Abstract
Systemic administration of perfluorocarbons (PFCs) reportedly attenuates acute lung injury induced by acid aspiration and phorbol myristate acetate. However, the effects of PFCs on ischemia–reperfusion (IR)-induced lung injury have not been investigated. Typical acute lung injury was induced in rats by 60 min of ischemia and 60 min of reperfusion in isolated and perfused rat lung model. Rat lungs were randomly assigned to receive PBS (control), 1 % FC-77, IR only, or IR with different doses of FC-77 (0.1 %, 0.5 %, or 1 %). Subsequently, bronchoalveolar lavage fluid (BALF), perfusate, and lung tissues were collected to evaluate the degree of lung injury. IR caused a significant increase in the following parameters: pulmonary arterial pressure, capillary filtration coefficient, lung weight gain, lung weight/body weight ratio, wet/dry lung weight ratio, and protein concentration in BALF. TNF-α and cytokine-induced neutrophil chemoattractant-1 concentrations in perfusate samples and MDA concentration and MPO activities in lung tissues were also significantly increased. Histopathology showed increased septal thickness and neutrophil infiltration in the lung tissues. Furthermore, NF-κB activity was significantly increased in the lungs. However, pretreatment with 1 % FC-77 prior to IR significantly attenuated the increases in these parameters. In conclusion, our results suggest that systemic FC-77 administration had a protective effect on IR-induced acute lung injury. These protective mechanisms may have been mediated by the inhibition of NF-κB activation and attenuation of subsequent inflammatory response.
KEY WORDS: ischemia–reperfusion, acute lung injury, perfluorocarbon, FC-77
INTRODUCTION
Perfluorocarbons (PFCs) are inert chemicals having high density and good oxygen-dissolving capacity; moreover, they are highly hydrophobic and lipophobic [1]. Because of these advantages, liquid ventilation with PFCs was initially shown to improve acute lung injury in animal models and a few human studies. Important mechanisms that attenuate lung inflammation include the distention of collapsed alveoli to improve lung compliance, improvement of oxygenation by increasing oxygen diffusion, and removal of inflammatory mediators from the alveolar regions of the lung [2–4]. However, two subsequent large clinical trials of patients with acute respiratory distress syndrome (ARDS) showed that liquid ventilation was not beneficial for improving survival [5, 6].
Ischemia–reperfusion (IR) lung injury is a common complication of lung transplantation procedures, pulmonary embolism, shock, and trauma, and it results in increased microvascular permeability and neutrophil infiltration in the lung tissue, damaged pulmonary endothelium, and pulmonary hypertension [7, 8]. Despite advances in intensive care, ARDS-associated morbidity and mortality remain high [7, 8]. Therefore, it is important to develop new strategies to treat these potentially reversible pulmonary injuries.
The results of a number of recent in vitro and in vivo studies have suggested that PFCs have anti-inflammatory effects that may directly modulate inflammatory cell function and decrease the production of inflammatory mediators [9–12]. Emulsified PFCs can be safely injected into the blood system of humans as a blood substitute [13]. Emulsified PFCs have also been used as part of management of a variety of diseases, including polytrauma, anemia, burns, hemorrhagic shock, toxic infectious shock, and blood vessel occlusion [14].
During the early stage of acute lung injury, the origin of activated neutrophils is intravascular and not intra-alveolar; therefore, intravascularly administered PFCs will make direct contact with inflammatory cells and may be a more potent means to decrease harmful inflammatory reactions. In addition, systemic FC-77 administration has been shown to attenuate acute lung injury induced by acid aspiration and phorbol myristate acetate (PMA) [15, 16]. Although PFCs reportedly limit the infarction size in IR heart injury [17, 18], the beneficial effects of FC-77 on IR-induced acute lung injury remain unclear. Therefore, the present study was designed to determine whether systemic FC-77 administration ameliorates acute lung injury induced by IR. In this experiment, we used a well-established model of isolated rat lung because it enabled us to measure the pulmonary filtration coefficient (K f), which is the most accurate indicator of pulmonary capillary permeability [19].
MATERIALS AND METHODS
Preparation of Isolated and Perfused Rat Lungs
We prepared the isolated and perfused rat lungs in situ as previously described [20, 21]. The animals used for this study were cared for in accordance with the guidelines of the National Institutes of Health (National Academy Press, 1996), and approval for our study protocol was obtained from the National Science Council and Animal Review Committee of the National Defense Medical Center. Male Sprague–Dawley rats (weight 250–350 g) were anesthetized with intraperitoneal injections of pentobarbital sodium (20–25 mg/rat). Tracheostomy was performed to enable ventilation with a rodent ventilator (7025; Ugo Basile, Comerio, VA, Italy). The rat lungs were ventilated with 5 % CO2 in air at 65–70 breaths/minute with a tidal volume of 2 mL. After a median sternotomy was performed, heparin (1 U/g of body weight) was injected into the right ventricle, from which 10 mL of blood was collected. This blood sample was mixed with 10 mL of normal saline containing 1.5 % human serum albumin. This was subsequently used as a perfusing fluid for the isolated lungs. For constant-flow perfusion of the isolated lungs, a cannula was inserted into the pulmonary artery via a right-ventricular puncture. A tight ligature was placed around the main trunk of the pulmonary artery. A wide-bore cannula was inserted into the left atrium via the left ventricle to divert pulmonary venous outflow into a reservoir. The wide-bore cannula was then fixed with a ligature at the apex of the heart. Another ligature was placed above the atrioventricular junction to prevent the flow of the perfusate into the ventricles. Both the pulmonary arterial pressure (PAP) and the pulmonary venous pressure (PVP) were recorded from side arms of the inflow and outflow cannula. A roller pump was used to provide constant perfusion flow at a rate of approximately 8–10 mL/min to stabilize PAP at 15–20 cmH2O. PVP was set at 4–6 cmH2O by adjusting the height of the venous reservoir. With the isolated perfused lungs remaining in situ, the whole rat was placed on an electronic balance. The digital signals of the electronic balance were converted to analog signals with a digital-to-analog converter and were recorded on an oscillograph recorder. Isolated lung preparations were used in this study only if they satisfied three criteria: (1) no leakage at the sites of cannula insertion, (2) no evidence of bleeding or edema, and (3) an isogravimetric state.
