Abstract
Purpose
This study was designed to evaluate the degree of microcirculatory abnormalities in patients with severe influenza A (H1N1) infection.
Methods
We assessed the sublingual microcirculation in seven consecutive patients with acute lung injury related to influenza A (H1N1) infection. The evaluation was carried out using sidestream dark field (SDF) imaging within the first 96 hr after the patients were admitted to the intensive care unit. Thenar oxygen saturation (StO2) was also measured with near-infrared spectroscopy (NIRS) during a vascular occlusion test. In addition, the Lung Injury Score (LIS) and the APACHE II and SOFA scores were recorded.
Results
All patients received invasive mechanical ventilation and at least one of the following adjuvant therapies: inhaled nitric oxide (n = 4), extracorporeal membrane oxygenation (n = 1), prone position (n = 4), recruitment maneuver (n = 3), and hydrocortisone 50 mg·hr−6 (n = 6). The median time from admission to microcirculatory assessment was 21 hr. Three patients had bacterial superinfection. The median LIS and PaO2/FiO2 were 2.5 (2.25-3.25) and 178 (158-212), respectively. Three subjects were treated with norepinephrine. During a vascular occlusion test, the microcirculation was moderately to severely compromised with a NIRS ascending slope of 2.39%·sec−1 (1.75-2.67%·sec−1), 66% (60-86%) of perfused small vessels in the sublingual microcirculation, and a microvascular flow index of 1.9 (1.3-2.6). The degree of microcirculatory abnormalities detected by the NIRS and SDF imaging techniques was correlated with the severity of the disease, as reflected by the SOFA and APACHE II scores.
Conclusions
The microcirculation as assessed by SDF imaging and NIRS techniques was compromised in patients with acute respiratory distress syndrome (ARDS) and influenza A (H1N1) infection.
Keywords: Influenza, Sequential Organ Failure Assessment, Sequential Organ Failure Assessment Score, H1N1 Infection, Lung Injury Score
Résumé
Objectif
Cette étude a été conçue afin d’évaluer le degré d’anomalies microcirculatoires chez des patients souffrant d’une infection grave à la grippe A (H1N1).
Méthode
Nous avons examiné la microcirculation sublinguale chez sept patients consécutifs souffrant d’une lésion pulmonaire aiguë liée à une infection à la grippe A (H1N1). L’évaluation a été réalisée à l’aide d’imagerie en champ noir à épi-illumination latérale (sidestream dark field – SDF) au cours des premières 96 h suivant l’admission des patients à l’unité des soins intensifs. La saturation thénarienne en oxygène (StO2) a également été mesurée par spectroscopie proche infrarouge (NIRS) pendant un test d’occlusion vasculaire. De plus, un Score de lésion pulmonaire (LIS) et les scores APACHE II et SOFA ont été enregistrés.
Résultats
Tous les patients ont subi une ventilation mécanique invasive et au moins l’une des thérapies adjuvantes suivantes: monoxyde d’azote inhalé (n = 4), oxygénation par membrane extracorporelle (n = 1), position ventrale (n = 4), manœuvre de recrutement (n = 3), et hydrocortisone 50 mg·h−6 (n = 6). Le temps moyen entre l’admission et l’évaluation microcirculatoire était de 21 h. Trois patients souffraient de surinfection bactérienne. Les LIS et PaO2/FiO2 étaient de 2,5 (2,25-3,25) et 178 (158-212), respectivement. Trois patients ont été traités avec de la norépinéphrine. Pendant le test d’occlusion vasculaire, la microcirculation a été compromise de façon modérée à grave avec une pente ascendante de NIRS de 2,39 %·sec−1 (1,75-2,67 %·sec−1), 66 % (60-86 %) de petits vaisseaux perfusés dans la microcirculation sublinguale, et un indice de débit microvasculaire de 1,9 (1,3-2,6). Le degré d’anomalies microcirculatoires détectées grâce aux techniques de NIRS et d’imagerie SDF était corrélé à la gravité de la maladie, comme l’ont reflété les scores SOFA et APACHE II.
Conclusion
La microcirculation, telle qu’analysée par des techniques d’imagerie SDF et de NIRS, a été compromise chez les patients souffrant de syndrome de détresse respiratoire aiguë (SDRA) et d’infection à la grippe A (H1N1).
