Abstract
The objective of this paper was to develop a prognostic index for severe complications among hospitalized patients with influenza A (H1N1) 2009 virus infection. We conducted a prospective observational cohort study of 618 inpatients with 2009 H1N1 virus infection admitted to 36 Spanish hospitals between July 2009 and February 2010. Risk factors evaluated included host-related factors and clinical data at admission. We developed a composite index of severe in-hospital complications (SIHC), which included: mortality, mechanical ventilation, septic shock, acute respiratory distress syndrome, and requirement for resuscitation maneuvers. Six factors were independently associated with SIHC: age >45 years, male sex, number of comorbidities, pneumonia, dyspnea, and confusion. From the β parameter obtained in the multivariate model, a weight was assigned to each factor to compute the individual influenza risk score. The score shows an area under the receiver operating characteristic (ROC) curve of 0.77. The SIHC rate was 1.9 % in the low-risk group, 10.3 % in the intermediate-risk group, and 29.6 % in the high-risk group. The odds ratio for complications was 21.8 for the high-risk group compared with the low-risk group. This easy-to-score influenza A (H1N1) 2009 virus infection risk index accurately stratifies patients hospitalized for H1N1 virus infection into low-, intermediate-, and high-risk groups for SIHC.
Keywords: Chronic Obstructive Pulmonary Disease, Influenza, Intensive Care Unit Admission, Seasonal Influenza, Influenza Epidemic
Introduction
The influenza A (H1N1) virus pandemic in 2009 shocked the health systems of many countries and raised great social alarm. Much of this alarm was due to the information emitted on the epidemiological and clinical characteristics of the infection: a high capacity for contagion and rapid spread [1, 2]. Unlike seasonal influenza, the infection affected mostly young people and fewer people aged >65 years [3]. Initially, it was regarded as a potentially severe disease, with a high mortality rate, above all in young people without comorbidities, who required intensive care [4–6]. Obesity and pregnancy were identified as risk factors [4, 7–9].
In this context, extraordinary measures were taken with respect to the care of hospitalized patients. For example, the isolation of patients together with thorough cleaning and protection of health care personnel, both steps aimed to limit contagion. Molecular microbiological determinations were used to provide an early diagnosis of the virus. The specific treatment of the virus was standardized for all infected patients admitted. Extraordinary resources were provided in order to increase intensive care unit (ICU) staff. However, we lacked specific tools for the early identification of adult patients hospitalized with a bad prognosis.
Although a large amount of information about the epidemiology and clinical management of influenza A (H1N1) 2009 virus infection has been obtained in a remarkably short period, a major gap exists in understanding disease severity and identifying at-risk populations. Most studies have focused on epidemiological aspects of the general population and on patients admitted to ICUs. Selecting the most seriously ill patients exclusively according to ICU admittance involves significant bias, due to the variability in ICU selection criteria [10, 11], especially if the initial alarm raised by the pandemic and the emphasis on critical care is taken into account. In addition, the information available on risk factors groups children and adults together. It is reasonable to suppose that serious risk factors could differ between children and adults and, in addition, the hospital care of children and adults is structured differently. No in-depth studies have been carried out to identify risk factors of severe evolution for adult patients hospitalized for influenza A (H1N1) 2009 virus infection in order to manage these patients.
The aim of this study was to identify risk factors present at admission in adult patients hospitalized due to influenza A (H1N1) 2009 virus infection during the period 2009–2010 that were independently associated with worse outcomes and, thereby, develop a prognostic influenza severity index.
Patients and methods
Setting and study design
A multicenter matched case–control study was carried out in 36 hospitals and primary care centers from seven Spanish regions (Andalusia, Catalonia, Castile and Leon, Madrid, Navarre, the Basque Country, and Valencia). Cases and controls were recruited between July 2009 and February 2010. A case was defined as a patient admitted to hospital for >24 h with influenza A (H1N1) virus infection laboratory-confirmed by real-time polymerase chain reaction (RT-PCR). Twenty-five patients aged ≥18 years were recruited from each of the 36 study hospitals, and were chosen by the systematic sampling of all patients admitted with laboratory-confirmed influenza A (H1N1) 2009 virus infection [12]. For the purpose of this article, only the prospective cohort of patients admitted to any of the participant hospitals were included (the cases) and the controls are not included. Therefore, for this study, based in our selection criteria, we included only adult cases (≥18 years) who were hospitalized selected from the 36 hospitals studied. We excluded patients who had nosocomial infection, defined as pandemic virus infection in a patient that appears ≥48 h after admission for another cause. All information collected was treated as confidential, in strict observance of legislation on observational studies. The study was approved by the Ethics Committees of the hospitals involved. Written informed consent was obtained from all patients included in the study.
