Abstract
Purpose
Liver retransplantation is the only therapeutic option for patients with graft failure after liver transplantation. The aim of this study is to evaluate the outcomes of pediatric retransplantation from living donor at a single center.
Methods
Between December 1998 to August 2015, retransplantation from a living donor was performed for 14 children (<18 years of age) at Kumamoto University Hospital. The characteristics of the retransplantation recipient and the clinicopathological factors between primary transplantation and retransplantation were analyzed to detect the prognostic factors.
Results
In retransplantation, the operative time was longer and the amount of blood loss was greater in comparison to primary transplantation. The 1-, 3-, and 5-year survival rates from the date of retransplantation were 85.7, 85.7, and 78.6%, respectively. The rates of re-laparotomy after primary transplantation, bile leakage and postoperative bleeding after retransplantation were higher than after primary transplantation. Among the three patients who died after retransplantation, the operative time, the rate of re-laparotomy after primary transplantation and the incidence of gastrointestinal complications were higher in comparison to the surviving patients.
Conclusion
Pediatric retransplantation from a living donor is an acceptable procedure that could save the lives of recipients with failing allografts when organs from deceased donors are scarce. To ensure good results, it is essential to make an appropriate assessment of the cardiopulmonary function and the infectious state of the patients before Re-LDLT.
Keywords: Pediatric, Living donor, Liver transplantation, Retransplantation
Introduction
Thousands of living donor liver transplantation (LDLT) procedures have been performed worldwide, and the surgical techniques and perioperative managements for liver transplantation have been well developed. Despite these improvements, graft loss still occurs, not only in deceased donor transplantation (DDLT) but also in LDLT.
Retransplantation represents the only therapeutic option for a patient with a failing allograft [1, 2]. Most previous studies regarding liver retransplantation have dealt with organs from deceased donors [3–8]. In Japan, the number of deceased donors is quite limited, even after the implementation of changes to the organ transplant law in 2010; furthermore, and there are far fewer pediatric donors than adult donors. Thus, retransplantation for pediatric donors naturally depends on living donors.
In our institution, DDLT procedures account for only 2% of all liver transplants. LDLT has a different background from DDLT for some inevitable reasons: (1) the limitation of living donor candidates, (2) ABO-blood type mismatching between the donor and the recipient, (3) small-for-size graft-related problems for the recipient, and (4) difficulties in vascular reconstruction. Moreover, the option of retransplantation from another living donor may make the issues more complicated, as described above.
We herein evaluate the outcomes of pediatric retransplantation from a living donor (Re-LDLT) and clarify the prognostic factors that are associated with improved outcomes.
Methods
Patients
From December 1998 to August 2015, primary LDLTs were performed in 157 children (<18 years of age) at Kumamoto University Hospital. During the same study period, 27 recipients developed graft failure after primary LDLT for various reasons. Graft failure was diagnosed based on the detection of uncontrollable hyperbilirubinemia, massive ascites due to hypoalbuminemia or PV obstruction, and repeated hepatic coma or GI bleeding. Among these patients with graft failure, 14 underwent Re-LDLT; retransplantation was contraindicated in 13 patients. Among them, 3 patients were registered on the DDLT waiting list, but a medically-appropriate donor could not be found (Fig. 1). The main cause for the contraindication of retransplantation among these patients was a poor general condition, such as uncontrollable infection, severe heart or respiratory failure, and irreversible brain damage (cerebral hemorrhage) (Table 2). Severe pulmonary failure included acute respiratory distress syndrome (ARDS) (PaO2/FiO2 ≤200), or pulmonary hypertension [PH; mean pulmonary artery pressure (PAP) ≥25 mmHg]. Uncontrollable infection was defined as a state of systemic inflammatory syndrome (SIRS) with an infection that did not improve with any antibacterial medication. The recurrence of malignant disease such as hepatoblastoma was an absolute contraindication for Re-LDLT. When the probability of recurrence could be predicted based on the primary disease—such as in cases of primary sclerosing cholangitis (PSC), progressive familial intrahepatic cholestasis type-1 (PFIC-1), and acute liver failure (ALF)—we repeatedly explained the possibility of recurrence to the patient and their family and obtained reliable informed consent. The characteristics between primary LDLT (n = 143) and Re-LDLT (n = 14) were analyzed to detect the factors that affected patient survival and evaluate the validity of Re-LDLT. The investigations were performed in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all of the patients and each operative procedure was approved by the institutional review board (IRB) of Kumamoto University.
Fig. 1.
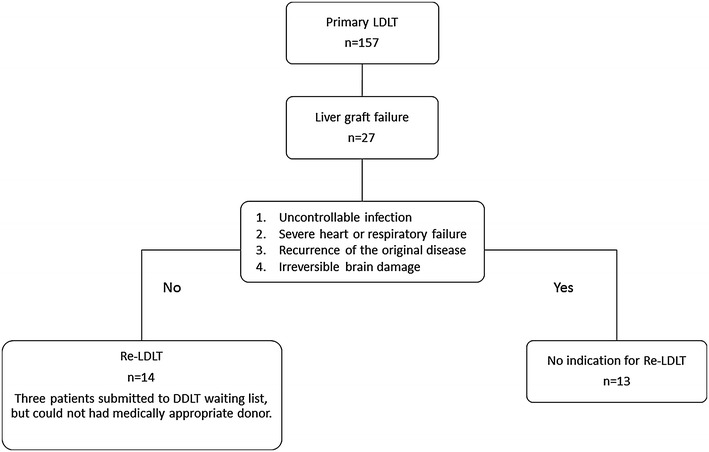
A flowchart of the period from the development of liver graft failure to Re-LDLT. Among the 157 LDLT cases, 27 recipients developed graft failure for various reasons. Among these patients with graft failure, 14 received Re-LDLT, while and retransplantation was contraindicated for 13 patients. Among the 14 Re-LDLT recipients, 3 patients were submitted to the DDLT waiting list, but a medically-appropriate donor could not be found
Table 2.
