Abstract
Purpose
We studied the risk factors for postoperative mortality between patients who underwent emergency or elective living-donor liver transplantation (LDLT).
Methods
Forty-seven patients underwent LDLT in our institute, 16 for emergencies and 31 as elective procedures. The emergency LDLT status was applied to cases in which the time period between referral to our institution and transplantation did not exceed 10 days, and in which liver failure was accompanied by the presence of any degree of hepatic encephalopathy.
Results
With regard to preoperative factors, age (P = 0.03), the model for end-stage liver disease score (P = 0.001), preoperative tracheal intubation (P = 0.001), ratio between arterial oxygen tension and fractional inspired oxygen (PaO2/FiO2 ratio) (P = 0.03), steroid therapy use (P = 0.001), lymphocyte count (P = 0.02), and cases requiring hemodiafiltration (P = 0.001) differed significantly between the two groups. Postoperative pneumonia occurred more frequently in emergency LDLT patients than in elective LDLT patients (P = 0.006). Invasive pulmonary aspergillosis (IPA) was the main cause of postoperative death in emergency LDLT patients, and, in a univariate analysis, a preoperative status of high serum (1 → 3)-β-d-glucan (>20 pg/ml, P = 0.001), advanced age (>52 years, P = 0.02), and a low PaO2/FiO2 ratio (<320, P = 0.01) were identified as factors predictive of IPA.
Conclusion
Careful perioperative management, including preoperative investigation of aspergillosis and empiric antibiotic therapy, should be considered for emergency LDLT patients who fulfill IPA risk factors.
Keywords: Emergency living donor liver transplantation, Postoperative pneumonia, Invasive pulmonary aspergillosis
Introduction
Liver transplantation (LT) is now accepted as a reliable treatment for patients with end-stage liver disease [1, 2]. Compared to liver transplantation from a deceased donor, living-donor liver transplantation (LDLT) may reduce the waiting time, particularly for patients suffering from hepatic encephalopathy [3]. The survival outcome of LDLT for patients with hepatic encephalopathy is controversial [4, 5]. LDLT using a right-lobe graft was shown to improve the overall survival rates of these patients [6, 7]. However, most of the previously reported causes of death of these patients were neurological damage and infections [8]. In this article, we reviewed the clinical outcomes of emergency LDLT in our institution to clarify the risk factors for postoperative mortality that may lead to improved survival rates for patients with emergency LDLT; namely, for those with hepatic encephalopathy irrespective of the presence of either acute liver failure or acute on chronic liver failure (ACLF).
Patients and methods
Forty-seven patients underwent LDLT at Yokohama City University Graduate School of Medicine, Japan, including one case of retransplantation. Sixteen patients underwent LDLT for emergency status and 31 for elective status. In this study, the emergency LT status was applied to cases in which the time period between referral to our institution and transplantation did not exceed 10 days, and in which liver failure was accompanied by the presence of any degree of hepatic encephalopathy. Hepatic encephalopathy was graded from 1 to 5 in accordance with the criteria described by the Japan Study Group for Fulminant Hepatitis: [9] grade 1 patients exhibit euphoria or depression, grade 2 exhibit drowsiness or confusion, grade 3 show somnolence but the ability to be roused, grade 4 are in a coma with response to painful stimuli, and grade 5 patients are in a deep coma with no response to painful stimuli.
The emergency indications included acute liver failure (n = 9) and ACLF (n = 7). The former was defined by the presence of hepatic encephalopathy as a consequence of severe liver damage without preexisting liver disease [10] and the latter as chronic end-stage liver disease [11]. The primary disease underlying acute liver failure was fulminant hepatitis, the etiology of which included unknown origins (n = 5), autoimmune hepatitis (AIH) (n = 3), and hepatitis B virus (HBV) (n = 1). The etiology of ACLF included HBV (n = 4), primary biliary cirrhosis (PBC) (n = 1), AIH (n = 1), and alcoholism (n = 1). The indications for elective LDLT in the 31 patients included hepatocellular carcinoma (n = 10), PBC (n = 7), HBV cirrhosis (n = 6), hepatitis C virus (HCV) cirrhosis (n = 3), AIH (n = 1), alcoholic cirrhosis (n = 1), and other diseases (n = 3).
