Abstract
Our aim was to clarify the significance of phenotype of circulating CD8 T+ cells on the outcome of ABO-incompatible (ABO-I) living donor liver transplantation (LDLT). Twenty-six recipients undergoing ABO-I LDLT and 92 undergoing ABO-compatible (ABO-C) LDLT were classified into three groups according to preoperative proportion of CD8 T+ cells: naive-dominant (group I), effector memory-dominant (group II), and effector-dominant (group III) recipients. The clinical courses were analyzed. The results showed that in ABO-C groups I and II and in ABO-I group I, effector cells remained above the pretransplant levels after tacrolimus administration. However, in ABO-C group III and ABO-I groups II and III, effector cells were down-regulated for a prolonged period, along with markedly decreased perforin expression and frequent life-threatening complications. ABO-I group II and group III recipients had higher infection rates. It was concluded that recipients with preexisting high effector CD8 T+ cells are unfavorable candidates for ABO-I LDLT.
Keywords: ABO-incompatible, CD8+ T cell phenotype, Infection, Liver transplant recipients
Introduction
ABO-incompatible (ABO-I) living donor liver transplantation (LDLT) has been often performed in Japan, because living donors are restricted to relatives or spouses. The rates of patient and graft survival are lower in ABO-I transplants than in ABO-compatible (ABO-C) transplants because of antibody-mediated rejection events, such as vascular thrombosis and ischemic bile duct complications. Recently, encouraging results have been achieved with new strategies to reduce posttransplant-specific hemagglutinin titers, including reinforced immunosuppression, splenectomy, and the use of rituximab [1, 2]. At our institution, we have developed local infusion therapy with methylprednisolone and prostaglandin E1 (PGE1) to ameliorate endothelial injury by antibody-mediated reactions. Methylprednisolone and PGE1 have been infused through the hepatic artery, and acceptable outcomes have been achieved in adult ABO-I LDLT with rituximab prophylaxis in 2006 [3].
The proportion of memory phenotype in T cells of recipients before transplantation can vary greatly according to exposure to environmental antigens and a decreasing ability to mediate effective immune responses to newly encountered antigens with increasing age [4]. In particular, a preexisting memory pool, produced by a host’s history of infection, is more likely to affect the course of infection and be a potential barrier for tolerance induction, a phenomenon termed heterologous immunity [5, 6]. Accordingly, it is very important to clarify how preexisting memory T cells affect the defense against repeated infections and pathogens that have not been encountered previously and repeat infections. Our previous studies have shown that the probability of survival and the frequency of infection after LDLT are closely related to the effector T cell population of circulating CD8+ T cells before LDLT [7] and that CD4+ cells play an important role in CD8+ T cell differentiation [8].
To improve the outcome of ABO-I LDLT, high-risk patients should be avoided, if they can be identified. Therefore, it is of great importance to investigate how the phenotypic and functional characteristics of circulating CD8+ effector T cells after LDLT may affect clinical outcomes. On the other hand, hierarchical clustering algorithms have been used to distinguish recipients so as to identify clusters of recipients with a similar proportion of naive, central/memory (CM), effector/memory (EM), and effector T cells. We studied the impact of phenotypic and functional analyses of CD8+ effector T cells on the clinical outcomes of ABO-I recipients and ABO-C recipients.
Patients and Methods
Patients and Graft
Our study involved 118 patients who had undergone standard LDLT from 2002 through 2007 at Kyoto University Hospital. Twenty-six patients underwent ABO-I LDLT. Their ages ranged from 19 to 67 years. The patients were followed-up from the time of transplantation until May 2007 or, if earlier, death. The median follow-up period was 2.7 years after LDLT (range, 6 months to 4.4 years). Written informed consent was obtained from all subjects before the start of the study, which was approved by the ethics committee of Kyoto University Hospital and was conducted in accordance with the Declaration of Helsinki of 1975 as revised in 1996.
Immunosuppression
All patients underwent standard LDLT [9]. The initial immunosuppression regimen after LDLT was tacrolimus with corticosteroids, using our standard procedure [7]. Methylprednisolone (10 mg/kg) was administered just before the start of graft reperfusion in all recipients. Afterward, in ABO-C recipients, 1 mg/kg of intravenous methylprednisolone was administered for 3 days and 0.5 mg/kg of intravenous methylprednisolone was administered for a further 3 days. Oral prednisolone, 0.3 mg/kg, was continued for 3 months. Tacrolimus was administered from day 1 according to our standard procedure [7]. If acute graft rejection was confirmed, recipients received a 3-day course of intravenous corticosteroid bolus therapy (10 mg/kg), the tacrolimus dosage was increased, and mycophenolate mofetil (MMF) was added if necessary. MMF was also added if patients could not tolerate calcineurin inhibitors.
Strategy for ABO-I LDLT
Figure 1a shows the scheme of immunosuppression regimens after LDLT for ABO-I recipients. Two types of local infusion therapy (portal vein infusion [PVI] and hepatic artery infusion [HAI]) were given for 3 weeks after LDLT. PVI was introduced in 2000, HAI combined with PVI (PVI + HAI) in 2001, and HAI without PVI in 2003. For HAI, an 18-G catheter designed for a central venous line was placed in the hepatic arterial branch of the recipient after hepatic artery anastomosis during the operation, which was not used for reconstruction. In addition to the basic immunosuppression, methylprednisolone (125 mg/day for the first week and 50 mg/day for the following 2 weeks) and PGE1 (0.01 μg/kg/min for 3 weeks) were administered immediately through the hepatic artery catheter. In PVI, PGE1, methylprednisolone, and mesilates (Ono, Osaka, Japan) were administered through a catheter into the portal vein [10]. In HAI, PGE1 and corticosteroids were administrated through a catheter into the hepatic artery [3, 10]. In the HAI + PVI protocol, PGE1 and corticosteroids were administered through a catheter into the hepatic artery and mesilates were administered through a catheter into the portal vein.