Data collection
A structured questionnaire was administered to patients by specifically trained personnel. We collected information on sociodemographic characteristics, pre-existing medical conditions, vaccinations, toxic habits, previous medications, exposure to social environments which could contribute to contagion, and the adoption of measures to prevent influenza. Variables on pre-existing medical conditions and vaccination were completed and verified by review of the medical records.
Possible predictive variables considered
The following demographic variables and pre-existing medical conditions were considered for this study: age, sex, previous hospital admission, history of pneumonia in the previous two years, obesity [body mass index (BMI) ≥ 30], morbid obesity (BMI ≥ 40), pregnancy in women aged 15–49 years, smoking, alcoholism, comorbidities (chronic obstructive pulmonary disease, asthma, other chronic respiratory diseases, cardiovascular disease, renal failure, diabetes, liver disease, HIV infection, disabling neurological disease, rheumatologic diseases, cancer, immunodeficiency, asplenia), vaccination, previous treatment, and clinical data at admission. For each vaccine, a case was considered to be vaccinated if they had received the vaccine at least 15 days before the onset of symptoms.
Outcomes
Severe in-hospital complications (SIHC) were the primary outcome of interest. This was a composite variable including: hospital mortality or requirement for mechanical ventilation or the presence of septic shock or acute respiratory distress syndrome (ARDS) or a requirement for resuscitation maneuvers during hospitalization. Shock was defined as systolic blood pressure below 90 mmHg without anti-hypertensive drugs and the need for vasopressive agents. Mechanical ventilation was included when it was needed for at least 24 h and always after influenza A diagnosis in the Emergency Department (ED). We did not include patients who received non-invasive positive pressure ventilation.
Statistical analysis
Univariate and multivariate logistic regression models were then constructed to identify the statistical significance of each risk factor. The dependent variable was SIHC, and the independent variables were factors with a significance of p < 0.15 in the univariate analysis. The odds ratio (OR) and 95 % confidence intervals (95 % CI) were calculated. The possible interaction between variables was also examined. The predictive accuracy of the model was determined by calculating the area under the receiver operating characteristic curve (AUC) for discrimination [13] and by comparing predicted and observed SIHC using the Hosmer–Lemeshow test for calibration [14]. Multilevel analysis with generalized estimated equations was carried out to determine whether the statistical significance of each predictive variable remained after adjusting for the Spanish regions.
To develop the influenza risk score, we first assigned a weight to each risk factor in relation to each β parameter based on the multivariate logistic regression model. Then, we added the weights of each of the risk factors presented by a patient. The predictive accuracy of the influenza risk score was determined by means of the AUC [13] and its calibration was tested by the Hosmer–Lemeshow test [14]. In addition, we attempted to validate the risk score by K-fold cross-validation [15, 16], which uses part of the available data to fit the model, and a different part to test it. That is, the model is validated in a random subsample which was not involved in the development of the model. This process is repeated sequentially for all partitions of the original sample. Thus, we split the data into K = 10 roughly equal-sized parts, we fitted the model with K − 1 parts of the data, and validated it by predicting the remaining kth part of the data. This procedure was repeated for each Kth part, until the ten groups were all used in the validation, meaning that all cases were used once in the validation of the risk score [15].
Once the influenza risk score was developed, we created three categories (low, intermediate, and high risk) in relation to the predicted SIHC. The performance of the index score categories was studied using a logistic regression model and the AUC, and the Cochran–Armitage test for trend was used to study the trend of the proportion of SIHC according to risk categories.
All effects were considered to be significant at p < 0.05, unless otherwise stated. All statistical analyses were performed using SAS for Windows statistical software, version 9.1 (SAS Institute, Inc., Carey, NC) and R© version 2.13.0 software.
Results
A total of 618 patients hospitalized with influenza A (H1N1) 2009 virus infection were included. Patient characteristics and outcomes are shown in Table 1. The mean age was 48.6 years [standard deviation (SD), 15.7], and 44.5 % were aged <46 years. Almost 40 % had no comorbidity, while 14.7 % had ≥3 comorbidities. Of the 320 women included, 48 (15 %) were pregnant. A total of 117 patients (22 %) were obese (BMI ≥ 30), of which 21 (17.9 %) were morbidly obese (BMI ≥ 40). The SIHC rate was 9.9 % (61/618).
Table 1.