Indications for emergency LDLT
We referred to the criteria of Chiba University, Japan, to determine the indications for three levels of emergency LDLT: [4] (1) subacute type (between 11 days and 8 weeks), (2) acute type with liver atrophy, and (3) acute type with no liver atrophy. The national guidelines were referred to for level 3 [4]; in other words, when conservative therapy was not found to be an effective treatment for liver failure, then LDLT was considered.
Preoperative management
Hemodiafiltration (HDF) was performed to treat hepatorenal syndrome, and, if hepatic encephalopathy above grade 2 was also present, high flow dialysate continuous hemodiafiltration (HFCHDF) [12, 13] was commenced to prevent irreversible brain damage. Plasma exchange (PE) was carried out to treat coagulopathy, with 0.1 l fresh frozen plasma/kg body weight given per PE session.
Chest and abdominal computed tomography (CT) were performed to evaluate the presence of lung disease and the degree of liver atrophy. Mechanical ventilation was performed if the arterial oxygen tension (PaO2) was less than 60 mmHg. The degree of brain edema was evaluated using cranial CT, and abnormal electrical activity of the brain was examined by electroencephalography (EEG). To avoid any incidental bleeding that might occur while applying this invasive technique, we did not monitor intracranial pressure. In ten ABO-incompatible cases, rituximab was administered, and PE was performed in two or three preoperative sessions to reduce the anti-donor blood type antibody titer [14].
Recipient operation
During the recipient operation, the whole liver was removed, and the branches of the vascular and biliary tree were divided and preserved for reconstruction. All LT procedures were performed using the piggyback technique without a venovenous bypass. A temporary portacaval shunt was placed during the anhepatic phase of the transplant in patients in whom circulatory instability was evident from the portal clamping test. A splenectomy was performed in ABO-incompatible cases, in cases of pancytopenia caused by splenomegaly, in patients with HCV infection as the primary disease, and when high portal vein pressure was diagnosed after liver graft reperfusion (>20 mmHg).
In ABO-incompatible cases, hepatic artery infusion (HAI) or portal vein infusion (PVI) was started immediately after liver graft reperfusion. HAI consisted of prostaglandin E1 (PGE1, 0.01 μg/kg/min) and methylprednisolone (initial dose 60–125 mg/day); PVI consisted of PGE1 (0.01 μg/kg/min), methylprednisolone (initial dose 62.5 mg/day), and gabexate mesilate (1000 mg/day) or nafamostat mesilate (150 mg/day). HAI or PVI continued for 2–3 weeks [15]. A Witzel tube jejunostomy was placed in the proximal jejunum with an 8 Fr enteral tube.
Postoperative management
All patients were treated with tacrolimus and steroids as immunosuppressants. Mycophenolate mofetil was frequently added to reduce the risk of renal dysfunction induced by tacrolimus. The whole-blood trough level of tacrolimus was adjusted to 10–12 ng/ml during the first postoperative week and tapered thereafter. Methylprednisolone (10 mg/kg) was administered twice intraoperatively, then tapered from 1 mg/kg/day on postoperative day 1–0.3 mg/kg/day at the end of the first postoperative month. If liver function stabilized, then steroids were discontinued from 2 months after LDLT. Steroid pulse therapy was performed if severe or moderate acute cellular rejection was diagnosed by liver biopsy.
Postoperative prophylactic antibiotic treatment included flomoxef sodium for 5 days and antifungal micafungin sodium for 7 days in all patients. Sulfamethoxazole/trimethroprim against Pneumocystis carinii was administered if a splenectomy was performed. Doppler echography was used three times daily during the patient’s ICU stay to monitor the blood flow of the reconstructed portal vein, hepatic artery, and hepatic vein, and once daily during regular hospitalization.
The intratracheal tube was extubated after surgery according to the patient’s good general and respiratory conditions. If patients did not meet these criteria by about 7 days after LT, a tracheostomy was considered. The patients were started on an enteral diet when contrast medium that had been administered intraoperatively reached the ileocecum.
