Abstract
Background
Obesity has been associated with compromised tissue oxygenation and reduced organ perfusion. The brain is critically dependent on oxygen delivery, and reduced brain tissue oxygen tension (PbtO2) may result in poor outcome after brain injury. We tested the hypothesis that obesity is associated with compromised PbtO2 after severe brain injury.
Methods
Patients with severe brain injury (GCS score ≤ 8) who underwent continuous PbtO2 monitoring were retrospectively identified from a prospective single-center database. Patients, were classified by body mass index (BMI = weight (kg)/m2) and were included if they were obese (BMI ≥ 30) or non-obese (BMI = <30).
Results
Sixty-nine patients (mean age 46.4 ± 17.0 years) were included. Mean daily PbtO2 was 25.8 (9.6) mmHg for the 28 obese and 31.8 (12.3) mmHg for the 41 non-obese patients (P = 0.03). Initial PbtO2 and mean daily maximum PbtO2 measurements also were significantly lower in obese patients than in non-obese patients. Univariate predictors of compromised PbtO2 (defined as minutes PbtO2 < 20 mmHg) included elevated BMI (P = 0.02), presence of ARDS (P < 0.01), mean PaO2 (P < 0.01), maximum FiO2 (P < 0.01), mean PaO2:FiO2 (P < 0.01), and mean CVP (P < 0.01). In multivariable analysis, BMI was significantly associated with compromised PbtO2 (P = 0.02). Sex, age, and mean CVP were also identified as significant predictors of compromised PbtO2; ARDS and PF ratio were not.
Conclusions
In patients with severe brain injury, obesity was found to be an independent predictor of compromised PbtO2. This effect may be mediated through obesity-related pulmonary dysfunction and inadequate compensatory mechanisms.
Keywords: Obesity, Brain oxygen, Brain injury
Introduction
Prevention of secondary neuronal injury is central to modern ICU care of acute brain injury. Brain tissue oxygen (PbtO2) monitors, placed directly into brain parenchyma, permit continuous bedside PbtO2 assessment and for quantification of hypoxic events in the brain. When used with an intracranial pressure (ICP) monitor PbtO2 monitors have enhanced the ability to detect secondary neuronal injury after severe brain injury. Observational clinical studies demonstrate a consistent association between reduced PbtO2 and poor outcome [1–3]. In addition clinical studies suggest that PbtO2 goal-directed therapy may be associated with improved outcomes among patients with traumatic brain injury (TBI) and subarachnoid hemorrhage (SAH) [4–6].
Brain oxygen tension (PbtO2) depends on numerous factors, one of which is pulmonary function [7, 8]. Obesity is linked to a wide variety of pulmonary diseases, including chronic obstructive pulmonary disease (COPD), asthma, obstructive sleep apnea (OSA), pulmonary embolic disease, and aspiration pneumonia; however, its impact on brain oxygenation remains unknown [9]. In the intensive care unit, obesity also is associated with lung dysfunction [10–12], ventilation–perfusion mismatch [13], metabolic derangements [9], and altered systemic oxygenation [14–16]. Obese trauma patients have been shown to have lower transcutaneous tissue oxygenation, reduced peripheral oxygen saturation and increased organ failure [17, 18].
Obesity is a known risk factor for cardiovascular and cerebrovascular disease. In SAH, hypertension, diabetes mellitus, hyperlipidemia, and ischemic stroke, all of which are associated with obesity, are associated with poor outcome [19]. Elevated body mass index (BMI) has been associated with the development of delayed cerebral ischemia (DCI) after SAH [20]. In this preliminary study, we examined whether obesity, defined by body mass index (BMI), is associated with reduced PbtO2 after severe acute brain injury.
Methods
Patients admitted to the Neurointensive Care Unit at the Hospital of the University of Pennsylvania were retrospectively identified from a prospective observational database (Brain Oxygen Monitoring Outcome study) with Institutional Review Board approval. The patients were selected using the following inclusion criteria: (1) diagnosis of severe brain injury with a Glasgow Coma Scale (GCS) ≤ 8 after initial resuscitation, (2) PbtO2 monitoring within 6 h of admission, (3) hemodynamic stability (i.e., did not require vasopressor agents during their ICU care), (4) need for mechanical ventilation, and (5) recorded height and weight data. Exclusion criteria included: (1) fixed and dilated pupils upon admission, (2) multiple or abdominal compartment syndrome, or (3) lack of recorded height and weight. Acute Physiology and Chronic Health Evaluation (APACHE II) [32] score was recorded on admission. Patients were classified as obese or non-obese according to their BMI calculated from actual measurements of their height and weight [(BMI = mass (kg)/surface area (m2)] performed on admission. Obesity was defined as a BMI ≥ 30 [33, 34].