During the months of March and April 2009, the identification of a new serotype of the influenza A (H1N1) virus in Mexico and the United States of America1 marked the beginning of the first pandemic to be declared by the World Health Organization (WHO) in the 21st century. The WHO registered more than 625,000 cases worldwide, with 15,174 deaths.2 Although it presented a global mortality rate similar to that of the seasonal influenza virus, this new strain of influenza A (H1N1) virus has some particularities that make it a specific challenge for the intensivist. First, certain groups of patients, who are usually not at risk of complications or death from seasonal influenza, are at special risk with the influenza A (H1N1) virus, including pregnant or postpartum women, asthmatic or obese patients, and young subjects.3–5 Second, this virus has a special tropism for the lower airways. Accordingly, acute viral pneumonitis, with or without bacterial superinfection, and airflow limitation occur frequently.6,7 For the most part, patients with severe influenza A (H1N1) are admitted to hospital for respiratory failure that progresses rapidly, usually within one day, into severe respiratory failure necessitating admission to the intensive care unit (ICU) and rescue ventilatory strategies.3,7,8 Such patients present with moderate to severe acute respiratory distress syndrome (ARDS) associated with high morbidity and mortality rates (15-40%).3,9,10
These pulmonary and remote alterations present many similarities to “bacterial” sepsis, even though they may occur in the absence of bacterial infection. The H1N1 infection is associated with a strong pulmonary inflammatory response with increased production of interleukin (IL)-10, IL-4, and interferon-gamma (IFN-γ).11 This pro-inflammatory response is not restricted to the lungs. Recently, Bermejo-Martin et al.12 reported that systemic levels of several pro-inflammatory cytokines were increased in patients hospitalized with H1N1 infection, especially in those who were critically ill. Remote organ dysfunction may also be observed, including elevated liver enzymes, rhabdomyolysis, renal failure, and shock.9
In sepsis, severe microcirculatory alterations have been observed.13 These alterations may have important implications, as the microcirculation is the principal site of oxygen exchange between blood and underlying tissues. Additionally, anomalies of the microcirculatory network have been consistently demonstrated as being associated with organ dysfunction and mortality.14,15 However, microcirculatory alterations have not yet been reported in patients with viral infections. We hypothesized that microcirculatory abnormalities may be present in patients with severe influenza A (H1N1) infection, participating in local and remote tissue injury. During the H1N1 pandemic in the fall of 2009, we evaluated the microcirculation of critically ill patients admitted to hospital with severe respiratory failure associated with influenza-like syndrome in order to evaluate the degree of microcirculatory dysfunction in patients with severe influenza A (H1N1) infection.
Patients and methods
All patients with acute respiratory failure associated with influenza-like illness who were admitted to the 35-bed ICU of a teaching hospital from October to December 2009 were screened for inclusion in the study. Influenza-like illness was defined as two or more of the following symptoms: fever, chills, myalgia, arthralgia, headache, and sore throat and/or cough in the absence of another known cause during the epidemic period of H1N1 infection. Acute lung injury (ALI) was defined as PaO2/FiO2 below 300 in the absence of signs of raised left atrial pressure.16,17 These patients were subsequently screened for influenza A (H1N1) virus infection in the upper and/or lower respiratory secretions (viral culture, polymerase chain reaction, or immunofluorescence) and classified as having confirmed, probable, or suspected infections according to the WHO and the Canadian National Microbiology Laboratory definitions (Canadian Critical Care Trials Group, 9 A.D. 29 /id;Public Health Agency of Canada, 2009 30 /id). Exclusion criteria included age < 18 yr, psychomotor agitation, reliance on non-invasive ventilatory support, oral bleeding, and H1N1 infection diagnosed for > 96 hr. The study was approved by the Erasme Hospital Ethical Committee, and all patients or their spouse or children gave informed consent.
General measurements
On the day of microcirculatory assessment, severity of illness was assessed using the Sequential Organ Failure Assessment (SOFA) score,18 the Acute Physiology and Chronic Health Evaluation (APACHE II) score,19 and the Lung Injury Score (LIS).20 Length of ICU stay, length of hospital stay, ICU mortality, 28-day mortality, and hospital mortality were recorded.
