Abstract
Pregnant women are at the highest risk to develop severe and even fatal influenza. The high vulnerability of women against influenza A virus infections during pregnancy was repeatedly highlighted during influenza pandemics including the pandemic of this century. In 2009, mortality rates were particularly high among otherwise healthy pregnant women. However, our current understanding of the molecular mechanisms involved in severe disease development during pregnancy is still very limited. In this review, we summarize the knowledge on the clinical observations in influenza A virus-infected pregnant women. In addition, knowledge obtained from few existing experimental infections in pregnant animal models is discussed. Since clinical data do not provide in-depth information on the pathogenesis of severe influenza during pregnancy, adequate animal models are urgently required that mimic clinical findings. Studies in pregnant animal models will allow the dissection of involved molecular disease pathways that are key to improve patient management and care.
Keywords: Influenza, Pregnancy, Pathogenesis, Animal models
Introduction
Influenza A viruses (IAV) belong to the major causative agents of respiratory infections posing a considerable burden for human health. The clinical course of IAV infections in healthy individuals may vary from mild self-limiting disease to severe disease, which requires hospitalization with occasional lethal outcome. The annual influenza epidemic which peaks during winter in temperate climates is estimated to cause >600 million cases globally, with 3 to 5 million cases of severe illness and up to 500,000 deaths per year worldwide (www.who.int). Since IAV have a zoonotic origin with an unlimited reservoir in aquatic birds, they may cross species barriers and transmit to humans leading to novel virus variants with an unpredictable outcome. Recently emerged avian IAV belonging to the H5N1 and H7N9 subtypes are responsible for high case fatality rates in humans and pose a future pandemic threat (www.who.int). The influenza pandemic of this century was caused by an H1N1 IAV in 2009—A(H1N1)pdm09—possessing genomes of avian, swine, and human origin [1]. It is estimated that it has caused ~200,000 respiratory and an additional ~80,000 cardiovascular deaths predominantly in people younger than 65 years of age [2].
Mortality rates upon IAV infections are highest among patients with underlying medical conditions, such as those with chronic pulmonary, cardiovascular, renal, hepatic, neuromuscular, hematologic, and metabolic disorders or in patients being obese, pregnant, or having immunodeficiencies [3–7]. Data from previous pandemics such as the 1918 H1N1 and the 1957 H2N2 pandemic as well as from seasonal influenza epidemics revealed that pregnant women have an increased risk in developing complications upon IAV infection [8–13]. The pandemic caused by A(H1N1)pdm09 highlighted again that especially pregnant women are at the highest risk to develop severe or even fatal influenza. Data from the USA show that 5 % of the A(H1N1)pdm09-related deaths were among pregnant women, although they make up only 1 % of the total population [9]. This unprecedented very severe disease outcome during pregnancy led to the revision of vaccination recommendations by the World Health Organization (WHO), setting pregnant women now as the highest priority to be vaccinated again influenza regardless of gestational age.
During pregnancy, women undergo various physiological changes which might contribute to disease severity upon IAV infection. These physiological changes include many metabolic, hemodynamic, and immunologic changes to allow growth and development of the fetus. Metabolic changes occur, e.g., in the glucose and lipid metabolism, and hemodynamic changes include an increase of plasma volume by 1000–1600 ml and alterations in the systemic coagulation system [14]. During pregnancy, there are also specific changes in both the upper and lower respiratory tract. In the upper respiratory tract, the mucosa of the nasopharynx and oropharynx changes, including hyperemia, edema, leakage of plasma into the stroma, glandular hypersecretion, and increased mucopolysaccharide content. All these physiological changes can result in nasal congestion [15]. The lower respiratory tract is altered due to the elevation of the diaphragm by up to 4 cm and a decrease in functional residual capacity. Functionally, there are changes in lung function, ventilation, and gas exchange, which lead to an increase in oxygen tension required for trans-placental oxygen transfer. Furthermore, changes in the cardiovascular system result in a decrease of pulmonary vascular resistance [15, 16]. All these physiological alterations might be further challenged by respiratory viral infections, such as IAV and, thus, contribute to disease severity during pregnancy.
Immunological changes comprise the altered differentiation status of immune cells in the uterus and the formation of a tolerogenic environment at the maternal/fetal interface that prevent rejection of the semi-allogeneic fetal tissues and thereby permit pregnancy. There are also systemic changes of the immune system, mainly caused by pregnancy hormones. These changes are reflected in increased maternal disease outcome not only upon IAV infections but also in patients with HIV, malaria, and toxoplasmosis [5–7, 17].