The characteristics of the graft failure patients who did not undergo Re-LDLT
| Era of graft failure | Patient no. | Gender | Age (years) | Primary disease | Survival period after LDLT (day) | Complication after LDLT | Contraindication for Re-LDLT |
|---|---|---|---|---|---|---|---|
| 1998–2009 | 1 | M | 15.8 | Primary hyperoxaluria | 319 | Peritonitis due to GI perforation | Uncontrollable infection |
| 2 | M | 5.3 | Hepatoblastoma | 1138 | Recurrence of the tumor | Recurrence of the tumor | |
| 3 | M | 1.3 | ALF | 21 | Cerebral hemorrhage | Irreversible cerebral damage | |
| 4 | F | 0.2 | ALF | 1 | Acute heart failure | Severe heart failure | |
| 5 | F | 0.7 | MTDP | 202 | Pulmonary hypertension | Pulmonary hypertension | |
| 6 | F | 12.0 | BA | 153 | Liver necrosis | Uncontrollable infection | |
| 2010–2015 | 7 | F | 7.2 | ALF | 1016 | Pneumoniae, VAHS | Severe respiratory failure |
| 8 | M | 0.8 | GSD IV | 406 | Pulmonary edema | Severe respiratory failure | |
| 9 | F | 0.3 | GSD IV | 41 | Acute heart failure | Severe heart failure | |
| 10 | F | 0.1 | ALF | 332 | Interstitial pneumoniae | Severe respiratory failure | |
| 11 | M | 4.1 | BA | 47 | Pneumoniae | Uncontrollable infection | |
| 12 | F | 0.6 | BA | 31 | Pneumoniae | Uncontrollable infection | |
| 13 | M | 0.5 | BA | 126 | GVHD | Uncontrollable infection |
BA biliary atresia, ALF acute liver failure, MTDP mitochondrial DNA depletion syndrome, GSD IV glycogen storage disease type IV, PA propionic acidemia, VAHS virus-associated hemophagocytic syndrome, GVHD graft versus host disease
Living donor selection in Re-LDLT
The selection criteria for living donor in the setting of Re-LDLT were the same as primary LDLT: age <65 years (approximately), the absence of severe systemic disease, hepatitis virus, and a recent history of malignant tumor.
The surgical procedure
The target graft-to-recipient weight ratio (GRWR) was 1–4% in all Re-LDLT cases. Hepatic artery anastomosis was performed using microsurgery techniques. The bile duct was reconstructed with choledochojejunostomy in 12 cases, and choledochocholedochostomy in 1 case. In most cases choledochojejunostomy was selected because of the shortness or strong adhesion of the recipient’s original bile duct. In 2 cases involving patients with a severe general condition, tube external choledochostomy was carried out to shorten the operative time. In 6 cases, a venous graft (such as a recipient external iliac vein, donor ovarian vein, or portal vein) from the resected graft liver was used for PV plasty because the length or thickness of the original PV was not sufficient for simple anastomosis. The venous graft was patched to enlarge the PV or anastomosed to the original PV or SMV to obtain an adequate length for anastomosis and front flow.
Immunosuppression
Tacrolimus and steroids were initially administered as baseline immunosuppressive agents for both primary LDLT and Re-LDLT. The target tacrolimus trough levels were 10–15 ng/mL for the first month, followed by 5–10 ng/mL for the next 2 months after LDLT. The intravenous administration of methylprednisolone was used for induction immunosuppressive therapy, which was switched to oral prednisolone at 1 week after LDLT. Prednisolone was gradually tapered and discontinued at 3 months after primary LDLT and 6 months after Re-LDLT, as long as the graft function was maintained. In cases in which adverse effects such as renal failure occurred, mycophenolate mofetil (MMF) or azathioprine were added as the third drug. In ABO-incompatible cases involving patients who were >2 years of age, rituximab (375 mg/m2) was administered 14 days before transplantation and MMF was added to the baseline immunosuppressive agents. For Re-LDLT, the basic immunosuppression protocol was the same as in primary LDLT; however, some modifications were applied depending on the patients’ general conditions and immunosuppressive state.
Postoperative management
In both primary LDLT and Re-LDLT, a daily ultrasound examination was performed to check the blood flow of the graft liver. Weekly surveillance cultures and infectious disease testing were carried out to detect infectious disease. Biopsy was conducted once a year after LDLT.
