Abstract
Cholesterol efflux is the key process protecting the vascular system from the development of atherosclerotic lesions. Various extracellular and intracellular events affect the ability of the cell to efflux excess cholesterol. To explore the possible pathways and processes that promote or inhibit cholesterol efflux, we applied a combined cheminformatic and bioinformatic approach. We performed a comprehensive analysis of published data on the various substances influencing cholesterol efflux and found 153 low molecular weight substances that are included in the Chemical Entities of Biological Interest (ChEBI) database. Pathway enrichment was performed for substances identified within the Reactome database, and 45 substances were selected in 93 significant pathways. The most common pathways included the energy-dependent processes related to active cholesterol transport from the cell, lipoprotein metabolism and lipid transport, and signaling pathways. The activators and inhibitors of cholesterol efflux were non-uniformly distributed among the different pathways: the substances influencing ‘biological oxidations’ activate cholesterol efflux and the substances influencing ‘Signaling by GPCR and PTK6’ inhibit efflux. This analysis may be used in the search and design of efflux effectors for therapies targeting structural and functional high-density lipoprotein deficiency.
Key Points
| We performed a comprehensive analysis of the various substances influencing cholesterol efflux, with pathway enrichment using the Reactome database. |
| The activators and inhibitors of cholesterol efflux are non-uniformly distributed among different pathways. |
| The substances influencing biological oxidation activate cholesterol efflux, and the substances influencing signaling by G protein-coupled receptors (GPCR) and non-receptor tyrosine kinase (PTK6) inhibit efflux. |
Reverse Cholesterol Transport
High-density lipoprotein (HDL) heterogeneity influences its atheroprotective effect via reverse cholesterol transport from macrophage to the liver [1]. Cholesterol efflux from a macrophage to the extracellular cholesterol acceptor is the first, and rate-limiting, step of reverse cholesterol transport [2, 3]. Four mechanisms of cholesterol efflux, namely aqueous diffusion, facilitated diffusion mediated by the scavenger receptor class B member 1 (SR-B1) receptor, and active unidirectional efflux mediated by the ATP binding cassette subfamily A member 1 (ABCA1) and the ATP binding cassette subfamily G member 1 (ABCG1) transporters are known [4]. ATP hydrolysis with concomitant conformational transition is required for cholesterol efflux by ABCA1 and ABCG1 transporters. The SR-B1 mediates cholesterol efflux by facilitated diffusion via hydrophobic tunnel within the molecule. Various HDL fractions and lipid-free apolipoprotein A1 (apoA-1) are able to accept cell-derived cholesterol with a different efficiency [2]. Cholesterol transport between intracellular compartments proceeds by both energy-dependent and energy-independent processes [5]. The energy-dependent vesicular traffic partly contributes to cholesterol flux between endoplasmic reticulum, plasma membrane (PM) and endocytic vesicles. The membrane contact sites and lipid transfer proteins are involved in non-vesicular lipid traffic [6–11]. Importantly, the PM cholesterol is the cholesterol that participates in the efflux to the extracellular acceptors [12].
Cholesterol efflux from the macrophage is clinically significant for two reasons. First, there is a significant relationship between the cholesterol efflux capacity (CEC) of apolipoprotein B (apoB)-depleted plasma and the manifestations of various cardiovascular events. The predictive significance of CEC for cardiovascular risk is stronger than for HDL cholesterol level [13–16]. The second reason is the positive effect of efflux stimulation on the regression of atherosclerotic plaques [15, 17, 18].
The molecular events in cellular cholesterol efflux, along with the contribution of various pathways, have been extensively studied; however, there is no systematic evaluation of the influence of various low molecular weight substances on cholesterol efflux as a process directed by both donor and acceptor participants. A combined cheminformatic and bioinformatic approach has been applied in the present review to classify and compare the known efflux effectors. Our work may be applicable in the targeted therapy of structural and functional HDL deficiency.
Effectors of Cholesterol Efflux
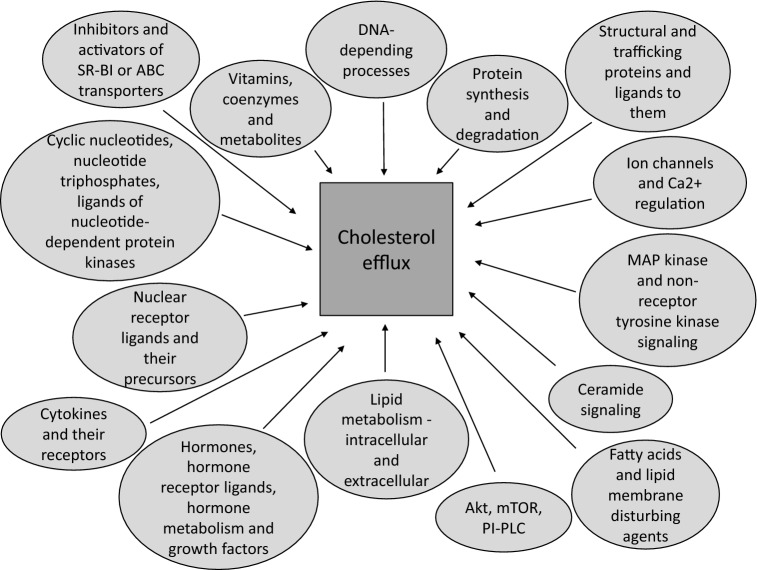
The PubMed database was initially searched using the term ‘cholesterol efflux’, and papers involving the use of low molecular weight substances were selected. This analysis of published data on the influence of low molecular weight substances on cholesterol efflux with various donors and acceptors revealed 191 substances with activating and inhibiting effects (Table 1). These substances were grouped into the following classes by means of small-molecule high-throughput screening (Fig. 1): (1) inhibitors and activators of SR-B1 receptors or ABC transporters, including sulfonylureas (inhibitors of ATP-sensitive K+ channels); (2) cyclic nucleotides, nucleotide triphosphates, ligands of nucleotide-dependent protein kinases; (3) nuclear receptor ligands and their precursors; (4) cytokines and their receptors; (5) hormones, hormone receptor ligands (excluding ligands of nuclear receptors), hormone metabolism and growth factors; (6) lipid metabolism—intracellular and extracellular; (7) fatty acids and lipid membrane-disturbing agents; (8) protein kinase B, mammalian target of rapamycin, phosphatidylinositol-phospholipase C; (9) ceramide signaling; (10) mitogen-activated protein kinase and non-receptor tyrosine kinase signaling; (11) ion channels and Ca2+ regulation; (12) protein synthesis and degradation; (13) structural and trafficking proteins and their ligands; (14) DNA-dependent processes; (15) other factors; (16) vitamins, coenzymes and metabolites; and (17) extracts, components of plants, and other natural sources. Overall, 153 substances were present in the Chemical Entities of Biological Interest (ChEBI) database [220].
Table 1.
Effect of some substances, drugs and natural extracts on cholesterol efflux in various cells for different cholesterol acceptors
| Substance used for cell treatment | Description of the substance | Cellsa | Acceptor of cholesterol | References |
|---|---|---|---|---|
| Inhibitors and activators of SR-B1 receptor or ABC transporters including sulfonylureas (inhibitors of ATP-sensitive K + channels) | ||||
| Stimulation | ||||
| Diphenoquinone | Supposedly an oxidized metabolite of probucol; inhibits calpain-mediated degradation of ABCA1 | THP-1, HEK293 expressing ABCA1 | ApoA-I | [19] |
| Glimepirideb | A sulfonylurea antidiabetic drug, inhibitor of ATP-sensitive K + channels | RAW 264.7 | HDL | [20] |
| Glyburideb (glibenclamide) | A sulfonylurea antidiabetic drug; a general inhibitor of ABC transporters, including ABCA1 | RAW 264.7 | HDL | [20] |
| IMB2026791 | An xanthone compound that enhances binding of apoA-I to ABCA1 | CHO, CHO expressing ABCA1, THP-1 cells | ApoA-I, HDL (CHO expressing ABCA1, THP-1 cells) | [21] |
| Spiroquinone | Supposedly an oxidized metabolite of probucol; inhibits calpain-mediated degradation of ABCA1 | THP-1, HEK293 expressing ABCA1 | ApoA-I | [19] |
| Inhibition | ||||
| BLT-1 - BLT-5 | Inhibitors of SR-B1; increases binding affinity of SR-B1 for HDL | ldlA-7 cells stably transfected to express SR-B1 | HDL | [22] |
| BLT-1, BLT-4 | Inhibitors of SR-B1; increases binding affinity of SR-B1 for HDL | RAW 264.7, 3T3 L-1-derived adipocytes | ApoA-I | [23, 24] |
| Compound 1 (methyl 3α-acetoxy-7α,12α-di[(phenylaminocarbonyl)amino]-5β-cholan-24-oate) | A novel inhibitor of ABCA1 | RAW 264.7 | ApoA-I, taurocholate, peptide 18A (i.e. 2F) | [25] |
| Compound 2 (N-[2-((4-nitrophenylaminocarbonyl)amino)ethyl]-N,N-di[2-((4-methylphenylsulfonyl)amino)ethyl]amine) | A novel inhibitor of ABCA1 | RAW 264.7 | ApoA-I | [25] |
| Glimepirideb | A sulfonylurea antidiabetic drug, inhibitor of ATP-sensitive K + channels | THP-1, HEK293 expressing ABCA1 | ApoA-I | [20] |
| Glyburideb (glibenclamide) | A sulfonylurea antidiabetic drug; a general inhibitor of ABC transporters, including ABCA1 | J774, RAW 264.7, THP-1, fibroblasts, SMC, HEK293 expressing ABCA1, 3T3 L-1-derived adipocytes | ApoA-I | [20, 23, 26, 27] |
| BHK-21, BHK-21 expressing SR-B1 | HDL | [20] | ||
| J774 | HDL3 | [28] | ||
| Wheat germ agglutinin | A lectin; inhibits generation of microparticle by ABCA1 | RAW 264.7 | No acceptor | [25] |
| Probucol | An inhibitor of ABCA1-mediated lipid efflux, lipid-lowering drug, an antioxidant, stimulates cellular lipids synthesis | J774, MPM | ApoA-I, ApoA-II (MPM), ApoE (MPM) | [29–31] |
| Astrocytes | ApoA-I, HDL, ApoE, conditioned medium | [32] | ||
| THP-1, WI-38 human fibroblast cells, MAC-T | ApoA-I | [19, 33, 34] | ||
| PSC833 | Inhibits ABCA1; a non-immunosuppressive cyclosporine not inhibiting calcineurin; an inhibitor of ABCB1 and ABCB4 | ABCA1-expressing BHK cells, THP-1 | ApoA-I | [35] |
| Cyclic nucleotides, nucleotide triphosphates, ligands of nucleotide-dependent protein kinases | ||||
| Stimulation | ||||
| 8-Br-cAMP | cAMP analog | RAW 264.7 | ApoA-I, ApoE2, ApoE3, ApoE4, HDL | [36] |
| J774, astrocytes | ApoA-I, HDL (astrocytes) | [23, 37] | ||
| A-769662 | Activator of AMPK | THP-1 | ApoA-I | [38] |
| ATP (up to 0.1–1 μM; inhibition over 1–10 μM) | Nucleoside triphosphate | RAW 264.7, ABCA1-expressing BHK cells | ApoA-I | [39] |
| ATP, 1 mM | Nucleoside triphosphate | Primary mouse type II pneumocytes | No acceptor | [40] |
| AICAR (5-aminoimidazole-4-carboxyamide ribonucleoside) | An activator of AMPK | J774 | HDL | [41] |
| cpt-cAMP (8-(4-chlorophenylthio)-cAMP) | cAMP analog | MPM, J774, L-cell | ApoA-I, HDL3 (J774) | [28–30] |
| st-Ht31 | PKA-anchoring inhibitor | ABCA1-expressing BHK cells, RAW 264.7 | No acceptor; also ApoA-I in a separate experiment | [42] |
| Inhibition | ||||
| Apyrase | ATP hydrolysis to AMP | RAW 264.7 and ABCA1-expressing BHK cells | ApoA-I | [39] |
| MDL-12330A | An inhibitor of adenylate cyclase | RAW 264.7 | ApoA-I | [43] |
| PKI | A PKA inhibitor | ABCA1-expressing BHK cells | ApoA-I | [44] |
| Oligomycin | An inhibitor of ATP synthase; inhibits mitochondrial respiration | THP-1 | ApoA-I | [45] |
| Sodium orthovanadate | A specific inhibitor of P-type ATPases and protein phosphotyrosine phosphatases | Fibroblasts, SMC | ApoA-I | [26] |
| Nuclear receptor ligands and their precursors | ||||
| Stimulation | ||||
| 9-cis-retinoic acid | A retinoid that activates RXRs and RARs | Astrocytes | ApoA-I, HDL | [46] |
| 13-cis-retinoic acid | A retinoid that is neither an RAR nor an RXR agonist | Astrocytes | ApoA-I, HDL | [46] |
| 13-hydroxy linoleic acid | Natural PPAR agonist | RAW 264.7 | ApoA-I | [47] |
| 22(R)-hydroxycholesterol | An oxysterol, natural LXR activator | hPBMC, mBMDM, RAW 264.7, THP-1 | ApoA-I, HDL (THP-1) | [48–51] |
| 22(R)-hydroxycholesterol with 9-cis-retinoic acid | LXR/RXR agonist | J774, MPM, astrocytes, primary mouse type II pneumocytes | ApoA-I, HDL (astrocytes, CaCo-2), no acceptor (CaCo-2) | [32, 40, 52–54] |
| 24(S),25-epoxycholesterol | An oxysterol, natural LXR activator | mBMDM, hPBMC | ApoA-I | [48] |
| 9-cis-β-carotene | A precursor for 9-cis-retinoic acid that stimulates cholesterol efflux | RAW 264.7, MPM | HDL | [55] |
| Acetyl-podocarpic dimer | LXR agonist | hPBMC, THP-1, primary human fibroblasts | ApoA-I | [51] |
| All-trans β-carotene | Vitamin A precursor | RAW 264.7 | HDL | [55] |
| All-trans retinoic acid (tretinoin) | A retinoid that activates RARs | Astrocytes | ApoA-I, HDL | [46] |
| Baicalin | PPARγ agonist | THP-1 | HDL2, HDL3 | [56] |
| Bezafibrate | A lipid-lowering fibrate drug, an agonist of PPARα | THP-1 | apoB-depleted plasma | [57] |
| E17110 | A novel benzofuran-2-carboxylate derivative with potential LXRβ agonist activity | RAW 264.7 | ApoA-I, HDL | [58] |
| Ethyl 2,4,6-trihydroxybenzoate | An LXR agonist isolated from Celtis biondii | THP-1 | HDL | [59] |
| Fenofibric acid | A fibrate; used for the treatment of dyslipidemia, a PPARα agonist | hPBMC | HDL | [60] |
| GW1929 | PPARγ agonist | THP-1 | HDL3, ApoA-I | [61] |
| GW3965 | LXR agonist | MPM, RAW 264.7, THP-1, Huh7.5 (hepatoma cells), 3T3 L-1-derived adipocytes, blastic plasmacytoid dendritic cell neoplasm cell line CAL-1 (a myeloid leukemia cell line) | ApoA-I, HDL2 (THP-1, CAL-1), HDL3 (THP-1) | [23, 62–66] |
| GW4064 | FXR agonist | THP-1 | No acceptor | [67] |
| Isosylibin A | A partial PPARγ agonist | THP-1 | ApoA-I | [68] |
| K-877 | Selective PPARα modulator | hPBMC | HDL | [60] |
| Methoprene | Synthetic selective RXR agonist | Astrocytes | ApoA-I, HDL | [46] |
| N-Acylthiadiazoline compound 2 (racemate or R enantiomer) | LXRβ agonist | MPM | ApoA-I | [64] |
| Pioglitazoneb | PPAR agonist | THP-1, RAW 264.7 | ApoA-I, HDL, HDL2 (THP-1), HDL3 (THP-1), human plasma (THP-1) | [68–71] |
| Rosiglitazone | Synthetic PPAR agonist | hPBMC, MPM, THP-1 | ApoA-I, HDL (THP-1), HDL2 (THP-1), HDL3 (THP-1) | [62, 72–74] |
| RAW 264.7 | HDL | [75] | ||
| TO-1317 (TO-901317) | LXR agonist | J774, MPM, RAW 264.7, THP-1, CaCo-2, MAC-T (ApoA-I) | ApoA-I, HDL, HDL3 (MPM), no acceptor (THP-1), taurocholate-phosphatidylcholine micelles (CaCo-2) | [34, 54, 59, 76–81] |
| Blastic plasmacytoid dendritic cell neoplasm cell line CAL-1 (a myeloid leukemia cell line) | ApoA-I, HDL2 | [66] | ||
| HepG2, human foreskin fibroblasts | ApoA-I | [82, 83] | ||
| Telmisartan | Angiotensin II receptor antagonist; also activates PPARγ | THP-1 | ApoA-I, HDL2, HDL3 | [70] |
| Tributyltin chloride | An organotin compound; an RXR activator | RAW 264.7 | ApoA-I | [76] |
| Wy14643 | PPARα activator | hPBMC | ApoA-I | [72] |
| Inhibition | ||||
| 15d-PGJ2 (15-Deoxy-delta(12,14)-prostaglandin J(2)) | PPARγ ligand | MPM | ApoA-I | [84] |
| Pioglitazoneb | PPAR agonist | MPM | ApoA-I | [84] |
| Troglitazone | PPARγ and, to a lesser extent, PPARα agonist | MPM | ApoA-I | [84] |
| TTNPB (4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid) | Synthetic selective RAR agonist | Astrocytes | ApoA-I, HDL | [46] |
| Cytokines and their receptors | ||||
| Stimulation | ||||
| Apelin-13 | An adipocytokine, a ligand for the cognate G-protein coupled receptor APJ | THP-1 | ApoA-I | [85] |
| CXCL5 | A chemokine that signals through the CXCR2 receptor | MPM | ApoA-I | [86] |
| IL-8-neutralizing antibody | IL-8 is a proinflammatory chemokine that induces chemotaxis and phagocytosis | THP-1 | ApoA-I | [87] |
