Abstract
The purpose of this paper was to prospectively characterize the clinical manifestations and outcomes of confirmed influenza A 2009 (H1N1) virus infection in immunosuppressed patients with hospital admission and compare them with those of a general population. A multicenter prospective cohort study was carried out. All adult patients admitted to 13 hospitals in Spain with confirmed influenza A 2009 (H1N1) virus infection from June 12, 2009 to November 11, 2009 were included. Risk factors for complicated influenza infection were studied in immunosuppressed patients. Overall, 559 patients were included, of which 56 were immunosuppressed, nine with solid or hematological malignancies, 18 with solid-organ transplant recipients, 13 with corticosteroid therapy, and six with other types of immunosuppression. Clinical findings at diagnosis were similar in both groups. Nineteen immunosuppressed patients had pneumonia (33.9%). Immunosuppressed patients with pandemic influenza had bacterial co-infection more frequently (17.9% vs. 6.4%, p = 0.02), specifically, gram-negative bacilli and Staphylococcus aureus infections. Mortality was higher in immunosuppressed patients (7.1% vs. 1.8%, p < 0.05). The only modifiable risk factor of complicated influenza A 2009 (H1N1) was delayed antiviral therapy. In immunosuppressed patients, influenza A 2009 (H1N1) virus infection has higher mortality than in non-immunosuppressed individuals. Bacterial co-infection is common in complicated cases.
Keywords: Influenza, Antiviral Therapy, Oseltamivir, Seasonal Influenza, Pandemic Influenza
Introduction
Advances in therapy for malignancies, autoimmune disorders, and end-stage organ diseases have lead to improved survival, but also to an increase in the number of immunosuppressed patients (ISP). These patients are at risk for opportunistic infections and also for community-acquired infections, such as respiratory virus infections, with considerable related morbidity and mortality [1–3].
In April 2009, a novel influenza A (H1N1) virus was identified and propragated throughout the world [4]. Clinical findings of influenza A 2009 (H1N1) virus infection were similar to those of seasonal influenza, with many patients having mild illness [5]. However, severe cases occurred, with over 18,000 patients dying throughout the world. Among hospitalized patients with severe influenza A 2009 (H1N1), 15–20% were immunosuppressed [6, 7]. Given the behavior of seasonal flu in ISP, a higher number of influenza-related complications in this population could be expected [3, 6, 8, 9]. However, data regarding clinical features and outcomes in non-human immunodeficiency virus (HIV) ISP are needed. The objective of this investigation was to prospectively characterize the clinical manifestations and outcomes of influenza A 2009 (H1N1) virus infection in ISP.
Methods
Setting, study subjects, and study design
This prospective cohorts observational study was conducted at 13 teaching hospitals belonging to the Spanish Network for Research in Infectious Diseases (REIPI). All patients older than 16 years of age admitted to the hospital with confirmed pandemic influenza A 2009 (H1N1) virus infection from June 12, 2009 to November 11, 2009 were included. Cases were detected on a daily basis by reviewing the microbiological reports. A confirmed case was defined in the presence of influenza-like illness with laboratory-confirmed pandemic influenza A 2009 (H1N1) virus infection. Pandemic influenza A 2009 (H1N1) virus testing was performed in each institution. The study was approved by the coordinator local ethics committee and informed consent was obtained from all subjects.
Clinical assessment and follow up
Microbiological studies, hospital and intensive care unit (ICU) admission criteria, and treatment decisions were not standardized and were made by attending physicians at each study center. Patients were seen during their hospital stay by one or more of the investigators at each participating hospital, who recorded the clinical data in a standardized, computer-assisted protocol. Data were collected on demographic characteristics, co-morbidities, body mass index (BMI), previous vaccination, clinical signs and symptoms, laboratory analysis, chest X-ray findings, antiviral and antibacterial therapy, concomitant and/or secondary infection, time to clinical stability, and outcomes, including readmissions (<30 days) and mortality. A long-term follow up visit took place one month after discharge. A senior investigator validated all data.
Definitions
Non-HIV ISP were considered as those patients with major immunosuppressive status different to HIV infection, such as solid-organ transplant and hematopoietic stem cell transplant (HSCT), active neoplasia, systemic chemotherapy, immunosuppressive therapy, asplenia, or congenital immunodeficiencies. Patients with corticosteroid treatment were considered as those with a dosage of prednisone >15 mg, or equivalent, during more than 15 days or with bolus. Neutropenic patients were considered as those with neutrophil cell count bellow 500/μl. Obesity was defined as a BMI ≥ 30 and morbidly obese as a BMI ≥40. Vaccinated patients included all individuals who had received the pneumococcal vaccine in the previous 5 years or the seasonal influenza vaccine in the previous year. Pneumonia was defined as the presence of a new infiltrate on a chest radiograph for which other causes were excluded and the severity of pneumonia was assessed according to the PSI score [10] and the CURB-65 score [11].
Bacterial co-infection was considered if bacteria were identified in blood and respiratory samples, or in cases with positive urinary antigen tests for Streptococcus pneumoniae and Legionella pneumophila serogroup 1. Complicated influenza was considered in cases of admission to ICUs, acute respiratory distress syndrome (ARDS), acute coronary event, shock, or death.
Overall mortality was defined as death from any cause within 30 days of diagnosis.