Induction of Lung Ischemia and Reperfusion
The lungs were then subjected to 60 min of ischemia by stopping ventilation and perfusion. After this period of ischemia, the lungs were reperfused for 60 min and ventilated with 5 % CO2–95 % air.
Microvascular Permeability
K f, which is an index of microvascular permeability to water, was determined from the lung weight change caused by increased venous pressure. During ventilation and lung perfusion, PVP was rapidly elevated by 10 cmH2O for at least 7 min. The slow, steady phase of weight gain as a function of time (ΔW/ΔT) was plotted on a semi-logarithmic paper. The slow component was then extrapolated to zero time to obtain the initial rate of transcapillary filtration. From this plot, K f was defined as the y-intercept (g min−1) divided by PVP (10 cmH2O) and lung weight. K f was expressed in units of grams per minute of cmH2O−1 × 100 g [20, 21].
Determinations of Lung Weight/Body Weight (LW/BW) and Wet/Dry Lung Weight (W/D) Ratios
After experiments, the right lung was removed from the hilar region and the wet weight was determined to calculate the LW/BW ratio. A part of the right upper lung lobe was placed in an oven at 60 °C for 48 h to determine the W/D lung weight ratio.
Protein Concentration in Bronchoalveolar Lavage Fluid (BALF)
BALF was obtained at the end of the experimental by irrigating the left lung with saline (2 × 2.5 mL). This fluid was centrifuged at 250×g for 10 min, and the protein concentration in the supernatant was determined using a BCA protein assay (Pierce, Rockford, IL, USA).
Measurement of TNF-α and Cytokine-Induced Neutrophil Chemoattractant (CINC)-1 Concentrations in the Perfusate
TNF-α and CINC-1 concentrations in the perfusate after the experiment were determined using an ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
Lung Malondialdehyde Concentration
Lung tissue was homogenized in a 1.15 % KCL solution. An aliquot (100 μL) of the homogenate was added to a reaction mixture containing 200 μL of 8.1 % thiobarbituric acid and 700 μL of distilled water. Samples were then boiled for 30 min at 100 °C and centrifuged at 3,000×g for 10 min. The absorbance of the supernatant was measured spectrophotometrically at 532 nm.
Lung Myeloperoxidase Activity
A part of the right lower lung lobe was freeze–thawed and sonicated three times. Homogenates were centrifuged at 15,000×g for 10 min at 4 °C. An aliquot (100 μL) of the supernatant was mixed with 900 μL of 50 mM phosphate buffer (pH 6.0) containing 0.167 mg/mL of o-dianisidine dihydrochloride and 0.0005 % hydrogen peroxide. One unit of peroxidase activity was defined as the amount of enzyme that decomposed 1 μmol of hydrogen peroxide per minute at 25 °C. Hydrogen peroxide decomposition was determined from the oxidation of o-dianisidine using an absorption coefficient of 11.3 mM cm at 460 nm.
NF-κB Activity
NF-κB activity was assessed by the nuclear translocation and DNA binding of the p65 subunit in lung tissues, using a commercially available ELISA kit (TransAM NF-κB p65; Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Histological Assessment
A part of the right lower lung lobe was stained with hematoxylin and eosin. The histopathologic assessment was performed by two pathologists blinded to the experimental condition. For each section, 10 random areas were examined at a magnification of ×400. Within each field, lung injury was scored according to (1) infiltration or aggregation of neutrophils in the airspace or vessel wall, and (2) thickness of the alveolar wall. Each assessment was graded on the following four-point scale: 0, 1, 2, or 3, for no, mild, moderate, or severe injury, respectively. The resulting two scores were added and presented as the lung injury score for that section [22].
Experimental Protocol
An isolated lung preparation was allowed to equilibrate for 20 min. We recorded baseline PAP, PVP, and weight change and determined the initial K f for 7 min. Then, all parameters were allowed to return to their baseline values for 10 min. Rat lungs were randomly assigned to receive PBS (control, n = 6), 1 % FC-77 (drug control, n = 6), IR only, or IR with different doses of FC-77 (0.1 %, 0.5 %, or 1 %; n = 6/per group). FC-77 (chemical formula, C8F18; purity, 100 %; 3M Company, St. Paul, MN, USA) was added to the reservoir (containing 20 mL of perfusate). Then the lungs were perfused and ventilated for 60 min following IR, and the K f measurement was repeated. Due to the hydrophobic property of FC-77, FC-77 was premixed with 3 mL of perfusate (drawn from the reservoir) and vortexed for several seconds before adding to the reservoir.
