Abstract
The objectives of this investigation were to analyze the clinical patterns, risk groups, prognostic factors, and mortality of infections caused by Aeromonas spp. This was a retrospective study of adult patients with Aeromonas spp. isolates attended at the Hospital del Mar in Barcelona, Spain, between January 2006 and December 2012. Epidemiological data, antimicrobial susceptibility, clinical patterns, underlying illnesses, type of infection, admission to the intensive care unit (ICU), number of episodes, coinfection, antimicrobial therapy, and evolution were analyzed. A total of 221 clinical samples from 204 patients were positive for Aeromonas spp. The mean age of the patients was 67.6 years. The main clinical form of presentation was gastrointestinal (78.4%). Malignancy was the main risk group in 69 (33.8%) patients, and 48 (23.5%) were previously healthy. Twenty-one patients (10.3%) were admitted to the ICU. Infections were acquired in the hospital in 52.5% of the patients, and 28.9% were polymicrobial. The overall mortality (after 1 year of follow-up from the first positive culture) was 26.5%. Univariate analysis identified an association between increased mortality and the following variables: age ≥80 years, hospitalization, admission to the ICU, malignancy, extraintestinal infection, and appropriate antimicrobial therapy. In the multivariate analysis, age ≥80 years [odds ratio (OR), 4.37 [95% confidence interval (CI), 1.68–11.35; p = 0.002]], admission to the ICU (OR, 6.59 [95% CI, 2.17–19.99; p = 0.001]), and malignancy (OR, 3.62 [95% CI, 1.32–9.90; p = 0.012]) were significantly associated with mortality. Aeromonas infections are mainly gastrointestinal. The 1-year follow-up mortality rate was high. Old age (age ≥80 years), admission to the ICU, and malignancy were identified as independent risk factors for mortality.
Keywords: Intensive Care Unit, Inflammatory Bowel Disease, Gastroenteritis, Soft Tissue Infection, Necrotizing Fasciitis
Introduction
Aeromonas spp. are Gram-negative rods, facultatively anaerobic, oxidase-positive, and non-spore-forming. Aeromonads are essentially ubiquitous in the microbial biosphere and they can be isolated from virtually every environmental niche where bacterial ecosystems exist, such as aquatic habitats, fish, foods, domesticated pets, birds, and natural soils [1–3]. The first classifications within the Aeromonas genus have been determined phenotypically (phenospecies), based on growth characteristics and biochemical tests. Nevertheless, there is great difficulty in identifying the different Aeromonas strains on the species level by these characteristics, due to the phenotypical heterogenicity and growing number of known species. One of the biggest steps forward in the taxonomic process has been the introduction and continuous use of genotypical methods (genospecies) [3–6]. With the use of molecular methods, 96% of the clinical isolates are represented by four species: A. caviae (29.9%), A. dhakensis (25.5%), A. veronii (22.0%), and A. hydrophila (18.0%) [7].
Aeromonas genus microorganisms are known as etiologic agents of a wide spectrum of diseases in humans [2–4, 7–10]: gastroenteritis, skin and soft tissue infection, and bacteremia are the most common manifestations. Other reported infections include pneumonia, liver abscesses, peritonitis, meningitis, endocarditis, urinary tract infections, otitis media, eye infections, and osteomyelitis. Infections occur mainly in immunosuppressed hosts (basically patients with cirrhosis, diabetes, or malignancy), but they may also develop in healthy individuals [3]. The role of Aeromonas species in some syndromes, such as bacterial gastroenteritis, is still speculative and the subject of much debate [3, 8, 11]. However, recently, the enteropathogenic role of Aeromonas has been re-evaluated and demonstrated [12].
Most of the data published refer to just a single clinical manifestation associated with Aeromonas spp. [13–16]. Epidemiological studies are very scarce, retrospective, and restricted to a few cases [7, 15]. We present an epidemiological study, undertaken in a single institution over an extended time period, with the aim of analyzing the different clinical presentations, prognostic factors, treatments, and outcomes associated with Aeromonas spp. infection. Patients were followed up for 1 year after the identification of microorganisms.
Materials and methods
Design, background, and samples
This retrospective study was carried out at the Hospital Universitari del Mar in Barcelona, a general hospital located on the Mediterranean coast with 440 beds, which covers a population of ≈450,000 inhabitants. All adult patients (aged over 18 years) with at least one positive culture for Aeromonas spp. during the period between 1st January 2006 and 31st December 2012 were reviewed and investigated. Clinical samples positive for Aeromonas spp. were identified from the Laboratori de Referència de Catalunya database.
Variables
The electronic medical records of all patients were examined. A specific data collection sheet was designed (Access 2010®), on which the following data were recorded: age, sex, clinical group (medical, surgical, or trauma), type of surgery (digestive tract, biliary, others), clinical form of presentation, underlying diseases, immunosuppressive treatment, isolation source, type of infections (nosocomial or community-acquired), intensive care unit (ICU) stay, number of microorganisms in the different samples analyzed, date of hospital admittance, date of hospital discharge, department of origin (outpatient consulting, emergency <48 h, hospital-admitted patient), antibiotic administered (appropriate, inappropriate, not administered), antimicrobial susceptibility tests, and clinical outcome at discharge (survivor or died for any reason). All patients were followed up for 1 year from the first positive culture. Information on mortality in this period, new identification of Aeromonas in case of readmission, and the source of the Aeromonas isolation were extracted from electronic databases.
Definitions
Clinical features of Aeromonas infections were classified into two major groups: (1) gastrointestinal: including Aeromonas spp. in stools and presence of acute diarrhea (acute gastroenteritis: >3 stools/day for less than 14 days) or chronic (chronic gastroenteritis: diarrhea >14 days), with or without associated symptoms (fever, abdominal pain, and/or pathological products); and (2) extraintestinal, with the following patterns, (2a) skin and soft tissue infection: Aeromonas spp. isolation in soft tissue abscesses, ulcers, and purulent exudate in surgical wound with pain, erythema, and edema (cellulitis, ulcers, and wound); (2b) bacteremia: Aeromonas spp. isolated in at least one blood culture; (2c) intraabdominal infection: Aeromonas spp. isolated from bile samples (cholangitis), or peritoneal exudate or intraabdominal abscess pus (peritonitis); and (2d) pulmonary infection: Aeromonas spp. isolation from sputum, tracheobronchial aspirate, or lung biopsy (pneumonia).
All of the patients were included in one comorbidity condition (even if they had more than one underlying illness), stratified as follows: transplant patients >> human immunodeficiency virus (HIV) infection >> hematological malignancy >> solid tumor >> autoimmune disorder >> chronic disease. Patients without underlying illnesses were classified as previously healthy. For statistical purposes, four groups were considered: malignancy, chronic disease, miscellaneous, and previously healthy.
