Abstract
Reported here are two cases of hantavirus pulmonary syndrome caused by Puumala virus infection, which rapidly resolved after initiation of corticosteroid treatment combined with continuous veno-venous hemodiafiltration. These cases emphasize the role of the inflammatory response in the pathogenesis of hantavirus pulmonary syndrome.
Keywords: Adult Respiratory Distress Syndrome, Hemorrhagic Fever With Renal Syndrome, Lung Injury Score, Nephropathia Epidemica, Hantavirus Pulmonary Syndrome
Introduction
Puumala virus (PUU) is a hantavirus that is endemic in northwestern Europe, the Balkans, and western Russia [1]. It is carried and spread by the bank vole. Puumala virus infection is usually either asymptomatic or causes a mild form of hemorrhagic fever with renal syndrome. The incidence in Finland is 19/100,000. The mortality rate is low (0.1–0.4%) [1]. The most common signs and symptoms are fever, headache, myalgia, back pain and gastrointestinal symptoms, often followed by an oliguric phase with proteinuria and hematuria, and later a polyuric phase. Transient visual impairment (myopia) is a pathognomonic sign. Hemorrhagic manifestations are present in about 10% of cases. However, mild mucosal bleeding is very common [1]. Spontaneous recovery usually takes place in 2–3 weeks [2].
Of the hospital-treated patients, about 5% require transient hemodialysis [1], and over 20% have pulmonary abnormalities. Most often these relate to both the inflammation-associated capillary permeability disorder and fluid retention caused by renal failure [3]. However, a few cases of acute non-cardiogenic pulmonary edema, similar to the hantavirus pulmonary syndrome (HPS) encountered on the American continent, have been described in Europe in association with PUU and other hantavirus species [4–7]. We describe two cases of PUU-associated HPS, in which administration of intravenous corticosteroids combined with continuous veno-venous hemodiafiltration (CVVHDF) was followed by rapid clinical improvement. Both patients gave their consent to publication of the data. The first day of fever is defined as day 0 post-onset of symptoms (POS).
Case reports
Case 1
A 72-year-old male patient was admitted to a district hospital in southern Finland in February 2003 with a 3-day history of fever, upper abdominal pain and melena. An infectious infiltrate was suspected in the right lung, and an enlarged spleen was detected on abdominal ultrasound. C-reactive protein (CRP) level was 94 mg/l, platelet count 84×109/l, leukocyte count 7.1×109/l, and hematocrit 37%. The qualitative urinalysis showed proteinuria. Intravenous antimicrobial agents were started for suspected community-acquired pneumonia. Urine output began to decrease, and serum creatinine increased from 89 to 295 μmol/l. Two blood cultures on consecutive days were negative. The patient became hypotensive, and oxygenation deteriorated.
The patient was transferred to a university hospital on day 7 POS. Echocardiography showed normal left ventricular function. The patient needed continuous ventilation with a positive airway pressure mask and 70% inspiratory oxygen fraction, and he was transferred to the intensive care unit (ICU). Diffuse bilateral perihilar infiltrates were present on chest radiograph (Fig. 1). CRP level was 77 mg/l, hematocrit 50%, platelet count 130×109/l, leukocyte count 46×109/l, creatinine 463 μmol/l, blood urea nitrogen 26.9 mmol/l, potassium 5.5 mmol/l, and lactate 3.9 mmol/l. Troponin-T and creatine-kinase MB mass were within normal limits.
Fig. 1.
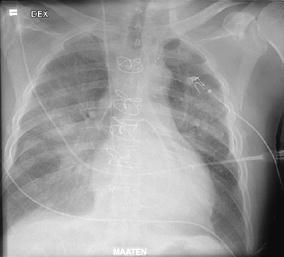
Chest radiograph of patient 1 on the day of intensive care unit admission (day 7 POS)
Due to poor oxygenation and oliguria, the patient required endotracheal intubation and CVVHDF. Initially, the ratio of arterial oxygen tension to inspired oxygen fraction (PaO2/FiO2 ratio) was 100 mmHg, and intermittent prone positioning was used for 3 days to improve gas exchange. Ceftriaxone (2 g/day) and fluconazole (200 mg/day) were given as antimicrobial treatment. The blood morphological examination showed both neutrophilia and lymphocytosis, including atypical lymphocytes, and a variable degree of maturation.