Understanding the molecular basis that mediates increased mortality upon IAV infection during pregnancy is key to improve patient management and care. Therefore, adequate animal models are required that reflect clinical findings and allow the identification of causative disease pathways. This knowledge will allow early diagnosis and the implementation of rapid intervention strategies to improve disease outcome during pregnancy. It is estimated that vaccination compliance among pregnant women is still below the WHO recommendations although it has been shown that vaccination during pregnancy protects mother and child [18, 19]. However, a thorough worldwide documentation of vaccination rates is still missing [20]. Thus, there is an urgent need to increase disease awareness among this most vulnerable group to improve patient management by early diagnosis as well as by increasing the currently poor vaccination compliance among pregnant women.
Here, we review current knowledge on the pathogenesis of IAV-associated complications during pregnancy in humans and in established animal models as a basis for mechanistic studies.
Influenza A virus pathogenesis in pregnant women
Pathology in IAV-infected pregnant women
Pregnant women have an increased risk of developing IAV-associated complications due to infection with seasonal, pandemic, or zoonotic influenza viruses. Several studies have shown that co-morbidities were present in ~30 % of the cases, of which a history of asthma was seen most frequently. Other co-morbidities included diabetes, cardiac diseases, hematological disorders, and low weight. Since two thirds of the complications in pregnant women could not be explained by other underlying medical conditions, pregnancy itself increased the risk of developing IAV-associated complications [21, 22]. Interestingly, the majority of pregnant women that develop severe influenza virus-associated disease were in their second or third trimester [21, 22].
Frequent symptoms in hospitalized pregnant women include fever, cough, respiratory distress, malaise, joint pain, headache, rhinorrhea, gastrointestinal symptoms, and the development of pneumonia. The latter can lead to the development of acute respiratory distress syndrome (ARDS) [12, 21, 23–26]. ARDS is a rapid onset of hypoxemic respiratory failure associated with bilateral radiographic opacities without congestive heart failure. There are many causes for the development of ARDS during pregnancy. Besides the IAV-mediated pneumonia, other causes include amniotic fluid embolism, pre-eclampsia, sepsis, and aspiration pneumonia [27]. Treatment of A(H1N1)pdm2009-associated ARDS included extracorporeal life support, also known as extracorporeal membrane oxygenation (ECMO), which resulted in a survival rate of ~70 % [27].
Extra-respiratory tract complications are occasionally described in pregnant women. These include the development of myocarditis after infection with A(H1N1)pdm09 [28, 29], and the development of an encephalopathy associated with a seasonal H3N2 IAV infection from which viral RNA was detected in cerebrospinal fluids [30].
Histopathology
As in other risk groups, the most common complication of IAV in pregnant women is the development of pneumonia and ARDS. Several studies describe the histopathology in pregnant and non-pregnant fatal cases during the A(H1N1)pdm09 pandemic. These studies did not reveal differences between the two groups, although this has not been extensively studied. In pregnant women, histopathological lesions were predominantly found in the respiratory tract, characterized by multifocal desquamation of the epithelial lining of the trachea and bronchus and inflammation of the submucosal glands. Within the lungs, there was evidence of diffuse alveolar damage, characterized by extensive fibrosis, hyaline membrane formation, thickening of the alveolar septa, type II pneumocyte hyperplasia, interstitial and alveolar edema, and an influx of many inflammatory cells [31–34]. Virus antigen was detected in ciliated epithelial cells as well as the submucosal glands of the bronchi and bronchioles. In the alveoli, virus antigen could be detected within the alveolar epithelial cells and alveolar macrophages [31, 32, 34]. In none of these cases, there was evidence of extra-respiratory tract replication.
Post-mortem histopathological examination of a 24-year-old otherwise healthy pregnant women who succumbed to A(H1N1)pdm2009 after developing ARDS [26] revealed severe lung damage with desquamated alveolar epithelial cells, destruction of the alveolar septae and interstitial inflammatory cells as described in other cases. Additional immunohistochemical staining identified inflammatory cells which mainly consisted of CD68+ macrophages and CD3+ T lymphocytes. Since the patient died 28 days after hospitalization and had received antiviral therapy, viral RNA could not be detected by in situ hybridization (Fig. 1).
Fig. 1.