Statistical analysis
The starting time for all of the survival analyses was the date of Re-LDLT. Failure was defined as death from any cause. For nonparametric data, the survival curve for each group was estimated using the Kaplan–Meier method and was compared using the log-rank test. The quantitative data were expressed as the mean ± standard error of the mean (SEM), and the qualitative data were expressed as the frequency and rate. All of the data were analyzed using the Mann–Whitney U test, the Chi squared test, or the log-rank test, as appropriate. P values of <0.05 were considered to indicate statistical significance. All of the statistical analyses were carried out using the SPSS 18 software program (IBM, Japan).
Results
The characteristics of the Re-LDLT patients
The characteristics of the patients undergoing Re-LDLT are listed in Table 1. The mean age of the Re-LDLT recipients was (range 0.8–15.8 years) and the mean period between primary LDLT and Re-LDLT was 1749 days (range 9–5072 days). The original liver disease at the first LDLT included biliary atresia (BA; n = 9, 64.2%), progressive familial intrahepatic cholestasis type-1 (PFIC-1; n = 2, 14.2%), acute liver failure (n = 1, 7.1%), Wilson’s disease (n = 1, 7.1%), and primary sclerosing cholangitis (PSC; n = 1, 7.1%). The two major causes of hepatic allograft failure were chronic rejection (n = 4, 28.6%) and vascular complications (n = 3, 21.4%). All cases of chronic rejection were confirmed by the pathological finding of the loss of the bile duct, obstructive foam cell arteriopathy, or both. The mean MELD score (>12 years of age) or PELD score (<12 years of age) of the patients was 20.3 points (range 2–60). The mean operative time was 864.9 min (range 415–1981 min) and the mean blood loss volume was 233.1 mL/kg (range 21–798 mL/kg). Three recipients (21.4%) expired after Re-LDLT due to peritonitis from gastrointestinal perforation (POD 11), pulmonary hypertension (POD 2), and infection (POD 1540). All of the expired cases underwent Re-LDLT during the first 12 years of the study period (1998–2009).
Table 1.
The characteristics of the patients undergoing Re-LDLT
| Era of Re-LDLT | Patient no. | Gender | Age (years) | Primary disease | Interval after primary LDLT (days) | BW (kg) | Cause of graft loss | MELD/PELD score | Operative time (min) | Bleeding (mL/kg) | Type of graft | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1998–2009 | 1 | M | 0.8 | BA | 9 | 7.3 | Vascular complication | 25 | 415 | 66 | Left lateral | Alive |
| 2 | F | 5.7 | BA | 782 | 16.3 | Sclerosing cholangitis | 2 | 587 | 21 | Left lateral | Alive | |
| 3 | M | 15.1 | BA | 52 | 38.6 | Vascular complication | 39 | 1163 | 104 | Left | Expired (POD11) | |
| 4 | F | 14.2 | BA | 4834 | 50.0 | Steatohepatitis | 18 | 1981 | 720 | Left | Expired (POD2) | |
| 5 | F | 15.8 | Wilson | 271 | 54.5 | Refractory acute rejection | 22 | 1000 | 118 | Right | Alive | |
| 6 | F | 12.3 | PFIC-1 | 3154 | 21.0 | Chronic rejection | 26 | 970 | 798 | Left | Expired (POD1540) | |
| 2010–2015 | 7 | M | 3.5 | ALF | 1090 | 13.0 | Chronic rejection | 2 | 946 | 372 | Left lateral | Alive |
| 8 | F | 7.7 | PSC | 854 | 17.0 | PSC recurrence | 60 | 607 | 377 | Left lateral | Alive | |
| 9 | F | 4.2 | BA | 861 | 13.0 | Refractory acute rejection | 8 | 990 | 177 | Left lateral | Alive | |
| 10 | M | 13.3 | PFIC-1 | 3521 | 30.0 | Steatohepatitis | 11 | 681 | 153 | Left | Alive | |
| 11 | F | 0.8 | BA | 109 | 6.3 | Chronic rejection | 24 | 601 | 67 | Left lateral | Alive | |
| 12 | M | 1.3 | BA | 252 | 8.6 | Biliary complication | 9 | 685 | 50 | Left lateral | Alive | |
| 13 | F | 14.8 | BA | 5072 | 43.0 | Chronic rejection | 28 | 609 | 146 | Left | Alive | |
| 14 | F | 10.3 | BA | 3635 | 41.0 | Vascular complication | 10 | 873 | 95 | Left | Alive |
BA biliary atresia, ALF acute liver failure, PFIC-1 progressive familial intrahepatic cholestasis type-1, FH fulminant hepatitis, Wilson Wilson’s disease, PSC primary, sclerosing cholangitis
The characteristics of the graft failure patients who did not undergo Re-LDLT
The characteristics of the patients for whom Re-LDLT was contraindicated are shown in Table 2. The contraindications for Re-LDLT were uncontrollable infection, severe heart or respiratory failure (containing pulmonary hypertension), recurrence of the original disease (hepatoblastoma), and irreversible brain damage (cerebral hemorrhage).
