Abstract
Fatty acids are a major fuel for the body and fatty acid oxidation is particularly important during fasting, sustained aerobic exercise and stress. The myocardium and resting skeletal muscle utilise long-chain fatty acids as a major source of energy. Inherited disorders affecting fatty acid oxidation seriously compromise the function of muscle and other highly energy-dependent tissues such as brain, nerve, heart, kidney and liver. Such defects encompass a wide spectrum of clinical disease, presenting in the neonatal period or infancy with recurrent hypoketotic hypoglycaemic encephalopathy, liver dysfunction, hyperammonaemia and often cardiac dysfunction. In older children, adolescence or adults there is often exercise intolerance with episodic myalgia or rhabdomyolysis in association with prolonged aerobic exercise or other exacerbating factors. Some disorders are particularly associated with toxic metabolites that may contribute to encephalopathy, polyneuropathy, axonopathy and pigmentary retinopathy. The phenotypic diversity encountered in defects of fat oxidation is partly explained by genotype/phenotype correlation and certain identifiable environmental factors but there remain many unresolved questions regarding the complex interaction of genetic, epigenetic and environmental influences that dictate phenotypic expression. It is becoming increasingly clear that the view that most inherited disorders are purely monogenic diseases is a naive concept. In the future our approach to understanding the phenotypic diversity and management of patients will be more realistically achieved from a polygenic perspective.
Keywords: Carnitine, Fatty Acid Oxidation, Spinal Muscular Atrophy, Bezafibrate, Pigmentary Retinopathy
Introduction
The oxidation of fatty acids plays a major role in energy production and during fasting provides up to 80 % of the total energy requirement. The major metabolic flux of long-chain fatty acids is through the beta-oxidation system of the mitochondria present in all cells except the mature erythrocyte (Kunau et al 1995). Long chain fatty acids act as an important source of respiratory fuel for many tissues especially skeletal and cardiac muscle. The brain is generally dependent on glucose for energy production and the “glucose sparing” effect of ketones during short term fasting is particularly important in infants. However in keto-adapted individuals the brain switches to utilise ketone bodies as its major energy source (Phinney 1983a).
Inherited disorders of fatty acid oxidation FAO result in a wide spectrum of clinical disease resulting from the interaction of genetic and numerous environmental factors that act together to produce the phenotype. The factors that conspire together to give rise to the diverse phenotypes will be discussed and a number of specific fatty acid oxidation disorders will be explored in some detail to illustrate the interplay of factors that may act together to produce such diverse phenotypes.
Inherited disorders of fatty acid oxidation
Inherited defects in 17 proteins directly affecting either carnitine dependent transport of long chain fatty acids or the process of β-oxidation itself have so far been described (Bennett et al 2000; Wanders et al 1999; Rinaldo et al 2002; Wanders et al 2010; Spiekerkoetter 2010; Houten and Wanders 2010). Several long chain acyl-CoA dehydrogenases involved in fatty acid oxidation including ACAD9, ACAD10 and ACAD11 have also been described but their role in the pathophysiology of human disease is not fully elucidated (Ensenaur et al 2005; Oey et al 2005, 2006; He et al 2011).
The biochemical consequences of a defect affecting the process of FAO can be categorized as follows: -
Inadequate supply of energy.
Accumulation of toxic metabolites.
Sequestration or loss of vital components.
Altered production of enzyme product, e.g. lack of surfactant in LCHAD/MTP/LCAD.
Loss of protein-protein interaction, e.g. SCHAD deficiency.
Inadequate supply of energy
Fatty acids are mobilised from adipose tissue in the fasted state and used for hepatic generation of ketone bodies which act as vital fuel to extra hepatic tissue with the additional effect of “sparing” glucose for other vital tissues, especially the brain. This “glucose sparing effect” is particularly important in infants where there is high proportional glucose utilisation in cerebral tissue. Hypoglycaemia is the inevitable consequence of an inability of the body to maintain normoglycaemia, as glycogen levels become exhausted and gluconeogenic pathways fail in the face of an inadequate supply of ketones.
In the fasting state hepatic gluconeogenesis and ketone body generation act synergistically to provide energy for both hepatic and extra hepatic tissues. Amino acids are the major substrates for hepatic gluconeogenesis which in turn relies on a steady supply of acetyl-CoA, NADH + H+ and ATP from fat oxidation to sustain this process. In the absence of a steady supply of these metabolites gluconeogenesis is compromised and hypoglycaemia follows (Fig. 1).
Fig. 1.
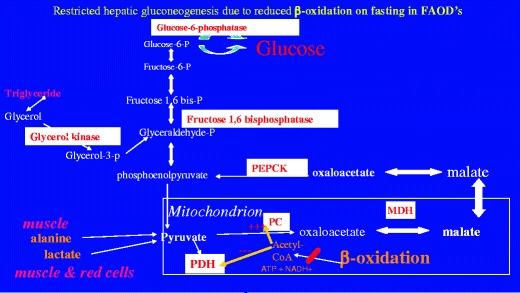
Interaction of β-oxidation and gluconeogenesis. β-oxidation generates ATP, NADH+ and acetyl-CoA and these stimulate pyruvate carboxylase PC and inhibit pyruvate dehydrogenase PDH. Mobilisation of alanine from muscle, and lactate produced, for example from anaerobic glycolysis in the red cell, are the major sources of pyruvate during catabolic stress. The conversion of pyruvate to oxaloacetate is a crucial step in the process of gluconeogenesis which is mediated through pyruvate carboxylase PC. Acetyl-CoA is the primary activator of this enzyme, which converts pyruvate to oxaloacetate. The equilibrium of the mitochondrial malate dehydrogenase MDH is displaced to favour the reduction of oxaloacetate to malate. ATP is used as a co-substrate for PC and phosphoenoylpuruvate carboxykinase PEPCK. Consequently in defects of fat oxidation this crucial early step of gluconeogenesis is compromised and this also contributes significantly to hypoglycaemia
Skeletal and cardiac muscle derive a significant proportion of their energy, even in the fed state, from β-oxidation of long chain fatty acids. Myocardial fat oxidation is severely reduced in patients with long-chain defects and these fatty acids are likely shunted into a slow-turnover compartment, predominantly reflecting esterification to triglycerides. The forced increased reliance of the myocardium on glucose appears in the long term to bring about a profound but poorly understood remodelling, mediated by major shifts in gene expression and resulting in hypertrophy of the myocardium (Ritchie and Delbridge 2006).
Reduced energy supply to skeletal muscle results in chronic hypotonia, muscle weakness and exercise intolerance. Similarly reduction of hepatic ATP availability for the vital and varied metabolic processes occurring within the hepatocyte frequently results in hyperammonaemia and resultant encephalopathy due to a failing urea cycle.
Accumulation of toxic metabolites
Approximately two thirds of all identified disease-associated gene variations are of the missense type with the potential to produce abnormal protein conformations due to misfolding. The fate of abnormal protein will, depending on a number of factors within the cell result in variable toxic effects within the putative fatty acid oxidation metabolone (Gregersen et al 2008; 2010a, b).
The potential toxicity effects are best divided up into those that affect the carnitine shuttle and those defects within the β-oxidation spiral itself.
Carnitine shuttle
The nature of the defects in primary carnitine transporter deficiency CTD (OCTN2, SLC22A5) and carnitine palmitoyltransferase type 1 (CPT1A) deficiency dictates that toxic metabolites are not produced within the mitochondria.
In severe carnitine acyl-carnitine translocase CACT and carnitine palmitoyl transferase type 2 (CPT2) deficiency the majority of tissue and plasma carnitine is ultimately as long-chain acylcarnitine species.
β-oxidation
Defects within the β-oxidation spiral generate, depending on the defect, a range of short, medium and long-chain acyl-CoA intermediates within the mitochondrion, some of which are reported to be particularly toxic, e.g. octanoyl-CoA in medium chain acyl-CoA dehydrogenase deficiency MCADD. These intermediates once transported back out of the mitochondria, undergo peroxisomal β-oxidation and/or ω-oxidation to produce a range of C4-C16 carboxylic and C4-C10 dicarboxylic acids including some unsaturated species. Short and medium chain carboxylic and dicarboxylic acids can re-enter the mitochondria where they can be oxidised in the normal way. However some carboxylic acid derivatives particularly C8-C10 and 3-hydroxy C12-C16 are reported to be toxic (Table 1).
Table 1.
