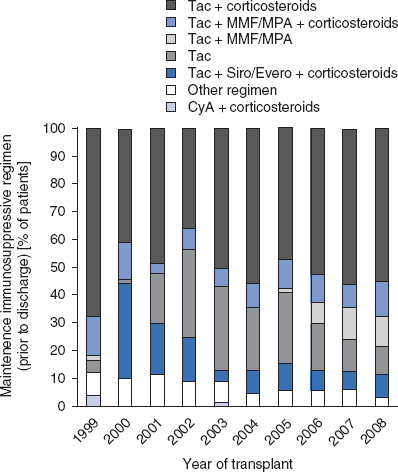
Immunosuppression use for maintenance therapy by regimen prior to discharge, 1999–2008, using data from the 2009 Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients annual report.[22] CyA = cyclosporine (ciclosporin); Evero = everolimus; MMF = mycophenolate mofetil; MPA = mycophenolate sodium; Siro = sirolimus; Tac = tacrolimus. Other regimen includes drug combinations that are different from the listed regimens.
Fig. 2.
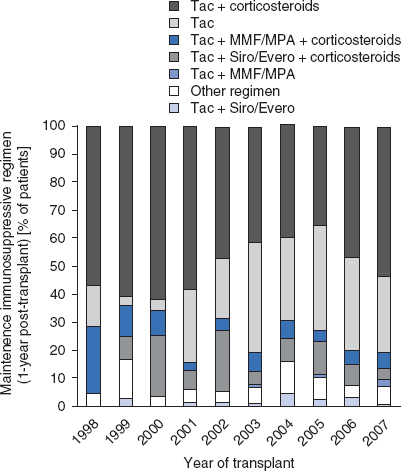
Immunosuppression use for maintenance therapy by regimen 1 year following transplantation, 1998–2007, using data from the 2009 Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients annual report.[22] Evero = everolimus; MMF = mycophenolate mofetil; MPA = mycophenolate sodium; Siro = sirolimus; Tac = tacrolimus. Other regimen includes drug combinations that are different from the listed regimens.
Fifty-seven of 198 (28.8%) patients received anti-rejection treatment 1-year post-transplantation in 2008.[22] Corticosteroids were used in the treatment of the majority (83%) of acute rejection episodes in 2008.[23] The use of antibodies for the treatment of corticosteroid-resistant rejection[22] in 2008 (figure 3) most commonly consisted of muromonab CD3 (53%), followed by rATG (12%) and alemtuzumab (12%).
Fig. 3.
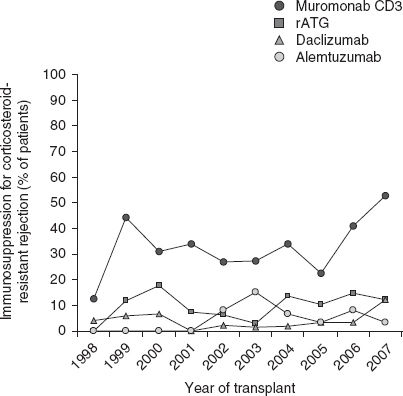
Immunosuppression use for corticosteroid-resistant rejection from transplant to 1 year following transplantation, 1998–2007, using data from the 2009 Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients annual report.[22] rATG = rabbit-antithymocyte globulin.
OPTN/SRTR patient and graft survival rates are shown in figures 4 and 5. Among the recipients of intestine grafts, the unadjusted 1-, 3-, 5-, and 10-year patient and graft survival rates were 89% and 79%, 72% and 59%, 58% and 40%, and 46% and 29%, respectively. Among recipients of combined liver-intestine grafts, unadjusted 1-, 3-, 5- and 10-year patient and graft survival rates were 63% and 59%, 58% and 55%, 58% and 53%, and 39% and 37%, respectively.
Fig. 4.
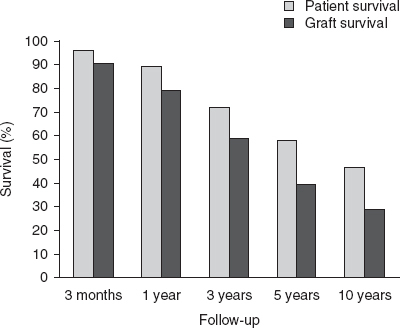
Unadjusted patient and graft survival for intestine-alone recipients, using data from the 2009 Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients annual report.[22]
Fig. 5.
