Abstract
Anti-MDA5 antibodies have been strongly associated with rapidly progressive interstitial lung disease (RP-ILD) in dermatomyositis (DM) patients, especially in the clinically amyopathic subset (CADM). We present a case of anti-MDA5 antibody-associated RP-ILD in a patient with arthritis but with no other clinical signs suggestive of DM or CADM successfully treated with a combination of cyclophosphamide, cyclosporine and corticoids. A review of the literature was also done. Despite its rarity, anti-MDA5 antibody-associated ILD should be suspected in cases of RP-ILD even without other signs of DM or CADM as prompt and aggressive treatment could improve prognosis.
Keywords: Anti-MDA5 antibodies, Anti-CADM-140 antibodies, Dermatomyositis, Clinically amyopathic dermatomyositis, Rapidly progressive interstitial lung disease, Diffuse alveolar damage
Introduction
Rapidly progressive interstitial lung disease (RP-ILD) is a feared complication of dermatomyositis, especially clinically amyopathic dermatomyositis (CADM) [1]. Anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibody, previously known as anti-CADM-140 antibody, has been related to RP-ILD in patients with dermatomyositis (DM) or CADM, and its presence has been associated with an unfavourable prognosis (90-day survival rate of 67%) [1, 2].
This clinical entity should be suspected in patients with DM or CADM presenting with RP-ILD. However, anti-MDA5 antibody-associated RP-ILD may be present in the absence of manifestations of muscle or skin disease [3, 4]. In those cases, diagnosis may be challenging and optimal treatment delayed.
We present a case of anti-MDA5 antibody-associated RP-ILD in a patient with arthritis but with no other clinical signs suggestive of DM or CADM.
Case report
A previously healthy 54-year-old Senegalese woman presented at the outpatient clinic with a 2-week onset of arthralgia and dyspnoea.
Initial physical examination revealed symmetrical arthritis of the ankles, wrists and metacarpophalangeal joints as well as “velcro-type” crackles in both lung bases. Basal oxygen saturation was over 98%. No skin disease was evidenced. The first laboratory findings showed a moderate elevation of inflammatory markers: C-reactive protein (CRP) of 10.8 mg/L and serum ferritin of 327.7 ng/mL. Creatine kinase and lactate dehydrogenase were normal. In addition, an immunological study showed rheumatoid factor (RF) activity (161.5 IU/mL) but all antinuclear antibodies (ANA), anti-DNA antibodies, antineutrophil cytoplasmic antibodies (ANCA), antiphospholipid antibodies (aPL) and anti-cyclic citrullinated peptides (aCCP) were negative. Serum complement factors were within normal levels. Hand X-ray was normal.
Based on the clinical suspicion of rheumatoid arthritis, and while waiting for a thoracic computed tomography (CT) to be done, medium doses of prednisone (30 mg/day) were started with rapid improvement of joint symptoms. However, a week after the initial evaluation the patient visited the clinic because of a rapid worsening of dyspnoea.
The patient now presented with severe respiratory failure (PaO2/FiO2 200) although mechanical ventilation was not required. A thoracic CT and a bronchoscopy with transbronchial biopsy were then performed. The thoracic CT revealed the presence of peripheral ground-glass opacities predominantly in the lung bases (Fig. 1). The lung biopsy showed signs of acute alveolar damage (Fig. 2). No germs were isolated either in bronchoscopy specimens or in blood and urine cultures. All viral serologies were negative (including HIV, hepatitis B and C virus, cytomegalovirus and Epstein–Barr virus). The most remarkable laboratory findings were: ESR of 50 mm, serum ferritin of 331 ng/mL and CRP 3.4 mg/L.
Fig. 1.
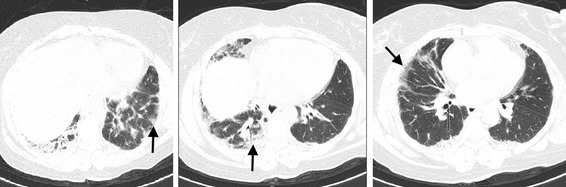
Thoracic CT showing peripheral ground-glass opacities predominantly in the lung bases (arrows)
Fig. 2.