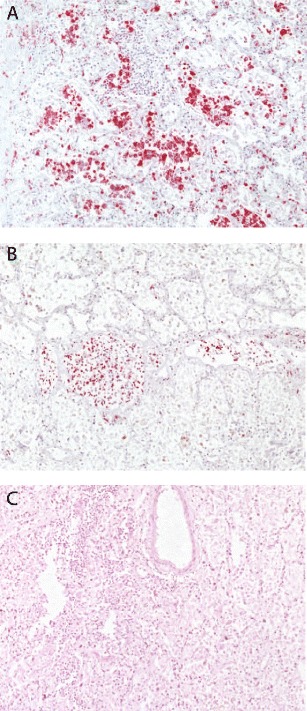
Post-mortem histopathological analysis of lung biopsy material obtained from a fatal pregnant case. Lung biopsy material was obtained from a 24-year-old pregnant woman without any known underlying diseases who succumbed to the 2009 H1N1 influenza virus infection after spontaneous expulsion of the fetus [26]. A severe alveolitis with numerous immunohistochemically (red) stained CD68+ macrophages (a) and CD3+ T lymphocytes (b) is observed. At this late stage of infection, influenza virus RNA was not detectable anymore by radioactive in situ hybridization (HE staining) (c). The histochemical stainings were performed according to protocols described before [35]
Post-mortem analysis of an H5N1 highly pathogenic avian virus (HPAIV) infected 4-month pregnant women showed similar diffuse alveolar damage characterized by focal desquamation of epithelial cells in the alveoli without any evidence of type II pneumocyte hyperplasia, influx of macrophages, neutrophils, and few lymphocytes. No lesions were described in any other parts of the respiratory tract. Virus antigen was detected in tracheal epithelial cells and alveolar epithelial cells. In contrast to reports on A(H1N1)pdm2009-infected pregnant women, upon H5N1 HPAIV infection, extra-respiratory virus spread to the placenta could be detected with virus-positive cytotrophoblastic and Hofbauer cells. There, lesions included scattered foci of syncytiotrophoblast necrosis, occasionally associated with dystrophic calcification. Moreover, virus was detected in the fetus with virus antigen-positive Kupffer cells of the liver and pneumocytes in the lungs. Virus in the fetal liver was not associated with histological lesions, but the fetal lung showed edema and a few scattered interstitial neutrophils [36].
However, the pathogenesis of IAV-associated complications in pregnant women is studied to a very limited extent. Important host factors for the pathogenesis of influenza in mammals including humans are the distribution of specific influenza virus receptors and nuclear transport proteins, the importin-α isoforms [37, 38] which largely determine the cell tropism of influenza viruses within the respiratory tract. Unfortunately, the impact of these cellular factors has not been studied during pregnancy yet. It is therefore currently unknown whether potential alterations in the distribution of receptors or importin-α isoforms might contribute to the increased risk of developing complications during pregnancy.
Immunopathology
Pregnancy causes the formation of an immune-tolerant environment in the uterus that prevents rejection of fetal tissue. Moreover, pregnancy has also profound effects on the peripheral immune system outside of the uterus that might interfere with the response against IAV infections [5, 6]. Endocrine adaptation to pregnancy, in particular the elevated levels of estradiol and progesterone, affect cytokine levels as well as their composition and function of peripheral leukocyte populations (reviewed in [6]). Numbers of peripheral neutrophils, monocytes, as well as myeloid and plasmacytoid dendritic cells (DC) are reduced [39]. Upon in vitro stimulation, both NK cells and CD4 T cells produce reduced amounts of inflammatory cytokines and chemokines. Interestingly, reduced cytokine production by CD4 T cells includes both TH1 (IFN-γ, TNF-α) and TH2 cytokines (IL-6, IL-13) [39] arguing against a shift in the TH1-TH2 balance during pregnancy as has been postulated from mouse studies [40]. DCs from pregnant women also display higher expression of costimulatory surface molecules, such as CD40, CD80, and CD86, than those of non-pregnant women suggesting a more mature status of DCs [41–43]. It should be mentioned that results on the phenotype and function of immune cells during pregnancy differ considerably between individual studies. Our understanding of immunity during pregnancy would clearly benefit from more systematic and comprehensive approaches using advanced methodology to better define and characterize immune cell subsets.
Despite these changes in the peripheral immune system, vaccination studies indicate that there is no general deficit in the adaptive immune response of pregnant women to influenza virus. Pregnant women still generate protective antibodies against IAV, which are further transferred to the fetus [44–47]. Moreover, pregnant women show diminished polyclonal T cell responses but enhanced virus-specific T cell responses following influenza vaccination [48]. Thus, despite significant changes in their peripheral immune system, pregnant women are still able to effectively mount an adaptive immune response against IAV.