The clinical and demographic parameters and surgical outcomes of the 16 emergency LDLT patients were compared with those of the 31 patients who underwent elective LDLT during the same period. The postoperative morbidities and survival rates were compared between the two groups, and the predictive factors for postoperative mortality among patients with emergency LDLT were also investigated.
Statistical analysis
Quantitative variables were given as the mean ± standard deviation and categorical variables as values and percentages. Chi-square testing was carried out for univariate analysis of categorical variables, and the significance level was set at a P value <0.05. The statistical software package, SPSS II for Windows (SPSS), was used for the analysis. To determine the cutoff levels of continuous variables, the receiver operating characteristic curve was used.
Results
Patient characteristics in emergency and elective LDLT
The patient characteristics from each group are summarized in Table 1. Preoperative factors including age (emergency, elective = 42.3 ± 14.4, 51.3 ± 8.0, respectively; P = 0.03), the model for end-stage liver disease (MELD) score (27.4 ± 7.6, 15.1 ± 6.6; P = 0.001), the percentage of cases requiring tracheal intubation (43.7, 0%; P = 0.001), the ratio between arterial oxygen tension and fractional inspired oxygen (PaO2/FiO2 ratio) (335.2 ± 110.2, 405.6 ± 73.7; P = 0.03), the percentage of cases requiring HDF (81.2, 0%; P = 0.001), steroid therapy in the 1 month before LDLT (68.7, 16.1%; P = 0.001), and the lymphocyte count (806 ± 856.3, 960 ± 533.5; P = 0.02) were significantly different between the two groups. Other variables including gender and ABO incompatibility did not differ significantly between the two groups. Concerning intraoperative factors, the two groups were similar in the duration of surgery, estimated volume of blood loss, warm ischemic time, cold ischemic time, and rates of splenectomy and of using a right lobe graft. Of the postoperative factors, the duration of admission for emergency LDLT was significantly longer than that for elective LDLT [93.6 ± 53.1, 57.3 ± 38.4 days, respectively (P = 0.02)].
Table 1.
Characteristics of the study population
| Factor | Emergent LDLT (n = 16) | Elective LDLT (n = 31) | P value |
|---|---|---|---|
| Preoperative factors | |||
| Females (%) | 9 (56.2) | 14 (45.1) | 0.47 |
| Age (years) | 42.3 ± 14.4 | 51.3 ± 8.0 | 0.03 |
| MELD score | 27.4 ± 7.6 | 15.1 ± 6.6 | 0.001 |
| ABO incompatibility | 2 (12.5) | 8 (25.8) | 0.29 |
| Tracheal intubation (performed) | 7 (43.7) | 0 (0) | 0.001 |
| PaO2/FiO2 ratio | 335.2 ± 110.2 | 405.6 ± 73.7 | 0.03 |
| HDF | 13 (81.2) | 0 (0) | 0.001 |
| Steroid (1 month) | 11 (68.7) | 5 (16.1) | 0.001 |
| Lymphocyte count | 806 ± 856.3 | 960 ± 533.5 | 0.02 |
| Intraoperative factors | |||
| Duration of operation (min) | 1085.4 ± 807.6 | 929.2 ± 164.3 | 0.45 |
| e-Blood loss (ml) | 6375.0 ± 8048.7 | 6257.1 ± 4926.8 | 0.95 |
| Warm ischemic time (min) | 61.3 ± 18.4 | 64.7 ± 18.6 | 0.55 |
| Cold ischemic time (min) | 137.4 ± 71.8 | 185.6 ± 96.2 | 0.06 |
| Splenectomy (performed) | 5 (31.2) | 15 (48.3) | 0.26 |
| Right lobe graft | 11 (68.7) | 22 (70.9) | 0.87 |
| Postoperative factors | |||
| Duration of admission (days) | 93.6 ± 53.1 | 57.3 ± 38.4 | 0.02 |
Data are presented as number of patients (percentages in parentheses) or mean ± SD
LDLT living donor liver transplantation, MELD model for end-stage liver disease, PaO 2 /FiO 2 ratio ratio between arterial oxygen tension and fractional inspired oxygen, HDF hemodiafiltration, e-Blood loss estimated blood loss
Postoperative short-term outcomes
A total of 14 types of complications occurred (Table 2). Twelve patients in the emergency LDLT group encountered a total of 24 complications and 20 patients in the elective LDLT group encountered a total of 32 complications. There was no significant difference in the frequency of postoperative complications between the two groups, including surgery and liver graft, and the central nervous system complications. Regarding preoperative hepatic coma, of 16 emergency LDLT patients, 6 patients had grade 2 encephalopathy, 5 had grade 3, and 5 had grade 4. All emergency LDLT patients who survived the perioperative period recovered without any degree of hepatic encephalopathy. Postoperative pneumonia occurred more frequently in emergency LDLT patients (n = 5, 31.2%) than in elective LDLT patients (n = 1, 3.2%; P = 0.006). A tracheotomy was also performed more frequently in emergency LDLT patients (n = 9, 56.2%) than in elective LDLT patients (n = 7, 22.5%; P = 0.02).