Characteristics and outcomes of patients hospitalized with influenza A (H1N1) virus infection (N = 618)
| n (%) | |
|---|---|
| Characteristics | |
| Age, years, mean (SD) | 48.60 (15.7) |
| Age, groups, years | |
| ≤45 years | 275 (44.5) |
| 46–65 years | 242 (39.2) |
| >65 years | 101 (16.3) |
| Female | 320 (51.8) |
| Hospitalized during the last year | 133 (22.1) |
| Previous hospitalization by influenza A (H1N1) virus infection | 3 (0.5) |
| Pneumonia in the last two years | 93 (16.3) |
| Obesity | |
| No | 414 (78) |
| 30 ≤ BMI ≤ 40 | 96 (18.1) |
| BMI ≥40 | 21 (4) |
| Pregnant women | 48 (7.8) |
| Toxic habit | |
| Smoking | |
| No | 308 (50.4) |
| Yes | 176 (28.8) |
| Ex-smoker | 127 (20.8) |
| Alcoholism | 44 (7.1) |
| Drugs | 13 (2.1) |
| Comorbidities | |
| Chronic obstructive pulmonary disease | 72 (11.7) |
| Asthma | 103 (16.8) |
| Others chronic pulmonary disease | 74 (13.2) |
| Chronic respiratory insufficiency | 54 (8.8) |
| Cardiovascular disease | 89 (14.5) |
| Renal failure | 40 (6.5) |
| Diabetes | 89 (14.5) |
| Liver disease | 33 (5.4) |
| AIDS | 19 (3.1) |
| AIDS/symptomatic infection by HIV | 21 (3.4) |
| Disabling neurological disease | 20 (3.3) |
| Cognitive deterioration | 8 (1.3) |
| Neuromuscular disease | 8 (2.1) |
| Convulsive event | 12 (3.1) |
| Rheumatologic disease | 21 (3.5) |
| Neoplasia | 62 (10.1) |
| Immunodeficiency | 10 (1.6) |
| Asplenia | 4 (0.7) |
| No. of comorbidities | |
| 0 | 240 (38.8) |
| 1 | 172 (27.8) |
| 2 | 115 (18.6) |
| >2 | 91 (14.7) |
| Vaccination | |
| Pandemic vaccine | 10 (1.8) |
| Seasonal influenza vaccine in last year | 174 (28.8) |
| 23-valent pneumococcal vaccine in last 5 years | 35 (6.3) |
| 7-valent conjugated pneumococcal vaccine in last 5 years | 10 (1.8) |
| Previous treatment | |
| Previous antibiotics | 229 (37.1) |
| Length of antibiotic treatment, days, mean (SD) | 1.14 (4.9) |
| Systemic corticosteroids in last 90 days | 94 (18) |
| Inhaled corticosteroids | 134 (21.8) |
| Clinical status at admission | |
| Multilobar and/or bilateral involvement | 35 (5.7) |
| Pneumonia | 174 (28.2) |
| Confusion | 37 (6.2) |
| Fever | 565 (92.8) |
| Dyspnea | 404 (67.9) |
| Outcomes | |
| Hospital mortality | 5 (1.3) |
| Shock | 22 (5.4) |
| Mechanical ventilation | 33 (5.3) |
| Acute respiratory distress syndrome | 19 (4.9) |
| Resuscitation maneuvers | 5 (1.3) |
SD, standard derivation
Data are given as frequency (percentage) unless otherwise stated. Percentages exclude patients with missing data
In the univariate analyses, several host-related factors, such as age, gender, smoking, comorbidities, and clinical data at admission, were significantly associated with the likelihood of SIHC (Table 2). Obesity was not associated with SIHC (OR, 1.39; 95 % CI, 0.72–2.66; p = 0.3275), and no pregnant woman developed SIHC.
Table 2.