| IL-10 | An anti-inflammatory cytokine | THP-1 | ApoA-I, HDL2, serum (FBS) | [88] |
| IL-12 with IL-18 | IL-12 and IL-18 synergize for the production of IFNγ | THP-1 | ApoA-I | [89] |
| IL-27 | An anti-inflammatory cytokine | THP-1 | ApoA-I | [90] |
| TGFβ | An anti-inflammatory cytokine | MPM from WT or apoE KO mice | ApoA-I, HDL | [91] |
| TNFαb | Proinflammatory cytokine | MPM | ApoA-I | [92] |
| Inhibition | ||||
| CCL2 | Pro-atherosclerotic chemokine | HCAEC, HUVEC | ApoA-I (HCAEC), HDL | [93] |
| IFNβ | Promotes atherogenesis in mice | mBMDM | ApoA-I | [94] |
| IL-1β | Pro-inflammatory cytokine | HepG2, primary mouse hepatocytes | ApoA-I | [95] |
| IL-6 | Pro-inflammatory cytokine | THP-1 | ApoA-I | [96] |
| INFγ | Pro-inflammatory cytokine, has a variety of proatherogenic effects | MPM, THP-1 | ApoA-I | [91, 97–99] |
| TNFαb | Pro-inflammatory cytokine | THP-1, HepG2, mouse primary hepatocytes, podocytes (kidney cells) | ApoA-I | [95, 96, 100] |
| TNF-like protein 1A (TL1A; TNFSF15) | Binds to DR3; highly expressed in atherosclerotic plaques | THP-1, hPBMC | ApoA-I | [99] |
| Visfatin (pre-B cell colony-enhancing factor 1) | A nicotinamide phosphoribosyltransferase | RAW 264.7 | ApoA-I, HDL | [101] |
| Hormones, hormone receptor ligands (excluding ligands of nuclear receptors), hormone metabolism and growth factors | ||||
| Stimulation | ||||
| 17β-estradiol | A steroid sex hormone | VSMC, MAC-T | ApoA-I, HDL (VSMC) | [34, 102] |
| Angiotensin-(1–7) | Produced by ACE2; ACE2-deficient mice have an increased risk of heart failure | THP-1, RAW 264.7 | ApoA-I or HDL (THP-1) | [43, 103] |
| Exendin-4 | A GLP-1 mimetic affecting insulin regulation | 3T3-L1 adipocytes | No acceptor | [104] |
| FGF-21 | Mitogenic and cell survival activities | THP-1 | ApoA-I, HDL | [105] |
| Ghrelin | An endocrine peptide mainly identified in stomach epithelium; stimulates food intake in humans | THP-1 | ND | [106] |
| GDP-15 | A 12-kDa secreted protein, also named macrophage inhibitory cytokine-1 | THP-1 | No acceptor | [107] |
| Hydrocortisone (i.e. cortisol) | A steroid hormone, stimulates gluconeogenesis, suppresses the immune system | MAC-T | ApoA-I | [34] |
| IGF-1 | Regulates metabolism, growth, and cell differentiation and survival | INS-1 cells originated from a rat insulinoma cell line | ND | [108] |
| Insulinb) | A peptide hormone, regulates glucose metabolism | MAC-T | ApoA-I | [34] |
| Progesterone | A steroid sex hormone | MAC-T | ApoA-I | [34] |
| Prolactin | A peptide hormone; initiates milk production | MAC-T | ApoA-I | [34] |
| Vildagliptin | An antidiabetic drug, an inhibitor of DPP-4, thus prolonging the half-life of GLP-1 | 3T3-L1 adipocytes | No acceptor | [104, 109] |
| Inhibition | ||||
| Adiponectin (Acrp30) | An adipokine secreted by adipocytes that functions as an insulin sensitizer | hPBMC | ApoA-I | [110] |
| Angiotensin-II | A peptide produced by the enzyme ACE; ACE inhibitors are used for the treatment of CVDs | THP-1 | ApoA-I or HDL | [103] |
| CRH | A peptide that links psychological stress to pathophysiologic responses | MPM | ApoA-I | [111] |
| Dexamethasone | A corticosteroid, agonist of GR | THP-1 | ApoA-I | [112] |
| EGF | Activates MAP kinases ERK1/2 | RAW 264.7 | ApoA-I | [113] |
| Hydrocortisone | A corticosteroid, agonist of GR | THP-1 | Human serum | [114] |
| Insulinb | A peptide hormone, regulates glucose metabolism | hPBMC, HepG2, HEK293 expressing ABCA1 | ApoA-I | [110, 115] |
| Raloxifene | A benzothiophene derivative that is used for the treatment of osteoporosis in postmenopausal women; a selective ER modulator: stimulates ER in bone and inhibits ER in the uterus and breast | THP-1, MPM | ApoA-I, HDL | [116] |
| Tamoxifen | A medication for treating breast cancer; a prodrug that is metabolized in the liver into an ER antagonist | THP-1, MPM | ApoA-I, HDL | [116] |
| Toremifene | A selective ER modulator; a medication for treating breast cancer | THP-1, MPM | ApoA-I, HDL | [116] |
| Lipid metabolism—intracellular and extracellular | ||||
| Stimulation | ||||
| Ibrolipim | An LPL activator | THP-1 | ApoA-I, HDL | [117] |
| MCC-147 | An inhibitor of ACAT | MPM | ApoA-I | [118] |
| Myriocin | An inhibitor of SPTLC1 | Primary human fibroblasts, mBMDM | ApoA-I | [119] |
| NTE-122 (trans-1,4-bis [[1 -cycIohexyI-З-(4-dimethyIamino phenyl)ureido]methyl]cyclohexane) | An inhibitor of ACAT | THP-1 | HDL | [120] |
| PLTPb | Transfers phospholipids between lipoproteins, remodels HDL | J774, BHK expressing ABCA1 | HDL, trypsinized HDL | [121] |
| BHK expressing ABCA1 | No acceptor, LDL, phospholipid vesicles | [121] | ||
| Pitavastatinb | Relatively lipophilic statin; type II statinc | Fu5AH | ApoA-I | [122] |
| Simvastatinb | Relatively lipophilic statin; type I statinc | THP-1 | ApoB-depleted plasma | [57] |
| Inhibition | ||||
| LPL | A secreted enzyme facilitating the hydrolysis of triglycerides in chylomicrons | THP-1 | ApoA-I | [123] |
| PLTPb | Transfers phospholipids between lipoproteins, remodels HDL | BHK expressing ABCA1 | ApoA-I | [121] |
| PCSK9 | A subtilisin family-serine protease that degrades LDL receptor in liver | MPM | ApoA-I | [53] |
| Simvastatinb (0.01 µM) | Relatively lipophilic statin; type I statinc | J774 | ApoA-I | [124] |
| THP-1, hPBMC | HDL | [125] | ||
| Atorvastatin (10 µM) | Relatively lipophilic statin; type II statinc | J774, RAW 264.7 | ApoA-I | [124, 126] |
| THP-1, hPBMC | HDL, ApoA-I (THP-1) | [125, 127] | ||
| Rosuvastatin (10 µM) | Relatively hydrophilic statin; type II statinc | J774 | ApoA-I | [124] |
| Pitavastatinb) (0.1 or 1 µM for J774, RAW—depends on the paper) | Relatively lipophilic statin; type II statinc | J774, MPM, RAW 264.7 | ApoA-I | [124, 126, 128] |
| Pravastatin | Relatively hydrophilic statin; type I statinc | 3T3-L1 adipocytes | No acceptor | [109] |
| Mevastatin (Compactin; 10 uM) | Relatively lipophilic statin; type I statinc | MPM | ApoA-I | [128] |
| Fatty acids and lipid membrane-disturbing agents | ||||
| Stimulation | ||||
| α-Linolenic acid conjugated to BSA | An omega-3 PUFA | THP-1 | No acceptor | [67] |
| Cholesterolb | GM3468A normal human skin fibroblasts, primary cerebellar astroglia | ApoA-I | [129, 130] | |
| Edelfosineb (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine) | An alkyl-phospholipid with amphiphilic properties | HepG2 | No acceptor (the compound itself might perform as the acceptor) | [131] |
| Erucylphosphocholine ([(Z)-docos-13-enyl] 2-(trimethylazaniumyl)ethyl phosphate) | An alkyl-phospholipid with amphiphilic properties | HepG2 | No acceptor (the compound itself might perform as the acceptor) | [131] |
| Miltefosine,b i.e. hexadecylphosphocholine (hexadecyl 2-(trimethylazaniumyl)ethyl phosphate) | An alkyl-phospholipid with amphiphilic properties | HepG2 | No acceptor (the compound itself might perform as the acceptor) | [131] |
| Imipramine | An amphipathic amine | MPM | ApoA-I | [132] |
| Perifosineb (1,1-dimethylpiperidin-1-ium-4-yl) octadecyl phosphate | An alkyl-phospholipid with amphiphilic properties | HepG2 | No acceptor (the compound itself might perform as the acceptor) | [131] |
| U18666A | An amphipathic amine | MPM | ApoA-I, HDL2 | [132] |
| Inhibition | ||||
| 1,2-dioleoyl-sn-glycero-3-phospho-rac-1-glycerol [a precursor of bis(monoacylglycero)phosphate (lysobisphosphatidic acid)] | Bis(monoacylglycero)phosphate (lysobisphosphatidic acid), a phospholipid highly abundant in the internal membranes of multivesicular late endosomes, in which it forms specialized lipid domains | RAW 264.7 | Mβ-CD, ApoA-I, HDL | [133] |
| Cholesterolb | MPM | ApoA-I, HDL2 | [132] | |
| Edelfosineb (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine) | An alkyl-phospholipid with amphiphilic properties | THP-1 | ApoA-I | [134] |
| Eicosapentaenoic acid [20:5(n-3)] | Omega-3 PUFA | RAW 264.7, THP-1 | ApoA-I | [49, 135] |
| Linoleic acid | 18:2 omega-6 PUFA | mBMDM | HDL | [136] |
| Miltefosineb, i.e. hexadecylphosphocholine [hexadecyl 2-(trimethylazaniumyl)ethyl phosphate] | An alkyl-phospholipid with amphiphilic properties | THP-1 | ApoA-I | [134] |
| Perifosineb (1,1-dimethylpiperidin-1-ium-4-yl) octadecyl phosphate | An alkyl-phospholipid with amphiphilic properties | THP-1 | ApoA-I | [134] |
| Oleic acid (18:1) | Monounsaturated fatty acid | J774, RAW 264.7 | ApoA-I | [49, 137] |
| Effectors of Akt, mTOR, PI-PLC | ||||
| Stimulation | ||||
| Akt1/2 kinase inhibitor | An inhibitor of Akt | BHK expressing ABCA1 | ApoA-I | [138] |
| DEPC (10-[4ʹ-(N,N-Diethylamino)butyl]-2-chlorophenoxazine hydrochloride) | An inhibitor of Akt; supresses mTORC1 activity | RAW 264.7, Min6, HepG2, BHK expressing ABCA1 | ApoA-I | [138] |
| BHK expressing ABCA1 | Mβ-CD | [138] | ||
| Ku-0063794 | mTOR inhibitor | ABCA1-expressing BHK cells | ApoA-I | [35] |
| LY294002 | An inhibitor of PI3 kinase; supresses mTORC1 activity | HepG2, HEK293 expressing ABCA1, BHK expressing ABCA1 | ApoA-I | [115, 138] |
| Rapamycin (at 10–100 nM; inhibition over 10 μM) | mTOR inhibitor | BHK expressing ABCA1 | ApoA-I | [35, 138] |
| Torin-1 | An inhibitor of mTORC1 | BHK expressing ABCA1 | ApoA-I | [138] |
| Inhibition | ||||
| PI-PLC | Hydrolyzes PIP2 to inositol triphosphate and diacylglycerol | RAW264.7, HEK293 expressing ABCA1 | ApoA-I | [139] |
| Ceramide signaling | ||||
| Stimulation | ||||
| C2-dihydroceramide | Ceramide analog that is not associated with apoptosis | CHO | ApoA-I | [140] |
| Ceramide | A lipid signaling molecule, a product of the digestion of sphingomyelin, an activator of cathepsin D (a lysosomal proteinase) | J774, CHO, CHO expressing ABCA1, HeLa expressing ABCA1 | ApoA-I | [140, 141] |
| MAPP [(1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol] | An inhibitor of alkaline ceramidase; elevates the level of endogenous ceramide | CHO | ApoA-I | [140] |
| MAP kinase and non-receptor tyrosine kinase signaling | ||||
| Stimulation | ||||
| PD98059b | An inhibitor of MAP kinases MEK1 and MEK2 | RAW 264.7, MPM | ApoA-I, HDL | [113, 142] |
| PP2 (i.e. AG 1879) | An inhibitor of Src family kinase | Jurkat cells (human acute T lymphocyte leukemia cell line) | ApoA-I | [143] |
| U0126 | An inhibitor of MAP kinases ERK1/2 | RAW 264.7, MPM | ApoA-I, HDL | [113, 142] |
| Inhibition | ||||
| AG490 | Inhibitor of JAK-2 | MAC-T | ApoA-I | [34] |
| PD98059b | An inhibitor of MAP kinases MEK1 and MEK2 | MAC-T | ApoA-I | [34] |
| Raf1 kinase inhibitor I, i.e. GW5074 [3-(3,5-Dibromo-4-hydroxybenzyliden)-5-iodo-1,3-dihydroindol-2-one] | Inhibits signaling through the MAPK cascade | HEK293 expressing ABCA1 | ApoA-I | [115] |
| Ion channels and Ca2 + regulation | ||||
| Stimulation | ||||
| BAY-K8644 | An agonist of plasma membrane L-type Ca2 + channels | ABCA1-expressing BHK cells | ApoA-I | [44] |
| Digoxin | A cardioactive glycoside that inhibits Na +/K + ATPase, activates the mevalonate pathway, and stimulates the mitochondrial respiratory chain and synthesis of ATP | H9c2 (rat cardiomyocyte cell line) | No acceptor, ApoA-I | [144] |
| Ouabain | A cardioactive glycoside that inhibits Na +/K + ATPase, activates the mevalonate pathway, and stimulates the mitochondrial respiratory chain and synthesis of ATP | H9c2 (rat cardiomyocyte cell line) | No acceptor, ApoA-I | [144] |
| Nifedipine | A calcium channel blocker | RAW 264.7 | ApoA-I, HDL | [145] |
| Inhibition | ||||
| BAPTA-AM | Intracellular Ca2 + chelator | ABCA1-expressing BHK cells, RAW 264.7 | ApoA-I | [44] |
| Benzamil (stimulation at 100 uM) | Blocks the epithelial sodium channel and sodium-calcium exchange | MAC-T | HDL | [34] |
| Cyclosporine A | Calcineurin inhibitor | ABCA1-expressing BHK cells, RAW 264.7, THP-1 | ApoA-I | [35, 44] |
| Disulphonic acid hydrate disodium salt | Chloride channel inhibitor | ABCA1-expressing BHK cells | ApoA-I | [44] |
| EDTA | Chelator of Ca2+ | ABCA1-expressing BHK cells | ApoA-I | [44] |
| EGTA | Chelator of Ca2+ | ABCA1-expressing BHK cells, RAW 264.7 | ApoA-I | [44] |
| Pimecrolimus | Calcineurin inhibitor | ABCA1-expressing BHK cells | ApoA-I | [35] |
| FK506 (tacrolimus) | Calcineurin inhibitor | ABCA1-expressing BHK cells, RAW 264.7 | ApoA-I | [35, 44] |
| W-7 | CaM antagonist (inhibits binding of Ca2 + -bound CaM with its substrates) | ABCA1-expressing BHK cells | ApoA-I | [44] |
| Protein synthesis and degradation | ||||
| Stimulation | ||||
| ALLN (Calpain inhibitor I) | Thiol protease inhibitors; increases ABCA1 level; reversibly blocks Ca-dependent neutral cysteine protease calpain I | THP-1 | ApoA-I | [146] |
| Bortezomib | A proteasome inhibitor | THP-1, RAW 264.7, MPM | ApoA-I, HDL | [147] |
| Chloroquine | A lysosomal inhibitor | HeLa expressing ABCA1 | ApoA-I | [24] |
| Epoxomicin | A proteasome inhibitor | THP-1, RAW 264.7, MPM | ApoA-I, HDL | [147] |
| MG132 | A proteasome inhibitor | THP-1, RAW 264.7, MPM | ApoA-I, HDL | [147] |
| Leupeptin | Thiol protease inhibitor; increases the ABCA1 level; inhibits serine and cysteine proteases (plasmin, trypsin, papain, calpain, and cathepsin B) | THP-1 | ApoA-I | [146] |
| Pepstatin A | An inhibitor of cathepsin D, a lysosomal proteinase | mBMDM, MPM, J774, CHO | ApoA-I | [141] |
| Inhibition | ||||
| Brefeldin A | Lactone antibiotic that alters the structure and function of the Golgi apparatus; inhibits protein processing through the Golgi | J774, RAW 264.7, human skin fibroblasts, 3T3 L-1-derived adipocytes | ApoA-I (J774, adipocytes), ApoE4 (RAW 264.7), HDL (fibroblasts), HDL3 (J774) | [23, 28, 148] |
| Cycloheximide | Protein synthesis inhibitor | J774, MPM | ApoA-I | [29, 118] |
| Monensin | Polyether antibiotic that alters the structure and function of the Golgi apparatus; inhibits protein processing through the Golgi | RAW 264.7, human skin fibroblasts | ApoE4 (RAW 264.7), HDL (fibroblasts) | [36, 148] |
| Structural and trafficking proteins and their ligands | ||||
| Stimulation | ||||
| Colchicine | Inhibits microtubule polymerization, a metabolic and transport inhibitor, mitotic poison | Human skin fibroblasts | Plasma, albumin-depleted plasma, and ApoA-I-depleted plasma | [149] |
| Caveolin-1 expression | Integral membrane protein that acts as a scaffolding protein | HepG2 stably transfected caveolin-1 | ApoA-I and plasma | [150] |
| FGIN-1-27 | A ligand for TSPO | THP-1 | ApoA-I, HDL | [151] |
| Flunitrazepam | A ligand for TSPO | THP-1 | ApoA-I | [151] |
| GGTI-298 | An inhibitor of prenyltransferase GGTase-I that post-translationally modifies proteins for association to the membrane | mBMDM, THP-1 | No acceptor, ApoA-I (mBMDM), HDL (mBMDM) | [152] |
| PK11195 | A ligand for TSPO | THP-1 | ApoA-I, HDL | [151] |