Table 2.
Postoperative complications
| Complication | Emergent LDLT | Elective LDLT | P value |
|---|---|---|---|
| Total number of complications | 24 | 32 | |
| Surgery- and liver graft-related | 6 (37.5) | 15 (48.3) | 0.47 |
| Biliary stricture | 2 (12.5) | 3 (9.6) | 0.77 |
| Biliary leakage | 1 (6.2) | 5 (16.1) | 0.33 |
| Hepatic artery stenosis | 0 (0) | 1 (3.2) | 0.46 |
| Hepatic vein stenosis | 0 (0) | 2 (6.4) | 0.29 |
| Primary nonfunction | 1 (6.2) | 0 (0) | 0.15 |
| Intraabdominal bleeding | 1 (6.2) | 0 (0) | 0.15 |
| Acute cellular rejection | 1 (6.2) | 4 (12.9) | 0.48 |
| Central nervous system-related | 4 (25.0) | 3 (9.6) | 0.16 |
| Cerebral hemorrhage | 2 (12.5) | 1 (3.2) | 0.21 |
| Central pontine myelinolysis | 1 (6.2) | 0 (0) | 0.15 |
| Tacrolimus-related convulsion | 1 (6.2) | 2 (6.4) | 0.97 |
| Respiratory system-related | 6 (37.5) | 5 (16.1) | 0.47 |
| Pneumonia | 5 (31.2) | 1 (3.2) | 0.006 |
| Others | 1 (6.2) | 4 (12.9) | 0.48 |
| Others | 8 (50.0) | 9 (29.0) | 0.15 |
| Cytomegalovirus infection | 7 (43.7) | 9 (29.0) | 0.31 |
| Acute cardiac infarction | 1 (6.2) | 0 (0) | 0.15 |
Data are presented as number of patients (percentages in parentheses)
LDLT living donor liver transplantation
Hospital mortality occurred in four patients in the emergency LDLT group (25%) and in one patient in the elective LDLT group (3.2%; P = 0.02) (Table 3). The causes of death were pneumonia (n = 3) and primary nonfunction (n = 1) in the emergency LDLT group and cerebral hemorrhage (n = 1) in the elective LDLT group.
Table 3.
Causes of hospital mortality
| Cause | Emergent LDLT (n = 16) | Elective LDLT (n = 31) | P value |
|---|---|---|---|
| Hospital mortality (all) | 4 (25.0) | 1 (3.2) | 0.02 |
| Cause of hospital mortality | |||
| Pneumonia | 3 (18.7) | 0 (0) | 0.01 |
| Cerebral hemorrhage | 0 (0) | 1 (3.2) | 0.46 |
| Primary nonfunction | 1 (6.2) | 0 (0) | 0.15 |
Data are presented as number of patients (percentages in parentheses)
LDLT living donor liver transplantation
Characteristics of pneumonia after LDLT
The incidence and causes of pneumonia after LDLT were investigated (Table 4). Six patients with postoperative pneumonia, five of whom were in the emergency LDLT group, required a respirator. Among the six patients who suffered from pneumonia, three (50%) were ABO incompatible, and all these patients underwent a splenectomy; the corresponding rates were similar among patients without pneumonia (41.4 and 17.0%, respectively). The pneumonia-causing pathogens were shown to be Aspergillus spp. (n = 3), methicillin-resistant Staphylococcus aureus (MRSA) (n = 1), and Pseudomonas aeruginosa (n = 1) in emergency LDLT patients and Enterococcus faecalis in the elective LDLT patient.