On day 8 POS, PUU-IgM was positive by μ-capture enzyme-linked immunosorbent assay based on baculovirus-expressed rN-antigen [8, 9]. PUU-IgG was detected with fluorescein isothiocyanate-conjugated sheep anti-human IgG, and demonstrated a granular fluorescence pattern associated with antibodies against the PUU nucleocapsid protein, typical of a recent infection [10, 11]. On day 10 POS, the PaO2/FiO2 ratio was still less than 200 mmHg with 14 mmHg pulmonary artery occlusion pressure, and intravenous methylprednisolone (120 mg/day in three doses) was started. On the following days, the PaO2/FiO2 ratio rapidly stabilized at over 300 mmHg. The evolution of the PaO2/FiO2 ratio and the lung injury score is shown in Fig. 2a,b. The patient required vasoactive support for 9 days. The minimum urine output was 65 ml on day 11 POS. CVVHDF was discontinued on day 16 POS.
Fig. 2.
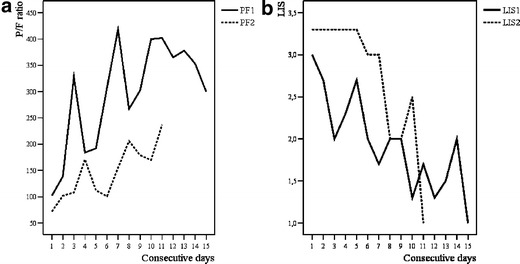
a Ratio of arterial oxygen tension (mmHg) to inspiratory oxygen fraction (P/F ratio) on consecutive days during the period of intensive care unit treatment for cases 1 (PF1, days 7–21 POS) and 2 (PF2, days 5–15 POS). Intravenous corticosteroid treatment (methylprednisolone 120 mg/day in three doses) was started on days 4 and 5 (days 10 and 9 POS) in cases 1 and 2, respectively. b Lung injury score (LIS) on consecutive days during the period of ICU treatment in cases 1 (LIS1, days 7–21 POS) and 2 (LIS2, days 5–15 POS). Intravenous corticosteroid treatment (methylprednisolone 120 mg/day in three doses) was started on days 4 and 5 (days 10 and 9 POS) in cases 1 and 2, respectively
The calculated dialysis and total fluid balances are shown in Table 1. The lowest platelet count was 59×109/l on day 14 POS, the highest leukocyte count 50.2×109/l on day 10 POS, and the highest CRP 97 mg/l on day 11 POS. The evolution of CRP values and platelet counts is shown in Table 2. Blood cultures taken on days 7 and 10 POS were negative. The patient was extubated on day 18 POS, and discharged from the ICU on day 21 POS. Methylprednisolone was tapered off and switched to oral prednisone. On day 22 POS, left laterobasal infiltration was still visible on chest radiograph. On that day, proteinuria was 0.6 g/24 h. The levels of C3 and C4 were normal, and there was no indication of C4 subtype insufficiency. The patient’s HLA genotype was B7 DRB1*15. The patient confirmed there were signs of vole infestation in the yard and shed of his home. He had not recently traveled abroad.
Table 1.