We also recorded global hemodynamic parameters, demographic data, major comorbidities, variables (in terms of respiration, mechanisms, and oxygenation), blood serum markers of organ function, and the presence of bacterial superinfection. In addition, we noted organ support therapy, such as vasopressors, renal replacement therapy, mechanical ventilation, and related adjuvant therapies (nitric oxide inhalation, steroids, prone position, recruitment maneuvers, and extracorporeal membrane oxygenation) received during the ICU stay.
Microcirculatory assessment
The microcirculation was assessed using sidestream dark field (SDF) imaging (MicroScan, MicroVision Medical, Amsterdam, The Netherlands) and near-infrared spectroscopy (NIRS) (InSpectra 650, Hutchinson, MN, USA).
The SDF imaging technique has been presented elsewhere in detail.13 In brief, five sequence images of 20 sec each were recorded from the lateral sublingual area by a video camera (5 X objective), stored and analyzed offline. The density, the proportion of perfused vessels, and the microvascular flow index (MFI) of the vessels were quantified using the semi-quantitative method.21
The NIRS technique has been detailed elsewhere.22 Briefly, a probe was placed over the skin of the thenar eminence to register the baseline tissue oxygen saturation (StO2) and the total hemoglobin index (THI), i.e., an estimate of the local tissue hemoglobin concentration. A three-minute vascular occlusive test was then performed with an arterial cuff around the arm inflated up to 50 mmHg above the systolic arterial pressure. The rate of tissue oxygen consumption (linear regression of the downslope) and the tissue oxygen consumption at the end of the first minute of ischemia (NirVO2) were measured during the ischemic phase. The vasoreactivity response to hypoxia was measured as the linear regression of the incremental StO2 upslope that followed the arterial cuff release. Finally, the difference between the maximal StO2 and THI following the reperfusion and the baseline values of these variables were computed as ΔStO2 and ΔTHI.23
Statistical considerations
Continuous variables are depicted as median (interquartile range, 25-75%) or as mean ± standard deviation (SD), and categorical variables are depicted as number (%). Parametric (Pearson correlation) or non-parametric (Spearman test) linear regression was performed to evaluate the relationships between microcirculation (NIRS and SDF imaging) variables and indices of severity.
Results
Among the 30 patients with influenza-like syndrome who were admitted to the ICU during the study period, seven patients were included for microcirculatory evaluation. Twenty-three patients were excluded for use of non-invasive mechanical ventilation (n = 16) precluding investigation of the sublingual microcirculation, age < 18 yr (n = 2), time from diagnosis > 96 hr (n = 2), and missed cases (n = 3). Three of the included patients had confirmed H1N1 infection, one probable and three suspected. At the very least, one comorbidity was found in each of the patients: immunosuppression (n = 4), pregnancy (n = 2), diabetes (n = 2), heart disease (n = 2), chronic obstructive pulmonary disease (n = 1), neoplasia (n = 1), chronic renal failure (n = 1), and cirrhosis (n = 1). Three patients had bacterial superinfection. All patients were on antibiotics and six were receiving oseltamivir at the time of the microcirculatory assessment—in the remaining patient, therapy was initiated just after the microcirculatory assessment. The main clinical and laboratory characteristics of the patients are detailed in Table 1. Three patients were in shock, requiring norepinephrine administration with a mean dose of 0.57 ± 0.55 μg·kg−1·min−1. The main hemodynamic data are shown in Table 2.
Table 1.
General medical characteristics of the patients who were studied on the day of the microcirculatory assessment
| Variables | Values |
|---|---|
| Sex (F/M) | 3/4 |
| Age (yr) | 50 (25-78) |
| Body mass index (kg·m-²) | 25 (23-28) |
| SOFA score (points) | 10 (8-14) |
| APACHE II score (points) | 24 (15-30) |
| Comorbidities (n/patient) | 2 (1-4) |
| Bacterial superinfection (n) | 3 |
| Use of antibiotics (n) | 7 |
| Use of oseltamivir (n) | 6 |
| Use of norepinephrine (n, dose in μg·kg−1·min−1) | 3 (0.57 ± 0.55) |
| Use of renal replacement therapy (n) | 2 |
| ICU length of stay (days) | 9 (7-17) |
| Hospital length of stay (days) | 20 (10-42) |
| ICU mortality (n) | 1 |
| 28-day mortality(n) | 2 |
| Hospital mortality (n) | 2 |
| Laboratory variables | |
| White blood cell count (cells·mm-³) | 11,300 (4,800-27,200) |
| Platelet count (x 10³·mm-³) | 203 (60-254) |
| C-reactive protein (mg·dL−1) | 14.0 (4.8-17.0) |
| LDH (U·L−1) | 714 (343-901) |
| Creatinine (mg·dL−1) | 1.1 (0.8-2.0) |
| Bilirubin (mg·dL−1) | 0.8 (0.7-2.1) |
| AST (U·L−1) | 48 (36-99) |
| ALT (U·L−1) | 23 (21-36) |
| Creatinine kinase (U·L−1) | 167 (54-870) |
SOFA = sequential organ failure assessment; APACHE = acute physiology and chronic health evaluation; LDH = lactate dehydrogenase; AST = aspartate aminotransferase; ALT = alanine aminotransferase
Table 2.
