Abstract
Amiodarone is a widely used and very potent antiarrhythmic substance. Among its adverse effects, pulmonary toxicity is the most dangerous without a causal treatment option. Due to a very long half-life, accumulation can only be prevented by strict adherence to certain dosage patterns. In this review, we outline different safe and proven dosing schemes of amiodarone and compare the incidence and description of pulmonary toxicity. Reason for this is a case of fatal pulmonary toxicity due to a subacute iatrogenic overdosing of amiodarone in a 74-year-old male patient with known severe coronary artery disease, congestive heart failure and ectopic atrial tachycardia with reduced function of kidneys and liver but without preexisting lung disease. Within 30 days, the patient received 32.2 g of amiodarone instead of 15.6 g as planned. Despite early corticosteroid treatment after fast exclusion of all other differential diagnoses, the patient died another month later in our intensive care unit from respiratory failure due to bipulmonal pneumonitis.
Keywords: Amiodarone pulmonary toxicity, Atrial fibrillation, Heart failure, Dosage patterns, Overdosage
Introduction
Amiodarone is the most potent antiarrhythmic drug to treat and prevent supraventricular and ventricular arrhythmias.
As an iodized benzofurane derivate it has multiple actions [1]
Despite various adverse effects (hyperthyreoidism, cornea deposits, photosensitisation of the skin, and disturbances in the intraventricular conduction) its reliable antiarrhythmic efficacy and low proarrhythmic potential make it widely used with virtue and safety proven in multiple clinical trials [2–11].
Within the spectrum of adverse events, amiodarone induced pulmonary toxicity (APT) causing interstitial pneumonitis, is the most dangerous without curative option [1]. It occurs dose-dependently, but also low dosages have been reported to cause severe pulmonary disorders within weeks after initiation of therapy [12].
Therefore, knowledge of and strict adherence to the recommended dosage especially during loading phases are essential [13, 14]. Due to the long elimination half-life (10–30 days up to 6 months) [15], its lipophilicity and large distribution volume, amiodarone re-circulates for about 3–10 months, promoting accumulation and overdosing. Initially higher loading doses are necessary, while high maintenance doses cause overdosing.
We report a subacute iatrogenic amiodarone overdose causing fatal interstitial pneumonitis within 1 month after initiation of treatment. We add a literature review of safe amiodarone dosing and incidence of APT.
Case Report
We report fatal APT due to subacute overdosing of amiodarone in a 74-year-old male patient with severe coronary artery disease, congestive heart failure, and ectopic atrial tachycardia with reduced renal and liver function but without preexisting lung disease.
Past Medical History
Long Term Medical History
The patient had severe coronary artery disease resulting in a diminished left ventricular function. A hemodynamically relevant ectopic atrial tachycardia was treated with sotalol (160 mg/d). The patient had reduced renal function and suffered from a chronically active hepatitis C without significant intrahepatic inflammation. No history of any pulmonary disease was known. The patient had ceased smoking in 1979.
Recent Medical History and Beginning of Amiodarone Therapy
One month prior to the present admission, the patient presented with an acute non ST-elevation myocardial infarction. Coronary angiography revealed a stenosis in one of the patient’s venous bypass-grafts, which was successfully dilated and stented.
Sotalol had been discontinued since it failed to control the arrhythmia and the renal function worsened progressively. The ectopic atrial tachycardia re-occurred permanently at a heart rate of 110–120 bpm, causing palpitations and aggravated left ventricular dysfunction as well as subjective dyspnoea. Therefore the antiarrhythmic treatment was changed to amiodarone. Due to reduced liver and kidney function, oral amiodarone was started with 800 mg/d.
Due to a widening of the QRS-complex from 140 ms to 180 ms after 8 days, the dosage was reduced to 200 mg/d for another 6 days, but after relapse of the tachycardia elevated again to 600 mg/d for the last 2 days in cardiologic treatment. Accompanied by an optimized programming of the CRT-ICD device, the arrhythmia was inhibited successfully and palpitations ceased.