The variables of the recipients with primary LDLT and Re-LDLT
The perioperative profiles of the primary LDLT and Re-LDLT patients are shown in Table 3. The Re-LDLT recipients were older than the primary LDLT recipients (8.5 vs. 4.3 years, P = 0.004). Along with their age, the body weight of the Re-LDLT recipients was higher than that of the primary LDLT recipients (25.7 vs. 16.7 kg, P = 0.013). In the Re-LDLT cases, the donor age was also higher than that of the primary LDLT cases (41.0 vs. 35.6 years, P = 0.032). There was no difference in the number of patients who were admitted to the ICU at the time of transplantation. In Re-LDLT, the operative time was longer (864.9 vs. 642.8 min, P = 0.008), and the amount of blood loss was much higher (233.1 vs. 66.1 mL/kg, P < 0.001) than in primary LDLT. The GRWR in patients undergoing Re-LDLT was also lower than that of primary LDLT (1.62 vs. 2.44%, P = 0.019). Despite the difficulty of the surgery, there was no significant difference in the postoperative hospital stay between patients undergoing primary LDLT and Re-LDLT. The rates of re-laparotomy (35.7 vs. 13.3%, P = 0.042), bile leakage (28.6 vs. 4.9%, P = 0.009) and postoperative bleeding from abdominal cavity (21.4 vs. 2.8%, P = 0.016) were significantly higher in Re-LDLT. There were no differences in the rates of vascular complications (14.3 vs. 15.4%, P = 1.000), bowel complications (14.3 vs. 2.8%, P = 0.09), infection (35.7 vs. 49.0%, P = 0.409), or rejection (57.1 vs. 36.4%, P = 0.154).
Table 3.
The variables of the recipients of primary LDLT and Re-LDLT
| Primary LDLT (n = 143) | Re-LDLT (n = 14) | P value | |
|---|---|---|---|
| Recipient age | 4.3 ± 0.44 | 8.5 ± 1.54 | 0.004 |
| Gender (M/F) | 71/72 (49.7%) | 5/9 (35.7%) | 0.405 |
| Primary disease (BA/non-BA) | 70/74 (49.0%) | 9/5 (64.3%) | 0.778 |
| Body weight (kg) | 16.7 ± 1.31 | 25.7 ± 4.48 | 0.013 |
| Donor relationship (parental/non-parental) | 135/8 (94.4%) | 12/2 (85.7%) | 0.220 |
| ABO incompatibility (Incompatible/others) | 25/118 (17.5%) | 3/11 (21.4%) | 0.717 |
| Donor age | 35.6 ± 0.73 | 41.0 ± 2.99 | 0.032 |
| Preoperative ICU care | 10 (7.0%) | 1 (7.1%) | 1.000 |
| Operative time (min) | 642.8 ± 14.39 | 864.9 ± 103.07 | 0.008 |
| Cold ischemic time (min) | 93.5 ± 6.16 | 180.1 ± 35.38 | 0.009 |
| Warm ischemic time (min) | 41.5 ± 0.69 | 38.8 ± 1.89 | 0.416 |
| Blood loss per body weight (mL/kg) | 66.1 ± 9.01 | 233.1 ± 66.17 | <0.001 |
| GRWR (%) | 2.44 ± 0.17 | 1.62 ± 0.26 | 0.019 |
| Duration of hospital stay (day) | 73.7 ± 5.10 | 60.6 ± 9.47 | 0.798 |
| Re-laparotomy after LDLT | 19 (13.3%) | 5 (35.7%) | 0.042 |
| Vascular complications | 22 (15.4%) | 2 (14.3%) | 1.000 |
| Hepatic arterial complications | 6 (4.2%) | 0 (0.0%) | 1.000 |
| Portal venous complications | 8 (5.6%) | 1 (7.1%) | 0.579 |
| Hepatic vein complications | 10 (7.0%) | 1 (7.1%) | 1.000 |
| Biliary complications | 19 (13.3%) | 5 (35.7%) | 0.042 |
| Bile leakage | 7 (4.9%) | 4 (28.6%) | 0.009 |
| Biliary stenosis | 15 (10.5%) | 2 (14.3%) | 0.651 |
| Bowel complications | 4 (2.8%) | 2 (14.3%) | 0.090 |
| Bowel perforation | 2 (1.4%) | 1 (7.1%) | 0.246 |
| Leakage | 2 (1.4%) | 0 (0.0%) | 1.000 |
| Ileus | 1 (0.7%) | 1 (7.1%) | 0.171 |
| Infection | 70 (49.0%) | 5 (35.7%) | 0.409 |
| Bacterial infection | 14 (9.8%) | 3 (21.4%) | 0.180 |
| Viral infection | 61 (42.7%) | 4 (28.6%) | 0.399 |
| Fungal infection | 7 (4.9%) | 2 (14.3%) | 0.185 |
| Rejection | 52 (36.4%) | 8 (57.1%) | 0.154 |
| Acute rejection | 40 (28.0%) | 7 (50.0%) | 0.123 |
| Chronic rejection | 16 (11.2%) | 2 (14.3%) | 0.664 |
| Postoperative bleeding | 4 (2.8%) | 3 (21.4%) | 0.016 |
The results are shown as the mean ± standard error of the mean or number (%)
The variables and postoperative complications associated with survival in the Re-LDLT recipients
The variables and postoperative complications that were associated with survival among the Re-LDLT recipients are shown in Table 4. The preoperative conditions of the surviving and expired patients did not differ to a statistically significant extent. The expired patients showed a longer operative time (1371.3 vs. 726.7 min, P = 0.024) and higher rates of re-laparotomy before Re-LDLT (100 vs. 18.2%, P = 0.027) and gastrointestinal complications after Re-LDLT (66.7 vs. 0%, P = 0.033) in comparison to the surviving patients.