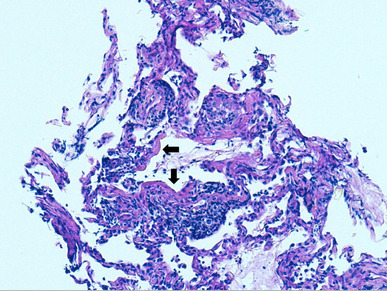
Transbronchial biopsy. Periodic acid–Schiff (PAS) staining. ×200. Lung parenchyma with destructuring, inflammatory infiltrate and presence of hyaline membrane remnants (arrows)
Based on the clinical suspicion of acute interstitial pneumonia, high doses of methylprednisolone (MP) (five pulses of 500 mg/day) followed by a tapering regimen of prednisone starting at 1 mg/kg and 1000 mg of cyclophosphamide (repeated after 15 days and then monthly for 6 months) were initiated. The patient showed slow but progressive clinical improvement and was discharged 1 month after admission.
Although initial clinical suspicion of the presence of anti-MDA5 antibodies was low, these were tested due to the rapidly progressive interstitial lung disease and a positive result was obtained. Cyclosporine (100 mg/12 h) was then added to the treatment. A panel of myositis-specific and myositis-associated antibodies were done showing negative results. This panel included anti-tRNA-synthetase (anti-Jo1, anti-PL7, anti-PL12, anti-OJ, anti-Ej, anti-KS), anti-Mi2, anti-SRP, anti-U1RNP, anti-U3RNP, anti-PmScl and anti-p155.
Ten months after therapy initiation, the patient remains asymptomatic with a daily dose of 7.5 mg of prednisone and 150 mg/12 h of cyclosporine. There were no cutaneous manifestations and muscle strength was normal during follow-up.
Discussion
According to the International Consensus Statement on Idiopathic Pulmonary Fibrosis of the American Thoracic Society and the European Respiratory Society [5], RP-ILD, including acute/subacute interstitial pneumonia, is a progressive deterioration associated with ILD within 3 months. In addition, anti-MDA5 antibodies have been strongly associated with RP-ILD in dermatomyositis patients, especially in the CADM subset of patients [2, 6].
The singularity as well as the controversial feature of the case of anti-MDA5 antibody-associated RP-ILD described here is the fact that the patient did not fulfil diagnostic criteria for DM or CADM as no evident cutaneous disease developed in the more than 6 months period following clinical onset.
We searched PubMed and EMBASE for English and Spanish language sources using the following keywords: anti-MDA5, anti-CADM-140, rapidly progressive interstitial lung disease and diffuse alveolar damage. We found only two cases of anti-MDA5 antibody-associated RP-ILD without typical clinical characteristics of DM or CADM. Tamai et al. [3] reported the case of a patient with a diagnosis of CADM-associated RP-ILD who presented with lung disease without cutaneous manifestations. However, a month after the onset of pulmonary symptoms, typical DM skin lesions developed. Ortíz-Santamaría et al. briefly reported a case of anti-MDA5 antibody-associated RP-ILD in a patient with cardiac involvement but without cutaneous findings [4].
It is already known that pulmonary disease can precede systemic symptoms in some autoimmune diseases [7, 8]. When ILD has a rheumatologic component such as specific autoantibodies or histological features, the term lung-dominant connective tissue disease (CTD) could be used [7]. Recently, the European Respiratory Society and the American Thoracic Society have jointly developed a set of classification criteria for interstitial pneumonia with autoimmune features (IPAF) [8]. Based on these criteria, our patient could be classified as having IPAF as she presented with interstitial lung disease and arthritis together with specific autoantibodies, without fulfilling classification criteria for any specific CTD.