Risk factors significantly associated with severe in-hospital complication in the univariate analyses (N = 618)
| Characteristics | Severe in-hospital complication, n (%) | Odds ratio (95 % CI) | p-value |
|---|---|---|---|
| Age, in groups | |||
| ≤45 years | 15 (5.5) | Ref. | |
| 46–65 years | 32 (13.2) | 2.6 (1.4–5) | 0.003 |
| >65 years | 14 (13.9) | 2.8 (1.3–6) | 0.009 |
| Sex | |||
| Female | 21 (6.6) | Ref. | |
| Male | 40 (13.4) | 2.2 (1.3–3.8) | 0.005 |
| Smoking | |||
| No | 20 (6.5) | Ref. | |
| Yes | 22 (12.5) | 2.1 (1.1–3.9) | 0.03 |
| Ex-smoker | 17 (13.4) | 2.2 (1.1–4.4) | 0.02 |
| No. of comorbidities | |||
| 0 | 19 (7.9) | Ref. | |
| 1 | 15 (8.7) | 1.1 (0.6–2.3) | 0.77 |
| 2 | 9 (7.8) | 1 (0.4–2.3) | 0.98 |
| >2 | 18 (19.8) | 2.9 (1.4–5.8) | 0.003 |
| Clinical data at admission | |||
| Multilobar and/or bilateral involvement | |||
| No | 54 (9.3) | Ref. | |
| Yes | 7 (20) | 2.5 (1–5.9) | 0.04 |
| Pneumonia | |||
| No | 37 (8.3) | Ref. | |
| Yes | 24 (13.8) | 1.8 (1–3) | 0.04 |
| Confusion | |||
| No | 49 (8.8) | Ref. | |
| Yes | 10 (27) | 3.9 (1.8–8.5) | 0.0007 |
| Fever | |||
| No | 9 (20.5) | Ref. | |
| Yes | 49 (8.7) | 0.4 (0.2–0.8) | 0.01 |
| Dyspnea | |||
| No | 6 (3.1) | Ref. | |
| Yes | 53 (13.1) | 4.7 (2–11) | 0.0005 |
CI, confidence interval; Ref., reference group
Percentages exclude patients with missing data. Only factors with a significance of p < 0.15 in the univariate analysis are presented
In the multivariate analysis, six factors were independently associated with SIHC: age, sex, number of comorbidities, and the presence of pneumonia, confusion, and dyspnea at admission (Table 3). The logistic model showed good discrimination, with an AUC value of 0.77. The model was also well calibrated, with a Hosmer–Lemeshow p-value of 0.9618. The statistical significance of each predictive variable remained after adjustment for Spanish regions.
Table 3.
Risk factors significantly associated with severe in-hospital complication in the multivariate analyses (N = 618)
| Risk factors | β parameter | Odds ratio (95 % CI) | p-value | Weight |
|---|---|---|---|---|
| Intercept | −4.91 | |||
| Age (years) | ||||
| >45 vs. ≤45 | 0.76 | 2.1 (1.1–4.2) | 0.03 | 1 |
| Sex | ||||
| Male vs. female | 0.88 | 2.4 (1.3–4.4) | 0.004 | 1 |
| No. of comorbidities | ||||
| ≥3 vs. <3 | 0.80 | 2.2 (1.2–44) | 0.02 | 1 |
| Pneumonia | 0.65 | 1.9 (1.1–3.5) | 0.03 | 1 |
| Confusion | 1.35 | 3.9 (1.6–9.1) | 0.002 | 2 |
| Dyspnea | 1.50 | 4.5 (1.95–11) | 0.0009 | 2 |
| AUC | 0.77 | |||
| Hosmer–Lemeshow p-valuea | 0.9618 | |||
CI, confidence interval; β parameter = estimated β coefficient; AUC, area under the receiver operating characteristic curve
All risk factors were examined jointly
aA significant value for the Hosmer–Lemeshow statistic indicates a significant deviation between predicted and observed outcomes
Based on the multivariate logistic model, a weight was assigned to each risk factor in relation to each β parameter (Table 3). By adding up the weights assigned to each predictive variable, an individual influenza risk score was given to each patient, ranging from 0 to 8, with a higher score corresponding to a higher likelihood of SIHC. The risk score was significantly associated with the likelihood of developing SIHC (OR, 2.11; 95 % CI, 1.68–2.64; p < 0.0001), and was well calibrated (Hosmer–Lemeshow o-value, 0.9603). The influenza risk score showed good discrimination (AUC, 0.76; 95 % CI, 0.71–0.82), in addition to the good results showed by the K-fold cross-validation, which had an AUC (95 % CI) of 0.74 (0.68–0.80) (Fig. 1).
Fig. 1.
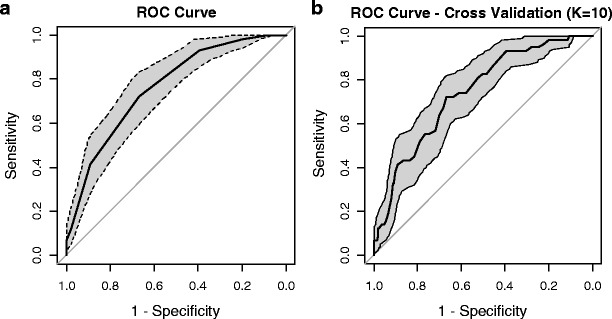
Receiver operating characteristic curve of predicting severe in-hospital complication according to the individual influenza risk score (a) and according to the 10-fold cross-validation model (b). a Area under the curve (AUC) [95 % confidence interval (CI)], 0.76 (0.71–0.82); b AUC (95 % CI), 0.74 (0.68–0.80)
Three risk categories were assigned using the influenza risk score (Table 4): low risk (0–2 points); intermediate risk (3–4 points); high risk (>4 points). The percentage of SIHC ranged from 1.9 % (95 % CI, 0.1–3.7) in patients classified as low risk to 29.6 % (95 % CI, 19.7–39.5) in patients classified as high risk (trend test, p < 0.001). The OR for the high-risk group was 21.8 compared with the low-risk group. The risk categories showed good discrimination, with an AUC value of 0.74.