Statistical Analysis
Results are expressed as mean ± SEM. Group comparisons were made by repeated measures one-way or two-way ANOVA, followed by post hoc comparisons using a Newman–Keuls test. Comparisons within each group for a given variable were made by paired Student’s t tests. A P value of <0.05 was considered statistically significant.
Results
PAP
PAP was significantly increased in the IR group than in the control group (Fig. 1a). Pretreatment with FC-77 at different concentrations tended to attenuate the increase in PAP induced by IR; however, the attenuation was significant only in the 1 % FC-77 group when compared with that in the IR group (P < 0.05).
Fig. 1.
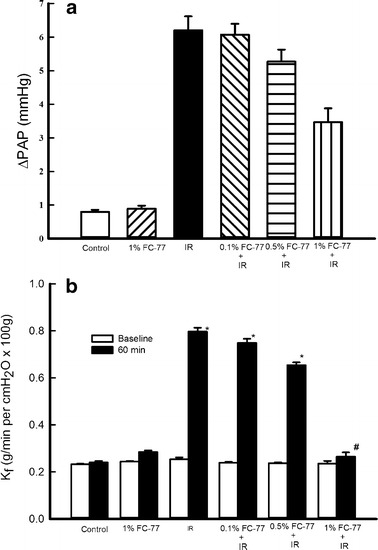
Effects of FC-77 pre-treatment on pulmonary hypertension and the pulmonary vasculature filtration coefficient (K f). a Ischemia–reperfusion (IR) caused a significant increase in pulmonary arterial pressure (ΔPAP). Pretreatment with 1 % FC-77 significantly attenuated the increase in PAP after IR. b IR significantly increased K f compared with that at baseline, although K f did not change after 120 min of perfusion in the control group. The increase in K f was significantly attenuated in the 1 % FC-77 group compared with that in the IR group. *P < 0.05 versus control group; # P < 0.05 versus IR group. Open squares, baseline; filled squares, 60 min.
Lung Filtration Coefficient
Figure 1b shows the microvascular permeability changes (expressed as K f) in the isolated rat lungs due to IR at different doses of FC-77. K f (P < 0.05) significantly increased after 120 min of IR, whereas K f did not change after 120 min of perfusion in the control group. Pretreatment with FC-77 at different concentrations tended to attenuate the increase in K f; however, significant attenuation was seen only in the 1 % FC-77 group when compared with that in the IR group (P < 0.05).
Lung Weight Gain
The lung weights in the control group remained essentially constant during the 120-min experimental period (Fig. 2). In contrast, IR caused a progressive increase in lung weight. The lung weight change expressed as the lung weight gain was significantly higher in the IR group than in the control group (P < 0.05). The decrease in lung weight gain was statistically significant in the 1 % FC-77 (P < 0.05) group, but not in the 0.1 % or 0.5 % FC-77 groups (P > 0.05), when compared with that in the IR group.
Fig. 2.
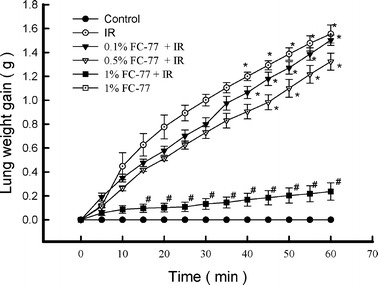
Changes in lung weight gain (LWG) during the experimental period. Ischemia–reperfusion (IR) caused a significant increase in LWG. Pretreatment with 1 % FC-77 significantly attenuated the increase in LWG after IR. *P < 0.05 versus control group; # P < 0.05 versus IR group.
LW/BW and Wet/Dry Ratios
The LW/BW and W/D ratios were significantly increased in the IR group compared with those in the control group (Fig. 3, P < 0.05). These ratios tended to decrease in the 0.1 % and 0.5 % FC-77 groups, albeit with no statistical significance when compared with those in the IR group. However, the LW/BW and W/D ratios significantly decreased in the 1 % FC-77group compared with those in the IR group (P < 0.05).
Fig. 3.
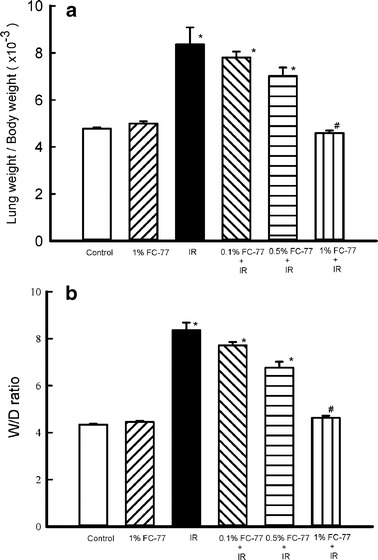
Changes in lung weight/body weight (LW/BW) and wet/dry (W/D) lung weight ratios. Ischemia–reperfusion (IR) caused significant increases in the LW/BW and W/D lung weight ratios. The increase in these ratios was significantly attenuated in the 1 % FC-77 group compared with that in the IR group. *P < 0.05 versus control group; # P < 0.05 versus IR group.
Protein Concentration in BALF
Protein concentration in BALF was significantly higher in the IR group than in the control group (Fig. 4, P < 0.05). A significant protective effect was observed in the 0.5 % and 1 % FC-77 groups (P < 0.05), but not in the 0.1 % FC-77 group (P > 0.05), when compared with the IR group.
Fig. 4.