After dismission from our department, the patient was given 600 mg amiodarone three times daily (1.8 g/d) over 13 days. Instead of the recommended total of 7.8 g amiodarone in this period, the patient received 23.4 g. The cumulative dose that the patient had received within 30 days was 32.2 g of amiodarone, 15.6 g more than planned (Fig. 1).
Fig. 1.
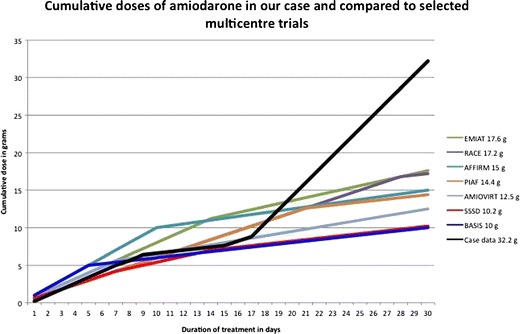
Cumulative doses of amiodarone within the first month in this case report in comparison to selected multicentre trials. Due to accidental overdosing after day 17, cummulative doses in this case rapidly exceeded the range of any multicentre trial. After 30 days, this resulted in a nearly doubled cumulative dose of amiodarone in this case (32.2 g) as compared to the highest cummulative dose in any multicentre trial (EMIAT 17.6 g)
Topical Admission with APT
Thirteen days after discharge from our department, the patient was re-admitted with progressive dyspnoea and an intermittent non-productive cough.
He presented orthopnoeic, but auscultation revealed no significant pathologic findings especially no heart murmurs or rales of the lung. Blood gas analysis under oxygen supply of 8 l/min showed a hypoxemia compensated by hyperventilation with an arterial oxygen saturation of 94 % (paO2 72 mmHg) and moderately decreased paCO2 levels (26 mmHg).
Cardiac enzymes were not elevated and the ECG showed no new changes.
Laboratory parameters revealed a slight leucocytosis of 12.000/μL, the C-reactive protein (CRP) was elevated to 12.2 mg/dL (<0.5 mg/dL), while procalcitonin remained normal, not indicating bacterial infection. In addition, all blood cultures and microbiological analyses of bronchioalveolar fluid remained sterile. Screening for pathogenic viruses including the influenza strain H1N1 virus was negative.
Chest x-ray and computed tomography of the chest showed a slight, diffuse bipulmonary interstitial infiltration. Pulmonary arterial embolism was excluded. No pleural effusion was confirmed by ultrasound. Bronchoscopy revealed no pus, no edema or other pathologic findings and cytopathologic examination of the broncho-alveolar fluid revealed unspecific granulocytic inflammation. Echocardiography did not reveal any changes compared to 1 month prior.
On admission, a broad-spectrum antibiotic therapy was initiated for suspected nosocomially acquired pneumonia and amiodarone medication was stopped immediately.
The Patient’s Respiratory Insufficiency Necessitated Noninvasive Ventilation Upon his Admission and After 3 Days he Required Intubation and Invasive Ventilation
Serologic signs of inflammation did not recede, leucocytosis and c-reactive protein remained unchanged after 4 days of effective broad spectrum antimicrobial treatment. Chest computed tomography showed rapidly progressive bipulmonary interstitial infiltrations in a diffuse ubiquitary pattern resembling adult respiratory distress syndrome (ARDS). (Figure 2) With all microbiological samples sterile, aggravation of the pulmonary infiltrations despite antibiotic and antimycotic therapy and low procalcitonin, bacterial pneumonia became unlikely.
Fig. 2.

Thoracic computered tomography of the reported patient, day three after admission demonstrates severe interstitial lung infiltrations as it is seen in ARDS. “Honeycombing”-like changes in lung structure are seen preferentially in the dorsal lung compartiments
Amiodarone toxicity was assumed and cortico-steroid treatment was initiated 3 days after admission (50, 30, 20 mg/d i.v. prednisolone in the first 3 weeks). Over the following 2 weeks, the respiratory situation of the patient further aggravated, especially the elimination of carbon dioxide was impaired. To enable a lung-protective ventilation with reduced tidal volumes (≤6 ml/kg) as recommended for ARDS, an interventional arterio-venous lung assist device (Novalung®) was implanted.