Table 4.
Validation of the influenza risk score: severe in-hospital complication by index score categories
| Risk group (points) | No. with SIHC/no. at risk | Percentage (95 % CI)a | Odds ratio (95 % CI) |
|---|---|---|---|
| Low risk (0–2) | 4/211 | 1.9 (0.1–3.7) | Ref. |
| Intermediate risk (3–4) | 30/291 | 10.3 (6.8–13.8) | 6 (2.1–17.1) |
| High risk (>4) | 24/81 | 29.6 (19.7–39.5) | 21.8 (7.3–65.3) |
| AUC | 0.74 |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; Ref., reference group
The low-risk group was considered as the reference group
a p < 0.001 for the Cochran–Armitage test for trend
Discussion
This study was able to derive and validate a 2009 H1N1 virus infection influenza risk score with acceptable validity, discriminative ability, and generalizability, using data from a large cohort of inpatients from 36 geographically distinct Spanish hospitals. The risk score has various strengths. We developed a clinical prediction tool with six variables using information on predictive factors, including host-related factors and clinical conditions. The tool can be readily assessed and computed by physicians using information readily available in the ED.
Our results suggest that males aged >45 years and those patients with more than two underlying chronic conditions have an increased risk. However, the greatest risk factors are associated with clinical factors at hospital admission, when a patient with dyspnea and confusion, and, for example, pneumonia, would have a probability of almost 30 % of developing SIHC. The main value of this predictive score is its ability to identify patients who need additional monitoring and more aggressive treatment after the first ED evaluation, either in the ICU, intermediate care units, or specialized regular wards, depending on the severity. The ED is the natural setting for the use of this severity score, but it could also be useful in outpatient services, as an adjunct to clinical judgment. It is easy and quick to apply. Patients with a score of >2 points should be taken to hospital and patients with a score of >4 points should be hospitalized on an emergency basis. However, in patients with a score of <3, the chances of severe complications would be slim and the patient could be managed at home.
The strengths of the study include the relatively large cohort of patients recruited from different settings during the influenza A (H1N1) 2009 pandemic and the amount of clinical information collected. The main limitation of this study comes from its original design as a case–control study, where only 25 patients were recruited from each participating center and followed until discharge. Likewise, some laboratory parameters were not collected. Although we performed cross-validation, external validation of our score is still required.
Many of the predictors incorporated in our severity score have been established as risk factors for influenza A (H1N1)-associated complications in earlier studies. Comorbidities have been associated with an increased risk of complications both in seasonal influenza [17, 18] and in the influenza A (H1N1) 2009 pandemic [3, 7, 19–21]. We found that the risk of SIHC only increases in patients with >2 comorbidities. In contrast to seasonal influenza, where the greatest risk occurs in patients aged ≥70 years [18, 22], in both this study and other reports [19], severe outcomes occurred in patients aged >45 years.
We found that men had a higher risk of SIHC than women: similar results were reported by a Chinese study [7]. Another previous study showed that male sex was an independent risk factor for prolonged RT-PCR positivity in cases infected by influenza A (H1N1) virus [23]. Men have been found to be at a higher risk than women for death due to pneumonia [24, 25] and for sepsis [26]. Patterns of inflammation, coagulation, and fibrinolysis biomarkers in men may explain the reduced survival [27].
A preliminary report of 32 patients with influenza A (H1N1) 2009 virus infection hospitalized in a Spanish ICU showed that pneumonia was associated with a relatively high case–fatality rate [28]. The rate of patients hospitalized in wards and the ICU with pneumonia in the 2009 pandemic was higher [3, 4, 8, 19, 29]. We sought to confirm that pneumonia was an independent risk factor for SIHC.
Severe obesity has been identified as a risk factor for influenza A (H1N1) 2009 virus infection [4, 6–8]. However, in accordance with other studies [3, 30], we found that obesity was not associated with a higher risk of SIHC. In fact, obesity has not been identified as a risk factor for seasonal influenza complications [31, 32]. However, the prevalence of obesity in our series was similar to that of the general Spanish population [33], while a previous Spanish study found a high prevalence of obesity in patients hospitalized for 2009 H1N1 virus infection [34]. Obese patients may have been under-represented in our sample because the recruiting process of hospitalized cases collected 25 cases in each center during the pandemic period, but without trying to be representative of all cases seen in each hospital. Further investigation is needed in order to clarify the association between obesity and severe influenza.