| DNA-depending processes | ||||
| Stimulation | ||||
| Etoposide (VP-16) | DNA topoisomerase II inhibitor | MPM | ApoA-I | [153] |
| Pyrrole-imidazole polyamide targeting ABCA1 promoter | A nuclease-resistant compound that inhibits the transcription factor by binding to the minor groove of DNA | RAW 264.7 | ApoA-I | [154] |
| Teniposide (VM-26) | DNA topoisomerase II inhibitor | MPM | ApoA-I | [153] |
| Inhibition | ||||
| Mithramycin A | A chemotherapeutic drug that binds to GC-rich DNA sequences and blocks the binding of the transcription factor Sp1 | RAW 264.7 | ApoA-I | [155] |
| Other factors | ||||
| Stimulation | ||||
| Aspirin (up to 0.5 mM; inhibition over 1 mM) | NSAID and an antiplatelet drug (antiaggregant) used in CVD | RAW 264.7 | ApoA-I | [63] |
| Doxazosin | α1-selective alpha blocker used to treat high blood pressure | RAW 264.7 | ApoA-I | [154] |
| EP 80317 | selective CD36 ligand | J774 | ApoA-I, HDL | [156] |
| IRAK1 and IRAK4 inhibitor | IRAK1 participates in signaling via toll-like receptors/IL-1R | THP-1 | ApoA-I, HDL | [157] |
| L. acidophilus bacteria strain K301, heat killed | A component of the human gut microflora; used as probiotics | THP-1 | ApoA-I | [158] |
| Paraoxonase-1 | An HDL-associated enzyme that contributes to the antioxidant and anti-inflammatory capacities of HDLs | J774, THP-1 | No acceptor, HDL3, ApoA-I (J774) | [159] |
| Fu5AH | HDL | [159] | ||
| Inhibition | ||||
| Arsenic trioxide | Chronic arsenic exposure is associated with an increased risk of CVD mortality | HepG2 | HDL | [160] |
| Celecoxib | COX-2-specific inhibitor for the treatment of pain and inflammation | THP-1 | ApoA-I | [161] |
| Chlamydia pneumoniae, viable | A Gram-negative obligate intracellular bacterium, a common cause of community-acquired pneumoniae | THP-1 | ApoA-I | [162] |
| CRP | CRP in plasma are elevated in numerous disease states; CRP possesses proinflammatory and proatherogenic properties | THP-1, hPBMC | ApoA-I, HDL (THP-1) | [163] |
| D-(+)-trehalose 6,6ʹ-dibehenate | Synthetic Clec4e (macrophage inducible Ca2 + -dependent lectin) ligand | mBMDM | HDL, serum | [164] |
| HSP65 | Binds to TCR and initiates immune responses, resulting in the production of proinflammatory cytokines | Jurkat cells (human acute T lymphocyte leukemia cell line), primary CD4 + T cells | ApoA-I | [143] |
| JNJ-26854165 (serdemetan) | A proposed drug, activates p53 | HEK293T; mantle cell lymphoma cell lines: MAVER-1, JeKo-1; multiple myeloma cell lines: OPM-2, U266 | ApoA-I | [165] |
| Low pH (pH 5.5–6.5 compared with pH7.5) | hPBMC | ApoA-I, HDL2, human plasma | [166] | |
| Low temperature | Human skin fibroblasts | Plasma, albumin-depleted plasma, and ApoA-I-depleted plasma | [149] | |
| RAW 264.7 | ApoA-I, HSA | [167] | ||
| mBMDM, primary hepatocytes | Mβ-CD | [168] | ||
| LPS (i.e. endotoxins) | A polysaccharide found in the outer membrane of Gram-negative bacteria that causes strong immune responses | MPM, THP-1 | ApoA-I | [97, 169] |
| Okadaic acid | An inhibitor of protein phosphatases that downregulates caveolin expression | Fibroblasts, SMC | ApoA-I | [26] |
| PAPP-A | A metalloproteinase detected in ruptured atherosclerotic plaques | THP-1 | ApoA-I, HDL | [50] |
| Ritonavir | A human immunodeficiency virus protease inhibitor | hPBMC, THP-1 | ApoA-I, HDL (THP-1) | [170] |
| Trypsin (pretreatment of the cells) | A protease | J774 | ApoA-I | [29] |
| Urotensin II | A vasoconstrictor peptide, a ligand of G protein-coupled receptor GPR14 | THP-1 | No acceptor | [171] |
| Vitamins, coenzymes and metabolites | ||||
| Stimulation | ||||
| 9-nitro oleic acid | Found in human plasma; is generated by nitration of oleic acid by peroxynitrite and acidified nitrite | J774 | HDL | [172] |
| Calcitriol [1,25-dihydroxyvitamin D3 or 1,25-(OH)2D3] | Hormonally active metabolite of vitamin D | THP-1 | ApoA-I | [173] |
| Citrulline | A precursor of arginine and a byproduct of arginine oxidation by nitric oxide synthase | hPBMC, THP-1 | ApoA-I, HDL | [174] |
| Coenzyme Q10 | A component of the electron transport chain and a natural antioxidant | hPBMC, THP-1, J774 | HDL | [175, 176] |
| Ethanol | Astrocytes, HepG2 (conditioned media) | ApoA-I, HDL, ApoE, conditioned medium | [32, 177] | |
| GSH (glutathione) | A tripeptide, a thiol antioxidant | J774 | HDL | [178] |
| Nicotinic acid (niacin) | Vitamin B3, lipid-lowering drug | MPM | HDL3 | [179], |
| 3T3-L1 adipocytes | ApoA-I | [180] | ||
| Spermidine | Endogenous polyamine that induces autophagy | VSMC | ApoA-I | [181] |
| Inhibition | ||||
| 7-Ketocholesterol (cholest-5-en-3beta-ol-7-one) | The major form of oxidized cholesterol that is present in oxidized LDL and atherosclerotic lesions | THP-1 | ApoA-I | [182] |
| Acetoacetate | A component of ketone bodies | RAW 264.7 | ApoA-I | [49] |
| Carbon monoxide | A component of the primary traffic emission; endogenously produced via heme degradation by heme oxygenase | J774 | HDL | [183] |
| Glucose, increased level (20–25 mM) | RAW 264.7, human glomerular endothelial cells | ApoA-I | [126, 184] | |
| Neopterin | A catabolic product of GTP, mainly synthesized by activated macrophages upon stimulation with IFNγ; a marker of inflammation | THP-1 | ApoA-I, HDL | [185] |
| Extracts, components of plants and other natural sources, hits from small-molecule high-throughput screening | ||||
| Stimulation | ||||
| Alpinetin (7-hydroxy-5-methoxyflavanone) | A plant flavonoid abundantly present in Alpinia katsumadai Hayata | THP-1, hPBMC | ApoA-I or HDL | [186] |
| Anthocyanins (cyanidin-3-O-beta-glucoside and peonidin-3-O-beta-glucoside) | Plant pigments; phenolic compound rich in plants | MPM | ApoA-I | [73] |
| Arctigenin | Antioxidant, antitumor and anti-inflammatory substance from Arctium lappa plant | THP-1 | ApoA-I, HDL2, HDL3 | [187] |
| α-Asarone | Isolated from Purple perilla extract; known as a component of Acorus tatarinowii herb | J774 | No acceptor | [188] |
| Astaxanthin | A carotenoid found in salmon, crab, and shrimp | RAW 264.7 | ApoA-I, HDL | [189] |
| BCD1 | A compound designed for ABCA1 induction based on the structure of rutaecarpine | RAW 264.7 | HDL | [190] |
| Betulin | A pentacyclic triterpenoid from the bark of yellow and white birch trees | RAW 264.7 | ApoA-I, HDL | [191] |
| Dihydrocapsaicin | A component of capsaicinoids of pepper | THP-1 | ApoA-I | [192] |
| Chrysin | A flavonoid that is widely present in honey, propolis, and plant extracts | RAW 264.7 | HDL | [75] |
| Curcumin | A polyphenol derived from the rhizome of turmeric (curcuma longa) | Adipocytes | ApoA-I | [193] |
| Dehydroxytrichostatin A (i.e. 9179B) | A compound found by screening of microbial secondary metabolites on the ability to induce ABCA1 | RAW 264.7 | ApoA-I | [194] |
| Diosgenin | A steroidal sapogenin present in a variety of plants, including fenugreek, yam root and soy bean | MPM, THP-1 | ApoA-I | [195] |
| Emodin | Anthraquinone derivative from the roots of Rheum palmatum | THP-1 | ApoA-I | [196] |
| Ethanolic extracts of Brazilian red propolis | Propolis, collected by honey bees from Dalbergia ecastophyllum (L) Taub. (Leguminosae) | THP-1 | ApoA-I | [197] |
| Hesperetin | One of the major citrus flavonoids | THP-1 | ApoA-I | [198] |
| Leoligin | The major lignan from edelweiss (Leontopodium nivale subsp. alpinum) | THP-1 | ApoA-I, human plasma | [199] |
| Marrubium vulgare extract | The plant is widely used in traditional medicine; extract is rich in phenolic compounds | THP-1 | HDL | [200] |
| Methyl protodioscin | A compound isolated from Dioscorea nipponica makino | THP-1, HepG2 | ApoA-I | [78] |
| Nagilactone B | A novel compound, suppresses atherosclerosis in apoE −/− mice | RAW264.7 | ApoA-I, HDL | [201] |
| Paeonol | A phenolic component purified from Paeonia suffruticosa (Cortex Moutan) used in traditional Chinese medicine | J774 | ApoA-I | [202] |
| Phellinus linteus polysaccharide extract (at 5–20 μg/mL; inhibition at 100 μg/mL) | An immunomodulatory agent with a molecular weight of 153 Kd | THP-1 | ApoA-I | [203] |
| Piperine | The pungent ingredient of black pepper | THP-1 | ApoA-I, human plasma | [71] |
| Pomegranate peel polyphenols | Gallic acid, ellagic acid, punicalagins are the main active substances | RAW 264.7 | ApoA-I | [204] |
| Protocatechuic acid | A metabolite of the flavonoid cyanidin-3-O-β-glucoside | MPM, THP-1 | ApoA-I, HDL | [205] |
| Purple perilla extract | Contains rosmarinic acid, methyl rosmarinic acid, caffeic acid, chlorogenic acid and luteolin | J774 | No acceptor | [188] |
| Rutaecarpine | A compound identified by screening of 20,000 compounds on the stimulation of the promoters of ABCA1 and CLA-1 (CD36 and lysosomal integral membrane protein II analogous 1) | RAW 264.7 | ApoA-I, HDL | [206] |
| Quercetin | A natural flavonoid found in red wine, fruits and other natural sources with antioxidant, anti-inflammatory and anti-atherosclerosis activities | J774, THP-1, RAW 264.7 | ApoA-I, HDL (J774, RAW 264.7) | [178, 207, 208] |
| Quercetin 7-O-sialic acid | Combines the cardioprotective effect of quercetin and N-acetylneuraminic acid | RAW 264.7 | ApoA-I, HDL | [208] |
| Resveratrol | A stilbenoid with cardioprotective and anti-inflammatory properties | THP-1 | Human plasma | [209] |
| Riccardin C | Non-sterol natural product isolated from liverworts | THP-1 | ApoA-I, no acceptor | [77] |
| Sage (Salvia plebeia) weed extract | Contains antioxidants royleanonic acid, hispidulin and eupatorin | J774 | No acceptor (just medium) | [210] |
| Saikosaponin A | One of the most active saikosaponins of Radix Bupleuri, a triterpenoid glycoside | THP-1 | ApoA-I, HDL | [74] |
| Salvianolic acid B | A compound isolated from the Danshen root (Salvia miltiorrhiza Bunge) | THP-1 | ApoA-I, HDL2, HDL3 | [62] |
| Sesame oil | Oil from Sesamum indicum | MPM | ApoA-I | [211] |
| Sesamin | The most abundant lignan in sesame oil | RAW 264.7 | HDL | [212] |
| Sesamol | A lignan found in sesame oil | MPM | ApoA-I | [211] |
| Tanshinone IIA | A lipophilic compound derived from Danshen (Salvia miltiorrhiza) | THP-1 | ApoA-I, HDL | [213] |
| VAO-PE | Unsaponifiable fraction of the oil contains tocopherols, squalene, sterols (schottenol and spinasterol) and phenols (ferulic, syringic and vanillic acid) | THP-1 | HDL, Ox-HDL pre-incubated with VAO-PE | [214] |
| Walnut oil | Walnuts contain high levels of PUFA, both linoleic acid and α-linolenic acid | THP-1 | No acceptor | [67] |
| Wogonin | A component of Scutellaria baicalensis Georgi extracts | J774 | No acceptor | [215] |
| Zerumbone | A cyclic sesquiterpene isolated from Zingiber zerumbet Smith | THP-1 | ApoA-I | [216] |
| Inhibition | ||||
| Cigarette smoke | Smoking a cigarette with a filter containing 14 mg of tar and 0.9 mg of nicotine was passed through 50 ml of culture medium | J774 | HDL | [217] |
| Nicotine | Considered a pro-atherogenic component in tobacco | hPBMC | ApoA-I | [218] |
ABC ATP-binding cassette, ABCA1 ATP binding cassette subfamily A member 1, ABCB1 ATP binding cassette subfamily b member 1, ABCB4 ATP binding cassette subfamily B member 4, ACAT acyl-CoA cholesterol acyltransferase, ACE angiotensin converting enzyme, ACE2 angiotensin-converting enzyme 2, Akt protein kinase B, AMP adenosine monophosphate, AMPK AMP-activated protein kinase, APJ apelin receptor, apoA-I apolipoprotein A-I, apoA-II apolipoprotein A-II, ApoE apolipoprotein E, ATP adenosine triphosphate, BHK baby hamster kidney cells, BHK-21 baby hamster kidney cell line 21, BLT block lipid transport, 8-Br-cAMP 8-bromoadenosine-cAMP, CaM calmodulin, cAMP adenosine 3′,5′-cyclic monophosphate, CCL2 CC-chemokine ligand 2, CHO Chinese hamster ovary cells, Clec4e c-type lectin domain family 4 member E, COX cyclooxygenase, COX-2 cyclooxygenase-2, CRH corticotropin-releasing hormone, CRP C-reactive protein, CVD cardiovascular disease, CXCR2 C-X-C chemokine receptor type 2, DPP-4 dipeptidyl peptidase 4, DR3 death receptor 3, EGF epidermal growth factor, ER estrogen receptor, ERK extracellular signal–regulated kinase, FGF-21 fibroblast growth factor 21, GDP-15 growth differentiation factor-15, GGTase-I geranylgeranyltransferase type-I, GLP-1 glucagon-like peptide 1, GR glucocorticoid receptor, GTP guanosine-5'-triphosphate, HCAEC primary human coronary artery endothelial cells, HDL high-density lipoprotein, HEK293 human embryonic kidney 293 cells, hPBMC human peripheral blood mononuclear cells, Huh7 cells human hepatocellular carcinoma cell line, HUVEC human umbilical vein endothelial cells, HSA human serum albumin, HSP65 heat shock protein 65, ldlA-7 LDL receptor-deficient Chinese hamster ovary cells, IFN interferon, IGF-1 insulin-like growth factor 1, IL interleukin, IL-1R IL-1 receptor, IRAK1 interleukin-1 receptor-associated kinase 1, IRAK4 inhibitor inhibitor of IL-1 receptor-associated kinase-4, JAK Janus kinase, KO knockout, LDL low-density lipoprotein, LPL lipoprotein lipase, LPS lipopolysaccharides, LXR liver X receptor, MAC-T immortalized bovine mammary secretory epithelial cells, MAP mitogen-activated protein, Mβ-CD methyl-β-cyclodextrin, hPBMC, mBMDM, MPM; [104]: DPP-4, GLP-1, mBMDM mouse bone marrow-derived macrophages, MEK mitogen-activated protein kinase kinase, MPM malignant pleural mesothelioma cells, mTOR mammalian target of rapamycin, mTORC1 mammalian target of rapamycin complex 1, NSAID nonsteroidal anti-inflammatory drug, Ox-HDL oxidised HDL, PAPP-A pregnancy-associated plasma protein A, PCSK9 proprotein convertase subtilisin/kexin type 9, PI3 phosphoinositide-3, PIP2 phosphatidylinositol 4,5-bisphosphate, PI-PLC phosphatidylinositol-specific phospholipase C, PKA protein kinase A, PLTP phospholipid transfer protein, PPAR peroxisome proliferator-activated receptor, PUFA polyunsaturated fatty acid, RAR retinoic acid receptor, RXR retinoid X receptor, SMC smooth muscle cells, SPTLC1 serine palmitoyltransferase long chain base subunit 1, SR-B1 scavenger receptor class B member 1, TCR T-cell receptor, TGF transforming growth factor, TNF tumor necrosis factor, TSPO translocator protein, VAO-PE virgin argan oil phenolic extract, VSMC vascular smooth muscle cells
aIn many cases, cells were treated with substances to differentiate to macrophages (e.g. by phorbol 12-myristate 13-acetate, macrophage colony-stimulating factor, or granulocyte/macrophage colony-stimulating factor), to induce expression of ABCA1 (e.g. by cpt-cAMP, TO-901317, or 22-OH + 9cRA), and transformed to foam cells (e.g. by Ac-LDL)
bThe same factor stimulates and inhibits, depending on the cells, acceptor, and cholesterol depletion
cStatin description is given according to McFarland et al. [219]
Fig. 1.