The patient additionally developed acute-on-chronic renal failure requiring hemodialysis.
Despite these measures and due to the severe underlying co-morbidities, the patient died after three more days in multi-organ failure, 62 days after initiation of amiodarone treatment.
Discussion
This case of severe APT is in many respects in line with earlier reports. The elderly male patient [16] with reduced left ventricular, renal and liver function [17] and low body fat and under agiotensin-inhibiting treatment [18] represents an at-risk group for APT. All his symptoms, the laboratory, radiologic and functional findings were as unspecific as reported previously [19] initially suggesting various differential diagnoses. In many cases, this further delayed the antiinflammatory treatment [12]. In our case, the obvious history of drastic overdosing of amiodarone quickly led to the diagnosis and enabled early antiinflammatory treatment. The daily dose of amiodarone in our case is unprecedented. No systematic experiences with such maintenance doses have been reported.
In the following, we review literature on APT, integrating the specifics of our case. Furthermore, we list different dosage schemes from official recommendations, multicentre trials for supraventricular and ventricular arrhythmias.
Adverse Effects of Amiodarone
Adverse reactions of amiodarone include hypo- or hyperthyreodism in 1–11 % [15] neurologic side effects in 3–35 % and microscopic corneal crystalline deposition (−90 %), sometimes diminishing visual acuity. Furthermore, unspecific hepatitis can occur in 4 % [15] while solely elevated liver enzymes are more common (15–30 %) [20]. Dermatological side effects include pseudocyanosis (4–9 %) and photo-sensibilization (25–75 %).
These symptoms should be meticulously assessed in regular office visits accompanying amiodarone treatment . Chest X-rays are recommended initially and annually as well as initial and symptom-oriented assessments of pulmonary function, especially of the diffusion capacity of the lung, in patients at risk [14]. Laboratory control of thyroid, liver and renal function semi-annually and regular ophthalmologic, dermatologic and neurologic examinations are recommended [14].
APT
APT, the most dangerous adverse effect of amiodarone, is known since 1980 [21] It is caused by direct toxic effects of the accumulated substance in the pulmonary tissue and immunologic reactions [1] leading to an unspecific diffuse interstitial pneumonitis. Direct toxic effects include the accumulation of phospholipids and the production of toxic oxygen radicals which show dose-dependency. The immunological reaction was found to be mediated by T-lymphocytes which are predominantly CD8 positive. It resembles hypersensitivity reactions and helps to explain the sudden onset of cases of severe APT after comparatively smalldoses of amiodarone [1].
Incidence of APT
Incidence and mortality of APT are reported inconsistently. Earlier reviews reported incidence up to 61 % with mortality between 1 % and 33 % [1]. Subsequent reports assumed incidence at 13 % [15]. Probably due to reduced maintenance doses, APT was observed less frequently in more recent reports ranging between 2.9 % [16] and 1.6 % [22]. Especially in critically ill intensive care patients, the side effects of amiodarone might be underestimated [19].
Patients with APT showed mortality of 9 % [23] or 7 % [16], slightly higher than the previously stated 5 % [1, 15].
A recent study reported an incidence of 1–2 % APT, fatal in 7 %, resulting in an overall incidence of fatal APT at <0.07 % [16].
This development might result from a “learning curve” in dosing amiodarone and detecting APT, since its incidence peaked around 1983 and declined afterwards [20].
Loading, Maintenance and Cumulative Dose and APT
Contrary to the overall rate of adverse events from amiodarone, the incidence of APT correlated with the maintenance rather than with the loading dose [23] while the cumulative dose was discussed controversially, from having no correlation to APT [24] to being stronger correlated than duration of therapy or amiodarone serum levels [25]. Due to wide interindividual differences in serum levels of amiodarone and desethylamiodarone with only poor correlation to dosage, therapeutic efficiacy and rate of adverse events, no toxic blood levels could be defined [24].
APT might occur early in treatment due to individual susceptibility with fatalities after <10 g of amiodarone in 14 days [12] or within the first month [26] and at low serum levels [25].