In previous influenza epidemics and pandemics, pregnancy has been associated with an increased risk of severe disease [35, 36]. Likewise, recent reports suggest that there is an increased hospitalization rate and severity of illness in pregnant women infected by influenza A (H1N1) 2009 virus [8, 9, 19], while a Chinese study found that pregnancy was an independent risk factor associated with severe illness [7]. However, in our series, none of the pregnant women hospitalized for 2009 H1N1 virus infection developed SIHC, similar to the results of a previous Spanish study [34] and a Canadian study [20] that did not identify pregnancy as a risk factor for ICU admission or death. Preventive measures carried out in Spain, together with a fast diagnosis, early evaluation, and early antiviral treatment, may explain the relatively low rate of severity in pregnant women infected with the influenza A (H1N1) 2009 virus.
We did not use ICU admission as a criterion for determining SIHC because the decision to admit a patient to the ICU depends on individual clinical judgment and local hospital practices, differences that could account for much of the variability in ICU admission [10, 11]. On the other hand, the risk of death from influenza A infection is not the same as the need for inpatient care. We considered in-hospital death, mechanical ventilation, septic shock, ARDS, and resuscitation maneuvers as endpoints, given their more objective nature as variables [10]. This is a major strength of our study.
Conclusions
Although the 2009 pandemic is over, gaining deeper knowledge of influenza A (H1N1) 2009 remains essential, as it will plan for the next, unavoidable pandemic and because, as in the 2010–2011 seasonal influenza in Spain [37], the influenza A (H1N1) virus might be one factor responsible for future seasonal influenza epidemics.
We identified risk factors for severe in-hospital complications (SIHC) in patients hospitalized due to influenza A (H1N1) virus infection and developed a clinical severity score that is very easy and simple to apply. The use of this score at diagnosis, or at diagnostic presumption, even with ambulatory patients, could assist decisions on care management. Early identification of the sickest patients could allow earlier interventions and, thus, potentially improve outcomes.
Acknowledgments
The other members of the CIBERESP Cases and Controls in Pandemic Influenza Working Group are:
Andalucía: Ernestina Azor (Médico Centinela), Miguel Angel Bueno (Complejo Hospitalario de Jaén), Manuel Carnero (Hospital Virgen de la Victoria), Jerónimo Carrillo (Médico Centinela), Fernández-Crehuet Joaquín (Hospital Virgen de la Victoria), Víctor Fuentes (Hospital Costa del Sol), Virtudes Gallardo (Servicio de Epidemiología), Mª Luisa Gómez (Complejo Hospitalario de Jaén), Reyes López (Hospital Infanta Elena de Huelva), José Ramón Maldonado (Hospital de Torrecárdenas), Marcial Mariscal (Complejo Hospitalario de Jaén), Belén Martínez (Complejo Hospitalario de Jaén), Jose Mª Mayoral (Servicio de Epidemiología), Áurea Morillo (Hospital Virgen del Rocío), Rosa Moyano (Médico Centinela), José Mª Navarro (Laboratorio de Referencia de Gripe), Juan Antonio Navarro (Médico Centinela), Salvador Oña (Hospital Carlos Haya), Esteban Pérez (Servicio de Epidemiología), Mª José Pérez (Hospital Virgen de Valme), Mercedes Pérez (Laboratorio de Referencia de Gripe), Juan Pedro Quesada (Complejo Hospitalario de Jaén), Jorge del Diego Salas (Hospital Virgen de la Victoria), María Sillero (Complejo Hospitalario de Jaén), Mª Carmen Ubago (Hospital Virgen de las Nieves), Manuel Vázquez (Médico Centinela), Francisco Zafra (Médico Centinela), Manuel Zarzuela (Hospital Puerta del Mar). Comunidad Valenciana: José Blanquer (Hospital Clínico de Valencia), María Morales (Hospital Doctor Peset), Red Centinela Sanitaria de la Comunidad Valenciana. Castilla y León: Demetrio Carriedo (Complejo Asistencial de León), José Javier Castrodeza (Dirección General de Salud Pública, Desarrollo e Innovación), Florentino Díez (Complejo Asistencial de León), Isabel