Table 4.
The variables that were associated with survival in Re-LDLT recipients (surviving vs. expired)
| Alive (n = 11) | Expired (n = 3) | P value | |
|---|---|---|---|
| Recipient age | 7.1 ± 1.71 | 13.8 ± 0.83 | 0.102 |
| Gender (M/F) | 4/7 (36.4%) | 1/2 (33.3%) | 1.000 |
| Primary disease (BA/non BA) | 7/4 (63.6%) | 2/1 (66.7%) | 1.000 |
| Body weight (kg) | 22.7 ± 5.03 | 36.5 ± 8.44 | 0.185 |
| Donor relationship (parental/non-parental) | 9/2 (81.8%) | 3/0 (100%) | 1.000 |
| ABO incompatibility(Incompatible/others) | 2/9 (18.1%) | 1/2 (33.3%) | 1.000 |
| Donor age | 40.2 ± 12.04 | 43.6 ± 1.25 | 0.436 |
| Preoperative ICU care | 1 (9.0%) | 0 (0.0%) | 1.000 |
| Interval from primary-LDLT (days) | 1496.0 ± 523.42 | 2680.0 ± 1400.64 | 0.697 |
| Operative time (min) | 726.7 ± 58.62 | 1371.3 ± 309.88 | 0.024 |
| Cold ischemic time (min) | 162.0 ± 36.66 | 246.7 ± 102.56 | 0.389 |
| Warm ischemic time (min) | 39.8 ± 2.21 | 35.0 ± 3.06 | 0.240 |
| Blood loss per body weight (mL/kg) | 149.2 ± 36.45 | 540.7 ± 219.59 | 0.102 |
| GRWR (%) | 1.78 ± 0.31 | 1.05 ± 0.26 | 0.243 |
| Duration of hospital stay (day) | 68.5 ± 9.17 | 32.0 ± 25.63 | 0.185 |
| Before Re-LDLT (between primary LDLT and Re-LDLT) | |||
| Re-laparotomy | 2 (18.2%) | 3 (100.0%) | 0.027 |
| Vascular complications | 3 (27.3%) | 2 (66.7%) | 0.505 |
| Biliary complications | 4 (36.4%) | 0 (0.0%) | 0.505 |
| Gastrointestinal complications | 2 (18.2%) | 1 (33.3%) | 1.000 |
| Infections | 4 (36.4%) | 1 (33.3%) | 1.000 |
| Rejections | 6 (54.5%) | 2 (66.7%) | 1.000 |
| Bleeding | 1 (9.1%) | 1 (33.3%) | 0.396 |
| After Re-LDLT | |||
| Re-laparotomy | 4 (36.4%) | 1 (33.3%) | 1.000 |
| Vascular complications | 1 (9.1%) | 1 (33.3%) | 0.396 |
| Biliary complications | 4 (36.4%) | 1 (33.3%) | 1.000 |
| Gastrointestinal complications | 0 (0.0%) | 2 (66.7%) | 0.033 |
| Infections | 4 (36.4%) | 1 (33.3%) | 1.000 |
| Rejections | 7 (63.6%) | 1 (33.3%) | 0.538 |
| Bleeding | 1 (9.1%) | 2 (66.7%) | 0.093 |
The results are shown as the mean ± standard error of the mean or number (%)
Patient survival among patients undergoing primary LDLT and Re-LDLT, including a comparison to previous studies from other countries
The survival of the patients who underwent primary LDLT and Re-LDLT is shown in Fig. 2. The 1-, 3- and 5-year survival rates from the date of primary LDLT were 90.4, 88.5, and 87.3%, respectively. In contrast, the 1-, 3-, and 5-year survival rates from the date of Re-LDLT were 85.7, 85.7, and 78.6%, respectively. Although the rate of patient survival after Re-LDLT was lower than that for primary LDLT, especially at 5 years, there were no significant differences between the 2 groups (P = 0.311). The results of the previous studies on retransplantation are summarized in Table 5 in comparison to our study. Although the outcomes of retransplantation as the era progressed, regardless of whether DDLT or LDLT was performed, the rates of vascular complications and mortality in the present study were lower in comparison to previous studies.
Fig. 2.
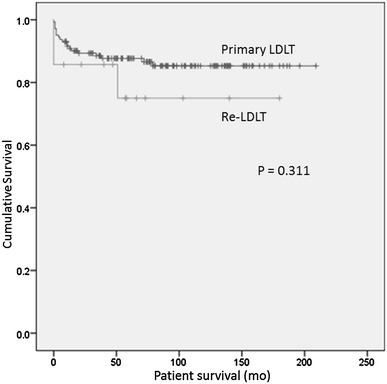
Kaplan–Meier survival curves showing the differences between primary LDLT and Re-LDLT. The 1-, 3-, and 5-year survival rates from the date of primary LDLT were 90.4, 88.5, and 87.3%, respectively. The 1-, 3-, and 5-year survival rates from the date of Re-LDLT were 85.7, 85.7, and 78.6%, respectively. There were no significant differences between the 2 groups (P = 0.311)
Table 5.