Toxic metabolites produced in various disorders and proposed biochemical effects and clinical consequences
| Disorder | Metabolite(s) | Toxicity effect | Clinical consequence | Reference |
|---|---|---|---|---|
| MCAD | Octanoic acid | Inhibition of choroid plexus organic anion transport | Cerebral accumulation of fatty acids with disruption of mitochondrial function | Kim et al 1992 |
| Decanoic acid | Uncouple oxidative phosphorylation | - Encephalopathy | Schuck et al 2009 | |
| Inhibition of complexes I-III, II-III, IV in liver (rat) | Scaini et al 2012 | |||
| MCAD | Octanoyl-CoA | ↑acyl-CoA:free CoA ratio | Inhibition of gluconeogenesis and intermediary metabolism liver dysfunction | Sauer et al 2008 |
| Elicit oxidative damage/reduce glutathione reserves inhibit cytochrome c oxidase activity | - Reye-like presentation | Schuck et al 2009 | ||
| MCAD | cis-4-decenoic acid | uncouples oxidative phosphorylation (rat brain) | encephalopathy | Schuck et al 2010 |
| Sharpe et al 1999 | ||||
| Defects affecting ETF or ETFDH | Short and medium chain carboxylic acids | Increased ROS generation | Encephalopathy | Scaini et al 2012 |
| C4-C10 | Inhibition of respiratory chain | Muscle weakness | Leipnitz et al 2003 | |
| Ethylmalonic acid (derived from butyryl-CoA) | Inhibit mitochondrial creatine kinase in cerebral cortex (rats) | |||
| LCHAD/MTP | 3-hydroxydodecanoic acid | Uncouple oxidative phosphorylation | Hepatic cholestasis, fibrosis, cirrhosis | Tyni et al 1996 |
| 3-hydroxytetradecanoic acid | HELLP | Ventura et al 1996 | ||
| 3-hydroxypalmitic acid | Ibdah et al 2000 | |||
| Tonin et al 2010 | ||||
| LCHAD/MTP | 3-hydroxydodecanoic acid | Interference with DHA metabolism in retinal pigment epithelium/neural membranes | Chorioretinopathy polyneuropathy, demyelination | Tyni et al 1996 |
| 3-hydroxytetradecanoic acid | Inhibition of respiratory chain | Rhabdomyolysis | Tein et al 1999 | |
| 3-hydroxypalmitic acid | Tyni et al 2002 | |||
| Ventura et al 1996 | ||||
| Ibdah et al 1998 | ||||
| Spiekerkoetter et al 2004a | ||||
| Tyni et al 2004 | ||||
| CPT2/CACT/VLCAD | Long chain acylcarnitines | Inhibition of N-acetylglutamate synthase | Hyperammonaemia | Mak et al 1986 |
| Generate ROS/lipid peroxidation | Cardiac arrhythmias | Sparagna et al 2000 | ||
| Uncouple oxidative phosphorylation | Corr and Yamanda 1995 | |||
| Detergent-like properties on sarcolemma | ||||
| CPT2/CACT/VLCAD | Long chain acyl-CoA’s | Inhibitition of ADP/ATP carrier | Organ failure | Ventura et al 2005 |
| Inhibition of oxidative phosphorylation | Ventura et al 2007 |
CTD OCTN2 transporter deficiency
CPT1 carnitine palmitoyltransferase type 1 deficiency
CACT carnitine-acylcarnitine translocase deficiency
CPT2 carnitine palmitoyltransferase type 2 deficiency
DHA docosahexaenoic acid
VLCAD very long-chain acyl-CoA dehydrogenase deficiency
LCHAD long chain 3-hydroxyacyl-CoA dehydrogenase deficiency
MTP mitochondrial trifunctional protein deficiency
MCAD medium chain acyl-CoA dehydrogenase deficiency
MADD multiple acyl-CoA dehydrogenase deficiency
RR-MADD riboflavin responsive multiple acyl-CoA dehydrogenase deficiency
Sequestration of vital components
Coenzyme A does not cross the mitochondrial membrane and there is a finite pool of coenzyme A within the mitochondria. Defects of β-oxidation lead to sequestration of coenzyme A in the form of acyl-CoA intermediates.
Primary defects of fatty acid oxidation, with the exception of CPT1 deficiency, invariably result in a reduction in total plasma L-carnitine concentration and/or an increase in the acyl: free L-carnitine ratio. Free L-carnitine facilitates the removal of unmetabolised acyl groups from the mitochondria with the freeing up of coenzyme A. Acylcarnitine is excreted by the kidneys with reduction in total L-carnitine. Acylcarnitines inhibit the carrier-mediated reabsorption of L-carnitine from the renal tubule, thus exacerbating L-carnitine depletion (Tein 2003).
In general it is difficult to evaluate the contribution of toxic metabolites in the context of human fatty acid oxidation defects to the overall pathology of disease, as in many fatty acid oxidation disorders there is a combination of energy deficit, potentially toxic metabolites and sequestration of vital components.
Altered enzyme product
Acute respiratory distress syndrome ARDS in both neonates and older patients has been described in both isolated long chain 3-hydroxyacyl-CoA dehydrogenase deficiency LCHADD and mitochondrial trifunctional protein MTP deficiency (Lundy et al 2003; Olpin et al 2005). Long chain acyl-CoA dehydrogenase LCAD and the MTP complex are expressed in human lung (Oey et al 2005). Surfactant abnormalities and mitochondrial dysfunction are two potential mechanisms by which MTP/LCHAD defects might predispose to ARDS. Accumulating fatty acid metabolites in patients with LCHAD/MTP defects might well alter the phospholipid components of surfactant and impair its function. A single case of respiratory distress has been linked to LCAD deficiency (Suhrie et al 2011). This might suggest a role for LCAD and MTP protein in substrate modification for the production of surfactant which contains several lipid components including palmitoylmyristoylphosphatidylcholine (Bernhard et al 2001; Ridsdale et al 2005). Synthesis of myristic acid would imply a need for fatty acid chain shortening.
Loss of protein-protein interaction
Patients with short chain 3-hydroxyacyl-CoA dehydrogenase SCHAD deficiency have been shown to present with hypoglycaemia precipitated by hyperinsulinaemia. This has been shown to be due to loss of inhibition of glutamate dehydrogenase by the SCHAD protein. This is an unusual example of a protein exhibiting dual function both as an enzyme and also through direct protein-protein interaction (Li et al 2010).
Phenotypic diversity in fatty acid oxidation disorders
Disorders of fatty acid oxidation result in a wide spectrum of clinical disease (Table 2). Even patients with the same mutation may present in entirely different ways, e.g. homozygous c.985A > G MCADD presenting as neonatal ketoacidosis with encephalopathy (personal observation), hypoketotic hypoglcaemia in infancy or absence of history of any related clinical disease in adults. The precise mechanisms underlying this diversity remain unclear, although recent advances in our knowledge of multiple gene expression which dictates the metabolome are providing some clues (Homuth et al 2012). Some FAOD’s do show significant genotype/phenotype correlation particularly with regard to the severe, as compared to the milder phenotypes of, e.g. CPT2, very long chain acyl-CoA dehydrogenase VLCAD and multiple acyl-CoA dehydrogenase MAD deficiency (Bonnefont et al 1996; Andresen et al 1999; Olsen et al 2003) see Table 3. However in many disorders there is a much poorer or virtually absent correlation, e.g. MCADD, CTD. Certainly there are many factors that are known to influence the phenotype such as frequency and duration of fasting, intercurrent infections, type of energy substrate, e.g. fat vs. carbohydrate, the extent and duration of exercise, exposure to certain drugs and protection by anti-oxidants and co-factors (Lucas et al 2011). However we still remain unable to explain why for example even relatively well controlled LCHADD (homozygous c.1528G > C) patients exhibit significant morbidity, develop chorioretinopathy and often neuropathy while a few reach adulthood with minimal retinal changes as the only manifestation of clinical disease (den Boer et al 2002; Olpin et al 2005).
Table 2.
Clinical features of fatty acid oxidation defects as presenting in various tissue/organs
| Tissue/organ | Disorder | Nature of clinical feature |
|---|---|---|
| CNS | CTD, CPT1, CACT, CPT2, VLCAD, LCHAD/MTP, MCAD, MADD | Reye-like encephalopathy, seizures, coma, death |
| Liver | CTD, CPT1, CACT, CPT2, VLCAD, LCHAD/MTP, MCAD, MADD | Hepatic dysfunction hyperammonaemia, increased liver enzymes, hepatosteatosis |
| Liver | CPT1 | Cholestatic jaundice, hyperlipidaemia, |
| Liver | LCHAD/MTP | Cholestatic jaundice, cirrhosis/fibrosis, maternal HELLP/AFLP |
| Heart | CTD, CPT2, CACT, VLCAD, LCHAD/MTP, MADD | Arrhythmias (anything from tachycardia to complete heart block), cardiomyopathy - dilated or hypertrophic |
| Skeletal muscle | CTD, CACT, CPT2, VLCAD, RR-MADD, MADD | Hypotonia, lipid deposition, weakness, exercise intolerance, myalgia, rhabdomyolysis |
| Nerves | LCHAD/MTP | Sensori-motor neuropathy, chorioretinopathy, axonopathy, demyelination, peripheral neuropathy |
| Lungs | LCHAD/MTP | Acute respiratory distress, respiratory failure |
| Parathyroid | LCHAD/MTP | Hypoparathyroidism – hypocalcaemia, hyperphosphataemia, low PTH |
| Kindney | CPT2, MADD (severe) | Renal cystic dysplasia |
| Kidney | CPT1 | Renal tubular acidosis |
CTD OCTN2 transporter deficiency
CPT1 carnitine palmitoyltransferase type 1 deficiency
CATR carnitine-acylcarnitine translocase deficiency
CPT2 carnitine palmitoyltransferase type 2 deficiency
VLCAD very long-chain acyl-CoA dehydrogenase deficiency
LCHAD long chain 3-hydroxyacyl-CoA dehydrogenase deficiency
MTP mitochondrial trifunctional protein deficiency
MCAD medium chain acyl-CoA dehydrogenase deficiency
MADD multiple acyl-CoA dehydrogenase deficiency
HELLP haemolysis, elevated liver enzymes, low platelets
AFLP acute fatty liver of pregnancy
Table 3.