Immunosuppressive treatment was defined as treatment with corticosteroids during the preceding 3 months, chemotherapy, or radiotherapy, or drugs administered to prevent organ rejection.
Patients who did not require hospital admission were defined as outpatients (including patients who visited the outpatient clinic or the emergency department for less than 48 h and discharged home).
Aeromonas spp. infection was defined as nosocomial when a positive culture was obtained 72 h after hospitalization with a clinical picture that motivated the admission different from the one related to the posterior isolation of Aeromonas and as community-acquired when it was identified within 72 h of hospital admission or in outpatients. Infection was judged to be polymicrobial if microorganisms other than Aeromonas spp. were also isolated in the same clinical sample.
Inappropriate antibiotic use was considered as the use of an antimicrobial agent ineffective against Aeromonas spp., while antimicrobial treatment was considered appropriate if it was active in vitro against the Aeromonas strain responsible for the infection and administered for at least 72 h.
Identification of isolates to the species level and antimicrobial susceptibilities
All strains (n = 221) were initially assigned to the genus Aeromonas, as they were resistant to the vibriostatic agent 0/129 (150 μg), Gram-negative, and oxidase-positive; they were facultative anaerobes which produce acid from glucose and mannitol but not from meso-inositol, and by lack of growth in 6.5% NaCl. Each strain was then identified to the species level by conventional methods and further verified by the ID32 GN System (bioMérieux Vitek Inc., Durham, NC, USA) or the Vitek 2 ID-GNB identification card (bioMérieux Inc., Durham, NC, USA). Antimicrobial susceptibilities of the isolates to a battery of antimicrobial agents were determined using the agar dilution method as described by the Clinical and Laboratory Standards Institute (CLSI) criteria [17]. The strains in the intermediate category were classified as resistant.
Statistical analysis
Continuous variables are reported as medians and interquartile range for non-parametric data (Wilcoxon test) or as means ± standard deviation (SD) for variables with normal distribution (Student’s t-test). Categorical variables were compared using the Chi-square test or Fisher’s exact test, where appropriate. To identify factors associated with mortality, we first performed a univariate analyses. Variables with p-values <0.05 were considered as potential risk factors and candidates for the model selection process. Multivariate logistic regression modeling was performed in a stepwise manner to establish independent predictors for mortality at 1 year of follow-up from the first isolation. Statistical significance was defined as p-values <0.05. Data analyses were performed using SPSS® version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
During the study period, a total of 221 clinical samples positive for Aeromonas spp. were identified to the phenospecies level in 204 patients. The number of patients with Aeromonas spp. infection identified every year are presented in Fig. 1. The clinical features of the 204 patients with Aeromonas spp. infection are summarized in Table 1. Gastroenteritis was diagnosed in 160 (78.4%) patients [including 101 (63.1%) patients with acute gastroenteritis and 59 (36.9%) with chronic diarrhea], peritonitis in 20 (9.8%) patients [including nine cases of secondary peritonitis, one case of spontaneous bacterial peritonitis, and nine cases of cholangitis), skin and soft tissue infection in 12 (5.9%) patients (of whom six cases with ulcers in legs and six with surgical or traumatic wound infection), bacteremia in 6 (2.9%) patients, and pneumonia in 6 (2.9%) patients. The patients ranged in age from 18 to 97 years (mean, 67.6 years). Seventy-four patients (36.3%) were aged <65 years old, 71 (34.8%) were between 65 and 79 years old, and 59 (28.9%) were >80 years old; 50.5% of the patients were male. Comorbidity conditions occurred in 76.5% of cases. Chronic disease (59, 28.9%) and solid tumor (58, 28.4%), of whom 33 (47.8%) had metastases, were the most frequent. Forty-eight (23.5%) were healthy. The complete list of 252 underlying illnesses (1.5% underlying illness per patient with comorbidities) is displayed in Table 2. Fifty-four (26.5%) were receiving immunosuppressive treatment.
Fig 1.
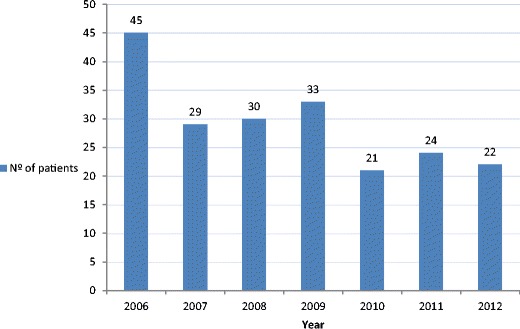
Annual distribution of Aeromonas spp. isolated from clinical samples recovered from 204 patients
Table 1.
Clinical features of 204 patients with Aeromonas spp. infections
| Extraintestinal group | |||||
|---|---|---|---|---|---|
| Variables (total no.) | Gastroenteritis, n = 160 | Intraabdominal, n = 20 | Skin and soft tissue, n = 12 | Lung, n = 6 | Bacteremia, n = 6 |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Gender | |||||
| Female (101) | 84 (52.5) | 6 (30.0) | 5 (41.7) | 2 (33.3) | 4 (66.7) |
| Male (103) | 76 (47.5) | 14 (70.0) | 7 (58.3) | 4 (66.7) | 2 (33.3) |