Fig. 1.
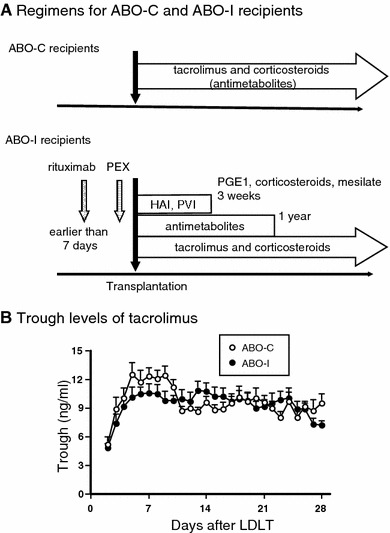
The scheme of immunosuppression regimen and the changes in trough levels of tacrolimus after LDLT in ABO-C and ABO-I recipients. a For a recipient of ABO-I LDLT, rituximab was administered once at a dose of 375 mg/m2 as prophylaxis before transplantation. PEX was performed, aiming for an antibody titer 8-fold less before transplantation. Local infusion therapy was continued for 3 weeks. Intravenous cyclophosphamide was administered for 2 weeks at a dose of 2 mg/kg/day and then MMF was administered (500 mg twice/day). MMF was continued for 1 year after transplantation. Basic immunosuppression consisting of tacrolimus and corticosteroids was the same for ABO-C LDLT. b There was no significant differences in trough levels of tacrolimus between ABO-C and ABO-I recipients. LDLT, living donor liver transplantation; HAI, hepatic artery infusion; PVI, portal vein infusion; PGE1, prostaglandin E1; PEX, plasmapheresis
Intravenous cyclophosphamide was administered for 2 weeks with a dose of 2 mg/kg/day, after which MMF was administered (500 mg twice/day). MMF was continued for 1 year after LDLT. Rituximab was administered once at a dose of 375 mg/m2 as prophylaxis before transplantation [3].
In our current policy for ABO-I, splenectomy is usually not performed. Our indications for splenectomy are (a) the future anti-hepatitis C viral treatment of recipients with thrombocytopenia, (b) thrombocytopenia or severe splenomegaly affecting quality of life or both, and (c) portal flow modulation for a small-for-size graft. Plasma exchange with blood type AB fresh-frozen plasma (0.5 unit/kg) was performed to achieve an antibody titer 8-fold or less before transplantation. After transplantation, plasma exchange was sustained until the antibody titer increased at least 256-fold.
Figure 1b shows the changes in tacrolimus trough levels after LDLT in ABO-C and ABO-I recipients. The trough levels were maintained at appropriate values in both groups of recipients and did not differ significantly between the groups.
Definition and Treatment of Rejection
In cases of clinical or laboratory signs of rejection, a liver biopsy was performed percutaneously. The specimens were graded according to the Banff criteria [11] as showing mild, moderate, or severe acute cellular rejection (ACR) or chronic rejection. Antibody-mediated rejection (AMR) in ABO-I LDLT was defined clinically and histologically separately from ACR and chronic rejection [12–14].
Definition of Surgical Complication
Surgical complications, such as vascular complications, biliary complications, and intestinal complications requiring radiological or surgical intervention were defined as surgical complications.
Definition of Infectious Complication
A bacterial, viral, or fungal infection was assumed if clinical or laboratory signs of acute infection or positive serologic markers or culture were found, as reported previously [15]. We used the criteria for sepsis defined by Bone [16].
Virology
Serum qualitative HCV-RNA was determined with the polymerase chain reaction method using a commercially available assay (Amplicor HCV; Roche Molecular Systems, Pleasanton, CA).
Tissue Typing
Serologic tissue typing was performed in all recipients for human leukocyte antigen (HLA)-A, HLA-B (Bw), HLA-C, HLA-DR, and HLA-DQ for class I and II loci.
Flow Cytometry
Heparinized venous blood samples were obtained 1 h before surgery, then at 0, 1, 3, 6, 12, 36, and 120 h, and every week or month following graft reperfusion for up to 15 months. A total of 2,550 samples from 118 recipients were analyzed.
The monoclonal antibodies used to stain cell-surface antigens were as previously reported [7]. The expression of interleukin (IL)-12 receptors was determined with R-phycoerythrin-conjugated anti-IL-12Rβ1 and IL-12Rβ2 antibodies (BD Biosciences, San Diego, CA). We analyzed the stained cells with a FACS Calibur flow cytometer by three- and four-color analysis, using CELL Quest software, version 3.3 (BD Biosciences).
Flow Cytometric Detection of Cytokine Production and Intracellular Staining for Perforin
Flow cytometric measurement of cytokine production was performed as described previously [17]. We measured intracellular perforin in CD8+ cells without previous stimulation.
Statistical Analysis
We performed hierarchical cluster analysis [18] using JMP 5 (SAS Institute Inc., Cary, NC) to identify clusters of recipients with similar proportions of naive, CM, EM, and effector T cells, as reported previously [7].
The posttransplant immune status was evaluated according to the following measure. To quantify changes in posttransplant alloreactive responses, the proportion of CD8+ T cell subsets (naive, CM, EM, effector) immediately before LDLT (pretransplant immune status) was subtracted from the proportion at various times after LDLT and expressed as % difference. This value reflects current immune status after LDLT. Similarly, the % difference was calculated for other variables, such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-12Rβ1+ cells, and perforin. With this assay, posttransplant immune status could be compared between recipients.