Statistical analysis
The results were analyzed using the statistical software package PASW Statistics, version 18.0.1. A descriptive statistical analysis was performed. Continuous variables are expressed as median and range or mean ± standard deviation. The Chi-square or Fisher’s exact tests were used for categorical variables and the Mann–Whitney test and Wilcoxon test for continuous variables, when appropriate. Statistical significance was established at α = 0.05. All reported p-values are two-tailed.
Results
A total of 559 patients with pandemic influenza A 2009 (H1N1) were included, of whom 56 (10%) were considered to be immunosuppressed (Fig. 1). The most common causes of immunosuppression were: solid or hematological malignancies in 19 cases (33.9%), of whom 5 (8.9%) were neutropenic and 3 (5.4%) received an HSCT; solid-organ transplant in 18 cases (32.1%); and corticosteroid therapy in 13 cases (23.2%) (Table 1).
Fig. 1.
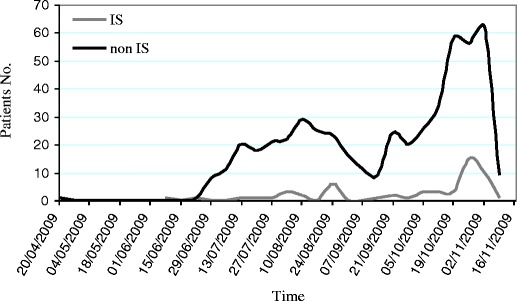
Patients with influenza A 2009 (H1N1) infection admitted to the hospital in several Spanish hospitals between April 20, 2009 and November 11, 2009
Table 1.
Causes of immunosuppression of patients with influenza A 2009 (H1N1) virus infection
| Solid-organ transplant recipients, no. (%) | 18 (32.1%) |
|---|---|
| - Kidney, no. | 11 |
| - Liver, no. | 4 |
| - Heart, no. | 1 |
| - Pancreas, no. | 1 |
| - Kidney–pancreas, no. | 1 |
| Oncohematological patients, no. (%) | 19 (33.9%) |
| Solid neoplasm receiving chemotherapy, no. | 12 |
| - Breast cancer, no. | 3 |
| - Lung cancer, no. | 3 |
| - Colorectal cancer, no. | 2 |
| - Glioblastoma, no. | 2 |
| - Gonadal tumor, no. | 1 |
| - Osteosarcoma, no. | 1 |
| Hematological malignancies, no. | 7 |
| - Leukemia, no. | 3 |
| - Hematopoietic steam cell transplantation, no. | 3 |
| - Essential thrombocytosis, no. | 1 |
| Corticosteroids continuous therapy patients, no. (%) | 13 (23.2%) |
| Chronic lung diseases, no. | 5 |
| - Chronic obstructive pulmonary disease, no. | 4 |
| - Cystic fibrosis, no. | 1 |
| Connective tissues diseases, no. | 4 |
| Adrenal insufficiency syndrome, no. | 2 |
| Other pathologies | 2 |
| Other situations | 6 (10.7%) |
| Asplenic patients | 3 |
| Multiple sclerosis | 2 |
| Combined variable immunodeficiency | 1 |
Besides immunosuppression, 98.2% of the ISP had at least one underlying medical condition versus 47.1% of non-ISP. Immunosuppressed patients had more frequent chronic renal and liver insufficiency, while those who were non-ISP were more frequently asthmatic, tobacco smokers, and obese (Table 2).
Table 2.
Demographic characteristics of patients with influenza A 2009 (H1N1) virus infection
| Variable | ISP, n (%) | Non-ISP, n (%) | p-value | RR (95% CI) |
|---|---|---|---|---|
| Age, years, median (range) | 46 (17–87) | 36 (16–83) | 0.003 | |
| Male sex | 36 (64.3) | 246 (48.9) | 0.029 | 1.7 (1.05–2.9) |
| Tobacco smoker | 10 (17.9) | 160 (32.1) | 0.028 | 0.4 (0.2–0.9) |
| Alcohol | 2 (3.6) | 28 (5.7) | >0.05 | |
| Underlying conditions | 55 (98.2) | 237 (47.1) | <0.001 | 50.3 (7–360.9) |
| - Chronic renal failure | 11 (19.6) | 12 (2.4) | <0.001 | 5.7 (3.4–9.4) |
| - Chronic liver insufficiency | 5 (8.9) | 16 (3.2) | 0.049 | 2.5 (1.1–5.6) |
| - Chronic heart disease | 5 (8.9) | 34 (6.8) | >0.05 | |
| - Chronic pulmonary disease | 11 (19.6) | 150 (29.8) | >0.05 | |
| - Chronic obstructive pulmonary disease | 5 (8.9) | 39 (7.8) | >0.05 | |
| - Asthma | 4 (7.1) | 98 (19.5) | 0.023 | 0.3 (0.1–0.9) |
| - Diabetes mellitus | 8 (14.3) | 47 (9.3) | >0.05 | |
| - Obesity | 3 (5.4) | 84 (16.7) | 0.026 | 0.3 (0.1–0.9) |
| Influenza vaccine 08/09 | 15 (33.3) | 33 (7.6) | <0.001 | 4.4 (2.6–7.7) |
| Influenza vaccine 09/10 | 5 (10.2) | 15 (3.3) | 0.034 | 2.78 (1.2–6.2) |
| Pneumococcal vaccine | 6 (13.6) | 8 (1.9) | <0.001 | 5.18 (2.6–10.1) |
ISP: immunosuppressed patients; non-ISP: non-immunosuppressed patients
Clinical manifestations in ISP
The median time from the onset of illness to hospital admission was 2 days (range 1–4). The most common symptoms were cough in 50 cases (89.3%) and fever in 34 (60.7%). Six patients (10.7%) were hypoxemic (SatO2 <90%). Nineteen ISP had pneumonia (33.9%). The severity of pneumonia was assessed at admission, with eight patients classified in the PSI risk groups IV–V (42.1%) and two patients with CURB-65 score >2 (10.5%). At influenza diagnosis, the laboratory findings in ISP that differed from previous basal values were leukocyte count/μl (7,272.3 ± 465.6 vs. 5,775.1 ± 687.1, p = 0.004), lymphocyte count/μl (1,671.1 ± 147.6 vs. 847.2 ± 104.3, p < 0.001), platelet count (208,938 ± 15,072 vs. 169,630 ± 14,599, p = 0.005), hematocrit (38.2% ± 0.8% vs. 36.1% ± 0.9%, p = 0.002), and natremia (139.4 ± 0.5 meq/l vs. 135.1 ± 0.8 meq/l, p < 0.001).