| Age group, years | |||||
| <65 (74) | 59 (36.9) | 4 (20.0) | 6 (50.0) | 3 (50.0) | 2 (33.3) |
| 65–79 (71) | 54 (33.8) | 9 (45.0) | 5 (41.7) | 3 (50.0) | 0 |
| ≥80 (59) | 47 (29.4) | 7 (35.0) | 1 (8.3) | 0 | 4 (66.7) |
| Comorbidity conditions | |||||
| Solid organ transplant (10) | 9 (5.6) | 0 | 1 (8.3) | 0 | 0 |
| HIV infection (8) | 6 (3.8) | 0 | 1 (8.3) | 0 | 1 (16.7) |
| Hematological malignancy (11) | 10 (6.3) | 0 | 1 (8.3) | 0 | 0 |
| Solid tumor (58) | 41 (25.6) | 11 (55.0) | 2 (16.7) | 3 (50.0) | 1 (16.7) |
| Autoimmune disorder (10) | 10 (6.3) | 0 | 0 | 0 | 0 |
| Chronic disease (59) | 48 (30.0) | 4 (20.0) | 2 (16.7) | 3 (50.0) | 2 (33.3) |
| Healthy (48) | 36 (22.5) | 5 (25.0) | 5 (41.7) | 0 | 2 (33.3) |
| Immunosuppressant drugs | |||||
| Yes (54) | 43 (26.9) | 3 (15.0) | 4 (33.3) | 3 (50.0) | 1 (16.7) |
| No (150) | 117 (73.1) | 17 (85.0) | 8 (66.7) | 3 (50.0) | 5 (83.3) |
| Type of patient | |||||
| Outpatient (56) | 49 (30.6) | 0 | 6 (50.0) | 1 (16.7) | 0 |
| Hospitalized (148) | 111 (69.4) | 20 (100.0) | 6 (50.0) | 5 (83.3) | 6 (100.0) |
| Medical (152) | 134 (83.8) | 3 (15.0) | 6 (50.0) | 4 (66.7) | 5 (83.3) |
| Traumatic (6) | 3 (1.9) | 0 | 3 (25.00) | 0 | 0 |
| Surgical (46) | 23 (14.4) | 17 (85.0) | 3 (25.0) | 2 (33.3) | 1 (16.7) |
| Type of surgery | |||||
| Digestive tract (23) | 12 (7.5) | 9 (45.0) | 1 (8.3) | 1 (16.7) | 0 |
| Hepatobiliary (13) | 3 (1.9) | 8 (40.0) | 1 (8.3) | 0 | 1 (16.7) |
| Others (10) | 8 (5.0) | 0 | 1 (8.3) | 1 (16.7) | 0 |
| ICU admission | |||||
| Yes (21) | 8 (5.0) | 6 (30.0) | 2 (16.7) | 3 (50.0) | 2 (33.3) |
| No (183) | 152 (95.0) | 14 (70.0) | 10 (83.3) | 3 (50.0) | 4 (66.7) |
| Number of samples with positive culture | |||||
| 1 (190) | 154 (96.3) | 17 (85.0) | 9 (75.0) | 6 (100.0) | 4 (66.7) |
| >1 (14) | 6 (3.8) | 3 (15.0) | 3 (25.0) | 0 | 2 (33.3) |
| Monomicrobial vs. polymicrobial | |||||
| Monomicrobial (145) | 137 (85.6) | 2 (10.0) | 3 (25.0) | 0 | 3 (50.0) |
| Polymicrobial (59) | 23 (14.4)a | 18 (90.0)b | 9 (75.0)c | 6 (100.0)d | 3 (50.0)e |
| Acquisition | |||||
| Nosocomial (107) | 87 (54.4) | 11 (55.0) | 5 (41.7) | 2 (33.3) | 2 (33.3) |
| Community (97) | 73 (45.6) | 9 (45.0) | 7 (58.3) | 4 (66.7) | 4 (66.7) |
| Antimicrobial treatment | |||||
| Appropriate (76) | 48 (30.0) | 14 (70.0) | 4 (33.3) | 6 (100.0) | 4 (66.7) |
| Not appropriate (44) | 33 (20.6) | 4 (20.0) | 5 (41.7) | 0 | 2 (33.3) |
| None (84) | 79 (49.4) | 2 (10.0) | 3 (25.0) | 0 | 0 |
| Outcome | |||||
| Survival (150) | 123 (76.9) | 13 (65.0) | 11 (91.7) | 1 (16.7) | 2 (33.3) |
| Mortality (54) | 37 (23.1) | 7 (35.0) | 1 (8.3) | 5 (83.3) | 4 (66.7) |
| During hospitalization (24) | 11 (6.9) | 4 (20.0) | 1 (8.3) | 5 (83.3) | 3 (50.0) |
| During follow-up (30) | 26 (16.3) | 3 (15.0) | 0 | 0 | 1 (16.7) |
ICU intensive care unit
a Clostridium difficile (n = 11), Campylobacter jejuni (n = 7), Blastocystis hominis (n = 3), Salmonella spp. (n = 2), C. botulinum (n = 1), Enterococcus casseliflavus (n = 1), Escherichia coli (n = 1)
b E. coli (n = 6), Klebsiella spp. (n = 4), Bacteroides fragilis (n = 3), Streptococcus viridans (n = 3), E. casseliflavus (n = 2), E. faecium (n = 2), other (n = 7)
c B. fragilis (n = 3), Enterobacter cloacae (n = 2), E. coli (n = 2), Pseudomonas aeruginosa (n = 2), other (n = 5)
d Candida spp. (n = 3), Pseudomonas spp. (n = 3), other (n = 5)
e E. coli (n = 2), E. faecium (n = 1), S. bovis (n = 1)
Table 2.
Underlying illnesses observed among 156 patients with Aeromonas infection (previously healthy patients excluded)
| Illness | No. of patientsa |
|---|---|
| Diabetes mellitus | 40 |
| Liver cirrhosis | 30 |
| Heart failure | 27 |
| COPD | 15 |
| End-stage renal disease | 15 |
| Autoimmune diseasesb | 11 |
| Solid organ transplant | 10 |
| HIV infection | 8 |
| Asthma | 5 |
| Solid malignancies | |
| Colonic | 16 |
| Gastric | 8 |
| Hepatobiliary | 7 |
| Other digestive tract malignancies | 9 |
| Gynecological malignancies | 6 |
| Urinary bladder malignancies | 7 |
| Other urological malignancies | 6 |
| Breast cancer | 6 |
| Lung cancer | 6 |
| Other solid malignancies | 4 |
| Hematological malignancies | |
| Chronic lymphatic leukemia | 5 |
| Other hematological malignanciesc | 9 |
| Miscellaneousd | 2 |
COPD Chronic obstructive pulmonary disease; HIV human immunodeficiency virus
aMost patients had multiple underlying illnesses
bRheumatoid arthritis: 4; Crohn’s disease: 4; ulcerative colitis: 3
cNon-Hodgkin lymphoma: 3; Hodgkin lymphoma: 1; acute myelomonocytic leukemia: 1; primary myelofibrosis: 1; multiple myeloma: 3
dSarcoidosis: 1; amyloidosis: 1
Regarding the distribution according to reason for admission, 152 (74.5%) patients were included in the medical group, 46 (22.5%) in the surgical group (digestive tract surgery in 23 and biliary tract in 13 patients), and 6 (2.9%) in the trauma group. Fifty-six patients (27.5%) were seen in the outpatient clinic or in the emergency department, while 148 (72.5%) were admitted to hospital with a median length of stay of 12 days (range 8–21). Among those 148 patients hospitalized, 21 (14.2%) required ICU admission.