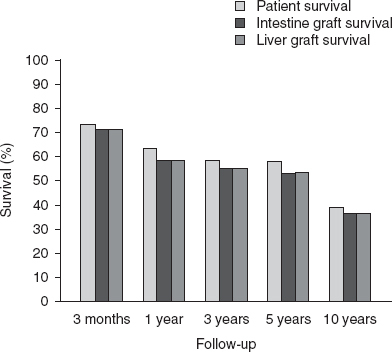
Unadjusted patient and graft survival for liver-intestine recipients using data from the 2009 Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients annual report.[22]
1.3 Single-Center Experience
In addition to the aggregate data of the Intestinal Transplant Registry (see section 1.1) and the SRTR (see section 1.2), single-center data have provided valuable information on long-term outcomes and center experience with the specific regimens. These data are summarized in table II.
Table II.
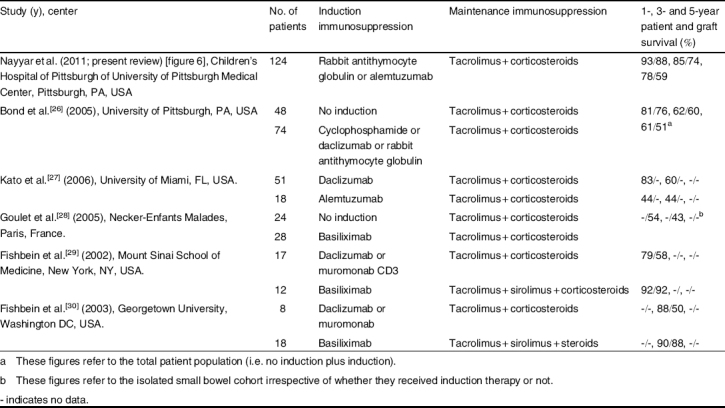
Single-center reports and outcomes after the use of various immunosuppressive regimens for pediatric intestinal transplantation
The current immunosuppressive protocol in place for intestinal transplantation at the Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center (CHP of UPMC) [Pittsburgh, PA, USA] includes induction with rATG followed by maintenance immunosuppression with tacrolimus and low dose corticosteroids. Lymphocyte ablation achieved with T-cell-depleting antibodies combined with minimization of post-transplant immunosuppression was introduced in 2001 and has significantly reduced rejection rates, lowered levels of immunosuppression, and advanced early and intermediate-term graft and patient survival in intestinal transplantation recipients.[26,31–34] T-cell-depleting antibodies primarily include rATG (Thymoglobulin®; Sangstat, Menlo Park, CA, USA) at a dose of 5 mg/kg, with alemtuzumab (Campath®; Genzyme Corporation, Cambridge, MA, USA) at a dose of 0.4 mg/kg (maximum of 30 mg) reserved for use in the sensitized patient or retransplantation. Results from the use of these agents have been promising — the rates of long-term patient and graft survival have significantly increased.[26–30]
At CHP, 213 children received 235 intestine-containing allografts between May 1990 and December 2009. Mean age at transplantation was 5.1 ± 5.3 years and mean follow-up time was 58.9 ± 53.4 months. The incidence of acute rejection post-transplantation was 79% in the cyclophosphamide plus daclizumab cohort (n = 39), and has decreased to 68% in the rATG (n = 104) cohort and 36% in the alemtuzumab (n = 20) cohort, compared with 82% in the cohort in which no induction agent (n = 50) was used. Twenty-eight of the 213 (13%) subjects developed evidence of chronic rejection. The frequency of opportunistic infections and PTLD has also decreased over time. The incidence of tissue infection with cytomegalovirus was 26% in the cyclophosphamide plus daclizumab cohort, decreasing to 13% in the rATG plus alemtuzumab cohort. PTLD associated with Epstein-Barr virus infection also decreased from 23% in the cyclophosphamide plus daclizumab cohort to 11% in rATG cohort. This reduction is a coalescence of many contributing factors, such as improved postoperative infection prophylaxis, serial monitoring of cytomegalovirus and Epstein-Barr virus viral loads, pre-emptive therapy with antiviral drugs, and reduction in immunosuppression when titers are elevated.
The overall patient and graft survival rates at CHP were 85% and 79%, respectively, at 1 year, 73% and 66% at 3 years, and 68% and 55% at 5 years after transplantation. The patient and graft survival rates in the current rATG plus alemtuzumab cohort (figure 6, table II) were 93% and 88%, respectively, at 1 year, 85% and 74% at 3 years, and 78% and 59% at 5 years after transplantation. The patient survival rate did not differ significantly by organ type (p = 0.50). The graft survival rates by organ type in the rATG plus alemtuzumab cohort were small bowel (48%), liver-small bowel (67%), and multivisceral (58%) at 5 years after transplantation; these differences were also not significant (p = 0.08).
Fig. 6.