Continuous variables between groups were compared using Student’s t-test and ANOVA. Proportions between groups were compared using Fisher’s exact test and the χ2 test. All statistical tests were two-sided, with significance defined as P < 0.05.
% Difference as the Measure for Evaluation of Posttransplant Immune Status
There are remarkable differences in the phenotype and function of T cells before and after LDLT among many heterogeneous recipients. From the analysis of our vast data bank, we have noticed that the posttransplant changes in phenotypic and functional properties of CD8+ T cells can be almost restored to pretransplant patterns. Therefore, the pretransplant value can be chosen as baseline. In order to compare posttransplant immune status in many recipients, it is particularly useful to estimate the magnitude of the additional immunologic load burden by liver transplantation in comparison with pretransplant immune status. As a measure, the proportion of the variables immediately before LDLT was subtracted from the proportion at various times after LDLT, and is expressed as % difference [19]. Similarly, the % difference was calculated for CD8+ T cell subsets and other variables such as IFN-γ, TNF-α, IL-12Rβ1+cells, perforin, and CD8+CD27−CD28− T cells.
Results
CD8+ T cell Subsets Before LDLT are Heterogeneous in Phenotype, Function, and Protective Capacity
Hierarchical Clustering by Preoperative CD8+CD45 Isoform Profiles
Table 1 shows the classification of recipients according to the hierarchical clustering of circulating CD8+ T cell-subpopulations before LDLT in the recipients undergoing ABO-I or ABO-C LDLT. Patients in group I were slightly but not significantly younger than those in groups II and III. There were significant differences in CD45 isoforms between the three groups. In ABO-I recipients, the pretransplantation mean proportion of naive T cells was 58.3% in group I (naive-dominant), 10.0% in group II, and 21.4% in group III. The CD8+ T cells in group II included the greatest numbers of EM T cells (EM dominant), and in group III they included the greatest number of effector T cells (effector-dominant). The proportion of CD8+ T cell subsets was not significantly different between ABO-I and ABO-C recipients. Also, the proportion or absolute number of effector T cells after LDLT was similar in ABO-I and ABO-C recipients (data not shown).
Table 1.
Characteristics in ABO-I or ABO-C recipients: phenotypic and functional characteristics of CD8+ T cell subsets before LDLT in three groups of ABO-I or ABO-C recipients
| Characteristic | Group I | Group II | Group III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ABO-I | ABO-C | P* | ABO-I | ABO-C | P | ABO-I | ABO-I | P | |
| n | 7 | 31 | 4 | 33 | 15 | 28 | |||
| Age (y) | 46 ± 12 | 47 ± 11 | 0.8591 | 56 ± 8 | 53 ± 9 | 0.5536 | 53 ± 11 | 53 ± 12 | 0.9509 |
| % Naïve T cells | 58.34 ± 14.38 | 53.44 ± 10.42 | 0.3010 | 9.99 ± 4.26 | 20.80 ± 11.99 | 0.0855 | 21.38 ± 10.97 | 21.22 ± 10.52 | 0.9641 |
| % CM T cells | 6.08 ± 3.30 | 7.56 ± 4.19 | 0.3885 | 9.82 ± 5.20 | 11.88 ± 6.04 | 0.5194 | 3.48 ± 2.43 | 5.88 ± 3.43 | 0.0207 |
| % EM T cells | 5.39 ± 3.07 | 7.01 ± 5.53 | 0.4610 | 25.61 ± 15.47 | 20.24 ± 7.21 | 0.2273 | 8.82 ± 4.49 | 7.13 ± 4.79 | 0.2660 |
| % Effector T cells | 17.51 ± 7.76 | 18.49 ± 11.98 | 0.7869 | 30.14 ± 12.86 | 24.25 ± 11.66 | 0.3509 | 47.19 ± 12.86 | 48.36 ± 12.77 | 0.7765 |
| % CD27−CD28− subsets | 13.04 ± 9.59 | 16.78 ± 8.90 | 0.4106 | 14.09 | 42.84 ± 17.40 | 48.65 ± 18.88 | 40.83 ± 18.34 | 0.3614 | |
| % IFN-γ | 13.10 ± 8.58 | 9.09 ± 3.76 | 0.1956 | 17.45 | 13.21 ± 8.00 | 25.44 ± 16.35 | 20.29 ± 16.36 | 0.6318 | |
| % TNF-α | 11.82 ± 9.62 | 7.98 ± 2.72 | 0.2711 | 10.62 | 13.33 ± 7.73 | 25.86 ± 13.07 | 21.08 ± 19.53 | 0.6981 | |
| % IL-12Rβ1 | 41.65 ± 15.25 | 45.72 ± 11.65 | 0.6280 | 74.85 ± 0.83 | 71.02 ± 13.53 | 0.7121 | 71.53 ± 9.61 | 73.05 ± 13.08 | 0.8303 |
| % Perforin | 19.36 ± 10.43 | 13.93 ± 7.49 | 0.2109 | 14.82 ± 0.21 | 23.66 ± 11.82 | 0.3414 | 35.88 ± 17.24 | 26.79 ± 8.16 | 0.1713 |
LDLT, living donor liver transplantation; ABO-I, ABO-incompatible; ABO-C, ABO-identical and compatible; CM T cells, central/memory T cell subsets, CD45RO+CCR7+; EM T cells, effector/memory T cell subsets, CD45RO+CCR7–; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor alpha; IL-12Rβ1, interleukin-12 receptor beta1
Values expressed are mean ± SD
* P values based on ANOVA
On the other hand, we analyzed these differentiation steps by extensively phenotyping CD8+ T cells with the differentiation markers CD28, CD27 [20], and CCR7 and by determining CD45RA versus CD45RO expression. In addition, IL-12Rβ1+ cells were measured because IL-12 plays an important role in promoting Th1-type immune responses and cell-mediated immunity [21]. The proportion of CD27−CD28− subsets, and IFN-γ, TNF-α, IL-12Rβ1, and perforin expression in ABO-I recipients were similar to those in ABO-C recipients.