Immunosuppressed patients had more frequently neutropenia, leukopenia, anemia, and renal insufficiency (serum creatinin > 1.5 mg/dl). The main clinical manifestations, and radiological and analytical findings are detailed in Table 3.
Table 3.
Clinical, laboratory, and radiological findings of immunosuppressed patients and non-immunosuppressed patients with influenza A 2009 (H1N1) infections
| Variable | ISP, n = 56 | Non-ISP, n = 503 | p-value | RR (95% CI) |
|---|---|---|---|---|
| Days since onset of symptoms, median (range) | 2 (1–14) | 3 (0–21) | >0.05 | |
| Rhinorrhea, no. (%) | 7 (12.5) | 110 (21.9) | >0.05 | |
| Sore throat, no. (%) | 11 (19.6) | 145 (28.8) | >0.05 | |
| Chills, no. (%) | 11 (19.6) | 126 (25) | >0.05 | |
| Cough, no. (%) | 50 (89.3) | 453 (90.1) | >0.05 | |
| Dyspnea, no. (%) | 20 (35.7) | 238 (47.3) | >0.05 | |
| Pleuritic chest pain, no. (%) | 7 (12.5) | 84 (16.7) | >0.05 | |
| Gastrointestinal disorders, no. (%) | 10 (17.8) | 124 (24.6) | >0.05 | |
| Myalgia/arthralgia, no. (%) | 27 (48.2) | 297 (59.1) | >0.05 | |
| Headache, no. (%) | 18 (32.1) | 164 (32.7) | >0.05 | |
| Expectoration, no. (%) | 27 (48.2) | 208 (41.3) | >0.05 | |
| Fever at admission (≥38°C), no. (%) | 34 (60.7) | 261 (51.8) | >0.05 | |
| Hypoxemia (Sat O2 <90), no. (%) | 6 (10.7) | 69 (13.7) | >0.05 | |
| Leukopenia (<4,000/mm3), no. (%) | 20 (35.7) | 59 (11.7) | <0.001 | 3.3 (2–5.5) |
| Leukocytosis (>12,000/mm3), no. (%) | 8 (14.3) | 66 (13.1) | >0.05 | |
| Neutropenia (<500/mm3), no. (%) | 5 (8.9) | 0 (0) | 0.001 | 10.9 (8.3–14.1) |
| Lymphopenia (<1,500/mm3 ), no. (%) | 43 (76.8) | 406 (80.7) | >0.05 | |
| Anemia (hematocrit <36%), no. (%) | 47 (83.9) | 110 (21.9) | <0.001 | 2.9 (1.7–4.6) |
| Thrombocytopenia (<150.103/mm3), no. (%) | 18 (32.1) | 123 (24.5) | >0.05 | |
| AST >40 U/l, no. (%) | 10 (17.9) | 88 (17.5) | >0.05 | |
| ALT >40 U/l, no. (%) | 9 (16.1) | 106 (21.1) | >0.05 | |
| Hyponatremia <135 mmol/l, no. (%) | 15 (26.8) | 125 (24.8) | >0.05 | |
| Serum creatine >1.5 mg/dl, no. (%) | 16 (28.6) | 59 (11.7) | 0.001 | 2.5 (1.5–4.2) |
| LDH > 350 U/l , no. (%) | 11 (19.6) | 115 (22.9) | >0.05 | |
| Glucose, mg/dl (mean ± SD) | 120.5 ± 8.5 | 118.7 ± 2.8 | >0.05 | |
| C-reactive protein >20 mg/l, no. (%) | 23 (41.1) | 231 (45.9) | >0.05 | |
| Pneumonia, no. (%) | 19 (33.9) | 203 (40.4) | >0.05 | |
| - Alveolar opacities, no. (%) | 17 (89.4) | 148 (72.9) | >0.05 | |
| - Multilobar infiltrates, no. (%) | 10 (52.6) | 117 (57.6) | >0.05 | |
| - PSI score, median (range) | 87 (45–146) | 46 (4–153) | <0.001 | |
| - CURB score, median (range) | 0.5 (0–3) | 0 (0–3) | >0.05 |
ISP: immunosuppressed patients; non-ISP: non-immunosuppressed patients; AST:alanine aminotransferase; ALT: aspartate aminotransferase
Bacterial co-infection
Bacterial co-infection was more frequent in ISP (17.9% vs. 6.4%, RR 2.7, 95% CI 1.4–4.9, p = 0.02). In cases of complicated influenza infection, the proportion of bacterial co-infection was 71.4% in ISP and 12.3% in non-ISP (RR 12.5, 95% CI 2.6–57.3, p < 0.001). The bacteria identified in each group of patients are detailed in Table 4. Immunosuppressed patients were more frequently co-infected with gram-negative bacilli (7.1% vs. 0.6%, RR 17.6, 95% CI 1.6–194.9, p = 0.002) and Staphylococcus aureus (3.6% vs. 0.2%, RR 11.9, 95% CI 2.7–52.1, p = 0.02).