Currently, there is only very limited information on the immune response against IAV in pregnant woman with severe IAV-associated complications. As stated before, complications in pregnant women are similar to those observed in other risk groups, arguing for potentially common mechanisms that mediate disease severity. There is evidence that immunopathology strongly contributes to severe courses of IAV infections [49]. High virus titers in the lung, which might represent a consequence of reduced production of type I and type III interferons in pregnant women [50], could cause excessive production of inflammatory cytokines and chemokines and massive infiltration of granulocytes and macrophages which subsequently cause severe tissue damage. Altered production of inflammatory cytokines as well as differences in the composition and response of peripheral immune cells in pregnant women could contribute to such an exacerbated response against IAV [6]. However, so far, there is no direct evidence supporting such a scenario. Patients with severe IAV infections show lower serum levels of IgG2. Healthy pregnant women also have low levels of serum IgG2, and levels are further reduced in pregnant women with severe IAV infections [51–53]. Yet again, the contribution of low serum IgG2 levels to severe courses of infection in pregnant women is not clear. Overall, it is currently not known why pregnant woman are more prone to develop severe courses of disease upon IAV infections.
Impact of maternal IAV infection on fetal development
IAV infections can also have an effect on the pregnancy outcome or the development of the fetus. IAV infections have been associated with an increase in perinatal mortality, preterm birth, cesarean section, and a low Apgar score 5 min after birth [9, 54–56]. In addition, infection with IAV within the first trimester of the pregnancy increases the risk by twofold on non-chromosomal congenital anomalies, such as neural tube defects, hydrocephaly, congenital heart defects, cleft lip, digestive system defects, and limb reduction defects [57]. Viral antigen has been detected in the amniotic fluid (H3N2 virus; [58]) and in fetal tissue (H5N1 virus; [36]). However, in the majority of cases, IAV could not be isolated from the placenta or fetus [31, 59, 60]. This suggests that the effect on pregnancy outcome and fetus might represent an indirect result of IAV infection of the mother, such as fever and cytokine and immune response [61]. Overall, there is still a lack of detailed knowledge regarding the impact of maternal IAV infection on pre- or even postnatal development in the offspring.
Animal models of influenza A virus pathogenicity during pregnancy
To gain more insight into the pathogenesis of IAV-associated complications during pregnancy, studies have been performed in small animal models, using mice, ferrets, and pigs. These experimental settings provide insights into events early after infection and mechanisms of diseases, which are difficult to study with materials from infected patients.
Mice
Experimental infections in pregnant BALB/c mice at a gestational age of 12–14 days have been performed with seasonal H1N1 and A(H1N1)pdm09 IAV as well as with H5N1 HPAIV. Intranasal infection with 104 or 105 egg infectious dose or intraperitoneal infection with 2 × 106 plaque forming units of pregnant mice with A(H1N1)pdm09 IAV resulted in a higher mortality compared to non-pregnant mice [62–64]. Virus titers in the lungs were higher and histological lesions were more severe in pregnant mice compared to non-pregnant mice at 5 days p.i. [63]. However, in another study, virus titers in bronchoalveolar lavage (BAL) fluids were comparable between pregnant and non-pregnant mice at 3 days p.i. [64]. Intranasal infection of pregnant mice with a seasonal H1N1 IAV did not result in increased mortality, although virus titers in the lung were higher and histological lesions were more severe compared to non-pregnant mice [63]. Virus transmission to extra-respiratory tract tissues, including the placenta and fetus, was not observed in any of these studies [62–64].
The importance of viral factors in the pathogenicity of IAV-associated disease during pregnancy was shown in a study which compared a wild type A(H1N1)pdm09 IAV to a mouse-adapted A(H1N1)pdm09 IAV that contained the D222G mutation in the hemagglutinin. Infection with the mouse-adapted virus resulted in increased mortality, higher virus titers in the lung, more influenza virus-infected cells in the bronchioles and alveoli when compared to pregnant mice infected with the wild type virus [62]. A study with H5N1 HPAIV showed that in pregnant mice, virus titers in the lungs were lower compared to virus titers in non-pregnant mice at 5 days p.i. However, in pregnant mice, H5N1 HPAIV spread to extra-respiratory organs and the virus could be detected in the placenta and fetus of intranasally infected mice [65].