Table 2 shows pretransplant characteristics in groups I, II, and III recipients undergoing ABO-I or ABO-C LDLT. There was no difference in the primary diseases or the number of recipients with a model for end-stage liver disease (MELD) score [22] greater than 20 between ABO-I and ABO-C recipients. There was no significant difference in frequency of HLA mismatch loci greater than 3 between ABO-I and ABO-C. The length of follow-up period for surviving recipients was similar in each subgroup.
Table 2.
Characteristics in ABO-I or ABO-C recipients
| Group | ABO-I recipients | ABO-C recipients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender (M/F) | Disease | MELD > 20 | HLA–MM > 3 | Follow-up (days) | Gender (M/F) | Disease | MELD > 20 | HLA–MM > 3 | Follow-up (days) | |
| I | 1/6 | 2 HCV, 2 HBV, 1 FHF, 2 PBC | 2 (29%) | 4 (57%) | 1034 ± 464 | 18/13 | 11 HCV, 6 HBV, 4 FHF, 4 PBC, 2 PSC, 1 BA, 1 LC, 1 alcoholic, 1 policystic liver | 9 (29%) | 13 (42%) | 1130 ± 247 |
| II | 3/1 | 2 HCV, 1 HBV, 1 alcoholic | 1 (25%) | 2 (50%) | 992 ± 693 | 23/10 | 13 HCV, 12 HBV, 2 FHF, 4 PBC, 1 PSC, 1 AIH, 1 Caroli | 9 (27%) | 16 (48%) | 1136 ± 308 |
| III | 6/9 | 5 HCV, 5 HBV, 2 PBC, 1 PSC, 1 Wilson’s disease, 1 policystic liver | 6 (40%) | 13 (87%) | 1022 ± 305 | 15/13 | 16 HCV, 6 HBV, 2 PBC, 2 BA, 1 HCC, 1 alcoholic | 6 (21%) | 21 (75%) | 1023 ± 375 |
ABO-I, ABO-incompatible; ABO-C, ABO-identical and compatible; MELD, model for end-stage liver disease score; HCV, hepatitis C virus; HBV, hepatitis B virus; FHF, fulminant hepatic failure; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; BA, biliary atresia; AIH, autoimmune hepatitis; HCC, hepatocellular carcinoma
Values are expresses as mean ± standard deviation. Follow-up days are given for surviving patients
Changes in Effector T Cells After ABO-I and ABO-C LDLT
Figure 2 shows changes in the proportion (top) and % difference (bottom) of CD8+ effector T cells after ABO-C and ABO-I LDLT in the three groups. After both types of LDLT, the proportion and % difference of CD8+ effector T cells (% effector difference) in the three groups promptly increased within 6 h following graft reperfusion and then down-regulated similarly to near or below preoperative levels after tacrolimus administration. The levels of up-regulation of effector T cells at 6 h decreased progressively from the highest in group I recipients to the lowest in group III recipients. After tacrolimus administration, in ABO-I and ABO-C recipients the proportion of CD8+ effector T cells remained at the lowest levels of approximately 20% throughout the posttransplant period. On the other hand, the % effector difference following tacrolimus administration was markedly different in group II between ABO-I and ABO-C LDLT. In both ABO-I and ABO-C recipients, the % effector difference in group I remained at baseline until day 12 and then increased to approximately 10% higher than baseline throughout the posttransplant period in response to posttransplant infection (data not shown). In ABO-C recipients, the % effector difference in group II remained at near or slightly higher than baseline until day 15 and then increased to approximately 5% by day 40. In ABO-I recipients, in contrast, the % effector difference in group II was significantly decreased by day 30 and then returned to baseline level. In group III recipients of either ABO-I or ABO-C, the % effector difference was similarly decreased to −15% for a prolonged period during the posttransplant period.
Fig. 2.