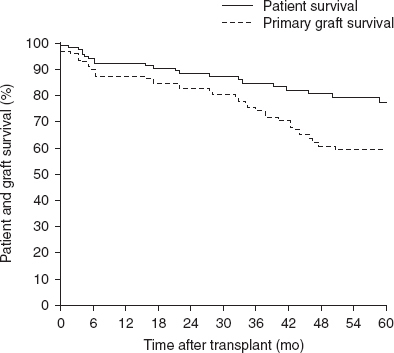
Patient and primary graft survival in the cohort of patients induced with rabbit antithymocyte globulin plus alemtuzumab at the Children’s Hospital of Pittsburgh (n = 124).
The University of Miami (Miami, FL, USA)[27] has reported their experience with the induction agents daclizumab and alemtuzumab. The factors associated with favorable survival were the use of induction with daclizumab, a multivisceral allograft with or without liver, the patient at home prior to transplant, and 1 year of age at transplant. Patients who received a multivisceral transplant had a lower mortality rate due to rejection, while older patients were associated with a lower risk of mortality following respiratory infections. Because of irreversible acute respiratory distress syndrome (ARDS)-like illness in three children induced with alemtuzumab, its use at their center since 2002 has been restricted to children 4 years of age and older.
Fishbein et al.[29,30] reported a decline in the incidence of early rejection in intestinal transplant recipients from 70% to 34% after the addition of sirolimus to their immunosuppressive regimen, consisting of the IL-2 receptor inhibitor, tacrolimus, and corticosteroids. There was also a significant increase (from 50% to 88%) in the 3-year actuarial graft survival rate with the use of sirolimus (table II). In subsequent reports,[29] sirolimus has been associated with anemia and wound dehiscence, and its role as a short-term adjunct to early tacrolimus immunosupression after intestinal transplantation or as a maintenance immunosuppressant agent remains to be defined.
Goulet et al.[28] described their experience with the induction agent basiliximab (Novartis, Basel, Switzerland) followed by maintenance immunosuppression with tacrolimus and corticosteroids. The overall 1- and 3-year graft survival rates were 58% and 52.5%, respectively; however, the graft survival for liver-small bowel allograft was significantly higher than for isolated small bowel allograft (table II). The rates of Epstein-Barr virus-related PTLD and cytomegalovirus disease were 15% and 19%, respectively, at their center. The important conclusions from this study included improved graft survival with the use of IL-2 receptor antibodies for induction as compared to those induced with tacrolimus and methylprednisolone alone; lower incidence and severity of acute rejection in recipients of liver-small bowel transplantation; no effect on survival after inclusion of the colon as a part of the allograft; and longer time required to achieve full enteral nutrition after combined liver-small bowel transplantation.
The Leuven multi-step protocol[35] was designed to enhance the generation of regulatory T cells that are hypothesized to play a major role in protection from rejection. The protocol involves donor and recipient intestine decontamination to prevent endotoxin translocation; protection of the mucosal intestinal barrier in both donors and recipients by administration of glutamine; a donor-specific blood transfusion at the time of transplantation to promote development of regulatory cells; induction with either IL-2 receptor antagonists or rATG; and maintenance immunosuppression with low-dose tacrolimus, azathioprine, and corticosteroids. Only seven patients have been treated with the Leuven pro-regulatory regimen and additional experience is required to evaluate the merits of the treatment.
2. Adverse Events
The improved efficacy achieved with tacrolimus-based regimens has led to a large cohort of surviving intestine transplant recipients. As a result, a growing population is being managed with long-term immunosuppression after intestinal transplantation. Minimizing the adverse effects of conventional immunosuppression, such as opportunistic infection, malignancy, and growth retardation, is of vital importance in this challenging pediatric population. Adverse effects of common immunosuppressant drugs used in pediatric intestinal transplantation are summarized in table I.
Current immunosuppressive strategies are aimed at reducing these adverse effects by evolving regimens that incorporate drug monitoring, combining different types of drugs in a novel way such that they complement each other, replacing one drug with another one exhibiting a different toxicity profile, and tailoring the immunosuppression according to appropriate clinical requirements.[17]
Lifelong therapeutic monitoring of tacrolimus blood concentrations is required to regulate drug levels and dosage in order to avoid suboptimal concentrations leading to graft rejection, or toxic levels potentially leading to adverse effects, including Epstein-Barr virus-related PTLD and nephrotoxicity. Also, in patients with refractory rejection despite optimal intensification of immunosuppression, the risk of over-immunosuppression, including opportunistic infections and malignancies, have to be balanced with the consequences of graft loss due to rejection. In some instances, enterectomy and re-transplantation[36] may be preferable to severe infectious or malignant adverse effects.