Pulmonary artery occlusion pressures (PAOP, mmHg), daily urine output (UO, ml/24 h), the calculated daily dialysis balance (DialBal, ml/24 h) and total fluid balance (TotBal, ml/24 h) in cases 1 and 2 on consecutive days POS during the period of ICU treatment (days 7–21 POS and 5–15 POS for cases 1 and 2, respectively)
| Case 1 | Case 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| POS | PAOP | UO | DialBal | TotBal | POS | PAOP | UO | DialBal | TotBal |
| 7 | 23 | 5 | 13 | ||||||
| 8 | 18 | 74 | −1120 | +877 | 6 | 14 | 108 | +4503 | |
| 9 | 16 | 86 | −220 | +2634 | 7 | 17 | 102 | −620 | +1918 |
| 10 | 14 | 124 | −260 | +1745 | 8 | 16 | 80 | −1130 | +1482 |
| 11 | 14 | 65 | −1380 | −227 | 9 | 16 | 147 | −3840 | −974 |
| 12 | 14 | 88 | −900 | +104 | 10 | 17 | 370 | −5360 | −3785 |
| 13 | 10 | 124 | −1510 | +42 | 11 | 17 | 1552 | −1290 | −1951 |
| 14 | 11 | 235 | −1700 | −568 | 12 | 16 | 3775 | −2061 | |
| 15 | 12 | 630 | −2920 | −2063 | 13 | 3835 | −1539 | ||
| 16 | 12 | 1322 | −610 | −1136 | 14 | 3120 | −288 | ||
| 17 | 15 | 2290 | −1197 | ||||||
| 18 | 12 | 3280 | −2719 | ||||||
| 19 | 5590 | −4911 | |||||||
| 20 | 4720 | −3813 | |||||||
Values in bold type indicate the day when corticosteroid treatment was started (days 10 and 9 POS for cases 1 and 2, respectively)
Table 2.
Platelet counts (PC, ×109/l) and C-reactive protein values (CRP, mg/l) in cases 1 and 2 on consecutive days POS during the period of ICU treatment (days 7–21 POS and 5–15 POS for cases 1 and 2, respectively)
| Case 1 | Case 2 | ||||
|---|---|---|---|---|---|
| POS | PC | CRP | POS | PC | CRP |
| 7 | 125 | 46 | 5 | 40 | 219 |
| 8 | 129 | 51 | 6 | 80 | 245 |
| 9 | 96 | 61 | 7 | 108 | 210 |
| 10 | 114 | 54 | 8 | 129 | 182 |
| 11 | 74 | 97 | 9 | 163 | 128 |
| 12 | 75 | 39 | 10 | 183 | 94 |
| 13 | 65 | 21 | 11 | 246 | 34 |
| 14 | 59 | 15 | 12 | 284 | 16 |
| 15 | 70 | 10 | 13 | 323 | 12 |
| 16 | 59 | 18 | 14 | 374 | 10 |
| 17 | 82 | 19 | |||
| 18 | 91 | 12 | |||
| 19 | 104 | 18 | |||
| 20 | 132 | 15 | |||
Values in bold type indicate the day when corticosteroid treatment was started (days 10 and 9 POS for cases 1 and 2, respectively)
Case 2
A 34-year-old male patient came to a university hospital in southern Finland in August 2004 for treatment of upper abdominal pain. His symptoms had started 3 days previously with high fever, fatigue, and myalgia. He had vomited once and had diarrhea in which he detected traces of blood. On day 4 POS, his CRP level was 245 mg/l, platelet count 46×109/l, leukocyte count 9.8×109/l, hematocrit 45%, sodium 126 mmol/l, and alanine aminotransferase 86 U/l. The chest radiograph showed bilateral perihilar infiltrates, and the patient was started on ceftriaxone (2 g/day) and levofloxacin (1 g/day). He received supplemental oxygen via nasal cannulae. Abdominal radiograph and ultrasound examinations were normal.
On day 5 POS the lung infiltrates had increased considerably (Fig. 3a,b), and the patient required continuous ventilation with a positive airway pressure mask. He became oliguric, proteinuria 1.7 g/24 h was detected, and his creatinine level increased from 87 to 255 μmol/l. The patient’s platelet count decreased to 40×109/l, and he experienced visual disturbances. He confirmed that 18 days before the onset of symptoms he had visited a summer cottage, where exposure to vole secretions was possible. He had not recently traveled abroad.
Fig. 3.