The characteristics reported in previous studies about retransplantation in pediatric recipients
| References | Year | Study period | Region | Treatment (%) | Sample size (n) | Complications (%) | Factors influencing patient survival | Early Re-Tx (<1 month) (%) | Survival rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||||||||
| Our cases | – | 1998–2015 | Japan | LDLT | 14 |
Vascular (14.3) Biliary (35.7) |
GI complications | 7.1 | 85.7 | 85.7 | 78.6 |
| Feier et al. [24] | 2016 | 1997–2013 | Brazil | LDLT | 18 |
Vascular (38.8) Biliary (16.6) |
Vascular complications | 38.9 | 70.6 | – | 58.6 |
| Urahashi et al. [10] | 2011 | 2001–2010 | Japan | LDLT | 6 |
Vascular (66.7) Biliary (14.3) |
– | 16.7 | 83.3 | – | 83.3 |
| Heffron et al. [3] | 2010 | 1997–2009 | USA |
LDLT (7.9) DDLT (92.1) |
34 |
Vascular (5.0) Biliary (16.0) |
– | 35.3 | 91.0 | 84.3 | – |
| Bourdeaux et al. [4] | 2008 | 1984–2005 | Belgium |
LDLT (4.4) DDLT (95.6) |
90 | – | Earlier Re-Tx (<1 month) | 64.4 | – | – | 66.0 |
| Ng et al. [5] | 2007 | 1996–2004 | Canada |
LDLT (6.9) DDLT (93.1) |
246 |
Vascular (13.0) Biliary (10.0) Bleeding (26.0) |
Younger donor age, reduced allograft, increased INR | 43.5 | 74.0 | – | – |
| Ogura et al. [9] | 2003 | 1990–2002 | Japan | LDLT | 28 | – | ICU care, earlier Re-Tx (<1 year), apheresis, T-Bil, Cre | 10.7 | 47.6 | – | 47.6 |
| Sieders et al. [18] | 2001 | 1982–2000 | USA | DDLT | 34 |
Vascular (27.0) Biliary (9.0) Bleeding (12.0) |
BA for the primary diagnosis | 70.6 | 70.0 | 63.0 | 52.0 |
| Achilleos et al. [17] | 1999 | 1983–1995 | UK | DDLT | 32 | – | Earlier Re-Tx (<1 month) | 46.9 | 63.0 | – | – |
| Hamada et al. [25] | 1995 | 1988–1993 | France | DDLT | 22 | – | – | – | – | – | – |
Discussion
Some previous studies have examined the perioperative clinical factors that are associated with poor outcomes after retransplantation in children [3–5]. However, most of the reports analyzed data from DDLT. Few studies have reported the outcomes of retransplantation from living donors [9, 10]. Since the first successful LDLT in 1989, the operation has been accepted throughout the world as a fundamental treatment option for patients with end-stage liver disease [11–13]. There has been a great deal of discussion about the advantages and disadvantages of LDLT [14–18]. Needless to say, the performance of such an invasive procedure in the living donor remains controversial [19]. In Japan, less than 50 DDLTs are performed each year [20]; thus, we are forced to rely on living donors, even at the time of retransplantation. It is therefore necessary to understand the relevance and adaptations of this procedure.
In general, the indications for Re-LDLT are as follows: (1) the patient’s general condition indicates that he or she might be able to withstand reoperation; and (2) the presence of an appropriate living donor. A Kyoto group reported that ICU care, a shorter interval between primary LDLT and Re-LDLT, hyperbilirubinemia, elevated creatinine, and apheresis were independently associated with poor outcomes after pediatric Re-LDLT [9]. In our institution, retransplantation was contraindicated in 13 patients due to a poor general condition. Most of these patients suffered from some fatal complications that affected the heart or respiratory functions (which affected their ability to withstand reoperation), or severe infection after primary LDLT. Recurrence of the primary malignant disease (such as hepatoblastoma) was also considered to be a contraindication due to the risk of recurrence in the second liver graft after Re-LDLT.
These factors, which might have shown the validity of our indication for Re-LDLT, did not reach statistical significance due to the limited number of Re-LDLT patients. In the Re-LDLT setting, the age of the recipient was found to have a negative impact on recipient survival. Even though the rate of mortality was higher among young recipients in previous reports [9], the survival rate of the younger patients in our study was good. The discrepancy in the results suggests the need for further consideration to determine more appropriate indications for Re-LDLT. There had been some discussions about the proper timing of retransplantation. Some reports have mentioned that later transplantation was better than earlier transplantation for improving patient survival after retransplantation [4, 7, 9]. Even though the period between primary LDLT and Re-LDLT was not found to influence survival in the present study, it might be important to try avoiding surgery in the acute phase after primary surgery as the patient would be less likely to withstand reoperation. The lower rate of early retransplantation in comparison to other studies might be attributed to our treatment protocol after primary LDLT.
In our Re-LDLT cases, the most common primary disease was BA, while the most common cause of graft loss was chronic rejection. This was consistent with previous reports. Although Sieders et al. [6] mentioned the fact that BA as a primary disease negatively influenced survival after retransplantation, it was not a significant factor in our cases. In one case involving a patient with PFIC-1, a primary liver graft with steatohepatitis was lost. PFIC refers to a group of familial cholestatic conditions caused by defects in the biliary epithelial transporters that usually leads to cirrhosis. The clinical course and outcomes of PFIC-1 recipients after LDLT are reported to be insufficient due to steatosis and fibrosis [21, 22]. In our case, the patient with PFIC-1 had developed steatohepatitis 8 years after primary LDLT. As PFIC-1 patients will require liver transplantation during the long-term progression of the disease, further strategy improvements and the elucidation of the mechanism of PFIC recurrence are crucial in LDLT for PFIC-1 patients.