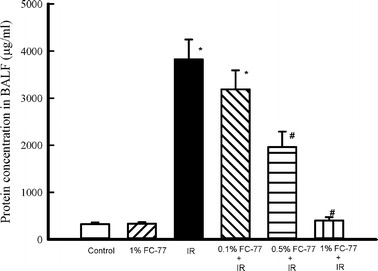
Effect of FC-77 on the protein concentration in bronchoalveolar lavage fluid (BALF). Ischemia–reperfusion (IR) caused a significant increase in the protein concentration in BALF. The increase in the BALF protein concentration was significantly attenuated in the 1 % FC-77 group compared with that in the IR group. *P < 0.05 versus control group; # P < 0.05 versus IR group.
TNF-α and CINC-1 Concentrations in Perfusates
TNF-α and CINC-1 concentrations in perfusates significantly increased in the IR group compared with those in the control group (P < 0.05). Although pretreatment with 0.5 % FC-77 tended to attenuate this increase, the attenuation was not statistically significant when compared with that in the IR group. On the other hand, the attenuation was significant in the 1 % FC-77 group when compared with that in the IR group (P < 0.05; Fig. 5).
Fig. 5.
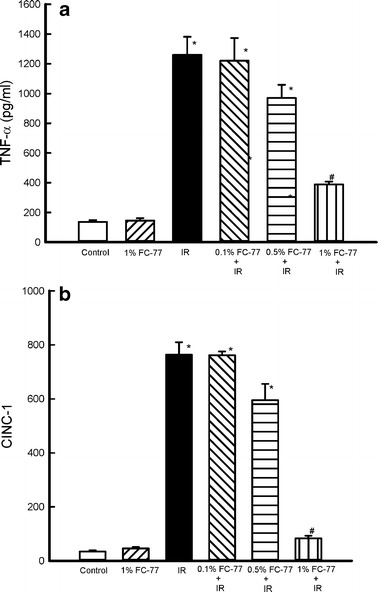
Effect of FC-77 on TNF-α and cytokine induced neutrophil chemoattractant (CINC)-1 concentrations in perfusates. TNF-α and CINC-1 concentrations in perfusates were significantly increased in the ischemia–reperfusion (IR) group compared with those in the control group. These increases were significantly attenuated in the 1 % FC-77 group compared with those in the IR group. *P < 0.05 versus control group; # P < 0.05 versus IR group.
MDA Concentration and MPO Activity in Lung Tissue
As shown in Fig. 6, MDA concentration and MPO activity in lung tissues significantly increased in the IR group compared with those in the control group (P < 0.05). Pretreatment with 0.1 % and 0.5 % FC-77 tended to attenuate these increases, although the attenuation was not statistically significant when compared with that in the IR group. However, MDA concentration and MPO activity were significantly attenuated in the 1 % FC-77 group compared with those in the IR group (P < 0.05).
Fig 6.
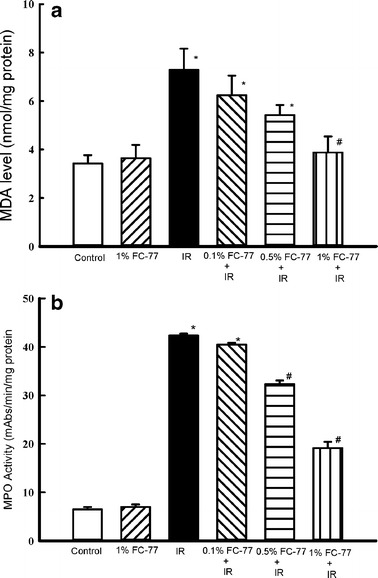
Effect of FC-77 on MDA concentration and MPO activity in lung tissue. Ischemia–reperfusion (IR) significantly increased MDA concentration and MPO activity in lung tissue. These increases were significantly attenuated in the 1 % FC-77 group compared with those in the IR group. *P < 0.05 versus control group; # P < 0.05 versus IR group.
NF-κB Activity
NF-κB activity in the lungs was significantly increased after IR (Fig. 7). NF-κB activity in the lungs tended to decrease in the 0.1 % and 0.5/% FC-77 groups compared with that in the IR group, although the difference was not statistically significant. However, the attenuation was significant in the 1 % FC-77 group when compared with that in the IR group (P < 0.05).
Fig. 7.
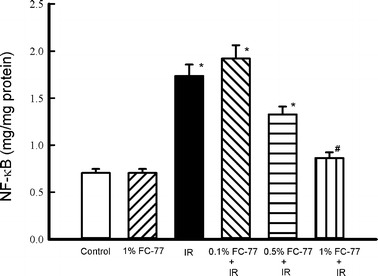
NF-κB p65 activity assay. NF-κB activity was significantly increased in the ischemia–reperfusion (IR) group compared with that in the control group. This increase in NF-κB activity was significantly attenuated in the 1 % FC-77 group compared with that in the IR group. *P < 0.05 versus control group; # P < 0.05 versus IR group.
Histopathological Findings
The control group showed normal lung tissue architecture and no inflammation (Fig. 8a), whereas the IR group exhibited septal thickening and marked inflammatory cell infiltration in the interstitium and alveoli (Fig. 8b). Lung inflammation was significantly lower in the 1 % FC-77 group than in the IR group (Fig. 8c). Lung injury scores also provided evidence that pretreatment with 1 % FC-77 significantly attenuated IR-induced acute lung injury (Fig. 8d; P < 0.05).