Disorders of fatty acid oxidation divided according to the presence or absence of significant residual protein function. Biochemical consequences of each defect with manifestation of clinical disease
| Severe fatty acid oxidation defects | ||
| Minimal/no functional protein/null mutations | ||
| Disorder | Biochemical consequence | Clinical disease |
| CTD, MCAD, CPT1 | Non/hypoketotic hypoglycaemia | Reye-like presentation, encephalopathy, seizures, coma, death. Moderate/severe hyperammonaemia, abnormal liver function |
| CPT2, CACT, VLCAD, MADD, LCHAD/MTP | Non-ketotic hypoglycaemia, inhibition of gluconeogenesis | Hepatic failure, severe hypotonia/rhabdomyolysis, cardiomyopathy/arrythmias, encephalopathy, seizures, coma, death |
| Severe metabolic acidiosis, hyperammonaemia, production of toxic metabolites, sequestration of Coenzyme A and carnitine | ||
| CPT2, MADD | Severely reduced ATP production, toxic metabolites | Congenital abnormalities due to disruption of intrauterine growth - renal cystic dysplasia, facial dysmorphism, rocker-bottom feet, muscular defects of anterior abdominal wall and abnormal genitalia (hypospadias and chordee) |
| LCHAD/MTP | Toxic 3-hydroxyacyl-metabolites | Maternal HELLP and acute fatty liver of pregnancy AFLP (affected foetus). |
| “Milder” disorder with reduced fatty acid oxidation | ||
| Significant residual protein function – many (not all) missense mutations | ||
| Disorder | Biochemical consequence | Clinical disease |
| VLCAD | Hypoketotic hypoglycaemia, | Reye-like episodes with seizures/encephalopathy |
| MADD | mild/moderate hyperammonaemia | |
| VLCAD | Reduced aerobic energy | Exercise intolerance, myalgia, muscle weakness, rhabdomyolysis - triggered by prolonged exercise, cold/heat stress or infection |
| CPT2 | production from long chain fat oxidation | |
| MTP | Chronic production of toxic 3-hydroxyacyl metabolites/reduced energy production | Sensori-motor peripheral neuropathy (mainly lower limbs), chorioretinopathy, axonopathy, demyelination, episodic rhabdomyolysis, respiratory failure |
| RR-MADD | Toxicity of multiple medium chain metabolites/reduced energy production – hypocarnitinaemia, ketoacidosis, secondary Coenzyme Q deficiency and reduced activity of respiratory chain complexes I, II-III and IV | Chronic hypotonia – weakness of neck, shoulder, hip, diaphragm/respiratory muscles. Progressive exercise intolerance, episodic rhabdomyolysis. |
| Cyclical vomiting, loss of appetite, weight loss. Acute confusion/encephalopathy | ||
CTD OCTN2 transporter deficiency
CPT1 carnitine palmitoyltransferase type 1 deficiency
CACT carnitine-acylcarnitine translocase deficiency
CPT2 carnitine palmitoyltransferase type 2 deficiency
VLCAD very long-chain acyl-CoA dehydrogenase deficiency
LCHAD long chain 3-hydroxyacyl-CoA dehydrogenase deficiency
MTP mitochondrial trifunctional protein deficiency
MCAD medium chain acyl-CoA dehydrogenase deficiency
MADD multiple acyl-CoA dehydrogenase deficiency
RR-MADD riboflavin responsive multiple acyl-CoA dehydrogenase deficiency
There is an increasing understanding of the influence of single nucleotide polymorphisms (SNPs) on the variation in functional activity of many of the genes involved in intermediary metabolism. Recent description of the peroxisome proliferators-activated receptor gamma-Co-activator-1α (PGC-1α) transcriptional complexes regulated through silent information regulator; ortholog of mammalian sirtuin (SIRT1) pathways may help to explain some of the regulatory processes that influence this phenotypic diversity (Abbott 2009; Rodgers et al 2008; Dominy et al 2011). It is becoming increasingly clear that the view that most inherited disorders are purely monogenic diseases is a naive concept. In future our approach to understanding the phenotypic diversity and management of patients will be more realistically achieved from a polygenic perspective (Rocha et al 2011a, b).
Individual disorders of fatty acid oxidation
Primary carnitine deficiency
CTD is a potentially lethal, autosomal recessive disorder characterised by early childhood onset cardiomyopathy, with or without weakness and hypotonia, recurrent hypoglycaemic hypoketotic seizures and/or coma, failure to thrive, and extremely low plasma and tissue carnitine concentrations (Tein et al 1990; Stanley et al 1991; Tein 2003). There is phenotypic variability in this disorder, many patients present during the first few years of life either with symptomatic cardiomyopathy and/or with repeated hypoketotic hypoglycaemia (Reye-like episodes). Some infants may present initially with relatively mild symptoms of muscle hypotonia, mild developmental delay, failure to thrive, delayed walking, repeated infections and poor growth. Other patients may present much later in childhood (4–12 years) with isolated cardiomyopathy which can present as sudden death. Occasionally older subjects have been described with mild ventricular fibrillation or adult onset lipid storage myopathy (Rijlaarsdam et al 2004; Vielhaber et al 2004). Both silent and symptomatic individuals have been described within the same pedigree (Spiekerkoetter et al 2003a) and asymptomatic adults are now well described (Vijay et al 2006). There is evidence to suggest that asymptomatic patients tend to have slightly higher residual carnitine transporter activity than clinically presenting patients (Rose et al 2011).
Clinical disease is generally explained by reduced energy production from long-chain fatty acids. Variability of the clinical phenotype however, is not fully understood although factors such as duration of fasting, composition of diet, infection, rates of endogenous carnitine synthesis and other environmental stressors such as drugs and cold exposure must play an important part (Rasmussen et al 2013). It is suggested that carriers of a single OCTN2 mutation may be at risk of clinical disease given sufficient stress, e.g. hypertension, valproic acid (Tein et al 1995; Tein 2003).
Carnitine palmitoyltransferase type 1 deficiency (CPT1A)
Carnitine palmitoyltranferase type 1 (CPT1) is an enzyme of the outer mitochondrial membrane that converts long chain fatty acyl molecules to their corresponding acylcarnitines which are then transported across the inner mitochondrial membrane for ß-oxidation in the mitochondrial matrix. There are three genes in the human genome that are known to encode related carnitine acyltransferases: CPT1A encodes the liver isoform of CPT1, CPT1B encodes muscle-type CPT1 and CPT1C, encoding CPT1 related protein expressed in brain (Price et al 2002). CPT1A is expressed in liver, kidney, lung, spleen, intestine, pancreas, ovary and fibroblasts. Only liver-type CPT1A deficiency has been recognised so far (Demaugre et al 1988; Falik-Borenstein et al 1992; Haworth et al 1992; Bergman et al 1994; Olpin et al 2001). Deficiency of CPT1A prevents the formation of long-chain acylcarnitines and the absence of these species, which are potent inhibitors of renal tubular free carnitine transport, results in a higher renal threshold for free carnitine (Stanley et al 1991). Patients often have characteristically high total plasma carnitine concentrations with more than 90 % in the free form. Patients with CPT1 deficiency usually present in infancy with recurrent episodes of hypoketotic hypoglycaemic, metabolic acidosis with raised transaminases, hepatomegaly, hepatosteatosis and mild to moderate hyperammonemia. Cholestatic jaundice has recently been described (Morris et al 2013). Acute fatty liver of pregnancy AFLP has also been reported in an Inuit female (Innes et al 2000). Death may occur during an acute presentation but surviving infants may suffer with severe developmental delay and intellectual impairment as a result of cerebral bioenergetic failure.
A range of cardiac abnormalities including tachycardia, bradycardia, arrhythmias, right bundle branch block and sudden cardiac arrest have been reported in neonates. Expression of CPT1A in fetal myocardium with persistence of expression into the neonatal period is proposed as an explanation (Weis et al 1994). Renal tubular acidosis has also been reported in a number of patients and this is explained by the expression of CPT1A in renal tubular epithelium with reliance on fat oxidation to power the high energy transport processes. Renal tubular acidosis appears to respond to medium chain triglyceride MCT supplementation but not to a low fat diet per se (Falik-Borenstein et al 1992; Olpin et al 2001). Hyperlipidaemia with raised triglycerides and/or cholesterol is also a feature of CPT1 deficiency, although hyperlipidaemia is rarely reported in other fat oxidation disorders (Falik-Borenstein et al 1992; Olpin et al 2001). The nature of a block on the outer mitochondrial membrane may specifically favour hepatic very low density lipoprotein synthesis with resultant hyperlipidaemia. Clinical presentation is explained primarily by lack of energy production from long chain fatty acids but with metabolic disturbance confined to the tissues where CPT1A is expressed.
A common CPT1A p.P479L (c.1436C > T) variant is prevalent in many Inuit populations and is present in its homozygous state in up to 65 % of the Inuit population in some Arctic costal areas. This indicates that this variant is unlikely to be clinically disadvantageous for most individuals, although it has been shown that the variant significantly reduces CPT1A activity and renders the enzyme partially insensitive to malonyl-CoA inhibition (Brown et al 2001; Morillas et al 2001; Greenberg et al 2009). It has been postulated that this variant may represent a selectively advantageous adaptation to a high marine fat diet and to a state of permanent keto-adaptation (Greenberg et al 2009).
Recent research suggests that the P479L variant in its homozygous state may increase the risk of hypoglycaemic events in neonates living a less traditional life-style on a more “westernised” diet, and to at least partially account for the higher infant mortality seen in these First Nations peoples. A reduced capacity to generate ketones as observed in patients with more “severe” mutations in the CPT1 gene is postulated as precipitating hypoglycaemia in these infants (Gessner et al 2010; Gillingham et al 2011).
Carnitine palmitoyltransferase type 2 (CPT2) deficiency
CPT2 is associated with the inner mitochondrial membrane and has a single ubiquitously expressed isoform. CPT2 deficiency has three phenotypes, a fatal neonatal-onset form with congenital abnormalities, a severe neonatal hepato-cardio-muscular form, and a mild myopathic adult form. The lethal neonatal form is characterised by non-ketotic hypoglycaemia, liver disease, hypotonia, cardiomyopathy and congenital abnormalities (Hug et al 1991). The infantile form with or without cardiac disease presents with liver, and skeletal muscle involvement with episodes of decompensation, primarily as non-ketotic hypoglycaemia occurring with fasting and/or intercurrent infection (Demaugre et al 1988). There is good correlation between the biochemical measures of enzyme activity, genotype and phenotype in severe infantile disease as compared to “mild” adult-onset myopathic presentation. However there is no such correlation within the myopathic group, despite significant phenotypic variability (Olpin et al 2003).