Table 4.
Respiratory management of post-LDLT pneumonia
| Case | LDLT | Age/gender | Primary disease | ABO | MELD | Splenectomy performed | Initial diagnosis of pneumonia (days after LDLT) | Pneumonia pathogen | Final diagnosis of IPA | Outcome | Months after LDLT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Emergent | 47/M | HBV-LC | Incompatible | 31.9 | (+) | 1 | Ps. aeruginosa | – | Survived | 29.9 |
| 2 | Emergent | 52/F | HBV-LC | Incompatible | 23.7 | (+) | 0 | MRSA | – | Died | 2.4 |
| 3 | Emergent | 52/F | FHF | Identical | 18.9 | (−) | 22 | A. fumigatus | Proven | Died | 3.1 |
| 4 | Emergent | 52/F | PBC | Identical | 36.5 | (−) | 1 | A. terreus | Probable | Died | 3.6 |
| 5 | Emergent | 53/F | FHF | Compatible | 20.2 | (−) | 0 | A. fumigatus | Proven | Survived | 57.5 |
| 6 | Elective | 66/M | HCC | Incompatible | 9.0 | (+) | 5 | E. faecalis | – | Survived | 26.3 |
M male, F female, HBV-LC hepatitis B virus-liver cirrhosis, FHF fulminant hepatic failure, PBC primary biliary cirrhosis, HCC hepatocellular carcinoma, MELD model for end-stage liver disease, LDLT living donor liver transplantation, Ps. aeruginosa Pseudmonous aeruginosa, MRSA methicillin-resistant Staphylococcus aureus, A. fumigatus Aspergillus fumigatus, A. terreus Aspergillus terreus, E. faecalis Enterococcus faecalis, IPA invasive pulmonary aspergillosis
Aspergillus spp. were the most common pneumonia pathogen, and in all three cases of infection, the pathogenesis developed as invasive pulmonary aspergillosis (IPA). IPA was defined as any LDLT recipient meeting the criteria for proven or probable IPA as described in a report from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Co-operative Group and the National Institute of Allergy and Infectious Disease Mycoses Study Group [16].
One IPA case was diagnosed from a resected lung specimen [17] and another at autopsy. The mean age of infected patients was 52.3 years, and the mean time from surgery to the initial IPA diagnosis was 7.6 ± 12.4 days. Although one IPA case has survived for 57.5 months after LDLT [17], the other two patients died 3.1 and 3.6 months, respectively, after LDLT.
The predictive factors for IPA were investigated by a univariate analysis among patients who had undergone an emergency LDLT, which was the only group in which IPA was encountered. The predictive preoperative factors were shown to be mature age (>52 years, P = 0.02), serum (1 → 3)-β-d-glucan levels above 20 pg/ml (P = 0.001), and a PaO2/FiO2 ratio of less than 320 (P = 0.01).
Long-term outcomes
The overall cumulative 3- and 5-year patient survival rates after emergency LDLT were 73.6 and 73.6%, respectively, and those of elective LDLT patients were 82.0 and 82.0%, respectively. The patient survival rate of the emergency LDLT group was therefore inferior to that of the elective LDLT group, although the difference was not significant (P = 0.39; Fig. 1).
Fig. 1.
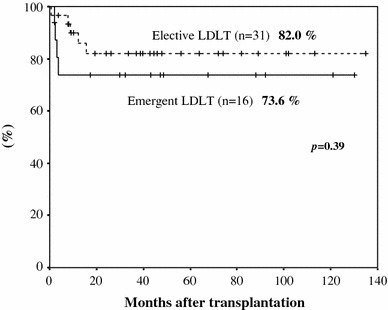
The patient survival rate of the emergency and elective LDLT patients. LDLT living donor liver transplantation
Discussion
LDLT is a timely and lifesaving procedure for fulminant hepatitis [5], but the long-term survival outcome is controversial. The complications of emergency LDLT were reported to be acute cellular rejection [5, 8], surgical complications [4], or opportunistic infection [5]. Our study showed that the frequency of surgical complications was not significantly different between the emergency LDLT and elective LDLT groups, but the rate of postoperative pneumonia was significantly higher in the former group. Thus, avoidance of respiratory complications is crucial for increasing the survival rates of the emergency LDLT group.