Histological lesions in the respiratory tract of A(H1N1)pdm09-inoculated pregnant mice were observed in the bronchioles and alveoli. The bronchiolar epithelium showed accumulation of mucous and detached cilia. In the alveoli, lesions were compatible with interstitial pneumonia associated with infiltration of lymphocytes and neutrophils [63]. Although histological lesions were not described in detail, the location of the lesions is consistent with those observed in humans. Taken together, although the number of studies is very limited, they show that virus replication, histological lesions, and mortality are increased in pregnant mice compared to non-pregnant mice. Future studies should reveal more insight into virus replication and associated lesions at different time points after infection to understand the dynamics within the mammalian host in time and the duration of infection and virus shedding.
Infection of mice has also been used to determine the consequences of pregnancy for the immune response against infection with pandemic A(H1N1)pdm09 [62–64]. Compared to infection of mice with seasonal H1N1 strains, infection with A(H1N1)pdm09 resulted in more severe interstitial pneumonia with massive cellular infiltrations, and lung pathology was further aggravated when pregnant mice were infected [63]. Compared to non-pregnant mice, pregnant mice infected with A(H1N1)pdm09 IAV demonstrated enhanced accumulation of neutrophils and macrophages in lung tissue and BAL fluid, and increased local NO production [64]. As expected, infection with A(H1N1)pdm09 was associated with increased levels of inflammatory cytokines in the lung when compared to infection with seasonal H1N1 IAV [63]. In comparison to non-pregnant mice, pregnant animals had enhanced levels of inflammatory cytokines (IL-1β, IL-6, TNF-α) as well as neutrophil- and monocyte-recruiting chemokines in BAL fluid and lung tissue, which was consistent with the fulminant accumulation of these cells in the lung [62, 64]. In contrast to the profound changes in the innate response to IAV infections, the adaptive response appeared to be less affected by pregnancy. Pregnant and non-pregnant mice generated similar frequencies of influenza virus-specific CD8+ T cells and these cells showed comparable activation, as indicated by CD69 expression, and accumulation in the infected lung [64]. Pregnant mice also mounted strong antibody responses against A(H1N1)pdm09, although in one study, titers of antibodies were reduced compared to non-pregnant mice [62, 64]. Overall, these studies indicate that disease severity in pregnant syngenically mated mice upon A(H1N1)pdm09 IAV infection is mainly due to enhanced cytokine production in the lung and massive recruitment of innate immune cells which results in severe tissue damage.
Ferrets
Intranasal inoculation of a seasonal H3N2 IAV in ferrets at early, mid, and late gestation resulted in virus replication within the respiratory tract comparable to that described before for non-pregnant ferrets. Virus was not transmitted to the placenta, umbilical cord, amnion, and chorion of the fetus [66, 67]. Of note, intracardial inoculation of H3N2 virus did result in virus transmission to the placenta and fetus, indicating that a high virus load viremia might be crucial for virus transmission to the fetus. Of note, in general, seasonal influenza viruses do not cause viremia in non-pregnant women [68].
To our knowledge, there are no studies performed in pregnant ferrets with recent seasonal IAV, pandemic A(H1N1)pdm09 IAV, or H5N1 HPAIV. Since the pathogenesis of IAV is extensively studied in this mammalian species, such studies would provide useful information on the pathogenesis of IAV infections during pregnancy. In addition, the pregnant ferret model could be used to study the efficacy of vaccines and antiviral therapies.
Pigs
Intranasal or intratracheal inoculation of young pregnant gilts, 85–90 days post insemination, with swine H1N2, swine H3N2, or A(H1N1)pdm09 IAV did not result in any clinical signs or gross pathological lesions 7 days post infection. In none of the inoculated animals (n = 5 per group), there was evidence for viremia or trans-placental virus transmission [69, 70]. However, another study did reveal trans-placental transmission of a swine H1N1 virus in one out of ten pigs [71].
Infection with swine H1N2, swine H3N2, and A(H1N1)pdm09 induced similar titers of influenza-specific antibodies and similar proliferative response of peripheral blood cells to stimulation with influenza virus. However, serum levels of IL-6, IL-10, and TNF-α were enhanced and sustained for several days in pregnant pigs infected with pandemic H1N1 suggesting that infection with this virus caused a stronger innate immune response [69, 70].
Conclusion
There is strong evidence that immune adaptation during pregnancy contributes to influenza disease severity. It was hypothesized that there is a contradictory demand for the maternal immune system to adapt to pregnancy in order to maintain the allogenic fetus and to simultaneously mount an immune response to clear viral infection [6]. However, available animal models reveal that the severity of the infection during pregnancy also depends on viral factors since viral pathogenicity is enhanced upon pandemic rather than seasonal IAV infection.