Mutation analysis in CPT2 deficiency has revealed numerous mutations but the common S113L mutation accounts for ∼50 % of myopathic disease (Taroni et al 1992; Verderio et al 1995; Taggart et al 1999; Deschauer et al 2005). Hypoglycaemia and cardiac involvement are generally not features of “mild” CPT2 deficiency.
A thermolabile CPT2 mutant SNP variant [p. Phe352Cys] (1055 T > G/F352C) has been recently described in the Japanese population and shown to be closely correlated with influenza associated encephalopathy (IEA), low blood ATP and predisposition to brain vascular invasion by virus (Kubota et al 2012; Yao et al 2011).
Very long-chain acyl-CoA dehydrogenase (VLCADD)
Very long-chain acyl-CoA dehydrogenase catalyses the initial rate-limiting step in mitochondrial long-chain fatty acid β-oxidation. Very long-chain acyl-CoA dehydrogenase deficiency is a clinically heterogeneous disease, and as with CPT2 deficiency there are three major phenotypes: a late onset myopathic form, a milder childhood form, usually with hypoketotic hypoglycaemia as the main presenting feature but often with exercise intolerance and rhabdomyolysis as a significant feature particularly in older children and young adults. There is also a severe infantile form, with early onset non-ketotic hypoglycaemia, high mortality, and high incidence of cardiomyopathy. However unlike the fatal neonatal-onset form of severe CPT2 deficiency where developmental abnormalities are often present (see Table 3), these are not reported in severe neonatal VLCADD. It is hypothesised that in fetal tissues the role of VLCADD is replaced by other ACAD’s thus permitting normal in utero development (He et al 2011). Overall VLCADD is one of the commonest fatty acid oxidation disorders that we encounter, second only to MCADD (Fig. 2). There is a good genotype/phenotype correlation between severe infantile disease and milder phenotypes (Andresen et al 1999).
Fig. 2.
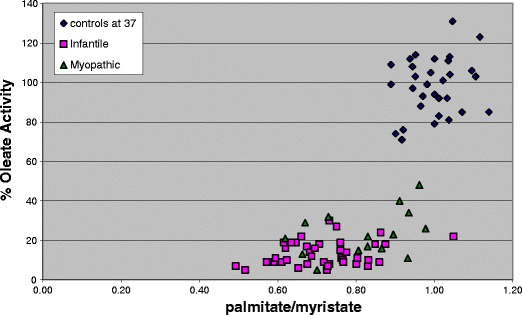
Fatty acid oxidation flux measured in fibroblasts at 37oC using [9,10-H3] myristate, palmitate and oleate as substrates in myopathic and infantile VLCADD as compared to controls. Each plotted value represents the mean result for individual patient or control cell lines assayed in duplicate in 2-5 separate assays. There is overlap between the infantile and milder myopathic phenotypes but all VLCADD cell lines are clearly separated from the controls
Triggerable myopathy
Fat oxidation and exercise
Resting skeletal muscle relies primarily on FAO for provision of energy but during work the predominant bioenergetic pathway depends on the type, intensity and duration of exercise (Essen et al 1977; Essen 1977; Spriet 2002; Wojtaszewski et al 2003). During the first 5 to 10 min of moderate exercise, high-energy phosphates are used but as these become depleted there is a switch to muscle glycogen. As the duration of moderate exercise increases both glucose and at first muscle triglycerides are used, but after approximately one hour, free fatty acids become increasingly important as the major fuel for muscle (Essen et al 1977). There is evidence that children and adolescents rely more heavily on fat metabolism during aerobic exercise than adults (Boisseau and Delmarche 2000). Thus milder defects of fat oxidation that may sustain energy production in tissues when demand on fatty acid oxidation is relatively low become rate limiting when demand is high (Orngreen et al 2004, 2005).
Myopathic VLCAD and CPT2 deficiency
The late-onset myopathic forms of both CPT2 and VLCAD deficiency are characterised by muscle pain and stiffness triggered by exercise, fasting, extremes of temperature and sometimes infection. Muscle biopsy is often unremarkable, although approximately 20 % of patients will show some lipid deposition. Together these two disorders represent the most common inherited metabolic causes of myalgia and rhabdomyolysis in children and adults. Clinically presenting cases of CPT2 are more common in males although the explanation that this is solely related to frequency and duration of aerobic exercise is debatable. A number of cases of myopathic VLCADD have a past history of isolated episodes of hypoglycaemia in infancy/early childhood and this is in contrast to myopathic CPT2 patients where hypoglycaemia in infancy/early childhood is very rarely reported. Some myopathic VLCADD patients appear to develop very poor exercise tolerance following clinical presentation as an adolescent/adult, although previously reporting relatively good exercise tolerance in childhood/early adolescence (personal observation).
Cases of symptomatic CPT2 “carriers” with ∼50 % residual fibroblast enzyme activity are also described although “synergistic heterozygocity” with the presence of mutation(s) in other genes has been demonstrated in some (Vladutiu et al 2000, 2002; Vladutiu 2001; Vockley et al 2000).
Defects of the mitochondrial trifunctional protein MTP
The MTP protein is a heterooctameric (α4β4) enzyme complex which catalyses the last three steps in β-oxidation of long chain fatty acids. The common c.1528G > C HADHA mutation results in isolated LCHADD whereas other mutations in the HADHA or HADHB result in deficiency of all three enzymes (Spiekerkoetter et al 2004b; Spiekerkoetter et al 2003b; Spiekerkoetter and Wood 2010).
Patients with MTP deficiency exhibit a wide clinical spectrum of disease with severe neonatal manifestation including cardiomyopathy and death, through moderate/severe infantile presentation with hepatic manifestations to mild peripheral polyneuropathy, episodic rhabdomyolysis and pigmentary retinopathy (Tyni et al 1998; Spiekerkoetter et al 2003b; Spiekerkoetter et al 2004a, b; Schaefer et al 1996; Chakrapani et al 2000). Hypoparathyroidism is a rare complication of both LCHADD and MTP deficiency (Tyni et al 1997).
Mild MTP deficiency is rare with an unusual phenotype having features in common with the hereditary sensory-motor neuropathies HMSN and spinal muscular atrophy SMA (Spiekerkoetter et al 2004a; Olpin et al 2005). There is a progressive peripheral polyneuropathy from infancy or early childhood affecting predominantly the lower limbs, but also symmetric weakness in wrist and finger extensors. There may be exercise intolerance, gait abnormalities with bilateral Achilles tendon contractures, planovalgus deformities and loss of vibrational sense. Some patients may develop respiratory failure often in association with an acute decompensation. Nerve conduction studies and sural nerve biopsy frequently show evidence of axonal degeneration and loss of normal myelin (Spiekerkoetter et al 2004a). There may be pigmentary retinopathy. Episodic rhabdomyolysis in association with exercise, illness or fasting, is usually encountered in this condition but this may not appear until many years after the initial neurological presentation (Spiekerkoetter et al 2004a, Olpin et al 2005). It has been proposed that the neuropathy and rhabdomyolysis seen in mild trifunctional protein deficiency is due to the specific toxic nature of the 3-hydroxacyl metabolites (Ibdah et al 1998; Schaefer et al 1996; Spiekerkoetter et al 2003b).
Riboflavin responsive multiple acyl-CoA dehydrogenase deficiency RR-MADD
Multiple acyl-CoA dehydrogenation deficiency (MADD) is a disorder of fatty acid, amino acid and choline oxidation caused by defects in any one of two flavoproteins, electron transfer flavoprotein (ETF) or ETF:ubiquinone oxidoreductase (ETF-QO) which affect some 14 dehydrogenases (Fig. 3). Patients are categorised into severe (MADD:S) or milder (MADD:M) forms depending on the nature of the defects in the genes (ETFA, ETFB and ETFDH) (Goodman et al 2002; Olsen et al 2003). Most patients do not respond to riboflavin supplementation.
Fig. 3.
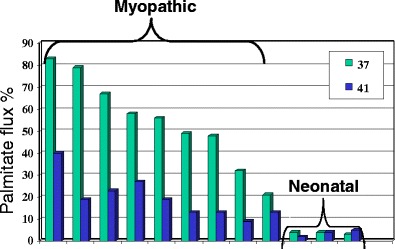
Fibroblast fatty acid oxidation flux using palmitate in 9 myopathic CPT2 deficient cell lines and 3 neonatal CPT2 deficient cell lines grown and assayed at 37oC and 41oC. The lower fatty acid oxidation flux in all the myopathic cell lines demonstrates the thermal instability of the CPT2 mutant protein at higher temperature. Neonatal “severe” CPT2 deficient cell lines show barely detectable flux at both temperatures. (Olpin et al 2003)
However a sub-set of MADD patients have been recently characterised who respond to pharmacological doses of riboflavin both clinically and biochemically (RR-MADD). These RR-MADD patients for the most part have been shown to have mutations in ETFQO and most mutations are situated around the ubiquinone binding pocket. (Olsen et al 2007; Gempel et al 2007). Riboflavin is the precursor of FAD, and riboflavin responsiveness results from the ability of FAD to act as a chemical chaperone that promotes folding of certain misfolded ETF-QO proteins, and thereby ameliorate or normalise disease symptoms (Cornelius et al 2012). Patients may present with cyclical vomiting, loss of appetite, progressive proximal muscle weakness particularly affecting neck, shoulder, hip and/or respiratory muscles but also chronic leg weakness and exercise intolerance with occasional rhabdomyolysis. Some patients may present with acute encephalopathy. There is also frequently an associated muscle ubiquinone deficiency with low respiratory chain complexes I, II/III and IV (Olsen et al 2007; Gempel et al 2007). The clinical response to pharmacological doses of riboflavin is usually rapid and striking. Most patients present as adolescents or young adults with an increased female: male ratio. Riboflavin intake in many diets often falls below the recommended daily intake and this may be particularly true for adolescent females (Gregory and Lowe 2000). RR-MADD may be considered as an example of the interaction of micronutrient availability and genetic predisposition.