Intracranial pressure (ICP; Camino; Integra Neuroscience, Plainsboro, NJ), brain temperature, and PbtO2 were continuously monitored using a Licox monitor (Integra Neuroscience) inserted at the bedside through a burr hole into the frontal lobe and secured with a triple-lumen bolt. Intracranial monitors were placed into brain parenchyma that appeared normal on admission head CT scan and ipsilateral to the worst pathology. After CT confirmation, PbtO2 probe function was confirmed by an oxygen challenge (a rise in PbtO2 of ≥2–5 mmHg at an FiO2 of 1.0 for 5 min). Intracranial monitors were removed once ICP and PbtO2 were within normal range without treatment for 24 h. Cerebral perfusion pressure (CPP) was calculated as mean arterial pressure [(MAP) − ICP].
Heart rate, arterial blood pressure, and central venous pressure (CVP) were recorded continuously in all patients using a bedside monitor (Component Monitoring System M1046-9090C: Hewlett Packard, Andover, MA). ICU flow sheets were reviewed for documentation of ICP, CPP, PbtO2, CVP, percent FiO2, and 24-h fluid balance. Daily mean, minimum, and maximum measurements of both arterial partial pressure of oxygen (PaO2) and fraction of inspired oxygen (FiO2) were recorded. Mean daily PaO2/FiO2 (PF ratios) were determined by averaging the PaO2 from all arterial blood gas samples performed that day and dividing them by the mean FiO2 obtained for respiratory flow sheets. Acute respiratory distress syndrome (ARDS) was defined as a PF ratio <200, bilateral lung infiltrates by radiography and a central venous pressure <18 mmHg. Acute lung injury (ALI) was defined as a PF ratio <300. Data on ventilator status and settings were not collected. Information on hemoglobin (Hb), serum sodium (Na), creatinine, glucose, blood urea nitrogen (BUN), arterial pH, PaO2, hospital length of stay, and whether a craniotomy was performed were obtained from online hospital patient records. Outcome was recorded as survival (dead or alive) at 30 days after brain injury.
General critical care and ICP management were performed as previously described [8]. Compromised brain oxygen (PbtO2 < 20 mmHg) was managed according to etiology. For example, elevated ICP was treated and systemic hypoxia was corrected if present. Abnormalities of metabolic supply (e.g., volume status or mean arterial pressure) or metabolic demand (e.g., pain, fever, seizures) were corrected. If these measures failed and the hemoglobin concentration was less than 10 g/dl, blood transfusion therapy was considered to augment oxygen delivery.
Patients were divided into two groups: obese (BMI ≥ 30) or non-obese (BMI < 30). To allow for intracranial probe equilibration, data from the first 6 h after PbtO2 monitor insertion were discarded. Following this 6-h window, initial PbtO2, mean daily PbtO2, minimum daily PbtO2, maximum daily PbtO2 were recorded. In addition, time (in minutes) of PbtO2 < 20 mmHg (brain tissue compromise) and PbtO2 < 10 mmHg (brain tissue hypoxia) were tabulated. Since episodes of reduced PbtO2 were recorded as single measurements in time on the ICU flow sheet and not continuous data, the event was assumed to occur for the total time until the next recorded value on the flow sheet (usually 15 min) or a time of 60 min, whichever was less. The mean daily PbtO2 was defined as the average of each patient’s PbtO2 measurements over 24 h. The total number of minutes that PbtO2 was compromised (PbtO2 < 20 mmHg) or that there was brain hypoxia (PbtO2 < 10 mmHg) was then normalized to the percent of total time monitored to adjust for patients who died early or who were monitored longer.
Statistical analysis was performed using SPSS 17 software (SPSS, Chicago, IL), with data summarized as the mean (standard deviation) unless otherwise stated. A P value < 0.05 was considered statistically significant and was two-sided unless otherwise specified. Kolmogorov–Smirnov and Shapiro–Wilk tests were employed to assess goodness of fit to determine normality. Univariate analysis of pooled data was performed using the Student t test and Wilcoxon Rank Sum (Mann–Whitney test) for continuous parametric and non-parametric variables, respectively, and the Chi-square test (or Fisher exact test) for categorical variables. Multivariable analyses using linear regression to identify predictors of compromised PbtO2 included all variables associated with compromised PbtO2 in univariate analysis (P < 0.1). Non-normally distributed continuous variables were log transformed.