2.1 Nephrotoxicity
Chronic renal failure is one of the leading causes of post-transplant morbidity and mortality after intestinal transplantation. Ojo et al.[37] conducted a population-based cohort analysis involving recipients of solid organ transplants using the SRTR database to evaluate the incidence of chronic renal failure, defined as glomerular filtration rate (GFR) <29 mL/min/1.73 m2 or end-stage renal disease. Chronic renal failure was associated with a 4-fold increased risk of death after transplantation, and 5-year survivors of intestinal transplantation had a 21% incidence of chronic renal failure, which is the highest incidence rate among non-renal transplant recipients.
Ueno et al.[38] assessed renal function after pediatric intestinal transplantation and found that calculated GFR dropped significantly to 81% of the pre-transplantation value from 138 to 102 mL/min/1.73 m2, and elevated tacrolimus levels predicted the subsequent development of renal impairment at 2 years after pediatric intestinal transplantation. The same group also reported that the mean creatinine clearance decreased from a pre-transplant value of 114 mL/min/1.73m2 to 49.6 mL/min/1.73m2, and also found a significant association between cumulative tacrolimus levels >4500 ng ⋅ day/mL and a decreased creatinine clearance at 2 years after adult intestinal transplantation.[39]
The optimal treatment strategy for pediatric intestinal transplantation recipients with calcineurin toxicity is unknown, but should include consideration of mycophenolate mofetil, sirolimus, or azathioprine.
2.2 Infection
Sepsis is the leading cause of mortality after intestinal transplantation. Unique challenges[9] in this patient population include increased frequency of blood stream infections with enteric pathogens due to translocation from intestinal pathology, and relatively higher levels of required maintenance immunosuppression. Bacterial, fungal, and viral infections are common[27,33] and require a high index of suspicion and early intervention with appropriate antibiotics or antiviral therapy, and investigation for gastrointestinal pathology. Since children are more likely to be seronegative for cytomegalovirus and Epstein-Barr virus, routine Epstein-Barr virus polymerase chain reaction surveillance in blood is recommended to ensure early detection, treatment, and monitoring of infection.
2.3 Post-Transplant Lymphoproliferative Disorder
The use of intense immunosuppression has led to a high incidence of PTLD, with the highest rates reported among solid-organ recipients. As a result of the use of optimal maintenance immunosuppression, and careful monitoring and pre-emptive treatment for Epstein-Barr virus, the current incidence of PTLD is decreasing. Abu-Elmagd et al.[40] recently reported that de novo malignancy developed in 15% of patients, with a median diagnosis time of 5.5 months (range 1–116 months) after visceral transplantation, with PTLD occurring in 13% and non-lymphoid cancer in 3.2%. Children had a significantly higher risk of developing PTLD (37 of 52 [71%]), and adults were more vulnerable to non-lymphoid cancer (10 of 13 [77%]). PTLD occurred within the first postoperative year in 71% of patients, and the intestinal allograft was the primary site in 71% of recipients. Reyes et al.[41] have shown that the odds ratio for the development of PTLD decreased to 0.39, and death or graft loss due to PTLD decreased to 0.36 following the implementation of aggressive monitoring and pre-emptive treatment of Epstein-Barr virus. Other treatment modalities for advanced PTLD include the use of rituximab and chemotherapy.
2.4 Hematologic Toxicity
Clinicians should be aware of the risks and presentations of cytopenias, thrombotic microangiopathy, and hemolytic uremic syndrome in pediatric intestinal transplantation patients. A subset of intestinal transplantation patients may be prone to developing immune dysregulations, which can range from asymptomatic auto-antibodies to hematologic abnormalities.
Botija and colleagues[42] observed autoimmune cytopenia in 6 of 49 (12.2%) patients at the median time of 10 months after intestinal transplantation. Warm autoimmune-type hemolytic anemia developed in three patients, cold autoimmune-type hemolytic anemia developed in one patient, and two patients presented with a mixed type of hemolytic anemia. All patients were successfully treated with high-dose corticosteroids, intravenous immunoglobulin, and plasmapheresis. Tacrolimus was switched to sirolimus in four of six (66%) patients. The authors concluded that the immunosuppressant drugs used, associated Epstein-Barr virus infection, PTLD, graft-versus-host disease, and the inclusion of the spleen as part of the multivisceral graft are important risk factors for the development of autoimmune cytopenia.