The assumption that maintenance doses ≤305 mg/d prevented APT [23] was disproved [26].
In conclusion, 200 mg/d of amiodarone are recommended for maintenance with respect to other more frequently occurring dose-dependent (gastrointestinal or dermatologic) side effects. If higher maintenance dosages are clinically needed, the risk-benefit ratio should be carefully assessed [23]
Diagnosis of APT
Unspecific dyspnea and unproductive cough are common initial symptoms in APT [23]. Laboratory parameters are nonspecific. As in our case, a slight leucocytosis and elevation of other parameters such as lactate dehydrogenase or KL-6, a mucin-like glycoprotein, might occur but are not pathognomonic [20].
Lung function tests can quantify the restriction leading to reduced lung volumes and a reduced diffusion capacity for carbon dioxide.
In our case, the disturbance in carbon dioxide elimination necessitated the arterio-venous lung assist device (Novalung®) while oxygen uptake became a limiting problem in the further course of treatment.
Chest x-rays show diffuse bilateral patchy infiltrates underestimating the extent of changes. There is a discrepancy, as initially in our case, between the x-rays and the computed tomography of the lung, which typically show distinct bilateral interstitial and/or alveolar infiltrates [27]. Probably due to the high iodine content, these infiltrates typically have high attenuation.
Non-conclusive bronchoscopic findings of unspecific inflammation are also in line with earlier reports [14].
Lack of pathognomonic findings in APT caused difficulties to ascertain the diagnosis and start an immunosuppressive treatment in earlier reports [20]. Differential diagnoses include bacterial or viral pneumonia, pulmonary embolism, ARDS and pulmonary edema.
This underlines the importance of medical history, which in our case revealed the drastic overdose of amiodarone and led to early treatment.
Therapy of APT
Only immediate and permanent discontinuation of amiodarone and administration of glucocorticoids can attenuate the concomitant inflammation [14].
Continuing or restarting amiodarone under steroid treatment resulted in high relapse rates [28]. These reports date back to an era when implantable cardioverter defibrillators were not widely available. The risk of APT needed to be balanced against the risk of uncontrolled hemodynamically relevant arrhythmias. The exact dose of corticosteroids for this indication is not systematically investigated. Recommendations range between 40 mg and 60 mg prednisolone per day [14]. Tapering schemes should individually consider the cumulative dose of amiodarone and may last 4 to 12 months.
In our case, we administered 50 mg prednisolone daily slowly tapering to 20 mg of prednisolone at day 21 of therapy, in accordance to previous cases [12].
Prevention of APT by Safe Dosage Patterns
Official recommendations of safe dosage patterns and limits
In supraventricular arrhythmias AHA/ACC/ESC suggest maintenance doses of 100–400 mg/d amiodarone after initiation of therapy with 600 mg/d over 1 month or 1000 mg/d for 1 week [13].
The FDA approved amiodarone in 1985 for ventricular arrhythmias with loading doses between 800 mg/d and 1600 mg/d over 1–3 weeks followed by 600–800 mg/d over 1 month and maintenance doses of 400 mg/d.
German reference dosing schemes differentiate intravenous amiodarone with up to 10–20 mg per kg bodyweight limited to 1 week from oral administration, recommending 600–1200 mg/d over 8–10 days initially, followed by 200–600 mg/d [29].
Standard American textbooks of cardiology recommend 800–1200 mg/d for 1–3 weeks, reducing to 400 mg/d for further 2–3 months and maintaining therapy with up to 300 mg/d [30].
The ESC textbook of cardiovascular medicine allows initial doses up to 1200–1800 mg/d of amiodarone intravenously for the in-patient until a total of 10 g is reached. In an out-patient setting this total loading dose is recommended to be reached with 600–800 mg amiodarone orally per day. Maintenance doses are limited to 200–400 mg/d [31].
Dosing schemes in randomized controlled trials in ventricular arrhythmias
Studies in ventricular arrhythmias utilize loading doses of 600 mg/d (SSSD [3]), 800 mg/d (AMIOVIRT [7]) 1000 mg/d (BASIS [2]) or 1200 mg/d (CIDS [8]) for 1 week.