Cholesterol efflux effectors grouped by signal and metabolic pathways. ABC transporter ATP-binding cassette transporter, Akt/mTOR protein kinase B/mammalian target of rapamycin, MAP kinase mitogen-activated protein kinase, PI-PLC phosphatidylinositol-specific phospholipase C, SR-B1 scavenger receptor class B member 1
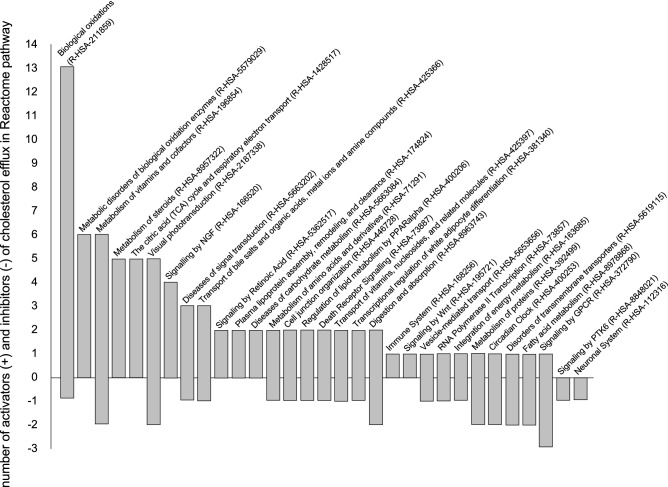
The subsequent Reactome database search [221] identified 67 substances, and 9 substances were excluded due to dual activating and inhibiting properties. Pathway enrichment was then performed for the remaining 58 substances using the standard Reactome tools with a ‘small molecules (chebi)’ key. The significant (p < 0.05) 93 pathways were selected, including 45 from 58 substances. The number of significant pathways was reduced to 31 by the replacement of pathways of very low level with higher-level (parent) pathways (Table 2). These pathways included the Neuronal System (R-HSA-112316); transcriptional regulation of white adipocyte differentiation (R-HSA-381340); the citric acid (TCA) cycle and respiratory electron transport (R-HSA-1428517); integration of energy metabolism (R-HSA-163685); metabolism of vitamins and cofactors (R-HSA-196854); biological oxidations (R-HSA-211859); fatty acid metabolism (R-HSA-8978868); regulation of lipid metabolism by peroxisome proliferator-activated receptor-α (PPARα; R-HSA-400206); metabolism of steroids (R-HSA-8957322); metabolism of amino acids and derivatives (R-HSA-71291); cell junction organization (R-HSA-446728); signaling by nerve growth factor (R-HSA-166520); signaling by Wnt (R-HSA-195721); visual phototransduction (R-HSA-2187338); signaling by GPCR (R-HSA-372790); signaling by retinoic acid (R-HSA-5362517); death receptor signaling (R-HSA-73887); signaling by PTK6 (R-HSA-8848021); disorders of transmembrane transporters (R-HSA-5619115); diseases of signal transduction (R-HSA-5663202); metabolic disorders of biological oxidation enzymes (R-HSA-5579029); diseases of carbohydrate metabolism (R-HSA-5663084); immune system (R-HSA-168256); plasma lipoprotein assembly, remodeling, and clearance (R-HSA-174824); transport of bile salts and organic acids, metal ions, and amine compounds (R-HSA-425366); transport of vitamins, nucleosides, and related molecules (R-HSA-425397); metabolism of proteins (R-HSA-392499); circadian clock (R-HSA-400253); vesicle-mediated transport (R-HSA-5653656); RNA polymerase II transcription (R-HSA-73857); and digestion and absorption (R-HSA-8963743). Importantly, the energy-dependent processes (R-HSA-1428517, R-HSA-163685, R-HSA-211859, R-HSA-5619115, R-HSA-5579029), lipoprotein metabolism and lipid transport (R-HSA-400206, R-HSA-8957322, R-HSA-174824, R-HSA-5653656) and signaling pathways (R-HSA-166520, R-HSA-195721, R-HSA-372790, R-HSA-5362517, R-HSA-73887, R-HSA-8848021, R-HSA-5663202) are included (Table 2).
Table 2.
Substances and pathways influencing cellular cholesterol efflux (ChEBI and Reactome pathway indexes are included)
| R-HSA-112316 Neuronal System | R-HSA-381340 Transcriptional regulation of white adipocyte differentiation | R-HSA-1428517 The citric acid (TCA) cycle and respiratory electron transport | R-HSA-163685 Integration of energy metabolism | R-HSA-196854 Metabolism of vitamins and cofactors | R-HSA-211859 Biological oxidations | R-HSA-8978868 Fatty acid metabolism | R-HSA-400206 Regulation of lipid metabolism by PPAR-α | R-HSA-8957322 Metabolism of steroids | R-HSA-71291 Metabolism of amino acids and derivatives | R-HSA-446728 Cell junction organization | R-HSA-166520 Signaling by NGF | R-HSA-195721 Signaling by Wnt | R-HSA-2187338 Visual phototransduction | R-HSA-372790 Signaling by GPCR | R-HSA-5362517 Signaling by retinoic acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activator | ||||||||||||||||
| 2981 Baicalin | ● | ● | ||||||||||||||
| 3086 Betulin | ● | ● | ||||||||||||||
| 3638 Chloroquine | ● | ● | ||||||||||||||
| 4551 Digoxin | ||||||||||||||||
| 4629 Diosgenin | ● | |||||||||||||||
| 4708 Doxazosin | ● | |||||||||||||||
| 6426 Leupeptin | ● | |||||||||||||||
| 15365 Aspirin | ||||||||||||||||
| 15367 All-trans retinoic acid (tretinoin) | ● | ● | ● | ● | ● | |||||||||||
| 15940 Nicotinic acid (niacin) | ● | ● | ||||||||||||||
| 16236 Ethanol | ● | |||||||||||||||
| 16243 Quercetin | ● | |||||||||||||||
| 16469 17β-estradiol | ● | ● | ||||||||||||||
| 16610 Spermidine | ● | |||||||||||||||
| 16856 GSH (glutathione) | ● | ● | ● | ● | ● | |||||||||||
| 17026 Progesterone | ● | ● | ||||||||||||||
| 17351 α-Linolenic acid | ● | ● | ● | ● | ||||||||||||
| 17579 All-trans β-carotene | ● | ● | ● | |||||||||||||
| 17650 Hydrocortisone (i.e. cortisol) | ● | ● | ||||||||||||||
| 17823 Calcitriol | ● | |||||||||||||||
| 18211 Citrulline | ● | |||||||||||||||
| 23359 Colchicine | ||||||||||||||||
| 27881 Resveratrol | ● | |||||||||||||||
| 36062 Protocatechuic acid | ● | |||||||||||||||
| 46245 Coenzyme Q10 | ● | ● | ||||||||||||||
| 47499 Imipramine | ||||||||||||||||
| 50122 Rosiglitazone | ● | ● | ● | ● | ||||||||||||
| 50648 9-cis-retinoic acid | ● | ● | ● | ● | ||||||||||||
| 63892 Zerumbone | ||||||||||||||||
| 65329 LY294002 | ||||||||||||||||
| 84612 cpt-cAMP | ● | |||||||||||||||
| Inhibitor | ||||||||||||||||
| 8772 Raloxifene | ||||||||||||||||
| 9635 Toremifene | ||||||||||||||||
| 15344 Acetoacetate | ● | |||||||||||||||
| 16196 Oleic acid | ● | ● | ● | ● | ● | |||||||||||
| 16551 D-(+)-trehalose 6,6ʹ-dibehenate | ||||||||||||||||
| 17245 Carbon monoxide | ● | ● | ||||||||||||||
| 25675 Oligomycin | ||||||||||||||||
| 28364 Eicosapentaenoic acid | ● | ● | ● | ● | ||||||||||||
| 30740 EGTA | ||||||||||||||||
| 34159 15d-PGJ2 | ● | |||||||||||||||
| 38545 Rosuvastatin | ● | |||||||||||||||
| 41423 Celecoxib | ● | |||||||||||||||
| 41774 Tamoxifen | ● | |||||||||||||||
| 41879 Dexamethasone |
| R-HSA-73887 Death receptor signaling | R-HSA-8848021 Signaling by PTK6 | R-HSA-5619115 Disorders of transmembrane transporters | R-HSA-5663202 Diseases of signal transduction | R-HSA-5579029 Metabolic disorders of biological oxidation enzymes | R-HSA-5663084 Diseases of carbohydrate metabolism | R-HSA-168256 Immune system | R-HSA-174824 Plasma lipoprotein assembly, remodeling, and clearance | R-HSA-425366 Transport of bile salts and organic acids, metal ions and amine compounds | R-HSA-425397 Transport of vitamins, nucleosides, and related molecules | R-HSA-392499 Metabolism of proteins | R-HSA-400253 Circadian Clock | R-HSA-5653656 Vesicle-mediated transport | R-HSA-73857 RNA Polymerase II Transcription | R-HSA-8963743 Digestion and absorption | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activator | |||||||||||||||
| 2981 Baicalin | ● | ● | |||||||||||||
| 3086 Betulin | ● | ● | |||||||||||||
| 3638 Chloroquine | ● | ● | |||||||||||||
| 4551 Digoxin | ● | ||||||||||||||
| 4629 Diosgenin | |||||||||||||||
| 4708 Doxazosin | |||||||||||||||
| 6426 Leupeptin | |||||||||||||||
| 15365 Aspirin | |||||||||||||||
| 15367 All-trans retinoic acid (tretinoin) | ● | ||||||||||||||
| 15940 Nicotinic acid (niacin) | ● | ||||||||||||||
| 16236 Ethanol | ● | ||||||||||||||
| 16243 Quercetin | ● | ||||||||||||||
| 16469 17β-estradiol | ● | ||||||||||||||
| 16610 Spermidine | ● | ||||||||||||||
| 16856 GSH (glutathione) | |||||||||||||||
| 17026 Progesterone | ● | ● | |||||||||||||
| 17351 α-Linolenic acid | ● | ● | ● | ● | ● | ||||||||||
| 17579 All-trans β-carotene | |||||||||||||||
| 17650 Hydrocortisone (i.e. cortisol) | |||||||||||||||
| 17823 Calcitriol | ● | ||||||||||||||
| 18211 Citrulline | |||||||||||||||
| 23359 Colchicine | ● | ||||||||||||||
| 27881 Resveratrol | |||||||||||||||
| 36062 Protocatechuic acid | ● | ||||||||||||||
| 46245 Coenzyme Q10 | |||||||||||||||
| 47499 Imipramine | ● | ||||||||||||||
| 50122 Rosiglitazone | ● | ||||||||||||||
| 50648 9-cis-retinoic acid | ● | ||||||||||||||
| 63892 Zerumbone | ● | ||||||||||||||
| 65329 LY294002 | ● | ||||||||||||||
| 84612 cpt-cAMP | |||||||||||||||
| Inhibitor | |||||||||||||||
| 8772 Raloxifene | ● | ||||||||||||||
| 9635 Toremifene | |||||||||||||||
| 15344 Acetoacetate | ● | ● | ● | ||||||||||||
| 16196 Oleic acid | ● | ● | ● | ||||||||||||
| 16551 D-(+)-trehalose 6,6ʹ-dibehenate | ● | ||||||||||||||
| 17245 Carbon monoxide | ● | ||||||||||||||
| 25675 Oligomycin | |||||||||||||||
| 28364 Eicosapentaenoic acid | ● | ● | ● | ||||||||||||
| 30740 EGTA | ● | ||||||||||||||
| 34159 15d-PGJ2 | |||||||||||||||
| 38545 Rosuvastatin | |||||||||||||||
| 41423 Celecoxib | |||||||||||||||
| 41774 Tamoxifen | |||||||||||||||
| 41879 Dexamethasone | ● | ● |
GPCR G protein-coupled receptor, NGF nerve growth factor, PPAR peroxisome proliferator-activated receptor, black circle (●) denotes the action of a particular substance
The distribution of activators and inhibitors between particular pathways is shown in Fig. 2. Importantly, the substances are distributed non-uniformly among different pathways; the ‘biological oxidations’ pathway includes mostly substances with an activating effect on cholesterol efflux (all-trans retinoic acid, ethanol, 17β-estradiol, progesterone, hydrocortisone, resveratrol), while signaling by the G protein-coupled receptor and protein tyrosine kinase 6 pathways include substances with an inhibiting effect (oleic and eicosapentaenoic acids). ‘Biological oxidations’ include biotransformation of xenobiotics and endogenous compounds in the liver, kidneys, gut and lungs. As far as chemicals that undergo functionalization, the electrophilic or nucleophilic species can be detrimental to biological systems. Electrophiles can react with electron-rich macromolecules such as proteins, DNA and RNA by covalent interaction, while nucleophiles have the potential to interact with biological receptors [221]. Thus, in addition to nuclear receptor ligands and their precursors activating cholesterol efflux and lipoprotein metabolism, and widely used in clinics (bezafibrate and fenofibric acid [222], pioglitazone [223], telmisartan [224]), targeting biological oxidation processes looks promising for the correction of inefficient reverse cholesterol transport in humans. For instance, the stimulating effect was described for chloroquine [225], diosgenin [226], 17β-estradiol [227], all-trans retinoic acid [228], ethanol [229], spermidine [230, 231], resveratrol [232] and 9-cis-retinoic acid [233].
Fig. 2.
The pathway-dependent distribution of activators and inhibitors in cholesterol efflux. The particular Reactome indexes are shown in brackets. GPCR G protein-coupled receptor, NGF nerve growth factor, PPARalpha peroxisome proliferator-activated receptor α, PTK6 non-receptor tyrosine kinase, Wnt combination of Wg (wingless) and Int
Conclusions
We performed a comprehensive analysis of the various substances influencing cholesterol efflux, with pathway enrichment using the Reactome database. The activators and inhibitors of cholesterol efflux are non-uniformly distributed among different pathways. The substances influencing biological oxidation activate cholesterol efflux, and the substances influencing signaling by GPCR and PTK6 inhibit efflux. This analysis may be useful in the targeted therapy of structural and functional HDL deficiency.
Compliance with Ethical Standards
Funding
No funding has been received for the conduct of this analysis or the preparation of this article.