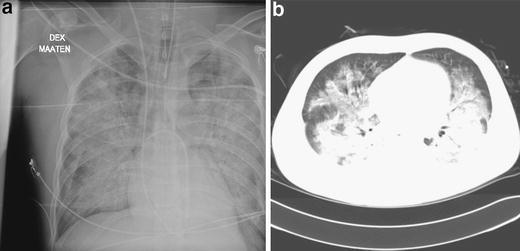
a Chest radiograph of patient 2 on the day of intensive care unit admission (day 5 POS). b High-resolution computerized tomography scan of patient 2 on the day of intensive care unit admission (day 5 POS)
The patient was transferred to the ICU due to respiratory insufficiency and renal failure. Oxygenation deteriorated rapidly, and he required endotracheal intubation and mechanical ventilation. The lowest PaO2/FiO2 ratio was 63 mmHg on day 6 POS, and intermittent prone positioning was continued until day 11 POS. The patient required a low dose of vasopressor support for 7 days. On day 5 POS, PUU-IgG- and PUU-IgM-antibodies were positive by the same methods described for case 1, and IgG-IFA showed the typical granular fluorescence pattern seen in the acute phase of immunity. Metabolic acidosis was detected, the patient’s creatinine level increased to 412 μmol/l and blood urea nitrogen to 23.8 mmol/l. CVVHDF was started on day 7 POS.
As the patient was severely ill, and a concomitant bacterial infection could not be ruled out, antibiotics were continued until CRP began to decrease on day 9 POS. The evolution of CRP values and platelet counts is shown in Table 2. Blood cultures taken on days 5 and 9 POS were negative. On day 9 POS the PaO2/FiO2 ratio was still 100–150 mmHg with 16 mmHg pulmonary artery occlusion pressure, and intravenous methylprednisolone (120 mg/day in three doses) was started. The evolution of the PaO2/FiO2 ratio and the lung injury score is shown in Fig 2a,b. The minimum urine output was 80 ml on day 8 POS. CVVHDF was discontinued on day 11 POS. The calculated dialysis and total fluid balances are shown in Table 1.
The patient was extubated on day 12 POS. He was discharged from the ICU on day 15 POS. On that day, left mediobasal and right basal atelectasis and slight bilateral pleural effusion were visible on chest radiograph. The methylprednisolone dose was tapered, and an oral preparation was substituted on day 16 POS. The patient was discharged home on day 18 POS. The patient lacked one of the C4B-type genes. His HLA genotype was B13,35 DRB1*01,07.
Discussion
We describe two cases of PUU-infected patients who presented with both renal and respiratory failure requiring renal replacement therapy and mechanical ventilation. Radiologically, both patients had alveolar infiltrates separate from pulmonary vascular congestion, and no prominent cardiomegaly. Nevertheless, fluid inevitably accumulated in the lung during the disease process. Most likely, both the corticosteroid treatment and the negative fluid balance achieved during CVVHDF treatment and recovery of renal function contributed to the subsequent improvement in gas exchange.
In HPS, a febrile prodrome is followed by a severe increase in pulmonary vascular permeability and myocardial depression. The syndrome progresses very rapidly from interstitial pulmonary edema to a clinical picture resembling adult respiratory distress syndrome (ARDS). The alveolar infiltrates are typically central or bibasilar, and pleural effusion is common [12]. The hantaviruses are not cytopathic, and the increased capillary permeability is likely caused by immunologic factors [13]. In pathological specimens of HPS-afflicted lungs, intra-alveolar edema and interstitial lymphoid cell infiltration are observed, the pneumocytes are preserved, and neutrophils are notably scarce [14, 15]. The endothelial cells are also largely intact, and the alveolar walls are thickened due to extravasation [16]. Activation of T-lymphocytes and cytokine production appear to be involved in both HPS and hemorrhagic fever with renal syndrome [13,17]. Terajima et al. [18] demonstrated that at the onset of pulmonary edema, HPS patients have abundant viremia which clears rapidly after the resolution of fever, while respiratory distress may persist.