More recent aspects of defects affecting fatty acid oxidation
Mutant proteins and future therapeutic options
The effect of increased temperature on fibroblast fatty acid oxidation flux can be demonstrated in both mild CPT2 (Fig. 4) and VLCAD deficiency (Olpin et al 2012). Several thermolabile CPT2 mutant SNP variants including [p. Phe352Cys] p. Val368Ile (V368l) has been recently described in the Japanese and Chinese population and shown to confer high susceptibility to influenza associated encephalopathy (IEA), low blood ATP and predisposition to brain vascular invasion by virus (Chen et al 2005; Kubota et al 2012; Yao et al 2011; Mak et al 2011). Treating fibroblasts with bezafibrate has been shown to upregulate ATP levels and may possibly offer a therapeutic option in patients infected with IEA (Yao et al 2011). The presence of the V368l variant was also reported as being overrepresented in myopathic CPT2 patients in the UK population (Olpin et al 2003). Bezafibrate also upregulates VLCAD mutant mRNA production and restores fat oxidation in VLCAD deficient cell lines (Gobin-Limballe et al 2007). Similarly bezafibrate has been shown to upregulate CPT2 activity in CPT2 deficient cells, both in vitro and in vivo, with improved clinical outcome (Djouadi et al 2005; Bonnefont et al 2010). Resveratrol and its synthetic analogues have been recently shown to offer great potential for the upregulation of mutant protein mediated through PGC-1α and SIRT1 pathways (Bastin et al 2011). This unstable nature of mutant protein is by no means confined to these disorders and is a well recognised feature of many mutant proteins (Gregersen et al 2008, 2010a, b; Yao et al 2011; Furuki et al 2006). As previously mentioned over two thirds of mutations are missense mutations many of which will result in unstable folding mutants but with potential for residual enzyme function. Studies using PTC124 and gentamicin have demonstrated the potential for the use of such compounds to promote readthrough and to increase functional activity of nonsense mutations (Tan et al 2011).
Fig. 4.
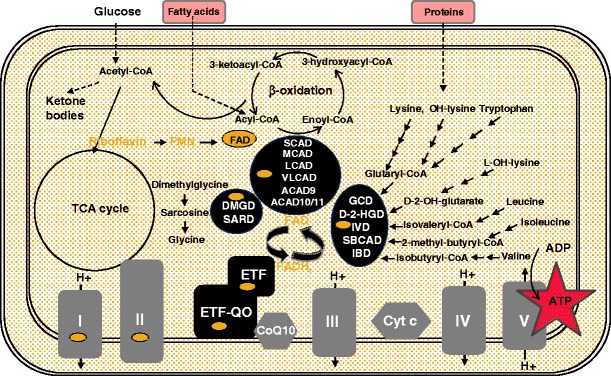
Mitochondrial FAD dependent pathways. FAD co-factor dependent acyl-CoA dehydrogenases, ETF, ETFDH and other FAD containing proteins including complexes I and II of the respiratory chain. Relationship of fatty acid oxidation, amino acid degradation and the respiratory chain. FMN flavin mononucleotide. FAD flavin adenine dinucleotide. ETF electron transfer flavoprotein. SCAD short chain acyl-CoA dehydrogenase. MCAD medium chain acyl-CoA dehydrogenase. LCAD long chain acyl-CoA dehydrogenase. VLCAD very long chain acyl-CoA dehydrogenase. ACAD9 acyl-CoA dehydrogenase 9. ACAD10/11 acyl-CoA dehydrogenase 10/11. GCD glutaryl-CoA dehydrogenase. D-2-HGD D-2-hydroxyglutaryl-CoA dehydrogenase. IVD isovaleryl-CoA dehydrogenase. SBCAD short branched chain acyl-CoA dehydrogenase. IBD isobutyryl-CoA dehydrogenase. DMGD dimethylglycine dehydrogenase. SARD sarcosine dehydrogenase
In general mutant proteins are recognised by molecular chaperones involved in cellular quality control and rapidly targeted for proteasomal degradation. If they can be rescued prior to being degraded by the cell there is potential for preserving enzyme function. A combination of upregulation of mutant protein, induction of readthrough mechanisms and enhanced protection by increasing either natural or artificial chaperones (e.g. riboflavin/FAD in ETFDH mutants) offers great potential for future therapeutic intervention in a wide range of inherited disorders.
Conclusion
Disorders of fatty acid oxidation exhibit a wide clinical spectrum of disease which are partly explained by genotype/phenotype correlation and identifiable environmental factors. However there remain many unresolved questions regarding the precise mechanisms that conspire to produce the phenotypic diversity that we encounter. Increased understanding of the gene regulatory mechanisms will hopefully improve our understanding of these disorders and these together with the environmental factors that influence the metabolome will serve to answer many of these unresolved questions. In time this will allow us to manage patients more effectively by tailoring therapies to achieve up or down regulation of metabolic pathways and to maximise mutant protein function.
Acknowledgements
I would like to thank Shirley Clark from Sheffield Children’s Hospital Department of Clinical Chemistry for her fatty acid oxidation studies and Dr Rikke K. J. Olsen Research Unit for Molecular Medicine, Aarhus University Hospital, Denmark for the diagram of mitochondrial flavoprotein metabolism
Conflict of interest
None.
Abbreviations
- AFLP
acute fatty liver of pregnancy
- CTD
OCTN2 transporter deficiency
- CPT1
carnitine palmitoyltransferase type 1 deficiency
- CATR
carnitine-acylcarnitine translocase deficiency
- CPT2
carnitine palmitoyltransferase type 2 deficiency
- FAD
flavin adenine dinucleotide
- FAO
fatty acid oxidation
- HELLP
haemolysis elevated liver enzymes, low platelets
- LCHAD
long chain 3-hydroxyacyl-CoA dehydrogenase deficiency
- MTP
mitochondrial trifunctional protein deficiency
- MCAD
medium chain acyl-CoA dehydrogenase deficiency
- MADD
multiple acyl-CoA dehydrogenase deficiency
- RR-MADD
riboflavin responsive multiple acyl-CoA dehydrogenase deficiency
- SCHAD
short chain 3-hydroxyacyl-CoA dehydrogenase deficiency
- VLCAD
very long-chain acyl-CoA dehydrogenase deficiency
References
- Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod Toxicol. 2009;27(3–4):246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Andresen BS, Olpin S, Poorthuis BJ, et al. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenasae deficiency. Am J Hum Genet. 1999;64:479–494. doi: 10.1086/302261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin J, Lopes-Costa A, Djouadi F. Exposure to resveratrol triggers pharmacological correction of fatty acid utilization in human fatty acid oxidation-deficient fibroblasts. Hum Mol Genet. 2011;20(10):2048–2057. doi: 10.1093/hmg/ddr089. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Rinaldo P, Strauss AW. Inborn errors of mitochondrial fatty acid oxidation. Crit Rev Clin Lab Sci. 2000;37:1–44. doi: 10.1080/10408360091174169. [DOI] [PubMed] [Google Scholar]
- Bergman AJ, Donckerwolcke RA, Duran M, et al. Rate-dependent distal renal tubular acidosis in carnitine palmitoyltransferase type I deficiency. Pediatr Res. 1994;36:582–588. doi: 10.1203/00006450-199411000-00007. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Haffmann S, Dombrowsky H, et al. Phosphatidylcholine molecular species in lung surfactant: composition in relation to respiratory rate and lung development. Am J Respir Cell Mol Biol. 2001;25:725–731. doi: 10.1165/ajrcmb.25.6.4616. [DOI] [PubMed] [Google Scholar]
- Boisseau N, Delmarche P. Metabolic and hormonal responses to exercise in children and adolescents. Sports Med. 2000;30:405–422. doi: 10.2165/00007256-200030060-00003. [DOI] [PubMed] [Google Scholar]
- Bonnefont J-P, Taroni F, Cavadini P, et al. Molecular analysis of carnitine palmitoyltransferase II deficiency with hepatocardiomuscular expression. Am J Hum Genet. 1996;58:971–978. [PMC free article] [PubMed] [Google Scholar]
- Bonnefont JP, Bastin J, Laforêt P, et al. Long-term follow-up of patients with myopathic form of carnitine palmitoyltransferase 2 deficiency. Clin Pharmacol Ther. 2010;88(1):101–108. doi: 10.1038/clpt.2010.55. [DOI] [PubMed] [Google Scholar]
- Brown NF, Mullur RS, Subramanian I, et al. Molecuar characterisation of L-CPT1 deficiency in six patients: insights into function of native enzyme. J Lipid Res. 2001;42:1134–1142. [PubMed] [Google Scholar]
- Chakrapani A, Olpin S, Cleary M, Walter JH, Wraith JE, Besley GTN. Trifunctional protein deficiency: Three families with significant maternal hepatic dysfunction in pregnancy not associated with E474Q mutation. J Inherit Metab Dis. 2000;23:826–834. doi: 10.1023/A:1026712719416. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mizuguchi H, Yao D, et al. Thermolabile phenotype of carnitine palmitoyltransferase II variations as a predisposing factor for influenza- associated encephalopathy. FEBS Lett. 2005;579(10):2040–2044. doi: 10.1016/j.febslet.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Cornelius N, Frerman FE, Corydon TJ, et al. Molecular mechanisms of riboflavin responsiveness in patients with ETF-QO variations and multiple acyl-CoA dehydrogenation deficiency. Hum Mol Genet. 2012;21(15):3435–3448. doi: 10.1093/hmg/dds175. [DOI] [PubMed] [Google Scholar]