Lacaille et al.[43] reported severe and potentially life-threatening dysimmune anemia and thrombocytopenia in six children after liver or small bowel transplantation, which was successfully treated with corticosteroids in three children, and rituximab in the other three. All children were on tacrolimus and low-dose alternate-day corticosteroids when dysimmune cytopenia developed. Dierickx et al.[44] reported three cases of intestinal transplantation-associated thrombotic microangiopathy, which was treated with tacrolimus withdrawal and plasmapheresis. Two of three patients were also receiving maintenance immunosuppression with sirolimus and corticosteroids. Paramesh et al.[45] reported two cases of thrombotic microangiopathy associated with combined sirolimus and tacrolimus immunosuppression after intestinal transplantation. One patient was treated with plasma exchange transfusions, reduction of tacrolimus dosage, and cessation of sirolimus, while the other was treated with plasma exchange transfusions, and replacement of tacrolimus and sirolimus with ciclosporin and mycophenolate mofetil, respectively. Humar et al.[46] reported two cases of hemolytic uremic syndrome after intestinal transplantation. One patient presented with renal failure; immunosuppression was changed from tacrolimus to ciclosporin, but renal function did not improve. A second patient presented with ischemic ulcers in the bowel mucosa secondary to occlusive thrombi in the allograft microcirculation, which was successfully managed by reducing the tacrolimus dose.
3. Special Considerations
3.1 Donor Pre-Conditioning
Donor pre-conditioning with muromonab CD3 (Don Mills, Toronto, ON, Canada), Minnesota antilymphocyte globulin, and methylprednisolone were used in the first successful liver-intestinal transplantation procedure in 1988 by Grant et al.[3] Other centers have used a combination of horse antithymocyte globulin (Fresenius, Munich, Germany), with or without murine anti-T-cell monoclonal antibody muromonab CD3 (Raritan, NJ, USA, and Novartis, Basel, Switzerland) for donor pre-conditioning.[47] A preliminary experimental study by Fryer et al.[48] in a rat model suggested that donor pre-treatment has the potential to reduce the incidence of acute cellular rejection by reducing the donor antigen load that is presented to the host. A significantly milder rejection with less arteritis and prolonged graft survival was seen in the allografts of the recipient macrophage-depleted group compared with non-depleted controls.
3.2 Treatment of Pre-Sensitized Patients
The first report to address the impact of a positive T-cell lymphocytotoxic crossmatch on intestinal allograft rejection and survival[49] found that 18% of intestinal recipients were harboring preformed anti-donor IgG lymphocytotoxic antibodies. Positive T-cell lymphocytotoxic crossmatch increased the frequency and severity of rejection after isolated intestinal transplantation. In our analysis of 103 consecutive primary small bowel transplantation recipients, 18% of recipients with a positive T-cell crossmatch and 21% of small-bowel transplantation recipients with complement-dependent cytotoxicity—anti-HLA panel reactive antibody (CDC-PRA) titers of >15% experienced graft loss.[50]
In an effort to improve post-transplant outcomes after intestinal transplantation, an intravenous immunoglobulin-based immunomodulation protocol for sensitized small bowel transplant candidates was studied by Gondolesi et al.[51] A stepwise protocol included level 1 treatment with intravenous immunoglobulin 200 mg/kg every other week for a total of two doses, level 2 treatment with intravenous immunoglobulin 500 mg/kg every other week for a total of two doses, level 3 treatment with intravenous immunoglobulin 1000 mg/kg with plasmapheresis three times per week if two venous access sites were available, otherwise mycophenolate mofetil (1 g twice daily), and level 4 treatment with rituximab 375 mg/m2 every other week for a total of four doses, with escalation to the next level of treatment if there was no significant decrease in PRA titers with the previous treatment. The transplant was performed regardless of the PRA titer if the crossmatch was negative. All transplanted patients responded to level 2 of therapy, and the mean pretreatment PRA titer in transplanted patients decreased from 66.2% to 25.5% at the end of treatment. Only one of six patients required level 4 treatment. This patient’s PRA titers remained >90%, representing treatment failure. The authors concluded that intravenous immunoglobulin treatment can desensitize patients on a waiting list, as evidenced by a decrease in PRA titers, resulting in excellent post-transplant outcomes. Although there was no significant decrease in the incidence of acute rejection between the two cohorts with low and high PRA, there was a significant decrease in the incidence of corticosteroid-resistant rejections.
Another potential innovative treatment is the use of the proteosomal inhibitor bortezomib (Velcade®; Millennium Pharmaceuticals, Cambridge, MA, USA), which could also provide a new option for desensitization of patients with a high level of donor-specific antibodies.[52] The efficacy of bortezomib as a part of desensitization protocols will require further study.