Table 4.
Bacterial co-infections in patients with influenza A 2009 (H1N1) virus infection
| Microorganisms | No. (%) | Samples (n) |
|---|---|---|
| Immunosuppressed patients | 10 (17.9) | |
| Streptococcus pneumoniae | 3 (5.4) | Sputum (1), antigenuria (2) |
| Staphylococcus aureus | 2 (3.6) | Sputum (1), blood: bronchoalveolar lavage (1) |
| Pseudomonas aeruginosa | 2 (3.6) | Sputum (2) |
| Acinetobacter baumannii | 1 (1.8) | Sputum (1) |
| Escherichia coli | 1 (1.8) | Blood (1) |
| Streptococcus sanguis | 1 (1.8) | Blood (1) |
| Non-immunosuppressed patients | 32 (6.4%) | |
| Streptococcus pneumoniae | 23 (4.6) | Sputum (3), blood (2), antigenuria (16), antigenuria: sputum (2) |
| Staphylococcus aureus | 1 (0.2) | Sputum (1) |
| Pseudomonas aeruginosa | 1 (0.2) | Sputum (1) |
| Acinetobacter baumannii | 1 (0.2) | Blood: bronchoalveolar lavage (1) |
| Haemophilus influenzae | 3 (0.6) | Sputum (3) |
| Moraxella catarrhalis | 2 (0.4) | Sputum (2) |
| Stenotrophomonas maltophilia | 1 (0.2) | Sputum (1) |
| Haemophilus parainfluenzae | 1 (0.2) | Blood (1) |
| Streptococcus pyogenes | 1 (0.2) | Pleural fluid (1) |
Treatment and clinical outcomes in ISP
All patients were treated with oseltamivir at a median of 5 days after the onset of symptoms (range 3–14 days), with a dosage of 75 mg bid in all but one patient (1.8%) that received 150 mg bid. One patient (1.8%) was treated with zanamivir. Forty-four patients (78.5%) received antibiotics, 28 (50%) corticosteroids, and two (3.6%) inotropic vasopressors.
The median duration of fever and cough were 2 days, respectively.
Seven patients (12.5%) had influenza-related complications. Of those, four patients (7.1%) were admitted to the ICU and mechanically ventilated, and four patients presented septic shock (Table 5).
Table 5.
Treatment and clinical outcomes of patients with influenza A 2009 (H1N1) virus infection
| Characteristics | Immunosuppressed patients, n = 56 | Non-immunosuppressed patients, n = 503 |
|---|---|---|
| Treatment | ||
| Antiviral therapy, no. (%) | 56 (100)* | 463 (92.1)* |
| - Time since onset of symptoms to antiviral therapy, days, median (range) | 5 (3–14) | 5.5 (4–11) |
| - Antiviral therapy within 48 h since onset of symptoms, no. (%) | 24 (42.9) | 169 (36.6) |
| Antibacterial therapy, no. (%) | 44 (78.5) | 351 (69.8) |
| - Antibacterial therapy within 4 h since hospital admission, no. (%) | 12 (27.3) | 90 (25.7) |
| - Duration, days, median (range) | 9 (1–23) | 6.5 (5–13) |
| Statins, no. (%) | 5 (8.9) | 25 (5.1) |
| Angiotensin-converting enzyme inhibitors, no. (%) | 4 (7.1) | 30 (6.1) |
| Inotropic vasopressor, no. (%) | 2 (3.6) | 23 (4.6) |
| Corticosteroids > 300 mg/day, no. (%) | 3 (5.3) | 47 (9.3) |
| - Duration in days, median (range) | 5 (13–14) | 5.5 (2–15) |
| Outcome | ||
| Time to clinical stability, days, median (range) | 3 (1–21) | 3 (1–21) |
| Length of hospital stay, days, median (range) | 2 (1–32) | 5 (1–98) |
| Complicated influenza infection, no. (%) | 7 (12.5%) | 71 (14.1%) |
| Intensive care unit admission, no. (%) | 4 (7.1%) | 64 (12.7%) |
| Invasive mechanical ventilation, no. (%) | 4 (7.1%) | 46 (9.1%) |
| Mortality, no. (%) | 4 (7.1%)** | 9 (1.8%)** |
*RR 1.09, 95% CI 1.06–1.1, p = 0.01
*RR 4.8, 95% CI 1.5–15.0, p = 0.01
Four ISP, all of them with pneumonia died. Mortality was higher in ISP than in non-ISP (7.1% vs. 1.8%, RR 4.8, 95% CI 1.5–15.0; p = 0.01).