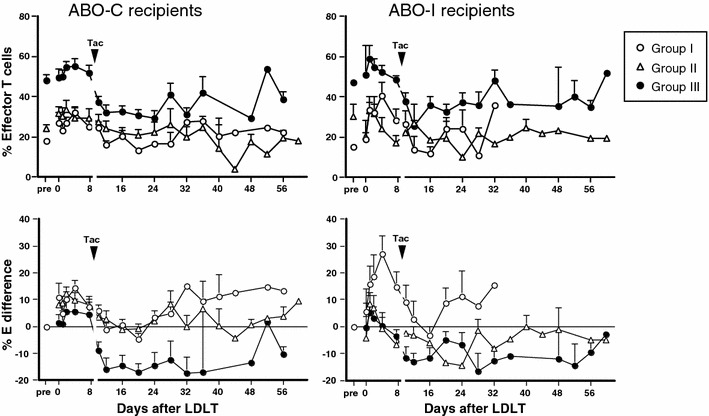
Changes in the proportion (top) and the difference (bottom) of CD8+ effector T cells after LDLT in 3 groups of ABO-C and ABO-I recipients. % E difference refers to % difference of CD8+ effector T cells. LDLT, living donor liver transplantation
Figure 3a shows changes in the % perforin difference of CD8+ effector T cells after LDLT in three groups of ABO-C and ABO-I recipients. The % perforin difference was similarly increased at 6 h in the three groups of ABO-C recipients, whereas in ABO-I recipients the increase at 6 h was the highest in group I, intermediate in group III, and the lowest in group II. After tacrolimus administration in ABO-C recipients, the % perforin difference in groups I and II remained near baseline until day 19, but had increased to approximately 10% greater than pretransplant value by day 33 in group I. In contrast, in group III recipients, the % perforin difference was transiently decreased from day 5 and then returned to pretransplant values by day 12. On the other hand, in ABO-I group I recipients, the % perforin difference remained at baseline from day 5 to day 12 after tacrolimus administration and then increased to greater than 20%, which corresponded to an increase in % effector difference in response to infection. In ABO-I group II recipients, the perforin difference remained near baseline throughout the posttransplant period. However, in ABO-I group III recipients, the % perforin difference was greatly decreased to approximately −20% by day 33. These results indicate that cytotoxic T lymphocyte (CTL) cytotoxicity in ABO-C group III recipients decreased transiently and then recovered by day 12, whereas in ABO-I group III recipients, CTL cytotoxicity decreased considerably throughout the posttransplant period. Figure 3b shows the results of flow cytometry in representative ABO-C and ABO-I group III recipients, specifically, an ABO-C recipient (a 54-year-old man) with hepatitis B-related liver cirrhosis and hepatocellular carcinoma undergoing LDLT and an ABO-I recipient (a 60-year-old man) with hepatitis B-related liver cirrhosis and hepatocellular carcinoma undergoing LDLT.
Fig. 3.
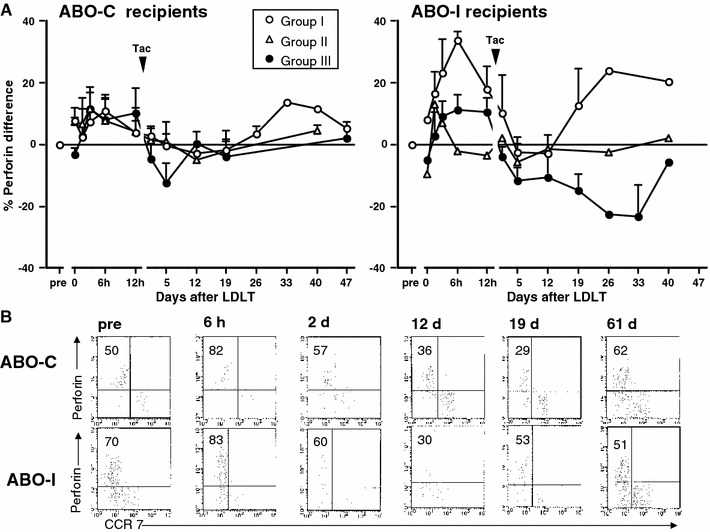
Changes in the % difference of perforin in CD8+ effector T cells after LDLT in three groups of ABO-C and ABO-I recipients. a The proportion of perforin expression is expressed as % of CD8+ T cells. b Flow cytometry of representative ABO-C and ABO-I group III recipients. Dot plots show double staining for perforin/CCR7. Perforin/CCR7 was gated on CD8+CD45RO−. Cells in the upper left are presented as %.
Tac, tacrolimus; LDLT, living donor liver transplantation
Frequencies of Infection, Rejection, Life-Threatening Complications, and Mortality After ABO-I and ABO-C LDLT
Figure 4 shows the comparison of posttransplant complication rates in ABO-I and ABO-C recipients. Most of bacterial infections were caused by Staphylococcus, Enterococccus, or Pseudomonas species, fungal infections by Candida and Aspergillus, and viral infection by cytomegalovirus. Overall, the incidence of infection, life-threatening complications (LTCs), and hospital mortality were significantly greater in ABO-I recipients than in ABO-C recipients. Interestingly, the ACR rates were similarly low in group I after both types of LDLT but were lower (0%) in ABO-I group II recipients than in ABO-C group II recipients. Three patients developed pathology-defined AMR and one patient developed clinical and fatal AMR after ABO-I transplantation without rituximab prophylaxis. No patients with rituximab prophylaxis developed AMR. In ABO-I recipients, the incidence of infection was significantly higher in group II (P = 0.0207), and the incidence of LTCs was significantly higher in group II (P = 0.0049) and group III (P = 0.0192). The hospital death rate was highest in group III of ABO-I recipients.
Fig. 4.
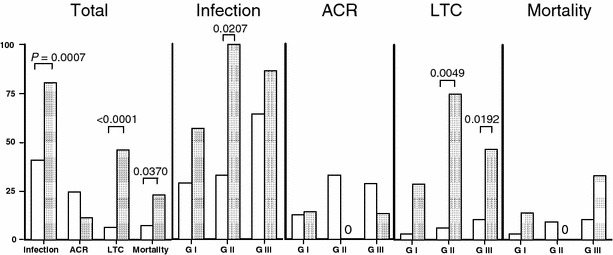
The frequencies of infection, ACR, LTCs, and hospital mortality after ABO-C LDLT and ABO-I LDLT. White bars show ABO-C LDLT, dark bars ABO-I LDLT.