Results
Patient Population
Two hundred and seventeen patients were screened for this study; 69 had measured height and weights. Of these patients, 21 were categorized as obese and 48 as non-obese. Thirty-five patients were admitted with TBI and 22 with aneurismal SAH. The remaining 12 patients were admitted with penetrating head trauma, arteriovenous malformation, brain tumor, or non-traumatic SDH. The clinical and radiographic characteristics of the patients included in the analysis are described in Table 1.
Table 1.
Comparison of clinical characteristics in obese or non-obese patients
| Characteristic | Obese (n = 28) | Non-obese (n = 41) | P value |
|---|---|---|---|
| BMI | 35.8 ± 4.9 | 22.0 ± 1.6 | P < 0.01 |
| Diagnosis | |||
| TBI | 10 (35.7) | 25 (61.0) | 0.11 |
| Aneurismal SAH | 11 (39.3) | 11 (26.8) | |
| Other (12) | 7 (25.0) | 5 (12.2) | |
| Duration of monitoring, mean (SD), hours | 140.5 ± 18.0 | 119.0 ± 12.9 | 0.32 |
| Age (years), mean (SD) | 51.6 (14.6) | 42.8 (17.7) | 0.03 |
| Sex | |||
| Female (%) | 12 (42.9) | 20 (48.8) | 0.63 |
| Male (%) | 16 (57.1) | 21 (51.2) | |
| Race | |||
| Caucasian (%) | 13 (46.4) | 29 (70.7) | 0.13 |
| African American (%) | 10 (35.7) | 10 (24.4) | |
| Other (%) | 5 (17.9) | 5 (4.9) | |
| Diabetes (%) | 12 (42.9) | 2 (4.9) | 0.01 |
| Admission Apache II, mean (SD) | 20.7 (4.8) | 21.0 (6.3) | 0.64 |
| Craniotomy (%) | 13 (46.4) | 17 (41.6) | 0.81 |
| Days of mechanical ventilation (SD) | 22.4 (18.8) | 15.1 (13.2) | 0.06 |
| Required tracheostomy | 12 (42.9) | 18 (43.9) | 1.00 |
| Chest trauma (%) | 7 (25.0) | 16 (39.0) | 0.30 |
| Pneumonia (%) | 10 (35.7) | 6 (14.6) | 0.08 |
| ARDS (%) | 5 (17.9) | 4 (9.8) | 0.47 |
| ICU length of stay, mean (SD), days | 17.7 (16.2) | 16.6 (16.1) | 0.79 |
| Hospital length of stay, mean (SD), days | 31.9 ± 20.4 | 25.7 ± 26.5 | 0.45 |
| 30-day mortality (%) | 6 (22.2) | 16 (42.1) | 0.17 |
Values in bold are statistically significant
Data are expressed as the mean ± standard deviation (SD) or number and (%). Entries in table are raw sample size (column percentage), unless otherwise specified
SAH traumatic subarachnoid hemorrhage, SDH acute subdural hematoma, IPH intraparenchymal hematoma or contusions, GCS Glasgow Coma Scale
Obese patients were significantly older than non-obese patients [51.6 (14.6) years compared to 42.8 (17.7); P = 0.03], and had a higher incidence of pre-injury diabetes mellitus (P = 0.01). Non-obese patients were more likely to have an admission diagnosis of trauma (P = 0.04). Admission diagnosis varied by sex; men were more likely to be admitted with trauma and women were more likely to be admitted with SAH (P = 0.04).
Obese patients tended toward longer periods of mechanical ventilation (P = 0.06) and a higher incidence of pneumonia (P = 0.08). There was no significant difference in the incidence of ARDS, chest trauma or tracheostomy placement between groups. Thirty-day mortality was similar between groups.
Physiological Variables
All patients underwent continuous ICP and PbtO2 monitoring with a mean duration of 127.7 (87.8) h. The physiological variables recorded during a patient’s course are listed in Table 2. Mean hemoglobin concentration was significantly higher in the obese group than in the non-obese group. There were no significant differences in the remainder of physiological and clinical variables between groups.