The severe and even fatal disease outcome observed in pregnant women is mostly studied and reflected in mouse models so far, although these studies are restricted to one mouse strain and infection at a gestational age of 12–14 days. The pregnant ferret and pig models may also provide complementary information on viral pathogenicity observed in humans although these models have only been used in single studies so far. In general, ferrets and to a lesser extent pigs, mimic IAV-mediated respiratory pathogenesis similar to that seen in humans. Therefore, pathogenesis studies in pregnant pigs and ferrets—which are both outbread—should be included in future studies.
In summary, future efforts in developing animal models that compare the pathogenesis of different IAV strains and associated pathogenesis, including virus replication, histological lesions, and immune responses in infected dams and their impact on the offspring are urgently required. These studies should reveal IAV replication dynamics and associated immune responses. In addition, these models could be used to study the pathogenesis during pregnancy of other emerging influenza viruses and evaluate vaccine or antiviral efficacy during pregnancy. Thus, these studies will allow an evidence-based risk assessment to increase disease awareness and to improve patient management and care.
Acknowledgments
This work was supported by research grants from the German Center for Infection Research (DZIF), the Fonds Nationale de la Recherche Luxembourg (FNR; AFR 6042498), Boehringer Ingelheim Fonds, and the German Research Foundation (DFG; GA1575/3; MI476/5). Debby van Riel is supported by a fellowship from the Netherlands Organisation for Scientific Research (NWO; contract number 91614115) and the Erasmus MC foundation.
References
- 1.Garten RJ, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawood FS, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12(9):687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 3.Van Kerkhove MD, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiore AE, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 5.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013;19(5):548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis. 2014;209(Suppl 3):S93–S99. doi: 10.1093/infdis/jiu020. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy JM, et al. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957-1958. Am J Public Health Nations Health. 1961;51:1182–1188. doi: 10.2105/AJPH.51.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen SA, Jamieson DJ. Influenza and pregnancy in the United States: before, during, and after 2009 H1N1. Clin Obstet Gynecol. 2012;55(2):487–497. doi: 10.1097/GRF.0b013e31824df23e. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen SA, Jamieson DJ. 2009 H1N1 influenza and pregnancy—5 years later. N Engl J Med. 2014;371(15):1373–1375. doi: 10.1056/NEJMp1403496. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14(1):95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers VL, et al. Presentation of seasonal influenza A in pregnancy: 2003–2004 influenza season. Obstet Gynecol. 2010;115(5):924–929. doi: 10.1097/AOG.0b013e3181da0c5e. [DOI] [PubMed] [Google Scholar]
- 13.Neuzil KM, et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 14.Gongora MC, Wenger NK. Cardiovascular complications of pregnancy. Int J Mol Sci. 2015;16(10):23905–23928. doi: 10.3390/ijms161023905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med. 2011;32(1):1–13. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Brito V, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med. 2011;32(1):121–132. doi: 10.1016/j.ccm.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13(1):23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 18.Olsen, S.J., et al. (2016) The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis. doi:10.1093/cid/ciw290 [DOI] [PMC free article] [PubMed]
- 19.Takeda S, et al. Influenza vaccination during pregnancy and its usefulness to mothers and their young infants. J Infect Chemother. 2015;21(4):238–246. doi: 10.1016/j.jiac.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Ding H, et al. Influenza vaccination coverage among pregnant women—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(36):1000–1005. doi: 10.15585/mmwr.mm6436a2. [DOI] [PubMed] [Google Scholar]
- 21.Lim C, et al. Influenza A(H1N1)pdm09 infection in pregnant and non-pregnant women hospitalized in Singapore, May–December 2009. Public Health. 2015;129(6):769–776. doi: 10.1016/j.puhe.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Louie JK, et al. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 23.Ergonul O, et al. Predictors of fatality in pandemic influenza A (H1N1) virus infection among adults. BMC Infect Dis. 2014;14:317. doi: 10.1186/1471-2334-14-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokolow LZ, et al. Severity of influenza and noninfluenza acute respiratory illness among pregnant women, 2010–2012. Am J Obstet Gynecol. 2015;212(2):202 e1–202 11. doi: 10.1016/j.ajog.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Varela MM, et al. Pandemic influenza A (H1N1) infection in pregnant and nonpregnant women in Spain (2009–2010) Jpn J Infect Dis. 2014;67(3):163–171. doi: 10.7883/yoken.67.163. [DOI] [PubMed] [Google Scholar]