Fourteen patients (6.9%) showed more than one positive culture for Aeromonas spp., with a total of 17 samples and nine different sources (in seven patients, Aeromonas spp. was recovered from different combinations of sources: bile + sputum; stool + urethral exudate; blood + bile + peritoneal exudate; blood + central venous catheter; stool + peritoneal exudate; abscess + surgical wound; and peritoneal exudate + surgical wound + abscess). In the remaining seven patients, Aeromonas was recovered sequentially from one source (stools in four patients with eight positive cultures, ulcers in two patients with five positive cultures, and one patient with two positive cultures from exudate of surgical wound). At least one blood culture was performed in 38 (23.7%) patients out of 160 patients with gastroenteritis and a positive fecal culture for Aeromonas spp. Of these, five (13.2%) patients developed bacteremia, for Enterococcus faecalis in two cases, Salmonella enterica typhimurium in two cases, and Staphylococcus aureus in one case. No patient developed bacteremia by Aeromonas spp. among patients with gastroenteritis. The infection was monomicrobial in 145 cases (71.1%) and polymicrobial in 59 patients (28.9%), mainly enteropathogens. Coinfection with more than one enteric pathogen occurred in 23 (14.4%) patients with gastroenteritis. The predominant microorganisms species found from stool cultures were Clostridium difficile (in 11 cases) and protozoa (in ten cases). The infection by Aeromonas spp. was considered community-acquired in 97 patients (47.5%) and nosocomial in 107 (52.5%). In 128 patients (62.7%), Aeromonas spp. was considered to be non-treated. Forty-four patients (21.6%) received treatment that was considered inappropriate, and 84 patients (41.2%) did not receive antibiotic treatment; in 76 patients (37.3%), the treatment was appropriate.
The results of in vitro susceptibility testing of clinical Aeromonas isolates against various antimicrobial agents are shown in Table 3. The most active agents against Aeromonas, with sensitivity rates exceeding 90%, were amikacin, aztreonam, cefepime, cefotaxime, ceftazidime, gentamicin, and tobramycin. Aeromonas showed 100% sensitivity to tigecycline. Aeromonas showed a high frequency of resistance to two classes of antimicrobial agents, including amoxicillin/clavulanic acid and ampicillin (each with a resistance rate exceeding 90%). Aeromonas had susceptibility rates ranging between 72.5 and 76% to ciprofloxacin, imipenem, and piperacillin + tazobactam.
Table 3.
In vitro susceptibilities of 221 clinical isolates of Aeromonas species
| Antimicrobial agent | No. of isolates | Susceptible, n (%) | Resistant, n (%) | Breakpoints | |
|---|---|---|---|---|---|
| S | R | ||||
| Amikacin | 207 | 203 (98.1) | 4 (1.9) | ≤8 | >16 |
| Co-amoxiclav | 162 | 10 (6.2) | 152 (93.8) | ≤4/2 | ≥8/2 |
| Ampicillin | 162 | 2 (1.2) | 160 (98.8) | ≤8 | ≥32 |
| Aztreonam | 159 | 156 (98.1) | 4 (1.9) | ≤8 | ≥32 |
| Cefepime | 208 | 203 (97.6) | 5 (2.4) | ≤8 | ≥32 |
| Cefotaxime | 187 | 182 (97.3) | 5 (2.7) | ≤8 | ≥32 |
| Cefoxitin | 65 | 40 (61.5) | 25 (38.5) | ≤8 | ≥32 |
| Ceftazidime | 201 | 194 (96.5) | 7 (3.5) | ≤8 | ≥32 |
| Cefuroxime | 63 | 51 (81) | 12 (19.0) | ≤8 | ≥32 |
| Ciprofloxacin | 211 | 160 (75.8) | 51 (24.2) | ≤1 | ≥4 |
| Cotrimoxazole | 174 | 146 (83.9) | 28 (16.1) | ≤2/38 | >4/76 |
| Gentamicin | 205 | 192 (93.7) | 22 (10.7) | ≤2 | >4 |
| Imipenem | 25 | 19 (76) | 7 (28.0) | ≤2 | >8 |
| Nalidixic acid | 38 | 23 (60.5) | 15 (39.5) | ≤16 | ≥32 |
| Piperacillin | 119 | 96 (80.7) | 32 (26.9) | ≤16 | ≥128 |
| Piperacillin + tazobactam | 109 | 79 (72.5) | 21 (27.5) | <16/4 | ≥128/4 |
| Tigecycline | 42 | 42 (100) | 0 | ≤1 | >2 |
| Tobramycin | 93 | 85 (91.4) | 8 (8.6) | ≤2 | >4 |
In-hospital and 1-year follow-up mortalities were 11.7% (24/204 patients) and 14.7% (30/204 patients), respectively, with an overall mortality of 26.5% (54/204 patients). Mortality during follow-up among patients with gastroenteritis was 2.5 times higher than in patients who died during hospitalization (16.3% vs. 6.9%). The highest mortality occurred among patients with lung infection (83.3%), all of them during hospitalization, and bacteremia (66.7%). Mortality of intraabdominal Aeromonas infection was 35% (20% during hospitalization and 15% during 1-year follow-up).
Univariate analysis was performed to identify variables associated with mortality. Age, age group, in-hospital patient, ICU stay, extraintestinal presentation, malignancy, and appropriate antimicrobial treatment were significantly associated with an increased mortality compared with survivors (Table 4).
Table 4.