3.3 Anti-Rejection Therapy
Rejection remains common after intestine transplantation.[53,54] Rejection episodes are treated with a 30 mg/kg dose of methylprednisolone given intravenously in three divided doses over 3 days, or as a 10 mg/kg bolus on day 1 followed by tapering doses of 5, 4, 3, and 2 mg/kg on days 2, 3, 4 and 5, respectively. Corticosteroid-resistant rejection is typically treated with rATG (Thymoglobulin®; Sangstat, Menlo Park, CA, USA) after premedication with methylprednisolone, diphenhydramine, and acetaminophen (paracetamol) to attenuate tumor necrosis factor-α- and IL-1-mediated cytokine release syndrome.
4. Conclusions
As a result of continued efforts spanning 2 decades, the 10-year patient survival for intestine-only recipients (46%) is greater than that for heart-lung recipients (29%) and lung-only recipients (28.6%) but lags behind that for other solid organ transplants (2009 OPTN/SRTR annual report).[22]
At experienced centers, 5-year patient and graft survival rates approach 80% and 60%, respectively. The use of induction immunosuppression is associated with better long-term patient survival; however, achieving improved long-term (>10 year) graft survival remains a clinical priority.
Further research and development to improve the efficacy and tolerability of existing immunosuppressant drugs and protocols in children with an emphasis on the avoidance of long-term adverse events and long-term maintenance of graft function will challenge the transplant community and provide our patients with sustained benefits after intestinal transplantation.
Acknowledgements
The authors would like to acknowledge the work of Dale Zecca (Administrative Assistant) and Christine Heiner (Scientific Writer in the Department of Surgery, Children’s Hospital of Pittsburgh of UPMC) in the review and preparation of this manuscript. No sources of funding were used to prepare this manuscript. The authors have no conflicts of interest to declare that are directly relevant to the content of this review.
References
- 1.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: report of a case. Clin Transplant. 1989;3:89–91. [Google Scholar]
- 2.Goulet O, Revillon Y, Jan D, et al. Small-bowel transplantation in children. Transplant Proc. 1990;22:2499–500. [PubMed] [Google Scholar]
- 3.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–4. doi: 10.1016/0140-6736(90)90275-A. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172(5):335–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222(3):270–80. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVille D, Goyet J, Mitchell A, et al. En block combined reduced liver and small bowel transplants: from large donors to small children. Transplantation. 2000;69:555–9. doi: 10.1097/00007890-200002270-00016. [DOI] [PubMed] [Google Scholar]
- 7.Bueno J, Abu-Elmagd K, Mazariegos G, et al. Composite liver-small bowel allografts with preservation of donor duodenum and hepatic biliary system in children. J Pediatr Surg. 2000;35:291–5. doi: 10.1016/S0022-3468(00)90027-7. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Elmagd K, Fung J, Bueno J, et al. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann Surg. 2000;232(5):680–7. doi: 10.1097/00000658-200011000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green M, Bueno J, Sigurdsson L, et al. Unique aspects of the infectious complications of intestinal transplantation. Curr Opin Organ Transpl. 1999;4:361–7. doi: 10.1097/00075200-199912000-00011. [DOI] [Google Scholar]
- 10.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of experience at a single center. Ann Surg. 2001;234(4):404–17. doi: 10.1097/00000658-200109000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes J, Mazariegos G, Bond G, et al. History of pediatric organ transplantation: pediatric intestinal transplantation: historical notes, principles and controversies. Pediatr Transplant. 2002;6(3):193–207. doi: 10.1034/j.1399-3046.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Elmagd K, Reyes J, Todo S, et al. Clinical intestinal transplantation: new perspectives and immunologic considerations. J Am Coll Surg. 1998;186(5):512–27. doi: 10.1016/S1072-7515(98)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinna AD, Weppler D, Nery JR, et al. Induction therapy for clinical intestinal transplantation: comparison of four different regimens. Transplant Proc. 2000;32(6):1193–4. doi: 10.1016/S0041-1345(00)01179-9. [DOI] [PubMed] [Google Scholar]