Risk factors for complicated influenza A 2009 (H1N1) infection were: pneumonia (100% vs. 24.5%, RR 1.5, 95% CI 1.1–2.2, p < 0.01), thrombocytopenia (71.5% vs. 26.5%, RR 5.2, 95% CI 1.1–24.6, p = 0.03), bacterial co-infection (71.4% vs. 10.2%, RR 11.5, 95% CI 2.5–51, p < 0.01), and early antiviral therapy (0% vs. 49%, RR 0.8, 95% CI 0.6–0.9, p = 0.015). All patients with complicated disease started antiviral therapy later than 3 days after the onset of symptoms. No other factors were associated with complicated influenza in terms of underlying diseases, age or sex, antecedent of vaccination, type of immunosuppression, and clinical findings.
Organ transplant recipients
Solid-organ transplant recipients received more frequently influenza and pneumococcal vaccine than the rest of the ISP (50% vs. 15.7%, RR 3, 95% CI 1.3–6.8, p = 0.007 and 27.8% vs. 2.6%, RR 3.1, 95% CI 1.7–6, p = 0.01, respectively) (Table 6).
Table 6.
Characteristics, clinical and laboratory findings, and outcome in different groups of immunosuppressed patients
| Variable | Transplant organ recipients, n = 18 | Oncohematological patients, n = 19 | Corticosteroids therapy, n = 13 |
|---|---|---|---|
| Age, years, mean ± SD | 44.3 ± 4.3 | 41.8 ± 2.7 | 52.3 ± 5.3 |
| Male sex, no. (%) | 15 (83.3) | 13 (68.4) | 6 (46.2) |
| Tobacco smoker, no. (%) | 1 (5.6) | 6 (31.6) | 2 (15.4) |
| Alcohol, no. (%) | 0 (0) | 2 (10.5) | 0 (0) |
| Underlying conditions | |||
| - Chronic renal failure, no. (%) | 11 (61.1)* | 0 (0) | 0 (0) |
| - Chronic liver insufficiency, no. (%) | 4 (22.2) | 0 (0) | 1 (7.7) |
| - Chronic heart disease, no. (%) | 2 (11.1) | 1 (5.3) | 2 (15.4) |
| - Chronic pulmonary disease, no. (%) | 3 (16.7) | 2 (10.5) | 6 (46.2)* |
| - Chronic obstructive pulmonary disease, no. (%) | 1 (5.6) | 0 (0) | 4 (30.8)* |
| - Asthma, no. (%) | 2 (11.1) | 1 (5.3) | 1 (7.7) |
| - Diabetes mellitus, no. (%) | 5 (27.8) | 2 (10.5) | 1 (7.7) |
| Influenza vaccine 08/09, no. (%) | 9 (50)* | 1 (5.3) | 4 (30.8) |
| Influenza vaccine 09/10, no. (%) | 3 (16.7) | 0 (0) | 1 (7.7) |
| Pneumococcal vaccine, no. (%) | 5 (27.8)* | 0 (0) | 0 (0) |
| Clinical findings | |||
| - Days since onset of symptoms, median (range) | 2 (1–14) | 2.5 (1–7) | 3 (1–10) |
| - Rhinorrhea, no. (%) | 3 (16.7) | 2 (10.5) | 2 (15.4) |
| - Sore throat, no. (%) | 3 (16.7) | 4 (21.1) | 2 (15.4) |
| - Chills, no. (%) | 5 (27.8) | 2 (10.5) | 3 (23.1) |
| - Cough, no. (%) | 15 (83.3) | 18 (94.7) | 11 (84.6) |
| - Dyspnea, no. (%) | 5 (27.8) | 5 (26.3) | 8 (61.5) |
| - Pleuritic chest pain, no. (%) | 1 (5.6) | 3 (15.8) | 1 (7.7) |
| - Gastrointestinal disorders, no. (%) | 5 (27.7) | 3 (15.7) | 0 (0) |
| - Myalgia/arthralgia, no. (%) | 8 (44.4) | 11 (57.9) | 4 (30.8) |
| - Headache, no. (%) | 8 (44.4) | 6 (31.6) | 4 (30.8) |
| - Expectoration, no. (%) | 1 (5.6) | 0 (0) | 0 (0) |
| Laboratory findings | |||
| Leukopenia (<4,000/mm3), no. (%) | 5 (27.7) | 14 (73.7)* | 1 (7.7) |
| Leukocytosis (>12,000/mm3), no. (%) | 1 (5.6) | 1 (5.3) | 4 (30.8) |
| Neutropenia (<500/mm3), no. (%) | 0 (0) | 5 (26.3)* | 0 (0) |
| Lymphopenia (<1,500/mm3), no. (%) | 17 (94.4)* | 16 (84.2) | 7 (53.8) |
| Anemia (Hematocrit <36%), no. (%) | 10 (55.6) | 12 (63.2) | 5 (38.5) |
| Thrombocytopenia (<150.103/mm3), no. (%) | 7 (38.9) | 9 (47.4) | 2 (15.4) |
| AST >40 U/l, no. (%) | 4 (22.2) | 4 (21.1) | 1 (7.7) |
| ALT >40 U/l, no. (%) | 1 (5.6) | 6 (31.6)* | 1 (7.7) |
| Hyponatremia <135 mmol/l, no. (%) | 6 (33.3) | 5 (26.3) | 3 (23.1) |
| Serum creatine >1.5 mg/dl, no. (%) | 12 (66.7)* | 1 (5.3) | 2 (15.4) |
| LDH > 350 U/l, no. (%) | 1 (5.6) | 8 (42.1) | 1 (7.7) |
| C-reactive protein >20 mg/l, no. (%) | 6 (33.3) | 8 (42.1) | 7 (53.8) |
| Radiological findings | |||
| Pneumonia, no. (%) | 5 (27.8) | 6 (31.6) | 4 (30.8) |
| - Alveolar opacities, no. (%) | 5 (100) | 5 (83.3) | 3 (75) |
| - Multilobar infiltrates, no. (%) | 3 (16.7) | 4 (21.1) | 2 (15.4) |
| - PSI score, median (range) | 103 (71–131) | 89 (48–146) | 57.5 (47–146) |
| - CURB score, median (range) | 1 (0–3) | 2 (0–2) | 0 (0–3) |
| Clinical outcomes | |||
| Days to clinical stability, median(range) | 2 (1–14) | 2.5 (1–7) | 3 (1–10) |
| Related complications, no. (%) | 1 (5.6) | 3 (15.8) | 3 (23.1) |
| Intensive care unit admission, no. (%) | 1 (5.6) | 0 (0) | 3 (23.1) |
| Septic shock, no. (%) | 0 (0) | 2 (10.5) | 0 (0) |
| Hospital stay, days, median (range) | 5 (2–28) | 6 (1–25) | 7 (3–32) |
| Death, no. (%) | 1 (5.6) | 3 (15.8) | 0 (0) |