Ventilatory and oxyhemodynamic variables at the time of the microcirculatory assessment
| Variables | Values |
|---|---|
| Ventilatory parameters | |
| LIS score (points) | 2.5 (2.25-3.25) |
| Static compliance (mL·cmH2O) | 30 (25-43) |
| Plateau pressure (cmH2O) | 25 (21-30) |
| PEEP (cmH2O) | 11 (6-16) |
| PaO2/FiO2 | 178 (158-212) |
| PaCO2 | 34 (20-45) |
| Tidal volume (mL·kg−1¥) | 5.9 (5.3-7.1) |
| Adjuvant therapy (n, %) | |
| Inhaled nitric oxide | 4 |
| ECMO | 1 |
| Prone position | 4 |
| Recruitment maneuver | 3 |
| Steroids | 6 |
| Hemodynamic parameters | |
| Heart rate (beats·min−1) | 82 (76-116) |
| Mean arterial pressure (mmHg) | 77 (71-90) |
| Mean pulmonary artery pressure (mmHg§) | 34 ± 7 |
| Central venous pressure (mmHg) | 14 (11-17) |
| Pulmonary artery occlusive pressure (mmHg*) | 26 ± 6 |
| Cardiac output (L·min−1#) | 5.3 (4.4-6.9) |
| Arterial lactate (mEq·L−1) | 1.8 (1.2-3.7) |
| S(C)VO2 (%) | 70.3 (59.4-74.1) |
| Hemoglobin (g·dL−1) | 9.6 (9.2-11) |
*Measured in three patients; §Measured in four patients; # Measured in five patients
¥ = predicted body weight. ECMO = extracorporeal membrane oxygenation; LIS = lung injury score; PEEP = positive end-expiratory pressure
All patients received mechanical ventilatory assistance and fulfilled the ALI/ARDS criteria by way of low lung compliance, hypoxemia, and high LIS. Adjuvant therapy for ARDS was frequently applied, particularly steroids, which were reserved for the most severe cases (Table 2). At the time of microcirculatory assessment, the median LIS of all patients was 2.50 (2.25-3.25) points.
The microcirculatory assessment was performed 21 (7-45) hr after admission to the ICU. Both SDF imaging and NIRS revealed that the microcirculation was moderately to severely compromised (Table 3 and Figure). The proportion of perfused small vessels was 66% (60-86%; normal > 90%) and the MFI was 1.9 points (1.3-2.6 points; normal > 2.5 points). The NIRS descending slope was −0.19%·sec−1 (−0.15-0.22%·sec−1; normal range −0.22 ± 0.06%·sec−1), ascending slope 2.39%·sec−1 (1.75-2.67% ·sec−1; normal range 4.8 ± 1.6%·sec−1), and nirVO2 110.6 U (80.1-120.2 U; normal range 171 ± 57 U).
Table 3.