Earlier smaller studies applied higher loading doses for this short initial phase with a maximum of 1600 mg/d amiodarone [23]. Other studies loaded for 2 weeks with 600 mg/d (GESICA [4]), 800 mg/d (EMIAT [6]) or 10 mg/kg body weight/d (CAMIAT [5]) and continued for 4 months (EMIAT [6]) to 1 year (AMIOVIRT [7]) with doubled maintenance doses (400 mg/d). Maintenance doses ranged between 200 mg/d and 300 mg/d [2, 3, 6] (s. Table 1/Fig. 1).
Dosing schemes in randomized controlled trials in supraventricular arrhythmias
Table 1.
Randomized controlled trials utilizing amiodarone for prevention of sudden cardiac death (SCD) in comparison to other antiarrhythmic drugs or to Implantable cardioverter defibrillator (ICD) and in comparison to usage of amiodarone in prevention of atrial fibrillation. Dosing schemes and incidence of adverse effects
| Trial | Year | Primary loading period | Secondary loading period | Maintenance dose | Adverse effects | Pulmonary toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| dose | duration | dose | duration | Incidence | Fatalities | |||||
| (mg) | (days) | (mg) | (%) | (%) | (%) | |||||
| Ventricular arrhythmia | ||||||||||
| Primary prevention of SCD | ||||||||||
| Comparison with other drugs | ||||||||||
| BASIS [2] | 1990 | 1000 | 5 | none | none | 200 | n/a | n/a | n/a | |
| PAT | 1992 | 800 | 7 | n/a | n/a | 100–400 | 30 | 0,3 | 0 | |
| SSSD [3] | 1993 | 600 | 7 | 400 | 7 | 200 | 9 | 1 | 0 | |
| GESICA [4] | 1994 | 600 | 14 | none | none | 300 | 6 | n/a | n/a | |
| STAT-CHF | 1995 | 800 | 4 | 300–400 | 18 | 3 | n/a | |||
| CAMIAT [5] | 1997 | 10 mg/kg | 14 | n/a | n/a | 200–400 | 26 | n/a | n/a | |
| EMIAT [6] | 1997 | 800 | 14 | 400 | 4 months | 200 | 53 | n/a | n/a | |
| Comparison with lCD | ||||||||||
| AMIOVIRT [7] | 2003 | 800 | 7 | 400 | 1 year | 300 | 48 | n/a | n/a | |
| SCD-HeFT | 2005 | 800 | 7 | n/a | n/a | 200–400 | 10 | n/a | n/a | |
| Secondary prevention of SCD | ||||||||||
| Comparison with ICD | ||||||||||
| CASH | 2000 | 1000 | 7 | n/a | n/a | 200–600 | 3 | 0 | 0 | |
| CIDS [8] | 2004 | > = 1200 | 7 | > = 400 | > = 10 weeks | > = 300 | n/a | 5,7 | n/a | |
| Supraventricular arrhythmia | ||||||||||
| Paroxysmal AF | ||||||||||
| CTAF | 2000 | 10 mg/kg | 14 | 300 | 4 weeks | 200 | 18 | 2 | 0 | |
| AFFIRM [9] | 2002 | l0g cumulative | > = 7 | none | none | 100–400 | 12 | 3,5 | 5,7 | |
| Persistent AF | ||||||||||
| PTAF [10] | 2000 | 600 | 21 | none | none | 200 | 25 | n/a | n/a | |
| RACE [11] | 2002 | 600 | 28 | none | none | 200 | n/a | n/a | n/a | |
| SAFE-T | 2003 | 800 | 14 | 600/300 | 2 weeks/1 year | 200 | n/a | 0,7 | 0 | |
| HOT CAFE | 2004 | 600 | 21 | 400 | until 12–16 g | 100–200 | 3 | 0 | 0 | |
| CAFE II | 2009 | 600 | 30 | 400 | 1 month | 200 | n/a | n/a | n/a | |
Amiodarone applied for supraventricular arrhythmias especially atrial fibrillation was dosed comparably to ventricular arrhythmias, but particularly high loading doses were not employed. Only the AFFIRM-study used a loading dose of 10 g within 7–10 days and then reduced it to maintenance doses of 300–400 mg/d, and in a subgroup of elderly patients with low body fat even to 100 mg/d [9]
Other studies started with 600 mg/d over 3 [10] or 4 weeks [11] before declining to 200 mg/d maintenance dose (Tables 1 and 2).