ACR, acute cellular rejection; LTC, life-threatening complication; LDLT, living donor liver transplantation
Table 3 shows the treatments for the ABO barrier, and the frequencies of rejection, LTCs, and hospital deaths for each patient of the three groups of ABO-I recipients. Three recipients underwent PVI, one underwent PVI + HAI, and the other 22 underwent HAI as a local infusion treatment. Ten recipients were given rituximab as prophylaxis. Splenectomy was performed in three recipients. Four of seven patients (57%) of group I, four of 4 (100%) in group II, and 13 of 15 (87%) of group III recipients had infectious complications. Septic shock was the most common LTC in ABO-I recipients. One recipient with pneumonia and two recipients with respiratory distress required respiratory support in the intensive care unit. There was no hospital deaths in group II, but one-third of group III recipients died in the hospital.
Table 3.
Characteristics and postoperative courses of patients undergoing ABO-I transplantation
| Patient | Age (gender) | Diseases | Treatments for ABO barrier | Infection | Rejection | Life-threatening complications | Hospital death (days posttransplant) |
|---|---|---|---|---|---|---|---|
| Group I | |||||||
| 1 | 41 F | PBC | HAI | – | AMR(C4d+) | – | – |
| 2 | 38 F | FHF | HAI | CMV | mod ACR | – | – |
| 3 | 33 F | HBV-LC, PVT | HAI | – | AMR(C4d+) | – | – |
| 4 | 65 F | HCV-LC | HAI, Rit | – | – | – | – |
| 5 | 61 F | HCV-LC, HCC | HAI, Rit | Bacteremia CMV | – | – | – |
| 6 | 43 F | PBC | HAI, Rit | Peritonitis | – | Hypovolemic shock at splenic vein rapture | 123 |
| 7 | 42 M | HBV-LC | HAI, Rit, splenectomy | Aspiration pneumonitis | – | DIC aspiration pnemonitis ARDS | – |
| Group II | |||||||
| 8 | 67 M | HCV-LC, HCC | PVI | Tuberculosis | – | Septic shock | – |
| 9 | 52 M | Alcoholic, LC | PVI & HAI | Peritonitis CMV | – | Septic shock | – |
| 10 | 56 F | HCV-LC, HCC | HAI, Rit, splenectomy | CMV | – | – | – |
| 11 | 49 M | Retransplantation | HAI, splenectomy | Bacteremia | – | DIC pneumonia encephalopathy | – |
| Group III | |||||||
| 12 | 60 M | HCV-LC, HCC | PVI | CMV | – | – | – |
| 13 | 51M | HCV-LC | HAI | Bacteremia | – | – | – |
| 14 | 24 F | Wilson’s disease | HAI | Bacteremia CMV | Mild ACR | Septic shock | – |
| 15 | 60M | HBV-LC, HCC | PVI | Cholangitis CMV | – | PVT biliary leakage septic shock | – |
| 16 | 56 F | HCV-LC, HCC | HAI | Peritonitis bacteremia | Mild ACR | Septic shock | 218 |
| 17 | 58 F | HBV-LC | HAI | Peritonitis | – | – | 185 |
| 18 | 38 F | HBV-LC | HAI, Rit | Pneumonia peritonitis | – | HAT septic shock | 63 |
| 19 | 67 F | HCV-LC, HCC | HAI | Pneumonia CMV | – | ARDS | – |
| 20 | 51 F | Policystic liver | HAI | Sepsis CMV | AMR (C4d+) | Septic shock | 67 |
| 21 | 56 F | PBC | HAI, Rit | – | – | – | – |
| 22 | 47M | PSC | HAI | Peritonitis CMV | – | – | – |
| 23 | 57 F | HCV-LC, HCC | HAI | Peritonitis CMV | AMR (fatal) | Hepatic necrosis | 49 |
| 24 | 64 F | PBC | HAI, Rit | Peritonitis CMV | – | – | – |
| 25 | 42M | HBVLC | HAI, Rit | Peritonitis | – | – | – |
| 26 | 59M | HBVLC | HAI, Rit | CMV tuberculosis | – | – | – |
HBV-LC, HBV liver cirrhosis; HCV-LC, HCV liver cirrhosis; HCC, hepatocellular carcinoma; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; HAI, hepatic artery infusion; PVI, portal vein infusion; Rit, rituximab prophylaxis; CMV, cytomegalovirus; ACR, acute cellular rejection; AMR, antibody-mediated rejection; PVT, portal vein thrombus; HAT, hepatic artery thrombus; DIC, disseminated intravascular coagulation; ARDS, adult respiratory distress syndrome
Discussion
Phenotypic and Functional Changes of CD8+ Effector T Cells Before and After LDLT
Pre-LDLT
The preexisting effector T cell proportion was lowest in ABO-I and ABO-C group I recipients but was significantly increased in groups II and III. In groups II and III, the up-regulation of effector T cells correlated positively with an increase in CD27−CD28−subsets, and in IFN-γ, TNF-α, IL-12Rβ1, and perforin expression, indicating that the circulating CD8+ effector cells were already functioning before LDLT.
After Tacrolimus Administration
The up-regulated effector T cells immediately after graft reperfusion were down-regulated to pretransplant levels by tacrolimus administered after 24 h in ABO-C group I and II recipients and in ABO-I group I recipients. In contrast, effector T cell levels decreased significantly to lower than those before transplant in ABO-C group III recipients and in ABO-I group II and III recipients. CD8+ effector T cells express perforin and granzyme and display extensively high levels of cytotoxic activity. Accordingly, the down-regulation of perforin expression associated with the depletion of effector T cells indicates cytotoxicity-deficiency, resulting in an acceleration of posttransplant infection.