*p < 0.05
LDH: lactate dehydrogenase
Influenza symptoms and signs did not differ from those of other ISP, with the exception of lymphopenia (94.4% vs. 68.4% RR 2.5, 95% CI 1.8–3.5, p < 0.001). Bacterial co-infection was detected in three patients (16.6%): S. aureus was isolated in two cases and Pseudomonas aeruginosa in one case.
Five patients had pneumonia (27.8%), with a median CURB-65 score of 1 (range 0–3). One patient with an S. aureus bacteremia died (5.5%) three days after transplantation.
Oncohematological patients
Oncohematological patients had more frequent laboratory disturbances, such as leukopenia (73.7% vs. 16.2%, RR 5.04, 95% CI 2.1–11.9, p < 0.001), neutropenia (26.3% vs. 0%, RR 3.64, 95% CI 2.3–5.7, p = 0.003), hypertransaminasemia (31.5% vs. 16.2%, RR 2.41, 95% CI 1.2–4.4, p = 0.04), and increased lactate dehydrogenase levels (42.1% vs. 5.4%, RR 7.8, 95% CI 1.8–33.1, p = 0.001) than the rest of the ISP (Table 6).
Six patients (31.6%) had pneumonia. Bacterial co-infection was present in four oncohematological patients (21.1%): S. pneumoniae in two cases, Escherichia coli in one patient, and Acinetobacter baumannii in another case. In 40% of neutropenic patients, a bacterial co-infection was detected. Three patients who received chemotherapy due to solid neoplasm, two of whom were neutropenic, died (15.8%). Mortality among neutropenic patients was higher than in non-neutropenic ISP (40% vs. 3.9%, RR 10.2, 95% CI 1.8–57.0, p = 0.03).
Corticosteroids continuous therapy patients
Nine of the thirteen patients with corticosteroid therapy had dyspnea (69.2%). Laboratory findings revealed fewer abnormalities than the rest of the ISP (Table 6). Bacterial co-infection was detected in three patients (23.1%): Streptococcus sanguis, P. aeruginosa, and S. pneumoniae. Four patients (30.8%) had pneumonia and three (23.1%) had influenza-related complications, all of them requiring ICU admission and mechanical ventilation. None of the patients died.
Discussion
Pandemic influenza A 2009 (H1N1) virus infection in ISP causes a more severe disease than in non-ISP. Globally, mortality is higher in ISP, but the severity of the illness depends on the type of immunosuppression. The only modifiable risk factor of complicated influenza infection was early instauration of antiviral therapy.
Seasonal influenza is a common cause of infection each winter in ISP, with a high rate of short- and long-term related complications, including viral pneumonia and secondary bacterial pneumonia [3, 8, 9].
Clinical features of pandemic influenza in the general population are similar to that of seasonal influenza, with a higher rate of primary viral pneumonia and gastrointestinal and neurological symptoms, especially in young children [7, 12, 13]. Peck et al. [14], in a study of seasonal influenza in HSCT recipients, observed that most patients presented upper respiratory symptoms with few systemic symptoms, such as fever or myalgia. Studies carried out in the transplant setting showed diverse results, with a high proportion of patients having low respiratory and systemic symptoms [1, 9]. The clinical findings of pandemic influenza A 2009 (H1N1) infection in different groups of ISP have been reported. A recent study reported that patients with cancer had fever and cough in almost all cases, with lower respiratory tract involvement occurring in 27% of them [15]. George et al. [16] reported 13 cases of influenza A 2009 (H1N1) infection occurring in HSCT recipients, with lower respiratory tract disease in 38.7% of the patients. In solid-organ transplant recipients, a retrospective study of 154 adults with influenza A showed similar clinical findings, with one-third of the patients having pneumonia [17]. To our knowledge, this is the first study that directly compares the clinical manifestation according to the presence of immunosuppression, and we observed that, although the clinical symptoms and signs were very similar, laboratory disturbances such as neutropenia, leukopenia, anemia, and renal insufficiency were more frequent in the ISP. Some of these, such as renal insufficiency or anemia, were present before the influenza episode and are probably related to the underlying disease and therapy of the ISP, while others, such as lymphopenia, thrombocytopenia, or anemia, significantly worsen after influenza infection, possibly due to an effect of the viral infection in this type of patient.