Univariate analysis of mortality and associated risk factors during the 1-year follow-up period in 204 patients with Aeromonas spp. infection
| Variable | Survivors, n = 150 (%) | Dead, n = 54 (%) | p-Value |
|---|---|---|---|
| Gender | |||
| Female | 78 (77.2) | 23 (22.8) | N.S. |
| Male | 72 (69.9) | 31 (30.1) | |
| Age group (years) | |||
| <65 | 61 (82.4) | 13 (17.6) | 0.02 |
| 65–79 | 53 (74.6) | 18 (25.4) | |
| ≥80 | 36 (61.0) | 23 (39.0) | |
| Comorbidity condition | |||
| Solid organ transplant | 9 (90.0) | 1 (10.0) | N.S. |
| HIV infection | 6 (75.0) | 2 (25.0) | |
| Hematological malignancy | 8 (72.7) | 3(27.3) | |
| Solid tumor | 35 (60.3) | 23 (39.7) | |
| Autoimmune disorder | 9 (90.0) | 1 (10.0) | |
| Chronic disease | 43 (72.9) | 16 (27.1) | |
| Previously healthy | 40 (83.3) | 8 (16.7) | |
| Comorbidity condition (regrouped) | |||
| Previously healthy | 40 (83.3) | 8 (16.7) | 0.03 |
| Malignancya | 43 (62.3) | 26 (37.7) | |
| Chronic disease | 43 (72.9) | 16 (27.1) | |
| Miscellaneousb | 24 (85.7) | 4 (14.3) | |
| Immunosuppressant drugs | |||
| No | 114 (76.0) | 36 (24.0) | N.S. |
| Yes | 36 (66.7) | 18 (33.3) | |
| Type of patient | |||
| Medical | 113 (74.3) | 39 (25.7) | N.S. |
| Surgical | 32 (69.6) | 14 (30.4) | |
| Traumatic | 5 (83.3) | 1 (16.7) | |
| Clinical presentation | |||
| Bacteremia | 2 (33.3) | 4 (66.7) | 0.001 |
| Gastroenteritis | 123 (76.9) | 37 (23.1) | |
| Intraabdominal infection | 13 (65.0) | 7 (35.0) | |
| Skin and soft tissue infection | 11 (91.7) | 1 (8.3) | |
| Lung infection | 1 (16.7) | 5 (83.3) | |
| Others | 27 (61.4) | 17 (38.6) | |
| Hospitalization | |||
| Outpatient | 48 (85.7) | 8 (14.3) | 0.015 |
| Hospitalized | 102 (68.9) | 46 (31.1) | |
| ICU | |||
| No | 142 (77.6) | 41 (22.4) | 0.001 |
| Yes | 8 (38.1) | 13 (61.9) | |
| Multiple episodes | |||
| No | 142 (74.7) | 48 (25.3) | N.S. |
| Yes | 8 (57.1) | 6 (42.9) | |
| Mono vs. poly | |||
| Monomicrobial | 108 (74.5) | 37 (25.5) | N.S. |
| Polymicrobial | 42 (71.2) | 17 (28.8) | |
| Acquisition | |||
| Community-acquired | 73 (75.3) | 24 (24.7) | N.S. |
| Nosocomial | 77 (72.0) | 30 (28.0) | |
| Antimicrobial treatment | |||
| Appropriate | 48 (63.2) | 28 (36.8) | <0.03 |
| Inappropriate | 36 (81.8) | 8 (18.2) | |
| None | 66 (78.6) | 18 (21.4) | |
b Miscellaneous: includes organ solid transplant (n = 10), HIV infection (n = 8), and autoimmune disorder (n = 10)
aMalignancy: includes solid tumors (n = 58) and hematological malignancies (n = 11)
Finally, the multivariate analysis disclosed three independent predictors of 1-year follow-up mortality among patients with infection by Aeromonas spp.: age ≥80 years [odds ratio (OR), 4.37 [95% confidence interval (CI), 1.68–11.35; p = 0.002]), admission to ICU (OR, 6.59 [95% CI, 2.17–19.99; p = 0.001]), and malignancy (OR, 3.62 [95% CI, 1.32–9.90; p = 0.012]) (Table 5).
Table 5.
Logistic regression analysis of independent risk factors related to mortality during the 1-year follow-up period in 204 patients with Aeromonas spp. infection
| Variable | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Low OR | High OR | |||
| <65 years | 1 | |||
| 65–79 years | 1.41 | 0.56 | 3.56 | 0.463 |
| ≥80 years | 4.37 | 1.68 | 11.35 | 0.002 |
| Previously healthy | 1 | |||
| Malignancy | 3.62 | 1.32 | 9.90 | 0.012 |
| Chronic diseases | 2.66 | 0.92 | 7.72 | 0.07 |
| Miscellaneous | 1.75 | 0.40 | 7.59 | 0.45 |
| Appropriate antimicrobial | 2 | 0.96 | 4.16 | 0.064 |
| ICU stay | 6.59 | 2.17 | 19.99 | 0.001 |
Discussion
We studied the features of Aeromonas spp. infection in a series of 204 adult patients; to our knowledge, this is the largest clinical series published so far. In humans, Aeromonas spp. is recognized as an opportunistic pathogen that can affect both immunocompromised and immunocompetent individuals [2–4]. In our series, 80% of patients had at least one underlying disease, with malignancy and chronic disease being the most common. Interestingly, 22% of patients in our study were previously healthy. In our cohort, gastroenteritis was the most prevalent infection. In several reported studies throughout the world, Aeromonas spp. has been isolated at a rate of 0.6–10% from patients with diarrhea, predominantly from infants and children [3, 7, 8, 11, 18]. In Spain, Aeromonas occupied the fourth place among microbiological causes of all gastrointestinal diseases reported each year during the period from 1997 to 2006 [19]. The high incidence of Aeromonas spp. gastroenteritis in our series of adult patients is not in agreement with data from France and countries of south-east Asia, which reported that Aeromonas-associated diarrhea was the third clinical form of presentation, with 19 and 15%, respectively [9, 20, 21]. This variation may be due to the geographical difference in that south-east of Asia is endemic for Aeromonas, but also other factors may explain these differences, such as dietetic habits, processing method adopted for isolation and identification of Aeromonas species, and the ecology of this microorganism [3, 4, 20, 22].
The role of Aeromonas as a gastrointestinal pathogen has been controversial, as 1–4% of asymptomatic individuals carry Aeromonas spp. in their gut [3, 4, 18]. Enterotoxins, cytotoxins, and hemolysins have been studied to elucidate a mechanism by which Aeromonas spp. produce diarrhea, but no convincing causal relationship has been established so far [23]. It is generally assumed that the virulence of Aeromonas is multifactorial and that only a subset of Aeromonas strains can cause gastroenteritis in humans. Moreover, the intestine is a complex ecosystem and in this complex “host-enteric pathogen ecosystem,” a variety of both direct and indirect interactions between enteric pathogens, their host, and the circumstances must be taken into account [12, 24, 25]. In the present series, up to 80% of patients with Aeromonas gastroenteritis suffered from severe comorbidities. Therefore, Aeromonas gastrointestinal tract involvement appears to develop in patients predisposed to bowel infection either due to local immune deficiency or by nutritional factors. Unfortunately, we did not explore the pathogenicity and infectivity at a genetic level to establish the role of Aeromonas as an important enteropathogen. The prevalence of coinfection in our series was 14.4% in patients with gastroenteritis, with C. difficile followed by protozoa as the most common pathogens. Thus, it is difficult, in mixed infections, to assess the true contribution of Aeromonas to disease. Bloodstream invasion in patients with gastroenteritis is extremely uncommon and occurs preceding or concurrent with the onset of bacteremia in 9–14% of cases [26]. In our series, no case was recorded, while five patients developed bacteremia in the course of gastroenteritis by other enteropathogens yielded in the same fecal sample. It is possible that Aeromonas requires a huge inoculum into the lumen of the gastrointestinal tract to spread to the bloodstream. The association of Aeromonas with inflammatory bowel disease (IBD) is of particular interest [10]. All of the patients with an autoimmune disorder (most of them with IBD) developed acute gastroenteritis as the clinical presentation of infection by Aeromonas. It is well known that Aeromonas has a variety of implications in patients with IBD, as a trigger of the IBD onset or of a relapse, or Aeromonas can cause gastroenteritis without any effect on the IBD course [10]. Remarkable findings among patients with Aeromonas-associated gastroenteritis in our cohort were that 72.5% had been hospitalized and 26.9% of patients received immunosuppressant drugs. In half of the patients with gastroenteritis, the infection was nosocomial. It’s possible that Aeromonas-associated diarrhea appeared as a consequence of proliferation and mucosal invasion of patients previously colonized, under aggressive therapies, and with certain comorbidity conditions.