Fig. 8.
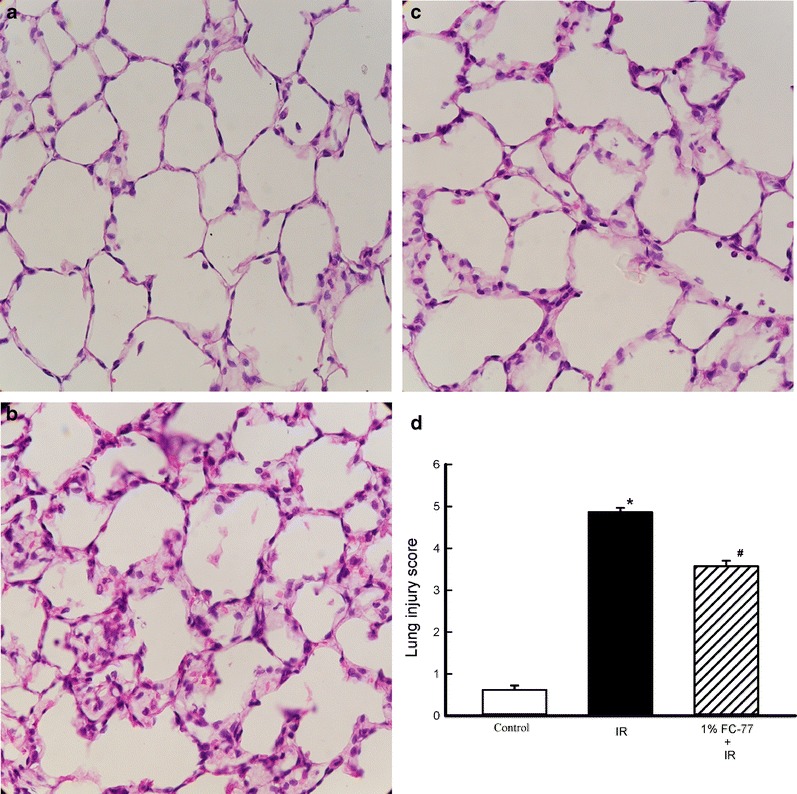
Histological appearance of lung tissue. a Control group (×400). b ischemia–reperfusion (IR) (×400). c 1 % FC-77 administration prior to IR (×400). Pretreatment with 1 % FC-77 significantly improved the lung pathology compared with that in the IR group. d Histopathological scores for lung injury. The lung injury scores were significantly decreased in the 1 % FC-77 group compared with those in the IR group. *P < 0.05 versus control group; # P < 0.05 versus IR group.
DISCUSSION
In this study, we demonstrated that pretreatment with FC-77 had beneficial effects on IR-induced increases in PAP, K f, lung weight gain, LW/BW ratio, W/D lung ratio, protein concentration in BALF, and TNF-α and CINC-1 concentrations in perfusate; and MDA concentration, MPO activity, and neutrophil infiltration in lung tissues. In addition, FC-77 pretreatment also inhibited NF-κB activation in the lungs. The protective effects of FC-77 may be mediated by the inhibition of NF-κB activation and attenuation of inflammatory responses in the lungs.
The major characteristic of IR-induced acute lung endothelial injury is an increase in pulmonary microvascular permeability. In this study, we demonstrated that FC-77 pretreatment could attenuate the increase in vascular permeability on the basis of evaluations of different variables, including K f, W/D lung ratio, LW/BW ratio, and protein concentration in BALF. These findings were consistent with those of previous reports where PFCs attenuated endothelial injury in acid and PMA-induced acute lung injury [15, 16].
Studies of IR-induced lung injury in animal models have confirmed that sequestration of neutrophils plays an important role in IR-induced lung injury [23]. Activation of neutrophils and their adhesion to endothelial cells are observed during the initial inflammatory response. Activated neutrophils that infiltrate the lung release reactive oxygen species, proteases, cytokines, and vasoconstricting lipids; upregulate the expression of adhesion molecules; and subsequently cause lung injury [23]. An in vitro study showed that PFCs could diffuse from the alveolar space into an adjacent pulmonary vascular endothelial layer that modulated neutrophil adhesion and decreased the number of activated neutrophils entering the injured lung [24]. However, whether PFCs had the same effects in vivo needed to be confirmed.
A previous in vitro study reported that PFCs decreased the production of hydrogen peroxide and superoxide anion by rabbit and piglet alveolar macrophages after chemical stimulation [12]. Steinhorn et al. also demonstrated that PFCs attenuated oleic acid-induced lung injury by reducing the production of reactive oxygen species [25]. PFCs have also been shown to inhibit neutrophil activation and chemotaxis via decreased Syk phosphorylation [26, 27]. Furthermore, systemic administration of PFCs can present a barrier function and prevent direct contact between neutrophils and endothelial cells in vessels [17]. In this study, these characteristics may have contributed to the capability of systemic FC-77 to attenuate MPO activity and lipid peroxidation in the lung and decrease the intensity of the inflammatory response. These results are in agreement with recent findings of decreased neutrophil accumulation, MPO activity, and oxidative damage in lung tissues by systemic PFC administration in a rat lung injury model [15].
TNF-α and IL-8 are early response mediators in the pathophysiology of acute lung injury and ARDS [28]. Previous investigations reported that anti-TNF-α or IL-8 antibodies significantly attenuated IR-induced acute lung injury [29, 30]. PFCs can reduce the production of various inflammatory cytokines in vitro and in vivo, such as TNF-α and IL-8 [9, 11, 31–34]. In this study, the decreased pulmonary inflammation may have been caused, in part, by decreased early production of TNF-α and IL-8.