Table 2.
Physiological and clinical variables observed during monitoring
| Physiologic or clinical variable | Obese (n = 28) | Non-obese (n = 41) | P value |
|---|---|---|---|
| ICP (mmHg) | |||
| Mean (SD) | 13 (11) | 17 (18) | 0.25 |
| Min (SD) | 1 (6) | 4 (11) | 0.21 |
| Max (SD) | 34 (18) | 43 (32) | 0.17 |
| CPP (mmHg) | |||
| Mean (SD) | 83 (19) | 75 (25) | 0.18 |
| Min (SD) | 36 (29) | 30 (21) | 0.39 |
| Max (SD) | 155 (113) | 144 (107) | 0.67 |
| Mean CVP (SD) cm H2O | 8 (1) | 7 (1) | 0.25 |
| Mean Hb (SD) g/dl | 11.8 (5.1) | 10.0 (1.2) | 0.03 |
| Mean 24-h fluid balance (ml) | 416 (616) | 466 (743) | 0.77 |
| Mean Na (mmol/l) | 138 (5) | 142 (2) | 0.47 |
| Mean osmolarity (mmol/l) | 308 (3) | 313 (9) | 0.63 |
| Mean creatinine (mg/dl) | 1.8 (0.8) | 1.6 (0.6) | 0.85 |
| Mean arterial pH | 7.43 (0.00) | 7.43 (0.01) | 1.00 |
| Admission blood glucose (mg/dl), mean (SD) | 151 (55) | 140 (54) | 0.40 |
Values in bold are statistically significant
Data are expressed as means ± standard deviation (SD)
P bt O 2 brain tissue oxygen pressure, ICP intracranial pressure, CPP cerebral perfusion pressure, CVP central venous pressure, Hb hemoglobin, Na sodium, min minimum, max maximum
Brain Oxygen
Obese patients had lower initial, mean and minimum PbtO2 values than non-obese patients (Table 3; Fig. 1), although there was no significant difference in mean PaO2 or mean FiO2 between the two groups. Obese patients experienced a longer cumulative duration of compromised PbtO2 (P = 0.02). Acute respiratory distress syndrome significantly predicted compromised PbtO2 (P = 0.004), while the incidence of chest trauma did not (P = 0.08). Other univariate predictors of compromised PbtO2 included mean daily PaO2, mean PF ratio, and mean, minimum and maximum daily FiO2. Mean, maximum, or minimum ICP was not associated with compromised PbtO2 values; CPP also was not associated with lower PbtO2 values. Mean CVP, maximum BUN, and arterial pH demonstrated an association with reduced PbtO2 values.
Table 3.
Brain oxygen and lung function in obese and non-obese patients
| Physiological variable (SD) | Obese n (SD) | Non-obese n (SD) | P value |
|---|---|---|---|
| Mean PaO2/FiO2 ratio | 290.8 (25.5) | 338.0 (16.4) | 0.11 |
| FiO2 | |||
| Daily minimum FiO2 | 55.0 (3.3) | 48.3 (1.7) | 0.06 |
| Daily mean FiO2 | 62.2 (3.8) | 59.0 (2.2) | 0.44 |
| Daily maximum FiO2 | 76.9 (3.1) | 73.6 (2.6) | 0.41 |
| PaO2 | |||
| Daily PaO2 | 178.2 (16.1) | 192.3 (11.6) | 0.47 |
| Daily minimum PaO2 | 96.0 (17.9) | 107.1 (10.8) | 0.58 |
| Daily maximum PaO2 | 338.6 (27.3) | 327.3 (18.2) | 0.72 |
| PbtO2 | |||
| Initial PbtO2 mmHg | 17.1 (2.6) | 33.6 (3.2) | <0.01 |
| Daily minimum PbtO2 mmHg (average min) | 15.1 (1.5) | 19.9 (2.1) | 0.09 |
| Daily mean PbtO2 mmHg (average mean) | 25.8 (1.8) | 31.8 (1.9) | 0.03 |
| Daily maximum PbtO2 mmHg (average max) | 44.6 (5.1) | 51.9 (2.8) | 0.18 |
| Percentage of time PbtO2 < 20 mmHg | 26.3 (29.1) | 13.9 (17.4) | 0.03 |
| Minutes hypoxic or compromised (PbtO2 < 20 mmHg) | 1780 (2386) | 779 (808) | 0.02 |
Values in bold are statistically significant
Data are expressed as mean ± standard deviation (SD)
max maximum, min minimum, P bt O 2 partial pressure of brain tissue oxygen, PaO 2 partial pressure of arterial oxygen, FiO 2 fraction of inspired oxygen
Fig. 1.