In comparison to primary LDLT cases, the donor age was higher and the graft size was smaller among Re-LDLT patients, which reflects the narrowing of donor selection. The operative time and bleeding volume in Re-LDLT were much higher in comparison to primary LDLT, reflecting the difficulty of the Re-LDLT procedure. Under such unfavorable conditions, the outcome of our Re-LDLT cases is good in comparison to previous studies. The main reason for this is the small number of vascular complications after Re-LDLT. Previous studies reported that the incidence of hepatic artery thrombosis (HAT) after pediatric retransplantation is up to 10% [23–25]. HAT might lead to fatal graft failure and should be avoided to allow the patient to survive the acute phase after Re-LDLT [26]. None of the Re-LDLT cases in our study involved the development of postoperative HAT; the introduction of microsurgical reconstruction might have contributed to this good result. In the Re-LDLT cases, re-laparotomy after primary LDLT and gastrointestinal complications after Re-LDLT had a negative impact on patient survival. Strong bowel adhesion caused by re-laparotomy after primary LDLT might have made the operative time longer and led to complications that affected feasibility, such as ileus or bowel perforation after Re-LDLT [27].
We should have been more careful in deciding the indications for Re-LDLT in the two patients who expired in the early period after Re-LDLT (cases 4 and 6). In case 4, the patient had pulmonary hypertension before Re-LDLT and had been treated by epoprostenol sodium (prostacyclin sodium, Flolan®). Based on the preoperative investigations for pulmonary hypertension, it was decided that the patient would be able to withstand the Re-LDLT operation. Death occurred due to pulmonary hypertension, which suggests the need for further study and appropriate assessment before operation. In case 6, the patient showed a poor nutritional condition due to intractable diarrhea, which developed due to the patient’s primary disease (PFIC-1). The patient’s condition may have led to vulnerability to infection after Re-LDLT. At present, the gastrointestinal symptoms of PFIC-1 are not cured even by liver transplantation; thus, we have to carefully judge whether Re-LDLT is an appropriate treatment for steatohepatitis after primary LDLT in PFIC-1 patients. After 3 expired cases, the indications for Re-LDLT in our institution had become strict and these circumstances reflected in the difference in the number of the patients for whom Re-LDLT was not indicated between the early and later (1998–2009, 2010–2015) periods of this study. Serious decisions regarding the indications for Re-LDLT must directly affect the outcomes after the operation.
In conclusion, pediatric Re-LDLT is an acceptable procedure when deceased organs are scarce, which has the potential to save the lives of LDLT recipients who present with allograft failure. The preoperative treatment of patients and the optimal timing of transplantation are important for facilitating LDLT. Thus, the general conditions of patients, especially with regard to their cardiopulmonary functions and infectious state should be appropriately assessed before Re-LDLT.
Author contribution
Kohei Miura primarily designed the study, analyzed data, and wrote the manuscript. Keita Shimata, Masaki Honda, and Takashi Kobayashi analyzed data. Seisuke Sakamoto, Toshifumi Wakai, Yasuhiko Sugawara, and Yukihiro Inomata designed the study, analyzed data, and wrote this manuscript. Seisuke Sakamoto is the corresponding author.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
References
- 1.D’Alessandro AM, Ploeg RJ, Knechtle SJ, Pirsch JD, Stegall MD, Hoffmann R, et al. Retransplantation of the liver—a seven-year experience. Transplantation. 1993;55:1083–1087. doi: 10.1097/00007890-199305000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Mora NP, Klintmalm GB, Cofer JB, Solomon H, Goldstein RM, Gonwa TA, et al. Results after liver retransplantation in a group of 50 regrafted patients: two different concepts of elective versus emergency retransplantation. Transpl Int. 1991;4:231–234. doi: 10.1111/j.1432-2277.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 3.Heffron TG, Pillen T, Smallwood G, Henry S, Sekar S, Solis D, et al. Liver retransplantation in children: the Atlanta experience. Pediatr Transplant. 2010;14:417–425. doi: 10.1111/j.1399-3046.2010.01304.x. [DOI] [PubMed] [Google Scholar]
- 4.Bourdeaux C, Brunati A, Janssen M, de Magnée C, Otte JB, Sokal E, et al. Liver retransplantation in children. A 21-year single-center experience. Transpl Int. 2009;22:416–422. doi: 10.1111/j.1432-2277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 5.Ng V, Anand R, Martz K, Fecteau A. Liver retransplantation in children: a SPLIT database analysis of outcome and predictive factors for survival. Am J Transpl. 2008;8:386–395. doi: 10.1111/j.1600-6143.2007.02056.x. [DOI] [PubMed] [Google Scholar]