NF-κB is a key transcription factor that is activated by a number of stimuli, including hypoxia, ischemia, inflammatory cytokines, chemokines, and oxygen radicals [35]. In addition, the promoter regions of many cytokines such as TNF-α and IL-8 are controlled by NF-κB. NF-κB activation amplifies an early inflammatory response and exacerbates tissue injury [35]. Previous studies demonstrated that inhibition of NF-κB activity decreased lung reperfusion injury in porcine and rabbit models [36, 37]. The inhibition was associated with the suppression of cytokine production and neutrophil infiltration [37]. PFCs have also been shown to inhibit NF-κB activation in LPS-stimulated macrophages, Chlamydophila pneumoniae-mediated pneumocytes, and models of respiratory syncytial virus-induced lung inflammation [15, 34, 38]. Therefore, our results are compatible with those studies.
Although the anti-inflammatory mechanisms of PFCs are not clear, most investigators initially considered that PFCs acted as a physical barrier on cell surfaces to prevent ligand–receptor-induced signal transduction and direct injury caused by inflammatory mediators [2–4]. Further investigations revealed that PFC particles were ingested early into phagocytic cells in a time-dependent manner, thus resulted in the formation of PFC-filled vacuoles [39]. These cellular inclusions may interfere with intracellular signaling pathways and prevent the activation of inflammatory cascades. Similarly, Fernandez et al. demonstrated that PFCs interfered with transmembrane signal transduction by decreasing Syk phosphorylation in neutrophils. PFCs were also reportedly incorporated into the cellular membranes of alveolar epithelial cells and erythrocytes; this stabilized the cellular membrane and prevented surface receptor activation [34, 40–42]. However, other important inflammatory pathways may be involved in the protective mechanisms of PFCs, and further investigations are required to clarify these pathways.
Under ischemic conditions, oxidative metabolism is impaired and ATP stores in the lung are rapidly depleted. The high oxygen solubility of PFCs provides a higher partial pressure of oxygen in the microcirculation, thereby enhancing the oxygen flux into tissues. It has been reported that PFCs are effective for preventing ischemic heart and brain injuries by enhancing ischemic myocardial and cerebral oxygen concentration [43, 44]. However, it is not clear if PFCs could oxygenate the ischemic rat lungs in this study. Additional studies are warranted to clarify this.
This study had several limitations. First, FC-77 was chosen because it is widely available. However, different PFCs have various pharmacological properties, and new-generation PFCs may be more effective in attenuating lung inflammation. Second, our observation period was 1 h; therefore, the long-term effects of PFCs on IR remained unknown. Studies with a longer experimental time will be necessary before extrapolating our results to the clinic. Finally, although isolated lung models are widely used to investigate various physiological phenomena, the influence of extrapulmonary organs was not tested. Additional studies in intact whole animals to explore these effects are necessary.
In summary, we demonstrated that rats treated with FC-77 prior to the induction of ischemia had attenuated IR-induced lung injury. The observed decrease in lung damage may have been mediated by the inhibition of NF-κB activity, inflammatory reactions, and neutrophil infiltration into the lung tissues. Systemic PFC administration is a new therapeutic approach. Our results provide evidence for the potential of prophylactic therapy with systemic PFCs to prevent reperfusion injury in lung transplantation.
Acknowledgments
This study was supported, in part, by NSC 98-2314-B-016-034-MY2 from the National Science Council of Taiwan; MAB101-63 from the Ministry of National Defense; grants 10132, 1032, and 10137 from Taoyuan Armed Forces General Hospital; and TSGH-102-066 from Tri-Service General Hospital, Taiwan.