Microcirculatory variables
| Variables | Values |
|---|---|
| Sidestream Dark Field | |
| Total vessel density (n·mm−1) | 14.8 (13.7–15.4) |
| Total perfused vessel density (n·mm−1) | 11.0 (10.7–11.9) |
| Small vessel density (n·mm−1) | 12.3 (11.9–12.6) |
| Small perfused vessel density (n·mm−1) | 8.4 (7.9–10.2) |
| Proportion of perfused all vessels (%) | 73 (67–87) |
| Proportion of perfused large vessels (%) | 99 (98–100) |
| Proportion of perfused small vessels (%, (normal range, > 90%) | 66 (60––86) |
| Microvascular flow index (points, normal range, > 2.5) | 1.9 (1.3–2.6) |
| Near-Infrared Spectroscopy | |
| Baseline StO2 (%, normal range, 83.8 ± 4.1%) | 80 (70–82) |
| Delta StO (%, normal range, 10.4 ± 2.8%) | 8 (8–10) |
| Baseline THI (U, normal range, 14.9 ± 2.0%) | 9.7 (8.8–10.4) |
| Delta THI (U, normal range, 3.6 ± 1.2%) | 2.0 (1.3–2.3) |
| NirVO2 (U, normal range, 179.41 ± 47.59%) | 110.6 (80.1–120.2) |
| Descending slope (%·sec−1, normal range, 0.21 ± 0.06·sec−1) | 0.19 (0.15–0.22) |
| Ascending slope (%·sec−1, normal range, 4.08 ± 1.31%·sec−1) | 2.39 (1.75–2.67) |
StO2 = tissue oxygen saturation; THI = total hemoglobin index; NirVO2 = tissue oxygen consumption at the end of the first minute of ischemia with the near-infrared spectroscopy vascular occlusion test
Figure.
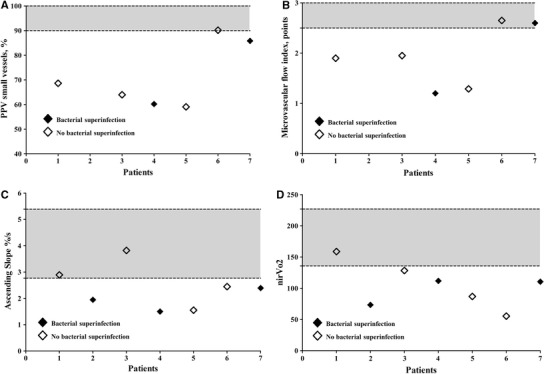
Sidestream dark field and near-infrared spectroscopy values of individual patients. Gray areas represent normal ranges (+2 standard deviation [SD] and -2 SD around mean). Panel A) Proportion of perfused vessels (PPV); Panel B) Microvascular flow index (MFI); Panel C) Ascending slope; Panel D) Near infrared oxygen consumption (NIR VO2)
The proportion of perfused small vessels and the MFI correlated negatively with the SOFA score (R² 0.67; P = 0.046 and R² 0.85; P = 0.009, respectively), while the NIRS ascending slope correlated negatively with the APACHE II score (R² 0.36; P = 0.05).
Discussion
Data from this series of seven patients demonstrates that patients with severe influenza A (H1N1) infection with ALI/ARDS have clinically significant microcirculatory abnormalities. In particular, both clinical (LIS) and microcirculatory insults were at least as severe in patients with only viral infection as in those with superimposed bacterial infection.
The microcirculation has a central role in the regulation of organ perfusion.24 In several studies and in different types of shock, the presence of microcirculatory dysfunction has been associated with poor outcomes, including a higher frequency of organ failure and mortality.14,15,25,26 In our small series of patients, the microcirculation was moderately to severely compromised, in line with previous studies of patients with severe sepsis and septic shock.14,22,23 For example, Creteur et al.22 reported NIRS ascending slope values of 2.3 ± 1.3%·sec−1 in patients with septic shock, a value similar to our findings. Skarda et al.23 found NirVO2values of 97 ± 36 U, again in the range seen in our patients. In addition, the proportion of perfused small vessels assessed by SDF imaging was 65% (53.1–68.9%) in patients with septic shock, similar to that reported by Sakr et al.14 As in sepsis, we observed a significant correlation between the microcirculatory abnormalities detected by the two methods (SDF imaging and NIRS) and the severity of disease as assessed by SOFA and APACHE II scores.
One of the most interesting findings was that bacterial superinfection was not mandatory for microvascular flow impairment to occur, although the small sample size limits the strength of this conclusion. Influenza A (H1N1) virus may induce microvascular alterations either directly or indirectly via the stimulation of host inflammatory factors. Administration of tumour necrosis factor (TNF), a pro-inflammatory cytokine, can lead to microcirculatory alterations in animals.27 This new influenza strain is more virulent than seasonal influenza strains and may, therefore, induce microcirculatory impairment by a direct viral effect. Using different mammalian models, Itoh et al.11 demonstrated that one of the first influenza A (H1N1) strains to be isolated was more lethal, persisted longer in the lower airways, caused more intense alveolitis, and induced higher concentrations of inflammatory cytokines in the lungs than a regular seasonal influenza strain. Compared with outpatients, the inflammatory response to severe influenza A (H1N1) is more pronounced in patients with severe disease who require hospitalization, and the response can be observed even in patients who do not have bacterial superinfection.12 The ability of the influenza and other acute respiratory viruses to provoke microvascular alterations—characterized by sludge of capillary flow associated with hypercoagulability and increased blood viscosity—has been suggested by others.28 Therefore, significant microcirculatory perfusion deficits could be an important pathophysiological mechanism in severe influenza A (H1N1) infections.