- 6.Sieders E, Peeters PM, TenVergert EM, de Jong KP, Porte RJ, Zwaveling JH, et al. Retransplantation of the liver in children. Transplantation. 2001;71:90–95. doi: 10.1097/00007890-200101150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Achilleos OA, Mirza DF, Talbot D, McKiernan P, Beath SV, Gunson BK, et al. Outcome of liver retransplantation in children. Liver Transpl Surg. 1999;5:401–406. doi: 10.1002/lt.500050505. [DOI] [PubMed] [Google Scholar]
- 8.Hamada H, Valayer J, Gauthier F, Yandza T, Takahashi H. Liver retransplantation in children. J Pediatr Surg. 1995;30:705–708. doi: 10.1016/0022-3468(95)90696-7. [DOI] [PubMed] [Google Scholar]
- 9.Ogura Y, Kaihara S, Haga H, Kozaki K, Ueda M, Oike F, et al. Outcomes for pediatric liver retransplantation from living donors. Transplantation. 2003;76:943–948. doi: 10.1097/01.TP.0000080068.22576.3B. [DOI] [PubMed] [Google Scholar]
- 10.Urahashi T, Mizuta K, Sanada Y, Wakiya T, Umehara M, Hishikawa S, et al. Pediatric liver retransplantation from living donors can be considered as a therapeutic option for patients with irreversible living donor graft failure. Pediatr Transpl. 2011;15:798–803. doi: 10.1111/j.1399-3046.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 11.Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368–75 (discussion 375–7). [DOI] [PMC free article] [PubMed]
- 12.Brölsch CE, Stevens LH, Whitington PF. The use of reduced-size liver transplants in children, including split livers and living related liver transplants. Eur J Pediatr Surg. 1991;1:166–171. doi: 10.1055/s-2008-1042480. [DOI] [PubMed] [Google Scholar]
- 13.Piper JB, Whitington PF, Woodle ES, Newell KA, Alonso EM, Emond JC, et al. Pediatric liver transplantation at the University of Chicago Hospitals. Clin Transpl. 1992;179–89. [PubMed]
- 14.Tanaka K, Kiuchi T. Living-donor liver transplantation in the new decade: perspective from the twentieth to the twenty-first century. J Hepatobiliary Pancreat Surg. 2002;9:218–222. doi: 10.1007/s005340200022. [DOI] [PubMed] [Google Scholar]
- 15.Malagó M, Testa G, Marcos A, Fung JJ, Siegler M, Cronin DC, et al. Ethical considerations and rationale of adult-to-adult living donor liver transplantation. Liver Transpl. 2001;7:921–927. doi: 10.1053/jlts.2001.28301. [DOI] [PubMed] [Google Scholar]
- 16.Strong RW, Lynch SV. Ethical issues in living related donor liver transplantation. Transpl Proc. 1996;28:2366–2369. [PubMed] [Google Scholar]
- 17.Shiffman M, Brown RS, Olthoff KM, Everson G, Miller C, Siegler M, et al. Living donor liver transplantation: summary of a conference at The National Institutes of Health. Liver Transpl. 2002;8:174–188. doi: 10.1053/jlts.2002.30981. [DOI] [PubMed] [Google Scholar]
- 18.Hibi T, Itano O, Shinoda M, Kitagawa Y. Liver transplantation for hepatobiliary malignancies: a new era of “Transplant Oncology” has begun. Surg Today. 2017;47:403–415. doi: 10.1007/s00595-016-1337-1. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi S, Soyama A, Nagai K, Miyazaki Y, Kurihara S, Hidaka M, et al. The donor advocacy team: a risk management program for living organ, tissue, and cell transplant donors. Surg Today. 2017 doi: 10.1007/s00595-017-1468-z. [DOI] [PubMed] [Google Scholar]
- 20.The Japanese Liver Transplantation Society Liver transplantation in Japan (part 1)—registry by the Japanese Liver Transplantation Society (in Japanese with English abstract) Jpn J Transpl. 2013;48:362–368. [Google Scholar]
- 21.Shneider BL. Progressive intrahepatic cholestasis: mechanisms, diagnosis and therapy. Pediatr Transpl. 2004;8:609–612. doi: 10.1111/j.1399-3046.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 22.Hori T, Egawa H, Takada Y, Ueda M, Oike F, Ogura Y, et al. Progressive familial intrahepatic cholestasis: a single-center experience of living-donor liver transplantation during two decades in Japan. Clin Transpl. 2011;25:776–785. doi: 10.1111/j.1399-0012.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 23.Newell KA, Millis JM, Bruce DS, Woodle ES, Cronin DC, Loss G, et al. An analysis of hepatic retransplantation in children. Transplantation. 1998;65:1172–1178. doi: 10.1097/00007890-199805150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Sieders E, Peeters PMJG, TenVergert EM, de Jong KP, Porte RJ, Zwaveling JH, et al. Graft loss after pediatric liver transplantation. Ann Surg. 2002;235:125–132. doi: 10.1097/00000658-200201000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshpande RR, Rela M, Girlanda R, Bowles MJ, Muiesan P, Dhawan A, et al. Long-term outcome of liver retransplantation in children. Transplantation. 2002;74:1124–1130. doi: 10.1097/00007890-200210270-00012. [DOI] [PubMed] [Google Scholar]
- 26.Feier F, da Fonseca EA, Candido HL, Pugliese R, Benavides MR, Neiva R, et al. Outcomes and technical aspects of liver retransplantation with living donors in children. Pediatr Transpl. 2016;20:813–818. doi: 10.1111/petr.12735. [DOI] [PubMed] [Google Scholar]
- 27.Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–685. doi: 10.1007/s00595-015-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