In ISP, respiratory viruses lead to prolonged clinical courses, with the risk of progression of the infection to the lower respiratory tract and increased mortality when compared to the immunocompetent population [2, 18]. The severity of seasonal influenza in ISP is variable according to the different studies published. Ljungman et al. [1], in a series of ISP with seasonal influenza, observed that most of the cases were mild. Schnell et al. [3], in a retrospective study of hospitalized ISP with seasonal influenza, reported that one-half of the patients had a pneumonia and one-third of patients were admitted to the ICU, with a 10% mortality rate. Other studies carried out in ISP with seasonal influenza report a mortality rate ranging from 0 to 25% [1, 8, 19]. Severe influenza A H1N1 infection has been associated with IgG2 deficiency [20]. In the present study, although there were no differences in some outcome variables, such as admission to ICUs, progression to lower respiratory tract or septic shock, the risk of death during the influenza episode was four times higher in the ISP. However, the severity and outcome of the infection deferred according to the degree of immunosuppression: patients with corticosteroid therapy presented less frequently analytical disturbances and complicated diseases with a better outcome, while oncohematological patients had higher mortality, especially those profoundly neutropenic.
The impact of corticosteroids on influenza severity and outcome are conflicting, since there are no randomized trials assessing this effect. High-dose steroids may prolong viral shedding in HSCT recipients [8, 21] and increase the risk of lower respiratory tract diseases in pediatric cancer patients. But in other studies, it has been suggested that progression to lower respiratory tract diseases may be reduced or not affected [8, 21]. In our experience, patients previously treated with corticosteroids showed a better outcome than other ISP and similar to that of non-ISP.
Although initially a low rate of bacterial co-infection was reported in cases of pandemic influenza A (H1N1) with severe pneumonia [6, 7], later studies showed that bacterial co-infection could be found in 28–55% of fatal cases [22, 23]. In the present study, the proportion of patients co-infected was higher in ISP, and this was especially important in those with complicated diseases, where three-quarters of cases presented a bacterial co-infection. The isolated bacteria were also different from non-ISP, with a higher proportion of gram-negative bacilli and S. aureus infections. Co-infection by these microorganisms is probably related to the close contact of these patients with healthcare facilities. The high frequency of co-infection in severe cases and the presence of “non-strict” community-acquired respiratory pathogens would determine, according to the local epidemiology, the need for using broad-spectrum antibacterial agents in severe cases of influenza A 2009 (H1N1) in ISP.
Delayed hospital admission and antiviral therapy have been associated with an unfavorable outcome in the general population and in solid-organ transplant recipients [17, 24, 25]. In the present study, all patients with complicated diseases started therapy beyond the first 48 h of symptoms. Indeed, this was the only modifiable risk factor of complicated influenza A 2009 (H1N1) infection in this population. Although the proportion of patients receiving antiviral therapy was much higher than that described in series of seasonal influenza [1, 9], an effort should be made to decrease the time of onset of antiviral therapy. For this reason, during epidemiological risk periods, ISP should be screened for symptoms and early influenza diagnosis and therapy should be considered in those cases with compatible syndromes or pneumonia.
Our study has several limitations. First, serological studies of atypical pathogens of community-acquired pneumonia could not be systematically done, and, therefore, co-infection by atypical microorganisms could not be ruled out. Second, susceptibility to oseltamivir was not performed, and, therefore, we cannot determine if oseltamivir resistance was associated with complicated diseases. Finally, as the outcome of influenza A 2009 (H1N1) infection was not previously known, it is possible that the decision of hospitalizing ISP was not only related to the severity of the underlying disease and to the degree of complications detected, but also to general caution regarding the management of these patients, and milder cases than in non-ISP patients would have been hospitalized.
In summary, influenza A 2009 (H1N1) virus infection in ISP causes a disease with clinical symptoms similar to those of non-ISP, but with higher related mortality, especially in neutropenic patients. Bacterial co-infection is frequent in complicated cases, with a high rate of gram-negative bacilli. Clinicians should have a high index of suspicion in pandemic or epidemic periods in order to initiate antiviral therapy early.
Acknowledgments
Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, Programa de Investigación Sobre Gripe A/H1N1 (Grant: GR09/0014), and Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, co-financed by the European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008). Dr. Viasus is the recipient of a research grant from the Institut d’Investigació Biomèdica de Bellvitge (IDIBELL).
We are indebted to Pilar Perez-Romero and Michael McConnell for the language revision of the manuscript.
Footnotes
Elisa Cordero and Teresa Aydillo contributed equally to this work.