The present study showed that the MELD score was higher in emergency LDLT patients than in elective LDLT patients. In addition, the preoperative lymphocyte count was significantly lower in patients who underwent an emergency LDLT, and they received more frequent preoperative immunosuppressive therapy than elective LDLT patients. Thus, emergency LDLT patients appear to suffer from more preoperative immunosuppressive conditions. Furthermore, emergency LDLT patients received tracheal intubation therapy at a higher frequency than elective LDLT patients and had a higher occurrence of postoperative respiratory infections.
IPA was shown to be the most frequent cause of postoperative infection in this study. Therefore, it is important that associated risk factors should be recognized at an early stage to enable empirical therapy to commence. Generally, most cases of IPA in LT recipients occur during the early period [18]. The frequency at which aspergillosis develops in the first 100 days after transplantation has previously been shown to range from 75 to 90% [19]. In this study, as all three patients developed IPA within 30 days after LDLT, it is conceivable that Aspergillus infection was already present during the preoperative period. Therefore, the risk factors for IPA were evaluated from the point of view of preoperative factors in this study.
Several risk factors for IPA have previously been reported, including dialysis requirements [20], increased immunosuppression [21], retransplantation [22], and preoperative steroid administration [21]. The presence of the Aspergillus antigen was also found to be useful for the detection of IPA [22], but false-positive reactions with fungus-derived antibiotics or other fungal genera are a matter of concern [23]. The polymerase chain reaction (PCR) assay of bronchoalveolar lavage fluid is an accurate test for detection of Aspergillus spp. DNA, but it does not differentiate between infection and colonization [24]. Therefore, the results must be considered cautiously in conjunction with diagnostic procedures.
In this study, a preoperative high (1 → 3)-β-d-glucan serum level (>20 pg/ml), advanced age (>52 years), and a low PaO2/FiO2 ratio (<320) in emergency LDLT patients were found to be potential predictive factors for IPA. Serum (1 → 3)-β-d-glucan detection is quick and straightforward, with results being obtainable within a day. Moreover, in a previous study, plasma (1 → 3)-β-d-glucan elevation was detected earlier than a positive fungal culture in half of recorded fungemic episodes [25]. Aging is known to be related to immunodeficiency [26] as a result of dysfunction of T cells. The pathogenesis of low preoperative PaO2/FiO2 ratios could be multifactorial, including atelectasis caused by pleural effusion, adult respiratory distress syndrome (ARDS), and hepatopulmonary syndrome (HPS), and these easily lead to pulmonary infection [27].
We showed that IPA occurred only in emergency LDLT patients, i.e., those with severe immunosuppression. Such patients should be considered a high-risk group for the development of IPA, requiring more careful management, including empiric antibiotic therapy. The preoperative administration of voriconazole [28] should be considered, whereas the administration of systemic immunosuppressants, such as tacrolimus and steroids, should be reduced as much as possible [16]. Although the perioperative improvement of nutrition is also necessary [29], preoperative ingestion was shown to be fundamentally difficult because emergency LDLT patients often experience a hepatic coma. Therefore, preoperative enteral nutrition using a nasointestinal feeding tube may be effective. Strict postoperative management can ensure the survival of some IPA patients, as reported in the present study. Thus, the rate of IPA complications can be controlled, and the prognosis of emergency LDLT patients might be further improved.
In conclusion, immunosuppression was stronger and the risk of postoperative pneumonia was higher in emergency LDLT patients compared with elective LDLT patients. IPA was the most frequent cause of hospital death in emergency LDLT patients, with preoperative high (1 → 3)-β-d-glucan serum levels, advanced age, and low PaO2/FiO2 ratios found to be predictive factors. Empiric antibiotic therapy should be considered for emergency LDLT patients who fulfill these factors to improve their outcomes and survival rates.
Conflict of interest
Kazuhisa Takeda and coauthors have no conflict of interest.