After tacrolimus administration in the ABO-I or ABO-C group I recipients, the capacities of CD8+ effector T cells in response to infections were maintained as fully functional populations capable of immediate synthesis of perforin. Upon encountering an antigen, these CD8+ T cells were highly cytolytic, even after immunosuppression. In contrast, in ABO-C group III and ABO-I groups II and III recipients, the cytotoxic ability of CD8+ effector T cells was significantly decreased and, thus, ensured an uncontrollable infection. In particular, such excessive down-regulation of cytotoxicity was associated with a markedly impaired ability of the recipient to eradicate the infection and an increased vulnerability to additional invading pathogens in group III. When the down-regulation of effector T cells continues for a prolonged period after LDLT, as shown in ABO-I group III recipients, with time the effector T cells seem to be driven to an “exhausted” phenotypic and functional state, culminating in the deletion of responding cells. Lastly, full exhaustion, indicating the complete loss of effector function, occurred in the terminal stage of recipients with life-threatening infection. This mechanism could operate to silence antiviral or bacterial T cell responses. Conversely, high bacterial and viral loads were associated with the ablation of CD8+ effector T cell responses and with impaired cytokine production, as proposed by Wherry et al. [23]. Thus, a marked down-regulation of effector T cells and perforin expression plays a greater role in determining survival probability. Furthermore, such functions of CD8+ effector T cells were determined by the preexisting CD8+ T cell differentiation phenotype before LDLT. These findings are consistent with the greater frequencies of donor-specific memory T cells in the peripheral blood at the time of transplantation being associated with poor allograft outcome [24].
The Relationship Between Infection and Rejection Related to Tacrolimus Immunosuppression
The frequencies of posttransplant infection, LTCs, and hospital mortality were significantly higher in ABO-I recipients than in ABO-C recipients. ABO-I recipients were more likely to develop life-threatening complications, mainly sepsis, after surgical complications. Once this occurs in ABO-I recipients, the outcome of surgical complications would be seriously compromised by a high rate of severe infection. Over immunosuppression, especially use of large amounts of corticosteroids, may also be one of the factors inducing severe infectious complication. Interestingly, in ABO-I group II and III recipients the frequencies of posttransplant infection were higher than in ABO-C group II and III recipients. However, the ACR rates tended to be suppressed in ABO-I group II and III recipients. These decreased ACR rates are due to tacrolimus administration inducing greater inhibition of both effector cell generation and CTL cytotoxicity in ABO-I group II and III recipients than in ABO-C recipients. In ABO-I group II and III recipients, the tacrolimus immunosuppression, although suppressing ACR development, contributes to a catastrophic effect on the development of infection. Accordingly, to avoid a prolonged down-regulation of effector T cells, tacrolimus should be adjusted to tailor the immunosuppression for each recipient.
Impact of Immunosuppression on Immune Status of ABO-I Group II
Based on our current experience, AMR can often be prevented by hepatic artery infusion therapy and rituximab prophylaxis. However, higher frequencies of effector T cells in the peripheral blood at the time of the transplantation, possibly with an extensive history of environmental exposure, were associated with poor allograft outcome. The reduction of posttransplant infection is a key to improve the outcome of ABO-I LDLT. Accordingly, it is of very high importance to prevent the marked down-regulation of effector T cells by a promising device to adjust immunosuppression.
Despite appropriate tacrolimus trough levels, differences in the down-regulation of effector T cells occurred between ABO-C and ABO-I recipients. This may provide important insights that the marked down-regulation of effector T cells is due to the highest negative sensitivity to tacrolimus administration. In this regard, there were marked differences in group II between ABO-C and ABO-I recipients. The effector T cells were not down-regulated after tacrolimus administration in ABO-C group II but were decreased significantly in ABO-I group II, indicating higher negative sensitivity to tacrolimus in this group. In ABO-I group II, the posttransplant course was more often compromised by LTCs, although there were no hospital mortalities. Immunosuppressive agents differ in their ability to control the generation and function of CD8+ memory T cells; for this purpose, calcineurin inhibitors are the most efficacious. However, the mechanism of the high negative sensitivity to tacrolimus in the recipients with preexisting effector T cells is unclear. On the other hand, the marked down-regulation of effector T cells was associated with a decrease in IFN-γ expression (data not shown). Experimental studies have found that in mice with high IFN-γ production, calcineurin-inhibitor therapy effectively induces long-term graft survival after cardiac allografting, whereas in IFN-γ-deficient mice the same therapy only marginally prolongs graft survival and induces a resistance to calcineurin inhibitors [25, 26]. Accordingly, it seems likely that CTL cytotoxicity cannot effectively respond to tacrolimus therapy during down-regulation of IFN-γ production in effector-enriched recipients. This indicates the importance of tailoring immunosuppressants for transplant recipients, particularly those with very high levels of preexisting immunological memory. However, there is no reliable approach to prevent the impairment of CTL cytotoxicity in transplantation. A promising strategy may be to meticulously adjust the minimum immunosuppression for each recipient. Because % effector difference was decreased by day 2, when reaching appropriate trough levels of tacrolimus, more accurate assessment of the recipient’s immune status may help to tailor immunosuppression for high-risk recipients and may prevent graft failure in selected patients.
Conclusions
In group I of both ABO-C and ABO-I recipients, the CD8+ T cells were characterized by preexisting lowest effector T cells and highest naive T cells. Our previous study showed that the CTL cytotoxity was promptly up-regulated in response to infection in group I, compared with those in groups II and III, indicating the ability to rapidly clear pathogens after LDLT, and that these activities may be dependent on the preexisting highest naive T cells [8]. Furthermore, group I of ABO-I or ABO-C recipients could successfully undergo LDLT without severe posttransplant complications. Accordingly, group III recipients may be unfavorable candidates for ABO-I LDLT under current immunosuppression regimens.