The increase in alveolocapillary permeability in acute lung injury results from the inflammatory response [19, 20]. Cortisol acts in concert with catecholamines to maintain vascular tone and endothelial integrity, and it suppresses the inflammatory cascade by down-regulating the production of proinflammatory molecules [21]. Animal studies on the efficacy of corticosteroids in acute lung injury have produced mixed results [22, 23]. The American–European consensus conference on ARDS stated that corticosteroids are not generally useful in the early management of sepsis and ARDS [19]. Early or prophylactic use of high-dose corticosteroid therapy in septicemia and ARDS may even be harmful [24]. The consensus conference acknowledged, however, that corticosteroids may be of value in treating certain ARDS variants [19]. Currently, there is no universally accepted drug treatment for the high permeability edema associated with acute lung injury/ARDS in humans [20]. An overaggressive inflammatory response may be an important factor in the outcome of ARDS patients [25]. Late corticosteroid treatment in sustained lung injury has been shown to inhibit proinflammatory activity and to improve lung function and possibly outcome [26].
In the series of seven patients with PUU-associated HPS described by Clement et al. [4], only one patient required mechanical ventilation. The course of mechanical ventilation was prolonged (20 days), but the patient made a full recovery. The treatment of the European HPS cases described by Launay et al. [5] and Klempa et al. [7] was also mainly supportive (i.e., no mechanical ventilation was required), and the patients recovered without sequelae. The patient with Dobrava virus-associated HPS reported by Schütt et al. [6] received both CVVHDF and prolonged mechanical ventilation (25 days), and eventually recovered. In a series of 16 Andes virus-associated HPS cases in Chile, five patients, all of whom were among the survivors, received corticosteroid treatment along with supportive treatment, but the efficacy of corticosteroid treatment could not be determined due to the low number of cases [27]. In the series of 14 HPS cases described by Hallin et al. [15], antimicrobial and supportive treatment was given. The two survivors who required mechanical ventilation were extubated after 5 days and made a full recovery.
Renal histology in nephropathia epidemica is characterized by tubulointerstitial nephritis with mixed interstitial cellular infiltration and edema, tubular epithelial changes and occasional medullar hemorrhages [28]. In a recent retrospective survey of mainly drug-induced acute interstitial nephritis, no effect of corticosteroid treatment on renal recovery was found as compared with supportive therapy alone [29].
The haplotype HLAB8DR3DQ2 is associated with a severe form of nephropathia epidemica [30]. This haplotype is also associated with a high level of TNF-α production [31] and deletion of the C4A gene [30]. The HLAB8DR3 haplotype is known to be associated with several chronic autoimmune diseases and immunologic abnormalities. Neither of our patients had that HLA haplotype, but one of them lacked one of the C4B genes, leading to lowered levels of C4B and possibly altered immune function.
As far as we know, this report is the first to describe the effects of corticosteroid treatment for PUU infection complicated by severe hypoxemia, consistent with the ARDS criteria. In our two patients, the implementation of intravenous corticosteroids combined with CVVHDF was associated with resolution of both respiratory failure and shock. We suggest that corticosteroid therapy is safe and may hasten recovery in severe cardiopulmonary forms of PUU infection.
Acknowledgement
This report was supported by a HUS research grant.
Appendix
Calculation of the lung injury score (LIS)
| Component of LIS | Value |
|---|---|
| Chest radiograph | |
| No alveolar consolidation | 0 |
| Alveolar consolidation in one quadrant | 1 |