References
- 1.Hong SK, Hwang S, Lee SG, Lee LS, Ahn CS, Kim KH, et al. Pulmonary complications following adult liver transplantation. Transplant Proc. 2006;38:2979–2981. doi: 10.1016/j.transproceed.2006.08.090. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura N, Okajima H, Ushigome H, Sakamoto S, Fujiki M, Okamoto M. Current status of organ transplantation in Japan and worldwide. Surg Today. 2010;40:514–525. doi: 10.1007/s00595-009-4214-3. [DOI] [PubMed] [Google Scholar]
- 3.Campsen J, Blei AT, Emond JC, Everhart JE, Freise CE, Lok AS, et al. Adult-to-Adult Living Donor Liver Transplantation Cohort Study Group. Outcomes of living donor liver transplantation for acute liver failure: the adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2008;14:1273–80. doi: 10.1002/lt.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui Y, Sugawara Y, Yamashiki N, Kaneko J, Tamura S, Togashi J, et al. Living donor liver transplantation for fulminant hepatic failure. Hepatol Res. 2008;38:987–996. doi: 10.1111/j.1872-034X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu CL, Fan ST, Lo CM, Wei WI, Yong BH, Lai CL, et al. Live donor liver transplantation for fulminant hepatic failure in children. Liver Transpl. 2003;9:1185–1190. doi: 10.1053/jlts.2003.50235. [DOI] [PubMed] [Google Scholar]
- 6.Liu CL, Fan ST, Lo CM, Yong BH, Fung AS, Wong J. Right-lobe live donor liver transplantation improves survival of patients with acute liver failure. Br J Surg. 2002;89:317–322. doi: 10.1046/j.0007-1323.2001.02035.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu CL, Fan ST, Lo CM, Wei WI, Yong BH, Lai CL, et al. Live-donor liver transplantation for acute-on-chronic hepatitis B liver failure. Transplantation. 2003;76:1174–1179. doi: 10.1097/01.TP.0000087341.88471.E5. [DOI] [PubMed] [Google Scholar]
- 8.Uemoto S, Inomata Y, Sakurai T, Egawa H, Fujita S, Kiuchi T, et al. Living donor liver transplantation for fulminant hepatic failure. Transplantation. 2000;70:152–157. [PubMed] [Google Scholar]
- 9.Nishizaki T, Hiroshige S, Ikegami T, Uchiyama H, Hashimoto K, Soejima Y, et al. Living-donor liver transplantation for fulminant hepatic failure in adult patients with a left-lobe graft. Surgery (St. Louis) 2002;131:S182–S189. doi: 10.1067/msy.2002.119574. [DOI] [PubMed] [Google Scholar]
- 10.Cariús LP, Pacheco-Moreira LF, Balbi E, Leal CR, Gonzalez AC, Agoglia LV, et al. Living donor liver transplantation for acute liver failure: a single center experience. Transplant Proc. 2009;41:895–897. doi: 10.1016/j.transproceed.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Escorsell Mañosa A, Mas Ordeig A. Acute on chronic liver failure. Gastroenterol Hepatol. 2010;33:126–134. doi: 10.1016/j.gastrohep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Yokoi T, Oda S, Shiga H, Matsuda K, Sadahiro T, Nakamura M, et al. Efficacy of high-flow dialysate continuous hemodiafiltration in the treatment of fulminant hepatic failure. Transfus Apher Sci. 2009;40:61–70. doi: 10.1016/j.transci.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Sekido H, Matsuo K, Takeda K, Ueda M, Morioka D, Kubota T, et al. Usefulness of artificial liver support for pretransplant patients with fulminant hepatic failure. Transplant Proc. 2004;36:2355–2356. doi: 10.1016/j.transproceed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Morioka D, Kumamoto T, Matsuo K, Tanaka K, Endo I, et al. A survival case of ABO-incompatible liver transplantation complicated with severe preoperative infection and subsequent overwhelming postsplenectomy infection. Transplant Proc. 2009;41:3941–3944. doi: 10.1016/j.transproceed.2009.02.094. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Tanaka K, Morioka D, Kumamoto T, Endo I, Togo S, et al. Pathogenesis in ABO incompatible liver transplantation: a clinicohistological evaluation of four patients. Clin Transpl. 