Aeromonas has also been implicated as a cause of various extraintestinal manifestations like peritonitis, cholangitis, skin and soft tissue infections, and others [15, 27–30]. Peritonitis caused by Aeromonas spp. is rare and, in our series, was the second cause of infection, together with cholangitis. In south-east Asia, Aeromonas peritonitis is more prevalent, presenting as secondary peritonitis, spontaneous bacterial peritonitis (SBP), or cholangitis. In this geographical area, the higher prevalence of cirrhosis caused by hepatitis B virus and biliary diseases, in association with dietary habits and wet climate combined with the higher frequency of Aeromonas intestinal carriage, could explain the observed difference with western countries and our series [15, 16, 22, 31].
Wound and skin and soft tissue infections caused by Aeromonas have had a very low incidence in our series. By contrast, in Lamy et al.’s study [9], this clinical presentation was the most prevalent. Individuals become infected with Aeromonas, subsequent to trauma and environmental exposure, as this organism has been found in a variety of aquatic environments and soil [14, 32–34]. In our series, these infections mainly occurred in patients with several comorbidities, whereas in the study of Lamy et al. [9], they mainly occurred in healthy patients. It was notable that these infections were predominantly polymicrobial, typically involving other Gram-negative bacilli. Surgical site infection due to Aeromonas spp. is rare. Only 21 cases had been reported up to 2009, and the infections have a probable endogenous source after abdominal or pelvic surgery [35]. No case of cellulitis or necrotizing fasciitis was observed in our series.
Pneumonia due to Aeromonas spp. is uncommon [3, 9, 13, 36]. Aeromonas spp. has been reported as a causative pathogen in cases of near-drowning-associated pneumonia [37]. In our ICU, we have attended 21 patients with acute respiratory failure after near-drowning during the period between 2006 and 2012, and no case of pneumonia by Aeromonas spp. was identified (data unpublished). In our series, pneumonia was related to aspiration of vomitus in patients with Aeromonas colonizing their gut [38]. The possibility that the infection was acquired either via the ventilator or as a cross-infection during hospitalization was dismissed because of the absence of a cluster of cases and because the infection was community-acquired. Interestingly, Aeromonas pneumonia was polymicrobial in all of the patients.
The prevalence of bacteremia due to Aeromonas spp. in Spain ranges between 0.3 and 12% [11, 15, 39]. The prevalence in our series was lower (2.94%), as also compared with other series reported in the last several years, both in south-east Asia and among westerners [9, 11, 20, 21, 36, 40, 41]. These epidemiological differences could be a consequence of variations in the ecology of Aeromonas and with differences in the fecal carriage rate of Aeromonas spp. in certain parts of south-east Asia that can be as high as 30%, whereas it is only 3% in Europe [42]. One-third of our cases, however, were healthy, and, among these, there was a predominance of very elderly patients. It has been suggested that contaminated lines, such as catheters, can also serve as a portal of entry to seed blood-borne infection [41]. In our series, only in one patient was Aeromonas bacteremia catheter-related.
The antibiotic susceptibility patterns of the clinical isolates in this study were similar to those reported previously [4, 43, 44]. Most of the isolates were not susceptible to ampicillin or co-amoxiclav, in agreement with what should be expected because this is a phenotypic characteristic of the genus Aeromonas, where only one species, A. trota, shows susceptibility and occasionally some other strains [43, 45]. More than 90% of the Aeromonas isolates demonstrated a high level of susceptibility to third- or fourth-generation cephalosporins, aminoglycosides, aztreonam, and tigecycline, indicating that these antibiotics would be the first choice for the empiric treatment of infections by Aeromonas. A quarter of strains were found to be resistant to imipenem, ciprofloxacin, and piperacillin + tazobactam, antibiotics widely used in the treatment of severe infections by Gram-negative bacteria. Multidrug-resistant isolates of Aeromonas spp. are of major concern. Recently, a study of different human and environmental sources indicated that Aeromonas is developing a high level of resistance to aminoglycosides, carbapenems, and third-generation cephalosporins [46].
Fatality rates range from 25 to 46% in cases of bacteremia and are higher than 50% among patients with pneumonia [2–4, 9, 13, 20–22, 31, 36, 47]. All of the patients with pneumonia died during hospitalization despite having been treated with appropriate antibiotics and 50% patients were admitted to the ICU. The high mortality may be a consequence of the development of acute respiratory distress syndrome (ARDS). A case of ARDS related to Aeromonas pneumonia successfully treated with antibiotics and extracorporeal membrane oxygenation (ECMO) has been recently reported [48]. Although about 30% of patients with Aeromonas peritonitis required treatment in the ICU, the in-hospital mortality for this condition was 20%. This relatively favorable outcome may be due to the fact that most of the patients received immediate drainage in addition to appropriate antibiotic therapy. The low mortality among patients with wound and skin and soft tissue infections was due to the absence of cases with fulminant cellulitis and necrotizing fasciitis [14, 49].
Multivariate analysis revealed three independent factors influencing mortality in patients with Aeromonas spp. infection. Age older than 80 years, ICU admission, and neoplastic disease were independent risk factors predicting unfavorable outcomes in patients with Aeromonas infection. Half of the patients over 80 years old died during the follow-up period after the isolation of Aeromonas due to conditions that were non-related to infection. Contaminated food is one of the transmission routes of Aeromonas spp. and the elderly are particularly susceptible to foodborne illness compared with healthy adults, many of whom are affected by chronic illness or advanced cancer [50].
The present study has some limitations. Firstly, all the isolates were obtained from a medical center and, therefore, the interpretation of our results may not be generalized to other areas. Secondly, this is a retrospective work based on phenotypic methods, which are less accurate than genotypic methods for precise Aeromonas identification. During the study period, there have been several shifts in the taxonomy of the genus Aeromonas, in particular, the A. hydrophila subspecies dhakensis [51, 52]. Nowadays, only molecular characterization using the sequences of housekeeping genes like rpoD or gyrB enable the proper identification of the Aeromonas spp. Another limitation is that the pathogenicity of Aeromonas in some cases might be debated because analyses of toxin production were not performed. Nevertheless, the main strength of this study is that the cohort was generated from the database of a large teaching referral center in a defined time period. Our study highlights that the isolation of Aeromonas spp. from clinical samples in humans is a marker of poor prognosis among those patients aged >80 years, with severe underlying disease, in particular with advanced malignancy, and acute illness requiring ICU admission.
Acknowledgements
The authors wish to thank Marta Gas, Secretary of the Intensive Care Unit, for her help with the database design, and Sergi Mojal, IMIM statistician, for his advice and support with the statistical analysis.