- 14.Pineda J, Mazariegos GV. Post-transplantation lymphoproliferative disorder (PTLD) in liver and small bowel transplant recipients. In: Dharnidharka VR, Green M, Webber SA, editors. Post-transplantation lymphoproliferative disorders. Berlin, Heidelberg: Springer-Varlag; 2010. pp. 153–62. [Google Scholar]
- 15.Intestinal Transplant Association (ITA) [online]. Available from URL: http://www.intestinaltransplant.org [Accessed 2011 Feb 15]
- 16.Parizhskaya M, Redondo C, Demetris A, et al. Chronic rejection of small bowel grafts: a pediatric and adult study on risk factors and morphologic progression. Pediatr Dev Pathol. 2003;6(3):240–50. doi: 10.1007/s10024-002-0039-4. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hussaini A, Tredger JM, Dhawan A. Immunosuppression in pediatric liver and intestinal transplantation: a closer look at the arsenal. J Pediatr Gas- troenterol Nutr. 2005;41:152–65. doi: 10.1097/01.mpg.0000172260.46986.11. [DOI] [PubMed] [Google Scholar]
- 18.Tredger JM, Brown NW, Dhawan A. Immunosuppression in pediatric solid organ transplantation: opportunities, risks, and management. Pediatr Transplantation. 2006;10:879–92. doi: 10.1111/j.1399-3046.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 19.Reyes J, Mazariegos GV, Abu-Elmagd K, et al. Intestinal transplantation under tacrolimus monotherapy after perioperative lymphoid depletion with rabbit antithymocyte globulin (thymoglobulin) Am J Transplant. 2005;5:1430–6. doi: 10.1111/j.1600-6143.2005.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debray D, Furlan V, Baudouin V, et al. Therapy for acute rejection in pediatric organ transplant recipients. Pediatr Drugs. 2003;5(2):81–93. doi: 10.2165/00128072-200305020-00002. [DOI] [PubMed] [Google Scholar]
- 21.LaCorcia G, Swistak M, Lawendowski C, et al. Polyclonal rabbit antithymocyte globulin exhibits consistent immunosuppressive capabilities beyond cell depletion. Transplantation. 2009;87(7):966–74. doi: 10.1097/TP.0b013e31819c84b8. [DOI] [PubMed] [Google Scholar]
- 22.2009 OPTN/SRTR annual report: transplant data 2009. Annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Rockville (MD): US Department of Health and Human Services, Health Resources and Services Administration, Health Care Systems Bureau, Division of Transplantation, 2009
- 23.Mazariegos GV, Steffick DE, Horslen S, et al. Intestine transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4):1020–34. doi: 10.1111/j.1600-6143.2010.03044.x. [DOI] [PubMed] [Google Scholar]
- 24.Tzakis AG, Kato T, Nishida S, et al. Alemtuzumab (Campath-1H) combined with tacrolimus in intestinal and multivisceral transplantation. Transplantation. 2003;75:1512–7. doi: 10.1097/01.TP.0000060250.50591.39. [DOI] [PubMed] [Google Scholar]
- 25.Tzakis AG, Kato T, Nishida S, et al. Preliminary experience with campath 1H (C1H) in intestinal and liver transplantation. Transplantation. 2003;75:1227–31. doi: 10.1097/01.TP.0000065192.53065.50. [DOI] [PubMed] [Google Scholar]
- 26.Bond GJ, Mazariegos GV, Sindhi R, et al. Evolutionary experience with immunosuppression in pediatric intestinal transplantation. J Pediatr Surg. 2005;40:274–9. doi: 10.1016/j.jpedsurg.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Tzakis AG, Selvaggi G, et al. Intestinal and multivisceral transplantation in children. Ann Surg. 2006;243(6):756–64. doi: 10.1097/01.sla.0000219696.11261.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulet O, Sauvat F, Ruemmele F, et al. Results of the paris program: ten years of pediatric intestinal transplantation. Transplant Proc. 2005;37:1667–70. doi: 10.1016/j.transproceed.2005.03.153. [DOI] [PubMed] [Google Scholar]
- 29.Fishbein TM, Florman S, Gondolesi G, et al. Intestinal transplantation before and after the introduction of sirolimus. Transplantation. 2002;73(10):1538–42. doi: 10.1097/00007890-200205270-00004. [DOI] [PubMed] [Google Scholar]
- 30.Fishbein TM, Kaufman SS, Florman SS, et al. Isolated intestinal transplantation: proof of clinical efficacy. Transplantation. 2003;76(4):636–40. doi: 10.1097/01.TP.0000083042.03188.6C. [DOI] [PubMed] [Google Scholar]
- 31.Starzl TE. Immunosuppressive therapy and tolerance of organ allografts. N Engl J Med. 2008;358(4):407–11. doi: 10.1056/NEJMe0707578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Zinkernagel RM. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;1(3):233–9. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abu-Elmagd KM, Costa G, Bond GJ, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg. 2009;250:567–81. doi: 10.1097/SLA.0b013e3181b67725. [DOI] [PubMed] [Google Scholar]