Conflict of interest
Dmitry Y. Litvinov, Eugeny V. Savushkin and Alexander D. Dergunov have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dergunov AD, Garaeva EA, Savushkin EV, Litvinov DY. Significance of lipid-free and lipid-associated ApoA-I in cellular cholesterol efflux. Curr Protein Pept Sci. 2017;18:92–99. doi: 10.2174/1389203717666160713150223. [DOI] [PubMed] [Google Scholar]
- 3.Litvinov DY, Savushkin EV, Garaeva EA, Dergunov AD. Cholesterol efflux and reverse cholesterol transport: experimental approaches. Curr Med Chem. 2016;23:3883–3908. doi: 10.2174/0929867323666160809093009. [DOI] [PubMed] [Google Scholar]
- 4.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillon AD, Latham CF, Miller EA. Vesicle-mediated ER export of proteins and lipids. Biochim Biophys Acta. 2012;1821:1040–1049. doi: 10.1016/j.bbalip.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder F, Atshaves BP, McIntosh AL, Gallegos AM, Storey SM, Parr RD, Jefferson JR, Ball JM, Kier AB. Sterol carrier protein-2: new roles in regulating lipid rafts and signaling. Biochim Biophys Acta. 2007;1771:700–718. doi: 10.1016/j.bbalip.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raychaudhuri S, Prinz WA. The diverse functions of oxysterol-binding proteins. Annu Rev Cell Dev Biol. 2010;26:157–177. doi: 10.1146/annurev.cellbio.042308.113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olkkonen VM. OSBP-related protein family in lipid transport over membrane contact sites. Lipid Insights. 2015;8:1–9. doi: 10.4137/LPI.S31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniele T, Schiaffino MV. Organelle biogenesis and interorganellar connections: better in contact than in isolation. Commun Integr Biol. 2014;7:e29587. doi: 10.4161/cib.29587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drin G, von Moser FJ, Copic A. New molecular mechanisms of inter-organelle lipid transport. Biochem Soc Trans. 2016;44:486–492. doi: 10.1042/BST20150265. [DOI] [PubMed] [Google Scholar]
- 11.Quon E, Beh CT. Membrane contact sites: complex zones for membrane association and lipid exchange. Lipid Insights. 2015;8:55–63. doi: 10.4137/LPI.S37190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes MP, Phillips MC, Rothblat GH. Efflux of cholesterol from different cellular pools. Biochemistry. 2000;39:4508–4517. doi: 10.1021/bi992125q. [DOI] [PubMed] [Google Scholar]
- 13.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogura M, Hori M, Harada-Shiba M. Association between cholesterol efflux capacity and atherosclerotic cardiovascular disease in patients with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2016;36:181–188. doi: 10.1161/ATVBAHA.115.306665. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt A, Rohatgi A. HDL cholesterol efflux capacity: cardiovascular risk factor and potential therapeutic target. Curr Atheroscler Rep. 2016;18:2. doi: 10.1007/s11883-015-0554-1. [DOI] [PubMed] [Google Scholar]
- 16.Rohatgi A. High-density lipoprotein function measurement in human studies: focus on cholesterol efflux capacity. Prog Cardiovasc Dis. 2015;58:32–40. doi: 10.1016/j.pcad.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chyu KY, Shah PK. HDL/ApoA-1 infusion and ApoA-1 gene therapy in atherosclerosis. Front Pharmacol. 2015;6:187. doi: 10.3389/fphar.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arakawa R, Tsujita M, Iwamoto N, Ito-Ohsumi C, Lu R, Wu CA, Shimizu K, Aotsuka T, Kanazawa H, Abe-Dohmae S, Yokoyama S. Pharmacological inhibition of ABCA1 degradation increases HDL biogenesis and exhibits antiatherogenesis. J Lipid Res. 2009;50:2299–2305. doi: 10.1194/jlr.M900122-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terao Y, Ayaori M, Ogura M, Yakushiji E, Uto-Kondo H, Hisada T, Ozasa H, Takiguchi S, Nakaya K, Sasaki M, Komatsu T, Iizuka M, Horii S, Mochizuki S, Yoshimura M, Ikewaki K. Effect of sulfonylurea agents on reverse cholesterol transport in vitro and vivo. J Atheroscler Thromb. 2011;18:513–530. doi: 10.5551/jat.7641. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Zhang Z, Xu Y, Feng T, Jiang W, Li Z, Hong B, Xie Z, Si S. IMB2026791, a xanthone, stimulates cholesterol efflux by increasing the binding of apolipoprotein A-I to ATP-binding cassette transporter A1. Molecules. 2012;17:2833–2854. doi: 10.3390/molecules17032833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieland TJ, Penman M, Dori L, Krieger M, Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc Natl Acad Sci USA. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard AD, Verghese PB, Arrese EL, Soulages JL. Characterization of apoA-I-dependent lipid efflux from adipocytes and role of ABCA1. Mol Cell Biochem. 2010;343:115–124. doi: 10.1007/s11010-010-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui HL, Grant A, Mukhamedova N, Pushkarsky T, Jennelle L, Dubrovsky L, Gaus K, Fitzgerald ML, Sviridov D, Bukrinsky M. HIV-1 Nef mobilizes lipid rafts in macrophages through a pathway that competes with ABCA1-dependent cholesterol efflux. J Lipid Res. 2012;53:696–708. doi: 10.1194/jlr.M023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyssenko NN, Brubaker G, Smith BD, Smith JD. A novel compound inhibits reconstituted high-density lipoprotein assembly and blocks nascent high-density lipoprotein biogenesis downstream of apolipoprotein AI binding to ATP-binding cassette transporter A1-expressing cells. Arterioscler Thromb Vasc Biol. 2011;31:2700–2706. doi: 10.1161/ATVBAHA.111.234906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fielding PE, Nagao K, Hakamata H, Chimini G, Fielding CJ. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry. 2000;39:14113–14120. doi: 10.1021/bi0004192. [DOI] [PubMed] [Google Scholar]
- 27.Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- 28.Favari E, Lee M, Calabresi L, Franceschini G, Zimetti F, Bernini F, Kovanen PT. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J Biol Chem. 2004;279:9930–9936. doi: 10.1074/jbc.M312476200. [DOI] [PubMed] [Google Scholar]
- 29.Sakr SW, Williams DL, Stoudt GW, Phillips MC, Rothblat GH. Induction of cellular cholesterol efflux to lipid-free apolipoprotein A-I by cAMP. Biochim Biophys Acta. 1999;1438:85–98. doi: 10.1016/S1388-1981(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 30.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 31.Tsujita M, Yokoyama S. Selective inhibition of free apolipoprotein-mediated cellular lipid efflux by probucol. Biochemistry. 1996;35:13011–13020. doi: 10.1021/bi960734h. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Zhang X, Kusumo H, Costa LG, Guizzetti M. Cholesterol efflux is differentially regulated in neurons and astrocytes: implications for brain cholesterol homeostasis. Biochim Biophys Acta. 2013;1831:263–275. doi: 10.1016/j.bbalip.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu CA, Tsujita M, Hayashi M, Yokoyama S. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem. 2004;279:30168–30174. doi: 10.1074/jbc.M403765200. [DOI] [PubMed] [Google Scholar]
- 34.Ontsouka CE, Huang X, Aliyev E, Albrecht C. In vitro characterization and endocrine regulation of cholesterol and phospholipid transport in the mammary gland. Mol Cell Endocrinol. 2017;439:35–45. doi: 10.1016/j.mce.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Nagao K, Maeda M, Manucat NB, Ueda K. Cyclosporine A and PSC833 inhibit ABCA1 function via direct binding. Biochim Biophys Acta. 2013;1831:398–406. doi: 10.1016/j.bbalip.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Smith JD, Miyata M, Ginsberg M, Grigaux C, Shmookler E, Plump AS. Cyclic AMP induces apolipoprotein E binding activity and promotes cholesterol efflux from a macrophage cell line to apolipoprotein acceptors. J Biol Chem. 1996;271:30647–30655. doi: 10.1074/jbc.271.48.30647. [DOI] [PubMed] [Google Scholar]
- 37.Guizzetti M, Chen J, Oram JF, Tsuji R, Dao K, Moller T, Costa LG. Ethanol induces cholesterol efflux and up-regulates ATP-binding cassette cholesterol transporters in fetal astrocytes. J Biol Chem. 2007;282:18740–18749. doi: 10.1074/jbc.M702398200. [DOI] [PubMed] [Google Scholar]
- 38.Kemmerer M, Wittig I, Richter F, Brune B, Namgaladze D. AMPK activates LXRalpha and ABCA1 expression in human macrophages. Int J Biochem Cell Biol. 2016;78:1–9. doi: 10.1016/j.biocel.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Lee JY, Karwatsky J, Ma L, Zha X. ABCA1 increases extracellular ATP to mediate cholesterol efflux to ApoA-I. Am J Physiol Cell Physiol. 2011;301:C886–C894. doi: 10.1152/ajpcell.00042.2011. [DOI] [PubMed] [Google Scholar]
- 40.Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L869–L878. doi: 10.1152/ajplung.00077.2003. [DOI] [PubMed] [Google Scholar]
- 41.Li D, Wang D, Wang Y, Ling W, Feng X, Xia M. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 2010;285:33499–33509. doi: 10.1074/jbc.M110.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma L, Dong F, Denis M, Feng Y, Wang MD, Zha X. Ht31, a protein kinase A anchoring inhibitor, induces robust cholesterol efflux and reverses macrophage foam cell formation through ATP-binding cassette transporter A1. J Biol Chem. 2011;286:3370–3378. doi: 10.1074/jbc.M110.173666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang B, Wang X, Yan F, Bian YF, Liu M, Bai R, Yang HY, Zhang NN, Yang ZM, Xiao CS. Angiotensin-(1–7) upregulates (ATP-binding cassette transporter A1) ABCA1 expression through cyclic AMP signaling pathway in RAW 264.7 macrophages. Eur Rev Med Pharmacol Sci. 2014;18:985–991. [PubMed] [Google Scholar]
- 44.Karwatsky J, Ma L, Dong F, Zha X. Cholesterol efflux to apoA-I in ABCA1-expressing cells is regulated by Ca2+-dependent calcineurin signaling. J Lipid Res. 2010;51:1144–1156. doi: 10.1194/jlr.M003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, Moore KJ, Perisic L, Maegdefessel L, Hedin U, Harper ME, Rayner KJ. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ Res. 2015;117:266–278. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Costa LG, Guizzetti M. Retinoic acid isomers up-regulate ATP binding cassette A1 and G1 and cholesterol efflux in rat astrocytes: implications for their therapeutic and teratogenic effects. J Pharmacol Exp Ther. 2011;338:870–878. doi: 10.1124/jpet.111.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kammerer I, Ringseis R, Biemann R, Wen G, Eder K. 13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages. Lipids Health Dis. 2011;10:222. doi: 10.1186/1476-511X-10-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouimet M, Wang MD, Cadotte N, Ho K, Marcel YL. Epoxycholesterol impairs cholesteryl ester hydrolysis in macrophage foam cells, resulting in decreased cholesterol efflux. Arterioscler Thromb Vasc Biol. 2008;28:1144–1150. doi: 10.1161/ATVBAHA.107.157115. [DOI] [PubMed] [Google Scholar]
- 49.Uehara Y., Engel T., Li Z., Goepfert C., Rust S., Zhou X., Langer C., Schachtrup C., Wiekowski J., Lorkowski S., Assmann G., von Eckardstein A. Polyunsaturated Fatty Acids and Acetoacetate Downregulate the Expression of the ATP-Binding Cassette Transporter A1. Diabetes. 2002;51(10):2922–2928. doi: 10.2337/diabetes.51.10.2922. [DOI] [PubMed] [Google Scholar]
- 50.Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS, Fu YC, Tang CK. PAPP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRalpha through the IGF-I-mediated signaling pathway. Atherosclerosis. 2012;222:344–354. doi: 10.1016/j.atherosclerosis.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Sparrow CP, Baffic J, Lam MH, Lund EG, Adams AD, Fu X, Hayes N, Jones AB, Macnaul KL, Ondeyka J, Singh S, Wang J, Zhou G, Moller DE, Wright SD, Menke JG. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J Biol Chem. 2002;277:10021–10027. doi: 10.1074/jbc.M108225200. [DOI] [PubMed] [Google Scholar]
- 52.Favari E, Zimetti F, Bortnick AE, Adorni MP, Zanotti I, Canavesi M, Bernini F. Impaired ATP-binding cassette transporter A1-mediated sterol efflux from oxidized LDL-loaded macrophages. FEBS Lett. 2005;579:6537–6542. doi: 10.1016/j.febslet.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 53.Adorni MP, Cipollari E, Favari E, Zanotti I, Zimetti F, Corsini A, Ricci C, Bernini F, Ferri N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis. 2017;256:1–6. doi: 10.1016/j.atherosclerosis.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Murthy S, Born E, Mathur SN, Field FJ. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J Lipid Res. 2002;43:1054–1064. doi: 10.1194/jlr.M100358-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Bechor Sapir, Zolberg Relevy Noa, Harari Ayelet, Almog Tal, Kamari Yehuda, Ben-Amotz Ami, Harats Dror, Shaish Aviv. 9-cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients. 2016;8(7):435. doi: 10.3390/nu8070435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu R, Lv Y, Wang J, Pan N, Zhang R, Wang X, Yu H, Tan L, Zhao Y, Li B. Baicalin promotes cholesterol efflux by regulating the expression of SR-BI in macrophages. Exp Ther Med. 2016;12:4113–4120. doi: 10.3892/etm.2016.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Triolo M, Annema W, de Boer JF, Tietge UJ, Dullaart RP. Simvastatin and bezafibrate increase cholesterol efflux in men with type 2 diabetes. Eur J Clin Invest. 2014;44:240–248. doi: 10.1111/eci.12226. [DOI] [PubMed] [Google Scholar]
- 58.Li N, Wang X, Liu P, Lu D, Jiang W, Xu Y, Si S. E17110 promotes reverse cholesterol transport with liver X receptor beta agonist activity in vitro. Acta Pharm Sin B. 2016;6:198–204. doi: 10.1016/j.apsb.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoang MH, Jia Y, Jun HJ, Lee JH, Lee DH, Hwang BY, Kim WJ, Lee HJ, Lee SJ. Ethyl 2,4,6-trihydroxybenzoate is an agonistic ligand for liver X receptor that induces cholesterol efflux from macrophages without affecting lipid accumulation in HepG2 cells. Bioorg Med Chem Lett. 2012;22:4094–4099. doi: 10.1016/j.bmcl.2012.04.071. [DOI] [PubMed] [Google Scholar]
- 60.Hennuyer N, Duplan I, Paquet C, Vanhoutte J, Woitrain E, Touche V, Colin S, Vallez E, Lestavel S, Lefebvre P, Staels B. The novel selective PPARalpha modulator (SPPARMalpha) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis. 2016;249:200–208. doi: 10.1016/j.atherosclerosis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Chai JT, Digby JE, Ruparelia N, Jefferson A, Handa A, Choudhury RP. Nicotinic acid receptor GPR109A is down-regulated in human macrophage-derived foam cells. PLoS ONE. 2013;8:e62934. doi: 10.1371/journal.pone.0062934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yue J, Li B, Jing Q, Guan Q. Salvianolic acid B accelerated ABCA1-dependent cholesterol efflux by targeting PPAR-gamma and LXRalpha. Biochem Biophys Res Commun. 2015;462:233–238. doi: 10.1016/j.bbrc.2015.04.122. [DOI] [PubMed] [Google Scholar]
- 63.Wang YH, Chen YF, Chen SR, Chen X, Chen JW, Shen XY, Mou YG, Liu PQ. Aspirin increases apolipoprotein-A-I-mediated cholesterol efflux via enhancing expression of ATP-binding cassette transporter A1. Pharmacology. 2010;86:320–326. doi: 10.1159/000321727. [DOI] [PubMed] [Google Scholar]
- 64.Molteni V, Li X, Nabakka J, Liang F, Wityak J, Koder A, Vargas L, Romeo R, Mitro N, Mak PA, Seidel HM, Haslam JA, Chow D, Tuntland T, Spalding TA, Brock A, Bradley M, Castrillo A, Tontonoz P, Saez E. N-Acylthiadiazolines, a new class of liver X receptor agonists with selectivity for LXRbeta. J Med Chem. 2007;50:4255–4259. doi: 10.1021/jm070453f. [DOI] [PubMed] [Google Scholar]