| Alveolar consolidation in two quadrants | 2 |
| Alveolar consolidation in three quadrants | 3 |
| Alveolar consolidation in four quadrants | 4 |
| Hypoxemia score PaO2/FiO2 (mmHg) | |
| ≥300 | 0 |
| 225–299 | 1 |
| 175–224 | 2 |
| 100–174 | 3 |
| <100 | 4 |
| Respiratory system compliance score (when ventilated) (ml/cmH2O) | |
| ≥80 | 0 |
| 60–79 | 1 |
| 40–59 | 2 |
| 20–39 | 3 |
| <19 | 4 |
| Positive end-expiratory pressure score (when ventilated) (cmH2O) | |
| <5 | 0 |
| 6–8 | 1 |
| 9–11 | 2 |
| 12–14 | 3 |
| ≥15 | 4 |
| Final value (obtained by dividing the aggregate sum by the number of components used) | |
| No lung injury | 0 |
| Acute lung injury | 0.1–2.5 |
| Adult respiratory distress syndrome | >2.5 |
References
- 1.Vapalahti O, Mustonen J, Lundkvist Å, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Review. Lancet Infect Dis. 2003;3:653–661. doi: 10.1016/S1473-3099(03)00774-6. [DOI] [PubMed] [Google Scholar]
- 2.Clement JP. Hantavirus. Antiviral Res. 2003;57:121–127. doi: 10.1016/S0166-3542(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 3.Kanerva M, Paakkala A, Mustonen J, Paakkala T, Lahtela J, Pasternack A. Pulmonary involvement in nephropathia epidemica: radiological findings and their clinical correlations. Clin Nephrol. 1996;46:369–378. [PubMed] [Google Scholar]
- 4.Clement J, Colson P, McKenna P. Hantavirus pulmonary syndrome in New England and Europe. N Engl J Med. 1994;331:545–546. doi: 10.1056/NEJM199408253310813. [DOI] [PubMed] [Google Scholar]
- 5.Launay D, Thomas C, Fleury D, Roueff S, Line M, Droz D, Vanhille P. Pulmonary-renal syndrome due to hemorrhagic fever with renal syndrome: an unusual manifestation of Puumala virus infection in France. Clin Nephrol. 2003;59:297–300. doi: 10.5414/cnp59297. [DOI] [PubMed] [Google Scholar]
- 6.Schütt M, Meisel H, Kruger D, Ulrich R, Dalhoff K, Dodt C. Life-threatening Dobrava hantavirus infection with unusually extended pulmonary involvement. Clin Nephrol. 2004;62:54–57. doi: 10.5414/cnp62054. [DOI] [PubMed] [Google Scholar]
- 7.Klempa B, Meisel H, Rath S, Bartel J, Ulrich R, Kruger D. Occurrence of renal and pulmonary syndrome in a region of northeast Germany where Tula hantavirus circulates. J Clin Microbiol. 2003;41:4894–4897. doi: 10.1128/JCM.41.10.4894-4897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vapalahti O, Lundkvist Å, Kallio-Kokko H, Paukku K, Julkunen I, Lankinen H, Vaheri A. Antigenic properties and diagnostic potential of Puumala virus nucleocapsid protein expressed in insect cells. J Clin Microbiol. 1996;34:119–125. doi: 10.1128/jcm.34.1.119-125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallio-Kokko H, Vapalahti O, Lundkvist Å, Vaheri A. Evaluation of Puumala virus IgG and IgM enzyme immunoassays based on recombinant baculovirus-expressed nucleocapsid protein for early nephropathia epidemica diagnosis. Clin Diagn Virol. 1998;10:83–90. doi: 10.1016/S0928-0197(97)10019-8. [DOI] [PubMed] [Google Scholar]
- 10.Vapalahti O, Kallio-Kokko H, Närvänen A, Julkunen I, Lundkvist Å, Plyusnin A, Lehväslaiho H, Brummer-Korvenkontio M, Vaheri A, Lankinen H. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early response. J Med Virol. 1995;46:293–303. doi: 10.1002/jmv.1890460402. [DOI] [PubMed] [Google Scholar]
- 11.Kallio-Kokko H, Leevelahti R, Brummer-Korvenkontio M, Lundkvist A, Vaheri A, Vapalahti O. Human immune response to Puumala virus glycoproteins and nucleocapsid protein expressed in mammalian cells. J Med Virol. 2001;65:605–613. doi: 10.1002/jmv.2079. [DOI] [PubMed] [Google Scholar]
- 12.Kim E, Lee K, Primack S, Yoon H, Byun H, Kim T, Suh G, Kwon O, Han J. Viral pneumonias in adults: radiologic and pathologic findings. RadioGraphics. 2002;22(Special issue):137–149. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. [DOI] [PubMed] [Google Scholar]
- 13.Peters C, Simpson G, Levy H. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med. 1999;50:531–545. doi: 10.1146/annurev.med.50.1.531. [DOI] [PubMed] [Google Scholar]