2010;24:747–751. doi: 10.1111/j.1399-0012.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 16.Ascioglu S, Rex JH, Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Morioka D, Matsuo K, Endo I, Sekido H, Moroboshi T, et al. A case of successful resection after long-term medical treatment of invasive pulmonary aspergillosis following living donor liver transplantation. Transpl Proc. 2007;39:3505–3508. doi: 10.1016/j.transproceed.2007.05.085. [DOI] [PubMed] [Google Scholar]
- 18.Collins LA, Samore MH, Roberts MS, Luzzati R, Jenkins RL, Lewis WD, et al. Risk factors for invasive fungal infections complicating orthotopic liver transplantation. J Infect Dis. 1994;170:644–652. doi: 10.1093/infdis/170.3.644. [DOI] [PubMed] [Google Scholar]
- 19.Fortún J, Martín-Dávila P, Moreno S, De Vicente E, Nuño J, Candelas A, et al. Liver risk factors for invasive aspergillosis in liver transplant recipients. Transplantation. 2002;8:1065–1070. doi: 10.1053/jlts.2002.36239. [DOI] [PubMed] [Google Scholar]
- 20.Shi-Chun L, Meng-Long W, Ning L, Wei L, Ping C, Jin-Ning L, et al. Emergent right lobe adult-to-adult living-donor liver transplantation for high model for end-stage liver disease score severe hepatitis. Transpl Int. 2010;23:23–30. doi: 10.1111/j.1432-2277.2009.00935.x. [DOI] [PubMed] [Google Scholar]
- 21.Osawa M, Ito Y, Hirai T, Isozumi R, Takakura S, Fujimoto Y, et al. Risk factors for invasive aspergillosis in living donor liver transplant recipients. Liver Transpl. 2007;13:566–570. doi: 10.1002/lt.21099. [DOI] [PubMed] [Google Scholar]
- 22.Rosenhagen M, Feldhues R, Schmidt J, Hoppe-Tichy T, Geiss HK. A risk profile for invasive aspergillosis in liver transplant recipients. Infection. 2009;37:313–319. doi: 10.1007/s15010-008-8124-x. [DOI] [PubMed] [Google Scholar]
- 23.Asano-Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H, et al. False-positive Aspergillus galactomannan antigenaemia after haematopoietic stem cell transplantation. J Antimicrob Chemother. 2008;61:411–416. doi: 10.1093/jac/dkm463. [DOI] [PubMed] [Google Scholar]
- 24.Hayette MP, Vaira D, Susin F, Boland P, Christiaens G, Melin P, et al. Detection of Aspergillus species DNA by PCR in bronchoalveolar lavage fluid. J Clin Microbiol. 2001;39:2338–2340. doi: 10.1128/JCM.39.6.2338-2340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwama A, Yoshida M, Miwa A, Obayashi T, Sakamoto S, Miura Y. Improved survival from fungaemia in patients with haematological malignancies: analysis of risk factors for death and usefulness of early antifungal therapy. Eur J Haematol. 1993;51:156–160. doi: 10.1111/j.1600-0609.1993.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 26.Cakman I, Rohwer J, Schütz RM, Kirchner H, Rink L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech Ageing Dev. 1996;87:197–209. doi: 10.1016/0047-6374(96)01708-3. [DOI] [PubMed] [Google Scholar]
- 27.Mandell MS. Hepatopulmonary syndrome and portopulmonary hypertension in the model for end-stage liver disease (MELD) era. Liver Transpl. 2004;10:S54–S58. doi: 10.1002/lt.20260. [DOI] [PubMed] [Google Scholar]
- 28.Martin T, Sharma M, Damon L, Kaplan L, Guglielmo BJ, Working M, et al. Voriconazole is safe and effective as prophylaxis for early and late fungal infections following allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2010;12:45–50. doi: 10.1111/j.1399-3062.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo F, Dickson ER, Pasha T, Kasparova P, Therneau T, Malinchoc M, et al. Impact of nutritional status on outcomes after liver transplantation. Transplantation. 2000;70:1347–1352. doi: 10.1097/00007890-200011150-00014. [DOI] [PubMed] [Google Scholar]