- 65.Bocchetta Simone, Maillard Patrick, Yamamoto Mami, Gondeau Claire, Douam Florian, Lebreton Stéphanie, Lagaye Sylvie, Pol Stanislas, Helle François, Plengpanich Wanee, Guérin Maryse, Bourgine Maryline, Michel Marie Louise, Lavillette Dimitri, Roingeard Philippe, le Goff Wilfried, Budkowska Agata. Up-Regulation of the ATP-Binding Cassette Transporter A1 Inhibits Hepatitis C Virus Infection. PLoS ONE. 2014;9(3):e92140. doi: 10.1371/journal.pone.0092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceroi A, Masson D, Roggy A, Roumier C, Chague C, Gauthier T, Philippe L, Lamarthee B, Angelot-Delettre F, Bonnefoy F, Perruche S, Biichle S, Preudhomme C, Macintyre E, Lagrost L, Garnache-Ottou F, Saas P. LXR agonist treatment of blastic plasmacytoid dendritic cell neoplasm restores cholesterol efflux and triggers apoptosis. Blood. 2016;128:2694–2707. doi: 10.1182/blood-2016-06-724807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Grieger JA, Kris-Etherton PM, Thompson JT, Gillies PJ, Fleming JA, Vanden Heuvel JP. Walnut oil increases cholesterol efflux through inhibition of stearoyl CoA desaturase 1 in THP-1 macrophage-derived foam cells. Nutr Metab (Lond). 2011;8:61. doi: 10.1186/1743-7075-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Rotter S, Ladurner A, Heiss EH, Oberlies NH, Dirsch VM, Atanasov AG. Silymarin constituents enhance ABCA1 expression in THP-1 macrophages. Molecules. 2015;21:E55. doi: 10.3390/molecules21010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozasa H, Ayaori M, Iizuka M, Terao Y, Uto-Kondo H, Yakushiji E, Takiguchi S, Nakaya K, Hisada T, Uehara Y, Ogura M, Sasaki M, Komatsu T, Horii S, Mochizuki S, Yoshimura M, Ikewaki K. Pioglitazone enhances cholesterol efflux from macrophages by increasing ABCA1/ABCG1 expressions via PPARgamma/LXRalpha pathway: findings from in vitro and ex vivo studies. Atherosclerosis. 2011;219:141–150. doi: 10.1016/j.atherosclerosis.2011.07.113. [DOI] [PubMed] [Google Scholar]
- 70.Nakaya K, Ayaori M, Hisada T, Sawada S, Tanaka N, Iwamoto N, Ogura M, Yakushiji E, Kusuhara M, Nakamura H, Ohsuzu F. Telmisartan enhances cholesterol efflux from THP-1 macrophages by activating PPARgamma. J Atheroscler Thromb. 2007;14:133–141. doi: 10.5551/jat.14.133. [DOI] [PubMed] [Google Scholar]
- 71.Wang L, Palme V, Rotter S, Schilcher N, Cukaj M, Wang D, Ladurner A, Heiss EH, Stangl H, Dirsch VM, Atanasov AG. Piperine inhibits ABCA1 degradation and promotes cholesterol efflux from THP-1-derived macrophages. Mol Nutr Food Res. 2017;61:1500960. doi: 10.1002/mnfr.201500960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 73.Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, Li Y, Chi D, Yu X, Zhao T, Han P, Xia X, Ling W. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J Biol Chem. 2005;280:36792–36801. doi: 10.1074/jbc.M505047200. [DOI] [PubMed] [Google Scholar]
- 74.He D, Wang H, Xu L, Wang X, Peng K, Wang L, Liu P, Qu P. Saikosaponin-a attenuates oxidized LDL uptake and prompts cholesterol efflux in THP-1 cells. J Cardiovasc Pharmacol. 2016;67:510–518. doi: 10.1097/FJC.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 75.Wang Shuai, Zhang Xue, Liu Mingyue, Luan Hong, Ji Yubin, Guo Peng, Wu Chongming. Chrysin inhibits foam cell formation through promoting cholesterol efflux from RAW264.7 macrophages. Pharmaceutical Biology. 2015;53(10):1481–1487. doi: 10.3109/13880209.2014.986688. [DOI] [PubMed] [Google Scholar]
- 76.Cui H, Okuhira K, Ohoka N, Naito M, Kagechika H, Hirose A, Nishimaki-Mogami T. Tributyltin chloride induces ABCA1 expression and apolipoprotein A-I-mediated cellular cholesterol efflux by activating LXRalpha/RXR. Biochem Pharmacol. 2011;81:819–824. doi: 10.1016/j.bcp.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 77.Tamehiro N, Sato Y, Suzuki T, Hashimoto T, Asakawa Y, Yokoyama S, Kawanishi T, Ohno Y, Inoue K, Nagao T, Nishimaki-Mogami T. Riccardin C: a natural product that functions as a liver X receptor (LXR)alpha agonist and an LXRbeta antagonist. FEBS Lett. 2005;579:5299–5304. doi: 10.1016/j.febslet.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 78.Ma W, Ding H, Gong X, Liu Z, Lin Y, Zhang Z, Lin G. Methyl protodioscin increases ABCA1 expression and cholesterol efflux while inhibiting gene expressions for synthesis of cholesterol and triglycerides by suppressing SREBP transcription and microRNA 33a/b levels. Atherosclerosis. 2015;239:566–570. doi: 10.1016/j.atherosclerosis.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 79.Wagner BL, Valledor AF, Shao G, Daige CL, Bischoff ED, Petrowski M, Jepsen K, Baek SH, Heyman RA, Rosenfeld MG, Schulman IG, Glass CK. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol Cell Biol. 2003;23:5780–5789. doi: 10.1128/MCB.23.16.5780-5789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, Inaba T. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/S0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 81.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Katsube A, Hayashi H, Kusuhara H. Pim-1L protects cell surface-resident ABCA1 from lysosomal degradation in hepatocytes and thereby regulates plasma high-density lipoprotein level. Arterioscler Thromb Vasc Biol. 2016;36:2304–2314. doi: 10.1161/ATVBAHA.116.308472. [DOI] [PubMed] [Google Scholar]
- 83.Low H, Mukhamedova N, Cui HL, McSharry BP, Avdic S, Hoang A, Ditiatkovski M, Liu Y, Fu Y, Meikle PJ, Blomberg M, Polyzos KA, Miller WE, Religa P, Bukrinsky M, Soderberg-Naucler C, Slobedman B, Sviridov D. Cytomegalovirus restructures lipid rafts via a US28/CDC42-mediated pathway, enhancing cholesterol efflux from host cells. Cell Rep. 2016;16:186–200. doi: 10.1016/j.celrep.2016.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang M, Li X. Activation of PPARgamma does not contribute to macrophage ABCA1 expression and ABCA1-mediated cholesterol efflux to apoAI. Biochem Biophys Res Commun. 2017;482:849–856. doi: 10.1016/j.bbrc.2016.11.123. [DOI] [PubMed] [Google Scholar]
- 85.Liu XY, Lu Q, Ouyang XP, Tang SL, Zhao GJ, Lv YC, He PP, Kuang HJ, Tang YY, Fu Y, Zhang DW, Tang CK. Apelin-13 increases expression of ATP-binding cassette transporter A1 via activating protein kinase C alpha signaling in THP-1 macrophage-derived foam cells. Atherosclerosis. 2013;226:398–407. doi: 10.1016/j.atherosclerosis.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 86.Rousselle A, Qadri F, Leukel L, Yilmaz R, Fontaine JF, Sihn G, Bader M, Ahluwalia A, Duchene J. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123:1343–1347. doi: 10.1172/JCI66580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y, Wang Z, Zhou L. Interleukin 8 inhibition enhanced cholesterol efflux in acetylated low-density lipoprotein-stimulated THP-1 macrophages. J Investig Med. 2014;62:615–620. doi: 10.2310/JIM.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 88.Halvorsen B, Holm S, Yndestad A, Scholz H, Sagen EL, Nebb H, Holven KB, Dahl TB, Aukrust P. Interleukin-10 increases reverse cholesterol transport in macrophages through its bidirectional interaction with liver X receptor alpha. Biochem Biophys Res Commun. 2014;450:1525–1530. doi: 10.1016/j.bbrc.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 89.Yu XH, Jiang HL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Ouyang XP, Lv YC, Jiang ZS, Zhang DW, Tang CK. Interleukin-18 and interleukin-12 together downregulate ATP-binding cassette transporter A1 expression through the interleukin-18R/nuclear factor-kappaB signaling pathway in THP-1 macrophage-derived foam cells. Circ J. 2012;76:1780–1791. doi: 10.1253/circj.CJ-11-1338. [DOI] [PubMed] [Google Scholar]
- 90.Fu H, Tang YY, Ouyang XP, Tang SL, Su H, Li X, Huang LP, He M, Lv YC, He PP, Yao F, Tan YL, Xie W, Zhang M, Wu J, Li Y, Chen K, Liu D, Lan G, Zeng MY, Zheng XL, Tang CK. Interleukin-27 inhibits foam cell formation by promoting macrophage ABCA1 expression through JAK2/STAT3 pathway. Biochem Biophys Res Commun. 2014;452:881–887. doi: 10.1016/j.bbrc.2014.08.120. [DOI] [PubMed] [Google Scholar]
- 91.Panousis CG, Evans G, Zuckerman SH. TGF-beta increases cholesterol efflux and ABC-1 expression in macrophage-derived foam cells: opposing the effects of IFN-gamma. J Lipid Res. 2001;42:856–863. doi: 10.1016/S0022-2275(20)31648-5. [DOI] [PubMed] [Google Scholar]
- 92.Edgel KA, Leboeuf RC, Oram JF. Tumor necrosis factor-alpha and lymphotoxin-alpha increase macrophage ABCA1 by gene expression and protein stabilization via different receptors. Atherosclerosis. 2010;209:387–392. doi: 10.1016/j.atherosclerosis.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 93.Sun RL, Huang CX, Bao JL, Jiang JY, Zhang B, Zhou SX, Cai WB, Wang H, Wang JF, Zhang YL. CC-chemokine ligand 2 (CCL2) suppresses high density lipoprotein (HDL) internalization and cholesterol efflux via CC-chemokine receptor 2 (CCR93) induction and p42/44 mitogen-activated protein kinase (MAPK) activation in human endothelial cells. J Biol Chem. 2016;291:19532–19544. doi: 10.1074/jbc.M116.714279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boshuizen Marieke C.S., Hoeksema Marten A., Neele Annette E., van der Velden Saskia, Hamers Anouk A.J., Van den Bossche Jan, Lutgens Esther, de Winther Menno P.J. Interferon-β promotes macrophage foam cell formation by altering both cholesterol influx and efflux mechanisms. Cytokine. 2016;77:220–226. doi: 10.1016/j.cyto.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 95.Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008;48:770–781. doi: 10.1002/hep.22423. [DOI] [PubMed] [Google Scholar]
- 96.Mao M, Lei H, Liu Q, Chen Y, Zhao L, Li Q, Luo S, Zuo Z, He Q, Huang W, Zhang N, Zhou C, Ruan XZ. Effects of miR-33a-5P on ABCA1/G1-mediated cholesterol efflux under inflammatory stress in THP-1 macrophages. PLoS One. 2014;9:e109722. doi: 10.1371/journal.pone.0109722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang XQ, Panousis CG, Alfaro ML, Evans GF, Zuckerman SH. Interferon-gamma-mediated downregulation of cholesterol efflux and ABC1 expression is by the Stat1 pathway. Arterioscler Thromb Vasc Biol. 2002;22:e5–e9. doi: 10.1161/01.atv.0000018287.03856.dd. [DOI] [PubMed] [Google Scholar]
- 98.Hao XR, Cao DL, Hu YW, Li XX, Liu XH, Xiao J, Liao DF, Xiang J, Tang CK. IFN-gamma down-regulates ABCA1 expression by inhibiting LXRalpha in a JAK/STAT signaling pathway-dependent manner. Atherosclerosis. 2009;203:417–428. doi: 10.1016/j.atherosclerosis.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 99.McLaren JE, Calder CJ, McSharry BP, Sexton K, Salter RC, Singh NN, Wilkinson GW, Wang EC, Ramji DP. The TNF-like protein 1A-death receptor 3 pathway promotes macrophage foam cell formation in vitro. J Immunol. 2010;184:5827–5834. doi: 10.4049/jimmunol.0903782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, Molina-David J, Ge M, Lassenius MI, Forsblom C, Lehto M, Groop PH, Kretzler M, Eddy S, Martini S, Reich H, Wahl P, Ghiggeri G, Faul C, Burke GW, III, Kretz O, Huber TB, Mendez AJ, Merscher S, Fornoni A. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest. 2016;126:3336–3350. doi: 10.1172/JCI85939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin Yu-Ting, Jian Deng-Yuan, Kwok Ching-Fai, Ho Low-Tone, Juan Chi-Chang. VISFATIN PROMOTES FOAM CELL FORMATION BY DYSREGULATING CD36, SRA, ABCA1, AND ABCG1 EXPRESSION IN RAW264.7 MACROPHAGES. Shock. 2016;45(4):460–468. doi: 10.1097/SHK.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Liu Y, Zhu L, Wang W, Wan Z, Chen F, Wu Y, Zhou J, Yuan Z. 17beta-estradiol promotes cholesterol efflux from vascular smooth muscle cells through a liver X receptor alpha-dependent pathway. Int J Mol Med. 2014;33:550–558. doi: 10.3892/ijmm.2014.1619. [DOI] [PubMed] [Google Scholar]
- 103.Liang B, Wang X, Bian Y, Yang H, Liu M, Bai R, Yang Z, Xiao C. Angiotensin-(1-7) upregulates expression of adenosine triphosphate-binding cassette transporter A1 and adenosine triphosphate-binding cassette transporter G1 through the Mas receptor through the liver X receptor alpha signalling pathway in THP-1 macrophages treated with angiotensin-II. Clin Exp Pharmacol Physiol. 2014;41:1023–1030. doi: 10.1111/1440-1681.12312. [DOI] [PubMed] [Google Scholar]
- 104.Mostafa AM, Hamdy NM, El-Mesallamy HO, Abdel-Rahman SZ. Glucagon-like peptide 1 (GLP-1)-based therapy upregulates LXR-ABCA1/ABCG1 cascade in adipocytes. Biochem Biophys Res Commun. 2015;468:900–905. doi: 10.1016/j.bbrc.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 105.Shang W, Yu X, Wang H, Chen T, Fang Y, Yang X, Zhou P, Nie F, Zhou Q, Zhou J. Fibroblast growth factor 21 enhances cholesterol efflux in THP-1 macrophage-derived foam cells. Mol Med Rep. 2015;11:503–508. doi: 10.3892/mmr.2014.2731. [DOI] [PubMed] [Google Scholar]
- 106.Cheng B, Wan J, Wang Y, Mei C, Liu W, Ke L, He P. Ghrelin inhibits foam cell formation via simultaneously down-regulating the expression of acyl-coenzyme A:cholesterol acyltransferase 1 and up-regulating adenosine triphosphate-binding cassette transporter A1. Cardiovasc Pathol. 2010;19:e159–e166. doi: 10.1016/j.carpath.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 107.Wu JF, Wang Y, Zhang M, Tang YY, Wang B, He PP, Lv YC, Ouyang XP, Yao F, Tan YL, Tang SL, Tang DP, Cayabyab FS, Zheng XL, Zhang DW, Zeng GF, Tang CK. Growth differentiation factor-15 induces expression of ATP-binding cassette transporter A1 through PI3-K/PKCzeta/SP1 pathway in THP-1 macrophages. Biochem Biophys Res Commun. 2014;444:325–331. doi: 10.1016/j.bbrc.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 108.Lyu J, Imachi H, Iwama H, Zhang H, Murao K. Insulin-like growth factor 1 regulates the expression of ATP-binding cassette transporter A1 in pancreatic beta cells. Horm Metab Res. 2016;48:338–344. doi: 10.1055/s-0035-1569272. [DOI] [PubMed] [Google Scholar]
- 109.Mostafa AM, Hamdy NM, Abdel-Rahman SZ, El-Mesallamy HO. Effect of vildagliptin and pravastatin combination on cholesterol efflux in adipocytes. IUBMB Life. 2016;68:535–543. doi: 10.1002/iub.1510. [DOI] [PubMed] [Google Scholar]
- 110.Park YM, Kashyap R, Major A, Silverstein RL. Insulin promotes macrophage foam cell formation: potential implications in diabetes-related atherosclerosis. Lab Invest. 2012;92:1171–1180. doi: 10.1038/labinvest.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho W, Kang JL, Park YM. Corticotropin-releasing hormone (CRH) promotes macrophage foam cell formation via reduced expression of ATP binding cassette transporter-1 (ABCA1) PLoS One. 2015;10:e0130587. doi: 10.1371/journal.pone.0130587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ayaori M, Sawada S, Yonemura A, Iwamoto N, Ogura M, Tanaka N, Nakaya K, Kusuhara M, Nakamura H, Ohsuzu F. Glucocorticoid receptor regulates ATP-binding cassette transporter-A1 expression and apolipoprotein-mediated cholesterol efflux from macrophages. Arterioscler Thromb Vasc Biol. 2006;26:163–168. doi: 10.1161/01.ATV.0000193513.29074.52. [DOI] [PubMed] [Google Scholar]
- 113.Zhou X, Yin Z, Guo X, Hajjar DP, Han J. Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J Biol Chem. 2010;285:6316–6326. doi: 10.1074/jbc.M109.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greco D, Favari E, Adorni MP, Zimetti F, Gatti R, Bernini F, Ronda N. Hydrocortisone directly promotes cholesterol accumulation in macrophages. Ann Rheum Dis. 2014;73:1274–1276. doi: 10.1136/annrheumdis-2013-204806. [DOI] [PubMed] [Google Scholar]
- 115.Nonomura K, Arai Y, Mitani H, Abe-Dohmae S, Yokoyama S. Insulin down-regulates specific activity of ATP-binding cassette transporter A1 for high density lipoprotein biogenesis through its specific phosphorylation. Atherosclerosis. 2011;216:334–341. doi: 10.1016/j.atherosclerosis.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 116.Fernandez-Suarez ME, Escola-Gil JC, Pastor O, Davalos A, Blanco-Vaca F, Lasuncion MA, Martinez-Botas J, Gomez-Coronado D. Clinically used selective estrogen receptor modulators affect different steps of macrophage-specific reverse cholesterol transport. Sci Rep. 2016;6:32105. doi: 10.1038/srep32105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen SG, Xiao J, Liu XH, Liu MM, Mo ZC, Yin K, Zhao GJ, Jiang J, Cui LB, Tan CZ, Yin WD, Tang CK. Ibrolipim increases ABCA1/G1 expression by the LXRalpha signaling pathway in THP-1 macrophage-derived foam cells. Acta Pharmacol Sin. 2010;31:1343–1349. doi: 10.1038/aps.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sugimoto K, Tsujita M, Wu CA, Suzuki K, Yokoyama S. An inhibitor of acylCoA: cholesterol acyltransferase increases expression of ATP-binding cassette transporter A1 and thereby enhances the ApoA-I-mediated release of cholesterol from macrophages. Biochim Biophys Acta. 2004;1636:69–76. doi: 10.1016/j.bbalip.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 119.Tamehiro Norimasa, Zhou Suiping, Okuhira Keiichiro, Benita Yair, Brown Cari E., Zhuang Debbie Z., Latz Eicke, Hornemann Thorsten, von Eckardstein Arnold, Xavier Ramnik J., Freeman Mason W., Fitzgerald Michael L. SPTLC1 Binds ABCA1 To Negatively Regulate Trafficking and Cholesterol Efflux Activity of the Transporter†. Biochemistry. 2008;47(23):6138–6147. doi: 10.1021/bi800182t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Azuma Y, Kawasaki T, Ikemoto K, Ohno K, Yamada T, Yamasaki M, Nobuhara Y. Effects of NTE-122, a novel acyl-CoA:cholesterol acyltransferase inhibitor, on cholesterol esterification and high-density lipoprotein-induced cholesterol efflux in macrophages. Jpn J Pharmacol. 1999;79:159–167. doi: 10.1254/jjp.79.159. [DOI] [PubMed] [Google Scholar]