Table 2.
Synoptic precautions for prevention of pulmonary toxicity by safe dosage of amiodarone
| Daily dose | Phase of therapy | Application form | Duration | |
|---|---|---|---|---|
| 1.8 g/d | Not used in any current dosing scheme as the maintenance dose. Experiences in this dosage level as initial loading dose are rare and date back to the l980s, when the incidence of pulmonary toxicity was observed more frequently (28). | not used | NA | NA |
| Initiation of treatment, initial loading phase: | ||||
| 1.2 g/d or 1.0 g/d | Loading dose in many centres and trials, usually limited to days (5–15 days). Should be monitored continuously. Higher doses do not bear electrophysiological benefits (26) while lower doses delay the control over the arrhythmia. Amiodarone should be administered orally as soon as control over the arrhythmia is achieved. | Initial saturation phase | p.o., i.v. in malabsorption or until life-threatening arrhythmia controlled | 5–15 days |
| 800 mg/d | Reduced loading dose in special indications, elderly patients or reduced organ functions (as in our case). | |||
| Intermittent loading phase, re-saturation doses: | ||||
| 600 mg/d | Usual maximum daily dose used outside a specialized arrhythmia monitoring ward or an intensive care unit. Intravenous application is unnecessary except for special indications (e.g. malabsorption). Transiently used, limited to several (4–8) weeks. This daily dose sometimes is employed for a re-saturation after relapse of an arrhythmia under a low maintenance dose. | Intermittent re-saturation, consecutive loading | p.o., i.v. in malabsorption | 4–8 weeks |
| 400 mg/d | Integrated in an initiation scheme for some weeks (see also loading scheme in our arrhythmia centre). A relapse of the arrhythmia might necessitate temporary re-elevation of dosing. In rare cases this might be the elevated maintenance dose under special indications. A dose of 400 mg/d that is obviously not limited to several weeks should be reason for further individual clarification with a rhythmologist. | Consecutive loading phase | p.o. | 4 weeks |
| Maintenance dose | ||||
| 200 mg/d | Normal maintenance dose. Higher maintenance doses need special indications (e.g. futile trials of dose reduction in the patient’s history). In these cases, it should be discussed whether an intermittent loading phase with subsequent lower maintenance dose might be more effective and less toxic. | Maintenance therapy | p.o. exclusively | maintenance |
| 100 mg/d | Reduced maintenance doses in elderly patients with reduced organ function. | Reduced dose | p.o. exclusively | |
Temporary Elevation of Dosage
Higher loading doses of amiodarone provide better antiarrhythmic control in ventricular arrhythmias, with 1000 mg/d being superior to 500 mg/d and to 125 mg/d [32]. In supraventricular arrhythmia, a high intravenous amiodarone dose (100 mg/h vs. 50 mg/h) provided a higher conversion rate and faster conversion [17]. These studies only observed 24 hours, cumulative or maintenance doses were not compared.
Higher loading doses of up to 4 g/d did not provide electrophysiological advantages compared to 1.2 g/d of amiodarone [33].
Re-elevating amiodarone dosage to 400 mg/d achieved rhythm control in cases of relapse of ventricular arrhythmia under 200 mg/d [34].
Lowest Possible Maintenance Doses
The lowest effective dose should be identified for every patient requiring long-term amiodarone treatment [14]. Disruption of amiodarone 1 month after conversion from atrial fibrillation resulted in 23 % less patients in sinus rhythm after 1 year than with amiodarone continued [35] without measurable effect on quality of life [35]. Therefore, continuation of treatment is only necessary in patients who do not tolerate arrhythmias or who are at risk for cardiac decompensation under tachycardia as in our case.