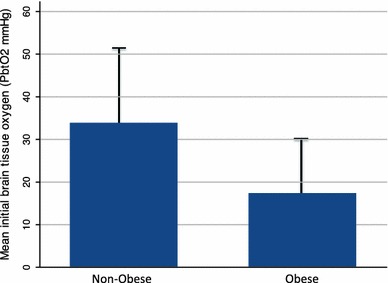
Histograms representing mean (SD) PbtO2 values according to the patients’ body mass (BMI) index at admission. Obese patients (BMI ≥ 30) had significantly lower mean daily PbtO2 values than non-obese patients (BMI < 30); P = 0.03
Obesity is an Independent Factor Associated with Compromised PbtO2
In multivariable analysis, BMI remained a significant predictor of compromised PbtO2 (P = 0.02). This effect persisted when controlling for the effects of univariate predictors (Table 4). Age also was an independent factor associated with compromised PbtO2; its predictive strength was similar to the effect of BMI. A greater mean CVP also was associated with compromised PbtO2.
Table 4.
Multivariable predictors of compromised PbtO2 (minutes spent with PbtO2 < 20 mmHg)
| Coefficient | Standard error | P value | |
|---|---|---|---|
| BMI | 64.4 | 28.9 | 0.03 |
| Age | −18.1 | 12.8 | 0.16 |
| Sex | 502.9 | 438.5 | 0.26 |
| ARDS | 1046.7 | 741.7 | 0.16 |
| Mean PaO2 | 4.4 | 4.7 | 0.35 |
| Mean PaO2:FiO2 | −2.6 | 3.2 | 0.42 |
| Mean daily CVP | 180.5 | 64.3 | <0.01 |
| Maximum daily ICP | 4.7 | 8.2 | 0.57 |
| APACHE II score | 21.1 | 38.5 | 0.59 |
| Constant | −2103.1 | 1736.0 | 0.23 |
Values in bold are statistically significant
Discussion
Obesity is a worldwide epidemic and its deleterious effects on cardiovascular health are well established. However, its effects on respiratory dysfunction and brain injury are less well studied. In this retrospective study of 69 patients with severe brain injury, we examined the relationship between obesity (defined by a BMI ≥ 30) and brain oxygenation. We hypothesized that obesity would compromise PbtO2, possibly through exacerbation of underlying pulmonary dysfunction. We found that initial PbtO2, mean daily PbtO2, and mean daily maximum PbtO2 measurements were significantly lower in obese patients than in non-obese patients. We observed a longer cumulative duration of compromised PbtO2 in obese patients. We demonstrated that obesity was associated with compromised PbtO2 independent of the effects of ICP, CPP, and PF ratio. Our findings suggest that obesity may be a risk factor for compromised PbtO2 after severe brain injury.
Altered Lung Function and Lower PbtO2 in Obese Patients
The precise reason(s) why PbtO2 was lower in the obese patients in our cohort remains unclear. There are many factors that may influence compromised PbtO2 but one possible mechanism is altered pulmonary function [7, 8]. Obesity affects control of the respiratory cycle, impairs respiratory muscle function, hampers gas exchange, and increases the risk of aspiration [21]. Airways resistance, metabolic demands, and work of breathing also are increased in obese patients; this may induce rapid shallow breathing, which exacerbates ventilation–perfusion mismatch [22, 23]. The intrapleural pressure at the lung base can exceed atmospheric pressure in the airway, causing bronchioles at the lung base to collapse [15, 24]. This further contributes to ventilation–perfusion mismatch [14, 25].
Obese patients in our study required prolonged mechanical ventilation and experienced a higher incidence of pneumonia. The association between obesity and lower respiratory tract infections is known [26]. Obesity has been identified as an independent risk factor of mortality among patients with H1N1 influenza virus [27]. The culprit mechanism may include obesity-related derangements of leptin and adiponectin, which have been linked to impaired immunity and response to infections [28].