- 121.Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transporter A1 and enhances cholesterol efflux from cells. J Biol Chem. 2003;278:52379–52385. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- 122.Zanotti I, Favari E, Sposito AC, Rothblat GH, Bernini F. Pitavastatin increases ABCA1-mediated lipid efflux from Fu5AH rat hepatoma cells. Biochem Biophys Res Commun. 2004;321:670–674. doi: 10.1016/j.bbrc.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 123.Kawashima RL, Medh JD. Down-regulation of lipoprotein lipase increases ABCA1-mediated cholesterol efflux in THP-1 macrophages. Biochem Biophys Res Commun. 2014;450:1416–1421. doi: 10.1016/j.bbrc.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Niesor EJ, Schwartz GG, Perez A, Stauffer A, Durrwell A, Bucklar-Suchankova G, Benghozi R, Abt M, Kallend D. Statin-induced decrease in ATP-binding cassette transporter A1 expression via microRNA33 induction may counteract cholesterol efflux to high-density lipoprotein. Cardiovasc Drugs Ther. 2015;29:7–14. doi: 10.1007/s10557-015-6570-0. [DOI] [PubMed] [Google Scholar]
- 125.Wang W, Song W, Wang Y, Chen L, Yan X. HMG-CoA reductase inhibitors, simvastatin and atorvastatin, downregulate ABCG1-mediated cholesterol efflux in human macrophages. J Cardiovasc Pharmacol. 2013;62:90–98. doi: 10.1097/FJC.0b013e3182927e7c. [DOI] [PubMed] [Google Scholar]
- 126.Chen WM, Sheu WH, Tseng PC, Lee TS, Lee WJ, Chang PJ, Chiang AN. Modulation of microRNA expression in subjects with metabolic syndrome and decrease of cholesterol efflux from macrophages via microRNA-33-mediated attenuation of ATP-binding cassette transporter A1 expression by statins. PLoS One. 2016;11:e0154672. doi: 10.1371/journal.pone.0154672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis. 2008;196:180–189. doi: 10.1016/j.atherosclerosis.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 128.Zanotti I, Poti F, Favari E, Steffensen KR, Gustafsson JA, Bernini F. Pitavastatin effect on ATP binding cassette A1-mediated lipid efflux from macrophages: evidence for liver X receptor (LXR)-dependent and LXR-independent mechanisms of activation by cAMP. J Pharmacol Exp Ther. 2006;317:395–401. doi: 10.1124/jpet.105.093930. [DOI] [PubMed] [Google Scholar]
- 129.Bielicki JK, Johnson WJ, Weinberg RB, Glick JM, Rothblat GH. Efflux of lipid from fibroblasts to apolipoproteins: dependence on elevated levels of cellular unesterified cholesterol. J Lipid Res. 1992;33:1699–1709. doi: 10.1016/S0022-2275(20)41392-6. [DOI] [PubMed] [Google Scholar]
- 130.Karten B, Campenot RB, Vance DE, Vance JE. Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J Biol Chem. 2006;281:4049–4057. doi: 10.1074/jbc.M508915200. [DOI] [PubMed] [Google Scholar]
- 131.Rios-Marco P, Jimenez-Lopez JM, Marco C, Segovia JL, Carrasco MP. Antitumoral alkylphospholipids induce cholesterol efflux from the plasma membrane in HepG2 cells. J Pharmacol Exp Ther. 2011;336:866–873. doi: 10.1124/jpet.110.172890. [DOI] [PubMed] [Google Scholar]
- 132.Feng Bo, Tabas Ira. ABCA1-mediated Cholesterol Efflux Is Defective in Free Cholesterol-loaded Macrophages. Journal of Biological Chemistry. 2002;277(45):43271–43280. doi: 10.1074/jbc.M207532200. [DOI] [PubMed] [Google Scholar]
- 133.Luquain-Costaz C, Lefai E, Arnal-Levron M, Markina D, Sakai S, Euthine V, Makino A, Guichardant M, Yamashita S, Kobayashi T, Lagarde M, Moulin P, Delton-Vandenbroucke I. Bis(monoacylglycero)phosphate accumulation in macrophages induces intracellular cholesterol redistribution, attenuates liver-X receptor/ATP-Binding cassette transporter A1/ATP-binding cassette transporter G1 pathway, and impairs cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33:1803–1811. doi: 10.1161/ATVBAHA.113.301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rios-Marco P, Marco C, Cueto FJ, Carrasco MP, Jimenez-Lopez JM. Pleiotropic effects of antitumour alkylphospholipids on cholesterol transport and metabolism. Exp Cell Res. 2016;340:81–90. doi: 10.1016/j.yexcr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 135.Hu YW, Ma X, Li XX, Liu XH, Xiao J, Mo ZC, Xiang J, Liao DF, Tang CK. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis. 2009;204:e35–e43. doi: 10.1016/j.atherosclerosis.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 136.Spartano NL, Lamon-Fava S, Matthan NR, Obin MS, Greenberg AS, Lichtenstein AH. Linoleic acid suppresses cholesterol efflux and ATP-binding cassette transporters in murine bone marrow-derived macrophages. Lipids. 2014;49:415–422. doi: 10.1007/s11745-014-3890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanter JE, Tang C, Oram JF, Bornfeldt KE. Acyl-CoA synthetase 1 is required for oleate and linoleate mediated inhibition of cholesterol efflux through ATP-binding cassette transporter A1 in macrophages. Biochim Biophys Acta. 2012;1821:358–364. doi: 10.1016/j.bbalip.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dong F, Mo Z, Eid W, Courtney KC, Zha X. Akt inhibition promotes ABCA1-mediated cholesterol efflux to ApoA-I through suppressing mTORC1. PLoS One. 2014;9:e113789. doi: 10.1371/journal.pone.0113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gulshan K, Brubaker G, Conger H, Wang S, Zhang R, Hazen SL, Smith JD. PI(4,5)P2 is translocated by ABCA1 to the cell surface where it mediates apolipoprotein A1 binding and nascent HDL assembly. Circ Res. 2016;119:827–838. doi: 10.1161/CIRCRESAHA.116.308856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Witting SR, Maiorano JN, Davidson WS. Ceramide enhances cholesterol efflux to apolipoprotein A-I by increasing the cell surface presence of ATP-binding cassette transporter A1. J Biol Chem. 2003;278:40121–40127. doi: 10.1074/jbc.M305193200. [DOI] [PubMed] [Google Scholar]
- 141.Haidar B, Kiss RS, Sarov-Blat L, Brunet R, Harder C, McPherson R, Marcel YL. Cathepsin D, a lysosomal protease, regulates ABCA1-mediated lipid efflux. J Biol Chem. 2006;281:39971–39981. doi: 10.1074/jbc.M605095200. [DOI] [PubMed] [Google Scholar]
- 142.Zhang L, Chen Y, Yang X, Yang J, Cao X, Li X, Li L, Miao QR, Hajjar DP, Duan Y, Han J. MEK1/2 inhibitors activate macrophage ABCG1 expression and reverse cholesterol transport-An anti-atherogenic function of ERK1/2 inhibition. Biochim Biophys Acta. 2016;1861:1180–1191. doi: 10.1016/j.bbalip.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 143.Luo T, Hu J, Xi D, Xiong H, He W, Liu J, Li M, Lu H, Zhao J, Lai W, Guo Z. Lck inhibits heat shock protein 65-mediated reverse cholesterol transport in T cells. J Immunol. 2016;197:3861–3870. doi: 10.4049/jimmunol.1502710. [DOI] [PubMed] [Google Scholar]
- 144.Campia I, Sala V, Kopecka J, Leo C, Mitro N, Costamagna C, Caruso D, Pescarmona G, Crepaldi T, Ghigo D, Bosia A, Riganti C. Digoxin and ouabain induce the efflux of cholesterol via liver X receptor signalling and the synthesis of ATP in cardiomyocytes. Biochem J. 2012;447:301–311. doi: 10.1042/BJ20120200. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Qian, Ma A Zhi Sha, Song Zhi Yuan, Wang Chan, Fu Xiao Dan. Nifedipine Enhances Cholesterol Efflux in RAW264.7 Macrophages. Cardiovascular Drugs and Therapy. 2013;27(5):425–431. doi: 10.1007/s10557-013-6472-y. [DOI] [PubMed] [Google Scholar]
- 146.Tang CK, Tang GH, Yi GH, Wang Z, Liu LS, Wan S, Yuan ZH, He XS, Yang JH, Ruan CG, Yang YZ. Effect of apolipoprotein A-I on ATP binding cassette transporter A1 degradation and cholesterol efflux in THP-1 macrophage-derived foam cells. Acta Biochim Biophys Sin (Shanghai) 2004;36:218–226. doi: 10.1093/abbs/36.3.218. [DOI] [PubMed] [Google Scholar]
- 147.Ogura M, Ayaori M, Terao Y, Hisada T, Iizuka M, Takiguchi S, Uto-Kondo H, Yakushiji E, Nakaya K, Sasaki M, Komatsu T, Ozasa H, Ohsuzu F, Ikewaki K. Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1980–1987. doi: 10.1161/ATVBAHA.111.228478. [DOI] [PubMed] [Google Scholar]
- 148.Mendez AJ. Monensin and brefeldin A inhibit high density lipoprotein-mediated cholesterol efflux from cholesterol-enriched cells. Implications for intracellular cholesterol transport. J Biol Chem. 1995;270:5891–900. [DOI] [PubMed]
- 149.Fielding CJ, Moser K. Evidence for the separation of albumin- and apo A-I-dependent mechanisms of cholesterol efflux from cultured fibroblasts into human plasma. J Biol Chem. 1982;257:10955–10960. doi: 10.1016/S0021-9258(18)33916-4. [DOI] [PubMed] [Google Scholar]
- 150.Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem. 2004;279:14140–14146. doi: 10.1074/jbc.M311061200. [DOI] [PubMed] [Google Scholar]
- 151.Taylor JM, Allen AM, Graham A. Targeting mitochondrial 18 kDa translocator protein (TSPO) regulates macrophage cholesterol efflux and lipid phenotype. Clin Sci (Lond). 2014;127:603–613. doi: 10.1042/CS20140047. [DOI] [PubMed] [Google Scholar]
- 152.Khan OM, Akula MK, Skalen K, Karlsson C, Stahlman M, Young SG, Boren J, Bergo MO. Targeting GGTase-I activates RHOA, increases macrophage reverse cholesterol transport, and reduces atherosclerosis in mice. Circulation. 2013;127:782–790. doi: 10.1161/CIRCULATIONAHA.112.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang L, Jiang M, Shui Y, Chen Y, Wang Q, Hu W, Ma X, Li X, Liu X, Cao X, Liu M, Duan Y, Han J. DNA topoisomerase II inhibitors induce macrophage ABCA1 expression and cholesterol efflux-an LXR-dependent mechanism. Biochim Biophys Acta. 2013;1831:1134–1145. doi: 10.1016/j.bbalip.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 154.Tsunemi A, Ueno T, Fukuda N, Watanabe T, Tahira K, Haketa A, Hatanaka Y, Tanaka S, Matsumoto T, Matsumoto Y, Nagase H, Soma M. A novel gene regulator, pyrrole-imidazole polyamide targeting ABCA1 gene increases cholesterol efflux from macrophages and plasma HDL concentration. J Mol Med (Berl). 2014;92:509–521. doi: 10.1007/s00109-013-1118-x. [DOI] [PubMed] [Google Scholar]
- 155.Zhao Y, Chen X, Yang H, Zhou L, Okoro EU, Guo Z. A novel function of apolipoprotein E: upregulation of ATP-binding cassette transporter A1 expression. PLoS One. 2011;6:e21453. doi: 10.1371/journal.pone.0021453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bujold K, Rhainds D, Jossart C, Febbraio M, Marleau S, Ong H. CD36-mediated cholesterol efflux is associated with PPARgamma activation via a MAPK-dependent COX-2 pathway in macrophages. Cardiovasc Res. 2009;83:457–464. doi: 10.1093/cvr/cvp118. [DOI] [PubMed] [Google Scholar]
- 157.Rana M, Kumar A, Tiwari RL, Singh V, Chandra T, Dikshit M, Barthwal MK. IRAK regulates macrophage foam cell formation by modulating genes involved in cholesterol uptake and efflux. BioEssays. 2016;38:591–604. doi: 10.1002/bies.201600085. [DOI] [PubMed] [Google Scholar]
- 158.Hong YF, Kim H, Kim HS, Park WJ, Kim JY, Chung DK. Lactobacillus acidophilus K301 inhibits atherogenesis via induction of 24 (S), 25-epoxycholesterol-mediated ABCA1 and ABCG1 production and cholesterol efflux in macrophages. PLoS One. 2016;11:e0154302. doi: 10.1371/journal.pone.0154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Berrougui H, Loued S, Khalil A. Purified human paraoxonase-1 interacts with plasma membrane lipid rafts and mediates cholesterol efflux from macrophages. Free Radic Biol Med. 2012;52:1372–1381. doi: 10.1016/j.freeradbiomed.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 160.Cheng TJ, Lin SW, Chen CW, Guo HR, Wang YJ. Arsenic trioxide suppresses liver X receptor beta and enhances cholesteryl ester transfer protein expression without affecting the liver X receptor alpha in HepG2 cells. Chem Biol Interact. 2016;258:288–296. doi: 10.1016/j.cbi.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 161.Voloshyna I, Kasselman LJ, Carsons SE, Littlefield MJ, Gomolin IH, De LJ, Reiss AB. COX-2-dependent and independent effects of COX-2 inhibitors and NSAIDs on proatherogenic changes in human monocytes/macrophages. J Investig Med. 2017;65:694–704. doi: 10.1136/jim-2016-000259. [DOI] [PubMed] [Google Scholar]
- 162.Zhao GJ, Mo ZC, Tang SL, Ouyang XP, He PP, Lv YC, Yao F, Tan YL, Xie W, Shi JF, Wang Y, Zhang M, Liu D, Tang DP, Zheng XL, Tian GP, Tang CK. Chlamydia pneumoniae negatively regulates ABCA1 expression via TLR2-Nuclear factor-kappa B and miR-33 pathways in THP-1 macrophage-derived foam cells. Atherosclerosis. 2014;235:519–525. doi: 10.1016/j.atherosclerosis.2014.05.943. [DOI] [PubMed] [Google Scholar]
- 163.Wang X, Liao D, Bharadwaj U, Li M, Yao Q, Chen C. C-reactive protein inhibits cholesterol efflux from human macrophage-derived foam cells. Arterioscler Thromb Vasc Biol. 2008;28:519–526. doi: 10.1161/ATVBAHA.107.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Clement M, Basatemur G, Masters L, Baker L, Bruneval P, Iwawaki T, Kneilling M, Yamasaki S, Goodall J, Mallat Z. Necrotic cell sensor Clec4e promotes a proatherogenic macrophage phenotype through activation of the unfolded protein response. Circulation. 2016;134:1039–1051. doi: 10.1161/CIRCULATIONAHA.116.022668. [DOI] [PubMed] [Google Scholar]
- 165.Jones RJ, Gu D, Bjorklund CC, Kuiatse I, Remaley AT, Bashir T, Vreys V, Orlowski RZ. The novel anticancer agent JNJ-26854165 induces cell death through inhibition of cholesterol transport and degradation of ABCA1. J Pharmacol Exp Ther. 2013;346:381–392. doi: 10.1124/jpet.113.204958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee-Rueckert M, Lappalainen J, Leinonen H, Pihlajamaa T, Jauhiainen M, Kovanen PT. Acidic extracellular environments strongly impair ABCA1-mediated cholesterol efflux from human macrophage foam cells. Arterioscler Thromb Vasc Biol. 2010;30:1766–1772. doi: 10.1161/ATVBAHA.110.211276. [DOI] [PubMed] [Google Scholar]
- 167.Ha JS, Ha CE, Chao JT, Petersen CE, Theriault A, Bhagavan NV. Human serum albumin and its structural variants mediate cholesterol efflux from cultured endothelial cells. Biochim Biophys Acta. 2003;1640:119–128. doi: 10.1016/S0167-4889(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 168.Wang MD, Franklin V, Sundaram M, Kiss RS, Ho K, Gallant M, Marcel YL. Differential regulation of ATP binding cassette protein A1 expression and ApoA-I lipidation by Niemann-Pick type C1 in murine hepatocytes and macrophages. J Biol Chem. 2007;282:22525–22533. doi: 10.1074/jbc.M700326200. [DOI] [PubMed] [Google Scholar]
- 169.Zhao GJ, Tang SL, Lv YC, Ouyang XP, He PP, Yao F, Chen WJ, Lu Q, Tang YY, Zhang M, Fu Y, Zhang DW, Yin K, Tang CK. Antagonism of betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol efflux through inhibiting nuclear factor-kappaB signaling pathway and miR-33 expression. PLoS One. 2013;8:e74782. doi: 10.1371/journal.pone.0074782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang X, Mu H, Chai H, Liao D, Yao Q, Chen C. Human immunodeficiency virus protease inhibitor ritonavir inhibits cholesterol efflux from human macrophage-derived foam cells. Am J Pathol. 2007;171:304–314. doi: 10.2353/ajpath.2007.060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Y, Wu JF, Tang YY, Zhang M, Li Y, Chen K, Zeng MY, Yao F, Xie W, Zheng XL, Zeng GF, Tang CK. Urotensin II increases foam cell formation by repressing ABCA1 expression through the ERK/NF-kappaB pathway in THP-1 macrophages. Biochem Biophys Res Commun. 2014;452:998–1003. doi: 10.1016/j.bbrc.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 172.Rosenblat Mira, Rom Oren, Volkova Nina, Aviram Michael. Nitro-Oleic Acid Reduces J774A.1 Macrophage Oxidative Status and Triglyceride Mass: Involvement of Paraoxonase2 and Triglyceride Metabolizing Enzymes. Lipids. 2016;51(8):941–953. doi: 10.1007/s11745-016-4169-2. [DOI] [PubMed] [Google Scholar]