- 26.Bowkalow S, et al. Severe H1N1-infection during pregnancy. Arch Gynecol Obstet. 2011;284(5):1133–1135. doi: 10.1007/s00404-011-1967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte AG. ARDS in pregnancy. Clin Obstet Gynecol. 2014;57(4):862–870. doi: 10.1097/GRF.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 28.Chan K, Meek D, Chakravorty I. Unusual association of ST-T abnormalities, myocarditis and cardiomyopathy with H1N1 influenza in pregnancy: two case reports and review of the literature. J Med Case Rep. 2011;5:314. doi: 10.1186/1752-1947-5-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ona MA, et al. A case of fatal fulminant myocarditis presenting as an acute ST-segment elevation myocardial infarction and persistent ventricular tachyarrhythmia associated with influenza A (H1N1) virus in a previously healthy pregnant woman. Cardiology. 2012;123(2):103–107. doi: 10.1159/000342076. [DOI] [PubMed] [Google Scholar]
- 30.Hakoda S, Nakatani T. A pregnant woman with influenza A encephalopathy in whom influenza A/Hong Kong virus (H3) was isolated from cerebrospinal fluid. Arch Intern Med. 2000;160(7):1041. doi: 10.1001/archinte.160.7.1041. [DOI] [PubMed] [Google Scholar]
- 31.Bal A, et al. Pathology and virology findings in cases of fatal influenza A H1N1 virus infection in 2009–2010. Histopathology. 2012;60(2):326–335. doi: 10.1111/j.1365-2559.2011.04081.x. [DOI] [PubMed] [Google Scholar]
- 32.Gill JR, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134(2):235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li HJ, et al. Critical influenza (H1N1) pneumonia: imaging manifestations and histopathological findings. Chin Med J. 2012;125(12):2109–2114. [PubMed] [Google Scholar]
- 34.Shelke VN, et al. Pathologic study of pandemic influenza A (H1N1) 2009 cases from India. Pathol Int. 2012;62(1):36–42. doi: 10.1111/j.1440-1827.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 35.Gabriel G, et al. Spread of infection and lymphocyte depletion in mice depends on polymerase of influenza virus. Am J Pathol. 2009;175(3):1178–1186. doi: 10.2353/ajpath.2009.090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu J, et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370(9593):1137–1145. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabriel G, et al. Differential use of importin-alpha isoforms governs cell tropism and host adaptation of influenza virus. Nat Commun. 2011;2:156. doi: 10.1038/ncomms1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Riel D, et al. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol. 2007;171(4):1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraus TA, et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol. 2012;32(2):300–311. doi: 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wegmann TG, et al. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 41.Della Bella S, et al. Incomplete activation of peripheral blood dendritic cells during healthy human pregnancy. Clin Exp Immunol. 2011;164(2):180–192. doi: 10.1111/j.1365-2249.2011.04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanders RL, et al. Plasmacytoid dendritic cells and CD8 T cells from pregnant women show altered phenotype and function following H1N1/09 infection. J Infect Dis. 2013;208(7):1062–1070. doi: 10.1093/infdis/jit296. [DOI] [PubMed] [Google Scholar]
- 43.Bachy V, Williams DJ, Ibrahim MA. Altered dendritic cell function in normal pregnancy. J Reprod Immunol. 2008;78(1):11–21. doi: 10.1016/j.jri.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Schlaudecker EP, et al. Pregnancy modifies the antibody response to trivalent influenza immunization. J Infect Dis. 2012;206(11):1670–1673. doi: 10.1093/infdis/jis592. [DOI] [PubMed] [Google Scholar]
- 45.Sperling RS, et al. Immunogenicity of trivalent inactivated influenza vaccination received during pregnancy or postpartum. Obstet Gynecol. 2012;119(3):631–639. doi: 10.1097/AOG.0b013e318244ed20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi K, et al. Relationship of Th1/Th2 cell balance with the immune response to influenza vaccine during pregnancy. J Med Virol. 2009;81(11):1923–1928. doi: 10.1002/jmv.21620. [DOI] [PubMed] [Google Scholar]