Other Potential Reasons Why PbtO2 is Lower in Obese Patients
Obesity-related systemic inflammation may contribute to compromised PbtO2. Obesity is an inflammatory state that may aggravate the post-traumatic inflammatory response [29] among the patients with TBI or exacerbate delayed cerebral ischemia in SAH patients [30, 31]. Adipocytes are biologically active and have been shown to secrete tumor necrosis factor alpha, transforming growth factor beta, and interferon gamma; these cytokines have been associated with poor outcome in both TBI and SAH [32–34].
Other factors that may explain the effect of obesity on compromised PbtO2 include inadequate compensatory mechanisms, difficulty with vascular access, and compromised drug delivery [35].
In our cohort, elevated central venous pressure was significantly associated with compromised PbtO2. Elevated central venous pressures may result from either an increase in central venous volume, or decreased venous compliance. This may reflect decreased systemic oxygenation due to heart failure, although we did not collect echocardiographic data. An alternate explanation is that elevated CVP may reflect increased PEEP. This may be associated with obesity as obese patients may require more PEEP to maintain alveoli recruitment. It is less likely that increased PEEP is related to acute lung injury since ARDS was not a significant predictor of compromised PbtO2 in multivariable analysis.
Obesity in Neurosurgical Patients
There is little study on the effects of obesity on neurosurgical patients. While there is an association between obesity and complications after spine surgery [36, 37]; one recent study suggests that obesity may not influence outcome among patients who undergo craniotomy [38]. We did not observe a relationship between PbtO2 and elevated ICP or increased risk of ARDS and in multivariable analysis in this study. We had hypothesized that obesity would affect ICP by altered intracranial compliance and/or decreased venous return, and so compromise PbtO2. Consistent with this hypothesis, induced abdominal compartment syndrome is associated with an increase in central venous pressure, internal jugular pressure, and thoracic transmural pressure that together contribute to increased ICP. Future studies measuring cerebral blood flow, cerebrovascular resistance, and autoregulation may shed light on the effect of obesity on intracranial compliance.
Methodological Limitation
Our study has several potential limitations. First, the data were examined retrospectively and this may bias our results. However, the data were collected prospectively and our patients were treated according to a standard practice guideline that may limit potential bias. Second, the study was performed on patients with several different forms of acute brain injury. This may obscure the effects of obesity in different types of brain injury. For example, risk factors for DCI, such as arterial vasospasm, were not accounted for in our analysis. However, we lacked sufficient power to analyze the effect of DCI in the subset of brain-injured patients with SAH. Third, our sample size was limited since not every patient admitted to our ICU had both measured height and weight assessments. This may introduce a selection bias although demographic characteristics did not differ between the study cohort and excluded patients. Recorded estimates of height and weight were available for some patients; however, we chose not to use this data since it is known that estimates by health care providers often are inaccurate. This issue is common to many studies in this field where inadequate documentation of height and weight measurements compromises sample size [39–41]. Fourth, we defined obesity using BMI. While this is a valid method to define obesity, percent body fat (% BF) or abdominal girth may be more sensitive markers of patient risk than BMI [42]. Finally, the study is not a pure observational study since patients received therapeutic interventions to correct a reduced PbtO2 or an increased ICP. It is possible that interventions themselves may have obscured the effect obesity had on PbtO2.
Conclusions
The findings of the current study suggest that obesity is associated with reduced PbtO2 in severely brain-injured patients. This adverse effect may be associated with acute respiratory insufficiency, exacerbation of obesity-related pulmonary dysfunction, or failure of compensatory mechanisms given chronic underlying metabolic and inflammatory changes. Whether this translates into worse outcome is not clear from our data. Further study is warranted to test this hypothesis.
Acknowledgments
We acknowledge the valuable contributions of the nurses in the Neurosurgical Intensive Care Unit at the Hospital of the University of Pennsylvania in caring for our patients as well as their help in data acquisition. This study is supported by Research Grants from Integra Neurosciences (PDLR) and the Mary Elisabeth Groff Surgical and Medical Research Trust (PDLR). PDL is a member of Integra Lifesciences’s Speaker’s Bureau.