All long-term amiodarone treatment needs meticulous follow-up exceeding the end of treatment.
Outlook on Class III Antiarrhythmic Drugs Without Risk of APT
The potential benefit of new class III antiarrhythmic drugs, such as dronedarone remains to be analyzed carefully. Although having less side effects it seems to bear less antiarrhythmic potency than amiodarone, which in turn will remain one of the most important antiarrhythmic drugs [18].
Conclusion
The level of daily amiodarone dose reported here is unprecedented. Its fatal outcome underlines the necessity to eliminate even the slightest doubt about amiodarone dosing. Respecting above mentioned dosage patterns and precautions amiodarone is safe. With loading doses reflecting the severity of the arrhythmia and the permanent effort to find the lowest possible maintenance dose and shortest duration of treatment, the incidence of adverse effects can be reduced. APT can be reduced to 1–2 % of the patients bearing mortality rates of <0.1 % of all patients. Meticulous follow-up can help to recognize the development of APT earlier and reduce its severity.
Perspectively, amiodarone will remain one of the most potent and widespread antiarrhythmic drugs beyond the introduction of new antiarrhythmic class III agents, so the knowledge of safe dosage patterns is essential to any clinically working physician. We hope to contribute to the awareness of safe dosage patterns with this case report and literature review.
References
- 1.Martin WJ, Rosenow EC. Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part 2) Chest. 1988;93:1242–8. doi: 10.1378/chest.93.6.1242. [DOI] [PubMed] [Google Scholar]
- 2.Burkart F, Pfisterer M, Kiowski W, Follath F, Burckhardt D. Effect of antiarrhythmic therapy on mortality in survivors of myocardial infarction with asymptomatic complex ventricular arrhythmias: Basel Antiarrhythmic Study of Infarct Survival (BASIS) J Am Coll Cardiol. 1990;16:1711–8. doi: 10.1016/0735-1097(90)90324-I. [DOI] [PubMed] [Google Scholar]
- 3.Navarro-López F, Cosin J, Marrugat J, Guindo J, Bayes de Luna A. Comparison of the effects of amiodarone versus metoprolol on the frequency of ventricular arrhythmias and on mortality after acute myocardial infarction. SSSD Investigators. Spanish Study on Sudden Death. Am J Cardiol. 1993;72:1243–8. doi: 10.1016/0002-9149(93)90291-J. [DOI] [PubMed] [Google Scholar]
- 4.Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) Lancet. 1994;344:493–8. doi: 10.1016/S0140-6736(94)91895-3. [DOI] [PubMed] [Google Scholar]
- 5.Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet. 1997;349:675–82. doi: 10.1016/S0140-6736(96)08171-8. [DOI] [PubMed] [Google Scholar]
- 6.Julian DG, Camm AJ, Frangin G, et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997;349:667–74. doi: 10.1016/S0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- 7.Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator:randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–12. doi: 10.1016/S0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–302. doi: 10.1161/01.CIR.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 9.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–94. doi: 10.1016/S0140-6736(00)03230-X. [DOI] [PubMed] [Google Scholar]
- 11.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 12.Kharabsheh S, Abendroth CS, Kozak M. Fatal pulmonary toxicity occurring within 2 weeks of initiation of amiodarone. Am J Cardiol. 2002;89:896–8. doi: 10.1016/S0002-9149(02)02213-0. [DOI] [PubMed] [Google Scholar]
- 13.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the ACC/AHA Task Force on Practice Guidelines and the ESC Committee for Practice Guidelines. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 14.Goldschlager N, Epstein AE, Naccarelli GV, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Hear Rhythm. 2007;4:1250–9. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JS, Podrid PJ. Side effects from amiodarone. Am Heart J. 1991;121:158–71. doi: 10.1016/0002-8703(91)90969-O. [DOI] [PubMed] [Google Scholar]