- 47.Zaman K, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 48.Kay AW, et al. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci U S A. 2014;111(40):14506–14511. doi: 10.1073/pnas.1416569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Jong MD, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forbes RL, et al. Pregnant women have attenuated innate interferon responses to 2009 pandemic influenza A virus subtype H1N1. J Infect Dis. 2012;206(5):646–653. doi: 10.1093/infdis/jis377. [DOI] [PubMed] [Google Scholar]
- 51.Chan JF, et al. The lower serum immunoglobulin G2 level in severe cases than in mild cases of pandemic H1N1 2009 influenza is associated with cytokine dysregulation. Clin Vaccine Immunol. 2011;18(2):305–310. doi: 10.1128/CVI.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon CL, et al. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50(5):672–678. doi: 10.1086/650462. [DOI] [PubMed] [Google Scholar]
- 53.Zheng R, et al. Imbalanced anti-H1N1 immunoglobulin subclasses and dysregulated cytokines in hospitalized pregnant women with 2009 H1N1 influenza and pneumonia in Shenyang, China. Hum Immunol. 2012;73(9):906–911. doi: 10.1016/j.humimm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205(1):10–18. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 55.Pierce M, et al. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ. 2011;342:d3214. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang PJ, et al. Clinical features and risk factors for severe and critical pregnant women with 2009 pandemic H1N1 influenza infection in China. BMC Infect Dis. 2012;12:29. doi: 10.1186/1471-2334-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luteijn JM, Brown MJ, Dolk H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum Reprod. 2014;29(4):809–823. doi: 10.1093/humrep/det455. [DOI] [PubMed] [Google Scholar]
- 58.McGregor JA, et al. Transplacental passage of influenza A/Bangkok (H3N2) mimicking amniotic fluid infection syndrome. Am J Obstet Gynecol. 1984;149(8):856–859. doi: 10.1016/0002-9378(84)90604-5. [DOI] [PubMed] [Google Scholar]
- 59.Meijer WJ, et al. High rate of chronic villitis in placentas of pregnancies complicated by influenza A/H1N1 infection. Infect Dis Obstet Gynecol. 2014;2014:768380. doi: 10.1155/2014/768380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramphal R, Donnelly WH, Small PA. Fatal influenzal pneumonia in pregnancy: failure to demonstrate transplacental transmission of influenza virus. Am J Obstet Gynecol. 1980;138(3):347–348. doi: 10.1016/0002-9378(80)90265-3. [DOI] [PubMed] [Google Scholar]
- 61.Edwards MJ. Influenza, hyperthermia, and congenital malformation. Lancet. 1972;1(7745):320–321. doi: 10.1016/S0140-6736(72)90327-3. [DOI] [PubMed] [Google Scholar]
- 62.Chan KH, et al. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One. 2010;5(10):e13757. doi: 10.1371/journal.pone.0013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HM, et al. The 2009 pandemic H1N1 influenza virus is more pathogenic in pregnant mice than seasonal H1N1 influenza virus. Viral Immunol. 2012;25(5):402–410. doi: 10.1089/vim.2012.0007. [DOI] [PubMed] [Google Scholar]
- 64.Marcelin G, et al. Fatal outcome of pandemic H1N1 2009 influenza virus infection is associated with immunopathology and impaired lung repair, not enhanced viral burden, in pregnant mice. J Virol. 2011;85(21):11208–11219. doi: 10.1128/JVI.00654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu L, et al. Highly pathogenic avian influenza H5N1 virus could partly be evacuated by pregnant BALB/c mouse during abortion or preterm delivery. Virol J. 2011;8:342. doi: 10.1186/1743-422X-8-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collie MH, et al. Association of foetal wastage with influenza infection during ferret pregnancy. Br J Exp Pathol. 1978;59(2):190–195. [PMC free article] [PubMed] [Google Scholar]
- 67.Sweet C, et al. The pregnant guinea-pig as a model for studying influenza virus infection in utero: infection of foetal tissues in organ culture and in vivo. Br J Exp Pathol. 1977;58(2):133–139. [PMC free article] [PubMed] [Google Scholar]
- 68.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26(Suppl 4):D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwit K, Pomorska-Mol M, Markowska-Daniel I. The influence of experimental infection of gilts with swine H1N2 influenza A virus during the second month of gestation on the course of pregnancy, reproduction parameters and clinical status. BMC Vet Res. 2014;10:123. doi: 10.1186/1746-6148-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwit K, Pomorska-Mol M, Markowska-Daniel I. Pregnancy outcome and clinical status of gilts following experimental infection by H1N2, H3N2 and H1N1pdm09 influenza A viruses during the last month of gestation. Arch Virol. 2015;160(10):2415–2425. doi: 10.1007/s00705-015-2518-8. [DOI] [PubMed] [Google Scholar]
- 71.Wallace GD, Elm JL., Jr Transplacental transmission and neonatal infection with swine influenza virus (Hsw1N1) in swine. Am J Vet Res. 1979;40(8):1169–1172. [PubMed] [Google Scholar]