References
- 1.Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med. 2009;37(6):2057–2063. doi: 10.1097/CCM.0b013e3181a009f8. [DOI] [PubMed] [Google Scholar]
- 2.van den Brink WA, van Santbrink H, Steyerberg EW, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000;46(4):868–876. doi: 10.1097/00006123-200004000-00018. [DOI] [PubMed] [Google Scholar]
- 3.van Santbrink H, vd Brink WA, Steyerberg EW, Carmona Suazo JA, Avezaat CJ, Maas AI. Brain tissue oxygen response in severe traumatic brain injury. Acta Neurochir (Wien) 2003;145(6):429–438. doi: 10.1007/s00701-003-0032-3. [DOI] [PubMed] [Google Scholar]
- 4.Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805–811. doi: 10.3171/jns.2005.103.5.0805. [DOI] [PubMed] [Google Scholar]
- 5.Narotam PK, Morrison JF, Nathoo N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg. 2009;111(4):672–682. doi: 10.3171/2009.4.JNS081150. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishna R, Stiefel M, Udoetuk J, et al. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;109(6):1075–1082. doi: 10.3171/JNS.2008.109.12.1075. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal G, Hemphill JC, Sorani M, et al. The role of lung function in brain tissue oxygenation following traumatic brain injury. J Neurosurg. 2008;108(1):59–65. doi: 10.3171/JNS/2008/108/01/0059. [DOI] [PubMed] [Google Scholar]
- 8.Oddo M, Nduom E, Frangos S, et al. Acute lung injury is an independent risk factor for brain hypoxia after severe traumatic brain injury. Neurosurgery. 2010;67(2):338–344. doi: 10.1227/01.NEU.0000371979.48809.D9. [DOI] [PubMed] [Google Scholar]
- 9.McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiology. Thorax. 2008;63(7):649–654. doi: 10.1136/thx.2007.086801. [DOI] [PubMed] [Google Scholar]
- 10.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87(3):654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus R, Gore CJ, Booth M, Owen N. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr. 1998;68(1):35–41. doi: 10.1093/ajcn/68.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000;85(1):91–108. doi: 10.1093/bja/85.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Yamane T, Date T, Tokuda M, et al. Hypoxemia in inferior pulmonary veins in supine position is dependent on obesity. Am J Respir Crit Care Med. 2008;178(3):295–299. doi: 10.1164/rccm.200801-113OC. [DOI] [PubMed] [Google Scholar]
- 15.Holley HS, Milic-Emili J, Becklake MR, Bates DV. Regional distribution of pulmonary ventilation and perfusion in obesity. J Clin Invest. 1967;46(4):475–481. doi: 10.1172/JCI105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedenstierna G. Gas exchange during anaesthesia. Br J Anaesth. 1990;64(4):507–514. doi: 10.1093/bja/64.4.507. [DOI] [PubMed] [Google Scholar]
- 17.Lehrman SG, Limann B, Koshy A, Aronow WS, Ahn C, Maguire G. Association of lung volumes with nocturnal oxygen saturation in obese persons: a possible role for therapeutic continuous positive airway pressure. Am J Ther. 2008;15(3):221–224. doi: 10.1097/MJT.0b013e3180a721f7. [DOI] [PubMed] [Google Scholar]
- 18.Belzberg H, Wo CC, Demetriades D, Shoemaker WC. Effects of age and obesity on hemodynamics, tissue oxygenation, and outcome after trauma. J Trauma. 2007;62(5):1192–1200. doi: 10.1097/01.ta.0000219701.07295.b8. [DOI] [PubMed] [Google Scholar]
- 19.Isozumi K. Obesity as a risk factor for cerebrovascular disease. Keio J Med. 2004;53(1):7–11. doi: 10.2302/kjm.53.7. [DOI] [PubMed] [Google Scholar]
- 20.Juvela S, Siironen J, Lappalainen J. Apolipoprotein E genotype and outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2009;110(5):989–995. doi: 10.3171/2008.11.JNS081266. [DOI] [PubMed] [Google Scholar]
- 21.Corrales-Medina VF, Valayam J, Serpa JA, Rueda AM, Musher DM. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis. 2011;15(1):e54–e57. doi: 10.1016/j.ijid.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax. 2008;63(8):738–746. doi: 10.1136/thx.2007.086843. [DOI] [PubMed] [Google Scholar]