- 16.Piccini JP, Berger JS, O’Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J. 2009;30:1245–53. doi: 10.1093/eurheartj/ehp100. [DOI] [PubMed] [Google Scholar]
- 17.Tuseth V, Jaatun HJ, Dickstein K. Amiodarone infusion in the treatment of acute atrial fibrillation or flutter: high versus low dose treatment. Heart. 2005;91:964–5. doi: 10.1136/hrt.2004.049171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–61. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 19.Ashrafian H, Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001;120:275–82. doi: 10.1378/chest.120.1.275. [DOI] [PubMed] [Google Scholar]
- 20.Camus P, Martin WJ, Rosenow EC. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25:65–75. doi: 10.1016/S0272-5231(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 21.Rotmensch HH, Liron M, Tupilski M, Laniado S. Possible association of pneumonitis with amiodarone therapy. Am Heart J. 1980;100:412–3. doi: 10.1016/0002-8703(80)90165-9. [DOI] [PubMed] [Google Scholar]
- 22.Amiodarone Trials Meta-Analysis Investigators Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350:1417–24. [PubMed] [Google Scholar]
- 23.Dusman RE, Stanton MS, Miles WM, et al. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990;82:51–9. doi: 10.1161/01.CIR.82.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Heger JJ, Prystowsky EN, Zipes DP. Relationships between amiodarone dosage, drug concentrations, and adverse side effects. Am Heart J. 1983;106:931–5. doi: 10.1016/0002-8703(83)90018-2. [DOI] [PubMed] [Google Scholar]
- 25.Rotmensch HH, Belhassen B, Swanson BN, et al. Steady-state serum amiodarone concentrations: relationships with antiarrhythmic efficacy and toxicity. Ann Intern Med. 1984;101:462–9. doi: 10.7326/0003-4819-101-4-462. [DOI] [PubMed] [Google Scholar]
- 26.Polkey MI, Wilson PO, Rees PJ. Amiodarone pneumonitis: no safe dose. Respir Med. 1995;89:233–5. doi: 10.1016/0954-6111(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 27.Kuhlman JE, Teigen C, Ren H, et al. Amiodarone pulmonary toxicity: CT findings in symptomatic patients. Radiology. 1990;177:121–5. doi: 10.1148/radiology.177.1.2399310. [DOI] [PubMed] [Google Scholar]
- 28.Rakita L, Sobol SM, Mostow N, Vrobel T. Amiodarone pulmonary toxicity. Am Heart J. 1983;106:906–16. doi: 10.1016/0002-8703(83)90015-7. [DOI] [PubMed] [Google Scholar]
- 29.Rote Liste 2011. Arzneimittelinformation für Deutschland. Frankfurt/Main: Rote Liste Service GmbH; 2011.
- 30.Libby P, Bonow RO, Zipes DP, Mann DL, eds. Braunwald’s heart disease: a textbook of cardiovascular medicine. 8th ed. Philadelhia: Saunders; 2008.
- 31.Camm J, Lüscher TF, Serruys PW. The ESC textbook of cardiovascular medicine. 2nd ed. New York: Oxford University Press; 2009.
- 32.Scheinman MM, Levine JH, Cannom DS, et al. Dose-ranging study of intravenous amiodarone in patients with life-threatening ventricular tachyarrhythmias. The Intravenous Amiodarone Multicenter Investigators Group. Circulation. 1995;92:3264–72. doi: 10.1161/01.CIR.92.11.3264. [DOI] [PubMed] [Google Scholar]
- 33.Kalbfleisch SJ, Williamson B, Man KC, et al. Prospective, randomized comparison of conventional and high dose loading regimens of amiodarone in the treatment of ventricular tachycardia. JACC. 1993;22:1723–9. doi: 10.1016/0735-1097(93)90603-X. [DOI] [PubMed] [Google Scholar]
- 34.Collaborative Group for Amiodarone Evaluation Multicenter controlled observation of a low-dose regimen of amiodarone for treatment of severe ventricular arrhythmias. Am J Cardiol. 1984;53:1564. doi: 10.1016/0002-9149(84)90580-0. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed S, Ranchor AV, Crijns HJGM, Van Veldhuisen DJ, Van Gelder IC, investigators ftC. Effect of continuous versus episodic amiodarone treatment on quality of life in persistent atrial fibrillation. Europace. 2010;12:785–91. [DOI] [PubMed]