Compliance with ethical standards
Conflict of interest
None declared.
Ethics statement
The study was approved by the Institutional Review Board of Parc de Salut Mar in Barcelona, Spain.
Consent statement
Not applicable given the non-interventional nature of the study, since data were retrospectively collected.
Consent to publish statement
Not applicable.
Accession number to microarray data
Not applicable.
Clinical trial registration number and date
Not applicable.
References
- 1.Senderovich Y, Ken-Dror S, Vainblat I, Blau D, Izhaki I, Halpern M. A molecular study on the prevalence and virulence potential of Aeromonas spp. recovered from patients suffering from diarrhea in Israel. PLoS One. 2012;7:1–6. doi: 10.1371/journal.pone.0030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. Sci World J. 2012;2012:625023. doi: 10.1100/2012/625023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker JL, Shaw JG. Aeromonas spp. clinical microbiology and disease. J Infect. 2011;62:108–118. doi: 10.1016/j.jinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Morris JG, Horneman A (2013) Aeromonas infections. UpToDate, Waltham, MA.. Available online at: http://www.uptodate.com/
- 6.Ormen O, Granum PE, Lassen J, Figueras MJ. Lack of agreement between biochemical and genetic identification of Aeromonas spp. APMIS. 2005;113:203–207. doi: 10.1111/j.1600-0463.2005.apm1130308.x. [DOI] [PubMed] [Google Scholar]
- 7.Figueras MJ, Beaz-Hidalgo R. Aeromonas infections in humans. In: Graf J, editor. Aeromonas. Norfolk: Caister Academic Press; 2015. [Google Scholar]
- 8.von Graevenitz A. The role of Aeromonas in diarrhea: a review. Infection. 2007;35:59–64. doi: 10.1007/s15010-007-6243-4. [DOI] [PubMed] [Google Scholar]
- 9.Lamy B, Kodjo A, colBVH Study Group. Laurent F. Prospective nationwide study of Aeromonas infections in France. J Clin Microbiol. 2009;47:1234–1237. doi: 10.1128/JCM.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lobatón T, Hoffman I, Vermeire S, Ferrante M, Verhaegen J, Van Assche G. Aeromonas species: an opportunistic enteropathogen in patients with inflammatory bowel diseases? A single center cohort study. Inflamm Bowel Dis. 2015;21:71–78. doi: 10.1097/MIB.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 11.Figueras MJ. Clinical relevance of Aeromonas sM503. Rev Med Microbiol. 2005;16:145–153. doi: 10.1097/01.revmedmi.0000184410.98677.8a. [DOI] [Google Scholar]
- 12.Teunis P, Figueras MJ. Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Front Microbiol. 2016;7:1395. doi: 10.3389/fmicb.2016.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao CM, Lai CC, Tsai HY, Wu CJ, Tang HJ, Ko WC, Hsueh PR. Pneumonia caused by Aeromonas species in Taiwan, 2004–2011. Eur J Clin Microbiol Infect Dis. 2013;32:1069–1075. doi: 10.1007/s10096-013-1852-6. [DOI] [PubMed] [Google Scholar]
- 14.Gold WL, Salit IE. Aeromonas hydrophila infections of skin and soft tissue: report of 11 cases and review. Clin Infect Dis. 1993;16:69–74. doi: 10.1093/clinids/16.1.69. [DOI] [PubMed] [Google Scholar]
- 15.Choi JP, Lee SO, Kwon HH, Kwak YG, Choi SH, Lim SK, Kim MN, Jeong JY, Choi SH, Woo JH, Kim YS. Clinical significance of spontaneous Aeromonas bacterial peritonitis in cirrhotic patients: a matched case–control study. Clin Infect Dis. 2008;47:66–72. doi: 10.1086/588665. [DOI] [PubMed] [Google Scholar]
- 16.Clark NM, Chenoweth CE. Aeromonas infection of the hepatobiliary system: report of 15 cases and review of the literature. Clin Infect Dis. 2003;37:506–513. doi: 10.1086/376629. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute (CLSI) (2006) Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI documents M2-A9 and M7-A7. CLSI, Wayne, PA
- 18.Ghenghesh KS, Ahmed SF, El-Khalek RA, Al-Gendy A, Klena J. Aeromonas-associated infections in developing countries. J Infect Dev Ctries. 2008;2:81–98. doi: 10.3855/T2.2.81. [DOI] [PubMed] [Google Scholar]
- 19.Epidemiological Surveillance System (2007) Epidemiological comment on reported diseases and Microbiological Information System, Spain. Year 2006. Boletín Epidemiol Semanal 15:109–114
- 20.Ko WC, Chuang YC. Aeromonas bacteremia: review of 59 episodes. Clin Infect Dis. 1995;20:1298–1304. doi: 10.1093/clinids/20.5.1298. [DOI] [PubMed] [Google Scholar]
- 21.Ko WC, Lee HC, Chuang YC, Liu CC, Wu JJ. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J Infect. 2000;40:267–273. doi: 10.1053/jinf.2000.0654. [DOI] [PubMed] [Google Scholar]
- 22.Rhee JY, Jung DS, Peck KR. Clinical and therapeutic implications of Aeromonas bacteremia: 14 years nation-wide experiences in Korea. Infect Chemother. 2016;48:274–284. doi: 10.3947/ic.2016.48.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chopra AK, Houston CW. Enterotoxins in Aeromonas-associated gastroenteritis. Microbes Infect. 1999;1:1129–1137. doi: 10.1016/S1286-4579(99)00202-6. [DOI] [PubMed] [Google Scholar]
- 24.Denny JE, Powell WL, Schmidt NW. Local and long-distance calling: conversations between the gut microbiota and intra- and extra-gastrointestinal tract infections. Front Cell Infect Microbiol. 2016;6:41. doi: 10.3389/fcimb.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17:173–183. doi: 10.1016/j.micinf.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Llopis F, Grau I, Tubau F, Cisnal M, Pallarés R. Epidemiological and clinical characteristics of bacteraemia caused by Aeromonas spp. as compared with Escherichia coli and Pseudomonas aeruginosa. Scand J Infect Dis. 2004;36:335–341. doi: 10.1080/00365540410020631. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz P, Fernández-Baca V, Peláez T, Sánchez R, Rodríguez-Créixems M, Bouza E. Aeromonas peritonitis. Clin Infect Dis. 1994;18:32–37. doi: 10.1093/clinids/18.1.32. [DOI] [PubMed] [Google Scholar]
- 28.Lin WT, Su SY, Lai CC, Tsai TC, Gau SJ, Chao CM. Peritonitis caused by Aeromonas species at a hospital in southern Taiwan. Intern Med. 2013;52:2517–2521. doi: 10.2169/internalmedicine.52.0180. [DOI] [PubMed] [Google Scholar]