*The other members of the Novel Influenza A(H1N1) Study Group are: Emilio García-Cabrera1, José Luis González-Fernández2, Manuel Gutiérrez-Cuadra2, María Victoria Sanjuan2, José Antonio Parra2, Alejandro Martín-Quirós3, María Romero-Gómez3, Guillermo Ruíz-Carrascoso3, Juan Carlos Figueira3, María Concepción Prados3, Jordi Niubo4, Antoni Campins5, José María Aguado6, Juan Vila6, Aroa Villoslada7, Mercedes García-Gasalla7, Laura Linares8, Irma Hoyo8, María Ángeles Marcos8, Tomás Pumarola8, Marina de Cueto9, Ángel Domínguez9, Juan Gálvez9, Laura Pérez-Martínez10, José R. Blanco10, Mercedes Sanz10, Luis Metola10, Valvenera Ibarra10, Lucía Ortega11, Rosario Lara12, Manuel Causse12, Juan Gutiérrez-Aroca12, Elisa Vidal12, Gemma Navarro13, Eva María González13.
References
- 1.Ljungman P, Andersson J, Aschan J, Barkholt L, Ehrnst A, Johansson M, Weiland O. Influenza A in immunocompromised patients. Clin Infect Dis. 1993;17:244–247. doi: 10.1093/clinids/17.2.244. [DOI] [PubMed] [Google Scholar]
- 2.Martino R, Rámila E, Rabella N, Muñoz JM, Peyret M, Portos JM, Laborda R, Sierra J. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis. 2003;36:1–8. doi: 10.1086/344899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnell D, Mayaux J, de Bazelaire C, Legoff J, Feuillet S, Scieux C, Andreu-Gallien J, Darmon M, Baruchel A, Schlemmer B, Azoulay E. Risk factors for pneumonia in immunocompromised patients with influenza. Respir Med. 2010;104:1050–1056. doi: 10.1016/j.rmed.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–470. [PubMed] [Google Scholar]
- 5.Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, Liang ZA, Liang L, Zhang SJ, Zhang B, Gu L, Lu LH, Wang DY, Wang C. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Hospitalized patients with novel influenza A (H1N1) virus infection—California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–541. [PubMed] [Google Scholar]
- 7.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 8.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 9.Vilchez RA, McCurry K, Dauber J, Lacono A, Griffith B, Fung J, Kusne S. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2:287–291. doi: 10.1034/j.1600-6143.2002.20315.x. [DOI] [PubMed] [Google Scholar]
- 10.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 11.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 13.Hackett S, Hill L, Patel J, Ratnaraja N, Ifeyinwa A, Farooqi M, Nusgen U, Debenham P, Gandhi D, Makwana N, Smit E, Welch S. Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet. 2009;374:605. doi: 10.1016/S0140-6736(09)61511-7. [DOI] [PubMed] [Google Scholar]
- 14.Peck AJ, Englund JA, Kuypers J, Guthrie KA, Corey L, Morrow R, Hackman RC, Cent A, Boeckh M. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redelman-Sidi G, Sepkowitz KA, Huang CK, Park S, Stiles J, Eagan J, Perlin DS, Pamer EG, Kamboj M. 2009 H1n1 influenza infection in cancer patients and hematopoietic stem cell transplant recipients. J Infect. 2010;60:257–263. doi: 10.1016/j.jinf.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 16.George B, Ferguson P, Kerridge I, Gilroy N, Gottlieb D, Hertzberg M. The clinical impact of infection with Swine flu (H1N1 09) strain of influenza virus in hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2011;17:147–153. doi: 10.1016/j.bbmt.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, Danziger-Isakov L, Stosor V, Estabrook M, Gantt S, Marr KA, Martin S, Silveira FP, Razonable RR, Allen UD, Levi ME, Lyon GM, Bell LE, Huprikar S, Patel G, Gregg KS, Pursell K, Helmersen D, Julian KG, Shiley K, Bono B, Dharnidharka VR, Alavi G, Kalpoe JS, Shoham S, Reid GE, Humar A. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10:521–526. doi: 10.1016/S1473-3099(10)70133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Englund JA, Sullivan CJ, Jordan MC, Dehner LP, Vercellotti GM, Balfour HH., Jr Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med. 1988;109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 19.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon CL, Johnson PD, Permezel M, Holmes NE, Gutteridge G, McDonald CF, Eisen DP, Stewardson AJ, Edington J, Charles PG, Crinis N, Black MJ, Torresi J, Grayson ML. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50:672–678. doi: 10.1086/650462. [DOI] [PubMed] [Google Scholar]
- 21.Boudreault AA, Xie H, Leisenring W, Englund J, Corey L, Boeckh M. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol Blood Marrow Transplant. 2011;17:979–986. doi: 10.1016/j.bbmt.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–1074. [PubMed] [Google Scholar]
- 23.Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, Deshpande C, Mollura DJ, Morens DM, Bray M, Travis WD, Taubenberger JK. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell A, Rodin R, Kropp R, Mao Y, Hong Z, Vachon J, Spika J, Pelletier L. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ. 2010;182:349–355. doi: 10.1503/cmaj.091823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee N, Cockram CS, Chan PK, Hui DS, Choi KW, Sung JJ. Antiviral treatment for patients hospitalized with severe influenza infection may affect clinical outcomes. Clin Infect Dis. 2008;46:1323–1324. doi: 10.1086/533477. [DOI] [PubMed] [Google Scholar]