- Corr PB, Yamanda KA. Selected metabolic alterations in the ischaemic heart and their contributions to arrhythmogenesis. Herz. 1995;20:156–168. [PubMed] [Google Scholar]
- Demaugre F, Bonnefont JP, Mitchell G, et al. Hepatic and muscular presentations of carnitine palmitoyl transferase deficiency: two distinct entities. Pediatr Res. 1988;24:308–311. doi: 10.1203/00006450-198809000-00006. [DOI] [PubMed] [Google Scholar]
- den Boer ME, Wanders RJ, Morris AA, et al. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: clinical presentation and follow-up of 50 patients. Pediatrics. 2002;109:99–104. doi: 10.1542/peds.109.1.99. [DOI] [PubMed] [Google Scholar]
- Deschauer M, Wieser T, Zierz S. Muscle carnitine palmitoyltransferase II deficiency: clinical and molecular genetic features and diagnostic aspects. Arch Neurol. 2005;62:37–41. doi: 10.1001/archneur.62.1.37. [DOI] [PubMed] [Google Scholar]
- Djouadi F, Aubey F, Schlemmer D, Bastin J. Peroxisome proliferator activated receptor delta (PPARdelta) agonist but not PPARalpha corrects carnitine palmitoyl transferase 2 deficiency in human muscle cells. J Clin Endocrinol Metab. 2005;90(3):1791–1797. doi: 10.1210/jc.2004-1936. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Gerhart-Hines Z, Puigserver P. Nutrient-dependent acetylation controls basic regulatory metabolic switches and cellular reprogramming. Cold Spring Harb Symp Quant Biol. 2011;76:203–209. doi: 10.1101/sqb.2012.76.010843. [DOI] [PubMed] [Google Scholar]
- Ensenaur R, He M, Willarg JM, et al. Human acyl-CoA dehydrogenase-9 plays a novel role in mitochondrial beta-oxidation of unsaturated fatty acids. J Biol Chem. 2005;280:32309–32316. doi: 10.1074/jbc.M504460200. [DOI] [PubMed] [Google Scholar]
- Essen B. Intramuscular substrate utilisation during prolonged exercise. Ann N Y Acad Sci. 1977;301:30–44. doi: 10.1111/j.1749-6632.1977.tb38183.x. [DOI] [PubMed] [Google Scholar]
- Essen B, Hagenfeldt L, Kaijser L. Utilisation of blood-borne and intramuscular substrates during continuous and intermittent exercise in man. J Physiol. 1977;265:489–506. doi: 10.1113/jphysiol.1977.sp011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falik-Borenstein ZC, Jordan SC, Saudubray JM, et al. Brief report: renal tubular acidosis in carnitine palmitoyltransferase type 1 deficiency. N Eng J Med. 1992;327:24–27. doi: 10.1056/NEJM199207023270105. [DOI] [PubMed] [Google Scholar]
- Furuki S, Tamura S, Matsumoto N, et al. Mutations in the peroxin Pex26p responsible for peroxisome biogenesis disorders of complementation group 8 impair its stability, peroxisomal localization, and interaction with the Pex1p x Pex6p complex. J Biol Chem. 2006;281(3):1317–23. doi: 10.1074/jbc.M510044200. [DOI] [PubMed] [Google Scholar]
- Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130:2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner BD, Gillingham MB, Birch S, Wood T, Koeller DM. Evidence for an association between infant mortality and a carnitine palmitoyltransferase 1A genetic variant. Pediatrics. 2010;126(5):945–951. doi: 10.1542/peds.2010-0687. [DOI] [PubMed] [Google Scholar]
- Gillingham MB, Hirschfeld M, Lowe S, et al. Impaired fasting tolerance among Alaska Native Children with a common Carnitine Palmitoyltransferase 1A sequence variant. Mol Genet Metab. 2011;104:261–264. doi: 10.1016/j.ymgme.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin-Limballe S, Djouadi F, Aubey F, et al. Genetic basis for correction of very-long-chain acyl-coenzyme A dehydrogenase deficiency by bezafibrate in patient fibroblasts: toward a genotype-based therapy. Am J Hum Genet. 2007;81(6):1133–1143. doi: 10.1086/522375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SI, Binard R, Woontner M, et al. Glutaric aciduria type II:gene structure and mutations of the electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO) gene. Mol Genet Metab. 2002;77:86–90. doi: 10.1016/S1096-7192(02)00138-5. [DOI] [PubMed] [Google Scholar]
- Greenberg CR, Dilling LA, Thompson GR, et al. The paradox of the carnitine palmitoyltransferase type Ia P479L variant in Canadian Aboriginal populations. Mol Genet Metab. 2009;96:201–207. doi: 10.1016/j.ymgme.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Gregersen N, Boss P. Protein misfolding and cellular stress: an overview. Methods Mol Biol. 2010a;648:3–23. doi: 10.1007/978-1-60761-756-3_1. [DOI] [PubMed] [Google Scholar]
- Gregersen N, Olsen RKJ. Disease mechanisms and protein structures in fatty acid oxidation defects. J Inher Metab Dis. 2010b;33:547–553. doi: 10.1007/s10545-010-9046-1. [DOI] [PubMed] [Google Scholar]
- Gregersen N, Andresen BS, Pedersen CB, Olsen RK, Corydon TJ, Boss P. Mitochondrial fatty acid oxidation defects - remaining challenges. J Inher Metab Dis. 2008;31:643–657. doi: 10.1007/s10545-008-0990-y. [DOI] [PubMed] [Google Scholar]
- Gregory J, Lowe S. National diet and nutrition survey of young people age 4–18 years. London: The Stationery Office; 2000. [Google Scholar]
- Haworth JC, Demaugre F, Booth FA, et al. Atypical features of hepatic form of carnitine palmitoyltransferase deficiency in a Hutterite family. J Pediatr. 1992;121:553–577. doi: 10.1016/S0022-3476(05)81143-6. [DOI] [PubMed] [Google Scholar]
- He M, Pei Z, Mohsen A-W, et al. Identification and characterisation of new long chain acyl-CoA dehydrogenases. Mol Genet Metab. 2011;102:418–429. doi: 10.1016/j.ymgme.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homuth G, Teumer A, Volker U, Nauck M. A description of large-scale metabolomics studies: increasing value by combining metabolomics with genome-wide SNP genotyping and transcriptional profiling. J Endocrinol. 2012;215:17–28. doi: 10.1530/JOE-12-0144. [DOI] [PubMed] [Google Scholar]
- Houten SM, Wanders RJA. A general introduction to the biochemistry of mitochondrial fatty acid oxidation. J Inher Metab Dis. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug G, Bove KE, Soukup S. Lethal neonatal multiorgan deficiency of carnitine palmitoyltransferase II. N Engl J Med. 1991;325:1862–1864. doi: 10.1056/NEJM199112263252607. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Tein I, Dionisi-Vicii C, et al. Mild trifunctional protein deficiency is associated with progressive neuropathy and myopathy and suggests a novel genotype-phenotype correlation. J Clin Invest. 1998;102:1193–1199. doi: 10.1172/JCI2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibdah JA, Yang Z, Bennett MJ. Liver disease in pregnancy and fetal fatty acid oxidation defects. Mol Genet Metab. 2000;71:182–189. doi: 10.1006/mgme.2000.3065. [DOI] [PubMed] [Google Scholar]
- Innes AM, Seargeant LE, Balachandra K. Hepatic carnitine palmitoyltrabnsferase deficiency presenting as maternal illness in pregnancy. Pediatr Res. 2000;47:43–45. doi: 10.1203/00006450-200001000-00010. [DOI] [PubMed] [Google Scholar]
- Kim TCS, Roe CR, Mann JD, Breese GR. Octanoic acid produces accumulation of monoamine acidic metabolites in the brain: interaction with organic anion transport at the choroid plexus. J Neurochem. 1992;58(4):1499–1503. doi: 10.1111/j.1471-4159.1992.tb11370.x. [DOI] [PubMed] [Google Scholar]
- Kubota M, Chida J, Hoshino H, et al. Thermolabile CPT II variants and low blood ATP levels are closely related to severity of acute encephalopathy in Japanese children. Brain Dev. 2012;34(1):20–27. doi: 10.1016/j.braindev.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Kunau WH, Dommes V, Schulz H. Beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prod Lipid Res. 1995;34:267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Leipnitz G, Schuck PF, Ribeiro CAJ, et al. Ethylmalonic acic inhibits mitochondrial creatine kinase activity from cerebral cortex of young rats in vitro. Neurochem Res. 2003;28:771–777. doi: 10.1023/A:1022874103630. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Palladino A, Narayan S, et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem. 2010;285(41):31806–31818. doi: 10.1074/jbc.M110.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas TG, Henriques BJ, Rodrigues JV, et al. Cofactors and metabolites as potential stabilizers of mitochondrial acyl-CoA dehydrogenases. Biochim Biophys Acta. 2011;1812(12):1658–1663. doi: 10.1016/j.bbadis.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Lundy CT, Shield JP, Kvittingen EA, Vinorum OJ, Trimble ER, Morris AAM. Acute respiratory distress syndrome in long-chain 3-hydroxyacyl-CoA dehydrogenase and mitochondrial trifunctional protein deficiencies. J Inher Metab Dis. 2003;26:537–541. doi: 10.1023/A:1025995813914. [DOI] [PubMed] [Google Scholar]