Acknowledgment
Supported in part by grants (nos. 13204041, 13307038, 15390394 and 17390350) from the Scientific Research Fund of the Ministry of Education, Science and Culture, Japan.
Abbreviations
- ABO-I
ABO-incompatible
- ABO-C
ABO-identical and compatible
- ACR
Acute cellular rejection
- CCR7
Chemokine receptor 7
- CM
Central/memory T cell subsets, CD45RO+CCR7+
- CTLs
Cytotoxic T lymphocytes
- EM
Effector/memory T cell subsets, CD45RO+CCR7–
- E
Effector T cell subsets, CD45RO–CCR7–
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HLA
Human leukocyte antigen
- IFN-γ
Interferon-gamma
- IL-12Rβ1
Interleukin-12 receptor beta1
- LDLT
Living donor liver transplantation
- LTC
Life threatening complication
- MMF
Mycophenolate mofetil
- N
Naive T cell subsets, CD45RO−CCR7+
- PGE1
Prostaglandin E1
- TNF-α
Tumor necrosis factor alpha
- % E difference
Where “% difference” refers to the difference in CD45 isoform proportion between preoperative and postoperative levels at different time points
- % difference of IFN-γ, IL-12Rβ1+ cells, TNF-α and perforin
Where “% difference” refers to the difference in their components between preoperative and postoperative levels at different time points
References
- 1.Hanto DW, Fecteau AH, Alonso MH, Valente JF, Whiting JF. ABO-incompatible liver transplantation with no immunological graft losses using total plasma exchange, splenectomy, and quadruple immunosuppression: evidence for accommodation. Liver Transpl. 2003;9(1):22–30. doi: 10.1053/jlts.2003.50011. [DOI] [PubMed] [Google Scholar]
- 2.Heffron T, Welch D, Pillen T, et al. Successful ABO-incompatible pediatric liver transplantation utilizing standard immunosuppression with selective postoperative plasmapheresis. Liver Transpl. 2006;12(6):972–978. doi: 10.1002/lt.20760. [DOI] [PubMed] [Google Scholar]
- 3.Egawa H, Ohmori K, Haga H, et al. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl. 2007;13(4):579–588. doi: 10.1002/lt.21092. [DOI] [PubMed] [Google Scholar]
- 4.Clambey ET, van Dyk LF, Kappler JW, Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunol Rev. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 5.Welsh RM, Selin LK. No one is naive: the significance of heterologous T cell immunity. Nat Rev Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 6.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, Ozawa K, Teramukai S, et al. Classification of human liver transplant recipients by the preoperative CD8+ T cell subpopulation and its relation to outcome. Liver Transpl. 2006;12:792–800. doi: 10.1002/lt.20705. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Uemoto S, Egawa H, et al. Cytotoxic T cell-mediated defense against infections in human liver transplant recipients. Liver Transpl. 2007;13:287–293. doi: 10.1002/lt.21065. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Inomata Y, Kaihara S. Living-donor Liver Transplantation. Surgical Techniques and Innovation. Barcelona, Spain: Prous Science; 2003. [Google Scholar]
- 10.Tanabe M, Shimazu M, Wakabayashi G, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002;73:1959–1961. doi: 10.1097/00007890-200206270-00021. [DOI] [PubMed] [Google Scholar]
- 11.Banff Working Group. Demetris AJ, Adeyi O, Bellamy CO, et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 12.Egawa H, Oike F, Buhler L, et al. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004;77:403–411. doi: 10.1097/01.TP.0000110295.88926.5C. [DOI] [PubMed] [Google Scholar]
- 13.Haga H, Egawa H, Shirase T, et al. Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation. Liver Transpl. 2004;6:16–27. doi: 10.1002/lt.20002. [DOI] [PubMed] [Google Scholar]
- 14.Haga H, Egawa H, Fujimoto Y, et al. Acute humoral rejection and C4D immunostaining in ABO blood type incompatible liver transplantation. Liver Transpl. 2006;12:457–464. doi: 10.1002/lt.20652. [DOI] [PubMed] [Google Scholar]
- 15.Uemoto S, Tanaka K, Fujita S, et al. Infectious complications in living related liver transplantation. J Pediatr Surg. 1994;29:514–517. doi: 10.1016/0022-3468(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 16.Bone RC. Let’s agree on terminology: definitions of sepsis. Crit Care Med. 1991;19:973–976. doi: 10.1097/00003246-199107000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry. J Clin Invest. 1997;99:173950. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everitt BS, Landau S, Leese M. Hierarchial clustering. In: Everitt BS, Landau S, Leese M, editors. Cluster analysis. 4. London: Arnold; 2001. pp. 55–89. [Google Scholar]
- 19.Uemoto S, Ozawa K, Egawa H, et al. Serial assessment of immune status by circulating CD8 effector T cell frequencies for posttransplant infectious complications. Clin Dev Immunol. 2008;718386. [DOI] [PMC free article] [PubMed]
- 20.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 22.Yu AS, Ahmed A, Keeffe EB. Liver transplantation: evolving patient selection criteria. Can J Gastroenterol. 2001;15:729–738. doi: 10.1155/2001/743019. [DOI] [PubMed] [Google Scholar]
- 23.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065X.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Hosiawa KA, Min W, et al. Cytokines regulate the pattern of rejection and susceptibility to cyclosporine therapy in different mouse recipient strains after cardiac allografting. J Immunol. 2003;171(7):3823–3836. doi: 10.4049/jimmunol.171.7.3823. [DOI] [PubMed] [Google Scholar]
- 26.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40–CD40 ligand interactions. J Immunol. 2001;166(5):3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]