Fernández (Complejo Asistencial de León), Silvia Fernandez (Complejo Asistencial de León), Ignacio Ledesma (Complejo Asistencial de León), Raul Ortiz de Lejarazu (Centro Nacional de Gripe de Valladolid), Juan Ortiz de Saracho (Hospital de El Bierzo), Alberto Pérez (Dirección General de Salud Pública, Desarrollo e Innovación), Ana Pueyo ( Complejo Asistencial de Burgos), Pedro Redondo (Servicio Territorial de Sanidad y Bienestar Social de León), Juan Ortiz (Hospital del Bierzo) , José Luis Viejo (Complejo Asistencial de Burgos). Cataluña: Alvar Agustí (Hospital Clínic de Barcelona), Ferrán Barbé (Hospital Arnau de Vilanova), Lluis Blanch (Hospital de Sabadell), Xavier Bonfill (Hospital de Sant Pau), Eva Borràs (Dirección General de Salud Pública), Carlos Bravo (Hospital Vall d’Hebrón), Francesc Calafell (Universitat Pompeu Fabra), Joan Cayla (Agencia de Salud Publica de Barcelona), Ignacio Garcia (Hospital Germans Trias i Pujol), Juan Jose Garcia (Hospital Sant Joan de Deu), Joaquim Gea (Hospital del Mar), Ned Hayes (Hospital Clínic, CRESIB), Juan Pablo Horcajada (Hospital del Mar), Joaquin López-Contreras (Hospital de Sant Pau), Ana Martínez (Dirección General de Salud Pública), Fernando Moraga (Hospital Vall d’Hebrón), Gemma Navarro (Hospital de Sabadell), Virginia Pomar (Hospital de Sant Pau), María Teresa Puig (Hospital de Sant Pau), Tomas Pumarola (Laboratorio de Referencia de Gripe), Antoni Rosell (Hospital de Bellvitge), Juan Ruiz (Hospital Germans Trias i Pujol), Marc Saez (Universidad de Girona), Núria Torner (Dirección General de Salud Pública), Antoni Torres (Hospital Clínic de Barcelona), Cecilia Tortajada (Agencia de Salud Publica de Barcelona), Antoni Trilla (Hospital Clínic de Barcelona), Ana Vilella (Hospital Clínic de Barcelona). Madrid: Carlos Álvarez (Hospital 12 de Octubre), Fernando Baquero (Hospital Universitario Ramón y Cajal), Rafael Cantón (Hospital Universitario Ramón y Cajal), Esther Córdoba (Area de Epidemiología de la Comunidad de Madrid), María Enríquez (Hospital 12 de Octubre), Juan Carlos Galán (Hospital Universitario Ramón y Cajal), Juan García (Area de Epidemiología de la Comunidad de Madrid), Ricard Génova (Area de Epidemiología de la Comunidad de Madrid), Elisa Gil (Area de Epidemiología de la Comunidad de Madrid), Susana Jiménez (Area de Epidemiología de la Comunidad de Madrid), Josefina López (Area de Epidemiología de la Comunidad de Madrid), Fernando Martín (Area de Epidemiología de la Comunidad de Madrid), María Luisa Martínez (Area de Epidemiología de la Comunidad de Madrid), José Ramón Paño (Hospital Universitario La Paz), Francisco Pozo (Hospital 12 de octubre), Ana Robustillo (Hospital Universitario Ramón y Cajal), Elena Rodriguez (Area de Epidemiología de la Comunidad de Madrid), María Romero (Hospital Universitario La Paz), Silvia Sánchez (Area de Epidemiología de la Comunidad de Madrid), MªAngeles Valdeón (Hospital Universitario Ramón y Cajal), Cenegundis Valdés (Area de Epidemiología de la Comunidad de Madrid). Navarra: Patricia Fanlo (Hospital Virgen del Camino), Francisco Gil (Hospital Virgen del Camino), Antonia Martinez (Instituto de Salud Pública), Leyre Martínez (Instituto de Salud Pública), María Ruiz (Hospital Virgen del Camino). País Vasco: Urko Aguirre (Hospital Galdakao), José María Antoñana (Hospital de Cruces), Javier Arístegui (Hospital Basurto), Itziar Astigarraga (Hospital de Cruces), Amaia Bilbao (Fundación Vasca de Innovación e Investigación Sanitarias), Alberto Caspelastegui (Hospital Galdakao), Gustavo Cilla (Hospital Donostia), Antonio Escobar (Hospital Basurto), Pedro Pablo España (Hospital Galdakao), Felipe Esteban (Hospital Txagorritxu), Carmen Garaizar (Fundación Vasca de Innovación e Investigación Sanitarias), Susana García (Hospital Galdakao), Javier Korta (Hospital Donostia), José Luis Lobo (Hospital Txagorritxu), Emilio Pérez Trallero (Hospital Donostia), Jose Ignacio Pijoan (Hospital de Cruces), José María Quintana (Hospital Galdakao), Mikel Santiago (Hospital de Cruces), Cristina Sarasqueta (Hospital Donostia), Eva Tato (Hospital Txagorritxu).