- 14.Duchin J, Koster F, Peters C, Simpson G, Tempest B, Zaki S, Ksiazek T, Rollin P, Nichol S, Umland E, et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- 15.Hallin G, Simpson S, Crowell R, James D, Koster F, Mertz G, Levy H. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit Care Med. 1996;24:252–258. doi: 10.1097/00003246-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Ketai L, Williamson M, Telepak R, Levy H, Koster F, Nolte K, Allen S. Hantavirus pulmonary syndrome: radiographic findings in 16 patients. Radiology. 1994;191:665–668. doi: 10.1148/radiology.191.3.8184043. [DOI] [PubMed] [Google Scholar]
- 17.Mori M, Rothman A, Kurane I, Montoya J, Nolte K, Norman J, Waite D, Koster F, Ennis F. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J Infect Dis. 1999;179:295–302. doi: 10.1086/314597. [DOI] [PubMed] [Google Scholar]
- 18.Terajima M, Hendershot J, III, Kariwa H, Koster F, Hjelle B, Goade D, DeFronzo M, Ennis F. High levels of viremia in patients with the Hantavirus pulmonary syndrome. J Infect Dis. 1999;180:2030–2034. doi: 10.1086/315153. [DOI] [PubMed] [Google Scholar]
- 19.Artigas A, Bernard G, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini J, Matthay M, Pinsky M, Spragg R, Suter P, Consensus Committee The American–European consensus conference on ARDS, Part 2. Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med. 1998;24:378–398. doi: 10.1007/s001340050585. [DOI] [PubMed] [Google Scholar]
- 20.Groeneveld A. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vasc Pharmacol. 2002;39:247–256. doi: 10.1016/S1537-1891(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 21.Riad M, Mogos M, Thangathurai D, Lumb P. Steroids. Curr Opin Crit Care. 2002;8:281–284. doi: 10.1097/00075198-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Metz C, Sibbald W. Anti-inflammatory therapy for acute lung injury. A review of animal and clinical studies. Chest. 1991;100:1110–1119. doi: 10.1378/chest.100.4.1110. [DOI] [PubMed] [Google Scholar]
- 23.Chiang C, Wu C, Perng W, Yan H, Yu C. Dexamethasone and pentastarch produce additive attenuation of ischaemia/reperfusion lung injury. Clin Sci. 2000;99:413–419. doi: 10.1042/CS20000081. [DOI] [PubMed] [Google Scholar]
- 24.Bone R, Fisher C, Jr, Clemmer T, Slotman G, Metz C. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987;92:1032–1036. doi: 10.1378/chest.92.6.1032. [DOI] [PubMed] [Google Scholar]
- 25.Meduri G, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1β and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 26.Meduri G, Headley S, Golden E, Carson S, Umberger R, Kelso T, Tolley E. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. A randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 27.Castillo C, Naranjo J, Sepulveda A, Ossa G, Levy H. Hantavirus pulmonary syndrome due to Andes virus in Temuco, Chile: clinical experience with 16 adults. Chest. 2001;120:548–554. doi: 10.1378/chest.120.2.548. [DOI] [PubMed] [Google Scholar]
- 28.Temonen M, Mustonen J, Helin H, Pasternack A, Vaheri A, Holthöfer H. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin Immunol Immunopathol. 1996;78:47–55. doi: 10.1006/clin.1996.0007. [DOI] [PubMed] [Google Scholar]
- 29.Clarkson M, Giblin L, O’Connel F, O’Kelly P, Walshe J, Conlon P, O’Meara Y, Dormon A, Campbell E, Donohoe J. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19:2778–2783. doi: 10.1093/ndt/gfh485. [DOI] [PubMed] [Google Scholar]
- 30.Mustonen J, Partanen J, Kanerva M, Pietilä K, Vapalahti O, Pasternack A, Vaheri A. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 1996;49:217–221. doi: 10.1038/ki.1996.29. [DOI] [PubMed] [Google Scholar]
- 31.Kanerva M, Vaheri A, Mustonen J, Partanen J. High-producer allele of tumour necrosis factor-alpha is part of the susceptibility MHC haplotype in severe Puumala virus-induced nephropathia epidemica. Scand J Infect Dis. 1998;30:532–534. doi: 10.1080/00365549850161629. [DOI] [PubMed] [Google Scholar]