- 34.Mazariegos GV, Squires RH, Sindhi RK. Current perspectives on pediatric intestinal transplantation. Curr Gastroenterol Rep. 2009;11:226–33. doi: 10.1007/s11894-009-0035-1. [DOI] [PubMed] [Google Scholar]
- 35.Pirenne J, Kawai M. Intestinal transplantation: evolution in immunosuppression protocols. Curr Opin Organ Transplant. 2009;14(3):250–5. doi: 10.1097/MOT.0b013e32832b2eb7. [DOI] [PubMed] [Google Scholar]
- 36.Mazariegos GV, Soltys K, Bond G, et al. Pediatric intestinal retransplantation: techniques, management, and outcomes. Transplantation. 2008;86(12):1777–82. doi: 10.1097/TP.0b013e3181910f51. [DOI] [PubMed] [Google Scholar]
- 37.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 38.Ueno T, Kato T, Gaynor J, et al. Renal function after pediatric intestinal transplant. Transplant Proc. 2006;38(6):1759–61. doi: 10.1016/j.transproceed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Ueno T, Kato T, Gaynor J, et al. Renal dysfunction following adult intestinal transplant under tacrolimus-based immunosuppression. Transplant Proc. 2006;38(6):1762–4. doi: 10.1016/j.transproceed.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 40.Abu-Elmagd KM, Mazariegos G, Costa G, et al. Lymphoproliferative disorders and de novo malignancies in intestinal and multivisceral recipients: improved outcomes with new outlooks. Transplantation. 2009;88(7):926–34. doi: 10.1097/TP.0b013e3181b7509c. [DOI] [PubMed] [Google Scholar]
- 41.Reyes J, Mazariegos GV, Bond GM, et al. Pediatric intestinal transplantation: historical notes, principles and controversies. Pediatr Transplant. 2002;6(3):193–207. doi: 10.1034/j.1399-3046.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 42.Botija G, Ybarra M, Ramos E, et al. Autoimmune cytopaenia after paediatric intestinal transplantation: a case series. Transpl Int. 2010;23(10):1033–7. doi: 10.1111/j.1432-2277.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 43.Lacaille F, Moesb N, Hugotc J-P. Severe dysimmune cytopenia in children treated with tacrolimus after organ transplantation. Am J Transplant. 2006;6:1072–6. doi: 10.1111/j.1600-6143.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 44.Dierickx D, Monbaliu D, De Rycke A, et al. Thrombotic microangiopathy following intestinal transplantation: a single center experience. Transplant Proc. 2010;42(1):79–81. doi: 10.1016/j.transproceed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Paramesh AS, Grosskreutz C, Florman SS, et al. Thrombotic microangiopathy associated with combined sirolimus and tacrolimus immunosuppression after intestinal transplantation. Transplantation. 2004;77(1):129–31. doi: 10.1097/01.TP.0000092522.36410.D0. [DOI] [PubMed] [Google Scholar]
- 46.Humar A, Jessurun J, Sharp HL. Hemolytic uremic syndrome in small bowel transplant recipients: the first two case reports. Transpl Int. 1999;12(5):387–90. doi: 10.1111/j.1432-2277.1999.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 47.Langnas AN, Shaw BW, Jr, Antonson DL, et al. Preliminary experience with intestinal transplantation in infants and children. Pediatrics. 1996;97(4):443–8. [PubMed] [Google Scholar]
- 48.Fryer J, Grant D, Jiang J, et al. Influence of macrophage depletion on bacterial translocation and rejection in small bowel transplantation. Transplantation. 1996;15:553–9. doi: 10.1097/00007890-199609150-00002. [DOI] [PubMed] [Google Scholar]
- 49.Bond G, Reyes J, Mazariegos G, et al. The impact of positive T-cell lymphocytotoxic crossmatch on intestinal allograft rejection and survival. Transplant Proc. 2000;32(6):1197–8. doi: 10.1016/S0041-1345(00)01181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sindhi R, AshokKumar C, Mazariegos G, et al. Immune monitoring in small bowel transplantation. Curr Opin Organ Transplant. 2010;15(3):349–56. doi: 10.1097/MOT.0b013e328339489c. [DOI] [PubMed] [Google Scholar]
- 51.Gondolesi G, Blondeau B, Aurette R, et al. Pretransplant immunomodulation of highly sensitized small bowel transplant candidates with intravenous immune globulin. Transplantation. 2006;81(12):1743–6. doi: 10.1097/01.tp.0000226078.94635.76. [DOI] [PubMed] [Google Scholar]
- 52.Everly MJ. Clin Transpl. 2009. A summary of bortezomib use in transplantation across 29 centers; pp. 323–37. [PubMed] [Google Scholar]
- 53.Ishii T, Mazariegos GV, Bueno J, et al. Exfoliative rejection after intestinal transplantation in children. Pediatr Transplant. 2003;7(3):185–91. doi: 10.1034/j.1399-3046.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 54.Nayyar N, Mazariegos G, Ranganathan S, et al. Pediatric small bowel transplantation. Semin Pediatr Surg. 2010;19(1):68–77. doi: 10.1053/j.sempedsurg.2009.11.009. [DOI] [PubMed] [Google Scholar]