- 29.Chao CM, Lai CC, Tang HJ, Ko WC, Hsueh PR. Biliary tract infections caused by Aeromonas species. Eur J Clin Microbiol Infect Dis. 2013;32:245–251. doi: 10.1007/s10096-012-1736-1. [DOI] [PubMed] [Google Scholar]
- 30.Tena D, González-Praetorius A, Gimeno C, Pérez-Pomata MT, Bisquert J. Infección extraintestinal por Aeromonas spp.: revisión de 38 casos. Enferm Infecc Microbiol Clin. 2007;25:235–241. doi: 10.1157/13100463. [DOI] [PubMed] [Google Scholar]
- 31.Lay CJ, Zhuang HJ, Ho YH, Tsai YS, Wang LS, Tsai CC. Different clinical characteristics between polymicrobial and monomicrobial Aeromonas bacteremia—a study of 216 cases. Intern Med. 2010;49:2415–2421. doi: 10.2169/internalmedicine.49.4117. [DOI] [PubMed] [Google Scholar]
- 32.Tang HJ, Lai CC, Lin HL, Chao CM. Clinical manifestations of bacteremia caused by Aeromonas species in Southern Taiwan. PLoS One. 2014;9:e91642. doi: 10.1371/journal.pone.0091642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semel JD, Trenholme G. Aeromonas hydrophila water-associated traumatic wound infections: a review. J Trauma. 1990;30:324–327. doi: 10.1097/00005373-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Vally H, Whittle A, Cameron S, Dowse GK, Watson T. Outbreak of Aeromonas hydrophila wound infections associated with mud football. Clin Infect Dis. 2004;38:1084–1089. doi: 10.1086/382876. [DOI] [PubMed] [Google Scholar]
- 35.Tena D, Aspíroz C, Figueras MJ, González-Praetorius A, Aldea MJ, Alperí A, Bisquert J. Surgical site infection due to Aeromonas species: report of nine cases and literature review. Scand J Infect Dis. 2009;41:164–170. doi: 10.1080/00365540802660492. [DOI] [PubMed] [Google Scholar]
- 36.Fraisse T, Lechiche C, Sotto A, Lavigne JP. Aeromonas spp. infections: retrospective study in Nîmes University Hospital, 1997–2004. Pathol Biol (Paris) 2008;56:70–76. doi: 10.1016/j.patbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Miyake M, Iga K, Izumi C, Miyagawa A, Kobashi Y, Konishi T. Rapidly progressive pneumonia due to Aeromonas hydrophila shortly after near-drowning. Intern Med. 2000;39:1128–1130. doi: 10.2169/internalmedicine.39.1128. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay C, Bhargava A, Ayyagari A. Aeromonas hydrophila and aspiration pneumonia: a diverse presentation. Yonsei Med J. 2003;44:1087–1090. doi: 10.3349/ymj.2003.44.6.1087. [DOI] [PubMed] [Google Scholar]
- 39.Campo C, Navarro V, Pérez C, Gutiérrez I, Alonso R. Aeromonas spp bacteremia: study of 12 cases and review of the literature. Enferm Infecc Microbiol Clin. 2001;19:161–164. doi: 10.1016/S0213-005X(01)72596-7. [DOI] [PubMed] [Google Scholar]
- 40.Lau SM, Peng MY, Chang FY. Outcomes of Aeromonas bacteremia in patients with different types of underlying disease. J Microbiol Immunol Infect. 2000;33:241–247. [PubMed] [Google Scholar]
- 41.Hsueh PR, Teng LJ, Lee LN, Yang PC, Chen YC, Ho SW, Luh KT. Indwelling device-related and recurrent infections due to Aeromonas species. Clin Infect Dis. 1998;26:651–658. doi: 10.1086/514587. [DOI] [PubMed] [Google Scholar]
- 42.Chan FK, Ching JY, Ling TK, Chung SC, Sung JJ. Aeromonas infection in acute suppurative cholangitis: review of 30 cases. J Infect. 2000;40:69–73. doi: 10.1053/jinf.1999.0594. [DOI] [PubMed] [Google Scholar]
- 43.Aravena-Román M, Inglis TJJ, Henderson B, Riley TV, Chang BJ. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob Agents Chemother. 2011;56:1110–1112. doi: 10.1128/AAC.05387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAuliffe GN, Hennessy J, Baird RW. Relative frequency, characteristics, and antimicrobial susceptibility patterns of Vibrio spp., Aeromonas spp., Chromobacterium violaceum, and Shewanella spp. in the Northern Territory of Australia, 2000–2013. Am J Trop Med Hyg. 2015;92:605–610. doi: 10.4269/ajtmh.14-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carnahan AM, Chakraborty T, Fanning GR, Verma D, Ali A, Janda JM, Joseph SW. Aeromonas trota sp. nov., an ampicillin-susceptible species isolated from clinical specimens. J Clin Microbiol. 1991;29:1206–1210. doi: 10.1128/jcm.29.6.1206-1210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esteve C, Alcaide E, Giménez MJ. Multidrug-resistant (MDR) Aeromonas recovered from the metropolitan area of Valencia (Spain): diseases spectrum and prevalence in the environment. Eur J Clin Microbiol Infect Dis. 2015;34:137–145. doi: 10.1007/s10096-014-2210-z. [DOI] [PubMed] [Google Scholar]
- 47.Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding Panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. 1998;27:332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- 48.Issa N, Napolitano LM. Aeromonas pneumonia in a trauma patient requiring extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: case report and literature review. Surg Infect (Larchmt) 2011;12:241–245. doi: 10.1089/sur.2010.037. [DOI] [PubMed] [Google Scholar]
- 49.Lee CY, Li YY, Huang TW, Huang TY, Hsu WH, Tsai YH, Huang JC, Huang KC. Synchronous multifocal necrotizing fasciitis prognostic factors: a retrospective case series study in a single center. Infection. 2016;44:757–763. doi: 10.1007/s15010-016-0932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lund BM. Microbiological food safety for vulnerable people. Int J Environ Res Public Health. 2015;12:10117–10132. doi: 10.3390/ijerph120810117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen PL, Lamy B, Ko WC. Aeromonas dhakensis, an increasingly recognized human pathogen. Front Microbiol. 2016;7:793. doi: 10.3389/fmicb.2016.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaz-Hidalgo R, Martínez-Murcia A, Figueras MJ. Reclassification of Aeromonas hydrophila subsp. dhakensis Huys et al. 2002 and Aeromonas aquariorum Martínez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst Appl Microbiol. 2013;36:171–176. doi: 10.1016/j.syapm.2012.12.007. [DOI] [PubMed] [Google Scholar]