Our study has several limitations. First, although we studied most of the severe cases in our institution, the sample size was relatively limited, and we were unable to perform some exploratory analyses of subgroups. Second, no control groups of patients with less severe H1N1 infection or other causes of ARDS were included. Third, although the examinations were performed quite early, it was impossible to standardize their timing, and there were no follow-up tests to study disease progression.
What then are the implications of our data? First, our data suggest that severe viral infections, like bacterial infections, can induce microcirculatory alterations. The study was not designed to evaluate whether the observed microcirculatory alterations are specific to H1N1 infection or whether they simply reflect the fact that critically ill patients with severe sepsis have disturbed microcirculation. Second, the finding of microcirculatory abnormalities and their correlation with disease severity using two different techniques suggests that endothelial dysfunction may play a role in the pathophysiology of severe H1N1 infections. These findings are in line with other studies in sepsis that relate the severity of microcirculatory abnormalities to the severity of disease.14,22,29
In conclusion, these data suggest that microcirculatory abnormalities are present in patients with acute lung injury associated with severe influenza A (H1N1) infection independently of any bacterial co-infection.
Acknowledgments
Funding
Departmental funds only.
Competing interests
None declared.
References
- 1.Centers for Disease Control and Prevention (CDC). Swine influenza A (H1N1) infection in two children–Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 400-2. [PubMed]
- 2.World Health Organization. WHO Pandemic (H1N1) 2009 - update 86. http://www.who int/csr/don/2010_02_5/en/index.html (accessed July 2010).
- 3.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 5.ANZIC Influenza Investigators. Webb SA, Pettila V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 6.Webb SA, Seppelt IM, ANZIC Influenza Investigators Pandemic (H1N1) 2009 influenza (“swine flu”) in Australian and New Zealand intensive care. Crit Care Resusc. 2009;11:170–172. [PubMed] [Google Scholar]
- 7.Rello J, Rodriguez A, Ibanez P, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 11.Itoh Y, Shinya K, Kiso M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016OC. [DOI] [PubMed] [Google Scholar]
- 14.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–1831. doi: 10.1097/01.CCM.0000138558.16257.3F. [DOI] [PubMed] [Google Scholar]
- 15.Trzeciak S, McCoy JV, Phillip Dellinger R, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artigas A, Bernard GR, Carlet J, et al. The American-European Consensus Conference on ARDS, part 2: Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157:1332–1347. doi: 10.1164/ajrccm.157.4.ats2-98. [DOI] [PubMed] [Google Scholar]
- 17.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707-10. [DOI] [PubMed]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 21.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33:1549–1556. doi: 10.1007/s00134-007-0739-3. [DOI] [PubMed] [Google Scholar]
- 23.Skarda DE, Mulier KE, Myers DE, Taylor JH, Beilman GJ. Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock. 2007;27:348–353. doi: 10.1097/01.shk.0000239779.25775.e4. [DOI] [PubMed] [Google Scholar]
- 24.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13–S19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147:91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Machiedo GW, Zaets SB, Berezina TL, et al. Trauma-hemorrhagic shock-induced red blood cell damage leads to decreased microcirculatory blood flow. Crit Care Med. 2009;37:1000–1010. doi: 10.1097/CCM.0b013e3181962d39. [DOI] [PubMed] [Google Scholar]
- 27.Vicaut E, Hou X, Payen D, Bousseau A, Tedgui A. Acute effects of tumor necrosis factor on the microcirculation in rat cremaster muscle. J Clin Invest. 1991;87:1537–1540. doi: 10.1172/JCI115165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogomolov BP, Deviatkin AV. Microcirculation and hemostasis in influenza and acute viral respiratory infections complicated with pneumonia (Russian) Ter Arkh. 2002;74:44–48. [PubMed] [Google Scholar]
- 29.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88–98. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]