Several case series have shown that anti-MDA5 antibody-associated RP-ILD is almost always associated with a clinical diagnosis of DM or CADM. In fact, anti-MDA5 antibodies have been associated with severe cutaneous manifestations [9]. Chen et al. reviewed 26 cases of positive anti-MDA5 antibody patients and the current literature, and found that in all cases patients presented with cutaneous manifestations [10]. More recently, Moghadam-Kia et al. described 16 cases of DM or CADM with positive anti-MDA5 antibodies, all of whom showed dermatologic abnormalities [11]. However, all these studies included patients with a clinical diagnosis of DM or CADM, so that the presence of cutaneous disease was mandatory.
It is possible that anti-MDA5 antibodies are not measured routinely in forms of ILD without classic DM symptoms, so that their prevalence in incomplete forms of DM or other autoimmune conditions could be underestimated.
The presence of anti-MDA5 antibodies has been strongly associated with RP-ILD in DM, especially in the CADM subtype [12]. A recent meta-analysis has shown they have a good sensitivity (83%) and specificity (86%) for identifying the risk of RP-ILD in this subset of patients [13]. Thus, anti-MDA5 antibodies have been proposed as a useful surrogate marker of disease activity [14–16]. Furthermore, it has been suggested that these antibodies could have a pathogenic role in lung injury [17]. According to this hypothesis, anti-MDA5 antibodies in our patient could have had a real pathogenic role rather than having been an epiphenomenon.
It has been suggested that serum ferritin could be a prognostic factor in anti-MDA5 antibody-associated DM [6, 18]. Gono et al. found that a serum ferritin cut-off value of 1600 ng/mL was the best indicator of survival among 14 anti-MDA5 antibody-associated ILD patients [6]. In their study, no death was reported during a 60-month follow-up among patients with ferritin levels < 500 ng/mL. Similar results were recently published by Fujiki et al. [18]. Although poor survival has been reported [1, 2], the clinical outcome of our patient was excellent. Interestingly, her serum ferritin levels were always < 500 ng/mL.
In our patient, the transbronchial biopsy revealed diffuse alveolar damage (DAD). This finding seems to be the pathological hallmark of anti-MDA5 antibody-associated RP-ILD [19]. Many diseases, including acute respiratory distress syndrome (ARDS), have been shown to cause DAD [20]. However, our patient did not fulfil the diagnostic criteria of ARDS and an exhaustive workup was carried out to exclude infections and neoplasms. Other CTDs were improbable and the patient was not taking any drugs.
Due to the severity and rapid course of the disease, we decided to use high parenteral doses of corticosteroids as well as cyclophosphamide. Corticosteroids are the first-choice treatment in DM-associated RP-ILD [21, 22]. Cyclophosphamide has been successfully used in other anti-MDA5 antibody-associated ILD patients [23]. Following detection of anti-MDA5 antibodies, cyclosporine was initiated as calcineurin inhibitors have been proposed as first-line therapy in anti-MDA5 antibody-associated RP-ILD [21, 22].
In summary, anti-MDA5 antibody-associated ILD should be suspected in cases of RP-ILD independently of extrapulmonary manifestations, as lung disease could precede systemic manifestations of DM.
Author contributions
All authors made substantial contributions to conception and design of the paper as well as participated in drafting the article. All authors gave final approval of the version to be submitted and any revised version.
Compliance with ethical standards
Informed consent
Informed consent was obtained from the patient described in the case report.
Contributor Information
Juan González-Moreno, Phone: 0034-637298301, Email: juan.glzmr@gmail.com.
Manuel Raya-Cruz, Email: mraya@hsll.es.
Ines Losada-Lopez, Email: ialosada@hsll.es.
Ana Paula Cacheda, Email: ana.cacheda@hsll.es.
Cristina Oliver, Email: colivert@hsll.es.
Bartomeu Colom, Email: bcolomo@hsll.es.