- Mak IT, Kramer JH, Weglicki WB. Potentiation of free radical-induced lipid peroxidative injury to sarcolemmal membranes by lipid amphiphiles. J Biol Chem. 1986;261:1153–1157. [PubMed] [Google Scholar]
- Mak CM, Lam CW, Fong NC, et al. Fatal viral infection-associated encephalopathy in two Chinese boys: a genetically determined risk factor of thermolabile carnitine palmitoyltransferase II variants. J Hum Genet. 2011;56(8):617–621. doi: 10.1038/jhg.2011.63. [DOI] [PubMed] [Google Scholar]
- Morillas M, Gómez-Puertas P, Roca R, et al. Structural model of the catalytic core of carnitine palmitoyltransferase I and carnitine octanoyltransferase (COT): mutation of CPT I histidine 473 and alanine 381 and COT alanine 238 impairs the catalytic activity. Biol Chem. 2001;276(48):45001–45008. doi: 10.1074/jbc.M106920200. [DOI] [PubMed] [Google Scholar]
- Morris AAM, Olpin SE, Bennett MJ, Santani A, Stahlschmidt J, McClean P. Cholestatic jaundice associated with carnitine palmitoyltransferase IA deficiency. J Inher Metab Dis. 2013;7:27–9. doi: 10.1007/8904_2012_135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oey NA, den Boer MEJ, Wijburg FA, et al. Long-chain fatty acid oxidation during early human development. Pediatr Res. 2005;57:755–759. doi: 10.1203/01.PDR.0000161413.42874.74. [DOI] [PubMed] [Google Scholar]
- Oey NA, Ruiter JP, Ijlst L, et al. Acyl-CoA dehydrogenase 9 (ACAD 9) is the long chain acyl-CoA dehydrogenase in human embryonic and fetal brain. Biochem Biophys Res Commun. 2006;346:33–37. doi: 10.1016/j.bbrc.2006.05.088. [DOI] [PubMed] [Google Scholar]
- Olpin SE, Allen JC, Bonham JR, et al. Features of carnitine palmitoyltransferase type I deficiency. J Inher Metab Dis. 2001;24:35–42. doi: 10.1023/A:1005694320063. [DOI] [PubMed] [Google Scholar]
- Olpin SE, Afifi A, Clark S, et al. Mutation and biochemical analysis in carnitine palmitoyltransferase type II (CPT II) deficiency. J Inher Metab Dis. 2003;26:543–557. doi: 10.1023/A:1025947930752. [DOI] [PubMed] [Google Scholar]
- Olpin SE, Clark S, Andresen BS, et al. Biochemical, clinical and molecular findings in LCHAD and general mitochondrial trifunctional protein deficiency. J Inher Metab Dis. 2005;28:533–544. doi: 10.1007/s10545-005-0533-8. [DOI] [PubMed] [Google Scholar]
- Olpin SE, Clark S, Scott C, et al. Diagnosing very long-chain acyl-CoA dehydrogenase deficiency (VLCADD) J Inher Metab Dis. 2012;35(suppl 1):S15–043. [Google Scholar]
- Olsen RK, Andresen BS, Christensen E, Bross P, Skovby F, Gregersen N. Clear relationship between ETF/ETFDH genotype & phenotype in patients with multiple acyl-CoA dehydrogenase deficiency. Human Mutat. 2003;22:12–23. doi: 10.1002/humu.10226. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Olpin SE, Andresen BS, et al. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Brain. 2007;130:2045–2054. doi: 10.1093/brain/awm135. [DOI] [PubMed] [Google Scholar]
- Orngreen MC, Norgaard MG, Sacchetti M, et al. Fuel utilisation in patients with very long-chain acyl-CoA dehydrogenase deficiency. Ann Neurol. 2004;56:279–283. doi: 10.1002/ana.20168. [DOI] [PubMed] [Google Scholar]
- Orngreen MC, Duno M, Ejstrup R, et al. Fuel utilisation in subjects with palmitoyltransferase 2 gene mutations. Ann Neurol. 2005;57:60–66. doi: 10.1002/ana.20320. [DOI] [PubMed] [Google Scholar]
- Phinney SD, Bistrian BR, Wolfe RR, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism. 1983a;32:757–768. doi: 10.1016/0026-0495(83)90105-1. [DOI] [PubMed] [Google Scholar]
- Price NT, van der Leij FR, Jackson VN, et al. A novel brain-expressed protein related to CPT1. Genomics. 2002;80:433–442. doi: 10.1006/geno.2002.6845. [DOI] [PubMed] [Google Scholar]
- Rasmussen J, Nielsen OW, Lund AM, Kober L, Djurhuus H. Primary carnitine deficiency and pivalic acid exposure causing encephalopathy and fatal cardiac events. J Inher Metab Dis. 2013;36:35–41. doi: 10.1007/s10545-012-9488-8. [DOI] [PubMed] [Google Scholar]
- Ridsdale R, Roth-Kleiner M, D’Ovidio F, et al. Surfactant palmitoylmyristoylphosphatidylcholine is a marker for alveolar size during disease. Am J Respir Crit Care Med. 2005;172:225–232. doi: 10.1164/rccm.200501-109OC. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam RS, van Spronsen FJ, Bink-Boelkens MT, et al. Ventricular fibrillation without overt cardiomyopathy as first presentation of organic cation transporter 2-deficiency in adolescence. Pacing Clin Electrophysiol. 2004;27:675–676. doi: 10.1111/j.1540-8159.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- Ritchie RH, Delbridge LM. Cardiac hypertrophy, substrate utilisation and metabolic remodelling: cause or effect? Clin Exp Pharmacol Physiol. 2006;33(1–2):156–166. doi: 10.1111/j.1440-1681.2006.04342.x. [DOI] [PubMed] [Google Scholar]
- Rocha H, Ferreira R, Carvalho J, et al. Characterisation of multiple acyl-CoA dehydrogenation defect through mitochondrial proteomics. J Inher Metab Dis. 2011a;34(Suppl 3):S149–037. [Google Scholar]
- Rocha H, Ferriera R, Carvalho J, et al. Characterisation of mitochondrial proteome in a severe case of ETF-QO deficiency. J Proteomics. 2011b;75:221–228. doi: 10.1016/j.jprot.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EC, Amat di San Filippo C, Erlingsson N, Ardon O, Pasquali M, Longo N. Genotype-phenotype correlation in Primary Carnitine Deficiency. Hum Mut. 2011;33:118–123. doi: 10.1002/humu.21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer SW, Okun JG, Hoffmann GF, Koelker S, Morath MA. Impact of short- and medium-chain organic acids, acylcarnitines, and acyl-CoAs on mitochondrial energy metabolism. Biochem Biophys Acta. 2008;1777:1276–1282. doi: 10.1016/j.bbabio.2008.05.447. [DOI] [PubMed] [Google Scholar]
- Scaini G, Simon KR, Tonin, et al. Toxicity of octanoate and decanoate in rat peripheral tissues: evidence of damage of bioenergetic dysfunction and oxidative damage induction in liver and skeletal muscle. Mol Cell Biochem. 2012;361:329–335. doi: 10.1007/s11010-011-1119-4. [DOI] [PubMed] [Google Scholar]
- Schaefer J, Jackson S, Dick DJ, Turnbull DM. Trifunctional enzyme deficiency: adult presentation of a usually fatal β-oxidation defect. Ann Neurol. 1996;40:597–602. doi: 10.1002/ana.410400409. [DOI] [PubMed] [Google Scholar]
- Schuck PF, Ferreira GC, Tonin AM, et al. Evidence that the major metabolites accumulating in medium chain acyl-CoA dehydrogenase deficiency disturb mitochondrial energy metabolism. Brain Res. 2009;1296:117–126. doi: 10.1016/j.brainres.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Schuck PF, Ferriera GC, Tahara EB, Klamt F, Kowaltowski AJ, Wajner M. cis-4-decenoic acid provokes mitochondrial bioenergetic dysfunction in rat brain. Life Sci. 2010;87:139–146. doi: 10.1016/j.lfs.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Sharpe MA, Clark JB, Heales SJR. Cytochrome C oxidase inhibition by cis-4-decenoic acid (C10:1): An important mechanism in medium chain acyl-CoA dehydrogenase (MCAD) deficiency? J Inher Metab Dis. 1999;22(1):23. [Google Scholar]
- Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2000;279:H2124–H2132. doi: 10.1152/ajpheart.2000.279.5.H2124. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U. Mitochondrial fatty acid oxidation disorders:clinical presentation of long-chain fatty acid oxidation defects before and after newborn screening. J Inher Metab Dis. 2010;33:527–532. doi: 10.1007/s10545-010-9090-x. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Wood P. Mitochondrial fatty acid oxidation disorders: pathophysiological studies in mouse models. J inher Metab Dis. 2010;33:539–546. doi: 10.1007/s10545-010-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter U, Huener G, Baykal T, et al. Silent and symptomatic primary carnitine deficiency within the same family due to identical mutations in the organic cation/carnitine transporter OCTN2. J Inher Metab Dis. 2003a;26:613–615. doi: 10.1023/A:1025968502527. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Sun B, Khuchua Z, Bennett MJ, Strauss AW. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to β-subunit mutations. Hum Mutat. 2003b;21:598–607. doi: 10.1002/humu.10211. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Bennett MJ, Ben-Zeev B, Strauss AW, Tein I. Peripheral neuropathy, episodic myoglobinuria, and respiratory failure in deficiency of the mitochondrial trifunctional protein. Muscle Nerve. 2004;29:66–72. doi: 10.1002/mus.10500. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Khuchua Z, Yue Z, Bennett MJ, Strauss AW. General mitochondrial trifunctional protein (TFP) deficiency as a result of either alpha- or beta-subunit mutations exhibits similar phenotypes because mutations in either subunit alter TFP complex expression and subunit turnover. Pediatr Res. 2004b;55:190–196. doi: 10.1203/01.PDR.0000103931.80055.06. [DOI] [PubMed] [Google Scholar]