- 173.Yin K, You Y, Swier V, Tang L, Radwan MM, Pandya AN, Agrawal DK. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 2015;35:2432–2442. doi: 10.1161/ATVBAHA.115.306132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Uto-Kondo H, Ayaori M, Nakaya K, Takiguchi S, Yakushiji E, Ogura M, Terao Y, Ozasa H, Sasaki M, Komatsu T, Sotherden GM, Hosoai T, Sakurada M, Ikewaki K. Citrulline increases cholesterol efflux from macrophages in vitro and ex vivo via ATP-binding cassette transporters. J Clin Biochem Nutr. 2014;55:32–39. doi: 10.3164/jcbn.13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yan X, Shen T, Jiang X, Tang X, Wang D, Li H, Ling W. Coenzyme Q10 consumption promotes ABCG1-mediated macrophage cholesterol efflux: a randomized, double-blind, placebo-controlled, cross-over study in healthy volunteers. Mol Nutr Food Res. 2015;59:1725–1734. doi: 10.1002/mnfr.201500186. [DOI] [PubMed] [Google Scholar]
- 176.Wang D, Yan X, Xia M, Yang Y, Li D, Li X, Song F, Ling W. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler Thromb Vasc Biol. 2014;34:1860–1870. doi: 10.1161/ATVBAHA.113.302879. [DOI] [PubMed] [Google Scholar]
- 177.Polo MP, de Bravo MG, de Alaniz MJ. Effect of ethanol on cell growth and cholesterol metabolism in cultured Hep G2 cells. Biochem Cell Biol. 2003;81:379–386. doi: 10.1139/o03-066. [DOI] [PubMed] [Google Scholar]
- 178.Rosenblat M., Volkova N., Khatib S., Mahmood S., Vaya J., Aviram M. Reduced glutathione increases quercetin stimulatory effects on HDL- or apoA1-mediated cholesterol efflux from J774A.1 macrophages. Free Radical Research. 2014;48(12):1462–1472. doi: 10.3109/10715762.2014.963574. [DOI] [PubMed] [Google Scholar]
- 179.Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121:1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu ZH, Zhao SP. Niacin promotes cholesterol efflux through stimulation of the PPARgamma-LXRalpha-ABCA1 pathway in 3T3-L1 adipocytes. Pharmacology. 2009;84:282–287. doi: 10.1159/000242999. [DOI] [PubMed] [Google Scholar]
- 181.Michiels CF, Kurdi A, Timmermans JP, De Meyer GR, Martinet W. Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis. 2016;251:319–327. doi: 10.1016/j.atherosclerosis.2016.07.899. [DOI] [PubMed] [Google Scholar]
- 182.Gaus K, Dean RT, Kritharides L, Jessup W. Inhibition of cholesterol efflux by 7-ketocholesterol: comparison between cells, plasma membrane vesicles, and liposomes as cholesterol donors. Biochemistry. 2001;40:13002–13014. doi: 10.1021/bi010833h. [DOI] [PubMed] [Google Scholar]
- 183.Petrick L, Rosenblat M, Aviram M. In vitro effects of exogenous carbon monoxide on oxidative stress and lipid metabolism in macrophages. Toxicol Ind Health. 2016;32:1318–1323. doi: 10.1177/0748233714558084. [DOI] [PubMed] [Google Scholar]
- 184.Yin QH, Zhang R, Li L, Wang YT, Liu JP, Zhang J, Bai L, Cheng JQ, Fu P, Liu F. Exendin-4 ameliorates lipotoxicity-induced glomerular endothelial cell injury by improving ABC transporter A1-mediated cholesterol efflux in diabetic apoE knockout mice. J Biol Chem. 2016;291:26487–26501. doi: 10.1074/jbc.M116.730564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yan JQ, Tan CZ, Wu JH, Zhang DC, Chen JL, Zeng BY, Jiang YP, Nie J, Liu W, Liu Q, Dai H. Neopterin negatively regulates expression of ABCA1 and ABCG1 by the LXRalpha signaling pathway in THP-1 macrophage-derived foam cells. Mol Cell Biochem. 2013;379:123–131. doi: 10.1007/s11010-013-1634-6. [DOI] [PubMed] [Google Scholar]
- 186.Jiang Z, Sang H, Fu X, Liang Y, Li L. Alpinetin enhances cholesterol efflux and inhibits lipid accumulation in oxidized low-density lipoprotein-loaded human macrophages. Biotechnol Appl Biochem. 2015;62:840–847. doi: 10.1002/bab.1328. [DOI] [PubMed] [Google Scholar]
- 187.Xu X, Li Q, Pang L, Huang G, Huang J, Shi M, Sun X, Wang Y. Arctigenin promotes cholesterol efflux from THP-1 macrophages through PPAR-gamma/LXR-alpha signaling pathway. Biochem Biophys Res Commun. 2013;441:321–326. doi: 10.1016/j.bbrc.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 188.Park SH, Paek JH, Shin D, Lee JY, Lim SS, Kang YH. Purple perilla extracts with alpha-asarone enhance cholesterol efflux from oxidized LDL-exposed macrophages. Int J Mol Med. 2015;35:957–965. doi: 10.3892/ijmm.2015.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iizuka M, Ayaori M, Uto-Kondo H, Yakushiji E, Takiguchi S, Nakaya K, Hisada T, Sasaki M, Komatsu T, Yogo M, Kishimoto Y, Kondo K, Ikewaki K. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J Nutr Sci Vitaminol (Tokyo). 2012;58:96–104. doi: 10.3177/jnsv.58.96. [DOI] [PubMed] [Google Scholar]
- 190.Li Y, Feng T, Liu P, Liu C, Wang X, Li D, Li N, Chen M, Xu Y, Si S. Optimization of rutaecarpine as ABCA1 up-regulator for treating atherosclerosis. ACS Med Chem Lett. 2014;5:884–888. doi: 10.1021/ml500131a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gui YZ, Yan H, Gao F, Xi C, Li HH, Wang YP. Betulin attenuates atherosclerosis in apoE−/− mice by up-regulating ABCA1 and ABCG1. Acta Pharmacol Sin. 2016;37:1337–1348. doi: 10.1038/aps.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hu YW, Ma X, Huang JL, Mao XR, Yang JY, Zhao JY, Li SF, Qiu YR, Yang J, Zheng L, Wang Q. Dihydrocapsaicin attenuates plaque formation through a PPARgamma/LXRalpha pathway in apoE(−/−) mice fed a high-fat/high-cholesterol diet. PLoS One. 2013;8:e66876. doi: 10.1371/journal.pone.0066876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dong SZ, Zhao SP, Wu ZH, Yang J, Xie XZ, Yu BL, Nie S. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol Cell Biochem. 2011;358:281–285. doi: 10.1007/s11010-011-0978-z. [DOI] [PubMed] [Google Scholar]
- 194.Xu Y, Xu Y, Bao Y, Hong B, Si S. Identification of dehydroxytrichostatin A as a novel up-regulator of the ATP-binding cassette transporter A1 (ABCA1) Molecules. 2011;16:7183–7198. doi: 10.3390/molecules16097183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lv YC, Yang J, Yao F, Xie W, Tang YY, Ouyang XP, He PP, Tan YL, Li L, Zhang M, Liu D, Cayabyab FS, Zheng XL, Tang CK. Diosgenin inhibits atherosclerosis via suppressing the MiR-19b-induced downregulation of ATP-binding cassette transporter A1. Atherosclerosis. 2015;240:80–89. doi: 10.1016/j.atherosclerosis.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 196.Fu X, Xu AG, Yao MY, Guo L, Zhao LS. Emodin enhances cholesterol efflux by activating peroxisome proliferator-activated receptor-gamma in oxidized low density lipoprotein-loaded THP1 macrophages. Clin Exp Pharmacol Physiol. 2014;41:679–684. doi: 10.1111/1440-1681.12262. [DOI] [PubMed] [Google Scholar]
- 197.Iio A, Ohguchi K, Maruyama H, Tazawa S, Araki Y, Ichihara K, Nozawa Y, Ito M. Ethanolic extracts of Brazilian red propolis increase ABCA1 expression and promote cholesterol efflux from THP-1 macrophages. Phytomedicine. 2012;19:383–388. doi: 10.1016/j.phymed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 198.Iio A, Ohguchi K, Iinuma M, Nozawa Y, Ito M. Hesperetin upregulates ABCA1 expression and promotes cholesterol efflux from THP-1 macrophages. J Nat Prod. 2012;75:563–566. doi: 10.1021/np200696r. [DOI] [PubMed] [Google Scholar]
- 199.Wang Limei, Ladurner Angela, Latkolik Simone, Schwaiger Stefan, Linder Thomas, Hošek Jan, Palme Veronika, Schilcher Nicole, Polanský Ondřej, Heiss Elke H., Stangl Herbert, Mihovilovic Marko D., Stuppner Hermann, Dirsch Verena M., Atanasov Atanas G. Leoligin, the Major Lignan from Edelweiss (Leontopodium nivale subsp. alpinum), Promotes Cholesterol Efflux from THP-1 Macrophages. Journal of Natural Products. 2016;79(6):1651–1657. doi: 10.1021/acs.jnatprod.6b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Berrougui H, Isabelle M, Cherki M, Khalil A. Marrubium vulgare extract inhibits human-LDL oxidation and enhances HDL-mediated cholesterol efflux in THP-1 macrophage. Life Sci. 2006;80:105–112. doi: 10.1016/j.lfs.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 201.Gui Y, Yao S, Yan H, Hu L, Yu C, Gao F, Xi C, Li H, Ye Y, Wang Y. A novel small molecule liver X receptor transcriptional regulator, nagilactone B, suppresses atherosclerosis in apoE-deficient mice. Cardiovasc Res. 2016;112:502–514. doi: 10.1093/cvr/cvw183. [DOI] [PubMed] [Google Scholar]
- 202.Zhao JF, Jim Leu SJ, Shyue SK, Su KH, Wei J, Lee TS. Novel effect of paeonol on the formation of foam cells: promotion of LXRalpha-ABCA1-dependent cholesterol efflux in macrophages. Am J Chin Med. 2013;41:1079–1096. doi: 10.1142/S0192415X13500730. [DOI] [PubMed] [Google Scholar]
- 203.Li XH, Li Y, Cheng ZY, Cai XG, Wang HM. The effects of phellinus linteus polysaccharide extracts on cholesterol efflux in oxidized low-density lipoprotein-loaded THP-1 macrophages. J Investig Med. 2015;63:752–757. doi: 10.1097/JIM.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 204.Zhao Shengjuan, Li Jianke, Wang Lifang, Wu Xiaoxia. Pomegranate peel polyphenols inhibit lipid accumulation and enhance cholesterol efflux in raw264.7 macrophages. Food & Function. 2016;7(7):3201–3210. doi: 10.1039/C6FO00347H. [DOI] [PubMed] [Google Scholar]
- 205.Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- 206.Xu Y, Liu Q, Xu Y, Liu C, Wang X, He X, Zhu N, Liu J, Wu Y, Li Y, Li N, Feng T, Lai F, Zhang M, Hong B, Jiang JD, Si S. Rutaecarpine suppresses atherosclerosis in ApoE−/− mice through upregulating ABCA1 and SR-BI within RCT. J Lipid Res. 2014;55:1634–1647. doi: 10.1194/jlr.M044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sun L, Li E, Wang F, Wang T, Qin Z, Niu S, Qiu C. Quercetin increases macrophage cholesterol efflux to inhibit foam cell formation through activating PPARgamma-ABCA1 pathway. Int J Clin Exp Pathol. 2015;8:10854–10860. [PMC free article] [PubMed] [Google Scholar]
- 208.Tian H, Liu Q, Qin S, Zong C, Zhang Y, Yao S, Yang N, Guan T, Guo S. Synthesis and cardiovascular protective efects of quercetin 7-O-sialic acid. J Cell Mol Med. 2017;21:107–120. doi: 10.1111/jcmm.12943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 209.Voloshyna I, Teboul I, Littlefield MJ, Siegart NM, Turi GK, Fazzari MJ, Carsons SE, DeLeon J, Reiss AB. Resveratrol counters systemic lupus erythematosus-associated atherogenicity by normalizing cholesterol efflux. Exp Biol Med (Maywood). 2016;241:1611–1619. doi: 10.1177/1535370216647181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park SH, Kim JL, Kang MK, Gong JH, Han SY, Shim JH, Lim SS, Kang YH. Sage weed (Salvia plebeia) extract antagonizes foam cell formation and promotes cholesterol efflux in murine macrophages. Int J Mol Med. 2012;30:1105–1112. doi: 10.3892/ijmm.2012.1103. [DOI] [PubMed] [Google Scholar]
- 211.Majdalawieh AF, Ro HS. Sesamol and sesame (Sesamum indicum) oil enhance macrophage cholesterol efflux via up-regulation of PPARgamma1 and LXRalpha transcriptional activity in a MAPK-dependent manner. Eur J Nutr. 2015;54:691–700. doi: 10.1007/s00394-014-0747-3. [DOI] [PubMed] [Google Scholar]
- 212.Liu Nan, Wu Chongming, Sun Lizhong, Zheng Jun, Guo Peng. Sesamin Enhances Cholesterol Efflux in RAW264.7 Macrophages. Molecules. 2014;19(6):7516–7527. doi: 10.3390/molecules19067516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu Z, Wang J, Huang E, Gao S, Li H, Lu J, Tian K, Little PJ, Shen X, Xu S, Liu P. Tanshinone IIA suppresses cholesterol accumulation in human macrophages: role of heme oxygenase-1. J Lipid Res. 2014;55:201–213. doi: 10.1194/jlr.M040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Berrougui Hicham, Cloutier Martin, Isabelle Maxim, Khalil Abdelouahed. Phenolic-extract from argan oil (Argania spinosa L.) inhibits human low-density lipoprotein (LDL) oxidation and enhances cholesterol efflux from human THP-1 macrophages. Atherosclerosis. 2006;184(2):389–396. doi: 10.1016/j.atherosclerosis.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 215.Chen CY, Shyue SK, Ching LC, Su KH, Wu YL, Kou YR, Chiang AN, Pan CC, Lee TS. Wogonin promotes cholesterol efflux by increasing protein phosphatase 2B-dependent dephosphorylation at ATP-binding cassette transporter-A1 in macrophages. J Nutr Biochem. 2011;22:1015–1021. doi: 10.1016/j.jnutbio.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 216.Zhu S, Liu JH. Zerumbone, a natural cyclic sesquiterpene, promotes ABCA1-dependent cholesterol efflux from human THP-1 macrophages. Pharmacology. 2015;95:258–263. doi: 10.1159/000381722. [DOI] [PubMed] [Google Scholar]
- 217.Rom O, Aviram M. Paraoxsonase2 (PON2) and oxidative stress involvement in pomegranate juice protection against cigarette smoke-induced macrophage cholesterol accumulation. Chem Biol Interact. 2016;259:394–400. doi: 10.1016/j.cbi.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 218.Zhang H, Li X, Qian Z. Regulation of macrophage cholesterol efflux and liver X receptor alpha activation by nicotine. Int J Clin Exp Med. 2015;8:16374–16378. [PMC free article] [PubMed] [Google Scholar]
- 219.McFarland AJ, Anoopkumar-Dukie S, Arora DS, Grant GD, McDermott CM, Perkins AV, Davey AK. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. 2014;15:20607–20637. doi: 10.3390/ijms151120607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hastings Janna, de Matos Paula, Dekker Adriano, Ennis Marcus, Harsha Bhavana, Kale Namrata, Muthukrishnan Venkatesh, Owen Gareth, Turner Steve, Williams Mark, Steinbeck Christoph. The ChEBI reference database and ontology for biologically relevant chemistry: enhancements for 2013. Nucleic Acids Research. 2012;41(D1):D456–D463. doi: 10.1093/nar/gks1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, Haw R, Jassal B, Korninger F, May B, Milacic M, Roca CD, Rothfels K, Sevilla C, Shamovsky V, Shorser S, Varusai T, Viteri G, Weiser J, Wu G, Stein L, Hermjakob H, D’Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2018;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tenenbaum A, Fisman EZ. Balanced pan-PPAR activator bezafibrate in combination with statin: comprehensive lipids control and diabetes prevention? Cardiovasc Diabetol. 2012;11:140. doi: 10.1186/1475-2840-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Khera AV, Cuchel M, Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kim TS, Rha SW, Kim SY, Park DG, Sung KC, Yoon MH, Kim KH, Lee HC, Kim WS, Kim YJ, Ahn JC, Rhee MY, Cha DH, Yoo BS, Park SH, Yoo KD, Jeon DW, Yoon YW, Cho SK, Oh YS. Efficacy and tolerability of telmisartan/amlodipine and rosuvastatin coadministration in hypertensive patients with hyperlipidemia: a phase III, multicenter, randomized, double-blind study. Clin Ther. 2019;41:728–741. doi: 10.1016/j.clinthera.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 225.Mirjafari H, Al-Husain A, Bruce IN. Cardiovascular risk factors in inflammatory arthritis. Curr Opin Lipidol. 2011;22:296–301. doi: 10.1097/MOL.0b013e3283488c50. [DOI] [PubMed] [Google Scholar]
- 226.Kim JK, Park SU. An update on the biological and pharmacological activities of diosgenin. EXCLI J. 2018;17:24–28. doi: 10.17179/excli2017-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab. 2018;15:45–55. doi: 10.1016/j.molmet.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jiang H, Badralmaa Y, Yang J, Lempicki R, Hazen A, Natarajan V. Retinoic acid and liver X receptor agonist synergistically inhibit HIV infection in CD4+ T cells by up-regulating ABCA1-mediated cholesterol efflux. Lipids Health Dis. 2012;11:69. doi: 10.1186/1476-511X-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Padro T, Munoz-Garcia N, Vilahur G, Chagas P, Deya A, Antonijoan RM, Badimon L. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients. 2018;10:E1237. doi: 10.3390/nu10091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Evans TD, Sergin I, Zhang X, Razani B. Target acquired: selective autophagy in cardiometabolic disease. Sci Signal. 2017;10:eaag2298. doi: 10.1126/scisignal.aag2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359:eaan2788. doi: 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 232.Reeskamp LF, Meessen ECE, Groen AK. Transintestinal cholesterol excretion in humans. Curr Opin Lipidol. 2018;29:10–17. doi: 10.1097/MOL.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 233.Parikh M, Patel K, Soni S, Gandhi T. Liver X receptor: a cardinal target for atherosclerosis and beyond. J Atheroscler Thromb. 2014;21:519–531. [PubMed] [Google Scholar]