References
- 1.Hozumi H, Fujisawa T, Nakashima R, et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med. 2016;121:91–99. doi: 10.1016/j.rmed.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheumatol. 2005;52:1571–1576. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 3.Tamai K, Tachikawa R, Otsuka K, Ueda H, Hosono Y, Tomii K. Early pulmonary involvement of anti-CADM-140 autoantibody-positive rapidly progressive interstitial lung disease preceding typical cutaneous symptoms. Intern Med. 2014;53:2515–2519. doi: 10.2169/internalmedicine.53.2769. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz-Santamaria V, Babot A, Ferrer C. Anti-MDA5-positive dermatomyositis: an emerging entity with a variable clinical presentation. Scand J Rheumatol. 2017;10:1–3. doi: 10.1080/03009742.2017.1340512. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 6.Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology. 2010;49:1713–1719. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 7.Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 2010;138:251–256. doi: 10.1378/chest.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer A, Antoniou KM, Brown KK, ERS/ ATS. Task Force on Undifferentiated Forms of. CTD-ILD et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 9.Chaisson NF, Paik J, Orbai AM, Casciola-Rosen L, Fiorentino D, Danoff S, Rosen A. A novel dermato-pulmonary syndrome associated with MDA-5 antibodies: report of 2 cases and review of the literature. Medicine (Baltimore) 2012;91:220–228. doi: 10.1097/MD.0b013e3182606f0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, Sun L. Utility of anti-melanoma differentiation associated gene 5 antibody measurement in identifying patients with dermatomyositis and high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 2013;65:1316–1324. doi: 10.1002/acr.21985. [DOI] [PubMed] [Google Scholar]
- 11.Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol. 2017;44:319–325. doi: 10.3899/jrheum.160682. [DOI] [PubMed] [Google Scholar]
- 12.Sontheimer RD. MDA5 autoantibody-another indicator of clinical diversity in dermatomyositis. Ann Transl Med. 2017;5:160. doi: 10.21037/atm.2017.03.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Wang Q, Yang F, et al. Anti-MDA5 antibody as a potential diagnostic and prognostic biomarker in patients with dermatomyositis. Oncotarget. 2017;8:26552–26564. doi: 10.18632/oncotarget.15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muro Y, Sugiura K, Hoshino K, Akiyama M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology. 2012;51:800–804. doi: 10.1093/rheumatology/ker408. [DOI] [PubMed] [Google Scholar]
- 15.Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. 2013;23:496–502. doi: 10.3109/s10165-012-0663-4. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176:395–402. doi: 10.1111/bjd.14882. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. 2013;23:496–502. doi: 10.3109/s10165-012-0663-4. [DOI] [PubMed] [Google Scholar]
- 18.Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. 2017;11:1–8. doi: 10.1080/14397595.2017.1318468. [DOI] [PubMed] [Google Scholar]
- 19.Chino H, Sekine A, Baba T, qt al Radiological and pathological correlation in anti-MDA5 antibody-positive interstitial lung disease: rapidly progressive perilobular opacities and diffuse alveolar damage. Intern Med. 2016;55:2241–2246. doi: 10.2169/internalmedicine.55.5774. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, Parambil JG. Acute interstitial pneumonia (AIP): relationship to Hamman–Rich syndrome, diffuse alveolar damage (DAD), and acute respiratory distress syndrome (ARDS) Semin Respir Crit Care Med. 2012;33:476–485. doi: 10.1055/s-0032-1325158. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima R, Hosono Y, Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus. 2016;25:925–933. doi: 10.1177/0961203316651748. [DOI] [PubMed] [Google Scholar]
- 22.Hoa S, Troyanov Y, Fritzler MJ, et al. Describing and expanding the clinical phenotype of anti-MDA5-associated rapidly progressive interstitial lung disease: case series of nine Canadian patients and literature review. Scand J Rheumatol. 2017;25:1–15. doi: 10.1080/03009742.2017.1334814. [DOI] [PubMed] [Google Scholar]
- 23.Goussot R, Theulin A, Goetz J, Sibilia J, Gottenberg JE, Meyer A. An arthro-dermato-pulmonary syndrome associated with anti-MDA5 antibodies. Jt Bone Spine. 2014;81:266. doi: 10.1016/j.jbspin.2014.01.005. [DOI] [PubMed] [Google Scholar]