- Spriet LL. Regulation of skeletal muscle fat oxidation during exercise in humans. Med Sci Sports Exerc. 2002;34:1477–1484. doi: 10.1097/00005768-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Stanley CA, DeLeeuw S, Coates PM, et al. Chronic cardiomyopathy and weakness or acute coma in children with a defect in carnitine uptake. Ann Neurol. 1991;30:709–716. doi: 10.1002/ana.410300512. [DOI] [PubMed] [Google Scholar]
- Suhrie KRS, Karunanidhi AK, Mohen WM, Reyes-Mugia MRM, Vockley JV. Long chain acyl-CoA dehydrogenase deficiency: a new inborn error of metabolism manifesting as congenital surfactant deficiency. J Inher Metab Dis. 2011;34(suppl 3):S149–038. [Google Scholar]
- Taggart RT, Smail D, Apolito C, Vladutiu D. Novel mutations associated with carnitine palmitoyltransferase II deficiency. Hum Mutat. 1999;13:210–220. doi: 10.1002/(SICI)1098-1004(1999)13:3<210::AID-HUMU5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Tan L, Narayan SB, Chen, et al. PTC124 improves readthrough and increases enzymatic activity of the CPT1A R160X nonsense mutation. J Inher Metab Dis. 2011;34:443–447. doi: 10.1007/s10545-010-9265-5. [DOI] [PubMed] [Google Scholar]
- Taroni F, Verderio E, Fiorucci S, et al. Molecular characterisation of inherited carnitine palmitoyltransferase II deficiency. Proc Natl Acad Sci U S A. 1992;89:8429–8433. doi: 10.1073/pnas.89.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tein I. Carnitine transport:pathophysiology and metabolism of known molecular defects. J Inher Metab Dis. 2003;26:147–169. doi: 10.1023/A:1024481016187. [DOI] [PubMed] [Google Scholar]
- Tein I, De Vivo DC, Biierman F, et al. Impaired skin fibroblast carnitine uptake in primary systemic carnitine deficiency manifested by childhood carnitine-responsive cardiomyopathy. Pediatr Res. 1990;28:247–255. doi: 10.1203/00006450-199009000-00020. [DOI] [PubMed] [Google Scholar]
- Tein I, DiMauro S, Xie Z-W, De Vivo DC. Heterozygotes for plasmalemmal carnitine transporter defect are at increased risk of valproic acid associated impairment of carnitine uptake in cultured human skin fibroblasts. J Inher Metab Dis. 1995;18:313–322. doi: 10.1007/BF00710422. [DOI] [PubMed] [Google Scholar]
- Tein I, Vajsar J, MacMillan L, Sherwood WG. Long chain L-3-hydroxyacyl-CoAenzyme A dehydrogenase deficiency neuropathy: Response to cod liver oil. Neurology. 1999;52:640–643. doi: 10.1212/WNL.52.3.640. [DOI] [PubMed] [Google Scholar]
- Tonin AM, Ferreira GC, Grings M, et al. Disturbance of mitochondrial energy homeostasis caused by the metabolites accumulating in LCHAD and MTP deficiencies in rat brain. Life Sci. 2010;86:825–831. doi: 10.1016/j.lfs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Tyni T, Majander A, Kalimo H, Pihko H. Pathology of skeletal muscle impaired respiratory chain function in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency with the G1528C mutation. Neuromuscul Disord. 1996;6:327–337. doi: 10.1016/0960-8966(96)00352-5. [DOI] [PubMed] [Google Scholar]
- Tyni T, Rapola J, Palotie A, Pihko H. Hypoparathyroidism in a patient with long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency caused by the G1528C mutation. J Pediatr. 1997;131(5):766–768. doi: 10.1016/S0022-3476(97)70111-2. [DOI] [PubMed] [Google Scholar]
- Tyni T, Kivela T, Lappi M, Summainen P, Nikoskelainen E, Pihko H. Ophthalmic findings in LCHAD deficiency caused by the G1528C mutation. Ophthaalmology. 1998;105:810–824. doi: 10.1016/S0161-6420(98)95019-9. [DOI] [PubMed] [Google Scholar]
- Tyni T, Johnson M, Eaton S, et al. Mitochondrial fatty acid beta-oxidation in the retinal pigment epithelium. Perdiatr Res. 2002;52:595–600. doi: 10.1203/00006450-200210000-00021. [DOI] [PubMed] [Google Scholar]
- Tyni T, Paetau A, Strauss AW, Middleton B, Kivela T. Mitochondrial fatty acid β-oxidation in human eye and brain: implications for retinopathy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Pediatr Res. 2004;56:744–750. doi: 10.1203/01.PDR.0000141967.52759.83. [DOI] [PubMed] [Google Scholar]
- Ventura FV, Ruiter JP, Ijlst L, Almeida IT, Wanders RJA. Inhibitory effect of 3-hydroxyacyl-CoA’s and other long-chain fatty acid β-oxidation intermediates on the oxidation phosphorylation system. J Inher Metab Dis. 1996;19:161–164. doi: 10.1007/BF01799419. [DOI] [PubMed] [Google Scholar]
- Ventura FV, Ruiter J, Ijlst L, de Almeida IT, Wanders RJ. Differential inhibitory effect of long-chain acyl-CoA esters on succinate and glutamate transport into rat liver mitochondria and its possible implications for long-chain fatty acid oxidation defects. Mol Genet Metab. 2005;86(3):344–52. doi: 10.1016/j.ymgme.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Ventura FV, Tavares de Almeida I, Wanders RJ. Inhibition of adenine nucleotide transport in rat liver mitochondria by long-chain acyl-coenzyme A beta-oxidation intermediates. Biochem Biophys Commun. 2007;352:873–878. doi: 10.1016/j.bbrc.2006.11.109. [DOI] [PubMed] [Google Scholar]
- Verderio E, Cavadini P, Montermini L, et al. Carnitine palmitoyltransferase II deficiency: structure of the gene and characterisation of two novel disease-causing mutations. Hum Mol Genet. 1995;4:19–129. doi: 10.1093/hmg/4.1.19. [DOI] [PubMed] [Google Scholar]
- Vielhaber S, Feistner H, Weis J, et al. Primary carnitine deficiency: adult onset lipid storage myopathy with a mild clinical course. J Clin Neurosci. 2004;11(8):919–924. doi: 10.1016/j.jocn.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Vijay S, Patterson A, Olpin S, et al. Carnitine transporter defect: Diagnosis in asymptomatic adult women following analysis of acylcarnitines in their newborn infants. J Inher Metab Dis. 2006;29:627–630. doi: 10.1007/s10545-006-0376-y. [DOI] [PubMed] [Google Scholar]
- Vladutiu GD. Heterozygosity: An expanding role in Proteomics. Mol Genet Metab. 2001;74:51–63. doi: 10.1006/mgme.2001.3240. [DOI] [PubMed] [Google Scholar]
- Vladutiu GD, Bennett MJ, Smail D, et al. A variable myopathy associated with heterozygosity for R503C mutation in the carnitine palmitoyltransferase II gene. Mol Genet Metab. 2000;70:134–141. doi: 10.1006/mgme.2000.3009. [DOI] [PubMed] [Google Scholar]
- Vladutiu GD, Bennett MJ, Nadine M, et al. Phenotypic variability among first-degree relatives with carnitine palmitoyltransferase II deficiency. Muscle and Nerve. 2002;26:492–498. doi: 10.1002/mus.10217. [DOI] [PubMed] [Google Scholar]
- Vockley J, Rinaldo P, Bennett MJ, Matern D, Vladutiu GD. Synergistic heterozygosity: disease resulting from multiple partial defects in one or more metabolic pathways. Mol Genet Metab. 2000;71:10–18. doi: 10.1006/mgme.2000.3066. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Vreken P, den Boer ME, Wijburg FA, van Gennip AH, Ijlst L. Disorders of mitochondrial fatty acid acyl-CoA beta-oxidation. J Inher Metab Dis. 1999;22:442–487. doi: 10.1023/A:1005504223140. [DOI] [PubMed] [Google Scholar]
- Wanders RJA, Ruiter JPN, IJlst L, Waterham HR, Houten S. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inher Metab Dis. 2010;33:479–494. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis BC, Esser V, Foster DW, et al. Rat hearts express two isoforms of mitochondrial carnitine palmitoyltransferase 1. J Biol Chem. 1994;269:18712–18715. [PubMed] [Google Scholar]
- Wojtaszewski JFP, McDonald C, Nielsen JN, et al. Regulation of 5′AMP-activated protein kinase activity and substrate utilisation in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Yao M, Yao D, Yamaguchi M, Chida J, Yao D, Kido H. Bezafibrate upregulates carnitine palmitoyltransferase II expression and promotes mitochondrial energy crisis dissipation in fibroblasts of patients with influenza-associated encephalopathy. Mol Genet Metab. 2011;104(3):265–272. doi: 10.1016/j.ymgme.2011.07.009. [DOI] [PubMed] [Google Scholar]