Sources of support
This study was supported by the Ministerio de Ciencia e Innovación Instituto de Salud Carlos III, Programa de Investigación Sobre Gripe A/H1N1 (grant GR09/0030), Agency for the Management of Grants for University Research (AGAUR grant number 2009/ SGR 42), Department of Health of the Basque Country, Osakidetza, and the thematic networks—CIBERESP and CIBERES—of the Instituto de Salud Carlos III.
Conflicts of interest
All authors declare that there are no conflicts of interest for any of the authors.
All authors had full access to the data.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- AUC
Area under the receiver operating characteristic curve
- BMI
Body mass index
- CI
Confidence intervals
- ED
Emergency department
- ICU
Intensive care unit
- OR
Odds ratio
- Ref.
Reference group
- RT-PCR
Real-time polymerase chain reaction
- SD
Standard deviation
- SIHC
Severe in-hospital complications
References
- 1.Miller MA, Viboud C, Balinska M, et al. The signature features of influenza pandemics—implications for policy. N Engl J Med. 2009;360:2595–2598. doi: 10.1056/NEJMp0903906. [DOI] [PubMed] [Google Scholar]
- 2.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Bautista E, Chotpitayasunondh T, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 3.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 6.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Feng Z, Uyeki TM, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;52:457–465. doi: 10.1093/cid/ciq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The ANZIC Influenza Investigators. Webb SA, Pettilä V, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 9.Louie JK, Acosta M, Jamieson DJ, California Pandemic (H1N1) Working Group et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 10.Angus DC, Marrie TJ, Obrosky DS, et al. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166:717–723. doi: 10.1164/rccm.2102084. [DOI] [PubMed] [Google Scholar]
- 11.McQuillan P, Pilkington S, Allan A, et al. Confidential inquiry into quality of care before admission to intensive care. BMJ. 1998;316:1853–1858. doi: 10.1136/bmj.316.7148.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domínguez A, Alonso J, Astray J, et al. Risk factors of influenza (H1N1) 2009 hospitalization and effectiveness of pharmaceutical and nonpharmaceutical interventions in its prevention: a case–control study. Rev Esp Salud Publica. 2011;85:3–15. doi: 10.1590/S1135-57272011000100002. [DOI] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 14.Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley Interscience; 1989. [Google Scholar]
- 15.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. New York: Springer; 2009. [Google Scholar]
- 16.Steyerberg EW. Clinical prediction models. New York: Springer; 2009. [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 18.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 19.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 20.Campbell A, Rodin R, Kropp R, et al. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ. 2010;182:349–55. doi: 10.1503/cmaj.091823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson LJ, Rutter PD, Ellis BM, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schanzer DL, Tam TW, Langley JM, et al. Influenza-attributable deaths, Canada 1990–1999. Epidemiol Infect. 2007;135:1109–1116. doi: 10.1017/S0950268807007923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan V, Angus DC, Griffin MF, et al. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 25.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 26.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 27.Reade MC, Yende S, D’Angelo G, et al. Differences in immune response may explain lower survival among older men with pneumonia. Crit Care Med. 2009;37:1655–1662. doi: 10.1097/CCM.0b013e31819da853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rello J, Rodríguez A, Ibañez P, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasoo S, Singh K, Trenholme GM. Predicting need for hospitalization of patients with pandemic (H1N1) 2009, Chicago, Illinois, USA. Emerg Infect Dis. 2010;16:1594–1597. doi: 10.3201/eid1610.091889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Díaz E, Rodríguez A, Martin-Loeches I, et al. Impact of obesity in patients infected with 2009 influenza A(H1N1) Chest. 2011;139:382–386. doi: 10.1378/chest.10-1160. [DOI] [PubMed] [Google Scholar]
- 31.Fiore AE, Shay DK, Broder K, Centers for Disease Control and Prevention et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 32.Ho YC, Wang JL, Wang JT, et al. Prognostic factors for fatal adult influenza pneumonia. J Infect. 2009;58:439–445. doi: 10.1016/j.jinf.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Martín A, Novalbos Ruiz JP, Martínez Nieto JM, et al. Life-style factors associated with overweight and obesity among Spanish adults. Nutr Hosp. 2009;24:144–151. [PubMed] [Google Scholar]
- 34.Santa-Olalla Peralta P, Cortes-García M, Vicente-Herrero M et al (2010) Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April–December 2009. Euro Surveill 15(38). pii: 19667 [DOI] [PubMed]
- 35.Harris JW. Influenza occurring in pregnant women. JAMA. 1919;72:978–980. doi: 10.1001/jama.1919.02610140008002. [DOI] [Google Scholar]
- 36.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14:95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Instituto de Salud Carlos III. Weekly report of the Spanish Influenza Surveillance System. Week 06/2011. In Spanish. Available online at: http://vgripe.isciii.es/gripe/documentos/20102011/boletines/grn062011.pdf