- 23.deJong AT, Gallagher MJ, Sandberg KR, et al. Peak oxygen consumption and the minute ventilation/carbon dioxide production relation slope in morbidly obese men and women: influence of subject effort and body mass index. Prev Cardiol. 2008;11(2):100–105. doi: 10.1111/j.1751-7141.2008.07591.x. [DOI] [PubMed] [Google Scholar]
- 24.Ray DE, Matchett SC, Baker K, Wasser T, Young MJ. The effect of body mass index on patient outcomes in a medical ICU. Chest. 2005;127(6):2125–2131. doi: 10.1378/chest.127.6.2125. [DOI] [PubMed] [Google Scholar]
- 25.Tucker DH, Sieker HO. The effect of change in body position on lung volumes and intrapulmonary gas mixing in patients with obesity, heart failure, and emphysema. Am Rev Respir Dis. 1960;82:787–791. doi: 10.1164/arrd.1960.82.6.787. [DOI] [PubMed] [Google Scholar]
- 26.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160(20):3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 27.Riquelme R, Jimenez P, Videla AJ, et al. Predicting mortality in hospitalized patients with 2009 H1N1 influenza pneumonia. Int J Tuberc Lung Dis. 2010;15(4):542–546. doi: 10.5588/ijtld.10.0539. [DOI] [PubMed] [Google Scholar]
- 28.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 29.Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14(7–8):485–492. doi: 10.2119/2008-00038.Nathan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaichana KL, Pradilla G, Huang J, Tamargo RJ. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg. 2010;73(1):22–41. doi: 10.1016/j.surneu.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 31.Gallia GL, Tamargo RJ. Leukocyte-endothelial cell interactions in chronic vasospasm after subarachnoid hemorrhage. Neurol Res. 2006;28(7):750–758. doi: 10.1179/016164106X152025. [DOI] [PubMed] [Google Scholar]
- 32.Pellettieri L, Carlson CA, Lindholm L. Is the vasospasm following subarachnoidal hemorrhage an immunoreactive disease? Experientia. 1981;37(11):1170–1171. doi: 10.1007/BF01989900. [DOI] [PubMed] [Google Scholar]
- 33.Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A. Possible role of the C-reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2006;18(1):68–72. doi: 10.1097/01.ana.0000181693.30750.af. [DOI] [PubMed] [Google Scholar]
- 34.Brown CV, Rhee P, Neville AL, Sangthong B, Salim A, Demetriades D. Obesity and traumatic brain injury. J Trauma. 2006;61(3):572–576. doi: 10.1097/01.ta.0000200842.19740.38. [DOI] [PubMed] [Google Scholar]
- 35.Joffe A, Wood K. Obesity in critical care. Curr Opin Anaesthesiol. 2007;20(2):113–118. doi: 10.1097/ACO.0b013e3280803d5f. [DOI] [PubMed] [Google Scholar]
- 36.Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144–157. doi: 10.3171/2010.3.SPINE09369. [DOI] [PubMed] [Google Scholar]
- 37.Schuster JM, Rechtine G, Norvell DC, Dettori JR. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine (Phila Pa 1976) 2010;35(9 Suppl):S125–S137. doi: 10.1097/BRS.0b013e3181d8342c. [DOI] [PubMed] [Google Scholar]
- 38.Schultheiss KE, Jang YG, Yanowitch RN, et al. Fat and neurosurgery: does obesity affect outcome after intracranial surgery? Neurosurgery. 2009;64(2):316–326. doi: 10.1227/01.NEU.0000336329.90648.17. [DOI] [PubMed] [Google Scholar]
- 39.Choban PS, Weireter LJ, Jr, Maynes C. Obesity and increased mortality in blunt trauma. J Trauma. 1991;31(9):1253–1257. doi: 10.1097/00005373-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Arbabi S, Wahl WL, Hemmila MR, Kohoyda-Inglis C, Taheri PA, Wang SC. The cushion effect. J Trauma. 2003;54(6):1090–1093. doi: 10.1097/01.TA.0000064449.11809.48. [DOI] [PubMed] [Google Scholar]
- 41.Brown CV, Neville AL, Rhee P, Salim A, Velmahos GC, Demetriades D. The impact of obesity on the outcomes of 1,153 critically injured blunt trauma patients. J Trauma. 2005;59(5):1048–1051. doi: 10.1097/01.ta.0000189047.65630.c5. [DOI] [PubMed] [Google Scholar]
- 42.Waisbren E, Rosen H, Bader AM, Lipsitz SR, Rogers SO, Jr, Eriksson E. Percent body fat and prediction of surgical site infection. J Am Coll Surg. 2010;210(4):381–389. doi: 10.1016/j.jamcollsurg.2010.01.004. [DOI] [PubMed] [Google Scholar]