References
- 1.Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology. 2005;33:47–63. doi: 10.1081/BIO-200046659. [DOI] [PubMed] [Google Scholar]
- 2.Hirschl RB, Pranikoff T, Wise C, Overbeck MC, Gauger P, Schreiner RJ, Dechert R, Bartlett RH. Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA: The Journal of the American Medical Association. 1996;275:383–389. doi: 10.1001/jama.1996.03530290053037. [DOI] [PubMed] [Google Scholar]
- 3.Papo MC, Paczan PR, Fuhrman BP, Steinhorn DM, Hernan LJ, Leach CL, Holm BA, Fisher JE, Kahn BA. Perfluorocarbon-associated gas exchange improves oxygenation, lung mechanics, and survival in a model of adult respiratory distress syndrome. Critical Care Medicine. 1996;24:466–474. doi: 10.1097/00003246-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Lange NR, Kozlowski JK, Gust R, Shapiro SD, Schuster DP. Effect of partial liquid ventilation on pulmonary vascular permeability and edema after experimental acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2000;162:271–277. doi: 10.1164/ajrccm.162.1.9908120. [DOI] [PubMed] [Google Scholar]
- 5.Hirschl RB, Croce M, Gore D, Wiedemann H, Davis K, Zwischenberger J, Bartlett RH. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2002;165:781–787. doi: 10.1164/ajrccm.165.6.2003052. [DOI] [PubMed] [Google Scholar]
- 6.Kacmarek RM, Wiedemann HP, Lavin PT, Wedel MK, Tutuncu AS, Slutsky AS. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2006;173:882–889. doi: 10.1164/rccm.200508-1196OC. [DOI] [PubMed] [Google Scholar]
- 7.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia–reperfusion-induced lung injury. American Journal of Respiratory and Critical Care Medicine. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 8.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. American Journal of Respiratory Cell and Molecular Biology. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang H, Kuo FC, Lai YS, Chou TC. Inhibition of inflammatory responses by FC-77, a perfluorochemical, in lipopolysaccharide-treated RAW 264.7 macrophages. Intensive Care Medicine. 2005;31:977–984. doi: 10.1007/s00134-005-2652-y. [DOI] [PubMed] [Google Scholar]
- 10.Kawamae K, Pristine G, Chiumello D, Tremblay LN, Slutsky AS. Partial liquid ventilation decreases serum tumor necrosis factor-alpha concentrations in a rat acid aspiration lung injury model. Critical Care Medicine. 2000;28:479–483. doi: 10.1097/00003246-200002000-00032. [DOI] [PubMed] [Google Scholar]
- 11.Nakata S, Yasui K, Nakamura T, Kubota N, Baba A. Perfluorocarbon suppresses lipopolysaccharide- and alpha-toxin-induced interleukin-8 release from alveolar epithelial cells. Neonatology. 2007;91:127–133. doi: 10.1159/000097130. [DOI] [PubMed] [Google Scholar]
- 12.Smith TM, Steinhorn DM, Thusu K, Fuhrman BP, Dandona P. A liquid perfluorochemical decreases the in vitro production of reactive oxygen species by alveolar macrophages. Critical Care Medicine. 1995;23:1533–1539. doi: 10.1097/00003246-199509000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Lowe KC. Engineering blood: synthetic substitutes from fluorinated compounds. Tissue Engineering. 2003;9:389–399. doi: 10.1089/107632703322066570. [DOI] [PubMed] [Google Scholar]
- 14.Maevsky E, Ivanitsky G, Bogdanova L, Axenova O, Karmen N, Zhiburt E, Senina R, Pushkin S, Maslennikov I, Orlov A, Marinicheva I. Clinical results of Perftoran application: present and future. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology. 2005;33:37–46. doi: 10.1081/BIO-200046654. [DOI] [PubMed] [Google Scholar]
- 15.Chang H, Li MH, Chen CW, Yan HC, Huang KL, Chu SJ. Intravascular FC-77 attenuates phorbol myristate acetate-induced acute lung injury in isolated rat lungs. Critical Care Medicine. 2008;36:1222–1229. doi: 10.1097/CCM.0b013e31816a04d3. [DOI] [PubMed] [Google Scholar]
- 16.Nader ND, Knight PR, Davidson BA, Safaee SS, Steinhorn DM. Systemic perfluorocarbons suppress the acute lung inflammation after gastric acid aspiration in rats. Anesthesia and Analgesia. 2000;90:356–361. doi: 10.1097/00000539-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj AK, Cobb MA, Virmani R, Gay JC, Light RT, Forman MB. Limitation of myocardial reperfusion injury by intravenous perfluorochemicals. Role of neutrophil activation. Circulation. 1989;79:645–656. doi: 10.1161/01.CIR.79.3.645. [DOI] [PubMed] [Google Scholar]
- 18.Rice HE, Virmani R, Hart CL, Kolodgie FD, Farb A. Dose-dependent reduction of myocardial infarct size with the perfluorochemical Fluosol-DA. American Heart Journal. 1990;120:1039–1046. doi: 10.1016/0002-8703(90)90115-E. [DOI] [PubMed] [Google Scholar]
- 19.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2004;286:L231–L246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 20.Chu SJ, Chang DM, Wang D, Chen YH, Hsu CW, Hsu K. Fructose-1,6-diphosphate attenuates acute lung injury induced by ischemia–reperfusion in rats. Critical Care Medicine. 2002;30:1605–1609. doi: 10.1097/00003246-200207000-00034. [DOI] [PubMed] [Google Scholar]
- 21.Li MH, Huang KL, Wu SY, Chen CW, Yan HC, Hsu K, Hsu CW, Tsai SH, Chu SJ. Baicalin attenuates air embolism-induced acute lung injury in rat isolated lungs. British Journal of Pharmacology. 2009;157:244–251. doi: 10.1111/j.1476-5381.2009.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu SY, Wu CP, Kang BH, Li MH, Chu SJ, Huang KL. Hypercapnic acidosis attenuates reperfusion injury in isolated and perfused rat lungs. Critical Care Medicine. 2012;40:553–559. doi: 10.1097/CCM.0b013e318232d776. [DOI] [PubMed] [Google Scholar]
- 23.Abraham E. Neutrophils and acute lung injury. Critical Care Medicine. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 24.Woods CM, Neslund G, Kornbrust E, Flaim SF. Perflubron attenuates neutrophil adhesion to activated endothelial cells in vitro. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2000;278:L1008–L1017. doi: 10.1152/ajplung.2000.278.5.L1008. [DOI] [PubMed] [Google Scholar]
- 25.Steinhorn DM, Papo MC, Rotta AT, Aljada A, Fuhrman BP, Dandona P. Liquid ventilation attenuates pulmonary oxidative damage. Journal of Critical Care. 1999;14:20–28. doi: 10.1016/S0883-9441(99)90004-7. [DOI] [PubMed] [Google Scholar]
- 26.Rossman JE, Caty MG, Rich GA, Karamanoukian HL, Azizkhan RG. Neutrophil activation and chemotaxis after in vitro treatment with perfluorocarbon. Journal of Pediatric Surgery. 1996;31:1147–1150. doi: 10.1016/S0022-3468(96)90105-0. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez R, Sarma V, Younkin E, Hirschl RB, Ward PA, Younger JG. Exposure to perflubron is associated with decreased Syk phosphorylation in human neutrophils. Journal of Applied Physiology. 2001;91:1941–1947. doi: 10.1152/jappl.2001.91.5.1941. [DOI] [PubMed] [Google Scholar]
- 28.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine & Growth Factor Reviews. 2003;14:523–535. doi: 10.1016/S1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 29.Khimenko PL, Bagby GJ, Fuseler J, Taylor AE. Tumor necrosis factor-alpha in ischemia and reperfusion injury in rat lungs. Journal of Applied Physiology. 1998;85:2005–2011. doi: 10.1152/jappl.1998.85.6.2005. [DOI] [PubMed] [Google Scholar]
- 30.Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993;365:654–657. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- 31.von der Hardt K, Schoof E, Kandler MA, Dotsch J, Rascher W. Aerosolized perfluorocarbon suppresses early pulmonary inflammatory response in a surfactant-depleted piglet model. Pediatric Research. 2002;51:177–182. doi: 10.1203/00006450-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt W, Koehne P, Wissel H, Graf S, Proquitte H, Wauer RR, Rudiger M. Intratracheal perfluorocarbons diminish LPS-induced increase in systemic TNF-alpha. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2008;294:L1043–L1048. doi: 10.1152/ajplung.00125.2007. [DOI] [PubMed] [Google Scholar]
- 33.Thomassen MJ, Buhrow LT, Wiedemann HP. Perflubron decreases inflammatory cytokine production by human alveolar macrophages. Critical Care Medicine. 1997;25:2045–2047. doi: 10.1097/00003246-199712000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Wissel H, Burkhardt W, Rupp J, Wauer RR, Rudiger M. Perfluorocarbons decrease Chlamydophila pneumoniae-mediated inflammatory responses of rat type II pneumocytes in vitro. Pediatric Research. 2006;60:264–269. doi: 10.1203/01.pdr.0000233033.82664.91. [DOI] [PubMed] [Google Scholar]
- 35.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 36.Long SM, Laubach VE, Tribble CG, Kaza AK, Fiser SM, Cassada DC, Kern JA, Kron IL. Pyrrolidine dithiocarbamate reduces lung reperfusion injury. The Journal of Surgical Research. 2003;112:12–18. doi: 10.1016/S0022-4804(03)00139-2. [DOI] [PubMed] [Google Scholar]
- 37.Ross SD, Kron IL, Gangemi JJ, Shockey KS, Stoler M, Kern JA, Tribble CG, Laubach VE. Attenuation of lung reperfusion injury after transplantation using an inhibitor of nuclear factor-kappaB. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2000;279:L528–L536. doi: 10.1152/ajplung.2000.279.3.L528. [DOI] [PubMed] [Google Scholar]
- 38.Haeberle HA, Nesti F, Dieterich HJ, Gatalica Z, Garofalo RP. Perflubron reduces lung inflammation in respiratory syncytial virus infection by inhibiting chemokine expression and nuclear factor-kappa B activation. American Journal of Respiratory and Critical Care Medicine. 2002;165:1433–1438. doi: 10.1164/rccm.2109077. [DOI] [PubMed] [Google Scholar]
- 39.Haufe D, Koenigshausen E, Knels L, Wendel M, Stehr SN, Koch T. Leukocyte antibacterial functions are not impaired by perfluorocarbon exposure in vitro. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2008;295:L134–L142. doi: 10.1152/ajplung.00338.2007. [DOI] [PubMed] [Google Scholar]
- 40.Haufe D, Luther T, Kotzsch M, Knels L, Koch T. Perfluorocarbon attenuates response of concanavalin A-stimulated mononuclear blood cells without altering ligand–receptor interaction. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2004;287:L210–L216. doi: 10.1152/ajplung.00432.2003. [DOI] [PubMed] [Google Scholar]
- 41.Koch T, Ragaller M, Haufe D, Hofer A, Grosser M, Albrecht DM, Kotzsch M, Luther T. Perfluorohexane attenuates proinflammatory and procoagulatory response of activated monocytes and alveolar macrophages. Anesthesiology. 2001;94:101–109. doi: 10.1097/00000542-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Obraztsov VV, Neslund GG, Kornbrust ES, Flaim SF, Woods CM. In vitro cellular effects of perfluorochemicals correlate with their lipid solubility. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2000;278:L1018–L1024. doi: 10.1152/ajplung.2000.278.5.L1018. [DOI] [PubMed] [Google Scholar]
- 43.Memezawa H, Katayama Y, Shimizu J, Suzuki S, Kashiwagi F, Kamiya T, Terashi A. Effects of fluosol-DA on brain edema, energy metabolites, and tissue oxygen content in acute cerebral ischemia. Advances in Neurology. 1990;52:109–118. [PubMed] [Google Scholar]
- 44.Mosca RS, Rohs TJ, Waterford RR, Childs KF, Brunsting LA, Bolling SF. Perfluorocarbon supplementation and postischemic cardiac function. Surgery. 1996;120:197–204. doi: 10.1016/S0039-6060(96)80288-1. [DOI] [PubMed] [Google Scholar]


